1. Introduction
The modification of the
N-substituent in classical opioid-type structures, such as 4,5-epoxymorphinans, morphinans, 6,7-benzomorphans, and 5-phenylmorphans, affects binding, selectivity, potency, and efficacy at opioid receptors. A variety of compounds have been synthesized based on the skeleton of (−)-
cis-
N-normetazocine, and most of the modifications happened in the
N-substituent and/or phenolic hydroxyl group [
1]. As part of ongoing efforts to develop safer analgesics, Pasquinucci et al. synthesized several series of (−)-
cis-
N-normetazocine derivatives by modifying the
N-substituent [
2]. In this context, LP1, a μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligand (
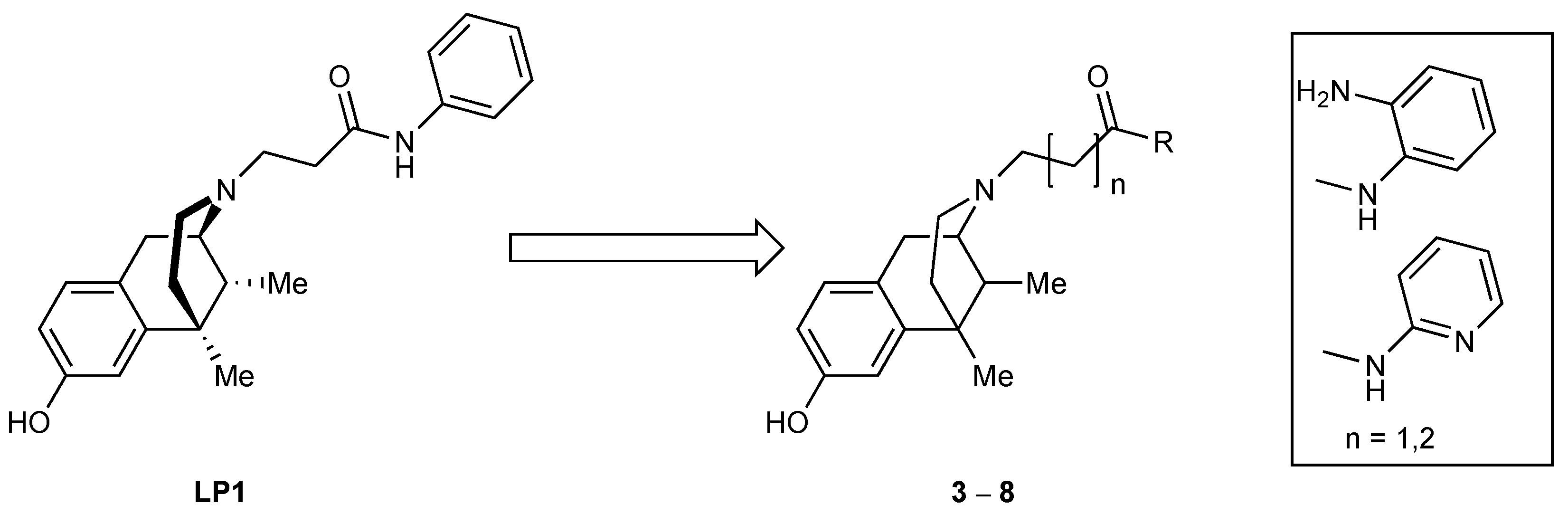
Figure 1) [
3], was found to be able to alleviate nociceptive pain and behavioral signs of persistent pain with low tolerance-inducing capability [
4,
5]. The LP1 ligand is featured by a
cis-(−)-
N-normetazocine skeleton with an
N-phenylpropanamide substituent that was demonstrated to have a crucial role. Building upon the related structure–activity relationship (SAR) information, other chemical series were synthesized in an effort to optimize the physicochemical and biological properties of the lead LP1. Since minor structural modifications often result in significant changes in the pharmacological profile of opioid ligands, we expanded our SARs evaluating the importance of the nature [
3], size [
6], hydrophobic [
7], electronic [
8], and steric [
9] properties of the
N-substituent in the achievement of the proper pharmacological fingerprint. These efforts provided several pharmacologically interesting compounds [
10,
11,
12].
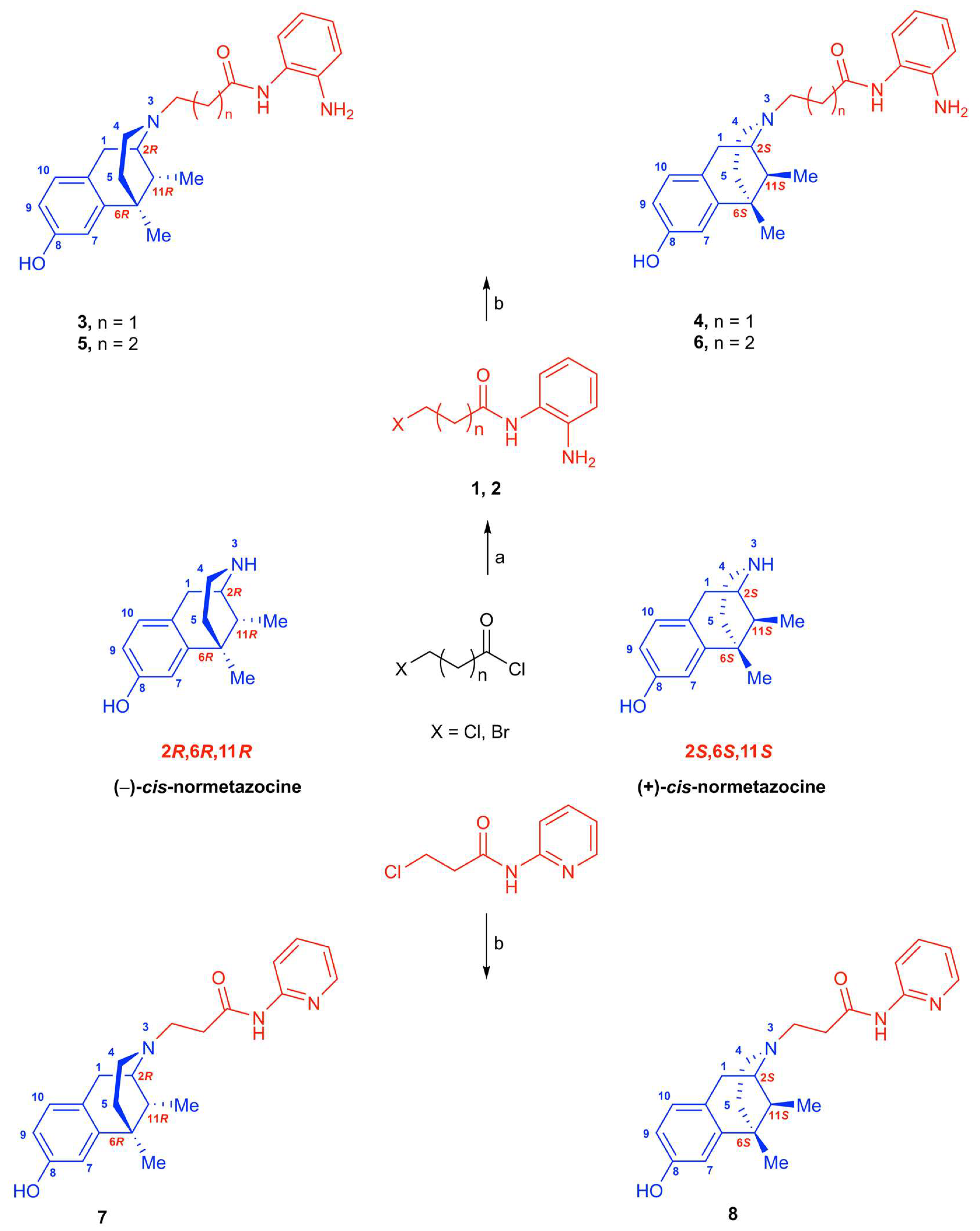
In this paper, we describe a representative strategy to obtain LP1 analogs by targeting the
N-substituent and to expand the SARs of this series of ligands
(Figure 1). The focus was on the key aromatic ring that was replaced by an electron-rich (compounds
3 and
5) or an electron-deficient (compounds
7) analog ring. Moreover, a methylene-extended alkyl linker was further inserted in compound
5. The
N-substituent modifications described were maintained in compounds
4,
6, and
8 with a
cis-(+)-
N-normetazocine skeleton to parallel these latter with levo compounds (
3,
5, and
7, respectively) and to evaluate the stereochemistry impact on the isomer–target interaction.
Here, we report on the in vitro and in vivo evaluations of new synthesized LP1 analogs. In order to investigate the affinity and selectivity of new compounds, competition binding assays on opioid and sigma receptors were performed. Selected compounds with significant inhibitory effects were further screened for their efficacy profile by ex vivo mouse vas deferens (MVD) assay. Finally, the antinociceptive effect of the most promising compound was determined by tail-flick and complete Freund’s adjuvant (CFA)–evoked hyperalgesia assays.
3. Discussion
Opioid analgesics relieve pain by activating opioid receptors (MOR, DOR, and KOR), and among them, MOR mediates the effects of most clinically relevant opioid analgesics. Several efforts have been made to promote the development of potent but safer opioid analgesics by the continuous exploring of the underlying mechanisms of MOR signaling. Among them, developing bifunctional or multifunctional drugs that simultaneously modulate MOR and one or more other targets has attracted attention [
2].
Here, we report on the chemistry and pharmacology of novel
N-normetazocine (benzomorphan)-based compounds, namely, (−)-
cis-
N-normetazocine- or (+)
-cis-
N-normetazocine-based analogs. The (−)-
cis-
N-normetazocine-based analog like LP1 has
N-phenylpropanamido substituent linked to the (−)-
cis-
N-normetazocine scaffold [
3]. The structure of the novel compounds differs from LP1 in the nature of the aromatic ring in
N-substituent, phenyl vs.
o-aminopyridine moiety or
o-phenylenediamine moiety, as well as in the length of the chain connecting
o-phenylenediamine moiety to the (−)-
cis-
N-normetazocine or (+)-
cis-
N-normetazocine (compounds
3–
8). However, the majority of the novel compounds reported here have a propanamido connecting chain (compounds
3,
4,
7, and
8).
In the first part of the work, the synthesis and receptor binding profiles of all of the above-mentioned novel compounds (
3–
8) were thoroughly described. In the receptor binding assay, the results indicated that compounds bearing
o-aminopyridine moiety or
o-phenylenediamine moiety, compounds
7 and
3, respectively, displayed high affinity in the 10 nM range for MOR. Additionally, compound
7 showed the highest affinity for both MOR and KOR. In comparison with LP1, compound
7 showed higher affinity for KOR. On the other hand, LP1 proved to have a higher affinity for both MOR and DOR in the previous studies carried out by our group [
3]. As stated above, LP1 is a (−)-
cis-
N-normetazocine-based compound bearing a phenylpropanamide substituent at the basic nitrogen [
3], whereas compounds
7 and
3 have
o-aminopyridine moiety or
o-phenylenediamine in the
N-substituent. Several previous studies have been intended to address the impacts of chemical modification on the
N-substituent of the normetazocine scaffold on opioid affinity, potency, and receptor selectivity for the three opioid receptor subtypes. In this regard, in a previous work where the primary amide substituent was changed from phenyl (LP1) to cyclohexyl ring, dramatic change in MOR affinity was observed, namely, a 60-fold increase in K
i value (K
i = 56 nM) [
3]. Furthermore, the replacement of phenyl with bulkier rings, such as 1- or 2-naphthyl, quinolines, isoquinoline, indoline, diphenylamine, tetrahydroquinoline, caused a shift in the MOR efficacy profile from agonist to antagonist [
6,
8]. In particular, the 1-naphthyl propanamide substituent at the basic nitrogen resulted in a selective and potent MOR antagonist with a K
i of 38 nM and an antagonist affinity (pA
2) of 8.6 nM [
6].
The
para electron-donating or electron-withdrawing groups’ insertion in the LP1 phenyl ring negatively altered the opioid binding profile in all obtained compounds, especially with respect to LP1 affinity for MOR, highlighting a negative steric hindrance. The LP1 analog with a
p-fluorophenyl in the
N-substituent only shared the functional profile of LP1, although it was less potent [
8]. Analogously, the phenyl ring alkylation (in various ways and positions) revealed that
para-methylation was unfavorable, while
ortho- and
meta-methylation was well tolerated [
9]. Indeed, these later compounds retained the opioid receptor affinity profile of LP1.
Next, in compounds with
N-methyl- or
N-ethyl-
N-phenyl-propanamide chains, the introduction of a secondary amide decreased their MOR affinity as a function of the steric bulk of the alkyl amide substituents (K
i = 65 and 136 nM, respectively) [
3]. Additionally, the introduction of a benzyl pendant at the amidic nitrogen, with and without simultaneous phenyl ring methylation, induced detrimental changes in the opioid binding affinity of all relative compounds, confirming an impediment in receptor interaction [
9].
On the other hand, only the amide functionality substitution with more flexible ethylamino and propylamino
N-substituents allowed all these LP1 analogs to reposition themselves relative to the (−)-
cis-
N-normetazocine nucleus, thus producing a different pharmacological profile at MOR, DOR, and KOR. Their second positive charge at the
N-substituent retained MOR affinity, but increased KOR affinity and dramatically reduced DOR affinity. For instance, the 2-methyl-phenyl-ethylamino LP1 analog displayed a full MOR agonist both in in vitro and in vivo assays [
7].
Shortening the length of the chain between the phenyl ring and the (−)-
cis-
N-normetazocine nucleus resulted in a dramatic loss of affinity for opioid receptors, as shown for the resulting acetamido analogs. On the other hand, the attempts to increase this distance failed to improve the MOR affinity in the
N-benzyl-propanamide analog of LP1 (Ki = 105 nM) [
3].
Likewise, the shorter and flexible
N-substituent in the (
R/
S)-2-methoxy-2-phenyl-ethyl derivative named LP2 maintained the opioid receptors’ affinity of LP1 compound but shifted its efficacy profile at DOR from antagonist to agonist, in comparison with LP1 [
10]. Interestingly, the (2
S)-2-methoxy-2-phenyl-ethyl analog, featured by the same pharmacodynamic profile, was endowed with functional selectivity, thus resulting in a biased MOR/DOR agonist [
11].
Taken together, the binding data of the novel compounds (3–8) reveal that (a) the aromatic ring is required for nanomolar affinity to MOR, although with a slight preference for the electron-deficient ring analog, the o-substituted pyridine; (b) the distance with (−)-cis-N-normetazocine nitrogen is important probably due to a misplacement of the aromatic ring, resulting in the loss of possible interaction; (c) compounds 3 and 7 showed reduced KOR/MOR selectivity with respect to LP1 due to a better KOR affinity profile; and (d) compounds with a (+)-cis-N-normetazocine skeleton are not able to bind σ1R and σ2R, probably due to unfavorable interactions of their N-substituents with the respective binding pockets. In addition, competition binding data corroborated the importance of an aromatic ring and its optimal distance by a (−)-cis-N-normetazocine nucleus for ligand–MOR interaction.
Although both electron-rich and electron-deficient rings bind MOR with similar K
i and profile of selectivity with respect to DOR and KOR, their different electronic properties differently stabilize MOR conformation with a consequently different signaling pathway. The (−)-
cis-
N-normetazocine nucleus, originated from morphine structure simplification, provides a rigid backbone represented by the aromatic ring, the saturated or morphan segment, and the nitrogen substituent identical to that of (−)-morphine. Moreover, as different properties of its
N-substituent affect the achievement of a specific affinity vs. opioid receptor subtypes and determine specific functional profiles [
1], various SARs have been conducted. First, May et al., in a series of
N-alkyl-substituted (−)-
cis-
N-normetazocines, demonstrated the correlation between their affinity values and chain elongation. Moreover, a switch of activity from agonist (
N-methyl) to agonist/antagonist (
N-ethyl, -propyl and -butyl) and back to agonist (
N-pentyl, -hexyl, -heptyl, and -octyl) until inactivity (
N-nonyl and -decyl) was detected [
14]. In a next series of
N-alkenyl-,
N-alkynyl-, and
N-cyanoalkyl-substituted analogs, an optimal chain length was assayed in affinity and potency profiles [
15]. To study the influence of
N-substituent lipophilicity/hydrophilicity in opioid receptor interaction, Metcalf et al. [
16] synthesized different compounds bearing at
N-substituent ester or carboxylic acid groups. Both ester and carboxylic acid derivatives showed K
i values for all opioid receptors in the micromolar range. Moreover, the spacer length of the
N-substituent changed in in vitro activity. In the
N-acid derivatives, the elongation of spacer length reduced activity, while in the N-ester series, it was observed as a switch of activity from antagonist to agonist.
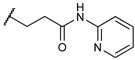
Compound
7 with
o-aminopyridine moiety in the
N-substituent of the (−)-
cis-
N-normetazocine scaffold showed a promising opioid property profile, which encouraged us to further investigate it in MVD and acute thermal and subchronic inflammatory pain assays. Compared with (D-Ala
2, MePhe
4, Gly
5-ol)-enkephalin (DAMGO), a highly selective MOR agonist peptide [
17], compound
7 showed a similar affinity for the MOR in a receptor binding assay. In MVD, compound
7 and DAMGO displayed comparable potency. It is noteworthy that naloxone’s K
e values obtained by the same assay were also comparable, indicating the MOR mediating action. Nonetheless, compound
7 showed a weak KOR- and no measurable DOR-mediated effect. Indeed, MVD hosts all opioid receptor subtypes with the highest reserve for DOR and the lowest for KOR [
18]. Therefore, compound
7 activates both MOR and KOR. Opioid ligands that activate MOR and KOR are used to manage mild to severe acute and chronic pains. Based on the MVD and competition binding assays’ results, the second task in the present study was to investigate the impact of compound
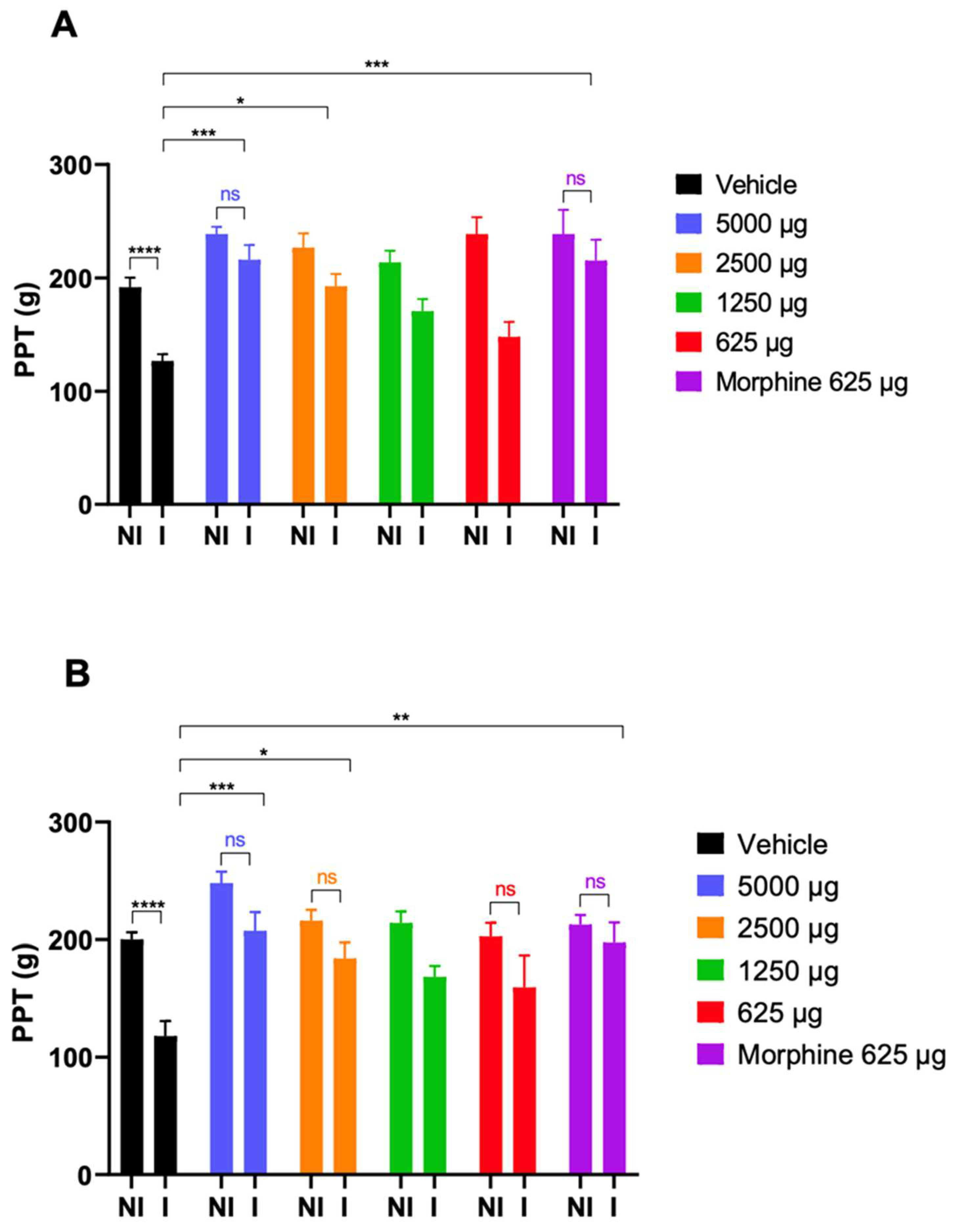
7 on pain evoked by thermal stimulus or complete Freund’s adjuvant (CFA) that models acute thermal or subchronic inflammatory pain, respectively [
19,
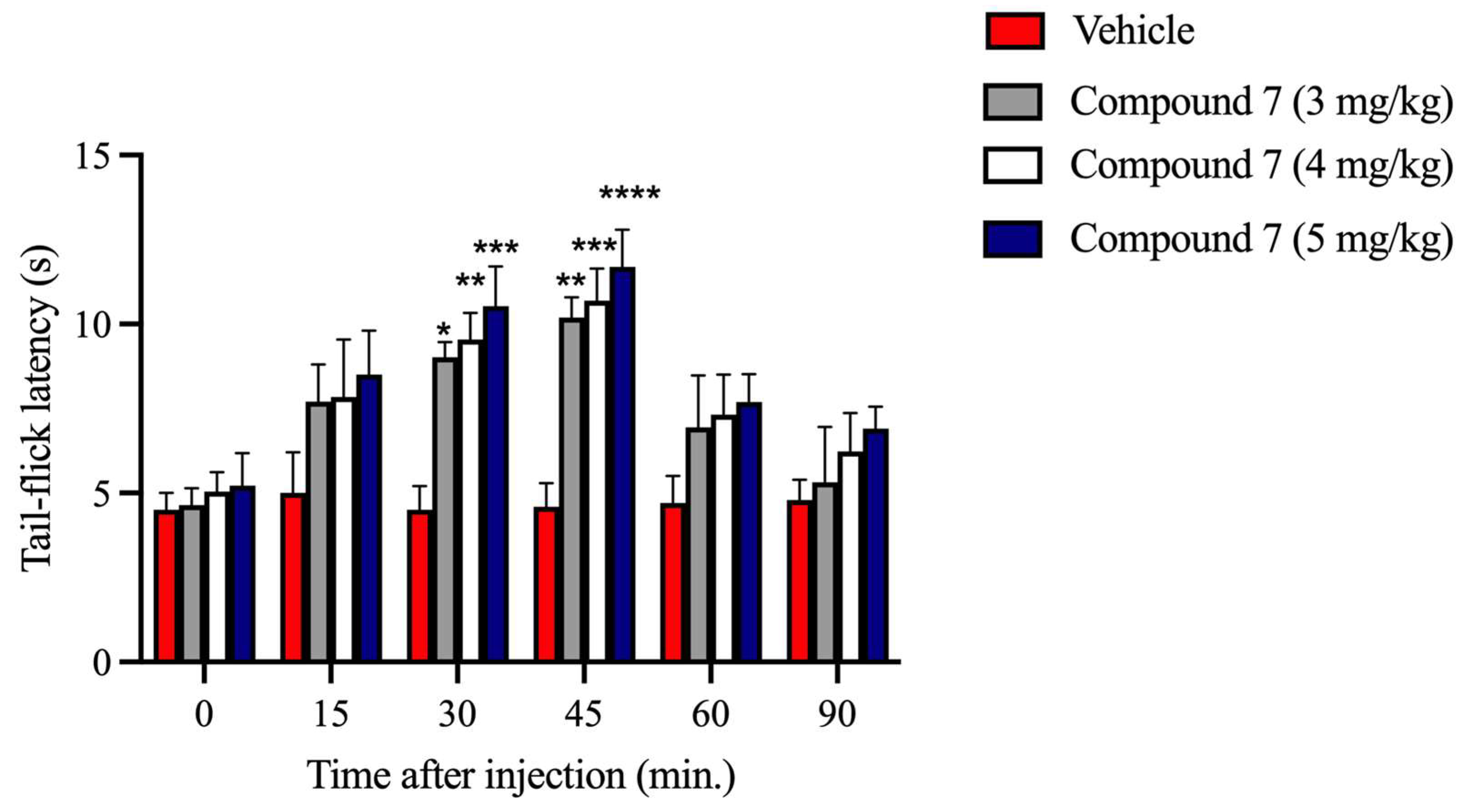
20]. The tail-flick test is one of the well-known pain assays for measuring the antinociceptive effect of opioids. In this pain model, compound
7 produced a dose-dependent antinociceptive effect that peaked at 30–45 min following i.p. injection. Likewise, it significantly attenuated the CFA-evoked hyperalgesia of inflamed paws at 30 and 60 min after s.c. administration. Previous works have identified that LP1, which shares a similar structure with compound
7, is able to address both nociception and behavioral signs of persistent pain conditions with low tolerance-inducing capability [
4,
5]. However, when we consider the receptor profiles of the two compounds, LP1 showed MOR and DOR binding preference, whereas compound
7 showed MOR and KOR binding preference. It means that both activate MOR, which is the main target of the majority of current opioid analgesics used in clinical practice and also causes opioid use disorder, which creates complex social problems that need solutions. One of the strategies to decrease opioid addiction is to decrease addiction liability by developing opioids with lower abuse potential. In this regard, previous works have shown that drugs activating KOR displayed lower abuse liability yet exerted an inverse effect on morphine-induced side effects [
21,
22]. This opioid property is likely hosted by compound
7, and to elucidate it, future studies are needed.
4. Materials and Methods
4.1. General Remarks
Reagent-grade chemicals were purchased as previously described [
23]. Melting points were determined in open capillary tubes with a Büchi 530 apparatus and are uncorrected. Optical rotations were determined in EtOH solution with a PerkinElmer 241 polarimeter.
1H and
13C NMR spectra were recorded at 200 and 500 MHz on Varian Inova spectrometers in CDCl
3 or DMSO-d
6. Chemical shifts δ are expressed in parts per million (ppm) with reference to tetramethylsilane as an internal standard. MALDI mass spectra were acquired in reflector mode by a 4800 MALDI TOF/TOF™ Analyzer (Applied Biosystems, Framingham, MA, USA). The excitation source is an Nd:YAG laser (wavelength of 355 nm) <500 ps pulse and 200 Hz repetition rate and working in positive-ion mode. The mass resolution of MALDI spectra was about 10,000 (full width at half maximum, FWHM), and the mass accuracy was 1–10 ppm for masses in the range
m/
z 200–1000 Da.
Trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenyldene] malononitrile (0.1 mmol in THF) was used as a matrix and mixed with appropriate volumes of samples dissolved in methanol (10 mg/mL concentration) at 1:1, 1:2, and 2:1 ratios (sample/matrix
v/
v). An amount of 1 µL of each sample/matrix mixture was spotted onto the MALDI sample holder and dried at 25 °C to allow matrix crystallization. The structural identification of MALDI peaks was mainly made based on empirical formulas. Elemental analyses (C, H, N) were performed on a Carlo Erba 1106 analyzer, and the results were within ±0.4% of the theoretical values.
4.2. Preparation of Amides 1 and 2
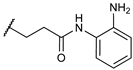
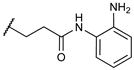
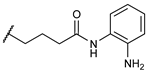
N-(2-aminophenyl)-3-bromopropanamide (1). o-Phenylenediamine (9.24 mmol, 3 eq) was dissolved in CH2Cl2 (10 mL), the solution was cooled at 0 °C under nitrogen atmosphere, and 3-bromopropionyl chloride (3.08 mmol, 1 eq) was added dropwise. After 3 h at RT, the reaction mixture was quenched with H2O and extracted with CH2Cl2. The organic phase was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel using CH2Cl2/EtOAc/EtOH (50:48:2 v/v), as solvents to obtain a white solid. Yield: 49%; Mp: 195–196 °C; TLC CH2Cl2/EtOAc/EtOH (50:48:2 v/v) Rf = 0.46; 1H NMR (200 MHz, CDCl3): δ = 7.25–7.20 (m, 2H); 7.14–7.06 (m, 1H); 6.84–6.77 (m, 1 H); 3.75 (t, 2H, J = 6.2 Hz); 2.99 (t, 2H, J = 6.2 Hz).
N-(2-aminophenyl)-4-chlorobutanamide (2). o-Phenylenediamine (9.24 mmol, 3 eq) was dissolved in CH2Cl2 (10 mL), the solution was cooled at 0 °C under nitrogen atmosphere, and 4-chlorobutyryl chloride (3.08 mmol, 1 eq) was added dropwise. After 3 h at RT, the reaction mixture was quenched with H2O and extracted with CH2Cl2. The organic phase was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel using CH2Cl2/EtOAc/EtOH (50:48:2 v/v), as solvents to obtain a white solid. Yield: 51%; Mp: 192–193 °C; TLC CH2Cl2/EtOAc/EtOH (50:48:2 v/v) Rf = 0.50; 1H NMR (200 MHz, CDCl3): δ = 7.18–7.17 (m, 1H); 7.06–7.03 (m, 1H); 6.79–6.77 (m, 2H), 3.66 (t, 2H, J = 6.0 Hz); 2.56 (t, 2H, J = 6.0 Hz); 2.18 (q, 2H, J = 6.0, 6.0 Hz).
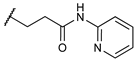
4.3. Preparation of Target Compounds 3–8
cis-(−)-N-normetazocine or cis-(+)-N-normetazocine (1 eq) was dissolved in DMF (5 ml), and the appropriate amide 1–2 or 3-chloro-N-pyridin-2-ylpropanamide (1.5 eq), NaHCO3 (1.5 eq), and a catalytic amount of KI were added. The reaction mixture was stirred for 72 h at 55 °C. After cooling, the reaction mixture was filtered and concentrated under vacuum to remove DMF. The resulting residue was purified by flash chromatography on a silica gel column using CH2Cl2/EtOH (95:5 v/v) as an eluent to obtain a dark solid.
N-(2-aminophenyl)-3-((2
R,6
R,11
R)-8-hydroxy-6,11-dimethyl-1,4,5,6-tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)propanamide (
3). Yield = 36%; Mp: 160–163 °C;
= –60.5° (c 1.004, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.22;
1H NMR (CDCl
3): δ = 10.88 (s, 1H, exchangeable in D
2O); 7.10–7.03 (m, 2H); 6.84–6.79 (m, 3H); 6.71–6.70 (m, 1H) 6.64–6.63 (m, 1H); 3.07–2.78 (m, 6H); 2.72–2.57 (m, 4H); 2.23–2.16 (m, 1H); 1.92–1.78 (m, 1H); 1.31 (t, 3H); 0.85 (d, 3H);
13C (CDCl
3): δ = 171.3; 154.6; 142.1; 140.5; 128.2; 126.9; 126.5; 124.5; 124.3; 118.9; 117.9; 113.0; 112.2; 57.8; 50.4; 44.8; 41.9; 36.2; 32.5; 29.7; 25.8; 24.4; 13.9. Mass:
m/
z calc C
23H
29N
3O
2 379.2 [M + H]
+; found 380.3. Anal (C
23H
29N
3O
2) C, H, N (
Table S1).
N-(2-aminophenyl)-3-((2
S,6
S,11
S)-8-hydroxy-6,11-dimethyl-1,4,5,6-tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)propanamide (
4). Yield = 35%; Mp = 160–163 °C;
= +61.0° (c 1.002, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.22;
1H NMR (CDCl
3): δ = 10.81 (s, 1H, exchangeable in D
2O); 7.03–6.96 (m, 2H); 6.86–6.74 (m, 3H); 6.64–6.63 (m, 1H); 6.57–6.53 (m, 1H); 3.00–2.71 (m, 6H); 2.64–2.50 (m, 4H); 2.15–2.09 (m, 1H); 1.86–1.71 (m, 1H); 1.24 (t, 3H); 0.78 (d, 3H);
13C (CDCl
3): δ = 171.5; 154.7; 142.3; 140.6; 128.4; 127.0; 126.7; 124.7; 124.5; 119.1; 118.1; 113.2; 112.3; 58.0; 51.0; 45.0; 42.0; 36.4; 32.7; 29.9; 26.0; 24.5; 14.1. Mass:
m/
z calc C
23H
29N
3O
2 379.2 [M + H]
+; found 380.2. Anal (C
23H
29N
3O
2) C, H, N (
Table S1).
N-(2-aminophenyl)-4-((2
R,6
R,11
R)-8-hydroxy-6,11-dimethyl-1,4,5,6tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)butanamide (
5). Yield = 38%; Mp = 155–158 °C;
= −58.7° (c 1.001, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.32);
1H NMR (DMSO
d6): δ = 11.18 (s, 1H, exchangeable in D
2O); 6.50–6.41 (m, 4H); 6.13–6.11 (m, 3H); 3.64 (br, 2H); 2.49–2.36 (m, 5H); 1.88–1.80 (m, 3H); 1.52–1.51 (m, 9H); 0.95–0.88 (m, 3H);
13C (DMSO
d6): δ = 169.9; 155.0; 144.6; 140.5; 121.2; 121.1; 121.0; 120.7; 120.4; 118.0;117.6; 112.6; 110.4; 60.1; 47.1; 40.6; 40.2; 39.8; 39.4; 30.8; 29.0; 25.3; 22.12; 10.8. Mass:
m/
z calc C
24H
31N
3O
2 393.2 [M + H]
+; found 394.4. Anal (C
24H
31N
3O
2) C, H, N (
Table S1).
N-(2-aminophenyl)-4-((2
S,6
S,11
S)-8-hydroxy-6,11-dimethyl-1,4,5,6tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)butanamide (
6). Yield = 60%; Mp = 157–160 °C;
= +57.7° (c 1.00, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.30);
1H NMR (DMSO
d6): δ = 11.18 (s, 1H, exchangeable in D
2O); 6.46–6.37 (m, 4H); 6.13–6.09 (m, 3H); 3.64 (br, 2H); 2.56–2.40 (m, 3H); 2.36–2.30 (m, 2H); 1.85–1.81 (m, 3H); 1.51–1.42 (m, 9H); 0.95–0.88 (m, 3H);
13C (DMSO
d6): δ = 170.5; 155.1; 144.6; 140.6; 121.3; 121.2; 121.1; 120.8; 120.5; 118.0;117.9; 11.8; 110.6; 60.2; 47.2; 40.7; 40.2; 39.7; 39.4; 30.8; 29.0; 25.4; 22.2; 10.4. Mass:
m/
z calc C
24H
31N
3O
2 393.2 [M + H]
+; found 394.4. Anal (C
24H
31N
3O
2) C, H, N (
Table S1).
3-((2
R,6
R,11
R)-8-hydroxy-6,11-dimethyl-1,4,5,6-tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)-N-(pyridin-2-yl)propanamide (
7). Yield = 50%; Mp = 205–206 °C;
= −60.4° (c 1.004, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.15;
1H NMR (DMSO-d
6): δ = 10.36 (s, 1H, exchangeable in D
2O); 8.72 (d, 1H); 8.25 (d, 1H); 7.99 (d, 1H); 7.34–7.32 (m, 1H); 6.94 (d, 1H); 6.64 (d, 1H); 6.58 (d, 1H); 3.64–3.41 (m, 6H); 3.05–2.76 (m, 4H); 1.98–183 (m, 2H); 1.28 (s, 3H); 0.78 (d, 3H).
13C (DMSO-d
6): δ = 177.7; 157.0; 152.0; 145.1; 141.5; 136.2; 128.9; 127.0; 124.6; 114.3; 112.7; 110.4; 55.0; 49.6; 46.1; 43.4; 41.9; 40.3; 39.9; 24.2; 22.3; 13.5. Mass:
m/
z calc C
22H
27N
3O
2 365.2 [M + H]
+; found 366.2. Anal (C
22H
27N
3O
2) C, H, N (
Table S1).
3-((2
S,6
S,11
S)-8-hydroxy-6,11-dimethyl-1,4,5,6-tetrahydro-2,6-methanobenzo[d]azocin-3(2H)-yl)-N-(pyridin-2-yl)propanamide (
8). Yield = 40%; Mp = 205–208 °C;
= +59.6° (c 1.003, EtOH); TLC CH
2Cl
2/EtOH (95:5
v/
v) R
f = 0.17;
1H NMR (DMSO-d
6): δ = 10.40 (s, 1H, exchangeable in D
2O); 8.75 (d, 1H); 8.29 (d, 1H); 8.03 (d, 1H); 7.38–7.36 (m, 1H); 6.98 (d, 1H); 6.66 (d, 1H); 6.63 (d, 1H); 3.41–3.25 (m, 6H); 3.09–2.80 (m, 4H); 2.02–1.87 (m, 2H), 1.32 (s, 3H); 0.82 (d, 3H).
13C (DMSO-d
6): δ = 177.4; 156.2; 152.0; 144.7; 141.1; 135.8; 128.5; 126.6; 124.3; 113.9; 112.3; 108.5; 54.6; 50.0; 46.1; 43.0; 39.9; 39.4; 39.1; 23.8; 21.9; 13.1. Mass:
m/
z calc C
22H
27N
3O
2 365.2 [M + H]
+; found 366.2. Anal (C
22H
27N
3O
2) C, H, N (
Table S1).
4.4. MOR, DOR, and KOR Radioligand Binding Assays
The radioligand binding assays and the data analysis were performed as previously reported [
23].
4.5. Radioligand Binding Assays for σ1R and σ2R
The radioligand binding assays and the data analysis were performed as previously reported [
24].
4.6. Isolated Organs: Mouse Vas Deferens (MVD)
Animals. Male NMRI mice (Toxicoop Zrt., Budapest, Hungary) weighing 35–45 g were kept in groups in a temperature- and humidity-controlled room under a 12 h light/dark cycle and with food and water available ad libitum. Each animal was used for one experiment. Experimental procedures were approved by the local ethical committee (IACUC) and conducted in accordance with international guidelines as well as the European Communities Council Directive and National Regulations (CEE Council 86/609 and DL 116/92).
Isolated mouse vas deferens. The preparation of MVD was carried out as previously described by Hughes et al. [
25] with minor modifications. Briefly, vasa deferentia were removed from the mice and unsheathed from the surrounding fat, connective tissue, and blood vessels. They were then suspended between an upper (ring) and a lower (straight) electrode positioned at 5.5 cm, in 5 mL organ baths containing Krebs solution (concentrations in mM: NaCl, 118.0; NaHCO
3, 25.0; KCl, 4.7; KH
2PO4, 1.2; glucose, 11.0; CaCl
2, 2.5; and MgSO
4, 1.2) aerated with a gas mixture of 95% O
2 and 5% CO
2 and at a constant temperature of 36 °C. The upper end of the isolated organ was attached to a transducer using a thread and connected to a computer via an amplifier (PowerLab 4/20, ADInstruments, Castle Hill, NSW, Australia). The resting tension was adjusted to 0.1 g. Electrical field stimulation was applied through trains of 10 Hz with 10 rectangular impulses at a 1 ms pulse width, 9 V/cm (i.e., supramaximal intensity), repeated with 0.1 Hz (Stimulator 88, Grass Medical Instruments, Quincy, MA, USA).
Experimental paradigms. The experimental design of MVD required a 50 min equilibration period under electrical field stimulation, during which Krebs solution was changed every 5 min. After the equilibration period, test compounds were added cumulatively, allowing a minimum of 2 min between doses. To determine the test compounds’ dissociation constant, Ke, vasa deferentia were preincubated with the antagonist naloxone, and without washing a single concentration of the agonist test, the compound was added.
Data analysis. The efficacy of each test compound to inhibit electrically evoked contraction was measured as the percentage change from baseline. The 50% inhibiting concentrations, IC
50, were determined by nonlinear regression (Hill slope, three parameters) of logarithmic dose–response curves using GraphPad Prism 8.01 software (San Diego, CA, USA). Each statistical analysis had a minimum of
n = 8 independent experiments. Data are presented as the mean ± SEM. K
e values were calculated using dose ratio and the single-dose method [
26]. Statistical analysis was performed with one-way ANOVA, followed by the Newman–Keuls post hoc test. Results were considered significantly different when the
p-value was less than 0.05.
4.7. In Vivo Pharmacology
Thermal acute pain model (tail-flick test). Male Swiss CD1 mice (Envigo Laboratories, S. Pietro al Natisone (UD)) weighing 25–30 g were housed at six per cage, kept at a constant room temperature (25 ± 1 °C), under a 12:12 h light-and-dark cycle with food and water ad libitum. Each mouse was used for only one experiment. Experimental procedures were approved by the local ethical committee (IACUC) and conducted in accordance with international guidelines as well as the European Communities Council Directive and National Regulations (CEE Council 86/609 and DL 116/92). The radiant heat tail-flick test consists of irradiation of the lower third of the tail with an infrared source (Ugo Basile, Comerio, Italy) [
27]. The day before the experiment, performed at room temperature (25 ± 1 °C), mice were habituated to the procedure for measuring the nociception threshold. The basal predrug latency was established between 3 and 6 s and was calculated as the average of the first three measurements, which were performed at 5 min intervals. A cutoff latency of 15 s was established to minimize damage to the tail. All tested compounds were dissolved in pyrogen-free isotonic saline (Baxter Healthcare, Deerfield, IL, USA) and DMSO (5%) and were administered to mice i.p. Post-treatment tail-flick latencies (TFLs) were determined at 15, 30, 45, 60, and 90 min after i.p. injection.
Inflammatory pain model (CFA-evoked hyperalgesia). Male Wistar rats (190–250 g) (Toxicoop Zrt., Budapest, Hungary) under brief isoflurane (Willy Rüsch GmbH, Böblingen, Germany) anesthesia received i.pl. injection of 0.15 mL complete Freund’s adjuvant (CFA) (source), a water-in-oil emulsion of killed mycobacterium, into the right hind paw. The experimental procedures were performed as described previously [
28].
Experimental paradigms of CFA-evoked hyperalgesia. The measurement of the effect of the test compound was carried out 4 days following i.pl. CFA injection as described previously [
28]. Briefly, the baseline (pretest compound) of the paw pressure threshold (PPT) of both inflamed and noninflamed paws was determined. Next, the drug or vehicle was s.c. administered at a volume of 5 mL/kg body weight. Then, the response threshold to mechanical pressure stimulation of inflamed and noninflamed paws was given as paw pressure thresholds (PPTs) and measured at 30 and 60 min post administration using an arbitrary cutoff weight of twice the baseline.