Abstract
As part of the valorization of agricultural waste into bioactive compounds, a series of structurally novel oleanolic acid ((3β-hydroxyolean-12-en-28-oic acid, OA-1)-phtalimidines (isoindolinones) conjugates 18a–u bearing 1,2,3-triazole moieties were designed and synthesized by treating an azide 4 previously prepared from OA-1 isolated from olive pomace (Olea europaea L.) with a wide range of propargylated phtalimidines using the Cu(I)-catalyzed click chemistry approach. OA-1 and its newly prepared analogues, 18a–u, were screened in vitro for their antibacterial activity against two Gram-positive bacteria, Staphylococcus aureus and Listeria monocytogenes, and two Gram-negative bacteria, Salmonella thyphimurium and Pseudomonas aeruginosa. Attractive results were obtained, notably against L. monocytogenes. Compounds 18d, 18g, and 18h exhibited the highest antibacterial activity when compared with OA-1 and other compounds in the series against tested pathogenic bacterial strains. A molecular docking study was performed to explore the binding mode of the most active derivatives into the active site of the ABC substrate-binding protein Lmo0181 from L. monocytogenes. Results showed the importance of both hydrogen bonding and hydrophobic interactions with the target protein and are in favor of the experimental data.
1. Introduction
Agricultural waste has emerged as a huge pool of fine chemicals that can be turned into high-value compounds with many pharmaceutical applications [1]. Triterpenic acids, such as Oleanolic Acid (OA-1, Figure 1) extracted from olive pomace [2], are typical examples of such high-value compounds. A number of studies have demonstrated that OA-1 has a wide range of biological activities, including anti-inflammatory [3], hepatoprotective [4], antioxidant [5], antitumor [6,7], anti-HIV [8], antidiabetic [9], and antiparasitic [10] effects. It has also been found that OA-1 and its derivatives have significant antibacterial activity with a wide range of MIC values [11,12,13]. The antibacterial activity of OA-1 is thought to be due to its ability to influence peptidoglycan structure and composition, gene expression, and biofilm formation, thereby preventing bacterial growth [14]. On the other hand, Nitrogen-containing heterocyclic compounds are omnipresent in bioactive molecules and are invaluable sources of therapeutic agents [15,16,17]. Among them, Phtalimidine (1-isoindolinone and C3 1-isoindolinone-derived) derivatives [18] play an important role in drug discovery due to their broad and abundant biological activities [19,20,21].Many of them have been prepared and examined as antihypertensive [22], antipsychotic [23,24,25], anti-inflammatory [26], anesthetic [27], and vasodilatory [28] agents. Antiviral [29,30,31,32], anticancer [33,34,35,36], antimicrobial [37,38], and anxiolytic [39] activities have also been observed in this class of structures (Figure 1). As a result, a lot of study has been completedover the past few decades to synthesize and to explore various therapeutic prospective of this moiety [18,40,41].
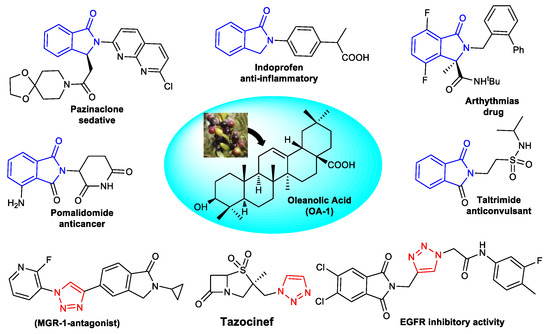
Figure 1.
Oleanolic acid (OA-1) and representative biologically active molecules including Isoindolinone and 1,2,3-Triazole.
Despite its multiple potential applications, OA-1 has not yet been developed as a medicine due to its instability and limited water solubility. To overcome these drawbacks, several studies have been designed to modify the structure of OA-1 with the hope of improving its physical properties to obtain better bioavailability and higher bioactivity. Among the plethora of strategies used, molecular hybridization, which is a new concept in drug design and development based on the combination of two different bioactive compounds to produce a new hybrid substance, has emerged as an essential tool for design and construction of novel hybrid molecules with improved biological activities [42,43,44,45,46,47,48]. Given the rising incidence of multidrug-resistant pathogens caused by the widespread use of antibiotics [49], the intriguing biological activities of isoindolinones and OA-1, and our current research interest in the valorization of agricultural waste into eco-efficient, bioactive products, we present here the synthesis of novel triazole-tethered isoindolinones oleanolic acid hybrids by means of click chemistry-mediated fusion between isoindolinones and oleanolic acid derivatives. To that end, a Cu(I)-catalyzed azide alkyne Huisgen 1,3-cycloaddition was used [50,51,52,53]. Moreover, we hope that the introduction of triazole linkers, known by their broad and abundant biological properties (antiviral [54], antioxidant [55,56], antimicrobial [57], anticancer [58,59], antimalarial [60,61]...) could contribute to the improvement of the overall biological activity of the hybrid molecules [62,63,64,65,66]. The antibacterial activity of OA-1 and the target compounds werescreened, followed by in silico molecular docking studies for the most potent derivatives, in order to obtaina better understanding about the interactions and binding mode in the active sites of the target protein.
2. Results and Discussion
2.1. Chemistry
2.1.1. Isolation of Oleanolic Acid OA-1
OA-1 (Figure 1) was isolated from olive pomace (Olea. europaea L.) cultivar, Chemlali, by using a solid–liquid and ultrasound-assisted extraction strategy, as previously described by us [67]. This method is low-cost, selective, and provides a large amount of OA-1 of around 6.8 g (3.4 mg/g DW)
2.1.2. Synthesis
The new hybrid molecules 18 were designed to include OA-1 on one hand and isoindolinones 6–8 and (±)-12–17 on the other, by connecting them via linker chains of different length. The Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) was chosen as the linking methodology. Starting from the naturally occurring triterpene OA-1, azidoacetyl 4, as the first precursor, was synthesized in three steps according to a previously reported procedure (Scheme 1) [68,69].
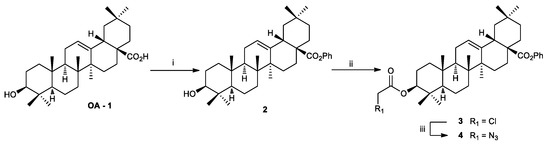
Scheme 1.
Synthesis of the azidoacetyl 4. Reagents and Conditions: (i) K2CO3, BnBr, DMF, rt; (ii) ClCH2COCl, DMAPcat., DCM, 0 °C; and (iii) NaN3, DMF, 75 °C.
The second precursors, namely N/O-propargylated isoindolinones 6–8, were also synthesized using previously published protocols [70,71,72,73,74,75,76]. Substrates 6a–g were prepared through two synthetic routes involving base-assisted N-alkylation of phtalimide 5a, hydantoin 5b, succinimide 5c, and saccharin 5d rings with propargyl bromide resulting in the isolation of the precursors 6a–d in well isolated yields (87 to 97%). Under the same conditions, deprotonation and then alkylation of N-hydroxyphthalimide5g afforded compound 6g in 67% isolated yield. Applied to isoindolinone 5a and successively but-3-yn-1-ol or but-3-yn-2-ol, the mitsunobu reaction [77] (Ph3P/DEAD/THF) provided isoindolinones 6e and 6f in 97% and 57% isolated yields, respectively (Scheme 2). Wishing to study the effect of the presence of a hydroxy and or acetoxy group on the antibacterial activity of our molecules, we subsequently prepared the hydroxylactams 7a and 7e, as well as the acetoxylactam 8g. In this sense, the selective reduction of propargylatedisoindolinones 6a and 6e was performed by using excess of NaBH4 (4 equiv) in a dry MeOH at 0 °C [78] leading to the hydroxylactams 7a and 7e in 95% and 50% isolated yields, respectively (Scheme 2). Using our recently reported one-pot reduction–acetylation protocol, imide 6g afforded the α-acetoxy-lactam 8g in a 98% overall yield (Scheme 2) [79].
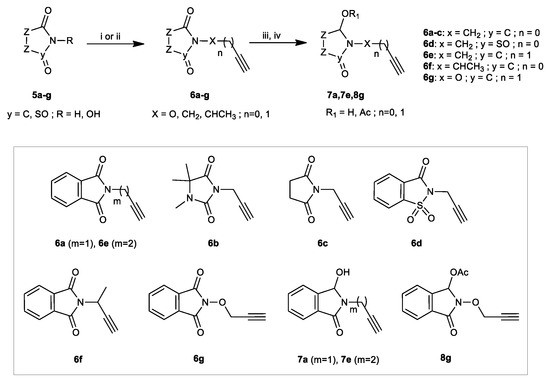
Scheme 2.
Straightforward synthesis of substrates 6-8. Reagents and Conditions for 6a–d and 6g: (i) imides (5a–d, R = H and 5g, R = OH) (1 equiv), NaH or K2CO3 (1.1 equiv), propargyl bromide (1.2 equiv), DMF, reflux; Reagents and Conditions for 6e and 6f: (ii) imide (5a, R = H) (1 equiv), but-3-yn-1-ol or but-3-yn-2-ol (1.2 equiv), Ph3P (1 equiv), DEADcat, THF, rt; Reagents and Conditions for 7a and 7e: (iii) imides 6a and 6e (1 equiv), NaBH4 (4 equiv), MeOH, 0–20 °C. Reagents and Conditions for 8g: (iv) (a) 6g (1 equiv), NaBH4 (4 equiv), MeOH, 0–20 °C; (b) Ac2O (2 equiv), Et3N (2 equiv), DMAPcat, DCM, rt.
Subsequently, phtalimidines(±)-12–17, the other precursors for the Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reaction, were obtained straightforwardly, starting with homophthalic acid (HPA-9), as previously described by our group [80,81,82]. Phtalimidines (±)-12a–f, as starting materials, were obtained by using our well-known 3-step sequence, including (i) esterification of diacid (HPA-9) under reflux in the presence of gaseous HCl, (ii) radical bromination of the obtained diester 10 (e.g., NBS, AIBNcat, CCl4 at reflux), and(iii) condensation of excess of primary amine (α-bromophthalate11, R-NH2, CH3CN, rt). Next, the C3-alkylated derivatives (±)-13 and (±)-14, were prepared by the deprotonation of the α-position of the nitrogen of phtalimidines (±)-12a–f with potassium carbonate, followed by the reaction of various halogenated electrophiles. Under these conditions, substrates (±)-13a–d and (±)-14b–f, were obtained in good to excellent isolated yields 87% to 90% for (±)-13a–d and 81% to 93% for (±)-14b–f (Scheme 3).
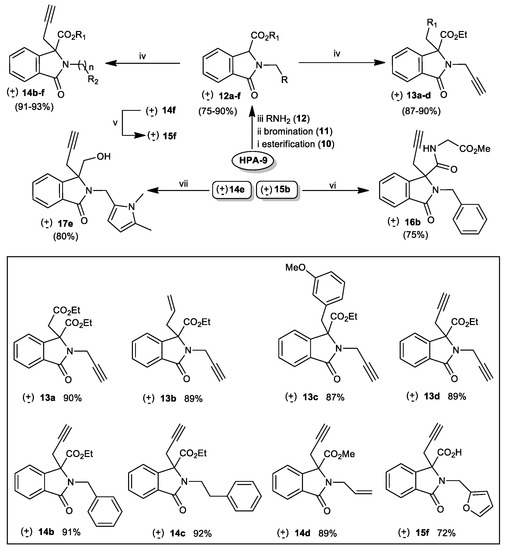
Scheme 3.
Straightforward synthesis of substrates (±)-12–17. Reagents and Conditions for synthesis of phtalimidines (±)-12: (i) homophtalic acid 9, gaseous HCl, reflux; (ii) ethyl homophtalate 10 (1 equiv), NBS (1.5 equiv), AIBNcat, CCl4, 60 °C. (iii) diethyl α-bromophthalate 11 (1 equiv), amine (2 equiv), CH3CN, rt; Reagents and Conditions for synthesis of phtalimidines (±)-13 and (±)-14:(iv) phtalimidines (±)-12a–f (1 equiv),K2CO3, alkylyl bromide (1.2 equiv), CH3CN, reflux; Reagents and Conditions for (±)-15f: (v) phtalimidine (±)-14f (1 equiv), NaOH (2 equiv), EtOH/H2O, rt then aqueous 1M HCl, 0 °C; Reagents and Conditions for (±)-16b: (vi) acid (±)-15b (1 equiv), EDCI (1 equiv), DMF, rt; Reagents and Conditions for (±)-17e: (vii) phtalimidine (±)-14e (1 equiv), LiBH4 (2 equiv), DCM, rt.
In order to evaluate the binding mode, particularly the effect of an hydroxy, amide, or carboxy group at C-3, on the antibacterial activity, acid (±)-15f, amide (±)-16b and alcohol (±)-17e were then prepared. The reduction of the ester group at C-3 of (±)-14e with LiBH4 in dichloromethane at room temperature gave phtalimidine alcohol (±)-17e in 80% isolated yield. Saponification of ester functions of esters (±)-14b and (±)-14f under standars conditions (excess of aqueous NaOH, EtOH, rt then diluted HCl at 0 °C) gave the corresponding carboxylic acids (±)-15b and (±)-15f in, respectively, 93% and 72% isolated yields. In order to obtain amide (±)-16b, acid (±)-15b was treated, in a next step, with methyl glycinate in the presence of EDCI/DMF at room temperature leading to compound (±)-16b in 75% isolated yield (Scheme 3) [83]. With azide 4 and propargylated phthalimidines 6–8 and (±)-12–17 in hand, we next directed our attention to explore the 1,3-dipolar cycloaddition to form the new hybrid molecules 18. Thus, as shown in Scheme 4, treatment of 4 and 6a used as a model for our study under the Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition (e.g., CuSO4.5H2O, sodium ascorbate (NaC6H7O6), DCM/H2O, rt 24 h) [84] resulted in the formation of the 1,2,3-triazole conjugate 18a in 84% isolated yield.
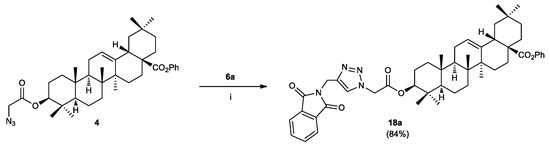
Scheme 4.
Click synthesis of 1,2,3-triazole conjugate 18a. Reagents and Conditions: (i) Phtalimidine 6a (1 equiv), azide 4 (1 equiv), CuSO4.5H2O (0.2 equiv), sodium ascorbate (0.4 equiv), DCM/H2O, rt, 24 h.
The above selected conditions were used with the rest of the substrates 6–8 and (±)-12–17, which cyclized to afford the hybrids molecules 18b–t with yields of 70 to 98% after silica gel column chromatography, and the results are summarized in Scheme 5.
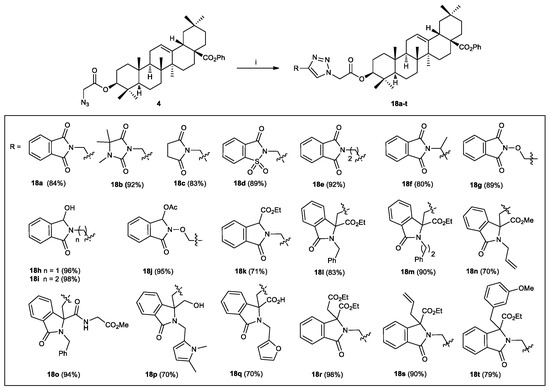
Scheme 5.
Click synthesis of 1,2,3-triazole hybrids 18a-t. Reagents and Conditions: (i) substrates 6–8, (±)-12–17 (1 equiv), azide 4 (1 equiv), CuSO4.5H2O (0.2 equiv), sodium ascorbate (0.4 equiv), DCM/H2O, rt, 24 h.
It is worth noting that in the presence of the C,N-bispropargylatedphtalimidine (±)-13d under standard conditions, the 1,3-dipolar cycloaddition became non-selective and lead to a mixture of non-separable products, including the mono-cycloaddition product on the C-propargylated alkyne, the mono-cycloaddition on the N-propargylated alkyne and the bis cycloadduct 18u. After rigorous investigations, it was observed that the double cycloaddition occurred seamlessly to give 18u in 80% yield when CuSO4.5H2O (0.2 equiv), sodium ascorbate (0.4 equiv), and 2 equivalents of 4 were used (Scheme 6).
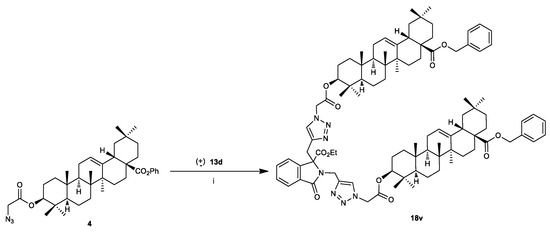
Scheme 6.
Click synthesis of 18u. Reagents and Conditions: (i) diproporgylated substrate (±)-13d, azide 4 (2 equiv), CuSO4.5H2O (0.2 equiv), sodium ascorbate (0.4 equiv), DCM/H2O, rt, 24.
2.2. Antibacterial Activity
All the newly synthesized compounds were evaluated in vitro for their antibacterial activity against the following human pathogen strains, two Gram-positive bacteria, Staphylococcus aureus ATCC 25923 and Listeria monocytogenes ATCC 19115, and two Gram-negative bacteria, Salmonella thyphimurium ATCC 14080 and Pseudomonas aeruginosa ATCC 27853. The activity of all the tested derivatives was compared with that of Tetracycline and Chlorhexidine used as standard reference antibiotics. The determination of the inhibition zone “IZ” (in mm), MIC and MBC (in µM) was carried out in this study. Overall, attractive results were obtained against certain strains. Indeed, the values of the IZ diameters, as a preliminary test, given in Table 1 for certain compounds were found to be in agreement with those of the MIC and MBC describedin Table 2. These results showed that the starting product OA-1 was active against S. aureus, S. thyphimurium, and P. aeruginosa, but inactive towards L. monocytogenes. In certain circumstances, the activity of this compound exceeds that of specific derivatives and reference antibiotics. It was discovered to be more active against S. aureus and P. aeruginosa than all of its derivatives 18a–u, but only slightly more active against S. typhimurium than 18f, 18k, and 18q. The results showed, on the other hand, that most of the tested compounds demonstrated a certain level of selectivity towards L. monocytogenes. For more details, the most interesting results in terms of MIC were noted with the derivatives 18a–h in addition to 18k, 18m, and 18q which showed good inhibitory effects on the growth of L. monocytogenes, compared to that of the reference antibiotics TET (MIC = 576.01 µM) and CHX (MIC = 253.24 µM). Thus, from a structural point of view, the compound 18g exhibits the highest activity (9.48 µM) towards this Gram-positive strain compared to the rest of the active compounds followed by derivatives 18d (9.56 µM) and 18h (9.89 µM). The observed high antibacterial activity of these compounds may be attributed to their sulfonyl and hydroxyl functional groups, as well as specific arrangements within their structures that enhance molecular interactions, binding affinity, or target recognition with this Gram-positive bacterium. It appears that the 3-hydroxyisoindolin-1-one fragment in compound 18h contributes to this activity compared to its analog 18a with an isoindoline-1,3-dione moiety (MIC = 12.4 μM). This finding shows that the additional hydroxyl group in 18h instead of the carbonyl function in 18a may account for the noted difference in activity. On the other hand, the higher activity of compound 18a against L. monocytogenes (Table 2), compared to that of its analog 18e allows to conclude that a single methylene binding the phthalimide to the trizole was better than two methylenes giving, perhaps, to this system freer rotation and, therefore, less possibility of interaction with the target proteins of the bacterium. Additionally, we noticed that when the methylene linker directly bonded to the phthalimide nitrogen atom in 18e (MIC = 39.01 µM and MBC = 2496.67 µM) is replaced by an oxygen atom in 18g (MIC = 9.48 µM and MBC = 155.65 µM), the antibacterial potential towards L. monocytogenes was much improved, hence the importance of this new linker (-O-CH2-) between phatlimide and triazole to better inhibit this strain. In addition, branching of the ethyl linker in 18e was found to significantly improve the MBC of its analog 18f (MBC = 624.16 µM). The additional methyl group in 18f compared to 18a, more than doubled the activity. Moreover, we noticed that compound 18a with an isoindoline-1,3-dione moiety (MIC = 12.4 µM) remains more active against L. monocytogenes than its analogue 18c with pyrrolidine-2,5-dione moiety (MIC = 21.13 µM). This finding serves as evidence that the antibacterial potential is affected by the additional aromatic ring. Interestingly, against P. aeruginosa, compound 18m exhibited the highest antibacterial activity (MIC = 42.79 µM and MBC = 171.18 µM) compared to the other active compound 18h (MIC = 158.41 µM and MBC = 633.66 µM) and also to the reference antibiotics. The results discussed above demonstrated the assumptions about the structural activity relationship (SAR) and also can be supported with some in silico studies.

Table 1.
Antibacterial activity of OA-1 and 18a–u expressed in the Zone of Inhibition (mm).

Table 2.
Antibacterial activity of OA-1 and 18a–u expressed in MIC and MBC (µM).
2.3. Molecular Docking Study
The molecular docking simulations applied to antibacterial agents tested in vitro gives a descriptive explanation of the ligand’s binding mode for the inhibition of the target bacterium [85,86].
The results of the in vitro antibacterial test which showed the sensitivity of L. monocytogenes for the synthesized compounds, prompted us to obtainfurther insight about the inhibitory effect involving the active site. Moreover, previous studies have provided significant information on the function of the cycloalternan pathway (cycloalternane: ligand co-complexed with ABC substrate-binding protein Lmo0181) and revealed the mechanism of regulators of the transcription repressor, open reading frame kinase (ROK). These studies, which have developed a structural overview, allow us to anticipate the role of the cycloalternan (CA) pathway in the metabolism of starch derivatives and prove its involvement in the pathogenesis of the ABC substrate-binding protein from L. monocytogenes. They suggest that CA plays a role in interspecific competition for resources potentially in the host’s gastrointestinal tract and create the methodological framework for characterizing bacterial systems of unknown function [87]. All these data motivated us to choose ‘ABC substrate-binding protein Lmo0181′ as the target receptor of the docking-complex in order to predict the inhibitory effect of compounds docked in the CA’s active site.
In this context, to pick up the mode of action of the tested derivatives for their antibacterial potentials, the molecular docking study has been used to determine the binding modes against ABC substrate-binding protein Lmo0181 from L. monocytogenes (PDB ID: 5F7V). As depicted in Table 3, the listed binding affinities of the formed complex were found to be in the range of −12.3 to −10.6 kcal/mol. Thus, from these results, it can be suggested that all tested compounds interact favorably with the target protein and especially for derivatives 18c, 18d, 18h, and 18k which showed the best binding scores (−12.3 to −11.6 kcal/mol) even better than the docked antibiotic “Tetracycline” (−11.1 kcal/mol) used as a reference in the in vitro test. Interestingly, as Figure 2 and Figure 3A–D show, the binding modes for these compounds demonstrate that each ligand was located inside the binding cavity, similar to the co-crystalized inhibitor. Therefore, the best docking score of compound 18h could be attributed to its correct orientation in the receptor cavity (Figure 3A) and it depends on its structure containing the 3-hydroxyisoindolin-1-one moiety, which could contribute to the stability of the receptor-ligand complex by the formation of three intermolecular conventional hydrogen bonds, two by its hydroxyl group with the residues Asn380 (2.55 Å) and Asp384 (2.12 Å), and another one by its carbonyl function with Thr75 (3.13 Å) amino acid (Figure 4C′). In addition, the stability of the complex is also perceptible through other hydrophobic interactions formed via the hydrocarbon skeleton of the molecule: Alkyl, Pi-Alkyl, and Pi-Pi as depicted in Table 4. The second best score was designated to compound 18d with −12.0 kcal/mol which is also involved in three conventional H-bonds formed through its benzo[d]isothiazol-3(2H)-one 1,1-dioxide fragment with Glu391 (3.27 Å) (3.38 Å) and its ester function with Trp271 (3.15 Å). As docking results of compound 18h, the derivative 18d also displayed some hydrophobic interactions (Figure 4B′). On another hand, besides other types of interactions, the ligand 18c (−11.7 kcal/mol) showed a single hydrogen bond through the carbonyl function of its pyrrolidine-2,5-dione pharmacophore with Trp271 (3.13 Å) (Figure 4A′), while the derivative 18k (−11.6 kcal/mol), besides forming an hydrogen bond with Trp271 (3.25 Å), displayed diverse interactions which additionally explain the stability of the docking complex, as well as its inhibitory effect towards L. monocytogenes (Figure 4D′). Attempt to validate QSAR model by using docking results demonstrated a high degree of correlation in terms of the ability to form hydrogen bonds, to display good binding scores and the antibacterial potentials of our synthesized compounds.

Table 3.
Binding energy of the docked compounds in the binding cavity of ABC substrate-binding protein Lmo0181 from L. monocytogenes.
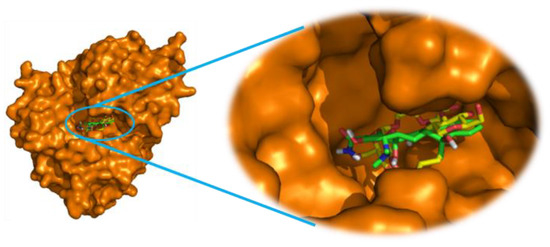
Figure 2.
The 3D binding modes of “Tetracycline” (green color) superimposed with theco-crystalized ligand “Cycloalternan” (yellow color) in the binding cavity of ABC substrate-binding protein Lmo0181 from L. monocytogenes.
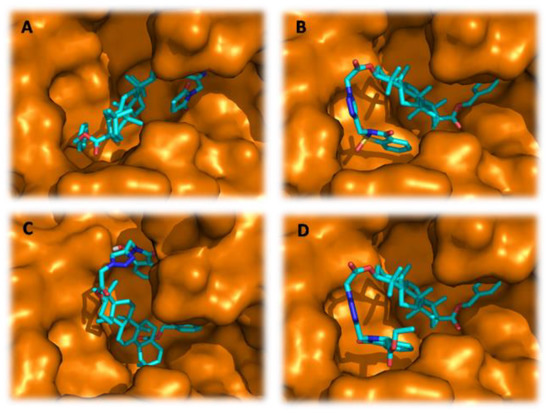
Figure 3.
The 3D binding modes of (A) derivative 18c; (B) derivative 18d; (C) derivative 18h; and (D) derivative 18k within the binding cavity of ABC substrate-binding protein Lmo0181 from L. monocytogenes.
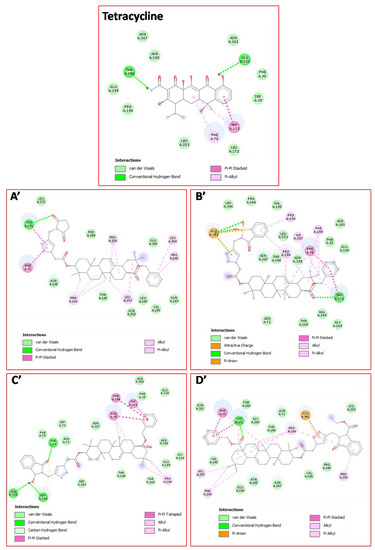
Figure 4.
2D binding modes of “Tetracycline”, (A′) derivative 18c; (B′) derivative 18d; (C′) derivative 18h; and (D′) derivative 18k within the binding cavity of ABC substrate-binding protein Lmo0181 from L. monocytogenes.

Table 4.
Docking results of derivatives with lowest binding energy score and interacting residues the binding cavity of ABC substrate-binding protein Lmo0181 from L. monocytogenes.
3. Materials and Methods
3.1. General Experimental Procedure
Commercially available compounds were used without further purification (suppliers: Thermo Fisher Scientific Inc., and Sigma-Aldrich Co. (Asnières-sur-Seine and Saint-Quentin-Fallavier, France)). All glass apparatus was oven dried and cooled under vacuum before use. Before their usage, precautions were taken to eliminate moisture by refluxing over CaH2 while distilling the solvents (CH3CN, CH2Cl2). Column chromatographies were carried out with a BUCHI Pure Flash/Prep C-850 chromatography system using puriFlash® packed columns. Distilled solvents were employed as eluents for column chromatography in all cases. Thin layer chromatographies (TLC) wererealized on sheets of silica gel 60 precoated with fluorescent indicator UV254 (Merck). Detection was performed by irradiation with a UV lamp and by using an ethanolic solution of p-anisaldehyde. Melting points were measured using a Stuart Scientific SMP10 apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer FT-IR Paragon 1000 spectrometer. 1H NMR and 13C NMR spectra were recorded on a Bruker AvanceIIITM 300 MHz spectrometer at room temperature (rt) with tetramethylsilane (TMS) serving as internal standard. Chemical shifts are expressed in parts per million (δ). Splitting patterns are designed, s, singlet; d, doublet; dd, doublet of doublets; t, triplet; m, multiplet; and br. s, broaden singlet. Employed abbreviations refers to Ph: Phenyl, Trz: Triazole, Hyd: Hydantoin, and Fur: Furane. Coupling constants (J) are reported in Hertz (Hz). Mass spectra (GC-MS) were obtained on a ThermoFinniganAutomass III spectrometer coupled with a gas chromatograph Trace GC 2000. An agilent 6530 Q-Tof MS system was used to conduct the measurement of high-resolution mass spectra (HRMS).
3.2. Chemistry
General Procedure for the Preparation of Compounds 18
CuSO4.5H2O (0.2 equiv.) and sodium ascorbate (0.4 equiv) were added to a mixture of equimolar amounts of azide 4 and propargylated phtalimidines 6–8 and (±)-12–17 in CH2Cl2 (1 mL) and H2O (1 mL). After stirring at room temperature for 24h, the resulting residue was concentrated under vacuum conditions and then extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine, dried using MgSO4, filtered, and concentrated. The residue was then purified by flash column chromatography using a mixture of cyclohexane/EtOAc as eluent.
The structures of all the synthesized compounds 18 were confirmed by spectroscopic analysis. For example, the IR spectrum of 18e shows a sharp intense band at 1714 cm−1 attributed to the four C=O functions. The 1H NMR spectrum of the same compound shows a singlet at δH 7.56 corresponding to the triazole proton and two triplets at δH 4.02 and 3.17 (J = 7.3 Hz) corresponding to the two methylene groups at the junction of the phthalimide and the triazole moities. The 13C NMR spectrum of 18e shows two characteristic signals at δC 177.5 and 168.2 corresponding to the C=O of the ester and the acetoxy groups, respectively, and an additional signal at δC 166.1 attributable to the two C=O moieties of the Phtalimide group was also observed.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((1,3-dioxoisoindolin-2-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18a), This derivative was isolated as a white solid in 84% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 84–86 °C; [α]D20 +57 (c 0.95 mg/mL, CH2Cl2); IR (νmax/cm−1) 2922.91 (CH str.), 1716.51 (4 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.90–7.83 (m, 2H, 2 × CHaro), 7.79–7.71 (m, 3H, 3 × CHaro), 7.41–7.30 (m, 5H, 5 × CHaro), 5.29 (t, J = 3.8 Hz, 1H, CH=C), 5.15–5.02 (m, 6H, NCH2Trz, TrzCH2CO2, OCH2Ph), 4.54 (dd, J = 10.8, 5.2 Hz, 1H, CHCO2), 2.91 (dd, J = 14.2, 4.4 Hz, 1H, C=CCHCH2), 2.05–1.72 (m, 4H, 2 × CH2), 1.70–1.55 (m, 8H, 4 × CH2), 1.50–1.33 (m, 4H, 2 × CH2), 1.30–1.20 (m, 4H, 2 × CH2), 1.18 (d, J = 3.7 Hz, 1H, CH), 1.12 (s, 3H, CH3), 1.07–1.01 (m, 1H, CH), 0.95–0.90 (m, 6H, 2 × CH3), 0.85 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.64 (s, 3H, CH3), 0.60 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3)δC 177.57 (C=O), 167.71 (C=O), 165.88 (2 × NC=O), 143.88 (Cq), 136.57 (2 × Cq), 134.23 (2 × CHaro), 132.19 (2 × Cq), 128.55 (2 × CHaro), 128.10 (2 × CHaro), 128.04 (CHaro), 124.55 (CHaro/Trz), 123.59 (2 × CHaro), 122.41 (CH=C), 83.97 (CHCO2), 66.05 (OCH2Ph), 55.27 (CH), 51.31 (TrzCH2CO2), 47.61 (CH), 46.87 (Cq), 45.98 (CH2), 41.80 (Cq), 41.50 (C=CCHCH2), 39.39 (Cq), 38.07 (CH2), 37.79 (Cq), 36.95 (Cq), 33.98 (NCH2Trz), 33.23 (CH3), 33.09 (CH2), 32.69 (CH2), 32.49 (CH2), 30.83 (Cq), 28.15 (CH3), 27.73 (CH2), 25.97 (CH3), 23.78 (CH3), 23.49 (2 × CH2), 23.16 (CH2), 18.22 (CH2), 16.98 (CH3), 16.54 (CH3), 15.41 (CH3); HRMS (+ESI) calculated for C50H63N4O6 [M + H]+: 815.4703, found: 815.4775.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 2,2,6a,6b,9,9,12a-heptamethyl-10-(2-(4-((3,4,4-trimethyl-2,5-dioxoimidazolidin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18b), This derivative was isolated as a white solid in 92% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 92–94 °C; [α]D20 +51 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1) 2923.14 (CH str.), 1770.86 (2 × C=O), 1711.07 (2 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.72 (s, 1H, CHaro/Trz), 7.38–7.28 (m, 5H, 5 × CHaro), 5.27 (t, J = 3.5 Hz, 1H, CH=C), 5.17–5.00 (m, 4H, TrzCH2CO2, OCH2Ph), 4.81 (s, 2H, NCH2Trz), 4.55 (dd, J = 9.9, 6.0 Hz, 1H, CHCO2), 2.94–2.83 (m, 4H, C=CCHCH2,NCH3), 2.02–1.78 (m, 4H, 2 × CH2),1.72–1.65 (m, 2H, CH2), 1.55–1.42 (m, 4H, 2 × CH2), 1.36 (s, 6H, 2 × COCH3CN), 1.30–1.19 (m, 10H, 5 × CH2), 1.16 (d, J = 3.7 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.04–1.00 (m, 1H, CH), 0.93–0.78 (m, 12H, 4 × CH3), 0.71 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3)δC 177.45 (C=O), 176.03 (NC=O), 165.81 (C=O), 154.68 (NC=O), 143.75 (Cq), 143.01 (Cq), 136.43 (Cq), 128.42 (2 × CHaro), 127.98 (2 × CHaro), 127.92 (CHaro), 124.38 (CHaro/Trz), 122.29 (CH=C), 83.79 (CHCO2), 65.93 (OCH2Ph), 61.39 (Cq), 55.17 (CH), 51.12 (TrzCH2CO2), 47.49 (CH), 46.73 (Cq), 45.84 (CH2), 41.68 (Cq), 41.36 (C=CCHCH2), 39.27 (Cq), 37.97 (CH2), 37.73 (Cq), 36.85 (Cq), 33.80 (NCH2Trz), 33.11 (CH3), 32.57 (CH2), 32.36 (CH2), 30.71 (Cq), 29.71 (CH2), 28.09 (CH3), 27.59 (CH2), 25.85 (CH3), 24.41 (NCH3), 23.65 (CH3), 23.38 (2 × CH2), 23.03 (CH2), 22.00 (2 × COCH3CN), 18.14 (CH2), 16.85 (CH3), 16.56 (CH3), 15.31 (CH3); HRMS (+ESI) calculated for C48H68N5O6 [M + H]+: 810.5125, found: 810.5169.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2,5-dioxopyrrolidin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18c), This derivative was isolated as a white solid in 83% yield; Rf (cyclohexane/EtOAc: 50/50) = 0.3; mp = 145–147 °C; [α]D20+108 (c 0.5 mg/mL, CH2Cl2); IR (νmax/cm−1) 2926.82 (CH str.), 1741.39 (C=O), 1721.47 (C=O), 1701.29 (2 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.71 (s, 1H, CHaro/Trz), 7.38–7.28 (m, 5H, 5 × CHaro), 5.27 (t, J = 3.6 Hz, 1H, CH=C), 5.17–4.99 (m, 4H, OCH2Ph, NCH2Trz), 4.80 (s, 2H, TrzCH2CO2), 4.54 (dd, J = 9.8, 6.1 Hz, 1H, CHCO2), 2.88 (dd, J = 13.6, 4.5 Hz, 1H, C=CCHCH2), 2.71 (s, 4H, 2 × CH2CON), 2.03–1.79 (m, 4H, 2 × CH2), 1.72–1.62 (m, 4H, 2 × CH2), 1.56–1.41 (m, 4H, 2 × CH2), 1.38–1.18 (m, 8H, 4 × CH2), 1.15 (d, J = 3.9 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH), 0.93–0.85 (m, 9H, 3 × CH3), 0.81 (s, 3H, CH3), 0.71 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3)δC 177.51 (C=O), 176.54 (2 × NC=O), 165.90 (C=O), 143.82 (Cq), 142.37 (Cq), 136.49 (Cq), 128.49 (2 × CHaro), 128.06 (2 × CHaro), 127.99 (CHaro), 124.72 (CHaro/Trz), 122.35 (CH=C), 83.91 (CHCO2), 66.00 (OCH2Ph), 55.24 (CH), 51.16 (TrzCH2CO2), 47.56 (CH), 46.79 (Cq), 45.91 (CH2), 41.75 (Cq), 41.43 (C=CCHCH2), 39.33 (Cq), 38.03 (CH2), 37.80 (Cq), 36.92 (Cq), 33.92 (NCH2Trz), 33.68 (CH2), 33.18 (CH3), 32.63 (CH2), 32.43 (CH2), 30.78 (Cq), 28.30 (2 × CH2CON), 28.16 (CH3), 27.66 (CH2), 25.93 (CH3), 23.73 (CH3), 23.46 (2 × CH2), 23.10 (CH2), 18.22 (CH2), 16.92 (CH3), 16.60 (CH3), 15.40 (CH3); HRMS (+ESI) calculated for C46H63N4O6 [M + H]+: 767.4703, found: 767.4751.
(4aS,6aS,6bR,10S,12aR)-benzyl 10-(2-(4-((1,1-dioxido-3-oxobenzo[d]isothiazol-2(3H)-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18d), This derivative was isolated as a white solid in 89% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 113–116 °C; [α]D20 +80 (c 0.7 mg/mL, CH2Cl2); IR (νmax/cm−1) 2921.46 (CH str.), 1725.07 (3 × C=O), 1181.72 (2 × S=O); 1H NMR (300 MHz, CDCl3)δH 8.12–7.78 (m, 5H, 5 × CHaro), 7.39–7.28 (m, 5H, 5 × CHaro), 5.27 (t, J = 3.69 Hz, 1H, CH=C), 5.21–4.97 (m, 6H, NCH2Trz, TrzCH2CO2, OCH2Ph), 4.53 (dd, J = 10.2, 5.6 Hz, 1H, CHCO2), 2.89 (dd, J = 13.7, 4.4 Hz, 1H, C=CCHCH2), 2.03–1.77 (m, 4H, 2 × CH2), 1.76–1.63 (m, 4H, 2 × CH2), 1.59–1.48 (m, 4H, 2 × CH2) 1.47–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 3.3 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.01 (m, 1H, CH), 0.93–0.87 (m, 6H, 2 × CH3), 0.83 (s, 3H, CH3), 0.78 (s, 3H, CH3), 0.65 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3)δC 177.56 (C=O), 165.77 (C=O), 158.59 (NC=O), 143.87 (Cq), 137.87 (Cq), 136.56 (2 × Cq), 135.09 (CHaro), 134.56 (CHaro), 128.54 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 127.31 (Cq), 125.48 (2 × CHaro), 122.40 (CHaro/Trz), 121.24 (CH=C), 84.00 (CHCO2), 66.04 (OCH2Ph), 55.28 (CH), 47.59 (CH), 46.85 (Cq), 45.97 (TrzCH2CO2), 45.94 (CH2), 41.79 (Cq), 41.49 (C=CCHCH2), 39.38 (Cq), 38.07 (CH2), 37.80 (Cq), 36.94 (Cq), 33.98 (NCH2Trz), 33.96 (CH2), 33.21 (CH3), 32.67 (CH2), 32.47 (CH2), 30.82 (Cq), 28.18 (CH3), 27.72 (CH2), 25.96 (CH3), 23.76 (CH3), 23.48 (2 × CH2), 23.15 (CH2), 18.22 (CH2), 16.97 (CH3), 16.59 (CH3), 15.38 (CH3); HRMS (+ESI) calculated for C49H63N4O7S [M + H]+: 851.4373, found: 851.4464.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(2-(1,3-dioxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18e), This derivative was isolated as a white solid in 92% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 94–96 °C; [α]D20 +65 (c 0.85 mg/mL, CH2Cl2); IR (νmax/cm−1) 2929.05 (CH str.), 1714.31 (4 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.86–7.79 (m, 2H, 2 × CHaro), 7.74–7.67 (m, 2H, 2 × CHaro), 7.56 (s, 1H, 1H, CHaro/Trz), 7.39–7.28 (m, 5H, 5 × CHaro), 5.27 (t, J = 3.6 Hz, 1H, CH=C), 5.14–4.99 (m, 4H, TrzCH2CO2, OCH2Ph), 4.54 (dd, J = 10.0, 5.9 Hz, 1H, CHCO2), 4.02 (t, J = 7.3 Hz, 2H, NCH2CH2Trz), 3.17 (t, J = 7.3 Hz, 2H, NCH2CH2Trz), 2.89 (dd, J = 14.0, 4.5 Hz, 1H, C=CCHCH2), 2.02–1.76 (m, 4H, 2 × CH2), 1.75–1.66 (m, 2H, CH2), 1.60–1.55 (m, 2H, CH2) 1.55–1.39 (m, 4H, 2 × CH2), 1.38–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 4.0 Hz, 1H, CH), 1.11 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH3), 0.92–0.86 (m, 9H, 3 × CH3), 0.81 (s, 3H, CH3), 0.71 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3)δC 177.55 (C=O), 168.28 (C=O), 166.13 (2 × NC=O), 144.80 (Cq), 143.86 (Cq), 136.54 (Cq), 134.08 (2 × CHaro), 132.17 (2 × Cq), 128.53 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 123.43 (2 × CHaro), 122.88 (CHaro/Trz), 122.40 (CH=C), 83.84 (CHCO2), 66.04 (OCH2Ph), 55.30 (CH), 51.20 (TrzCH2CO2), 47.60 (CH), 46.84 (Cq), 45.96 (CH2), 41.79 (Cq), 41.48 (C=CCHCH2), 39.38 (Cq), 38.08 (CH2), 37.84 (Cq), 37.45 (NCH2CH2Trz), 36.96 (Cq), 33.96 (CH2), 33.21 (CH3), 32.68 (CH2), 32.47 (CH2), 30.81 (Cq), 28.19 (CH3), 27.71 (CH2), 25.96 (CH3), 24.96 (NCH2CH2Trz), 23.76 (CH3), 23.49 (2 × CH2), 23.14 (CH2), 18.25 (CH2), 16.96 (CH3), 16.64 (CH3), 15.43 (CH3); HRMS (+ESI) calculated for C51H65N4O6 [M + H]+: 829.4859, found: 829.4959.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(1-(1,3-dioxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18f), This derivative was isolated as a white solid in 80% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 82–84 °C; [α]D20 +40 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1)2942.80 (CH str.), 1776.94 (C=O), 17113.95 (3 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.84–7.77 (m, 3H, 3 × CHaro), 7.73–7.66 (m, 2H, 2 × CHaro), 7.38–7.28 (m, 5H, 5 × CHaro), 5.81 (q, J = 7.3 Hz, 1H, NCHCH3), 5.27 (t, J = 3.6 Hz, 1H, CH=C), 5.21–5.00 (m, 4H, TrzCH2CO2, OCH2Ph), 4.53 (dd, J = 10.5, 5.4 Hz, 1H, CHCO2), 2.89 (dd, J = 13.6, 4.4 Hz, 1H, C=CCHCH2), 2.06–1.89 (m, 2H, CH2), 1.86 (d, J = 7.3 Hz, 3H, NCHCH3), 1.84–1.76 (m, 2H, CH2), 1.74–1.63 (m, 4H, 2 × CH2) 1.58–1.44 (m, 4H, 2 × CH2), 1.42–1.18 (m, 8H, 4 × CH2),1.16 (d, J = 3.7 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.01 (d, J = 10.7 Hz, 1H, CH), 0.93–0.87 (m, 6H, 2 × CH3), 0.84 (s, 3H, CH3), 0.78 (s, 3H, CH3), 0.64 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.54 (C=O), 167.76 (C=O), 165.67 (2 × NC=O), 147.82 (Cq), 143.85 (Cq), 136.53 (2 × Cq), 134.13 (2 × CHaro), 132.05 (Cq), 128.53 (2 × CHaro), 128.08 (2 × CHaro), 128.02 (CHaro), 123.84 (CHaro/Trz), 123.40 (2 × CHaro), 122.39 (CH=C), 83.94 (CHCO2), 66.03 (OCH2Ph), 55.26 (CH), 51.32 (TrzCH2CO2), 47.58 (CH), 46.83 (Cq), 45.94 (CH2), 42.62 (NCHCH3), 41.77 (Cq), 41.46 (C=CCHCH2), 39.35 (Cq), 38.05 (CH2), 37.78 (Cq), 36.93 (Cq), 33.95 (CH2), 33.21 (CH3), 32.65 (CH2), 32.45 (CH2), 30.81 (Cq), 28.15 (CH3), 27.70 (CH2), 25.95 (CH3), 23.75 (CH3), 23.46 (2 × CH2), 23.13 (CH2), 18.42 (NCHCH3), 18.21 (CH2), 16.95 (CH3), 16.58 (CH3), 15.40 (CH3); HRMS (+ESI) calculated for C51H65N4O6 [M + H]+: 829.4859, found: 829.4942.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(((1,3-dioxoisoindolin-2-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18g), This derivative was isolated as a white solid in 89% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 107–109 °C; [α]D20 +77 (c 0.7 mg/mL, CH2Cl2); IR (νmax/cm−1) 2945.81 (CH str.), 1791.82 (C=O), 1729.79 (3 × C=O); 1H NMR (300 MHz, CDCl3)δH 8.00 (s, 1H, CHaro/Trz), 7.82–7.69 (m, 4H, 4 × CHaro), 7.38–7.28 (m, 5H, 5 × CHaro), 5.39 (s, 2H, OCH2Trz), 5.28 (t, J = 3.5 Hz, 1H, CH=C), 5.17 (s, 2H, OCH2Ph), 5.12–4.99 (m, 2H, TrzCH2CO2), 4.58 (t, J = 7.9 Hz, 1H, CHCO2), 2.89 (dd, J = 13.9, 4.3 Hz, 1H, C=CCHCH2), 2.09–1.73 (m, 4H, 2 × CH2) 1.71–1.61 (m, 6H, 3 × CH2), 1.52–1.43 (m, 2H, CH2), 1.42–1.18 (m, 8H, 4 × CH2), 1.16 (d, J = 3.7 Hz, 1H, CH), 1.11 (s, 3H, CH3), 1.05–1.00 (m, 1H, CH), 0.94–0.82 (m, 12H, 4 × CH3), 0.75 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3)δC 177.56 (C=O), 165.87 (C=O), 163.56 (2 × NC=O), 143.86 (Cq), 142.13 (Cq), 136.54 (Cq), 134.61 (2 × CHaro), 128.95 (2 × Cq), 128.53 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 126.09 (CHaro/Trz), 123.73 (2 × CHaro), 122.39 (CH=C), 84.05 (CHCO2), 70.52 (OCH2Trz), 66.04 (OCH2Ph), 55.30 (CH), 51.30 (TrzCH2CO2), 47.60 (CH), 46.84 (Cq), 45.95 (CH2), 41.79 (Cq), 41.47 (C=CCHCH2), 39.37 (Cq), 38.09 (CH2), 37.87 (Cq), 36.96 (Cq), 33.96 (CH2), 33.21 (CH3), 32.67 (CH2), 32.46 (CH2), 30.82 (Cq), 28.22 (CH3), 27.70 (CH2), 25.96 (CH3), 23.76 (CH3), 23.51 (2 × CH2), 23.14 (CH2), 18.25 (CH2), 16.96 (CH3), 16.68 (CH3), 15.44 (CH3); HRMS (+ESI) calculated for C50H63N4O7 [M + H]+: 831.4652, found: 831.4703.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((1-hydroxy-3-oxoisoindolin-2-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18h), This derivative was isolated as a white solid in 96% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 88–90 °C; [α]D20 +24 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1) 3350 (OH), 2928.90 (CH str.), 1699.10 (3 × C=O); 1H NMR (300 MHz, CDCl3)δH7.77 (s, 1H, CHaro/Trz), 7.70 (d, J = 7.4 Hz, 1H,CHaro), 7.61–7.40 (m, 3H, 3 × CHaro), 7.39–7.27 (m, 5H, 5 × CHaro), 5.92 (s, 1H, CHOH), 5.25 (t, J = 3.8 Hz, 1H, CH=C), 5.17–4.98 (m, 4H, TrzCH2CO2,OCH2Ph), 4.90 (d, J = 13.4 Hz, 1H, NCH2bTrz), 4.70 (d, J = 15.4 Hz, 1H, NCH2aTrz), 4.49 (dd, J = 10.5, 5.5 Hz, 1H, CHCO2), 2.89 (dd, J = 14.0, 4.4 Hz, 1H, C=CCHCH2), 2.19–1.77 (m, 4H, 2 × CH2), 1.76–1.61 (m, 4H, 2 × CH2), 1.53–1.38 (m, 4H, 2 × CH2), 1.37–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 4.4 Hz, 1H, CH), 1.09 (s, 3H, CH3), 1.03–0.99 (m, 1H, CH), 0.92–0.86 (m, 6H, 2 × CH3), 0.79 (s, 3H, CH3), 0.76 (s, 3H, CH3), 0.62 (s, 3H, CH3), 0.57 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.53 (C=O), 167.40 (NC=O), 165.89 (C=O), 144.25 (Cq), 143.80 (Cq), 136.49 (Cq), 132.42 (Cq), 131.46 (Cq), 129.65 (2 × CHaro), 128.50 (2 × CHaro), 128.04 (2 × CHaro), 127.99 (CHaro), 123.60 (2 × CHaro), 123.26 (CHaro/Trz), 122.35 (CH=C), 83.93 (CHCO2), 81.59 (CHOH), 66.00 (OCH2Ph), 55.19 (CH), 51.25 (TrzCH2CO2), 47.53 (CH), 46.79 (Cq), 45.91 (CH2), 41.73 (Cq), 41.42 (C=CCHCH2), 39.32 (Cq), 37.99 (CH2), 37.73 (Cq), 36.87 (Cq), 34.49 (NCH2Trz), 33.92 (CH2), 33.18 (CH3), 32.61 (CH2), 32.42 (CH2), 30.77 (Cq), 28.09 (CH3), 27.66 (CH2), 25.93 (CH3), 23.73 (CH3), 23.42 (2 × CH2), 23.10 (CH2), 18.15 (CH2), 16.91 (CH3), 16.51 (CH3), 15.35 (CH3); HRMS (+ESI) calculated for C50H64N4O6Na [M+Na]+: 839.4718, found: 839.4752.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-(2-(1-hydroxy-3-oxoisoindolin-2-yl)ethyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18i), This derivative was isolated as a white solid in 98% yield; Rf (cyclohexane/EtOAc: 40/60) = 0.5; mp = 111–113 °C; [α]D20 +40 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1)3350 (OH), 2931.46 (CH str.), 1729.50 (3 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.68 (d, J = 7.4 Hz, 1H, CHaro/Trz), 7.59–7.40 (m, 4H, 4 × CHaro), 7.37–7.28 (m, 5H, 5 × CHaro), 5.83 (d, J = 6.6 Hz, 1H, CHOH), 5.39 (br. s, 1H, OH), 5.27 (t, J = 3.7 Hz, CH=C), 5.14–4.98 (m, 4H, TrzCH2CO2,OCH2Ph), 4.52 (t, J = 8.0 Hz, 1H, CHCO2), 3.92 (t, J = 6.7 Hz, 2H, NCH2CH2Trz), 3.17 (t, J = 6.8 Hz, 2H, NCH2CH2Trz), 2.89 (dd, J = 13.8, 4.5 Hz, 1H, C=CCHCH2), 2.04–1.79 (m, 4H, 2 × CH2), 1.74–1.67 (m, 2H, CH2), 1.53–1.47 (m, 2H, CH2), 1.47–1.35 (m, 4H, 2 × CH2), 1.31–1.21 (m, 8H, 4 × CH2),1.16 (d, J = 3.8 Hz, 1H, CH), 1.11 (s, 3H, CH3), 1.04–1.00 (m, 1H, CH), 0.92–0.85 (m, 9H, 3 × CH3), 0.79 (s, 3H, CH3), 0.72 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.57 (C=O), 167.70 (C=O), 166.06 (NC=O), 144.23 (Cq), 143.87 (Cq), 136.52 (Cq), 132.26 (CHaro), 131.67 (2 × Cq), 129.63 (CHaro), 128.53 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 123.48 (2 × CHaro), 123.20 (CHaro/Trz), 122.38 (CH=C), 84.10 (CHCO2), 82.51 (CHOH), 66.04 (OCH2Ph), 55.27 (CH), 51.39 (TrzCH2CO2), 47.59 (CH), 46.83 (Cq), 45.95 (CH2), 41.78 (Cq), 41.46 (C=CCHCH2), 39.36 (Cq), 38.93 (NCH2CH2Trz), 38.06 (CH2), 37.84 (Cq), 36.94 (Cq), 33.95 (CH2), 33.22 (CH3), 32.65 (CH2), 32.46 (CH2), 30.82 (Cq), 28.20 (CH3), 27.70 (CH2), 25.97 (CH3), 24.87 (NCH2CH2Trz), 23.76 (CH3), 23.49 (2 × CH2), 23.13 (CH2), 18.24 (CH2), 16.95 (CH3), 16.65 (CH3), 15.44 (CH3); HRMS (+ESI) calculated for C51H67N4O6 [M + H]+: 831.5016, found: 831.5037.
Methyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-oxoisoindoline-1-carboxylate (18j), This derivative was isolated as a white solid in 95% yield; Rf (cyclohexane/EtOAc: 50/50) = 0.5; mp = 86–88 °C; [α]D20 +52 (c 0.9 mg/mL, CH2Cl2); IR (νmax/cm−1) 2926.94 (CH str.), 1732.76 (4 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.96 (s, 1H, CHaro/Trz), 7.79 (d, J = 7.8 Hz, 1H, CHaro), 7.64–7.43 (m, 3H, 3 × CHaro), 7.38–7.30 (m, 5H, 5 × CHaro), 7.03 (s, 1H, CH3CO2CH), 5.33 (d, J = 1.7 Hz, 2H, OCH2Trz), 5.28 (t, J = 4.0 Hz, 1H, CH=C), 5.17 (s, 2H, OCH2Ph), 5.13–4.99 (m, 2H, TrzCH2CO2), 4.57 (dd, J = 9.4, 6.6 Hz, 1H, CHCO2), 2.90 (dd, J = 14.0, 4.4 Hz, 1H, C=CCHCH2), 2.17 (s, 3H, CH3CO2), 1.51–1.38 (m, 4H, 2 × CH2), 1.32–1.23 (m, 8H, 4 × CH2), 1.16 (d, J = 3.7 Hz, 1H, CH), 1.11 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH), 0.93–0.85 (m, 9H, 3 × CH3), 0.83 (s, 3H, CH3), 0.73 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.56 (C=O), 170.81 (C=O), 165.92 (C=O), 165.66 (NC=O), 143.88 (Cq), 142.91 (Cq), 138.67 (Cq), 136.56 (Cq), 133.51 (CHaro), 130.71 (CHaro), 129.34, (Cq), 128.54 (2 × CHaro), 128.10 (2 × CHaro), 128.04 (CHaro), 125.85 (CHaro), 124.22 (CHaro), 124.06 (CHaro/Trz), 122.41 (CH=C), 84.01 (CHCO2), 80.82 (CH3CO2CH), 66.05 (OCH2Ph), 62.01 (OCH2Trz), 55.31 (CH), 51.27 (TrzCH2CO2), 47.61 (CH), 46.85 (Cq), 45.96 (CH2), 41.80 (Cq), 41.49 (C=CCHCH2), 39.39 (Cq), 38.09 (CH2), 37.87 (Cq), 36.97 (Cq), 33.98 (CH2), 33.22 (CH3), 32.69 (CH2), 32.48 (CH2), 30.83 (Cq), 28.22 (CH3), 27.72 (CH2), 25.97 (CH3), 23.77 (CH3), 23.52 (2 × CH2), 23.15 (CH2), 21.18 (CH3CO2), 18.26 (CH2), 16.97 (CH3), 16.66 (CH3), 15.45 (CH3); HRMS (+ESI) calculated for C52H67N4O8 [M + H]+: 875.4914, found: 875.4983.
Ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18k), This derivative was isolated as a white solid in 71% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 91–93 °C; [α]D20 +47 (c 0.95 mg/mL, CH2Cl2); IR (νmax/cm−1) 2943.22 (CH str.), 1702.18 (4 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.82 (d, J = 7.3 Hz, 1H, CHaro), 7.74 (s, 1H, CHaro/Trz), 7.65–7.45 (m, 3H, 3 × CHaro), 7.39–7.29 (m, 5H, 5 × CHaro), 5.43–5.34 (m, 1H, OCH2bPh), 5.31–5.23 (m, 2H, CH=C, OCH2aPh), 5.14–5.00 (m, 4H, NCH2bTrz, CHCO2CH2CH3, TrzCH2CO2), 4.62–4.46 (m, 2H, CHCO2, NCH2aTrz), 4.40–4.20 (m, 2H, CH3CH2O), 2.91 (dd, J = 13.2, 4,1 Hz, 1H, C=CCHCH2), 2.07–1.72 (m, 4H, 2 × CH2), 1.70–1.55 (m, 8H, 4 × CH2), 1.54–1.37 (m, 4H, 2 × CH2), 1.33 (t, J = 7.1 Hz, 3H, CH3CH2O), 1.28–1.21 (m, 4H, 2 × CH2),1.16 (d, J = 3.7 Hz, 1H, CH), 1.09 (s, 3H, CH3), 1.05–0.96 (m, 1H, CH), 0.93–0.86 (m, 6H, 2 × CH3), 0.82 (s, 3H, CH3), 0.74 (s, 3H, CH3), 0.61–0.52 (m, 6H, 2 × CH3); 13C NMR (75 MHz,CDCl3) δC 177.58 (C=O), 168.50 (C=O), 168.08 (C=O), 165.81 (NC=O), 143.78 (Cq), 139.72 (Cq), 135.56 (2 × Cq), 132.20 (CHaro), 129.32 (CHaro), 128.92 (Cq) 128.55 (2 × CHaro), 128.09 (2 × CHaro), 128.04 (CHaro), 124.52 (CHaro), 124.05 (CHaro), 123.16 (CHaro/Trz), 122.40 (CH=C), 83.96 (CHCO2), 66.05 (OCH2Ph), 62.45 (CH3CH2O), 62.01 (CHCO2CH2CH3), 61.15 (NCH2Trz), (55.23 (CH), 51.29 (TrzCH2CO2), 47.58 (CH), 46.85 (Cq), 45.95 (CH2), 41.78 (Cq), 41.48 (C=CCHCH2), 39.37 (Cq), 38.03 (CH2), 37.76 (Cq), 36.92 (Cq), 33.98 (CH2), 33.22 (CH3), 32.66 (CH2), 32.48 (CH2), 30.83 (Cq), 28.13 (CH3), 27.71 (CH2), 25.96 (CH3), 23.77 (CH3), 23.47 (2 × CH2), 23.14 (CH2), 18.20 (CH2), 16.97 (CH3), 16.47 (CH3), 15.40 (CH3), 14.33 (CH3CH2O); HRMS (+ESI) calculated for C53H69N4O7 [M + H]+: 873.5122, found: 873.5161.
Ethyl2-benzyl-1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18l), This derivative was isolated as a white solid in 83% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 105–106 °C; [α]D20+13 (c 0.7 mg/mL, CH2Cl2); IR (νmax/cm−1) 2927.79 (CH str.), 1710 (2 × C=O), 1700.90 (2 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.78 (d, J = 7.5 Hz, 1H, CHaro), 7.60–7.39 (m, 5H, 5 × CHaro), 7.36–7.27 (m, 6H, 6 × CHaro), 7.25–7.20 (m, 1H, CHaro), 6.18 (d, J = 9.4 Hz, 1H, CHaro), 5.27 (t, J = 3.7 Hz, 1H, CH=C), 5.13–5.00 (m, 2H, OCH2Ph), 4.94–4.62 (m, 4H, 2 × CH2, TrzCH2CO2, NCH2Ph), 4.53–4.43 (m, 1H, CHCO2), 3.90–3.74 (m, 3H, CH3CH2bO, CCH2Trz), 3.53 (dqd, J = 14.4, 7.1, 5.0 Hz, 1H, CH3CH2aO), 2.89 (dd, J = 13.6, 4.4 Hz, 1H, C=CCHCH2), 2.09–1.71 (m, 4H, 2 × CH2), 1.70–1.48 (m, 10H, 5 × CH2),1.47–1.27 (m, 6H, 3 × CH2), 1.25 (t, J = 7.5 Hz, 3H, CH3CH2O), 1.18 (d, J = 9.3 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH), 0.93–0.87 (m, 9H, 3 × CH3), 0.78 (s, 3H, CH3), 0.68 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC177.56 (C=O), 169.99 (C=O), 169.38 (C=O), 165.84 (NC=O), 143.87 (Cq), 143.76 (Cq), 140.77 (Cq), 137.17 (Cq), 136.54 (2 × Cq), 132.35 (CHaro), 129.37 (3 × CHaro), 128.53 (4 × CHaro), 128.11 (2 × CHaro), 128.04 (CHaro), 127.69 (CHaro), 124.06 (CHaro), 123.08 (CHaro/Trz), 122.41 (CH=C), 122.00 (CHaro), 83.72 (CHCO2), 70.76 (Cq), 66.05 (OCH2Ph), 62.33 (CH3CH2O), 55.28 (CH), 50.94 (TrzCH2CO2), 47.61 (CH), 46.84 (Cq), 45.96 (CH2), 44.87 (NCH2Ph), 41.79 (Cq), 41.47 (C=CCHCH2), 39.38 (Cq), 38.08 (CH2), 37.86 (Cq), 36.95 (Cq), 33.97 (CH2), 33.22 (CH3), 32.68 (CH2), 32.47 (CH2), 30.82 (Cq), 29.85 (CCH2Trz), 28.18 (CH3), 27.71 (CH2), 25.96 (CH3), 23.76 (CH3), 23.48 (2 × CH2), 23.15 (CH2), 18.26 (CH2), 16.96 (CH3), 16.65 (CH3), 15.43 (CH3), 13.61 (CH3CH2O); HRMS (+ESI) calculated for C60H75N4O7 [M + H]+: 963.5591, found: 963.5641.
Ethyl1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxo-2-phenethylisoindoline-1-carboxylate (18m), This derivative was isolated as a white solid in 90% yield; Rf (cyclohexane/EtOAc: 70/30) = 0.5; mp = 93–95 °C; [α]D20 +73 (c 0.75 mg/mL, CH2Cl2); IR (νmax/cm−1) 2936.05 (CH str.), 1708.51 (4 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.77 (d, J = 7.4 Hz, 1H, CHaro), 7.62–7.54 (m, 2H, 2 × CHaro), 7.53–7.45 (m, 1H, CHaro), 7.40–7.27 (m, 9H, 9 × CHaro), 7.25–7.18 (m, 1H, CHaro), 6.53 (d, J = 18.8 Hz, 1H, CHaro), 5.27 (t, J = 3.7 Hz, 1H, CH=C), 5.12–5.01 (m, 2H, OCH2Ph), 4.88 (qd, J = 17.43, 17.43, 17.42, 2.38 Hz, 2H, TrzCH2CO2), 4.52–4.43 (m, 1H, CHCO2), 4.28–4.10 (m, 2H, CH3CH2O), 4.03–3.93 (m, 1H, CCH2bTrz), 3.87–3.77 (m, 1H, CCH2aTrz), 3.67 (t, J = 7.9 Hz, 2H, NCH2CH2Ph), 3.18–2.98 (m, 2H, NCH2CH2Ph), 2.90 (dd, J = 13.7, 4.5 Hz, 1H, C=CCHCH2), 2.05–1.73 (m, 4H, 2 × CH2), 1.71–1.52 (m, 8H, 4 × CH2), 1.49–1.24 (m, 8H, 4 × CH2), 1.20 (t, J = 7.1 Hz, 3H, CH3CH2O), 1.16 (d, J = 8.6 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.00 (d, J = 3.9 Hz, 1H, CH), 0.93–0.84 (m, 9H, 3 × CH3), 0.76 (s, 3H, CH3), 0.68 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3)δC 177.55 (C=O), 170.31 (C=O), 169.22 (C=O), 165.83 (NC=O), 143.86 (Cq), 143.28 (Cq), 142.99 (Cq), 140.92 (Cq), 139.24 (Cq), 136.54 (2 × Cq), 132.29 (CHaro), 129.61 (CHaro), 129.05 (2 × CHaro), 128.68 (2 × CHaro), 128.54 (2 × CHaro), 128.11 (2 × CHaro), 128.04 (CHaro), 126.54 (CHaro), 123.81 (CHaro), 122.84 (CHaro/Trz), 122.41 (CH=C), 122.24 (CHaro), 83.79 (CHCO2), 71.45 (Cq), 66.05 (OCH2Ph), 62.77 (CH3CH2O), 55.27 (CH), 51.06 (TrzCH2CO2), 47.60 (CH), 46.85 (Cq), 45.96 (CH2), 44.25 (NCH2CH2Ph), 41.79 (Cq), 41.48 (C=CCHCH2), 39.37 (Cq), 38.06 (CH2), 37.83 (Cq), 36.94 (Cq), 34.36 (NCH2CH2Ph), 33.97 (CH2), 33.22 (CH3), 32.68 (CH2), 32.47 (CH2), 30.82 (Cq), 30.69 (CCH2Trz), 28.17 (CH3), 27.71 (CH2), 25.96 (CH3), 23.77 (CH3), 23.48 (2 × CH2), 23.15 (CH2), 18.25 (CH2), 16.96 (CH3), 16.65 (CH3), 15.39 (CH3), 14.09 (CH3CH2O); HRMS (+ESI) calculated for C61H77N4O7 [M + H]+: 977.5748, found: 977.5807.
Methyl2-allyl-1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18n), This derivative was isolated as a white solid in 70% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 98–100 °C; [α]D20 +80 (c 0.7 mg/mL, CH2Cl2); IR (νmax/cm−1) 2935.43 (CH str.), 1710.09 (4 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.75 (d, J = 7.4, 1.3 Hz, 1H, CHaro), 7.61–7.43 (m, 3H, 3 × CHaro), 7.37–7.29 (m, 5H, 5 × CHaro), 6.55 (d, J = 21.3 Hz, 1H, CHaro), 5.98–5.81 (m, 1H, CH=CH2), 5.36–5.16 (m, 3H, CH=C, CH=CH2), 5.14–4.99 (m, 2H, OCH2Ph), 4.98–4.77 (m, 2H, TrzCH2CO2), 4.53–4.35 (m, 2H, CHCO2, NCH2bCH=CH2), 4.11–4.00 (m, 1H, NCH2aCH=CH2), 3.88 (qd, J = 15.72, 15.71, 15.71, 4.49 Hz, 2H, CCH2Trz), 3.65 (s, 3H, CH3O), 2.89 (dd, J = 13.5, 4.3 Hz, 1H, C=CCHCH2), 2.03–1.73 (m, 4H, 2 × CH2), 1.72–1.62 (m, 4H, 2 × CH2), 1.61–1.57 (m, 2H, CH2), 1.51–1.43 (m, 2H, CH2),1.41–1.18 (m, 8H, 4 × CH2), 1.16 (d, J = 3.8 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH), 0.94–0.82 (m, 9H, 3 × CH3), 0.77 (s, 3H, CH3), 0.69 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.56 (C=O), 170.80 (C=O), 168.70 (C=O), 165.87 (NC=O), 143.86 (Cq), 142.38 (Cq), 140.86 (Cq), 136.53 (2 × Cq), 133.11 (CH=CH2), 132.35 (CHaro), 129.61 (CHaro), 128.53 (2 × CHaro), 128.10 (2 × CHaro), 128.04 (CHaro), 124.03 (CHaro), 122.91 (CHaro/Trz), 122.40 (CH=C), 121.93 (CHaro) 118.54 (CH=CH2), 83.80 (CHCO2), 70.35 (Cq), 66.04 (OCH2Ph), 55.28 (CH), 53.10 (CH3O), 51.04 (TrzCH2CO2), 47.60 (CH), 46.84 (Cq), 45.95 (CH2), 44.15 (NCH2CH=CH2), 41.78 (Cq), 41.47 (C=CCHCH2), 39.37 (Cq), 38.07 (CH2), 37.86 (Cq), 36.94 (Cq), 33.96 (CH2), 33.22 (CH3), 32.68 (CH2), 32.46 (CH2), 30.82 (Cq), 30.02 (CCH2Trz), 28.16 (CH3), 27.70 (CH2), 25.95 (CH3), 23.76 (CH3), 23.47 (2 × CH2), 23.14 (CH2), 18.25 (CH2), 16.95 (CH3), 16.64 (CH3), 15.42 (CH3); HRMS (+ESI) calculated for C55H71N4O7 [M + H]+: 899.5278, found: 899.5327.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2-benzyl-1-((2-methoxy-2-oxoethyl)carbamoyl)-3-oxoisoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18o), This derivative was isolated as a white solid in 94% yield; Rf (cyclohexane/EtOAc: 40/60) = 0.5; mp = 120–122 °C; [α]D20 +41 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1) 3300 (NH), 2931.02 (CH str.), 1726.01 (3 × C=O), 1677.77 (C=O); 1H NMR (300 MHz, CDCl3) δH 7.72 (d, J = 7.4 Hz, 1H, CHaro), 7.63–7.39 (m, 6H, 6 × CHaro), 7.36–7.27 (m, 6H, 6 × CHaro), 7.25–7.19 (m, 1H, CHaro), 6.32 (d, J = 11.7 Hz, 1H, CHaro), 5.86 (br. s, 1H, NH), 5.27 (t, J = 3.8 Hz, 1H, CH=C), 5.12–4.76 (m, 5H, NCH2bPh,TrzCH2CO2, OCH2Ph), 4.65 (dd, J = 15.2, 6.7 Hz, 1H, NCH2aPh), 4.55–4.44 (m, 1H, CHCO2), 3.96 (d, J = 15.6 Hz, 1H, CCH2bTrz), 3.85–3.56 (m, 5H, CCH2aTrz, NHCH2bCO2, CH3O), 3.28–3.14 (m, 1H, NHCH2aCO2), 2.89 (dd, J = 13.8, 4.4 Hz, 1H, C=CCHCH2), 2.04–1.79 (m, 4H, 2 × CH2), 1.69–1.63 (m, 2H, CH2), 1.52–1.46 (m, 2H, CH2), 1.44–1.31 (m, 4H, 2 × CH2), 1.29–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 4.0 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.04–1.00 (m, 1H, CH), 0.92–0.87 (m, 9H, 3 × CH3), 0.78 (s, 3H, CH3), 0.68 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.55 (C=O), 169.83 (NHC=O), 169.18 (2 × C=O), 165.83 (NC=O), 144.31 (Cq), 143.85 (Cq), 141.05 (Cq), 137.54 (Cq), 136.52 (Cq), 132.81 (CHaro), 130.95 (Cq), 129.66 (CHaro), 129.42 (2 × CHaro), 128.83 (2 × CHaro), 128.52 (2 × CHaro), 128.09 (2 × CHaro), 128.02 (CHaro), 127.83 (CHaro), 124.19 (CHaro), 123.54 (CHaro), 122.63 (CHaro/Trz), 122.39 (CH=C), 83.73 (CHCO2), 71.66 (Cq), 66.04 (OCH2Ph), 55.27 (CH), 52.34 (CH3O), 50.98 (TrzCH2CO2), 47.60 (CH), 46.83 (Cq), 45.94 (CH2), 44.84 (NCH2Ph), 41.78 (Cq), 41.46 (C=CCHCH2), 41.29 (NHCH2CO2), 39.36 (Cq), 38.07 (CH2), 37.86 (Cq), 36.94 (Cq), 33.95 (CH2), 33.20 (CH3), 32.67 (CH2), 32.46 (CH2), 30.81 (Cq), 29.81 (CCH2bTrz), 28.16 (CH3), 27.69 (CH2), 25.95 (CH3), 23.75 (CH3), 23.47 (2 × CH2), 23.13 (CH2), 18.24 (CH2), 16.95 (CH3), 16.64 (CH3), 15.41 (CH3); HRMS (+ESI) calculated for C61H75N5O8Na[M+Na]+: 1028.5508, found: 1028.5584.
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-(2-(4-((2-((1,5-dimethyl-1H-pyrrol-2-yl)methyl)-1-(hydroxymethyl)-3-oxoisoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)acetoxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (18p), This derivative was isolated as a yellow solid in 70% yield; Rf (cyclohexane/EtOAc: 40/60) = 0.5; mp = 85–87 °C; [α]D20 +64 (c 0.85 mg/mL, CH2Cl2); IR (νmax/cm−1) 3400 (OH), 2923.75 (CH str.), 1726.83 (C=O), 1678.10 (2 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.78 (d, J = 7.5 Hz, 1H, CHaro), 7.63–7.40 (m, 3H, 3 × CHaro), 7.39–7.28 (m, 6H, 6 × CHaro), 6.44 (d, J = 16.9 Hz, 1H, CHaro), 6.16 (t, J = 2.6 Hz, 1H, CHaro), 5.84 (d, J = 3.3 Hz, 1H, CHaro), 5.27 (t, J = 3.7 Hz, 1H, CH=C), 5.19–4.79 (m, 5H, NCH2bHyd, TrzCH2CO2, OCH2Ph), 4.64–4.45 (m, 2H, NCH2aHyd, CHCO2), 3.82–3.70 (m, 2H, CH2OH), 3.53–3.36 (m, 5H, CH3N, CCH2Trz), 2.89 (dd, J = 14.0, 4.6 Hz, 1H, C=CCHCH2), 2.18 (s, 3H, NCCH3), 1.99–1.81 (m, 4H, 2 × CH2), 1.74–1.65 (m, 2H, CH2), 1.59–1.56 (m, 2H, CH2), 1.37–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 2.8 Hz, 1H, CH), 1.11 (s, 3H,CH3), 1.54–1.39 (m, 4H, 2 × CH2), 1.05–1.00 (m, 1H, CH), 0.93–0.86 (m, 9H, 3 × CH3), 0.79 (s, 3H, CH3), 0.69 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.55 (C=O), 168.51 (C=O), 165.93 (NC=O), 146.14 (Cq), 143.86 (Cq), 141.70 (Cq), 136.53 (Cq), 132.29 (Cq), 132.10 (CHaro), 130.78 (Cq), 128.90 (CHaro), 128.52 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 127.19 (Cq), 123.92 (CHaro), 123.09 (CHaro/Trz), 122.39 (CH=C), 121.92 (CHaro), 108.56 (CHaro), 105.93 (CHaro), 83.83 (CHCO2), 70.12 (Cq), 66.72 (CH2OH), 66.04 (OCH2Ph), 55.29 (CH), 51.05 (TrzCH2CO2), 47.60 (CH), 46.84 (Cq), 45.96 (CH2), 41.79 (Cq), 41.47 (C=CCHCH2), 39.38 (Cq), 38.08 (CH2), 37.87 (Cq), 36.95 (Cq), 35.98 (NCH2Hyd), 33.96 (CH2), 33.21 (CH3), 32.68 (CH2), 32.46 (CH2), 30.88 (CH3N), 30.81 (Cq), 29.82 (CCH2Trz), 28.19 (CH3), 27.71 (CH2), 25.96 (CH3), 23.75 (CH3), 23.49 (2 × CH2), 23.14 (CH2), 18.26 (CH2), 16.96 (CH3), 16.67 (CH3), 15.44 (CH3), 12.66 (NCCH3); HRMS (+ESI) calculated for C58H75N5O6Na [M+Na]+: 960.5610, found: 960.5708.
1-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-2-(furan-2-ylmethyl)-3-oxoisoindoline-1-carboxylic acid (18q), This derivative was isolated as a yellow solid in 60% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.4; mp = 81–83 °C; [α]D20 +76 (c 0.5 mg/mL, CH2Cl2); IR (νmax/cm−1) 2924.69 (CH str.), 1724.90 (3 × C=O), 1711 (C=O); 1H NMR (300 MHz, CDCl3) δH 7.79 (d, J = 7.4 Hz, 1H, CHaro), 7.55–7.27 (m, 10H, 10 × CHaro), 6.34 (dd, J = 6.9, 2.8 Hz, 2H, 2 × CHaro), 5.27 (t, J = 4.2 Hz, 1H, CH=C), 5.23–4.78 (m, 6H, NCH2Fur, TrzCH2CO2, OCH2Ph), 4.58–4.35 (m, 2H, CHCO2, CCH2bTrz), 3.51 (s, 1H, CCH2aTrz), 2.89 (dd, J = 14.1, 4.5 Hz, 1H, C=CCHCH2), 2.05–1.80 (m, 4H, 2 × CH2), 1.73–1.61 (m, 4H, 2 × CH2), 1.57–1.44 (m, 4H, 2 × CH2), 1.43–1.19 (m, 8H, 4 × CH2), 1.16 (d, J = 3.7 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.05–1.00 (m, 1H, CH), 0.93–0.86 (m, 9H, 3 × CH3), 0.80 (s, 3H, CH3), 0.72 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.53 (C=O), 171.42 (C=O), 168.26 (C=O), 165.83 (NC=O), 150.45 (Cq), 144.47 (Cq), 143.85 (Cq), 142.66 (CHaro), 136.51 (2 × Cq), 132.25 (Cq), 131.88 (CHaro), 128.56 (2 × CHaro), 128.51 (2 × CHaro), 128.08 (2 × CHaro), 128.02 (CHaro), 123.91 (CHaro), 122.68 (CHaro/Trz), 122.37 (CH=C), 110.65 (CHaro), 108.94 (CHaro), 83.87 (CHCO2), 68.76 (Cq), 66.02 (OCH2Ph), 55.28 (CH), 51.71 (TrzCH2CO2),47.59 (CH), 46.82 (Cq), 45.94 (CH2), 41.77, (Cq), 41.46 (C=CCHCH2), 39.36 (Cq), 38.07 (CH2), 37.86 (Cq), 37.06 (NCH2Fur), 36.94 (Cq), 33.94 (CH2), 33.20 (CH3), 32.66 (CH2), 32.44 (CH2), 30.80 (Cq), 29.80 (CCH2Trz), 28.18 (CH3), 27.69 (CH2), 25.94 (CH3), 23.74 (CH3), 23.48 (2 × CH2), 23.12 (CH2), 18.24 (CH2), 16.94 (CH3), 16.65 (CH3), 15.42 (CH3); HRMS (+ESI) calculated for C56H69N4O8[M + H]+: 925.5071, found: 925.5109.
Ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-1-(2-ethoxy-2-oxoethyl)-3-oxoisoindoline-1-carboxylate (18r), This derivative was isolated as a white solid in 98% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 97–99 °C; [α]D20 +55 (c 1 mg/mL, CH2Cl2); IR (νmax/cm−1) 2936.99 (CH str.), 1710.10 (5 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.88–7.77 (m, 2H, 2 × CHaro), 7.61–7.45 (m, 3H, 3 × CHaro), 7.39–7.28 (m, 5H, 5 × CHaro), 5.27 (t, J = 3.7 Hz, 1H, CH=C), 5.15–4.85 (m, 6H, NCH2Trz, TrzCH2CO2, OCH2Ph), 4.53 (dd, J = 9.5, 6.5 Hz, 1H, CHCO2), 4.26–4.05 (m, 2H, CH3CH2CO2C), 4.03–3.92 (m, 2H, CH3CH2CO2CH2), 3.52 (d, J = 17.1 Hz, 1H, CCH2bCO2), 3.13 (dd, J = 17.0, 2.6 Hz, 1H, CCH2aCO2), 2.89 (dd, J = 14.2, 4.4 Hz, 1H, C=CCHCH2), 2.01–1.80 (m, 4H, 2 × CH2), 1.78–1.63 (m, 4H, 2 × CH2), 1.62–1.54 (m, 4H, 2 × CH2), 1.54–1.27 (m, 8H, 4 × CH2), 1.25 (d, J = 4.6 Hz, 1H, CH), 1.19 (td, J = 7.1, 2.5 Hz, 3H, CH3CH2CO2CH2), 1.11–1.03 (m, 6H, CH3CH2CO2C, CH3), 1.02–0.98 (m, 1H, CH), 0.92–0.83 (m, 9H, 3 × CH3), 0.80 (s, 3H, CH3), 0.69 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.55 (C=O), 169.08 (C=O), 169.40 (C=O), 168.92 (C=O), 165.91 (NC=O), 143.85 (Cq), 143.57 (Cq), 136.54 (2 × Cq), 132.40 (2 × CHaro), 131.20 (Cq), 129.52 (CHaro), 128.53 (2 × CHaro), 128.09 (2 × CHaro), 128.03 (CHaro), 123.74 (CHaro), 122.40 (CHaro/Trz+ CH=C), 83.83 (CHCO2), 69.23 (Cq), 66.03 (OCH2Ph), 62.77 (CH3CH2CO2C), 61.12 (CH3CH2CO2CH2), 55.28 (CH), 51.17 (TrzCH2CO2), 47.59 (CH), 46.84 (Cq), 45.95 (CH2), 41.78 (Cq), 41.47 (C=CCHCH2), 40.38 (CCH2bCO2), 39.37 (Cq), 38.07 (CH2), 37.83 (Cq), 36.94 (NCH2Trz),36.92 (Cq), 33.96 (CH2), 33.21 (CH3), 32.67 (CH), 32.46 (CH2), 30.81 (Cq), 28.18 (CH3), 27.70 (CH2), 25.95 (CH3), 23.76 (CH3), 23.48 (2 × CH2), 23.14 (CH2), 18.23 (CH2), 16.96 (CH3), 16.63 (CH3), 15.40 (CH3), 14.00 (CH3CH2CO2C, CH3CH2CO2CH2); HRMS (+ESI) calculated for C57H75N4O9 [M + H]+: 959.5489, found: 959.5543.
Ethyl1-allyl-2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxoisoindoline-1-carboxylate (18s), This derivative was isolated as a white solid in 90% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 100–102 °C; [α]D20 +53 (c 1.5 mg/mL, CH2Cl2); IR (νmax/cm−1) 2943.31 (CH str.), 1731.34 (2 × C=O), 1701.17 (2 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.93 (s, 1H, CHaro/Trz), 7.80 (d, J = 7.4 Hz, 1H, CHaro), 7.62–7.44 (m, 3H, 3 × CHaro), 7.39–7.28 (m, 5H, 5 × CHaro), 5.26 (t, J = 3.7 Hz, 1H, CH=C), 5.17–4.67 (m, 9H, NCH2Trz, TrzCH2CO2, OCH2Ph, CH=CH2, CH=CH2), 4.55 (t, J = 8.0 Hz, 1H, CHCO2), 4.18–4.01 (m, 2H, CH3CH2O), 3.26–3.11 (m, 2H, CH2CH=CH2), 2.89 (dd, J = 13.6, 4.4 Hz, 1H, C=CCHCH2), 2.05–1.78 (m, 6H, 3 × CH2), 1.75–1.62 (m, 4H, 2 × CH2),1.57–1.50 (m, 2H, CH2), 1.46–1.23 (m, 8H, 4 × CH2), 1.20 (d, J = 3.1 Hz, 1H, CH), 1.14 (dt, J = 7.1, 3.5 Hz, 3H, CH3CH2O), 1.10 (s, 3H, CH3), 1.04–0.99 (m, 1H, CH), 0.93–0.85 (m, 9H, 3 × CH3), 0.82 (s, 3H, CH3), 0.73 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.58 (C=O), 169.97 (C=O), 169.28 (C=O), 165.87 (NC=O), 144.46 (Cq), 143.85 (Cq), 143.60 (Cq), 136.55 (Cq), 132.35 (CH=CH2), 131.49 (Cq), 129.82 (CHaro), 129.27 (CHaro), 128.54 (2 × CHaro), 128.10 (2 × CHaro), 128.04 (CHaro), 125.88 (CHaro), 123.69 (CHaro), 122.42 (CHaro/Trz, CH=C), 120.29 (CH=CH2), 83.89 (CHCO2), 72.31 (Cq), 66.05 (OCH2Ph), 62.57 (CH2), 55.30 (CH), 51.23 (TrzCH2CO2), 47.60 (CH), 46.85 (Cq), 45.95 (CH2), 41.79 (Cq), 41.48 (C=CCHCH2), 39.38 (Cq), 38.09 (CH2), 37.86 (Cq), 37.77 (CH2CH=CH2), 37.02 (NCH2Trz), 36.96 (Cq), 33.97 (CH2), 33.22 (CH3), 32.69 (CH2), 32.48 (CH2), 30.82 (Cq), 28.22 (CH3), 27.71 (CH2), 25.96 (CH3), 23.77 (CH3), 23.51 (2 × CH2), 23.15 (CH2), 18.25 (CH2), 16.97 (CH3), 16.69 (CH3), 15.43 (CH3), 14.03 (CH3CH2O); HRMS (+ESI) calculated for C56H73N4O7 [M + H]+: 913.5435, found: 913.5466.
Ethyl2-((1-(2-(((3S,4aR,6aR,6bS,8aS,12aS,14aR,14bR)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-1-(3-methoxybenzyl)-3-oxoisoindoline-1-carboxylate (18t), This derivative was isolated as a white solid in 79% yield; Rf (cyclohexane/EtOAc: 60/40) = 0.5; mp = 100–102 °C; [α]D20 +70 (c 1.5 mg/mL, CH2Cl2); IR (νmax/cm−1) 2936.46 (CH str.), 1702.26 (4 × C=O); 1H NMR (300 MHz, CDCl3) δH 7.75, (s, 1H, CHaro/Trz), 7.69 (d, J = 7.5 Hz, 1H,CHaro), 7.63–7.53 (m, 2H, 2 × CHaro), 7.50–7.42 (m, 1H, CHaro), 7.40–7.28 (m, 5H, 5 × CHaro), 6.86 (td, J = 7.9, 2.5 Hz, 1H, CHaro), 6.56 (d, J = 7.8 Hz, 1H, CHaro), 6.16 (t, J = 6.3 Hz, 1H, CHaro), 5.99 (s, 1H, CHaro), 5.27 (t, J = 3.9 Hz, 1H, CH=C), 5.10–4.85 (m, 4H, TrzCH2CO2, OCH2Ph), 4.86 (s, 2H, NCH2Trz), 4.54 (t, J = 7.9 Hz, 1H, CHCO2), 4.12–4.01 (m, 2H, CH3CH2O), 3.83 (d, J = 14.7 Hz, 1H, CCH2b3-OMePh), 3.57 (d, J = 14.7 Hz, 1H, CCH2a3-OMePh), 3.49 (s, 3H, CH3O), 2.89 (dd, J = 13.8, 4.4 Hz, 1H, C=CCHCH2), 2.18–1.72 (m, 4H, 2 × CH2), 1.70–1.61 (m, 4H, 2 × CH2), 1.58–1.45 (m, 4H, 2 × CH2), 1.43–1.18 (m, 8H, 4 × CH2), 1.16 (d, J = 3.7 Hz, 1H, CH), 1.10 (s, 3H, CH3), 1.08–1.02 (m, 3H, CH3CH2O), 1.01–0.97 (m, 1H, CH), 0.93–0.79 (m, 12H, 4 × CH3), 0.72 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (75 MHz,CDCl3) δC 177.52 (C=O), 169.96 (C=O), 169.28 (C=O), 165.83 (NC=O), 158.96 (Cq), 143.95 (Cq), 143.81 (Cq), 136.50 (Cq), 135.19 (Cq), 132.01 (CHaro), 131.55 (2 × Cq), 129.28 (CHaro), 128.86 (CHaro), 128.50 (2 × CHaro), 128.06 (2 × CHaro), 127.99 (CHaro), 125.74 (CHaro), 123.71 (CHaro), 122.89 (CHaro), 122.38 (CH=C), 122.17 (CHaro/Trz), 114.87 (CHaro), 113.00 (CHaro), 83.81 (CHCO2), 72.80 (Cq), 66.00 (OCH2Ph), 62.64 (CH3CH2O), 55.25 (CH), 54.97 (CH3O), 51.14 (TrzCH2CO2), 47.55 (CH), 46.80 (Cq), 45.91 (CH2), 41.74 (Cq), 41.43 (C=CCHCH2), 39.54 (CCH23-OMePh), 39.33 (Cq), 38.04 (CH2), 37.81 (Cq), 37.65 (NCH2Trz), 36.90 (Cq), 33.92 (CH2), 33.18 (CH3), 32.64 (CH2), 32.43 (CH2), 30.78 (Cq), 28.17 (CH3), 27.66 (CH2), 25.92 (CH3), 23.73 (CH3), 23.45 (2 × CH2), 23.10 (CH2), 18.20 (CH2), 16.92 (CH3), 16.63 (CH3), 15.38 (CH3), 13.84 (CH3CH2O); HRMS (+ESI) calculated for C61H77N4O8 [M + H]+: 993.5697, found: 993.5744.
Ethyl1,2-bis((1-(2-(((3S,4aR,6aR,6bS,8aS,12aR,14aS,14bS)-8a-((benzyloxy)carbonyl)-4,4,6a,6b,11,11,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl)oxy)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-3-oxo-2,3-dihydro-1H-isoindole-1-carboxylate (18u), This derivative was isolated as a white solid in 80% yield; Rf (cyclohexane/EtOAc: 50/50) = 0.4; mp = 143–145 °C; [α]D20 +63 (c 0.9 mg/mL, CH2Cl2); IR (νmax/cm−1) 2938.94 (CH str.), 1728.35 (6 × C=O); 1H NMR (300 MHz, CDCl3)δH 7.92 (d, J = 6.6 Hz, 1H,CHaro), 7.73–7.61 (m, 2H, 2 × CHaro), 7.46–7.28 (m, 12H, 12 × CHaro), 6.31 (d, J = 16.1 Hz, 1H,CHaro), 5.26 (t, J = 3.6 Hz, 2H, 2 × CH=C), 5.24–4.99 (m, 7H, TrzCH2aCO2, TrzCH2CO2, 2 × OCH2Ph), 4.85 (t, J = 3.8 Hz, 2H, 2 × CHCO2), 4.67 (dd, J = 15.8, 4.5 Hz, 1H, TrzCH2aCO2), 4.60–4.33 (m, 2H, NCH2Trz), 4.30–4.05 (m, 3H, CCH2bTrz,CH3CH2O), 3.89 (dd, J = 15.3, 4.3 Hz, 1H, CCH2aTrz), 2.92 (dd, J = 13.6, 4.4 Hz, 2H, 2 × C=CCHCH2), 2.05–1.74 (m, 8H, 4 × CH2), 1.72–1.62 (m, 8H, 4 × CH2), 1.60–1.51 (m, 8H, 4 × CH2), 1.48–1.24 (m, 16H, 8 × CH2),1.21 (t, J = 3.6 Hz, 3H, CH3CH2O), 1.17–1.14 (m, 2H, 2 × CH), 1.11 (s, 6H, 2 × CH3), 1.04–1.00 (m, 2H, 2 × CH), 0.94–0.80 (m, 24H, 8 × CH3), 0.77–0.67 (m, 6H, 2 × CH3), 0.61 (s, 6H, 2 × CH3); 13C NMR (75 MHz,CDCl3) δC 177.55 (2 × C=O), 169.68 (C=O), 169.61 (C=O), 166.61 (C=O), 166.04 (NC=O), 144.52 (Cq), 143.85 (2 × Cq), 142.98 (Cq), 140.25 (Cq), 136.53 (Cq), 132.45 (CHaro), 130.98 (Cq), 129.39 (CHaro), 128.53 (4 × CHaro), 128.09 (4 × CHaro), 128.03 (2 × CHaro), 126.01 (CHaro), 123.51 (2 × CHaro/Trz), 123.26 (CHaro), 122.38 (2 × CH=C), 84.31 (CHCO2), 83.33 (CHCO2), 72.40 (Cq), 66.04 (2 × OCH2Ph), 62.92 (CH3CH2O), 55.24 (2 × CH), 51.03 (2 × TrzCH2CO2), 47.59 (2 × CH), 46.84 (2 × Cq), 45.97 (2 × CH2), 41.80 (2 × Cq), 41.48 (2 × C=CCHCH2), 39.37 (2 × Cq), 38.08 (2 × CH2), 37.74 (Cq), 37.23 (NCH2Trz), 36.95 (2 × Cq), 33.96 (2 × CH2), 33.23 (2 × CH3), 32.68 (2 × CH2), 32.46 (2 × CH2), 30.81 (2 × Cq), 30.11 (CCH2Trz), 28.20 (2 × CH3), 27.72 (2 × CH2), 25.96 (2 × CH3), 23.76 (2 × CH3), 23.50 (4 × CH2), 23.14 (2 × CH2), 18.27 (2 × CH2), 16.98 (2 × CH3), 16.63 (2 × CH3), 15.46 (2 × CH3), 14.10 (CH3CH2O); HRMS (+ESI) calculated for C95H125N7O11Na [M+Na]+: 1562.9329, found: 1562.9335.
All 1H, 13C NMR and HRMS spectra are provided as Supplementary Materials.
3.3. Antibacterial Activity
In the present study, the antimicrobial activity of the starting product OA-1 and its derivatives 18a–u was screened by the agar disc diffusion method according to the protocol described by Dbeibia et al. (2022) [88] against four bacteria, namely Staphylococcus aureus ATCC 25923, Listeria monocytogenes ATCC 19115, Salmonella thyphimurium ATCC 14,080, and Pseudomonas aeruginosa ATCC 27853. The inoculums of the microorganisms were adjusted to 0.1 at OD600 and then streaked onto Muller Hinton (MH) agar plates using a sterile cotton mop. Sterile filter discs (diameter 6 mm, Biolife Italy) were placed at the surface of the appropriate agar mediums and 20 µL of the product (10 mg/mL) was dropped onto each disc. Tetracycline and chlorhexidine (10 mg/mL; 20 μL/disc) were used as reference antibiotics. After incubation at 37 °C for 24 h, the antibacterial activities were evaluated by measuring an inhibition zone formed around the disc. Each assay was performed in triplicate. The minimum inhibitory concentration (MIC) was evaluated as recommended by Dbeibia et al. (2022) [88]. Briefly, serial dilutions of the synthesized compounds (3.9–2000 µg/mL) were filled in 96 U bottomed-wells plates (Nunc, Roskilde, Denmark) with MH broth and the target bacteria. The treated plates were left to incubateovernight at 37 °C. The MIC was reported as the lowest concentration of the sample that did not allow the growth of microorganisms and do not show visible turbidity of the broth medium. The minimum bactericidal concentration (MBC) was evaluated by transferring 10 µL from the well showing no bacteria growth after MIC assay, on MH agar. After a 24 h period of incubation at 37 °C, the bacterial growth was examined and the MBC was determined as the lowest concentration of the sample having bactericidal activity.
3.4. Molecular Docking Procedure
Molecular docking studies were performed by using Auto Dock 4.2 program package [89]. The optimization of all the geometries of ligands was carried out by ACD (3D viewer) software (http://www.filefacts.com/acd3d-viewer-freeware-info (accessed on 18 January 2023)) and the three dimensional structure of PDB (PDB: 5F7V) [87] was obtained from the RSCB protein data bank (https://www.rcsb.org/ (accessed on 18 January 2023)). Before docking, the water molecules have been erased and the missing hydrogens in additionto Gasteiger charges were then added to the system during the receptor preparation input file. Then, the AutoDock Tools were used for the preparation of all ligands and protein files (PDBQT). The pre-calculation of grid maps was performed by Auto Grid for saving a lot of time during the docking procedure and the docking calculation was carried out by a grid per map with 40 × 40 × 40 A˚ points ofall PDB used in additionto the grid-point spacing of 0.375 A˚, that was centered on the receptor structure in order to determine the active site andthe visualization and analysis of interactions were performed using Discovery Studio 2017R2 (https://www.3dsbiovia.om/products/collaborative-science/biovia-discovery-studio/, accessed on 5 June 2023). Further, all molecular docked models for the cavities 3D were prepared via PyMOL viewer v. 0.99 [90].
3.5. Statistical Analysis
Statistical analysis was carried out using Graph Pad Prism 7.0 (Graph Pad Software Inc., CA, USA). The experimental data of the antibacterial activity expressed in the inhibition zone are presented as mean ± standard error of the mean (SEM). Student’s t-test was used to assess the difference between two groups. For significant differences among three or more groups, one-way ANOVA with post hoc analysis was performed.
4. Conclusions
In summary, oleanolic acid (OA-1) isolated from olive pomace (Olea europaea L.) was used as a starting material to prepare a series of new (OA-1)-phtalimidines coupled 1,2,3-triazole derivatives by application of the Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reaction. The designed cycloadducts were assessed for their antibacterial activity against two Gram-positive (S. aureus and L. monocytogenes) and two Gram-negative (S. thyphimurium and P. Aeruginosa) strains. Significant antibacterial activities were observed notably against L. Monocytogenes. Interestingly, the derivative 18g exhibited the highest activity toward this strain (MIC = 9.48 µmol/L) followed by 18d (MIC = 9.56 µmol/L) and 18h (MIC = 9.89 µmol/L), compared to tetracycline and chlorhexidine (reference antibiotics). The in silico molecular docking study revealed that the selected lead compounds 18c, 18d, 18h, and 18k can fit well into the binding cavity of the ABC substrate-binding protein Lmo0181 from L. monocytogenes. These results constitute a basis for future works of optimization and expansion of the antibacterial spectrum, especially of derivatives of (OA-1)-phthalimidines against Gram-positive bacteria. This also makes it possible to better understand the mechanism of action and the resistance potential of these strains against this new series of semi-synthetic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28124655/s1.
Author Contributions
Conceptualization, Methodology and Data curation, G.L.; Software, Visualization, Validation, M.H.; Writing—Original draft preparation, G.L. and M.H.; Conducting the testing of antibacterial activity, A.D. (Amal Dbeibia) and A.M.; Validation, A.M. and A.H.H.; Supervision, A.R., A.M.L. and A.D. (Adam Daïch); Writing- Reviewing and Editing, H.B.J. and M.O. The manuscript was a collaborative effort by all authors. The final version of the manuscript has received approval from all the authors.All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Campus France, the Ministry of Higher Education and Scientific Research of Tunisia and Le Havre Normandie University, France via Hubert Curien-Utique program (PHC-Utique) (Tunisien code: 22G1202; Frensh code 47661YE). It was also funded by King Saud University, Riyadh, Saudi Arabia for the Researchers Supporting Project number (RSP202317).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The source data underlying tables and figures are available from the authors upon request.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number RSP2023R17, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no financial competing interest.
Sample Availability
Samples of the compounds are available from the authors upon request.
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the evolution, approaches and perspectives on alternative uses. Global. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, E.-J.; Ku, S.-K.; Song, K.-S.; Bae, J.-S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, Q.; Bo, S.; Zeng, X.; Wei, Y.; Lei, S.; Xiao, X.; Xiao, J.; Wang, Z. Advances in research on hepatoprotective activity and synthesis of oleanolic acid derivatives. J. Appl. Biopharm. Pharmacokinet. 2015, 3, 27–33. [Google Scholar] [CrossRef]
- Ghafoor, K. Antioxidant properties of oleanolic acid from grape peel. Agro-Food Ind. Hi Tech. 2014, 25, 54–57. [Google Scholar]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosisin HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef]
- Yamai, H.; Sawada, N.; Yoshida, T.; Seike, J.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Kondo, K.; Bando, Y.; Ohnishi, Y.; et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro andsuppress experimental metastasis in vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef]
- Chen, F.F.; Wang, J.T.; Zhang, L.X.; Xing, S.F.; Wang, Y.X.; Wang, K.; Deng, S.L.; Zhang, J.Q.; Tang, L.; Wu, H.S. Oleanolic acid derivative DKS26 exerts antidiabetic and hepatoprotective effects in diabetic mice and promotes glucagon-like peptide-1 secretion and expression in intestinal cells. Br. J. Pharmacol. 2017, 174, 2912–2928. [Google Scholar] [CrossRef]
- Melo, T.S.; Gattass, C.R.; Soares, D.C.; Cunha, M.R.; Ferreira, C.; Tavares, M.T.; Saraiva, E.; Parise-Filho, R.; Braden, H.; Delorenzi, J.C. Oleanolic acid (OA) as an antileishmanial agent: Biological evaluation and in silico mechanistic insights. Parasitol. Int. 2016, 65, 227–237. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Hichri, F.; Ben Jannet, H.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
- Chouaïb, K.; Hichri, F.; Nguir, A.; Daami-Remadi, M.; Elie, N.; Touboul, D.; Ben Jannet, H. Semi-synthesis of new antimicrobial esters from the natural oleanolic and maslinic acids. Food Chem. 2015, 183, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lim, Y.K.; Choi, M.H.; Cho, E.; Bang, I.S.; Kim, J.M.; Ahn, S.J.; Kook, J.K. Antimicrobial mechanism of oleanolic and ursolic acids on Streptococcus mutans UA159. Curr. Microbiol. 2018, 75, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; Piccionello, A.P.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; et al. New tricyclic systems as photosensitizers towards triple negative breast cancer cells. Arch. Pharm. Res. 2022, 45, 806–821. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Savela, R.; Méndez-Gálvez, C. Isoindolinone synthesis via one-pot type transition metal catalyzed C-C bond forming reactions. Chem. Eur. J. 2021, 27, 5344–5378. [Google Scholar] [CrossRef]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef]
- Thakur, K.; Singh, G. A comprehensive review on SAR and activities of Isoindolinone. Eur. J. Mol. Clin. Med. 2020, 7, 3658–3668. [Google Scholar]
- Othman, I.M.; Gad-Elkareem, M.A.; El-Naggar, M.; Nossier, E.S.; Amr, A.E.G.E. Novel phthalimide based analogues: Design, synthesis, biological evaluation, and molecular docking studies. J. Enzym. Inhib. Med. Chem. 2019, 34, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Ferland, J.-M.; Demerson, C.A.; Humber, L.G. Synthesis of derivatives of isoindole and of pyrazino[2,1-a]isoindole. Can. J. Chem. 1985, 63, 361–365. [Google Scholar] [CrossRef]
- Zhuang, Z.-P.; Kung, M.-P.; Mu, M.; Kung, H.F. Isoindol-1-one analogues of 4-(2‘-methoxyphenyl)-1-[2‘-[N-(2‘‘-pyridyl)-p-iodobenzamido]ethyl]piperazine (p-MPPI) as 5-HT1A receptor ligands. J. Med. Chem. 1998, 41, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.; Hadler, D.; Hofmann, S. Randomized, double-blind, placebo-controlled trial of the efficacy and tolerability of a new isoindoline derivative (DN-2327) in generalized anxiety. Hum. Psychopharmacol. 1997, 12, 445–452. [Google Scholar] [CrossRef]
- Norman, M.H.; Minick, D.J.; Rigdon, G.C. Effect of linking bridge modifications on the antipsychotic profile of some phthalimide and isoindolinone derivatives. J. Med. Chem. 1996, 39, 149–157. [Google Scholar] [CrossRef]
- Meng, X.B.; Han, D.; Zhang, S.N.; Guo, W.; Cui, J.R.; Li, Z.J. Synthesis and anti-inflammatory activity of N-phthalimidomethyl 2,3-dideoxy- and 2,3-unsaturated glycosides. Carbohydr. Res. 2007, 342, 1169–1174. [Google Scholar] [CrossRef]
- Marushiiri, A. 2-(Omega-Alkylaminoalkyl)- and 2-(Omega-diAlkylaminoalkyl)-3-(4-x-Benzylidene)- Phthalimidines. J.P.S. Patent 5,946,268, 15 March 1984. [Google Scholar]
- Achinami, K; Ashizawa, N; Kobayasui, F. Alkylidene Isoindolinone Derivative and Vasodilator Containing Same Derivative As Active Ingredient. J.P.H. Patent 03,133,955, 07 June 1991.
- De Clercq, E. Toward Improved Anti-HIV Chemotherapy: Therapeutic Strategies for Intervention with HIV Infections. J. Med. Chem. 1995, 38, 2491–2517. [Google Scholar] [CrossRef]
- Pendrak, I.; Barney, S.; Wittrock, R.; Lambert, D.M.; Kingsbury, W.D. Synthesis and Anti-HSV Activity of A-Ring-Deleted Mappicine Ketone Analog. J. Org. Chem. 1994, 59, 2623–2625. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Ravindranath, K.R.; Viswanathan, N. Mappicine, a minor alkaloid from Mappiafoetidamiers. J. Chem. Soc. Perkin Trans. 1974, 1, 1215–1217. [Google Scholar] [CrossRef]
- Mertens, A.; Zilch, J.H.; Konig, B.; Schafer, W.; Poll, T.; Kampe, W.; Seidel, S.; Leser, U.; Leinert, H. Selective non-nucleoside HIV-1 reverse transcriptase inhibitors. New 2,3-dihydrothiazolo[2,3-a]isoindol-5(9bH)-ones and related compounds with anti-HIV-1 activity. J. Med. Chem. 1993, 36, 2526–2535. [Google Scholar] [CrossRef]
- Rezaei, Z.; Moghimi, S.; Javaheri, R.; Asadi, M.; Mahdavi, M.; Shabani, S.; Edraki, N.; Firuzi, O.; Safavi, M.; Amini, M.; et al. Synthesis and biological evaluation of 1, 3, 4-thiadiazole linked phthalimide derivatives as anticancer agents. Lett. Drug Des. Discov. 2017, 14, 1138–1144. [Google Scholar] [CrossRef]
- Taylor, E.C.; Zhou, P.; Jenning, L.D.; Mao, Z.; Hu, B.; Jun, J.-G. Novel synthesis of a conformationally-constrained analog of DDATHF. Tetrahedron Lett. 1997, 38, 521–524. [Google Scholar] [CrossRef]
- Armoiry, X.; Aulagner, G.; Facon, T. Lenalidomide in the treatment of multiple myeloma: A review. J. Clin. Pharm. Ther. 2008, 33, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Lee, K.R. Isohericenone, a new cytotoxic isoindolinone alkaloid from Hericiumerinaceum. J. Antibiot. 2012, 65, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.M.; El-masry, A.H.; Mohamed, N.A.; Awad, G.E.; Habib, B.S. Synthesis, characterization and antimicrobial activity of some novel isoindole-1,3-dione derivatives. Der. Pharm. Chem. 2013, 5, 97–108. [Google Scholar]
- Dabholkar, V.V.; Udawant, D.; Jaiswar, R.; Gopinathan, A. Novel 2-(5-(substituted-benzyl)-1,3,4-thiadiazol-2-yl)-substituted-isoindoline-1,3-dione derivatives—Their one pot synthesis and Antimicrobial evaluation. J. Chem. Sci. 2019, 9, 274–280. [Google Scholar]
- Takahashi, I.; Kawakami, T.; Hirano, V.; Yokota, H.; Kitajima, H. Novel phthalimidine synthesis. Mannich condensation of o-phthalaldehyde with primary amines using 1,2,3-1H-benzotriazole and 2-mercaptoethanol as dual synthetic auxiliaries. Synlett 1996, 4, 353–355. [Google Scholar] [CrossRef]
- Klaus, S.; Thomas, M. The chemistry of isoindole natural products. Beilstein. J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef]
- Kovtunenko, V.A.; Voitenko, Z.V. The chemistry of isoindoles. Russ. Chem. Rev. 1994, 63, 997–1018. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; et al. Concept of hybrid drugs and recent advancements in anticancer hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef] [PubMed]
- Marinho, J.A.; Guimarães, D.S.M.; Glanzmann, N.; de Almeida Pimentel, G.; da Costa Nunes, I.K.; Pereira, H.M.G.; Navarro, M.; de PillaVarotti, F.; da Silva, A.D.; Abramo, C. In vitro and in vivo antiplasmodial activity of novel quinoline derivative compounds by molecular hybridization. Eur. J. Med. Chem. 2021, 215, 113271. [Google Scholar] [CrossRef]
- Hosseini-Zare, M.S.; Sarhadi, M.; Zarei, M.; Thilagavathi, R.; Selvam, C. Synergistic effects of curcumin and its analogs with other bioactive compounds: A comprehensive review. Eur. J. Med. Chem. 2021, 210, 113072. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef]
- Cars, O.; Nordberg, P. Antibiotic resistance–The faceless threat. Int. J. Risk Saf. Med. 2005, 17, 103–110. [Google Scholar]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Mashayekh, K.; Shiri, P. An overview of recent advances in the applications of click chemistry in the synthesis of bioconjugates with anticancer activities. Chem. Select. 2019, 4, 13459–13478. [Google Scholar] [CrossRef]
- Brennan, J.L.; Hatzakis, N.S.; Tshikhudo, T.R.; Dirvianskyte, N.; Razumas, V.; Patkar, S.; Vind, J.; Svendsen, A.; Nolte, R.J.; Rowan, A.E.; et al. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjug. Chem. 2006, 17, 1373–1375. [Google Scholar] [CrossRef]
- Zaia, J. The 2022 Nobel Prize in Chemistry for the development of click chemistry and bioorthogonal chemistry. Anal. Bioanal. Chem. 2023, 415, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Huang, T.; Dick, A.; Meuser, M.E.; Zalloum, W.A.; Chen, C.H.; Ding, X.; Gao, P.; Cockin, S.; Lee, K.-H.; et al. Design, synthesis andstructure-activity relationships of 4-phenyl-1H-1, 2, 3-triazole phenylalaninederivatives as novel HIV-1 capsid inhibitors with promising antiviral activities. Eur. J. Med. Chem. 2020, 190, 112085–112103. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, C.P.; Sangwan, J. Regioselective synthesis, antibacterial, and antioxidant activities of ester-linked 1, 4-disubstituted 1, 2, 3-triazoles. Monatsh. Chem. 2020, 151, 807–819. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Chahal, M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1, 4-disubstituted 1, 2, 3-triazoles. Monatsh. Chem. 2021, 152, 1001–1012. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Gao, F.; Wang, Y.L.; Zhang, F.; Bai, L.Y.; Deng, J.L.; Wang, Q.; Fan, Y.L. Design, synthesis, and evaluation of tetraethylene glycol-tetheredisatin–1, 2, 3-triazole–coumarin hybrids as novel anticancer agents. J. Heterocycl. Chem. 2019, 56, 1127–1132. [Google Scholar] [CrossRef]
- Endoori, S.; Gulipalli, K.C.; Bodige, S.; Ravula, P.; Seelam, N. Design, synthesis, anticancer activity, and in silico studies of novel imidazo[1, 2-a]pyridinebased 1 H-1, 2, 3-triazole derivatives. J. Heterocycl. Chem. 2021, 58, 1311–1320. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, L.; Huang, X.; Wang, X.; Gong, M. Chalcone hybrids and theirantimalarial activity. Arch. Pharm. 2020, 353, 1900350–1900360. [Google Scholar] [CrossRef]
- Santos, B.M.D.; Gonzaga, D.T.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R. Plasmodium falciparum knockout for the GPCR-like PfSR25 receptor displays greatersusceptibility to 1, 2, 3-triazole compounds that block malaria parasite development. Biomolecules 2020, 10, 1197–1210. [Google Scholar] [CrossRef]
- Vagish, C.B.; Sudeep, P.; Jayadevappa, H.P.; Ajay Kumar, K. 1, 2, 4-Triazoles: Synthetic and medicinal perspectives. Int. J. Curr. Res. 2020, 12, 12950–12960. [Google Scholar] [CrossRef]
- Bivacqua, R.; Romeo, I.; Barreca, M.; Barraja, P.; Alcaro, S.; Montalbano, A. HSV-1 Glycoprotein D and Its Surface Receptors: Evaluation of Protein–Protein Interaction and Targeting by Triazole-Based Compounds through In Silico Approaches. Int. J. Mol. Sci. 2023, 24, 7092. [Google Scholar] [CrossRef] [PubMed]
- BezerraMorais, P.A.; Javarini, C.L.; Valim, T.C.; Francisco, C.S.; de Freitas Ferreira, L.C.; TrancosoBottocim, R.R.; Neto, Á.C.; Júnior, V.L. Triazole: A New Perspective in Medicinal Chemistry and Material Science. Curr. Org. Chem. 2022, 26, 1691–1702. [Google Scholar] [CrossRef]
- Bivacqua, R.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Andrei, G.; Barraja, P.; Montalbano, A. Insight into non-nucleoside triazole-based systems as viral polymerases inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: A brief review. Future J. Pharm. Sci. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3, 5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, L.; Li, L.; Liu, J.; Li, H.; Zhang, L.; Chen, L.; Cheng, K.; Zheng, M.; Wen, X.; et al. Identification of pentacyclic triterpenes derivatives as potent inhibitors against glycogen phosphorylase based on 3D-QSAR studies. Eur. J. Med. Chem. 2011, 46, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, X.; Dong, J.; Chen, D.; Liu, J.; Zhang, L.; Wen, X. Synthesis and evaluation of novel oleanolic acid derivatives as potential antidiabetic agents. Chem. Biol. Drug Des. 2014, 83, 297–305. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Hawker, C.J. A general approach to sequence-controlled polymers using macrocyclic ring opening metathesis polymerization. J. Am. Chem. Soc. 2015, 137, 8038–8041. [Google Scholar] [CrossRef]
- Rubio-Ruiz, B.; Perez-Lopez, A.M.; Sebastián, V.; Unciti-Broceta, A. A minimally-masked inactive prodrug of panobinostat that is bioorthogonally activated by gold chemistry. Bioorg. Med. Chem. 2021, 41, 116217. [Google Scholar] [CrossRef]
- Rateb, H.S.; Ahmed, H.E.; Ahmed, S.; Ihmaid, S.; Afifi, T.H. Discovery of novel phthalimide analogs: Synthesis, antimicrobial and antitubercular screening with molecular docking studies. EXCLI J. 2016, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Hadley, M.; Ustinova, K.; Pavlicek, J.; Knox, T.; Noonepalle, S.; Tavares, M.T.; Zimprich, C.A.; Zhang, G.; Robers, M.B.; et al. Discovery of a new isoxazole-3-hydroxamate-based histone deacetylase 6 inhibitor SS-208 with antitumor activity in syngeneic melanoma mouse models. J. Med. Chem. 2019, 62, 8557–8577. [Google Scholar] [CrossRef] [PubMed]
- Anwar, U.; Casaschi, A.; Grigg, R.; Sansano, J.M. Palladium catalysed queuing processes. Part 2: Termolecular cyclization–anion capture employing carbon monoxide as a relay switch with in situ generated vinylstannanes. Tetrahedron 2001, 57, 1361–1367. [Google Scholar] [CrossRef]
- Padula, D.; Mazzeo, G.; Santoro, E.; Scafato, P.; Belviso, S.; Superchi, S. Amplification of the chiroptical response of UV-transparent amines and alcohols by N-phthalimide derivatization enabling absolute configuration determination through ECD computational analysis. Org. Biomol. Chem. 2020, 18, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Kumara Swamy, K.C.; Bhuvan Kumar, N.N.; Balaraman, E.; Pavan Kumar, K.V.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef]
- Kumari Sahu, A.; Unnava, R.; Shit, S.; Saikia, A.K. In(OTf)3-catalyzed one-pot tandem mannich and conia-ene cyclization reaction of N-propargyl amido alcohols with 1,3-dicarbonyl compounds: An approach to construct tetrahydro-1H-pyrrolo[2,1-a]isoindolone-1,1-dicarboxylate and its application. J. Org. Chem. 2020, 85, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Szemes, F.; Fousse, A.; Bousquet, T.; Ben Othman, R.; Othman, M.; Dalla, V. Towards simplifying the chemistry of N-acyliminium ions: A one-pot protocol for the preparation of 5-acetoxy pyrrolidin-2-ones and 2-acetoxy N-alkoxycarbonyl pyrrolidines from imides. Synthesis 2006, 5, 875–879. [Google Scholar] [CrossRef]
- Ben Othman, R.; Othman, M.; Coste, S.; Decroix, B. One-pot enyne metathesis / diels–alder reaction for the construction of highly functionalized novel polycyclic aza-compounds. Tetrahedron 2008, 64, 559–567. [Google Scholar] [CrossRef]
- Rammah, M.M.; Othman, M.; Ciamala, K.; Strohmann, C.; Rammah, M.B. Silver-catalyzed spirolactonization: First synthesis of spiroisoindole-γ-methylene-γ-butyrolactones. Tetrahedron 2008, 64, 3505–3516. [Google Scholar] [CrossRef]
- Chortani, S.; Othman, M.; Lawson, A.M.; Romdhane, A.; Ben Jannet, H.; Knorr, M.; Brieger, L.; Strohmann, C.; Daïch, A. Aza-heterocyclic frameworks through intramolecular π-system trapping of spiro-N-acyliminiums generated from isoindolinone. New J. Chem. 2021, 45, 2393–2403. [Google Scholar] [CrossRef]
- Pesquet, A.; Marzag, H.; Knorr, M.; Strohmann, C.; Lawson, A.M.; Ghinet, A.; Dubois, J.; Amaury, F.; Daïch, A.; Othman, M. Access to 3-spiroindolizines containing an isoindole ring through intra-molecular arylation of spiro-N-acyliminium species: A new family of potent farnesyltransferase inhibitors. Org. Biomol. Chem. 2019, 17, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Yadava, P.; Kaushik, C.P.; Mukesh Kumar, M.; Kumar, A. Phthalimide/Naphthalimide containing 1,2,3-triazole hybrids: Synthesis and antimicrobial evaluation. J. Mol. Struct. 2023, 1276, 134688. [Google Scholar] [CrossRef]
- Horchani, M.; Hajlaoui, A.; Harrath, A.H.; Mansour, L.; Ben Jannet, H.; Romdhane, A. New pyrazolo-triazolo-pyrimidine derivatives as antibacterial agents: Design and synthesis, molecular docking and DFT studies. J. Mol. Struct. 2020, 1199, 127007. [Google Scholar] [CrossRef]
- Horchani, M.; Edziri, H.; Harrath, A.H.; Ben Jannet, H.; Romdhane, A. Access to new schiff bases tethered with pyrazolopyrimidinone as antibacterial agents: Design and synthesis, molecular docking and DFT analysis. J. Mol. Struct. 2022, 1248, 131523. [Google Scholar] [CrossRef]
- Light, S.H.; Cahoon, L.A.; Halavaty, A.S.; Freitag, N.E.; Anderson, W.F. Structure to function of an alpha-glucan metabolic pathway that promotes Listeria monocytogenes pathogenesis. Nat. Microbiol. 2016, 2, 16202. [Google Scholar] [CrossRef]
- Dbeibia, A.; Taheur, F.B.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2022, 29, 1021–1028. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrö; Schrödinger, Inc.: New York, NY, USA, 2010.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).