Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups
Abstract
1. Introduction
2. Results
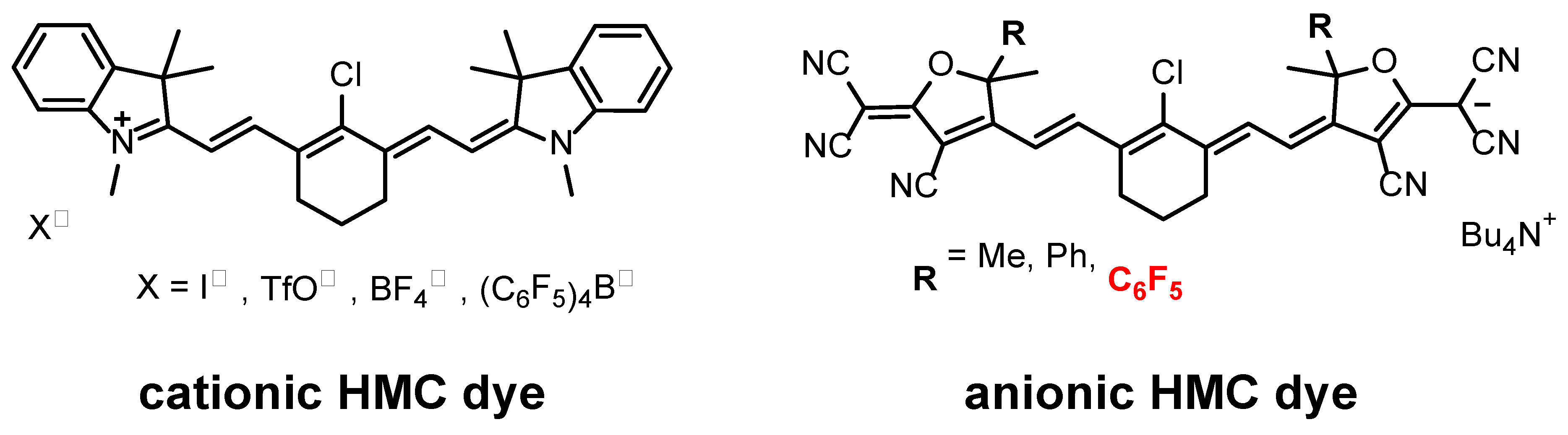
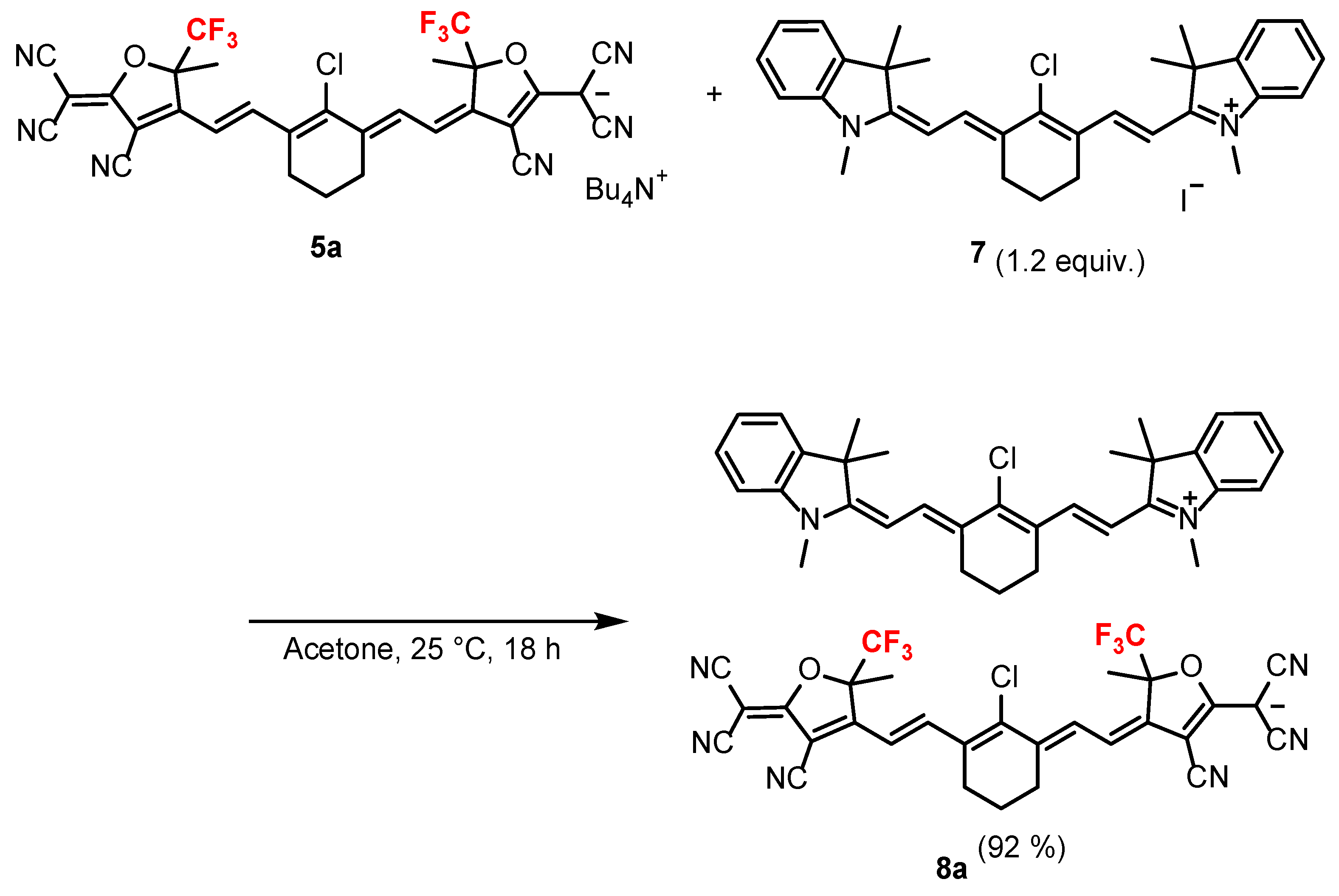
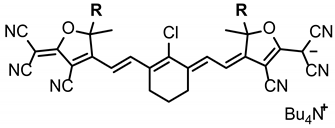
2.1. Synthesis of the Anionic Dye with Trifluoromethyl Groups 5a and 8a
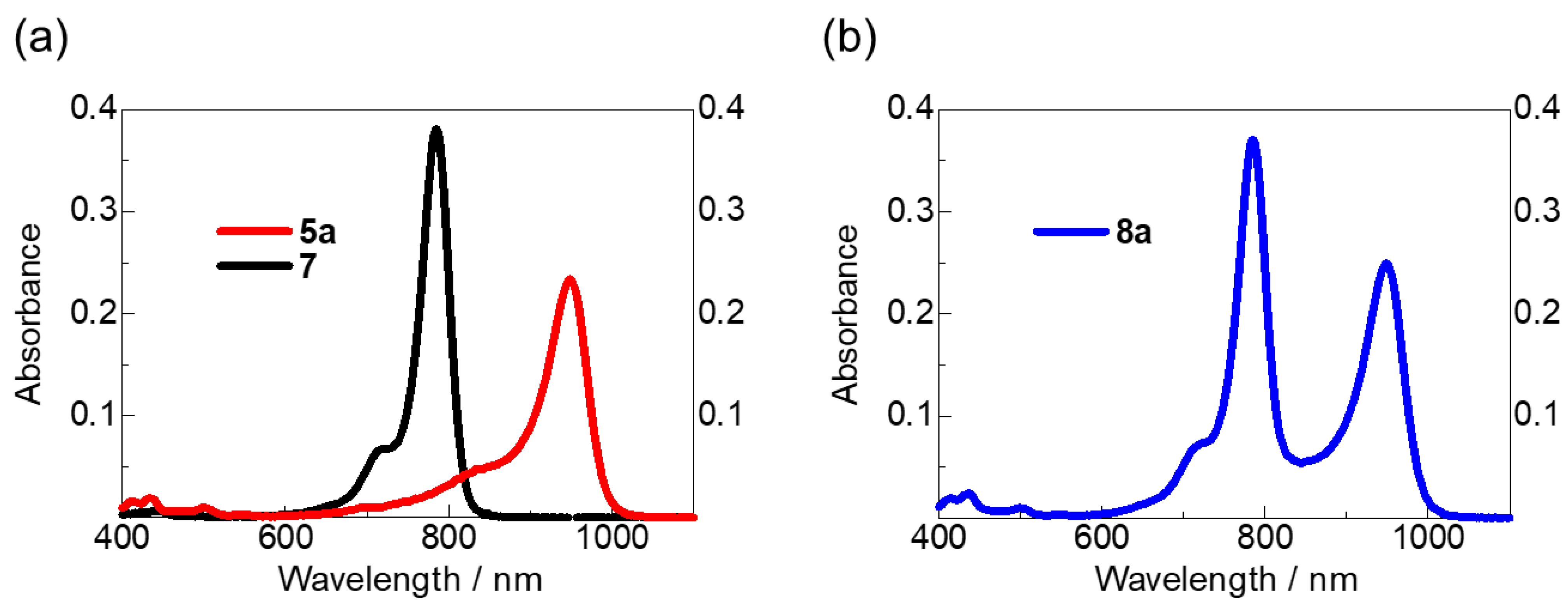
2.2. UV–vis–NIR Spectra and CV Measurements of Anionic HMC Dye 5a and Cyanine–Cyanine Mixed Dye 8a
2.3. The Photostabilities of Anionic HMC Dye 5a and Cyanine−Cyanine Mixed Dye 8a
3. Experimental Section
3.1. Measurements
3.2. Materials
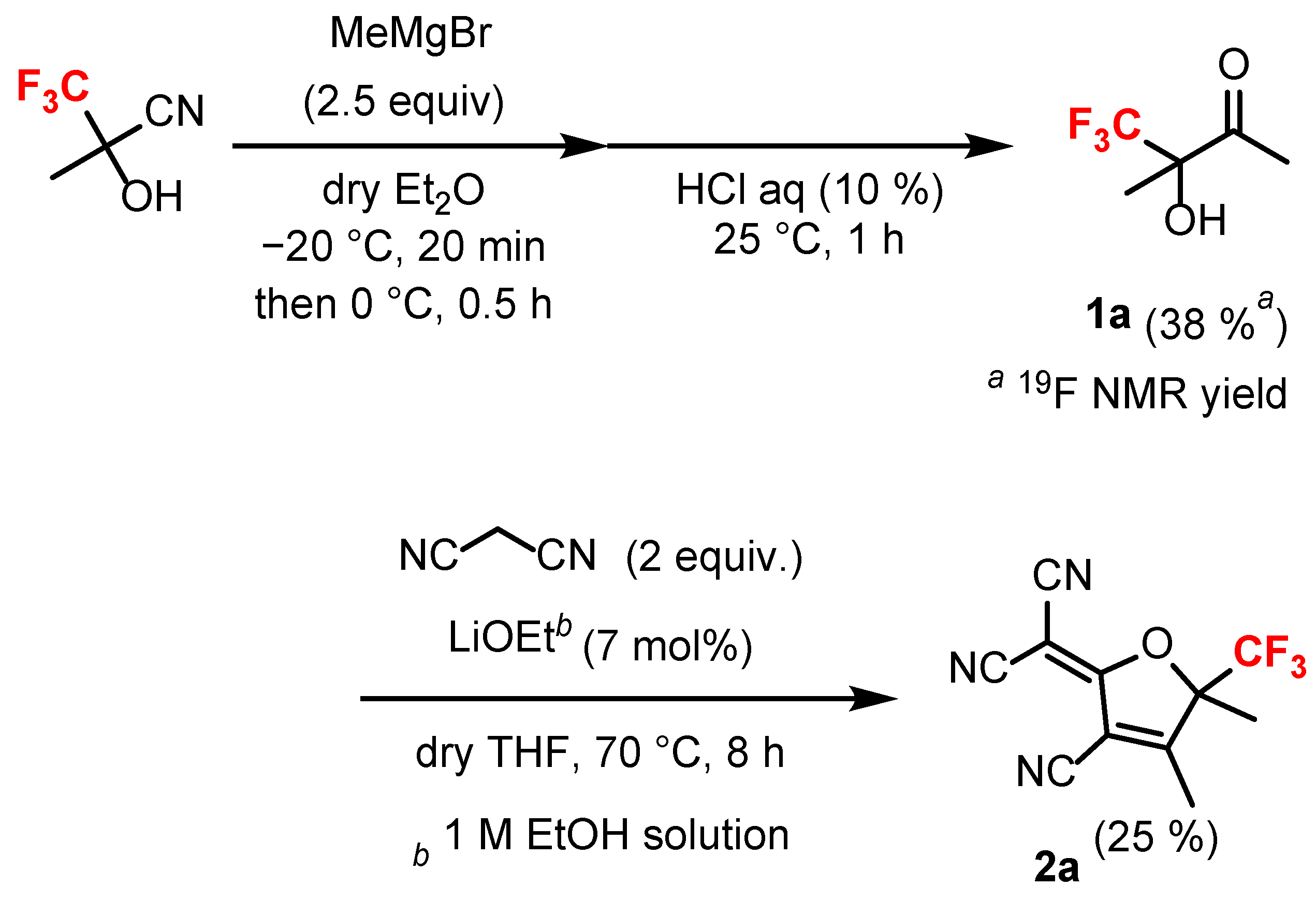
3.3. Synthesis of 4,4,4-Trifluoro-3-hydroxy-3-methylbutan-2-one (1a) [41]
3.4. Synthesis of 2-(2-Cyano-3,4-dimethyl-4-(trifluoromethyl)cyclopent-2-en-1-ylidene)malononitrile (2a) [42]
- First, a mixture of 1a (0.601 g, 3.847 mmol) and malononitrile (0.510 g, 7.724 mmol) was dissolved in an anhydrous THF (4 mL) under an argon atmosphere. Subsequently, 0.269 mL of lithium ethoxide (1.0 M in EtOH, 0.269 mmol) was added dropwise to the solution. The reaction mixture was stirred under reflux for 8 h and then concentrated using a rotary evaporator. The residue was extracted with CH2Cl2 (30 mL × 3), washed with brine, and the combined organic layers were dried over anhydrous Na2SO4. After the organic solvent was concentrated, the crude product was purified via column chromatography on silica gel using CH2Cl2 as the solvent, followed by washing with methanol to produce 2-(2-cyano-3,4-dimethyl-4-(trifluoromethyl)cyclopent-2-en-1-ylidene)malononitrile (2a) with a yield of 25% (0.239 g).
- White solid; Yield 25%; m.p. = 160.1–161.0 °C; Rf 0.49 (CH2Cl2); IR (KBr) 2233 (C≡N) cm−1; HRMS (ESI) found: m/z 254.0555. Calc. for C16H12N3O: 254.0541; 1H NMR (CDCl3) δ 1.84 (s, 3H, –CH3), 2.46 (s, 3H, –CH3); 13C NMR (CDCl3) δ 14.7 (s), 17.6 (s), 62.6 (s), 96.2 (q, J = 32.89 Hz), 107.9 (s), 109.1 (s), 109.5 (s), 109.7 (s), 121.6 (q, J = 285.94 Hz), 172.0 (s), 173.8 (s); 19F NMR (CDCl3) δ −77.23 (s, 3F) (see S2–S4 in the Supplementary Materials).
3.5. Synthesis of (E)-2-Chloro-3-(hydroxymethylene)cyclohex-1-ene-1-carbaldehyde (3) [44,48]
- Phosphoryl chloride (1.90 mL, 20.119 mmol) was added dropwise to DMF (4 mL) at 0 °C under an argon atmosphere, and the reaction mixture was stirred for 30 min. A DMF (1 mL) solution of cyclohexanone (0.514 g, 5.080 mmol) was slowly added to the mixture at 0 °C, followed by stirring for 30 min. After the reaction mixture was heated to 55 °C, it was stirred for 4 h. Next, 150 mL of water and crushed ice was added to the reaction mixture, and the blend was stored in a refrigerator overnight. The precipitate obtained was filtered and washed with water. The yield of the prepared solid, name (E)-2-chloro-3-(hydroxymethylene)cyclohex-1-ene-1-carbaldehyde (3), was 67% (0.595 g).
- Yellow solid; Yield 67%; m.p. = 143.0–145.0 °C; IR (KBr) 1620 (C=O) cm−1; HRMS (ESI) found: m/z 173.0370. Calcd for C8H9ClO2: 173.0369; 1H NMR (DMSO-d6) δ 1.57 (quin, J = 6.17 Hz, 2H, –CH2CH2CH2–), 2.35 (s, 4H, –CH2CH2CH2–), 7.56 (br s, 1H, vinly H), 10.1 (br s, 1H, –CHO), 10.8 (br s, 1H, –OH); 13C NMR (DMSO-d6) δ 20.0 (s), 23.7 (s), 146.1 (s) (see S5 and S6 in the Supplementary Materials).
3.6. Synthesis of Sodium ((Z)-4-((E)-2-(2-Chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (4a)
- A mixture of 2a (0.205 g, 0.810 mmol), 3 (0.069 g, 0.401 mmol), and sodium acetate (0.075 g, 0.890 mmol) in acetic anhydride (8 mL) was stirred at 25 ℃ overnight under an argon atmosphere. The reaction mixture was added to hexane (200 mL) and Et2O (15 mL), and the precipitate was filtered. The dark green solid crude product, namely sodium ((Z)-4-((E)-2-(2-chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (4a) (0.273 g), was used for subsequent reactions without further purification.
3.7. Synthesis of Tetrabutylammonium ((Z)-4-((E)-2-(2-Chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (5a)
- A mixture of 4a (0.273 g, 0.411 mmol) and tetrabutylammonium iodide (0.039 g, 0.452 mmol) in acetone (7 mL) was stirred at 25 °C for 2 h under an argon atmosphere. After the solvent was removed under low pressure, the residue was purified via column chromatography on silica gel using a CH2Cl2–methanol (100:1 (v/v)) solvent to obtain tetrabutylammonium ((Z)-4-((E)-2-(2-chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (5a) with a yield of 13% (0.047 g).
- Dark green solid; Yield 13%; Tdt 238.3 °C; Rf 0.46 (CH2Cl2/methanol = 20/1); IR (KBr) 2214 (C≡N) cm−1; HRMS (ESI) found: m/z 641.0933. Calc. for C30H16ClF6N6O2: Bu4N, 641.0927; 1H NMR (Acetone-d6) δ 0.98 (td, J = 7.31, 1.35 Hz, 12H, –CH2CH2CH2CH3 × 4), 1.44 (sex, J = 7.31 Hz, 8H, –CH2CH2CH2CH3 × 4), 1.77–1.80 (m, 2H, –CH2CH2CH2–), 1.80–1.86 (m, 8H, –CH2CH2CH2CH3 × 4), 1.93 (s, 6H, –CH3 × 2), 2.59–2.73 (m, 4H, –CH2CH2CH2–), 3.41–3.45 (m, 8H, –CH2CH2CH2CH3 × 4), 6.24 (d, J = 13.91 Hz, 2H, vinyl H), 8.58 (d, J = 13.91 Hz, 2H, vinyl H); 13C NMR (Acetone-d6) δ 13.9 (s), 19.7 (s), 20.4 (s), 21.6 (s), 24.4 (s), 26.9 (s), 49.6 (s), 59.4 (s), 85.7 (s), 92.9 (q, J = 31.63 Hz), 109.4 (s), 114.0 (s), 114.6 (s), 124.0 (q, J = 282.50 Hz), 131.4(s), 137.9 (s), 141.7 (s), 149.4 (s), 156.6 (s), 177.2 (s); 19F NMR (Acetone-d6) δ −80.29 (s, 6F) (see S7–S9 and S18 in the Supplementary Materials).
3.8. Synthesis of 1,2,3,3-Tetramethyl-3H-indol-1-ium Iodide (6) [47]
- To an acetonitrile solution (5 mL) of 2,3,3-trimethyl-3H-indole (0.7883 g, 4.950 mmol), iodomethane was added (1.417 g, 9.980 mmol), and the mixture was stirred at 40 °C for 1 day. After the reaction mixture was poured into diethyl ether (75 mL), the precipitate was filtered to 1,2,3,3-tetramethyl-3H-indol-1-ium iodide (3) (1.180 g, 82%).
- Pale pink solid; Yield 82%; m.p. = 248.0–252.0 °C; IR (KBr) 1609 (C=N) cm−1; HRMS (ESI) found: m/z 174.1283 Calc. for C12H16IN: [M-I]+, 174.1254; 1H NMR (DMSO-d6) δ 1.54 (s, 6H, –CH(CH3)(CH3)), 2.79–2.80 (m, 3H, CH3), 3.99 (s, 3H, -NCH3), 7.58–7.65 (m, 2H, aryl H × 2), 7.81–7.88 (m, 1H, aryl H), 7.89–7.95 (m, 1H, aryl H); 13C NMR (DMSO-d6) δ 14.5 (s), 21.7 (s), 34.9 (s), 53.9 (s), 115.1 (s), 123.3 (s), 128.8 (s), 129.2 (s), 141.6 (s), 142.1 (s), 195.9 (s) (see S10 and S11 in the Supplementary Materials).
3.9. Synthesis of 2-((E)-2-((E)-2-Chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,3,3-trimethyl-3H-indol-1-ium Iodide (7) [46]
- (E)-2-chloro-3-(hydroxymethylene)cyclohex-1-ene-1-carbaldehyde (3) (0.085 g, 0.492 mmol) was added to a N,N-dimethylformamide solution (3 mL) of 1,2,3,3-tetramethyl-3H-indol-1-ium iodide (6) (0.3036 g, 1.001 mmol), and the mixture was stirred at 120 °C for 5 h. The reaction mixture was poured into ice water (100 mL), stirred for 30 min, and thereafter refrigerated for 30 min. The resulting precipitate was collected using suction filtration. The residue was purified using silica gel chromatography (dichloromethane/methanol = 25/1) to yield 2-((E)-2-((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,3,3-trimethyl-3H-indol-1-ium iodide (7) (0.229 g, 76%).
- Yellow-green solid; Yield 76%; Tdt 244.0 °C; Rf 0.25 (CH2Cl2/methanol = 25/1); IR (KBr) 1550 (C=N) cm−1; HRMS (ESI) found: m/z 483.2561 Calc. for C32H36N2Cl: [M − I−]+, 483.2567; 1H NMR (CDCl3) δ 1.77 (s, 12H, –C(CH3)2 × 2), 1.96–1.99 (m, 2H, –CH2CH2CH2–), 2.75–2.81 (m, 4H, –CH2CH2CH2–), 3.76 (s, 6H, NCH3 × 2), 6.25 (d, J = 13.91 Hz, 2H, vinyl H×2), 7.17–7.24 (m, 4H, Aryl H), 7.34–7.39 (m, 4H, Aryl H), 8.34 (d, J = 13.91 Hz, 2H, vinyl H×2); 13C NMR (CDCl3) δ 20.8 (s), 27.0 (s), 28.2 (s), 32.8 (s), 49.3 (s), 102.0 (s), 110.9 (s), 122.2 (s), 125.4 (s), 128.1 (s), 129.0 (s), 141.1 (s), 143.0 (s), 144.4 (s), 150.6 (s), 173.0 (s) (see S12, S13 and S18 in the Supplementary Materials).
3.10. Synthesis of 2-((E)-2-((E)-2-Chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,3,3-trimethyl-3H-indol-1-ium ((Z)-4-((E)-2-(2-chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (8a)
- A mixture of 7 (0.008 g, 0.013 mmol) and 5a (0.009 g, 0.0010 mmol) in acetone (2 mL) was stirred at 25 ℃ overnight under an argon atmosphere. After the solvent was removed under low pressure, the residue was purified via column chromatography on silica gel using a CH2Cl2–methanol (200:1 (v/v)) solvent to obtain 2-((E)-2-((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,3,3-trimethyl-3H-indol-1-ium ((Z)-4-((E)-2-(2-chloro-3-((E)-2-(4-cyano-5-(dicyanomethylene)-2-methyl-2-(trifluoromethyl)-2,5-dihydrofuran-3-yl)vinyl)cyclohex-2-en-1-ylidene)ethylidene)-3-cyano-5-methyl-5-(trifluoromethyl)-4,5-dihydrofuran-2-yl)dicyanomethanide (8a) with a yield of 92% (0.011 g).
- Red glossy solid; Yield 92%; Tdt 201.3 °C; Rf 0.55 (CH2Cl2/methanol = 10/1); IR (KBr) 1550 (C=N), 2214 (C≡N) cm−1; HRMS (ESI) found: m/z 483.2566. Calc. for C32H36ClN2: [M]+, 483.2567, m/z 641.0931. Calc. for C30H16ClN6O2F6: [M]−, 641.0927; 1H NMR (Acetone-d6) δ 1.78 (s, 12H, –CH3 × 4), 1.83–1.88 (m, 2H, –CH2CH2CH2–), 1.93 (m, 6H, –CH3 × 2), 1.94–1.99 (m, 2H, –CH2CH2CH2–), 2.55–2.71 (m, 4H, –CH2CH2CH2–), 2.77 (t, J = 5.17 Hz, 4H, –CH2CH2CH2–), 3.80 (s, 3H, -NCH3 × 2), 6.24 (d, J = 13.91 Hz, 2H, vinyl H), 6.41 (d, J = 14.39 Hz, 2H, vinyl H), 7.32 (t, J = 6.73 Hz, 2H, aryl H), 7.41–7.49 (m, 4H, aryl H), 7.62 (d, J = 7.18 Hz, 2H, aryl H), 8.44 (d, J = 13.91 Hz, 2H, vinyl H), 8.55 (d, J = 14.39 Hz, 2H, vinyl H); 13C NMR (Acetone-d6) δ 19.6 (s), 21.6 (s), 26.9 (s), 27.0 (s), 28.1 (s), 32.0 (s), 49.6 (s), 50.1 (s), 85.8 (s), 92.8 (q, J = 31.32 Hz), 102.5 (s), 109.3 (s), 112.0 (s), 113.9 (s), 114.5 (s), 114.6 (s), 123.2 (s), 123.9 (q, J = 285.67 Hz), 126.2 (s), 127.4 (s), 130.0 (s), 131.3 (s), 141.6 (s), 142.1 (s), 144.1 (s), 144.7 (s), 149.4 (s), 150.1 (s), 156.7 (s), 174.3 (s), 177.1 (s); 19F NMR (Acetone-d6) δ −80.25 (s, 6F) (see S14–S16 and S18 in the Supplementary Materials).
3.11. Electrochemical Measurements of the Dyes
3.12. Methods for Evaluating Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tian, Y.; Yin, D.; Yan, L. J-aggregation strategy of organic dyes for near-infrared bioimaging and fluorescent image-guided phototherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1831. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, X.; Wang, M.; Li, C.; Jia, T.; Zhao, X. Recent advances in the development of near-infrared organic photothermal agents. Chem. Eng. J. 2021, 417, 128844. [Google Scholar] [CrossRef]
- Li, B.; Zhao, M.; Zhang, F. Rational design of near-infrared-ii organic molecular dyes for bioimaging and biosensing. ACS Materials Lett. 2020, 2, 905–917. [Google Scholar] [CrossRef]
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-infrared materials: The turning point of organic photovoltaics. Adv. Mater. 2022, 34, 2107330. [Google Scholar] [CrossRef]
- Bar, N.; Chowdhury, P. A brief review on advances in rhodamine b based chromic materials and their prospects. ACS Appl. Electron. Mater. 2022, 4, 3749–3771. [Google Scholar] [CrossRef]
- Uno, K.; Kim, D.; Bucevicius, J.; Bossi, M.L.; Belov, V.N.; Hell, S.W. Synthesis, structure–property relationships and absorbance modulation of highly asymmetric photochromes with variable oxidation and substitution patterns. Org. Chem. Front. 2022, 9, 6295–6304. [Google Scholar] [CrossRef]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 2020, 59, 9841–9855. [Google Scholar] [CrossRef]
- Abdollahi, A.; Mamaqani, H.R.; Razavi, B.; Kalajahi, M.S. Photoluminescent and chromic nanomaterials for anticounterfeiting technologies: Recent advances and future challenges. ACS Nano 2020, 14, 14417–14492. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, Q.; Tai, Y.-A.A.; Wang, Y. Applications of Cellulose Nanomaterials in Stimuli-Responsive Optics. J. Agric. Food Chem. 2020, 68, 12940–12955. [Google Scholar] [CrossRef]
- Naren, G.; Li, S.; Andreasson, J. A simplicity-guided cocktail approach toward multicolor fluorescent systems. Chem. Commun. 2020, 56, 3377–3380. [Google Scholar] [CrossRef]
- Ikeya, M.; Katada, G.; Ito, S. Tunable mechanochromic luminescence of 2-alkyl-4-(pyren-1-yl)thiophenes: Controlling the self-recovering properties and the range of chromism. Chem. Commun. 2019, 55, 12296–12299. [Google Scholar] [CrossRef] [PubMed]
- Traverse, C.J.; Young, M.; Suddard-Bangsund, J.; Partrick, T.; Bates, M.; Chen, P.; Wingate, B.; Lunt, S.Y.; Anctil, A.; Lunt, R.R. Anions for near-infrared selective organic salt photovoltaics. Sci. Rep. 2017, 7, 16399. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Suddard-Bangsund, J.; Patrick, T.J.; Pajares, N.; Traverse, C.J.; Barr, M.C.; Lunt, S.Y.; Lunt, R.R. Organic heptamethine salts for photovoltaics and detectors with near-infrared photoresponse up to 1600 nm. Adv. Optical Mater. 2016, 4, 1028–1033. [Google Scholar] [CrossRef]
- Funabiki, K.; Mase, H.; Hibino, A.; Tanaka, N.; Mizuhata, N.; Sakuragi, Y.; Nakashima, A.; Yoshida, T.; Kubota, Y.; Matsui, M. Synthesis of a novel heptamethine–cyanine dye for use in near-infrared active dye-sensitized solar cells with porous zinc oxide prepared at low temperature. Energy Environ. Sci. 2011, 4, 2186–2192. [Google Scholar] [CrossRef]
- Peters, A.; Branda, N.R. Enantioselective michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J. Am. Chem. Soc. 2003, 125, 3404–3405. [Google Scholar] [CrossRef]
- Wang, Y.; Kublitski, J.; Xing, S.; Dollinger, F.; Spoltore, D.; Benduhn, J.; Leo, K. Narrowband organic photodetectors-towards miniaturized, spectroscopic sensing. Mater. Horiz. 2022, 9, 220–251. [Google Scholar] [CrossRef]
- Zhang, H.; Jenatsch, S.; Jonghe, J.D.; Nuesch, F.; Steim, R.; Veron, A.C.; Hany, R. Transparent organic photodetector using a near-infrared absorbing cyanine dye. Sci. Rep. 2015, 5, 9439. [Google Scholar] [CrossRef]
- Wang, S.; Yan, X.; Cheng, Z.; Zhang, H.; Liu, Y.; Wang, Y. Highly efficient near-infrared delayed fluorescence organic light emitting diodes using a phenanthrene-based charge-transfer compound. Angew. Chem. Int. Ed. 2015, 54, 13068–13072. [Google Scholar] [CrossRef]
- Hao, Q.; Li, Z.J.; Bai, B.; Zhang, X.; Zhong, Y.W.; Wan, L.J.; Wang, D. A covalent organic framework film for three-state near-infrared electrochromism and a molecular logic gate. Angew. Chem. Int. Ed. 2021, 60, 12498–12503. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, K.; Huang, S.; Zhang, H.; Wang, Y.Y. Organic crystals with near-infrared amplified spontaneous emissions based on 2’-hydroxychalcone derivatives: Subtle structure modification but great property change. Angew. Chem. Int. Ed. 2015, 54, 8369–8373. [Google Scholar] [CrossRef]
- Cai, K.; Xie, J.; Zhao, D. Equivalent circuits of a self-assembled monolayer-based tunnel junction determined by impedance spectroscopy. J. Am. Chem. Soc. 2014, 136, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, N.G.; Braga, C.A.; Camera, V.S.; Duarte, R.C.; Rodembusch, F.S. Near-infrared fluorophores based on heptamethine cyanine dyes: From their synthesis and photophysical properties to recent optical sensing and bioimaging applications. Asian J. Org. Chem. 2022, 11, e202200095. [Google Scholar] [CrossRef]
- Feng, L.; Chen, W.; Ma, X.; Liu, S.H.; Yin, J. Near-infrared heptamethine cyanines (Cy7): From structure, property to application. Org. Biomol. Chem. 2020, 18, 9385–9397. [Google Scholar] [CrossRef]
- Broadwater, D.; Medeiros, H.C.D.; Bates, M.; Roshanzadeh, A.; Teoh, S.T.; Ogrodzinski, M.P.; Borhan, B.; Luont, R.R.; Lunt, S.Y. Counterion tuning of near-infrared organic salts dictates phototoxicity to inhibit tumor growth. ACS Appl. Mater. Interfaces 2022, 14, 53511–53522. [Google Scholar] [CrossRef]
- She, Z.; Chen, J.; Sun, L.; Zeng, F.; Wu, S. An NO-responsive probe for detecting acute inflammation using NIR-II fluorescence/optoacoustic imaging. Chem. Commun. 2022, 58, 13123–13126. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Matikonda, S.S.; Usama, S.M.; Furusawa, A.; Kato, T.; Stackova, L.; Klan, P.; Kobayashi, H.; Schnermann, M.J. Cyanine phototruncation enables spatiotemporal cell labeling. J. Am. Chem. Soc. 2022, 144, 11075–11080. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Wang, S.; Fan, Z.; Ma, Y.; Yin, Y.; Wang, W.; Xi, R.; Meng, M. A tumor-targeting near-infrared heptamethine cyanine photosensitizer with twisted molecular structure for enhanced imaging-guided cancer phototherapy. J. Am. Chem. Soc. 2021, 143, 20828–20836. [Google Scholar] [CrossRef]
- Naim, W.; Novelli, V.; Nikolinakos, I.; Barbero, N.; Dzeba, I.; Grifoni, F.; Ren, Y.; Alnasser, T.; Velardo, A.; Borrelli, R.; et al. Transparent and colorless dye-sensitized solar cells exceeding 75% average visible transmittance. JACS Au. 2021, 1, 409–426. [Google Scholar] [CrossRef]
- Li, D.H.; Smith, B.D. Supramolecular mitigation of the cyanine limit problem. J. Org. Chem. 2022, 87, 5893–5903. [Google Scholar] [CrossRef]
- Sato, R.; Machida, S.; Sohmiya, M.; Sugahara, Y.; Guegan, R. Intercalation of a cationic cyanine dye assisted by anionic surfactants within Mg−Al layered double hydroxide. ACS Omega 2021, 6, 23837–23845. [Google Scholar] [CrossRef]
- Qi, Y.; Ndaleh, D.; Meador, W.E.; Delcamp, J.H.; Hill, G.; Pradhan, N.R.; Dai, Q. Interface passivation of inverted perovskite solar cells by dye molecules. ACS Appl. Energy Mater. 2021, 4, 9525–9533. [Google Scholar] [CrossRef]
- Guralchuk, G.Y.; Katrunov, I.K.; Grynyov, R.S.; Sorokin, A.V.; Yefimova, S.L.; Borovoy, I.A.; Malyukin, Y.V. Anomalous surfactant-induced enhancement of luminescence quantum yield of cyanine dye J-aggregates. J. Phys. Chem. C 2008, 112, 14762–14768. [Google Scholar] [CrossRef]
- Li, Z.; Syed, A.A.; Zhao, P.; Yang, J.C.; Sharma, R.; Ensley, T.R.; Matichak, J.D.; Davydenko, I.; Jang, S.H.; Hagan, D.J.; et al. Cationic polyelectrolyte for anionic cyanines: An efficient way to translate molecular properties into material properties. J. Am. Chem. Soc. 2019, 141, 17331–17336. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Krokos, E.; Bouit, P.A.; Delgaro, J.L.; Guldi, D.M.; Martin, N. Efficient light harvesting anionic heptamethine cyanine–[60] and [70]fullerene hybrids. Energy Environ. Sci. 2011, 4, 679–684. [Google Scholar] [CrossRef]
- Bouit, P.A.; Piazza, E.D.; Rigaut, S.; Guennic, B.L.; Aronica, C.; Toupet, L.; Andraud, C.; Maury, O. Stable near-infrared anionic polymethine dyes: Structure, photophysical, and redox properties. Org. Lett. 2008, 10, 4159–4162. [Google Scholar] [CrossRef]
- Li, Y.; Ma, T.; Jiang, H.; Li, W.; Tian, D.; Zhu, J.; Li, Z. Anionic cyanine J-type aggregate nanoparticles with enhanced photosensitization for mitochondria-targeting tumor phototherapy. Angew. Chem. Int. Ed. 2022, 61, e202203093. [Google Scholar]
- Arisawa, Y.; Kubota, Y.; Inuzuka, T.; Funabiki, K. Photostability and halochromic properties of near-infrared absorbing anionic heptamethine cyanine dyes. ChemistrySelect 2022, 7, e202104213. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Tofighi, S.; Sharma, R.; Ensley, T.R.; Jang, S.H.; Hagan, D.J.; Stryland, E.W.V.; Jen, A.K.-Y. Zwitterionic cyanine–cyanine salt: Structure and optical properties. J. Phys. Chem. C 2016, 120, 15378–15384. [Google Scholar] [CrossRef]
- Li, Z.; Mukhopadhyay, S.; Jang, S.H.; Bredas, J.L.; Jen, A.K.-Y. Supramolecular assembly of complementary cyanine salt J-aggregates. J. Am. Chem. Soc. 2015, 137, 11920–11923. [Google Scholar] [CrossRef]
- Bouit, P.-A.; Rauh, D.; Neugebauer, S.; Delgado, J.L.; Pizza, E.D.; Rigaut, S.; Maury, O.; Andraud, C.; Dyakonov, V.; Martin, N.A. A “cyanine−cyanine” salt exhibiting photovoltaic properties. Org. Lett. 2009, 11, 4806–4809. [Google Scholar] [CrossRef]
- Tarrant, P.; Taylor, R.E. Fluoroolefins. VII. The synthesis of 2-trifluoromethyl-1,3-butadiene1. J. Org. Chem. 1959, 24, 1888–1890. [Google Scholar] [CrossRef]
- Liu, S.; Haller, M.A.; Ma, H.; Dalton, L.R.; Jang, S.-H.; Jen, A.K.-Y. Focused microwave-assisted synthesis of 2,5-dihydrofuran derivatives as electron acceptors for highly efficient nonlinear optical chromophores. Adv. Mater. 2003, 15, 603–607. [Google Scholar] [CrossRef]
- He, M.; Leslie, T.M.; Sinicrop, J.A. α-Hydroxy Ketone Precursors Leading to a Novel Class of Electro-optic Acceptors. Chem. Mater. 2002, 14, 2393–2400. [Google Scholar] [CrossRef]
- Carrera, M.; De Coen, L.; Coppens, M.; Dermaut, W.; Stevens, C.V. A Vilsmeier Chloroformylation by Continuous Flow Chemistry. Org. Process Res. Dev. 2020, 24, 2260–2265. [Google Scholar] [CrossRef]
- Mukherjee, A.; Saha, P.C.; Das, R.S.; Bera, T.; Guha, S. Acidic pH-Activatable Visible to Near-Infrared Switchable Ratiometric Fluorescent Probe for Live-Cell Lysosome Targeted Imaging. ACS Sens. 2021, 6, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Uehashi, Y.; Ajioka, S.; Kubota, Y.; Inuzuka, T.; Funabiki, K. Vapochromism of indolenine-based heptamethine cyanine dye adsorbed on silica gel. New J. Chem. 2023, 47, 5262–5269. [Google Scholar] [CrossRef]
- Burdette, M.K.; Jenkins, R.; Bandera, Y.P.; Jones, H.; Foulger, I.K.; Dickey, A.; Nieminen, A.-L.; Foulger, S.H. Click-Engineered, Bioresponsive, and Versatile Particle–Protein–Dye System. ACS Appl. Bio Mater. 2019, 2, 3183–3193. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical redox agents for organometallic chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]


 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dye | R | λmax (nm) a | ε (M−1 cm−1) a | Eox (V vs. SCE) b | HOMO (eV) | λonsetabs (nm) a | HOMO–LUMO Gap (eV) c | LUMO (eV) d | Reference |
| 5a | CF3 | 948 | 230,000 | 0.57 | −4.97 | 1080 | 1.15 | −3.82 | This study |
| 8a | ― | 785 949 | 370,000 250,000 | ― | ― | ― | ― | ― | |
| 5b | C6F5 | 934 | 310,000 | 0.43 | −4.83 | 1053 | 1.18 | −3.65 | [37] |
| 5c | Ph | 920 | 330,000 | 0.34 | −4.74 | 1033 | 1.20 | −3.54 | |
| 5d | Me | 906 | 260,000 | 0.30 | −4.70 | 1024 | 1.21 | −3.49 | |
| 7 | ― | 785 | 380,000 | ― | ― | ― | ― | ― | |
| Dye | R | Residual Rates (%) a | Reference | |
|---|---|---|---|---|
| Anion | Cation | |||
| 5a | CF3 | 80 | ― | This study |
| 8a | ― | 35 | 75 | |
| 5b | C6F5 | 79 | ― | [37] |
| 5c | Ph | 62 | ― | |
| 5d | Me | 24 | ― | |
| 7 | ― | ― | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuoka, H.; Kubota, Y.; Inuzuka, T.; Funabiki, K. Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups. Molecules 2023, 28, 4650. https://doi.org/10.3390/molecules28124650
Masuoka H, Kubota Y, Inuzuka T, Funabiki K. Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups. Molecules. 2023; 28(12):4650. https://doi.org/10.3390/molecules28124650
Chicago/Turabian StyleMasuoka, Hiroki, Yasuhiro Kubota, Toshiyasu Inuzuka, and Kazumasa Funabiki. 2023. "Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups" Molecules 28, no. 12: 4650. https://doi.org/10.3390/molecules28124650
APA StyleMasuoka, H., Kubota, Y., Inuzuka, T., & Funabiki, K. (2023). Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups. Molecules, 28(12), 4650. https://doi.org/10.3390/molecules28124650







