An Integrated Computational Analysis of High-Risk SNPs in Angiopoietin-like Proteins (ANGPTL3 and ANGPTL8) Reveals Perturbed Protein Dynamics Associated with Cancer
Abstract
1. Introduction
2. Results
2.1. Missense SNP Datasets
2.2. Determination of High-Risk Missense SNPs
2.3. Conservation Analysis and Effect of the High-Risk SNPs on Protein Structure Stability
2.4. Prediction of Functional Ligand-Binding Sites
2.5. Prediction of PTM Sites
2.6. High-Risk SNPs Located at PTM and Ligand-Binding Sites
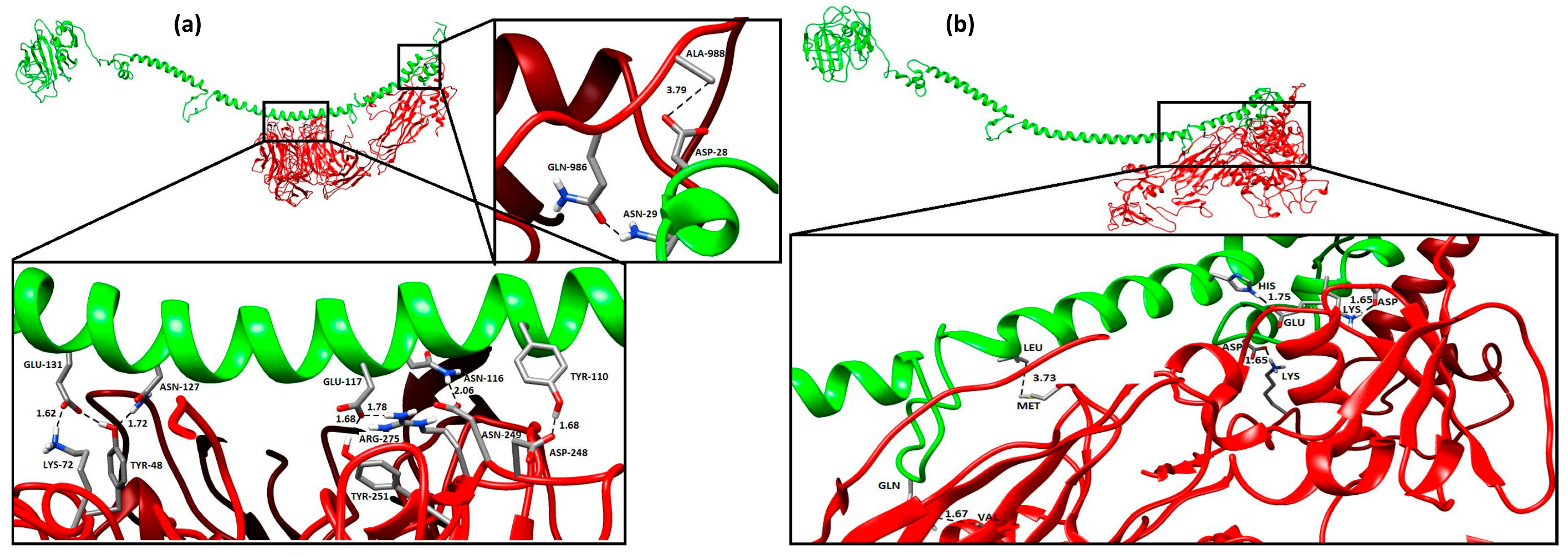
2.7. ANGPTL 3D Structures, Protein–Protein Interactions, and Molecular Docking
2.7.1. Tertiary Structure and Protein–Protein Interaction Analysis
2.7.2. Protein–Protein Docking Using ANGPTL Proteins
2.8. High-Risk nsSNPs Associated with Cancer
2.9. Structure Comparison between Wild-Type and Mutated Proteins
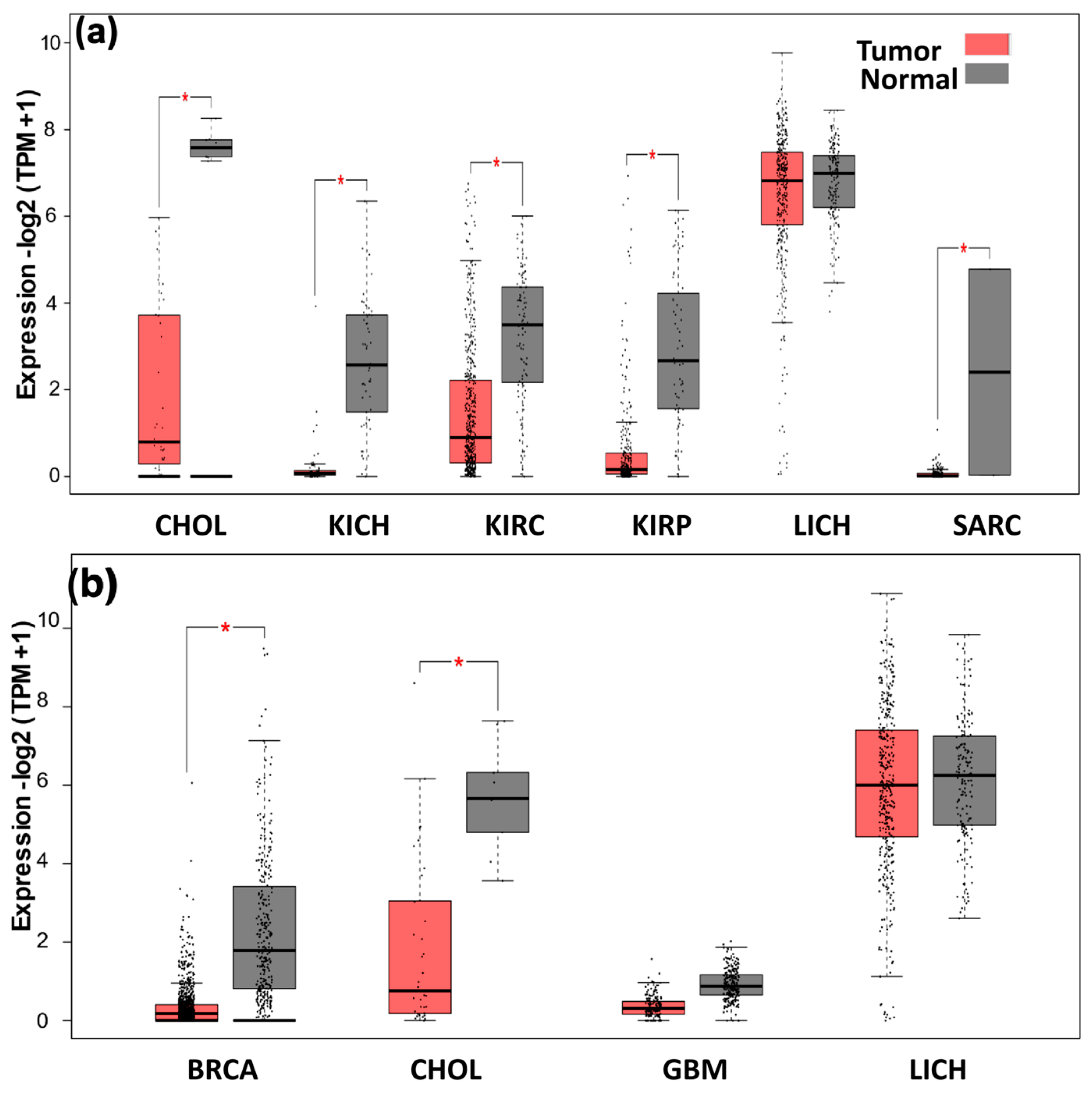
2.10. Expression Analysis of ANGPTL3 and C19orf80
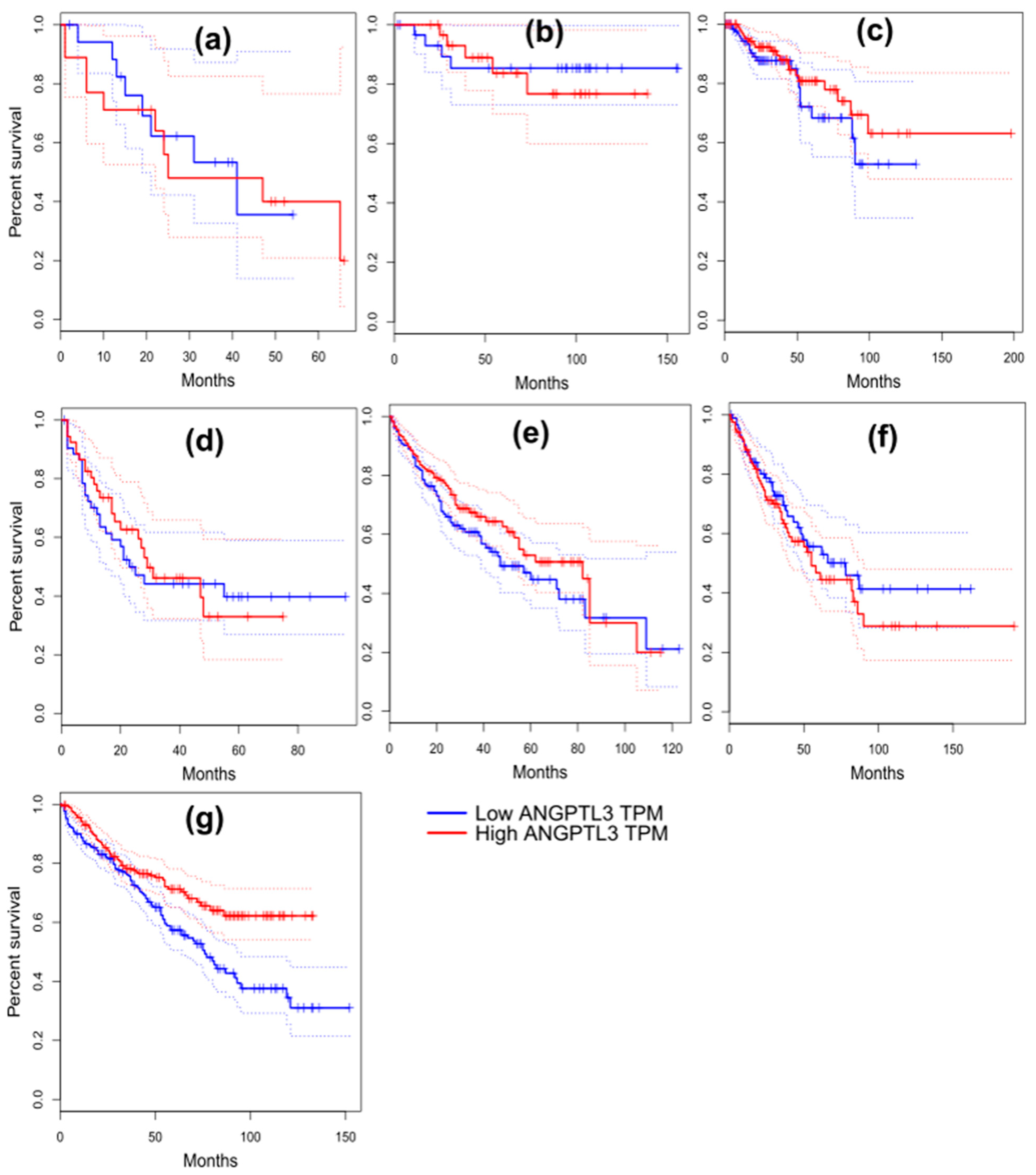
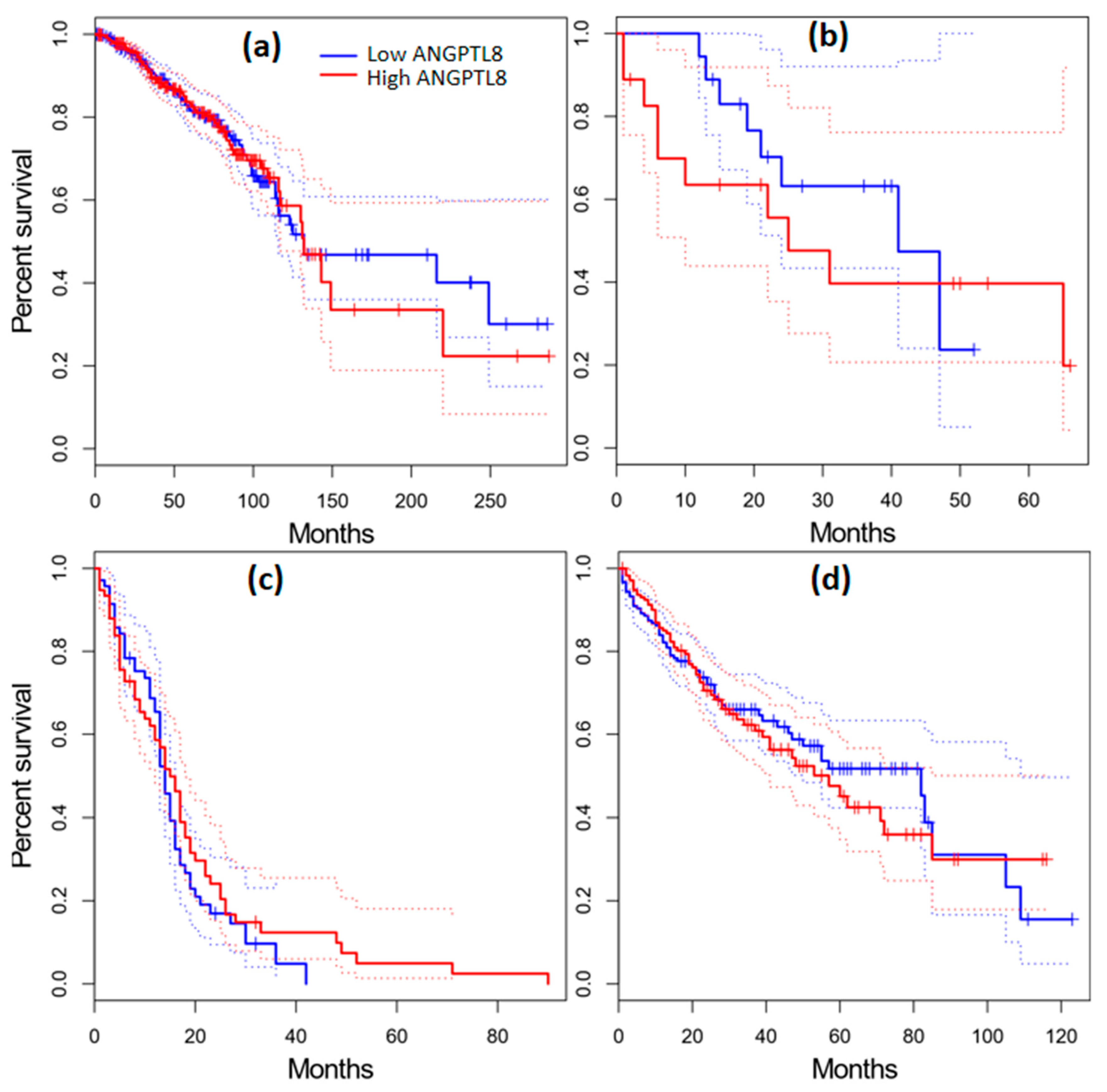
2.11. Survival Analysis in Cancer Patients
2.12. Association between Mutation and Expression Level of ANGPTL3 and C19orf80 with Other Genes
3. Discussion
4. Material and Methods
4.1. Retrieval of Protein Sequence Datasets and Identification of High-Risk SNPs
4.2. Conservation Pattern of High-Risk SNPs in ANGPTL3 and ANGPTL8
4.3. Prediction of Changes in Protein Stability Induced by High-Risk SNPs in ANGPTL3 and ANGPTL8
4.4. Structural Consequences of High-Risk nsSNPs
4.5. Tertiary Structure and Ligand-Binding Site Prediction
4.6. Prediction of PTMs Sites
4.6.1. Phosphorylation, Kinase-Specific Phosphorylation, and O-Glycosylation Sites
4.6.2. Ubiquitylation Sites
4.6.3. Palmitoylation and Methylation Sites
4.6.4. Acetylation and Sumoylation Site
4.7. Protein–Protein Interactions (PPIs) and Prediction of High-Risk SNPs Associated with Cancer
4.8. Protein Structure Preparation and Docking
4.9. Comparative Modeling and Visualization of Wild-Type Proteins and Their Mutated Structures
4.10. Analysis of Survival Rate and Gene Expression Analysis
4.11. Analysis of the Correlation between Expression Level and Mutation in ANGPTL3 and C190orf80 Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of cancer metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Chiodoni, C.; Colombo, M.P.; Sangaletti, S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010, 29, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Gerarduzzi, C.; Hartmann, U.; Leask, A.; Drobetsky, E. The matrix revolution: Matricellular proteins and restructuring of the cancer microenvironment. Cancer Res. 2020, 80, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Angiopoietin-like proteins: A comprehensive look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Oike, Y.; Ito, Y.; Maekawa, H.; Morisada, T.; Kubota, Y.; Akao, M.; Urano, T.; Yasunaga, K.; Suda, T. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood 2004, 103, 3760–3765. [Google Scholar] [CrossRef]
- Okazaki, H.; Hirakawa, S.; Shudou, M.; Nakaoka, Y.; Shirakata, Y.; Miyata, K.; Oike, Y.; Hashimoto, K.; Sayama, K. Targeted overexpression of Angptl6/angiopoietin-related growth factor in the skin promotes angiogenesis and lymphatic vessel enlargement in response to ultraviolet B. J. Dermatol. 2012, 39, 366–374. [Google Scholar] [CrossRef]
- Endo, M. The Roles of ANGPTL Families in Cancer Progression. J. UOEH 2019, 41, 317–325. [Google Scholar] [CrossRef]
- Yan, Q.; Jiang, L.; Liu, M.; Yu, D.; Zhang, Y.; Li, Y.; Fang, S.; Li, Y.; Zhu, Y.-H.; Yuan, Y.-F.; et al. ANGPTL1 Interacts with Integrin α1β1 to Suppress HCC Angiogenesis and Metastasis by Inhibiting JAK2/STAT3 Signaling. Cancer Res. 2017, 77, 5831–5845. [Google Scholar] [CrossRef]
- Yang, L.; Sun, R.; Wang, Y.; Fu, Y.; Zhang, Y.; Zheng, Z.; Ji, Z.; Zhao, D. Expression of ANGPTL2 and its impact on papillary thyroid cancer. Cancer Cell Int. 2019, 19, 204. [Google Scholar] [CrossRef]
- Koyama, T.; Ogawara, K.; Kasamatsu, A.; Okamoto, A.; Kasama, H.; Minakawa, Y.; Shimada, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015, 4, 759–769. [Google Scholar] [CrossRef]
- Luo, M.; Peng, D. ANGPTL8: An important regulator in metabolic disorders. Front. Endocrinol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Rozario, L.T.; Sharker, T.; Nila, T.A. In silico analysis of deleterious SNPs of human MTUS1 gene and their impacts on subsequent protein structure and function. PLoS ONE 2021, 16, e0252932. [Google Scholar] [CrossRef] [PubMed]
- Khoruddin, N.A.; Noorizhab, M.N.; Teh, L.K.; Mohd Yusof, F.Z.; Salleh, M.Z. Pathogenic nsSNPs that increase the risks of cancers among the Orang Asli and Malays. Sci. Rep. 2021, 11, 16158. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Nakata, J.; Kinoshita, K. Distribution of single-nucleotide variants on protein–protein interaction sites and its relationship with minor allele frequency. Protein Sci. 2016, 25, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Suri, M.; Evers, J.M.G.; Laskowski, R.A.; O’Brien, S.; Baker, K.; Clayton-Smith, J.; Dabir, T.; Josifova, D.; Joss, S.; Kerr, B.; et al. Protein structure and phenotypic analysis of pathogenic and population missense variants in STXBP1. Mol. Genet. Genom. Med. 2017, 5, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Alodaib, A.; Sobreira, N.; Gold, W.A.; Riley, L.G.; Van Bergen, N.J.; Wilson, M.J.; Bennetts, B.; Thorburn, D.R.; Boehm, C.; Christodoulou, J. Whole-exome sequencing identifies novel variants in PNPT1 causing oxidative phosphorylation defects and severe multisystem disease. Eur. J. Hum. Genet. 2016, 25, 79–84. [Google Scholar] [CrossRef]
- Abid, K.; Trimeche, T.; Mili, D.; Msolli, M.A.; Trabelsi, I.; Nouira, S.; Kenani, A. ANGPTL4 variants E40K and T266M are associated with lower fasting triglyceride levels and predicts cardiovascular disease risk in type 2 diabetic Tunisian population. Lipids Health Dis. 2016, 15, 63. [Google Scholar] [CrossRef]
- Hanson, R.L.; Leti, F.; Tsinajinnie, D.; Kobes, S.; Puppala, S.; Curran, J.E.; Almasy, L.; Lehman, D.M.; Blangero, J.; Duggirala, R.; et al. The Arg59Trp variant in ANGPTL8 (betatrophin) is associated with total and HDL-cholesterol in American Indians and Mexican Americans and differentially affects cleavage of ANGPTL3. Mol. Genet. Metab. 2016, 118, 128–137. [Google Scholar] [CrossRef]
- Degn, K.; Beltrame, L.; Dahl Hede, F.; Sora, V.; Nicolaci, V.; Vabistsevits, M.; Schmiegelow, K.; Wadt, K.; Tiberti, M.; Lambrughi, M.; et al. Cancer-related Mutations with Local or Long-range Effects on an Allosteric Loop of p53. J. Mol. Biol. 2022, 434, 167663. [Google Scholar] [CrossRef]
- Lim, S.W.; Tan, K.J.; Azuraidi, O.M.; Sathiya, M.; Lim, E.C.; Lai, K.S.; Yap, W.-S.; Afizan, N.A.R.N.M. Functional and structural analysis of non-synonymous single nucleotide polymorphisms (nsSNPs) in the MYB oncoproteins associated with human cancer. Sci. Rep. 2021, 11, 24206. [Google Scholar] [CrossRef] [PubMed]
- Doss, C.G.P.; Nagasundaram, N.; Chakraborty, C.; Chen, L.; Zhu, H. Extrapolating the effect of deleterious nsSNPs in the binding adaptability of flavopiridol with CDK7 protein: A molecular dynamics approach. Hum. Genom. 2013, 7, 10. [Google Scholar] [CrossRef]
- Cherra, S.J.; Kulich, S.M.; Uechi, G.; Balasubramani, M.; Mountzouris, J.; Day, B.W.; Chu, C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010, 190, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, H.; Wu, J.; Huang, Q.; Liu, J.O.; Yu, L. Arginine68 is an essential residue for the C-terminal cleavage of human Atg8 family proteins. BMC Cell Biol. 2013, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zou, Z.; Becker, N.; Anderson, M.; Sumpter, R.; Xiao, G.; Kinch, L.; Koduru, P.; Christudass, C.S.; Veltri, R.W.; et al. XEGFR-mediated beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 2013, 154, 1269. [Google Scholar] [CrossRef]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef]
- Hu, W.; Tang, C.; Chen, H.; Zhao, J.; Jin, L.; Kang, L.; Wu, Y. Correlations between angiopoietin-2 gene polymorphisms and lung cancer progression in a Chinese Han population. J. Cancer 2019, 10, 2935–2941. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Beaulieu, M.E.; Soucek, L. MYC, MYCL, and MYCN as therapeutic targets in lung cancer. Expert Opin. Ther. Targets 2020, 24, 101–114. [Google Scholar] [CrossRef]
- Ye, J.; Wang, J.; Tan, L.; Yang, S.; Xu, L.; Wu, X.; Deng, H.; Tan, H. Expression of protein TARBP1 in human hepatocellular carcinoma and its prognostic significance. Int. J. Clin. Exp. Pathol. 2015, 8, 9089–9096. [Google Scholar]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Fei, L.; Chang, L.; Yongyu, L.; Jianhui, J.; Yanan, L.; Yi, R. Protocadherin alpha 3 inhibits lung squamous cell carcinoma metastasis and epithelial-mesenchymal transition. Genes Genom. 2022, 44, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Nyswaner, K.; Torres-Ayuso, P.; Brognard, J. The protein kinase MAP3K19 phosphorylates MAP2Ks and thereby activates ERK and JNK kinases and increases viability of KRAS-mutant lung cancer cells. J. Biol. Chem. 2020, 295, 8470–8479. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. DbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhou, M.; Cui, Y. nsSNPAnalyzer: Identifying disease-associated nonsynonymous single nucleotide polymorphisms. Nucleic Acids Res. 2005, 33, W480–W482. [Google Scholar] [CrossRef]
- Calabrese, R.; Capriotti, E.; Fariselli, P.; Martelli, P.L.; Casadio, R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum. Mutat. 2009, 30, 1237–1244. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Ferrer-Costa, C.; Gelpí, J.L.; Zamakola, L.; Parraga, I.; de la Cruz, X.; Orozco, M. PMUT: A web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 2005, 21, 3176–3178. [Google Scholar] [CrossRef]
- Casper, C.; Fitzmaurice, C. Infection-related cancers: Prioritising an important and eliminable contributor to the global cancer burden. Lancet Glob. Heal. 2016, 4, e580–e581. [Google Scholar] [CrossRef]
- Venselaar, H.; te Beek, T.A.H.; Kuipers, R.K.P.; Hekkelman, M.L.; Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform. 2010, 11, 548. [Google Scholar] [CrossRef]
- Lu, H.C.; Herrera Braga, J.; Fraternali, F. PinSnps: Structural and functional analysis of SNPs in the context of protein interaction networks. Bioinformatics 2016, 32, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- De Baets, G.; Van Durme, J.; Reumers, J.; Maurer-Stroh, S.; Vanhee, P.; Dopazo, J.; Schymkowitz, J.; Rousseau, F. SNPeffect 4.0: On-line prediction of molecular and structural effects of protein-coding variants. Nucleic Acids Res. 2012, 40, D935–D939. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lee, T.Y.; Liang, H.K.; Huang, C.M.; Wang, T.Y.; Yang, Y.H.; Chu, C.H.; Da Huang, H.; Ko, M.T.; Hwang, J.K. KinasePhos 2.0: A web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 2007, 35, W588–W594. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar]
- Petersen, B.; Petersen, T.N.; Andersen, P.; Nielsen, M.; Lundegaard, C. A generic method for assignment of reliability scores applied to solvent accessibility predictions. BMC Struct. Biol. 2009, 9, 51. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; De Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: φ,ψ and Cβ deviation. Proteins Struct. Funct. Genet. 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; Van Dijk, M.; De Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]



| Purpose | Tool Used | ANGPTL3 | ANGPTL8 | ||
|---|---|---|---|---|---|
| Effect of SNP on protein | nsSNP analyzer | Pathological | Neutral | Pathological | Neutral |
| 83 | 135 | 12 | 71 | ||
| SNP&GO | 115 | 103 | 38 | 45 | |
| PROVEAN | 67 | 151 | 30 | 53 | |
| PMUT | 78 | 140 | 4 | 79 | |
| Total high-risk SNPs | Predicted by 3 or >3 | 67 | 12 | ||
| Protein stability upon SNP | I-Mutant | Decrease | Increase | Decrease | Increase |
| 60 | 7 | 6 | 1 | ||
| No. of conserved residues | ClustalO | 50 | All | ||
| Ligand binding | FTsite and COACH | 8 | 0 | ||
| Phosphorylation sites | Netphos 2.0 | 49 | 8 | ||
| Glycosylation sites | YinOYang | 8 | 5 | ||
| Ubiquitination sites (≥2) | iUbiq-lys, CKSAAP UbSite, BDM-PUB | 0 | 1 | ||
| Palmitoylation sites | CSS-PALM 3.0 | 0 | 1 | ||
| Methylation sites | PMes | 7 | 0 | ||
| Acetylation sites | PAIL and ASEB | 15 | 3 | ||
| Sumoylation sites | SUMOplotTM SUMOhydro and SUMOsp | 3 | 0 | ||
| Total PTM sites | - | 82 | 18 | ||
| Protein | High-Risk SNPs | Conserved (100%) | I-Mutant Analysis | PTM Site | Ligand-Binding Site | Associated Cancer Type | ||
|---|---|---|---|---|---|---|---|---|
| DDG | Stability | Reliability Index | ||||||
| ANGPTL3 | L57H | Yes | −0.44 | Decrease | 5 | - | - | Kidney |
| T64K | Yes | −2.41 | Decrease | 8 | Yes | Yes | - | |
| T64R | Yes | −1.33 | Decrease | 3 | - | Yes | - | |
| S292P | Yes | −1.41 | Increase | 2 | Yes | - | - | |
| F295L | Yes | −2.25 | Decrease | 6 | - | - | Lung | |
| L309F | Yes | −0.18 | Decrease | 6 | - | - | Endometrium | |
| K319M | Yes | −0.15 | Increase | 3 | - | - | Skin | |
| Y321D | Yes | −0.32 | Decrease | 4 | Yes | - | - | |
| R332L | Yes | −1.64 | Decrease | 9 | - | - | Large intestine | |
| R332Q | Yes | −1.59 | Decrease | 9 | - | - | Large intestine | |
| S348C | Yes | −1.27 | Decrease | 2 | Yes | - | Esophagus | |
| Y358H | Yes | −0.35 | Decrease | 6 | Yes | - | - | |
| G409R | Yes | 0.14 | Decrease | 5 | CNS | |||
| S446P | No | −1.63 | Increase | 3 | Yes | - | - | |
| S447I | No | −1.69 | Decrease | 6 | Yes | - | - | |
| ANGPTL8 | P23L | No | 0.06 | Increase | 3 | - | - | Large intestine |
| R85W | No | −0.17 | Decrease | 7 | - | - | Large intestine | |
| R138S | No | −1.18 | Decrease | 9 | - | - | Breast | |
| E148D | Yes | −0.65 | Decrease | 7 | - | - | Liver | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.; Begum, F.; Nyamai, D.W.; Jalal, N.; Shaw, P. An Integrated Computational Analysis of High-Risk SNPs in Angiopoietin-like Proteins (ANGPTL3 and ANGPTL8) Reveals Perturbed Protein Dynamics Associated with Cancer. Molecules 2023, 28, 4648. https://doi.org/10.3390/molecules28124648
Iqbal S, Begum F, Nyamai DW, Jalal N, Shaw P. An Integrated Computational Analysis of High-Risk SNPs in Angiopoietin-like Proteins (ANGPTL3 and ANGPTL8) Reveals Perturbed Protein Dynamics Associated with Cancer. Molecules. 2023; 28(12):4648. https://doi.org/10.3390/molecules28124648
Chicago/Turabian StyleIqbal, Sajid, Farida Begum, Dorothy Wavinya Nyamai, Nasir Jalal, and Peter Shaw. 2023. "An Integrated Computational Analysis of High-Risk SNPs in Angiopoietin-like Proteins (ANGPTL3 and ANGPTL8) Reveals Perturbed Protein Dynamics Associated with Cancer" Molecules 28, no. 12: 4648. https://doi.org/10.3390/molecules28124648
APA StyleIqbal, S., Begum, F., Nyamai, D. W., Jalal, N., & Shaw, P. (2023). An Integrated Computational Analysis of High-Risk SNPs in Angiopoietin-like Proteins (ANGPTL3 and ANGPTL8) Reveals Perturbed Protein Dynamics Associated with Cancer. Molecules, 28(12), 4648. https://doi.org/10.3390/molecules28124648







