Unlocking the Potential of Deep Eutectic Solvents for C–H Activation and Cross-Coupling Reactions: A Review
Abstract
1. Introduction
- ▪ Type I: composed of a metal chloride and a quaternary ammonium salt.
- ▪ Type II: similar to type I, but with hydrated metal halides instead of non-hydrated ones.
- ▪ Type III: composed of a hydrogen bond donor (HBD), such as alcohols, amino acids, or amides, and a quaternary ammonium salt.
- ▪ Type IV: composed of a transition-metal salt and HBDs.
- ▪ Type V: composed solely of non-ionic components.
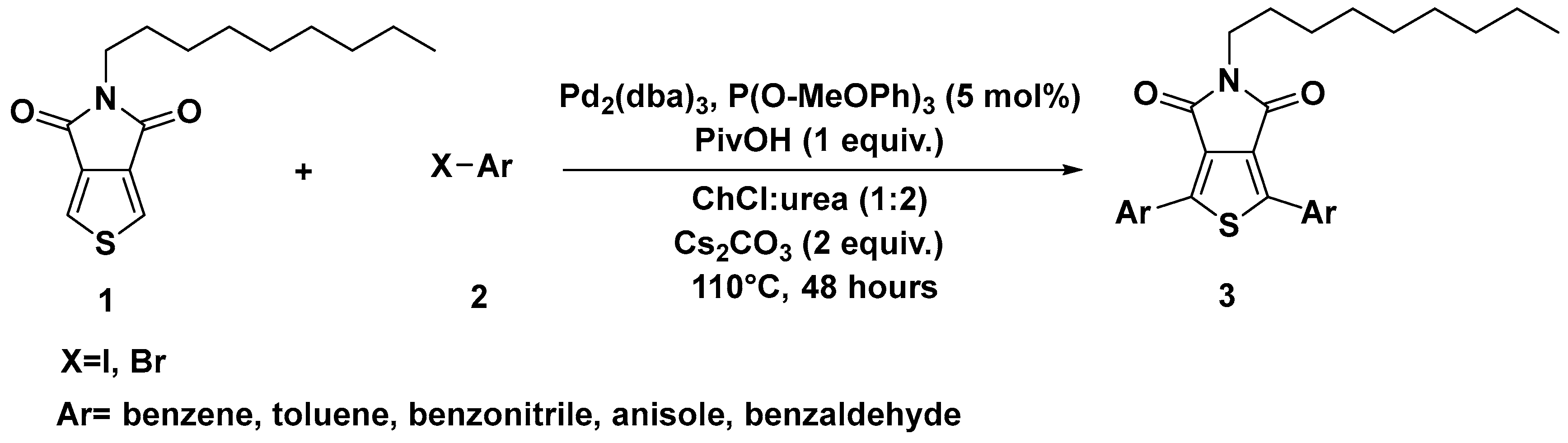
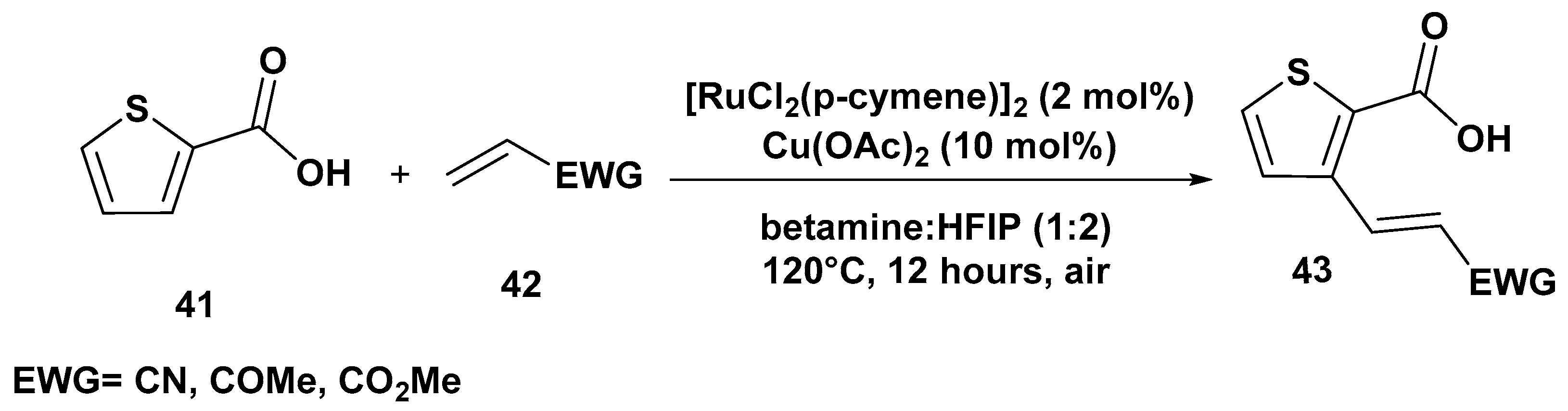
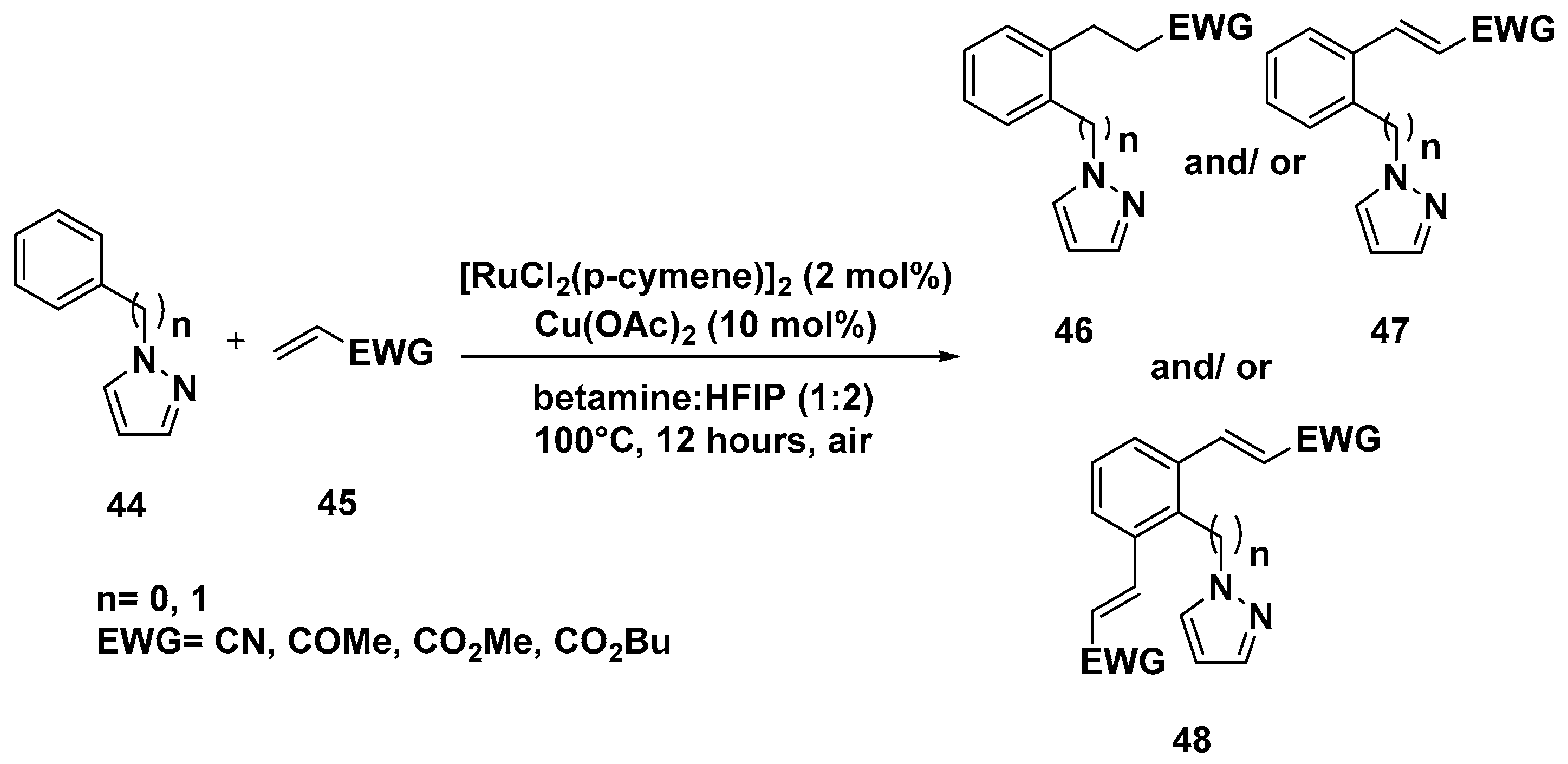
2. C–H Activation Reactions
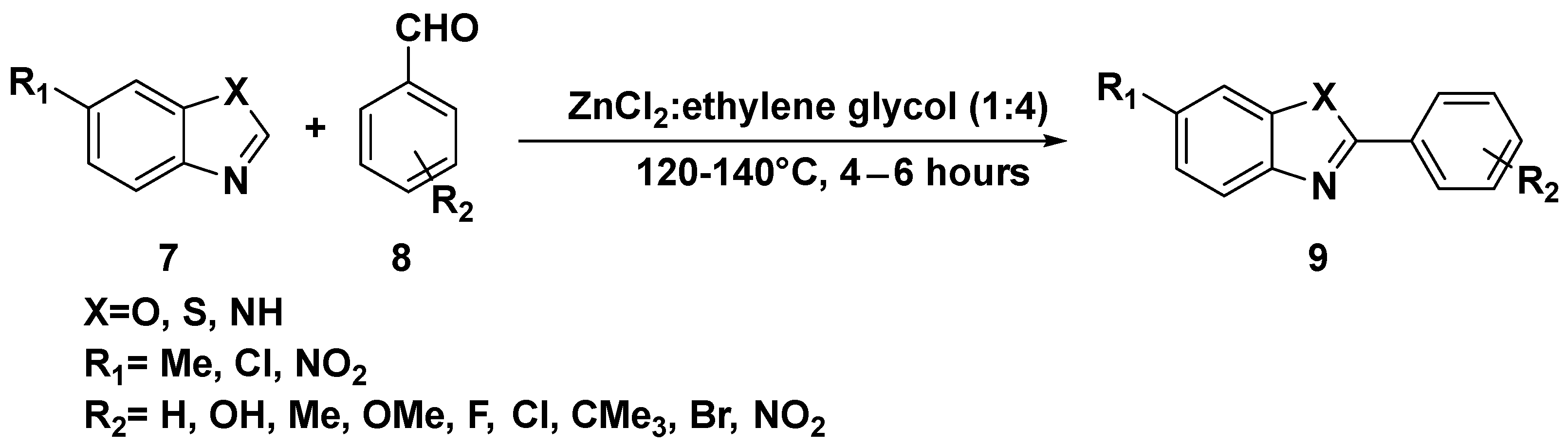
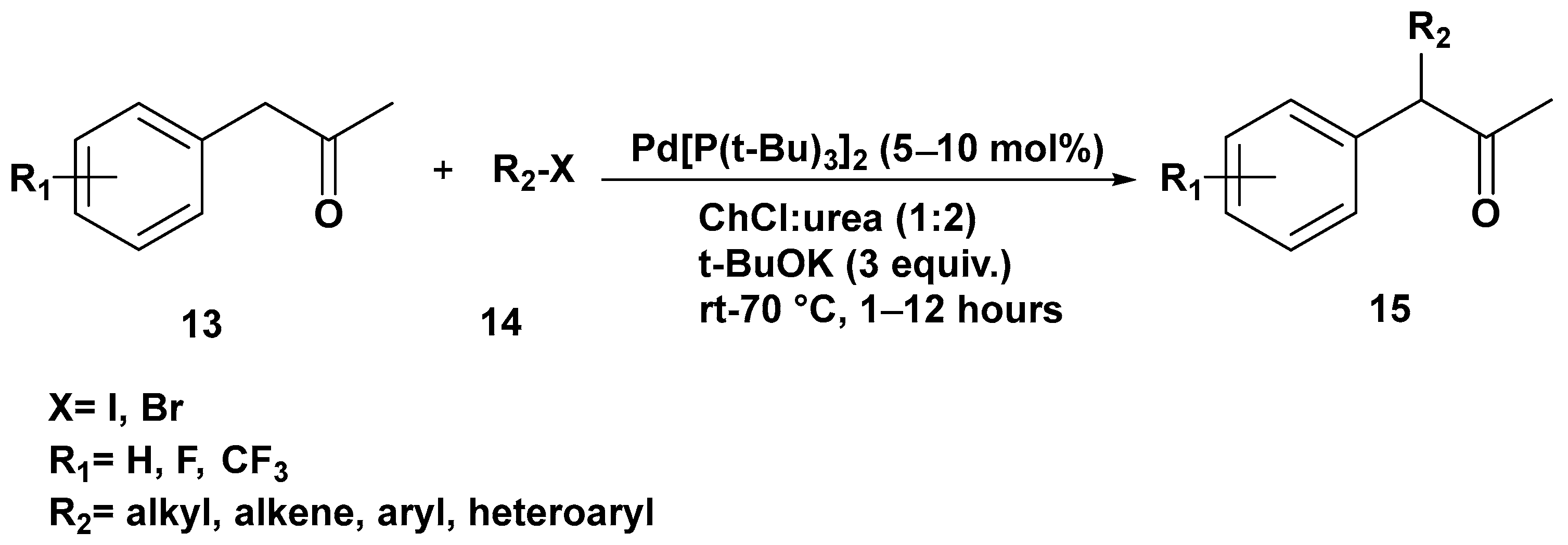
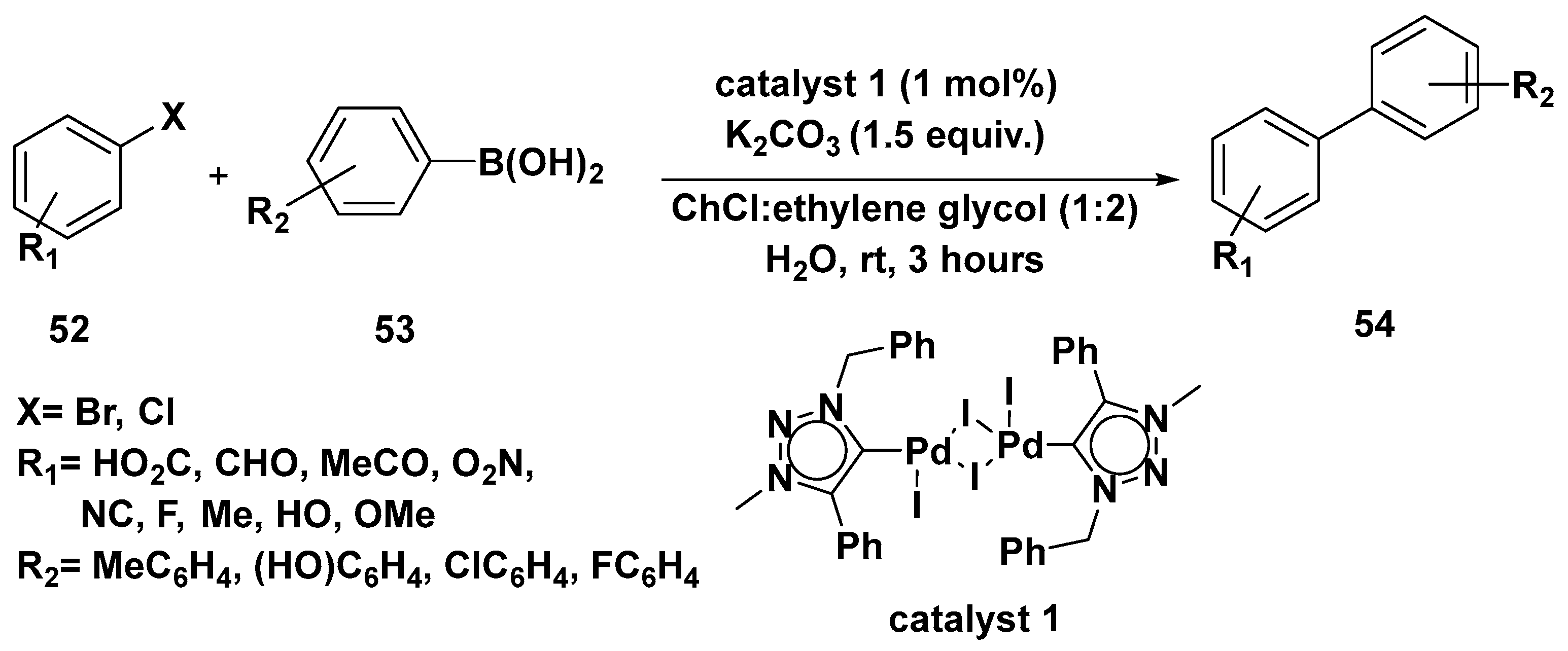
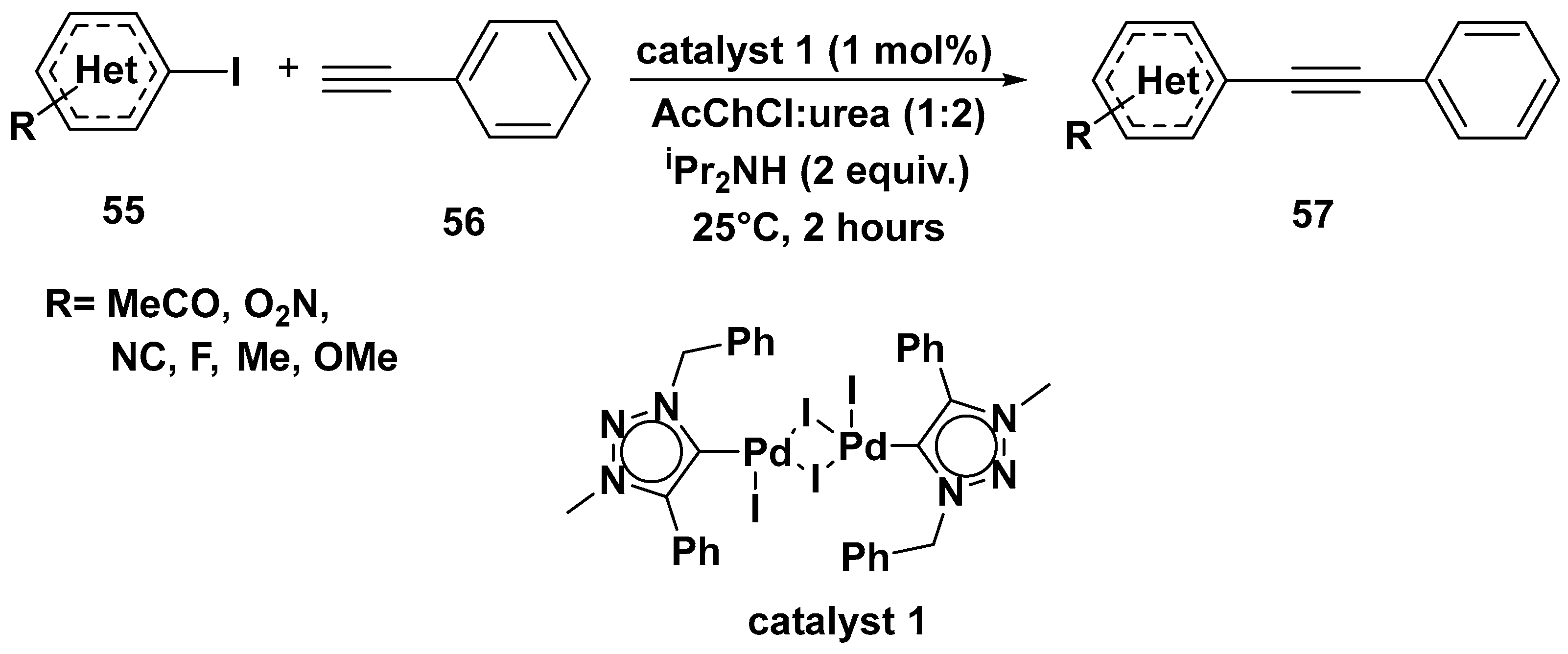
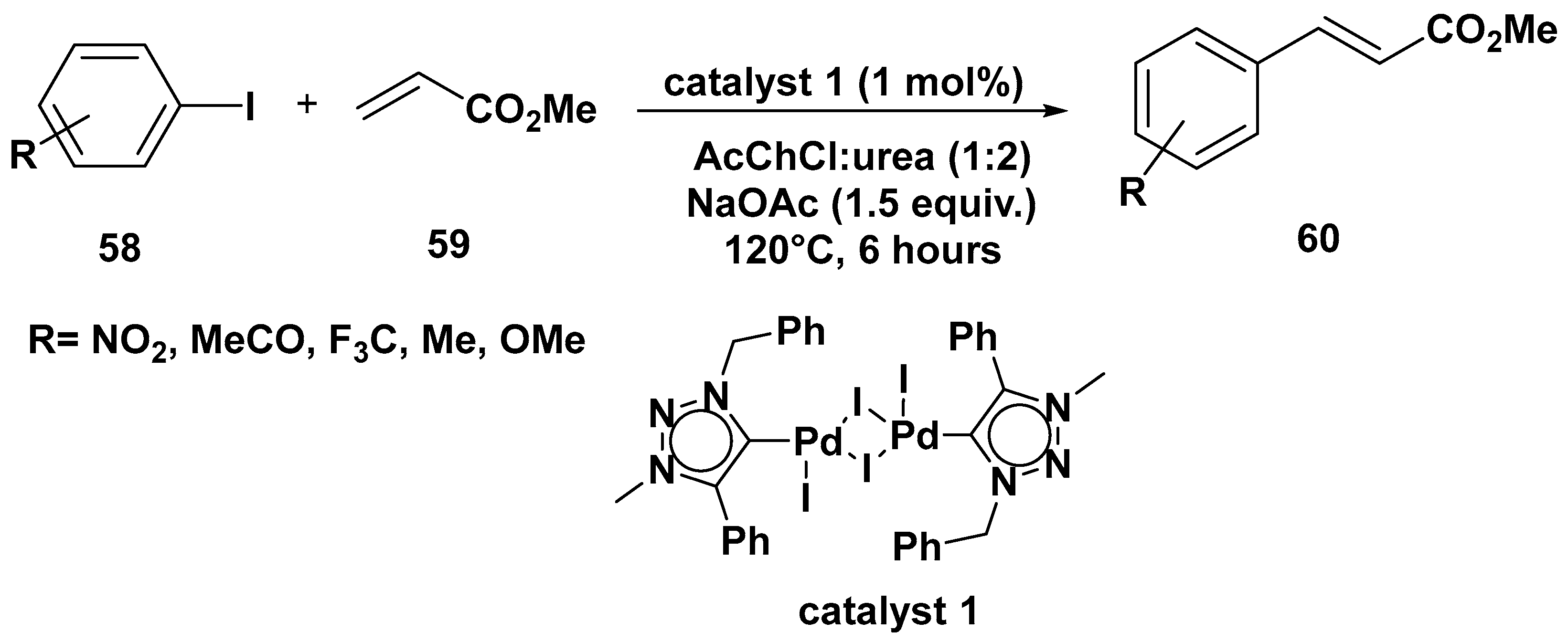
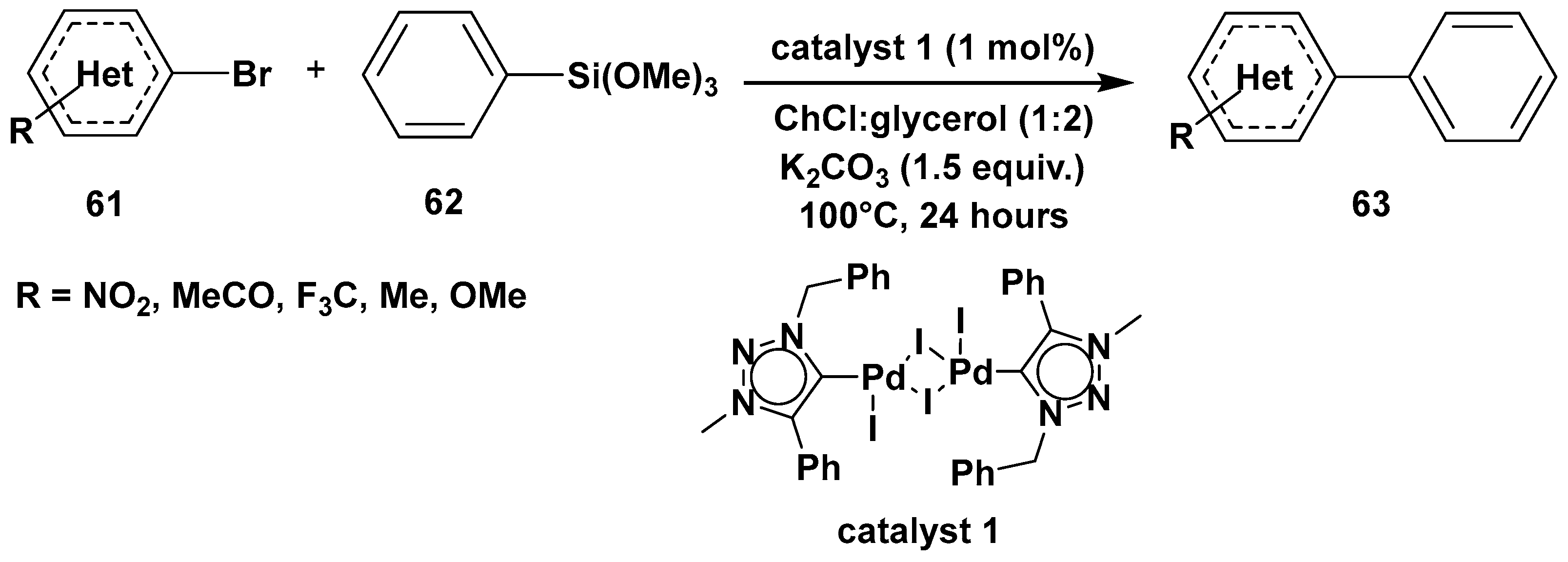
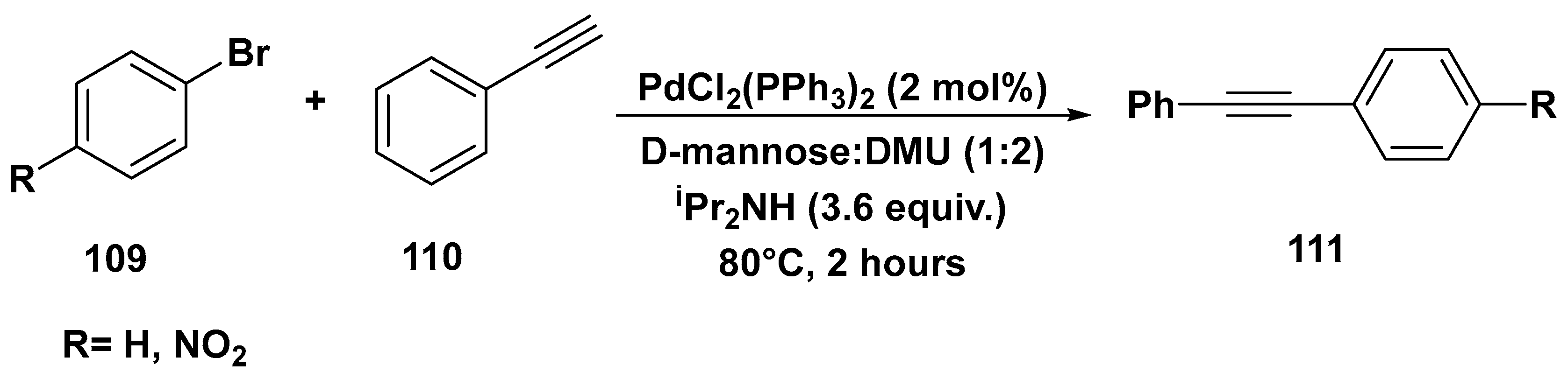
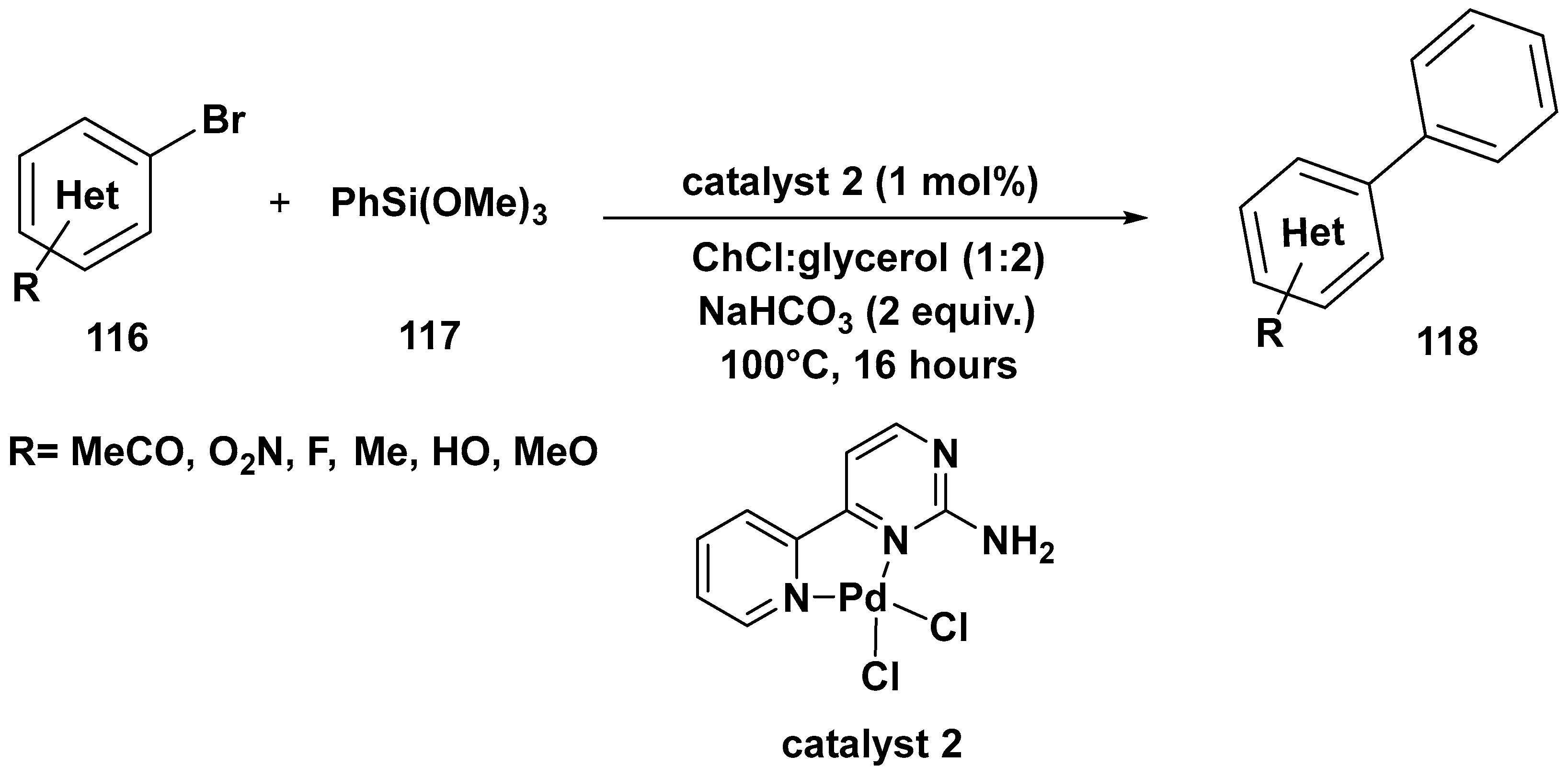
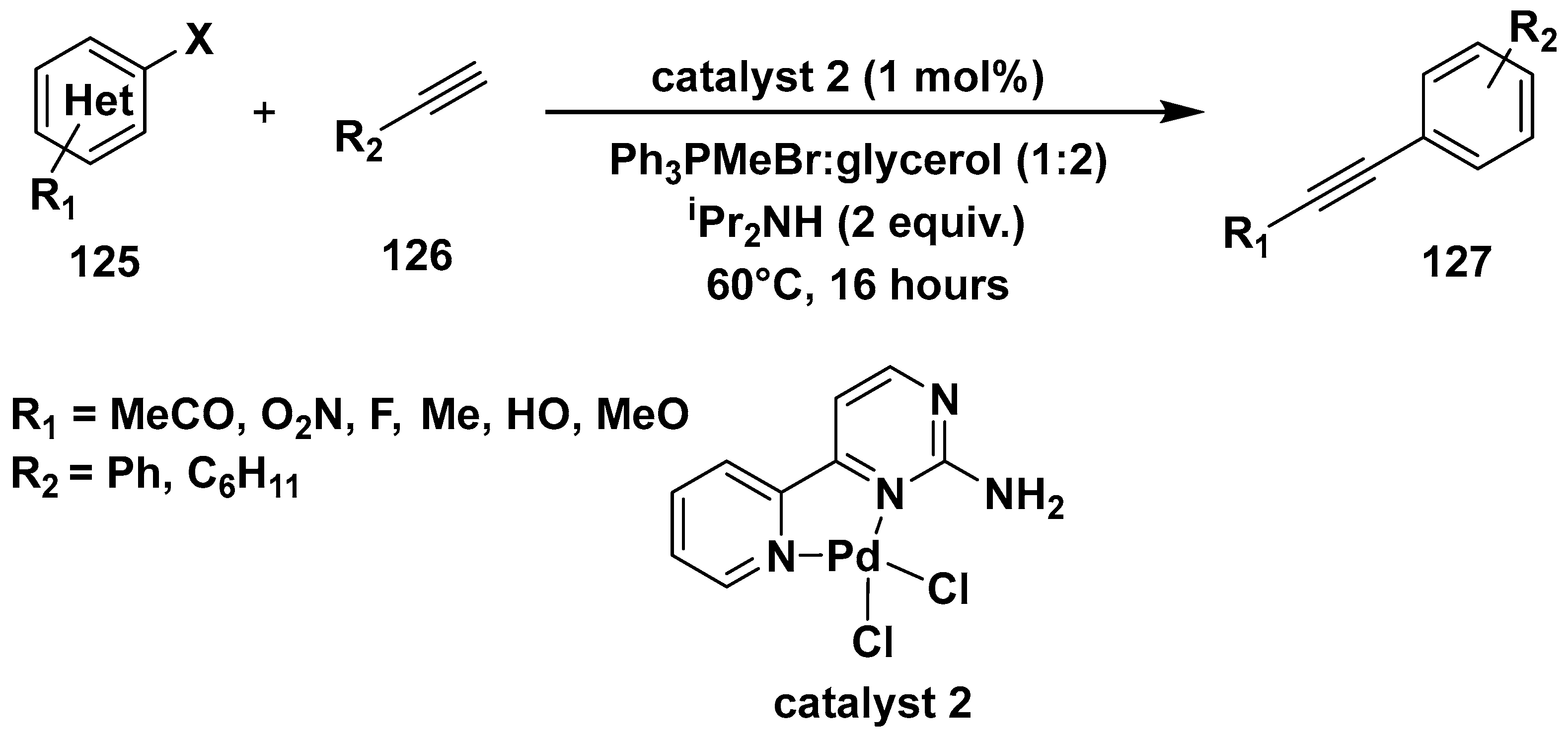
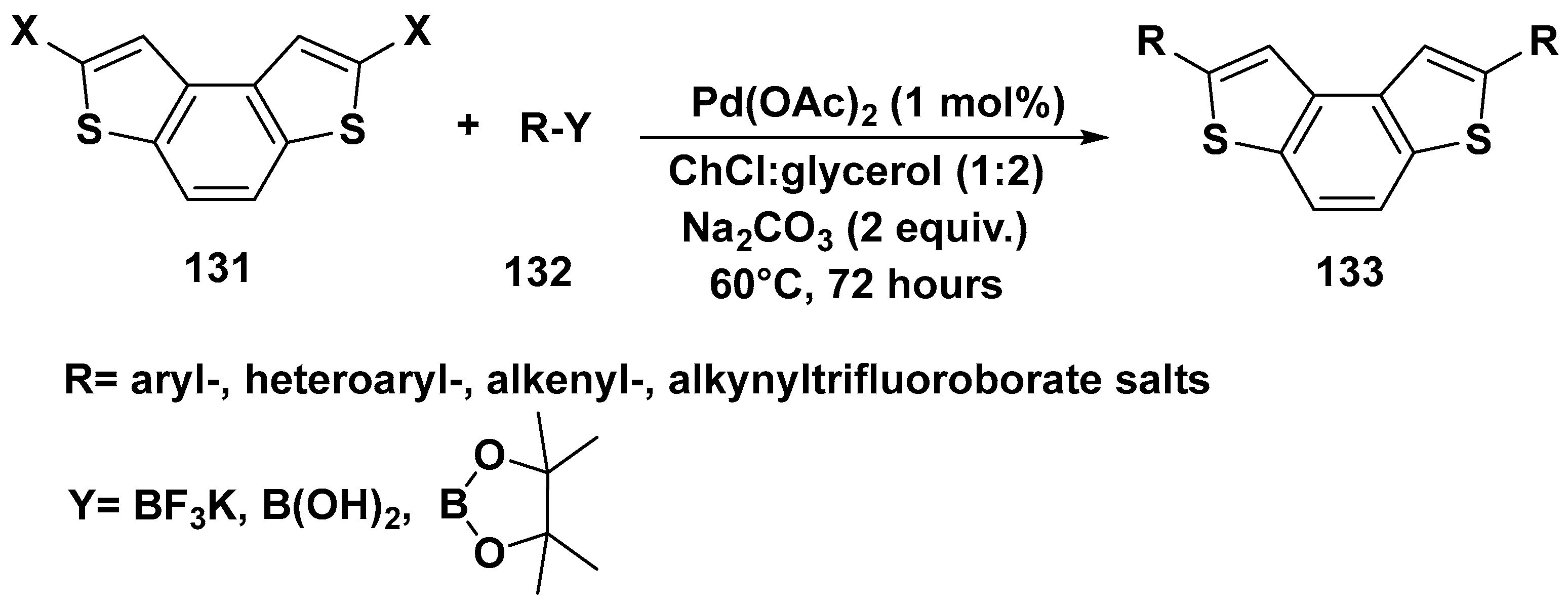
3. Cross-Coupling Reactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laird, T. Green Chemistry Is Good Process Chemistry. Org. Process Res. Dev. 2012, 16, 1–2. [Google Scholar] [CrossRef]
- Kemeling, G.M. Editorial: Solvent Choices and Sustainable Chemistry. ChemSusChem 2012, 5, 2291–2292. [Google Scholar] [CrossRef] [PubMed]
- Burrow, C.J.; Harper, J.B.; Sander, W.; Tantillo, D.J. Solvation E Ff Ects in Organic Chemistry. J. Org. Chem. 2022, 87, 1599–1601. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, F. Green Chemistry: Principle and Its Application. In Proceedings of the 2nd International Conference on Advancement in Engineering, Applied Science and Management (ICAEASM-2017), New Delhi, India, 2 July 2017. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Memorandum of Understanding of the 12 Principles of Green Chemistry; American Chemical Society Green Chemistry Institute: Washington, DC, USA, 2010; pp. 29–30. [Google Scholar]
- Seltzer, K.M.; Pennington, E.; Rao, V.; Murphy, B.N.; Strum, M.; Isaacs, K.K.; Pye, H.O.T. Reactive Organic Carbon Emissions from Volatile Chemical Products. Atmos. Chem. Phys. 2021, 21, 5079–5100. [Google Scholar] [CrossRef] [PubMed]
- Amelio, A.; Genduso, G.; Vreysen, S.; Luis, P.; Van Der Bruggen, B. Guidelines Based on Life Cycle Assessment for Solvent Selection during the Process Design and Evaluation of Treatment Alternatives. Green Chem. 2014, 16, 3045–3063. [Google Scholar] [CrossRef]
- Jing, A.; Kumar, V.; Kannan, K. Environmental Chemistry and Ecotoxicology A Review of Environmental Occurrence, Toxicity, Biotransformation and Biomonitoring of Volatile Organic Compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Roy, W.R. Environmental Impact of Solvents. Handb. Solvents 2014, 2, 361–385. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Choi, Y.; Kim, D. Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality. Atmosphere 2021, 12, 637. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Type V Deep Eutectic Solvents: Design and Applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep Eutectic Solvents in Separations: Methods of Preparation, Polarity, and Applications in Extractions and Capillary Electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent Developments in Deep Eutectic Solvents in Chemical Sciences. Mon. Fur Chem. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- El Abbouchi, A.; Koubachi, J.; Brahmi, N. El Direct Arylation and Suzuki-Miyaura Coupling of Imidazo [1,2-a]Pyridines Catalyzed by (SIPr) Pd (Allyl) Cl Complex Under. Mediterr. J. Chem. 2019, 9, 347–354. [Google Scholar] [CrossRef]
- Gambouz, K.; El Abbouchi, A.; Nassiri, S.; Suzenet, F.; Bousmina, M.; Akssira, M.; Guillaumet, G.; El Kazzouli, S. “On Water” Palladium Catalyzed Direct Arylation of 1H-Indazole and 1H-7-Azaindazole. Molecules 2020, 25, 2820. [Google Scholar] [CrossRef] [PubMed]
- Koubachi, J.; El Kazzouli, S.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. New and Efficient Palladium(0)-Mediated Microwave-Assisted Direct C3 Alkenylation of Imidazo[1,2-a]Pyridines. Synthesis 2008, 16, 2537–2542. [Google Scholar] [CrossRef]
- El Kazzouli, S.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Synthesis and Functionalization of Imidazo[1,2-a]Pyridines and Imidazo[1,2-a]Pyrimidines on Solid Phase Using Suzuki-Miyaura Cross-Coupling Reactions. Lett. Org. Chem. 2012, 9, 118–127. [Google Scholar] [CrossRef]
- Berteina-raboin, S.; Mouaddib, A. Regioselective Palladium-Catalyzed Arylation and Heteroarylation of Imidazo [1,2-a] Pyridines. Synlett 2006, 19, 3237–3242. [Google Scholar] [CrossRef]
- Lavrard, H.; Popowycz, F. Regioselective Late-Stage C-3 Functionalization of Pyrazolo-[3,4-b] Pyridines. Synthesis 2018, 50, 998–1006. [Google Scholar] [CrossRef]
- Faarasse, S.; El Kazzouli, S.; Naas, M.; Jouha, J.; Suzenet, F.; Guillaumet, G. “On Water” Direct C-3 Arylation of 2H-Pyrazolo[3,4-b]Pyridines. J. Org. Chem. 2017, 82, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Faarasse, S.; El Kazzouli, S.; Suzenet, F.; Guillaumet, G. Palladium-Catalyzed C3-Arylations of 1H- and 2H-Pyrazolo[4,3-b]Pyridines on Water. J. Org. Chem. 2018, 83, 12847–12854. [Google Scholar] [CrossRef] [PubMed]
- Boujdi, K.; El Brahmi, N.; Graton, J.; Dubreuil, D.; Collet, S.; Mathé-Allainmat, M.; Akssira, M.; Lebreton, J.; El Kazzouli, S. A Regioselective C7 Bromination and C7 Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Arylation of 4-Substituted NH-Free Indazoles. RSC Adv. 2021, 11, 7107–7114. [Google Scholar] [CrossRef] [PubMed]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Ben-Yahia, A.; Naas, M.; El Kazzouli, S.; Essassi, E.M.; Guillaumet, G. Direct C-3-Arylations of 1H-Indazoles. Eur. J. Org. Chem. 2012, 36, 7075–7081. [Google Scholar] [CrossRef]
- Faarasse, S.; El Brahmi, N.; Guillaumet, G.; El Kazzouli, S. Ring of the 6, 5-Fused Heterocyclic Systems: An Overview. Molecules 2021, 26, 5763. [Google Scholar] [CrossRef]
- Koubachi, J.; El Brahmi, N.; Guillaumet, G.; El Kazzouli, S. Oxidative Alkenylation of Fused Bicyclic Heterocycles. Eur. J. Org. Chem. 2019, 2019, 2568–2586. [Google Scholar] [CrossRef]
- Naas, M.; El Kazzouli, S.; Essassi, E.M.; Bousmina, M.; Guillaumet, G. Palladium-Catalyzed Oxidative Direct C3- and C7-Alkenylations of Indazoles: Application to the Synthesis of Gamendazole. Org. Lett. 2015, 17, 4320–4323. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.W.; Mo, L.P.; Zhang, Z.H. Recent Advances in the Application of Deep Eutectic Solvents as Sustainable Media as Well as Catalysts in Organic Reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Punzi, A.; Coppi, D.I.; Matera, S.; Capozzi, M.A.M.; Operamolla, A.; Ragni, R.; Babudri, F.; Farinola, G.M. Pd-Catalyzed Thiophene-Aryl Coupling Reaction via C-H Bond Activation in Deep Eutectic Solvents. Org. Lett. 2017, 19, 4754–4757. [Google Scholar] [CrossRef]
- Shariatipour, M.; Salamatmanesh, A.; Jadidi Nejad, M.; Heydari, A. Imidazole-Aryl Coupling Reaction via C[Sbnd]H Bond Activation Catalyzed by Palladium Supported on Modified Magnetic Reduced Graphene Oxide in Alkaline Deep Eutectic Solvent. Catal. Commun. 2020, 135, 105890. [Google Scholar] [CrossRef]
- Naser, J.; Mjalli, F.; Jibril, B.; Al-Hatmi, S.; Gano, Z. Potassium Carbonate as a Salt for Deep Eutectic Solvents. Int. J. Chem. Eng. Appl. 2013, 4, 114–118. [Google Scholar] [CrossRef]
- Tran, P.H.; Thi Hang, A.H. Deep Eutectic Solvent-Catalyzed Arylation of Benzoxazoles with Aromatic Aldehydes. RSC Adv. 2018, 8, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Links, D.A.; Liu, S.; Chen, R.; Guo, X.; Yang, H.; Deng, G.; Li, C. Iron-Catalyzed Arylation of Benzoazoles with Aromatic Aldehydes Using Oxygen. Green Chem. 2012, 14, 1577–1580. [Google Scholar] [CrossRef]
- Teo, Y.C.; Riduan, S.N.; Zhang, Y. Iodine-Mediated Arylation of Benzoxazoles with Aldehydes. Green Chem. 2013, 15, 2365–2368. [Google Scholar] [CrossRef]
- Zhu, F.; Tao, J.; Wang, Z. Palladium-Catalyzed C−H Arylation of (Benzo)Oxazoles or (Benzo)Thiazoles with Aryltrimethylammonium Tri Fl Ates. Org. Lett. 2015, 17, 4926–4929. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, K.; Kim, S.E.; Cho, H.J.; Lee, J.; Kim, Y.; Kim, M. Copper-Catalyzed Selective Arylations of Benzoxazoles with Aryl Iodides. J. Org. Chem. 2015, 80, 3670–3676. [Google Scholar] [CrossRef]
- Steinberg, D.F.; Turk, M.C.; Kalyani, D. Nickel-Catalyzed C−H Arylation of Benzoxazoles and Oxazoles: Benchmarking the Influence of Electronic, Steric and Leaving Group Variations in Phenolic Electrophiles. Tetrahedron 2017, 73, 2196–2209. [Google Scholar] [CrossRef]
- D’Amico, F.; Papucci, C.; Franchi, D.; Reginato, G.; Calamante, M.; Zani, L.; Dessì, A.; Mordini, A. Sustainable Pd-Catalyzed Direct Arylation of Thienyl Derivatives with (Hetero)Aromatic Bromides under Air in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2022, 10, 3037–3047. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora Japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Chen, X.; Engle, K.M.; Wang, D.H.; Jin-Quan, Y. Palladium(II)-CataIyzed C-H Aetivation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef] [PubMed]
- Vitale, P.; Cicco, L.; Perna, F.M.; Capriati, V. Introducing Deep Eutectic Solvents in Enolate Chemistry: Synthesis of 1-Arylpropan-2-Ones under Aerobic Conditions. React. Chem. Eng. 2021, 6, 1796–1800. [Google Scholar] [CrossRef]
- Vitale, P.; Tacconelli, S.; Perrone, M.G.; Malerba, P.; Simone, L.; Scilimati, A.; Lavecchia, A.; Dovizio, M.; Marcantoni, E.; Bruno, A.; et al. Synthesis, Pharmacological Characterization, and Docking Analysis of a Novel Family of Diarylisoxazoles as Highly Selective Cyclooxygenase-1 (COX-1) Inhibitors. J. Med. Chem. 2013, 56, 4277–4299. [Google Scholar] [CrossRef]
- Perrone, M.G.; Vitale, P.; Panella, A.; Fortuna, C.G.; Scilimati, A. General Role of the Amino and Methylsulfamoyl Groups in Selective Cyclooxygenase(COX)-1 Inhibition by 1,4-Diaryl-1,2,3-Triazoles and Validation of a Predictive Pharmacometric PLS Model. Eur. J. Med. Chem. 2015, 94, 252–264. [Google Scholar] [CrossRef]
- Saavedra, B.; Ramón, D.J. Deep Eutectic Solvent as a Sustainable Medium for C-C Bond Formation Via Multicomponent Radical Conjugate Additions. ACS Sustain. Chem. Eng. 2021, 9, 7941–7947. [Google Scholar] [CrossRef]
- Nomura, K.; Terwilliger, P. Self-Dual Leonard Pairs Use of Deep Eutectic Solvents as Catalyst: A mini-review. Green Process. Synth. 2019, 8, 355–372. [Google Scholar]
- Teja, C.; Nawaz Khan, F.R. Choline Chloride-Based Deep Eutectic Systems in Sequential Friedländer Reaction and Palladium-Catalyzed Sp3 CH Functionalization of Methyl Ketones. ACS Omega 2019, 4, 8046–8055. [Google Scholar] [CrossRef]
- Schlepphorst, C.; Maji, B.; Glorius, F. Ruthenium-NHC Catalyzed α-Alkylation of Methylene Ketones Provides Branched Products through Borrowing Hydrogen Strategy. ACS Catal. 2016, 6, 4184–4188. [Google Scholar] [CrossRef]
- Marset, X. Palladium-Catalysed Csp3-H Functionalisation of Unactivated 8-Aminoquinoline Amides in Deep Eutectic Solvents. Org. Biomol. Chem. 2022, 20, 7071–7075. [Google Scholar] [CrossRef]
- Larrosa, M.; Heiles, S.; Becker, J.; Spengler, B.; Hrdina, R. C-H Bond Arylation of Diamondoids Catalyzed by Palladium(II) Acetate. Adv. Synth. Catal. 2016, 358, 2163–2171. [Google Scholar] [CrossRef]
- Marset, X.; Khoshnood, A.; Sotorríos, L.; Gómez-Bengoa, E.; Alonso, D.A.; Ramón, D.J. Deep Eutectic Solvent Compatible Metallic Catalysts: Cationic Pyridiniophosphine Ligands in Palladium Catalyzed Cross-Coupling Reactions. ChemCatChem 2017, 9, 1269–1275. [Google Scholar] [CrossRef]
- Marset, X.; Guillena, G.; Ramón, D.J. Deep Eutectic Solvents as Reaction Media for the Palladium-Catalysed C−S Bond Formation: Scope and Mechanistic Studies. Chem. Eur. J. 2017, 23, 10522–10526. [Google Scholar] [CrossRef] [PubMed]
- González-Gallardo, N.; Saavedra, B.; Guillena, G.; Ramón, D.J. A Jackpot C-H Activation Protocol Using Simple Ruthenium Catalyst in Deep Eutectic Solvents. Green Chem. 2022, 24, 4941–4951. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How Polar Are Choline Chloride-Based Deep Eutectic Solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Rehwinkel, H.; Schmees, N.; Schäcke, H.; Edman, K.; Wissler, L.; Reichel, A.; Jaroch, S. Discovery of New Selective Glucocorticoid Receptor Agonist Leads. Bioorg. Med. Chem. Lett. 2017, 27, 437–442. [Google Scholar] [CrossRef]
- Bosanac, T.; Hickey, E.R.; Ginn, J.; Kashem, M.; Kerr, S.; Kugler, S.; Li, X.; Olague, A.; Schlyer, S.; Young, E.R.R. Substituted 2H-Isoquinolin-1-Ones as Potent Rho-Kinase Inhibitors: Part 3, Aryl Substituted Pyrrolidines. Bioorg. Med. Chem. Lett. 2010, 20, 3746–3749. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, S.; Ann, J.; Ha, H.; Yoo, Y.D.; Kim, Y.H.; Hwang, J.Y.; Hur, K.H.; Jang, C.G.; Pearce, L.V.; et al. Discovery of Dual-Acting Opioid Ligand and TRPV1 Antagonists as Novel Therapeutic Agents for Pain. Eur. J. Med. Chem. 2019, 182, 111634. [Google Scholar] [CrossRef]
- Shabir, G.; Saeed, A.; El-Seedi, H.R. Phytochemistry Natural Isocoumarins: Structural Styles and Biological Activities, the Revelations Carry on …. Phytochemistry 2021, 181, 112568. [Google Scholar] [CrossRef]
- Saeed, A. Isocoumarins, Miraculous Natural Products Blessed with Diverse Pharmacological Activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties as a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Brown, A.W.; Fisher, M.; Tozer, G.M.; Kanthou, C.; Harrity, J.P.A. Sydnone Cycloaddition Route to Pyrazole-Based Analogs of Combretastatin A4. J. Med. Chem. 2016, 59, 9473–9488. [Google Scholar] [CrossRef] [PubMed]
- Delaye, P.-O.; Pénichon, M.; Boudesocque-Delaye, L.; Enguehard-Gueiffier, C.; Gueiffier, A. Natural Deep Eutectic Solvents as Sustainable Solvents for Suzuki –Miyaura Cross-Coupling Reactions Applied to Imidazo-Fused Heterocycles. SynOpen 2018, 2, 306–311. [Google Scholar] [CrossRef]
- Enguehard-Gueiffier, C.; Gueiffier, A. Recent Progress in the Pharmacology of Imidazo[1,2-a]Pyridines. Mini Rev. Med. Chem. 2007, 7, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Singh, D.; Kaur, G.; Mor, S.; Putta, V.P.R.K.; Polina, S.; Malakar, C.C.; Singh, V. In(OTf)3 Assisted Synthesis of β-Carboline C-3 Tethered Imidazo[1,2-a]Azine Derivatives. New J. Chem. 2017, 41, 1082–1093. [Google Scholar] [CrossRef]
- Sun, B.; Xu, T.; Zhang, L.; Zhu, R.; Yang, J.; Xu, M.; Jin, C. Metal-Free Regioselective Alkylation of Imidazo[1,2-a]Pyridines with N -Hydroxyphthalimide Esters under Organic Photoredox Catalysis. Synlett 2020, 31, 363–368. [Google Scholar] [CrossRef]
- Marset, X.; Saavedra, B.; González-Gallardo, N.; Beaton, A.; León, M.M.; Luna, R.; Ramón, D.J.; Guillena, G. Palladium Mesoionic Carbene Pre-Catalyst for General Cross-Coupling Transformations in Deep Eutectic Solvents. Front. Chem. 2019, 7, 700. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The Role of Water in Deep Eutectic Solvent-Base Extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Chen, W.; Fu, L.; Mu, T. Water Absorption by Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2019, 21, 2601–2610. [Google Scholar] [CrossRef]
- Dilauro, G.; García, S.M.; Tagarelli, D.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Bioinspired Suzuki–Miyaura Coupling Reactions Using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents. ChemSusChem 2018, 11, 3495–3501. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C. Palladium-Catalyzed Heck and Suzuki Coupling in Glycerol. Chem. Pap. 2007, 61, 228–232. [Google Scholar] [CrossRef]
- Wolfson, A.; Snezhko, A.; Meyouhas, T.; Tavor, D. Glycerol Derivatives as Green Reaction Mediums. Green Chem. Lett. Rev. 2012, 5, 7–12. [Google Scholar] [CrossRef]
- Chahdoura, F.; Pradel, C.; Gõmez, M. Palladium Nanoparticles in Glycerol: A Versatile Catalytic System for C-X Bond Formation and Hydrogenation Processes. Adv. Synth. Catal. 2013, 355, 3648–3660. [Google Scholar] [CrossRef]
- Edwards, G.A.; Trafford, M.A.; Hamilton, A.E.; Buxton, A.M.; Bardeaux, M.C.; Chalker, J.M. Melamine and Melamine-Formaldehyde Polymers as Ligands for Palladium and Application to Suzuki-Miyaura Cross-Coupling Reactions in Sustainable Solvents. J. Org. Chem. 2014, 79, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Ferlin, F.; Luciani, L.; Ackermann, L.; Vaccaro, L. Biomass-Derived Solvents as Effective Media for Cross-Coupling Reactions and C-H Functionalization Processes. Green Chem. 2017, 19, 1601–1612. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Monopoli, A.; Dell’Anna, M.M.; Latronico, M.; Cotugno, P.; Nacci, A. Ionic Liquids in Palladium-Catalyzed Cross-Coupling Reactions. Top. Organomet. Chem. 2015, 51, 237–286. [Google Scholar] [CrossRef]
- McNulty, J.; Capretta, A.; Wilson, J.; Dyck, J.; Adjabeng, G.; Robertson, A. Suzuki Cross-Coupling Reactions of Aryl Halides in Phosphonium Salt Ionic Liquidunder Mild Conditions. Chem. Commun. 2002, 17, 1986–1987. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Montingelli, F. Pd Nanoparticles as Efficient Catalysts for Suzuki and Stille Coupling Reactions of Aryl Halides in Ionic Liquids. ChemInform 2005, 36, 6040–6044. [Google Scholar] [CrossRef]
- Ning, Y.; Xue, Y.; Zhaofu, F.; Yongdan, L.; Yuan, K.; Dyson, P.J. Solvent-Enhanced Coupling of Sterically Hindered Reagents and Aryl Chlorides Using Functionalized Ionic Liquids. Organometallics 2009, 28, 937–939. [Google Scholar] [CrossRef]
- Azanza Perea, J.R.; Honorato Pérez, J.M.; Cuena Boy, R. Diflunisal. Rev. Med. Univ. Navarra 1982, 26, 253–254. [Google Scholar] [CrossRef]
- Thiyagamurthy, P.; Khan, F.R.N. A Base-Free Pd-Precatalyst Mediated Suzuki-Miyaura and Sonogashira Cross-Coupling in Deep Eutectic Solvents. ChemistrySelect 2020, 5, 2610–2617. [Google Scholar] [CrossRef]
- Donnell, A.F.; Michoud, C.; Rupert, K.C.; Han, X.; Aguilar, D.; Frank, K.B.; Fretland, A.J.; Gao, L.; Goggin, B.; Heather Hogg, J.; et al. Benzazepinones and Benzoxazepinones as Antagonists of Inhibitor of Apoptosis Proteins (IAPs) Selective for the Second Baculovirus Iap Repeat (BIR2) Domain. J. Med. Chem. 2013, 56, 7772–7787. [Google Scholar] [CrossRef] [PubMed]
- Marset, X.; Pérez, J.M.; Ramón, D.J. Cross-Dehydrogenative Coupling Reaction Using Copper Oxide Impregnated on Magnetite in Deep Eutectic Solvents. Green Chem. 2016, 18, 826–833. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, Properties, and Applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.B.; Huang, W.L.; Zhen, X.; Li, Y.M. Synthesis and Biological Evaluation of Tetrahydroisoquinoline Derivatives as Potential Multidrug Resistance Reversal Agents in Cancer. Chin. Chem. Lett. 2008, 19, 169–171. [Google Scholar] [CrossRef]
- Chelopo, M.P.; Pawar, S.A.; Sokhela, M.K.; Govender, T.; Kruger, H.G.; Maguire, G.E.M. Anticancer Activity of Ruthenium(II) Arene Complexes Bearing 1,2,3,4-Tetrahydroisoquinoline Amino Alcohol Ligands. Eur. J. Med. Chem. 2013, 66, 407–414. [Google Scholar] [CrossRef]
- Gitto, R.; Caruso, R.; Pagano, B.; De Luca, L.; Citraro, R.; Russo, E.; De Sarro, G.; Chimirri, A. Novel Potent Anticonvulsant Agent Containing a Tetrahydroisoquinoline Skeleton. J. Med. Chem. 2006, 49, 5618–5622. [Google Scholar] [CrossRef]
- Grunewald, G.L.; Dahanukar, V.H.; Caldwell, T.M.; Criscione, K.R. Examination of the Role of the Acidic Hydrogen in Imparting Selectivity of 7-(Aminosulfonyl)-1,2,3,4-Tetrahydroisoquinoline (SK&F 29661) Toward Inhibition of Phenylethanolamine N-Methyltransferase vs the R2-Adrenoceptor. J. Med. Chem. 1997, 2623, 3997–4005. [Google Scholar]
- Ashford, M.E.; Nguyen, V.H.; Greguric, I.; Pham, T.Q.; Keller, P.A.; Katsifis, A. Synthesis and in Vitro Evaluation of Tetrahydroisoquinolines with Pendent Aromatics as Sigma-2 (Σ2) Selective Ligands. Org. Biomol. Chem. 2014, 12, 783–794. [Google Scholar] [CrossRef]
- Kang, J.H. Salsolinol, a Tetrahydroisoquinoline-Derived Neurotoxin, Induces Oxidative Modification of Neurofilament-L: Protection by Histidyl Dipeptides. BMB Rep. 2011, 36, 488–492. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Karimi, S.; Shekaari, H. Hydrophilic Role of Deep Eutectic Solvents for Clean Synthesis of Biphenyls over a Magnetically Separable Pd-Catalyzed Suzuki-Miyaura Coupling Reaction. J. Mol. Liq. 2021, 324, 115078. [Google Scholar] [CrossRef]
- Nam, N.N.; Dang, H.; Do, K.; The, K.; Trinh, L.; Lee, N.Y. Design Strategy and Application of Deep Eutectic Solvents for Green Synthesis of Nanomaterials. Nanomaterials 2023, 13, 1164. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of Deep Eutectic Solvents in the Extraction and Separation of Target Compounds from Various Samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Shekaari, H.; Karimi, S. Pd Supported on Clicked Cellulose-Modified Magnetite-Graphene Oxide Nanocomposite for C-C Coupling Reactions in Deep Eutectic Solvent. Carbohydr. Polym. 2021, 251, 117109. [Google Scholar] [CrossRef]
- Nuri, A.; Vucetic, N.; Smått, J.H.; Mansoori, Y.; Mikkola, J.P.; Murzin, D.Y. Synthesis and Characterization of Palladium Supported Amino Functionalized Magnetic-MOF-MIL-101 as an Efficient and Recoverable Catalyst for Mizoroki–Heck Cross-Coupling. Catal. Lett. 2020, 150, 2617–2629. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Bahadorikhalili, S.; Lijan, H.; Ansari, S.; Hamedifar, H.; Mahdavi, M. Synthesis and Characterization of γ-Fe2O3@SiO2-(CH2)3-PDTC-Pd Magnetic Nanoparticles: A New and Highly Active Catalyst for the Heck/Sonogashira Coupling Reactions. New J. Chem. 2019, 43, 8930–8938. [Google Scholar] [CrossRef]
- Messa, F.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V.; Salomone, A. Sustainable Ligand-Free Heterogeneous Palladium-Catalyzed Sonogashira Cross-Coupling Reaction in Deep Eutectic Solvents. ChemCatChem 2020, 12, 1979–1984. [Google Scholar] [CrossRef]
- Di Carmine, G.; Abbott, A.P.; D’Agostino, C. Deep Eutectic Solvents: Alternative Reaction Media for Organic Oxidation Reactions. React. Chem. Eng. 2021, 6, 582–598. [Google Scholar] [CrossRef]
- Ramesh, D.; Vijayakumar, B.G.; Kannan, T. Therapeutic Potential of Uracil and Its Derivatives in Countering Pathogenic and Physiological Disorders. Eur. J. Med. Chem. 2020, 207, 112801. [Google Scholar] [CrossRef]
- Pałasz, A.; Ciez, D. In Search of Uracil Derivatives as Bioactive Agents. Uracils and Fused Uracils: Synthesis, Biological Activity and Applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Paris, J.; Ríos-Lombardía, N.; Morís, F.; Gröger, H.; González-Sabín, J. Novel Insights into the Combination of Metal- and Biocatalysis: Cascade One-Pot Synthesis of Enantiomerically Pure Biaryl Alcohols in Deep Eutectic Solvents. ChemCatChem 2018, 10, 4417–4423. [Google Scholar] [CrossRef]
- Burda, E.; Hummel, W.; Gröger, H. Modular Chemoenzymatic One-Pot Syntheses in Aqueous Media: Combination of a Palladium-Catalyzed Cross-Coupling with an Asymmetric Biotransformation. Angew. Chem. Int. Ed. 2008, 47, 9551–9554. [Google Scholar] [CrossRef]
- Borchert, S.; Burda, E.; Schatz, J.; Hummel, W.; Gröger, H. Combination of a Suzuki Cross-Coupling Reaction Using a Water-Soluble Palladium Catalyst with an Asymmetric Enzymatic Reduction towards a One-Pot Process in Aqueous Medium at Room Temperature. J. Mol. Catal. B Enzym. 2012, 84, 89–93. [Google Scholar] [CrossRef]
- Gauchot, V.; Kroutil, W.; Schmitzer, A.R. Highly Recyclable Chemo/Biocatalyzed Cascade Reactions with Ionic Liquids: One-Pot Synthesis of Chiral Biaryl Alcohols. Chem. A Eur. J. 2010, 16, 6748–6751. [Google Scholar] [CrossRef]
- Nejrotti, S.; Antenucci, A.; Pontremoli, C.; Gontrani, L.; Barbero, N.; Carbone, M.; Bonomo, M. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7, 47449–47461. [Google Scholar] [CrossRef]
- Mukai, S.; Yamada, Y. Catalyst Recycling in the Suzuki Coupling Reaction: Toward a Greener Synthesis in the Pharmaceutical Industry. Knowledge 2022, 3, 1. [Google Scholar] [CrossRef]
- Afewerki, S.; Franco, A.; Balu, A.M.; Tai, C.W.; Luque, R.; Córdova, A. Sustainable and Recyclable Heterogenous Palladium Catalysts from Rice Husk-Derived Biosilicates for Suzuki-Miyaura Cross-Couplings, Aerobic Oxidations and Stereoselective Cascade Carbocyclizations. Sci. Rep. 2020, 10, 6407. [Google Scholar] [CrossRef]
- Yoon, B.; Yen, C.H.; Mekki, S.; Wherland, S.; Wai, C.M. Effect of Water on the Heck Reactions Catalyzed by Recyclable Palladium Chloride in Ionic Liquids Coupled with Supercritical CO2 Extraction. Ind. Eng. Chem. Res. 2006, 45, 4433–4435. [Google Scholar] [CrossRef]
- Ilgen, F.; König, B. Organic Reactions in Low Melting Mixtures Based on Carbohydrates and L-Carnitine—A Comparison. Green Chem. 2009, 11, 848–885. [Google Scholar] [CrossRef]
- Rajagopal, R.; Srinivasan, K.V. Ultrasound Promoted Para-Selective Nitration of Phenols in Ionic Liquid. Ultrason. Sonochem. 2003, 10, 41–43. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Novak, Z.; Szabo, A.; Repasi, J.; Sonogashira, A.K. Coupling of Aryl Halides Catalyzed by Palladium on Charcoal The Palladium-Catalyzed Coupling of Terminal Acet- Ylenes with Aryl and Vinyl Halides (the Sonogashira Reaction) Is One of the Important and Widely Used Carbon—Carbon Bond-Forming. J. Org. Chem. 2003, 68, 3327–3329. [Google Scholar] [PubMed]
- Dakin, L.A.; Langille, N.F.; Panek, J.S. Synthesis of the C1′-C11′ Oxazole-Containing Side Chain of Leucascandrolide A. Application of a Sonogashira Cross-Coupling. J. Org. Chem. 2002, 67, 6812–6815. [Google Scholar] [CrossRef] [PubMed]
- Dilauro, G.; Cicco, L.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Pd-Catalyzed Reductive Mizoroki-Heck Reaction Strategy for the One-Pot Synthesis of Functionalized Oxygen Heterocycles in Deep Eutectic Solvents. Eur. J. Org. Chem. 2023, 26, e202200814. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Hay, M.B. Recent Advances in the Stereoselective Synthesis of Tetrahydrofurans. Tetrahedron 2007, 63, 261–290. [Google Scholar] [CrossRef] [PubMed]
- Lorente, A.; Lamariano-Merketegi, J.; Albericio, F.; Álvarez, M. Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea. Chem. Rev. 2013, 113, 4567–4610. [Google Scholar] [CrossRef] [PubMed]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef]
- Xu, F.; Zacuto, M.J.; Kohmura, Y.; Rosen, J.; Gibb, A.; Alam, M.; Scott, J.; Tschaen, D. Asymmetric Synthesis of Highly Functionalized Tetrahydropyran DPP-4 Inhibitor. Org. Lett. 2014, 16, 5422–5425. [Google Scholar] [CrossRef]
- Saavedra, B.; González-Gallardo, N.; Meli, A.; Ramón, D.J. A Bipyridine-Palladium Derivative as General Pre-Catalyst for Cross-Coupling Reactions in Deep Eutectic Solvents. Adv. Synth. Catal. 2019, 361, 3868–3879. [Google Scholar] [CrossRef]
- Wu, W.; Chen, S.; Tsai, F. Recyclable and Highly Active Cationic 2,20-Bipyridyl Palladium(II) Catalyst for Suzuki Cross-Coupling Reaction in Water. Tetrahedron Lett. 2008, 47, 9267–9270. [Google Scholar] [CrossRef]
- Chen, S.; Wu, W.; Tsai, F. Hiyama Reaction of Aryl Bromides with Arylsiloxanes Catalyzed by a Reusable Palladium(II)/Cationic Bipyridyl System in Water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Tsai, F. Palladium(II)/Cationic 2,2’-Bipyridyl System as a Highly Efficient and Reusable Catalyst for the Mizoroki-Heck Reaction in Water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef]
- Kerner, C.; Straub, S.; Sun, Y.; Thiel, W.R. A Rapid and Additive-Free Ruthenium-Catalyzed Reductive Amination of Aromatic Aldehydes. Eur. J. Org. Chem. 2016, 2016, 3060–3064. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Reetz, M.T.; Westermann, E. Phosphane-Free Palladium-Catalyzed Coupling Reactions: The Decisive Role of Pd Nanoparticles. Angew. Chem. Int. Ed. 2000, 39, 165–168. [Google Scholar] [CrossRef]
- Imperato, G.; Vasold, R.; König, B. Stille Reactions with Tetraalkylstannanes and Phenyltrialkylstannanes in Low Melting Sugar-Urea-Salt Mixtures. Adv. Synth. Catal. 2006, 348, 2243–2247. [Google Scholar] [CrossRef]
- Pelliccioli, V.; Dilauro, G.; Grecchi, S.; Arnaboldi, S.; Graiff, C.; Perna, F.M.; Vitale, P.; Licandro, E.; Aliprandi, A.; Cauteruccio, S.; et al. Ligand-Free Suzuki–Miyaura Cross-Coupling Reactions in Deep Eutectic Solvents: Synthesis of Benzodithiophene Derivatives and Study of Their Optical and Electrochemical Performance. Eur. J. Org. Chem. 2020, 2020, 6981–6988. [Google Scholar] [CrossRef]
- Krishna, T.; Reddy, T.; Kalita, D. nickel-catalyzed suzuki—Miyauracross- coupling reactions: One-pot synthesis of 2-arylthiophes. Rasayan J. Chem. 2020, 13, 2438–2444. [Google Scholar] [CrossRef]
- Allaka, T.R.; Varala, R.; Anireddy, J. Synthesis and anti-cancer activity of novel 3-aryl thiophene-2-carbaldehydes and their aryl /heteroaryl chalcone derivatives. Rasayan J. Chem. 2018, 9, 30–39. [Google Scholar]
- Xie, S.; Li, D.; Huang, H.; Zhang, F.; Chen, Y. Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation. J. Am. Chem. Soc. 2019, 141, 16237–16242. [Google Scholar] [CrossRef]

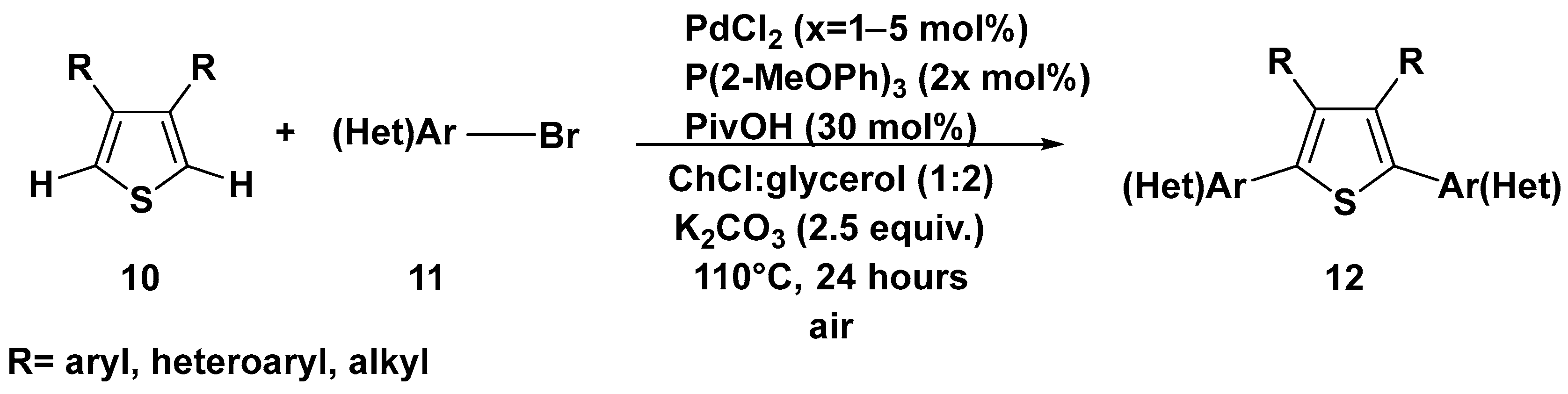



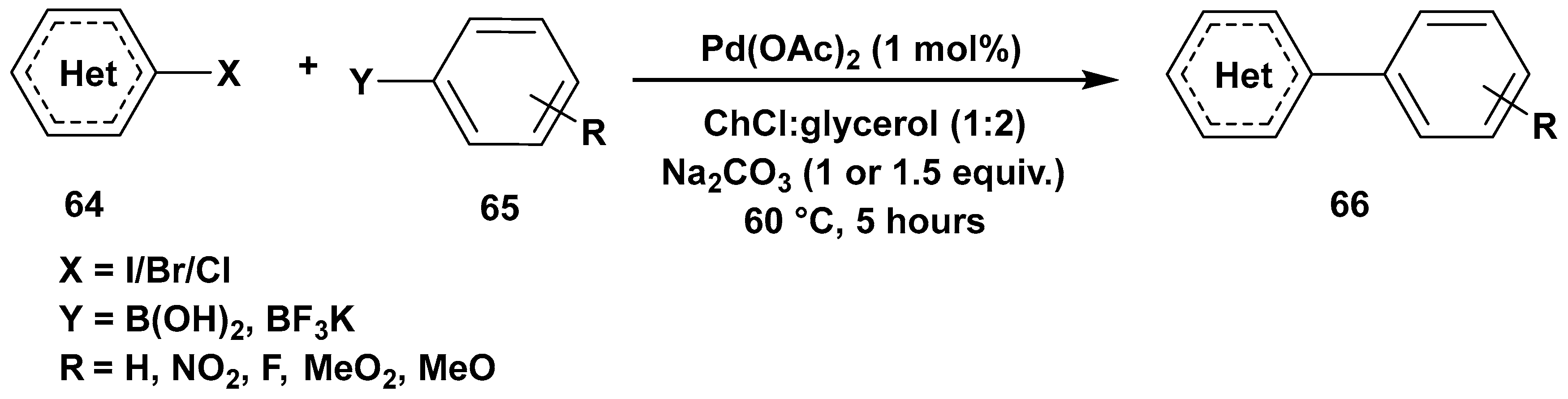
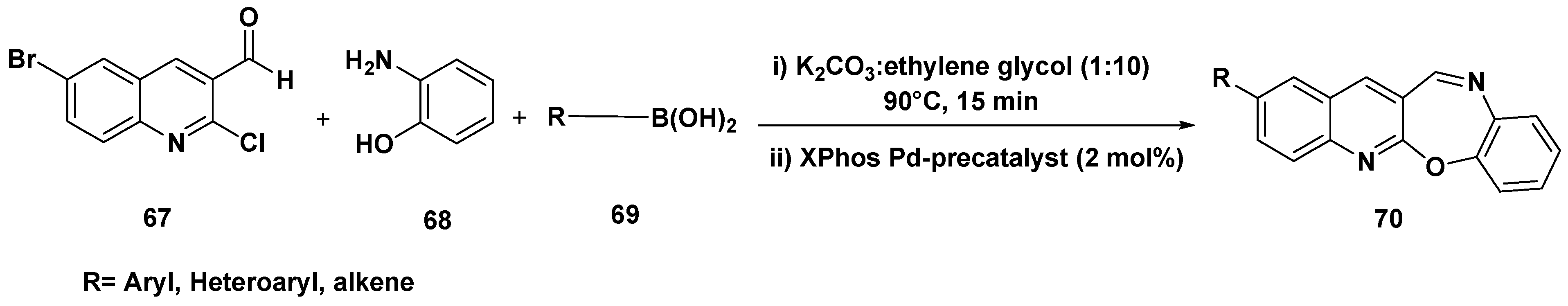

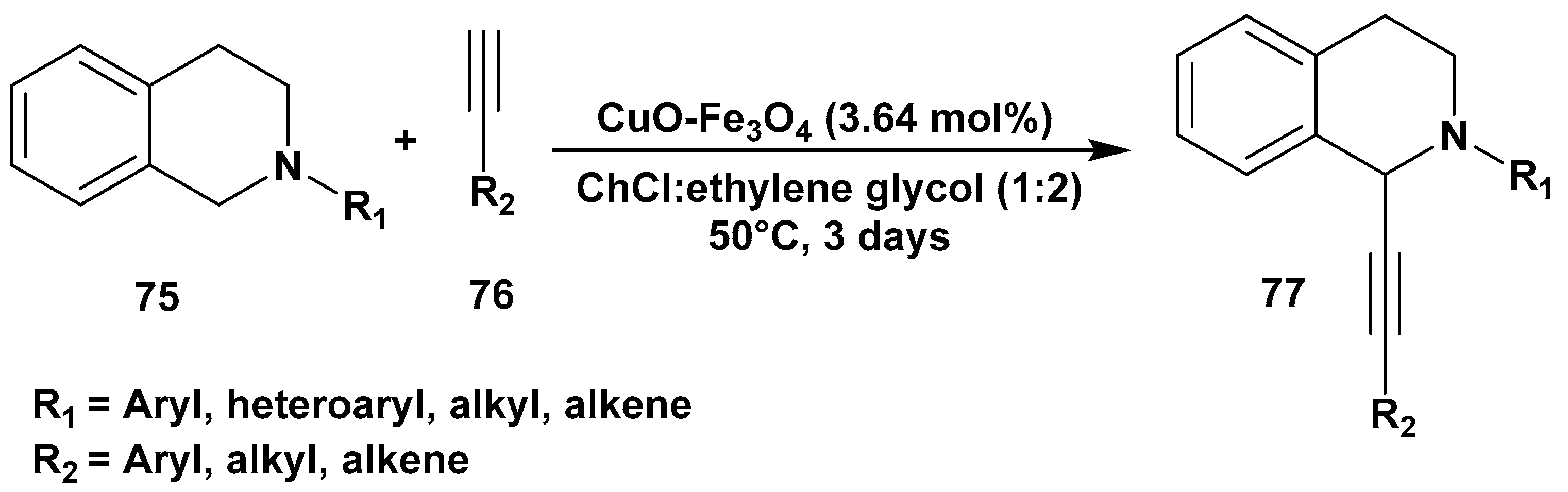
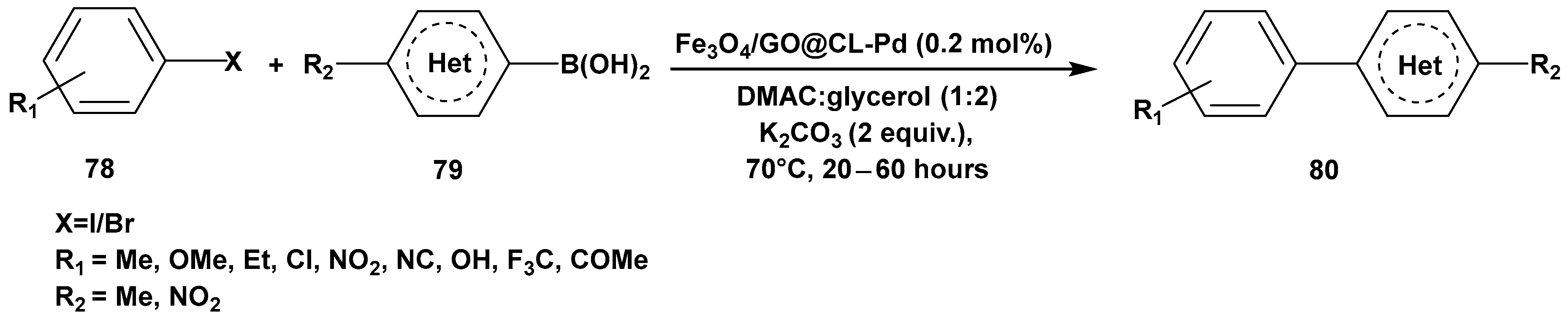
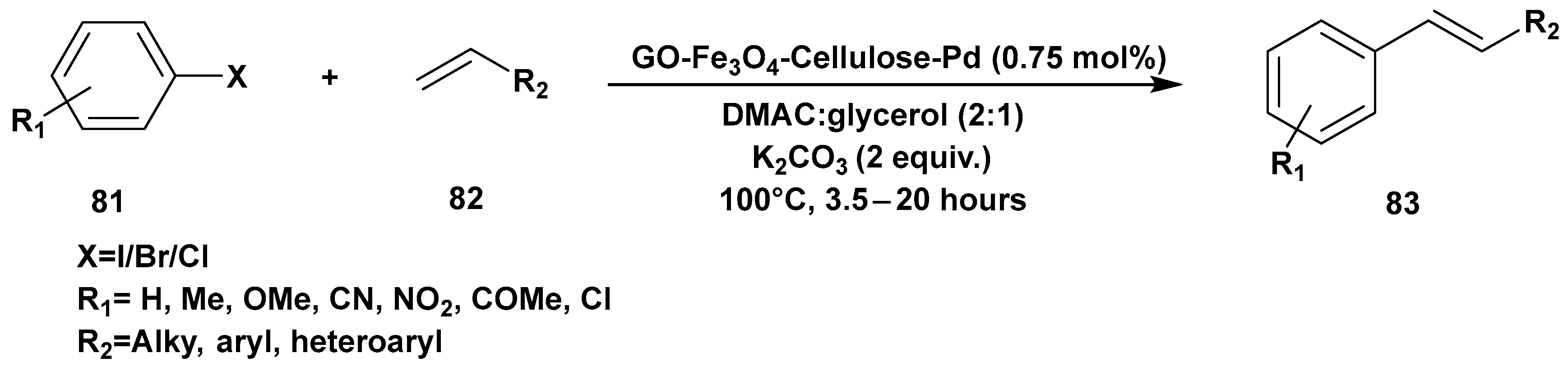
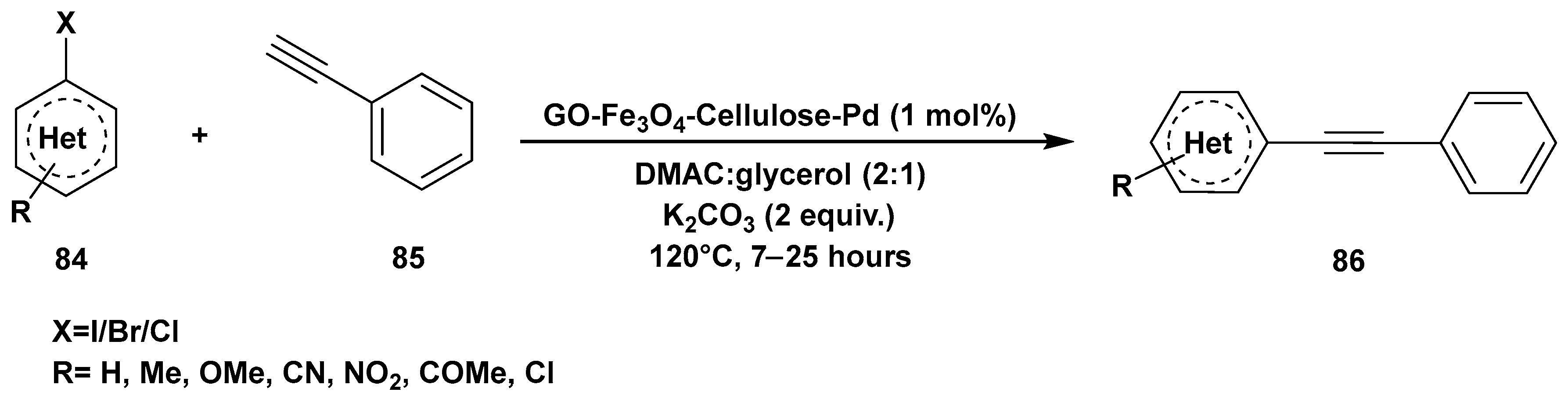
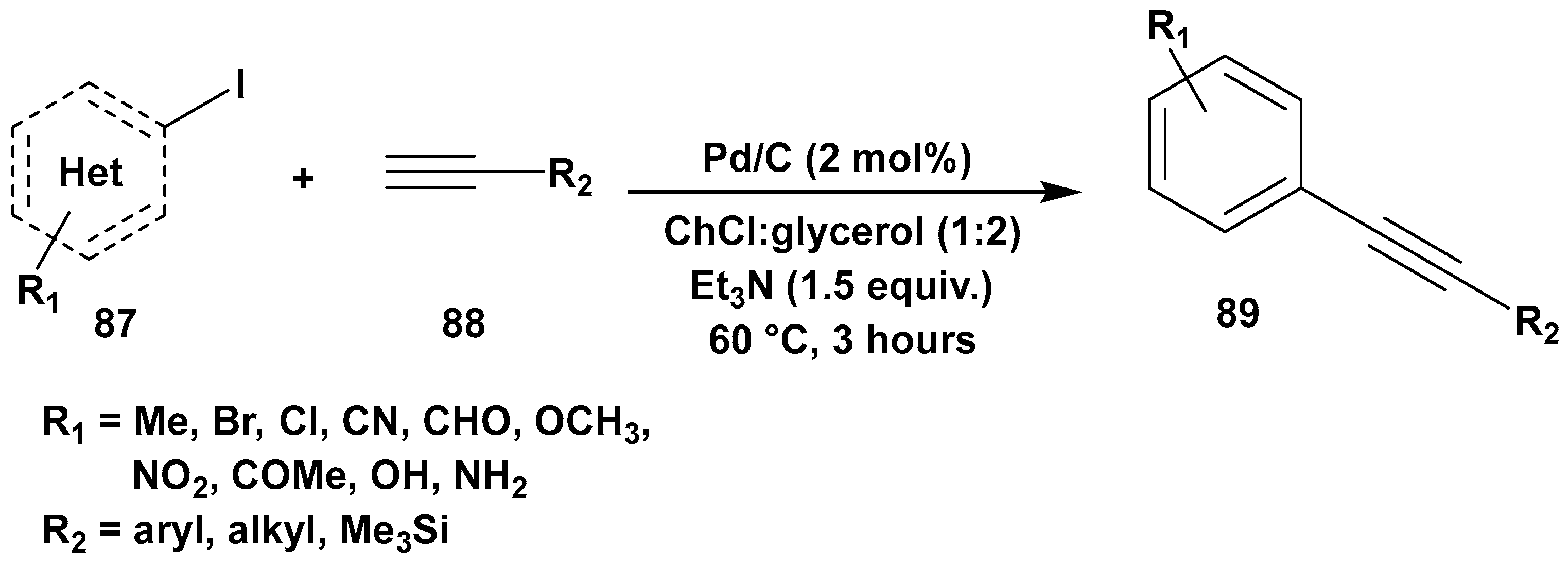
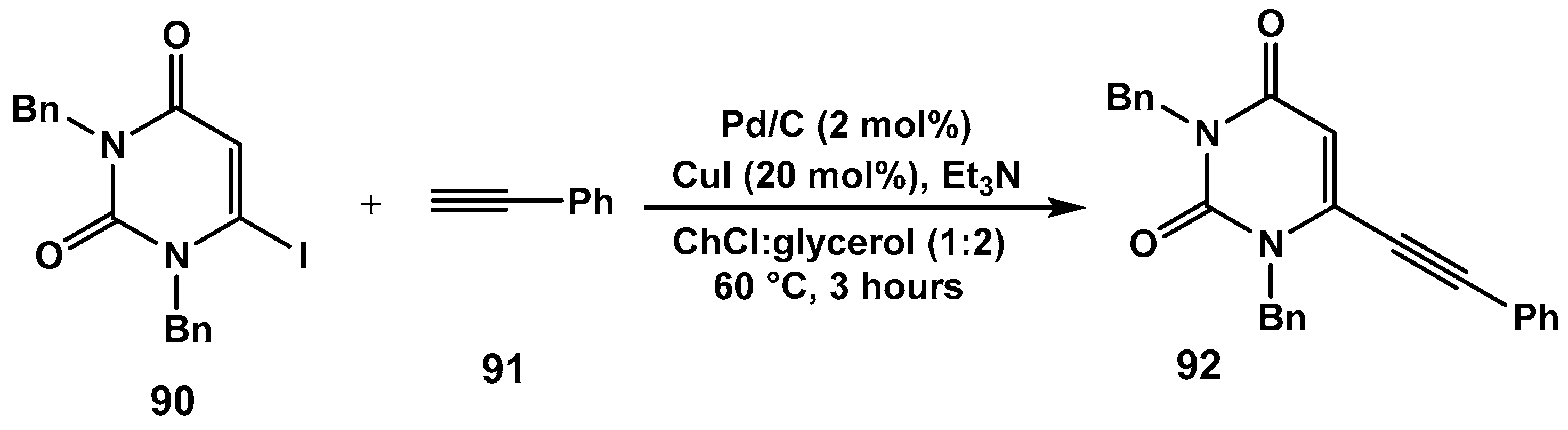
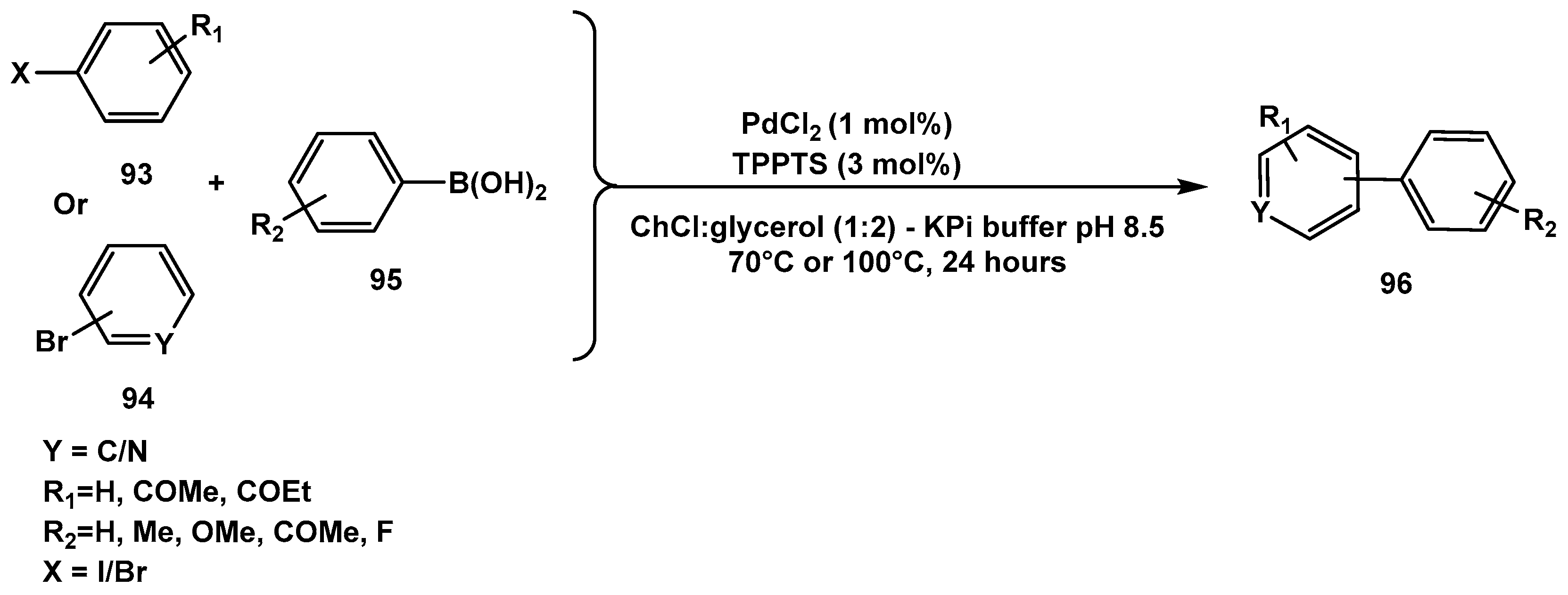
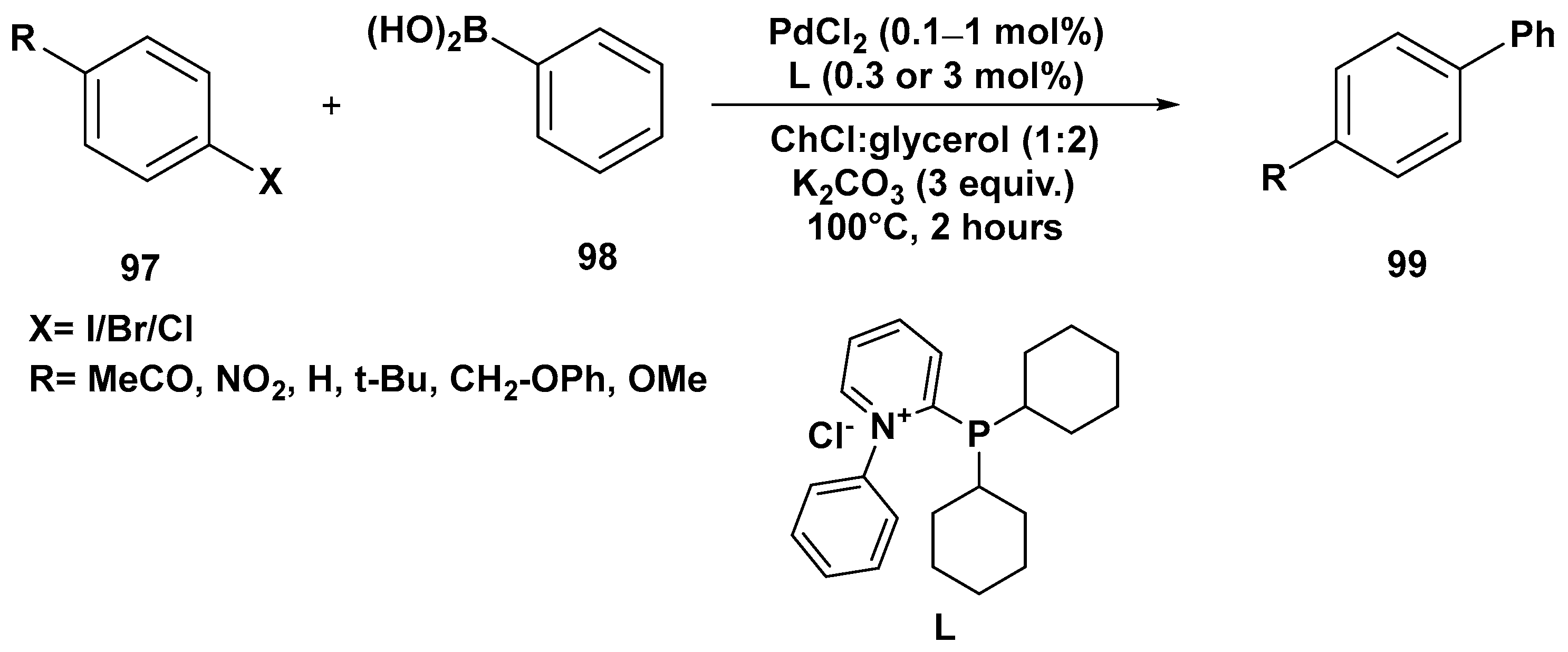

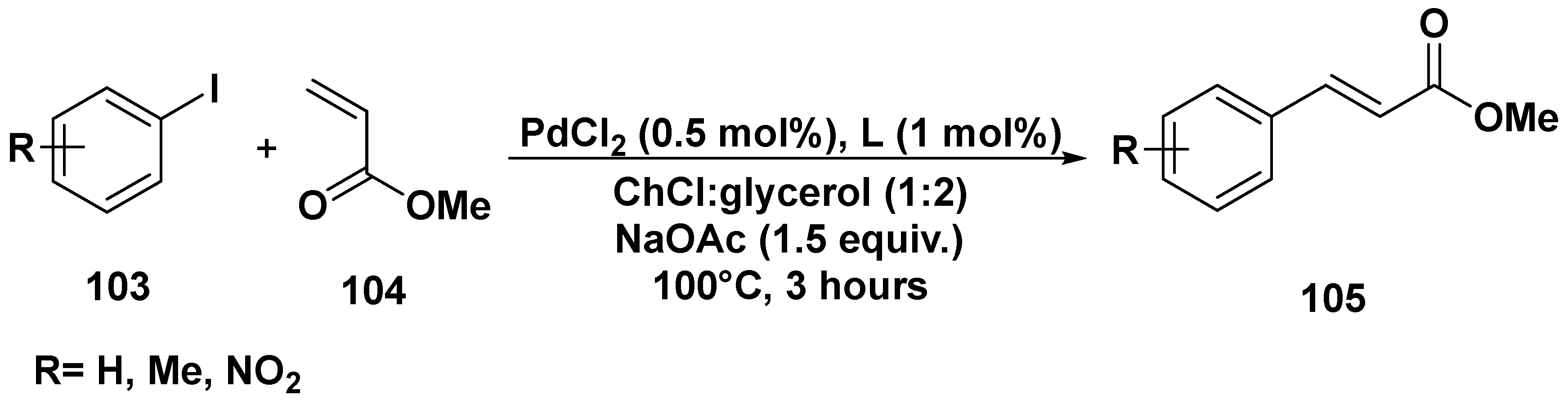

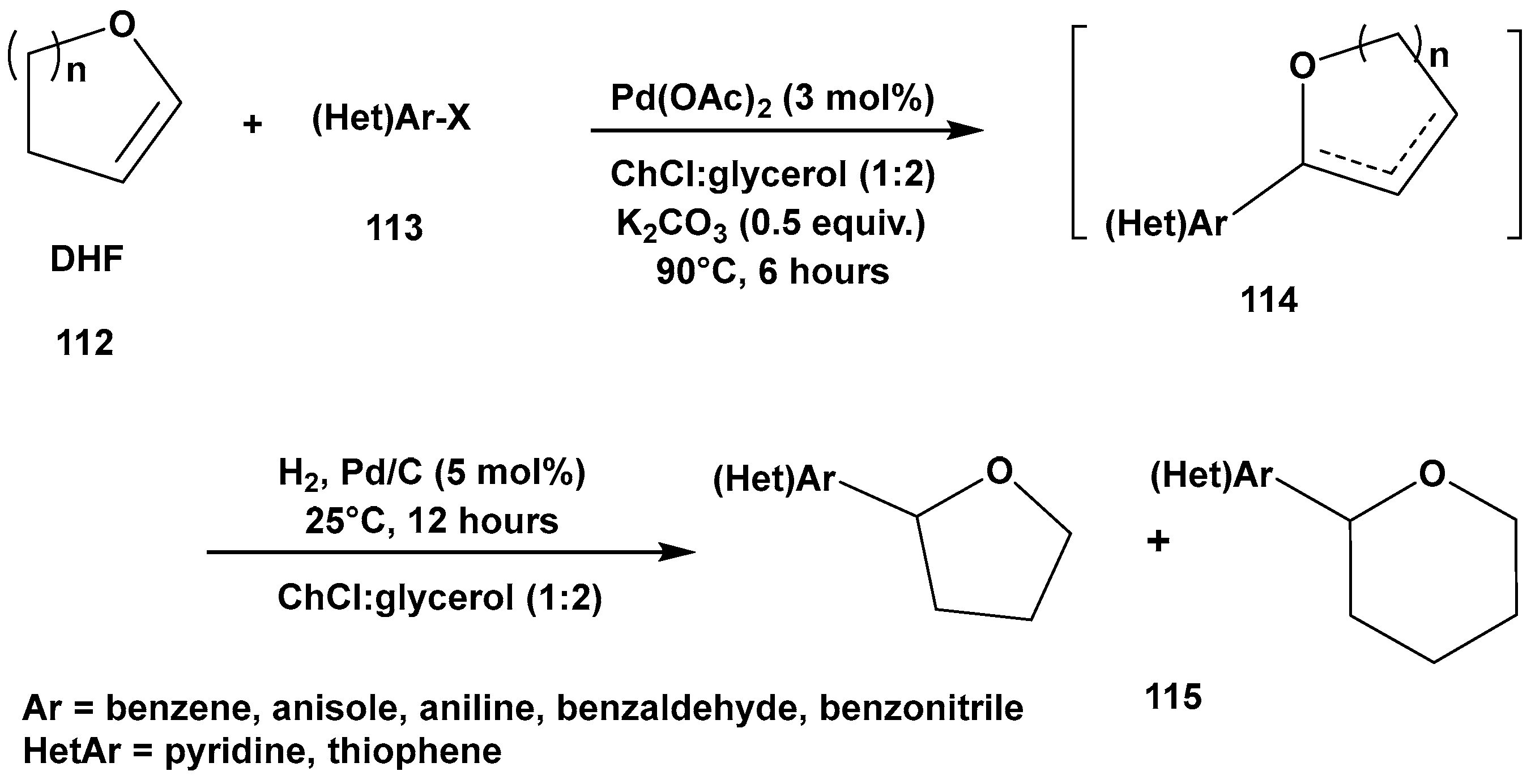


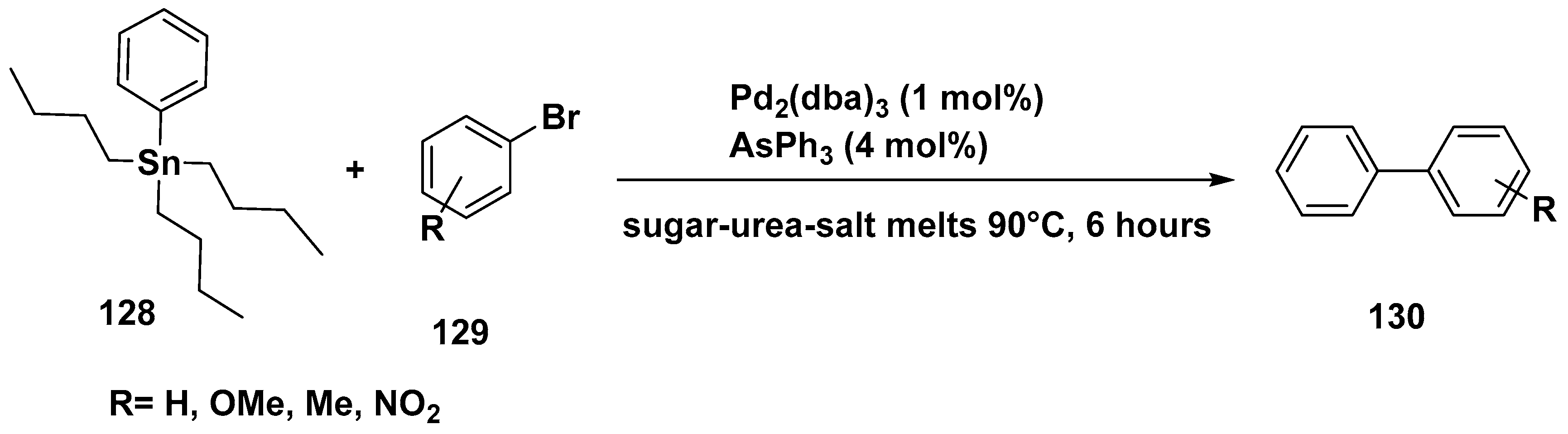
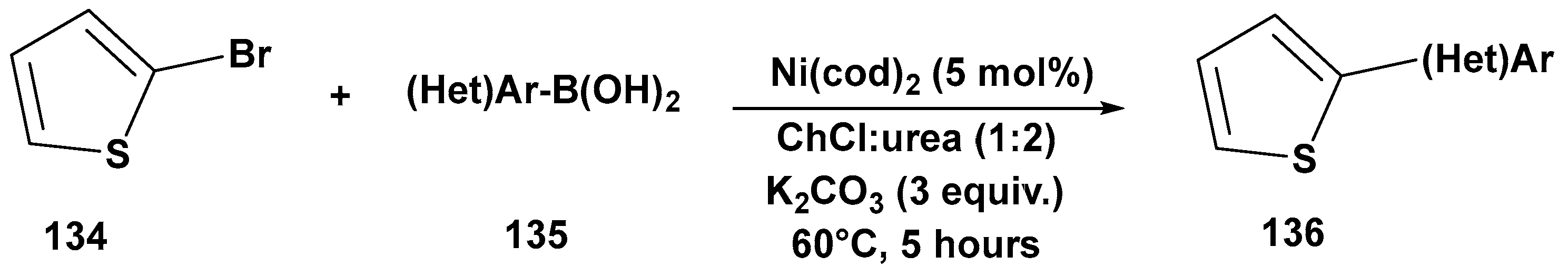
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Baraka, Y.; Hamdoun, G.; El Brahmi, N.; El Kazzouli, S. Unlocking the Potential of Deep Eutectic Solvents for C–H Activation and Cross-Coupling Reactions: A Review. Molecules 2023, 28, 4651. https://doi.org/10.3390/molecules28124651
El Baraka Y, Hamdoun G, El Brahmi N, El Kazzouli S. Unlocking the Potential of Deep Eutectic Solvents for C–H Activation and Cross-Coupling Reactions: A Review. Molecules. 2023; 28(12):4651. https://doi.org/10.3390/molecules28124651
Chicago/Turabian StyleEl Baraka, Yassine, Ghanem Hamdoun, Nabil El Brahmi, and Saïd El Kazzouli. 2023. "Unlocking the Potential of Deep Eutectic Solvents for C–H Activation and Cross-Coupling Reactions: A Review" Molecules 28, no. 12: 4651. https://doi.org/10.3390/molecules28124651
APA StyleEl Baraka, Y., Hamdoun, G., El Brahmi, N., & El Kazzouli, S. (2023). Unlocking the Potential of Deep Eutectic Solvents for C–H Activation and Cross-Coupling Reactions: A Review. Molecules, 28(12), 4651. https://doi.org/10.3390/molecules28124651





