Recent Advances in Regioselective C–H Bond Functionalization of Free Phenols
Abstract
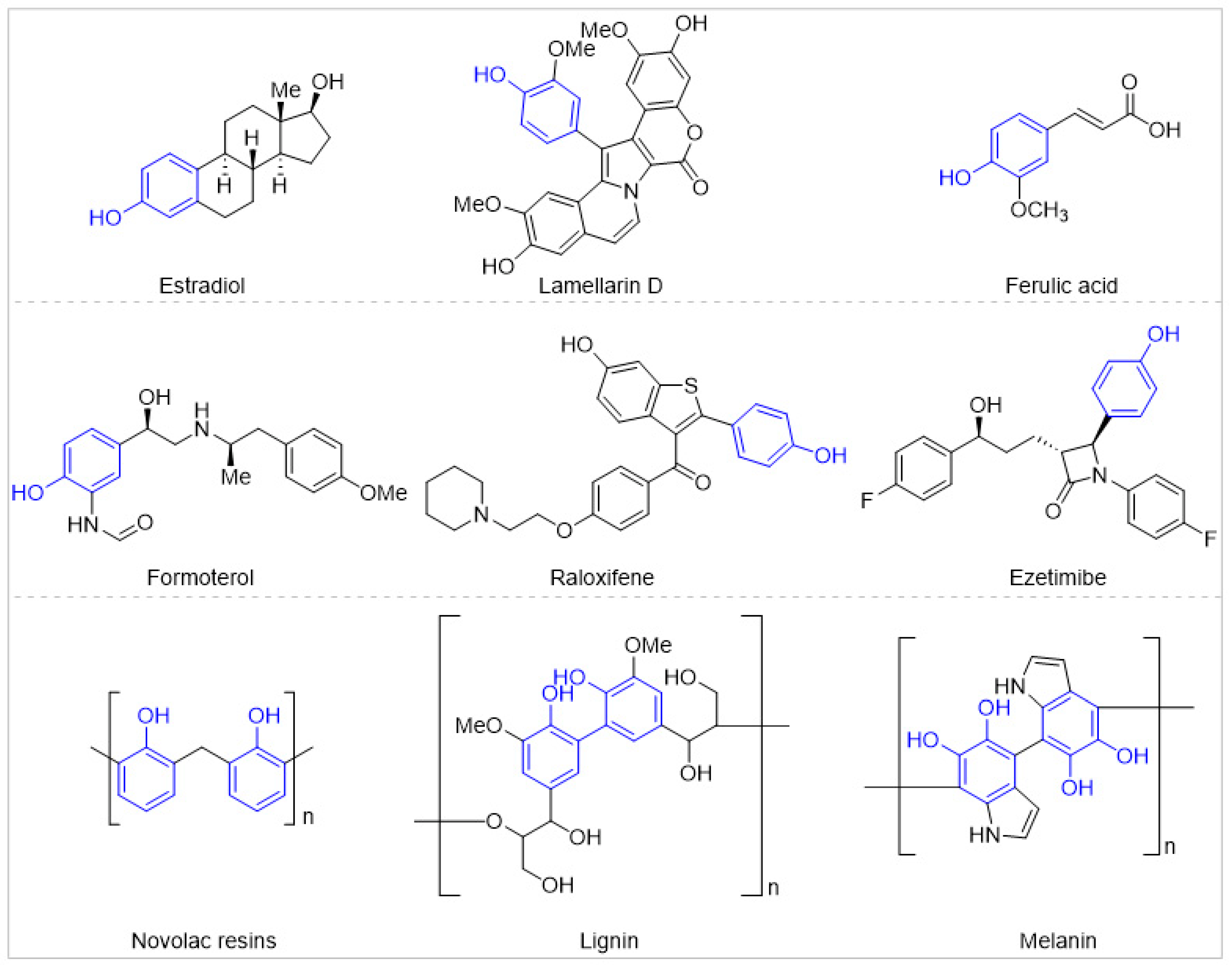
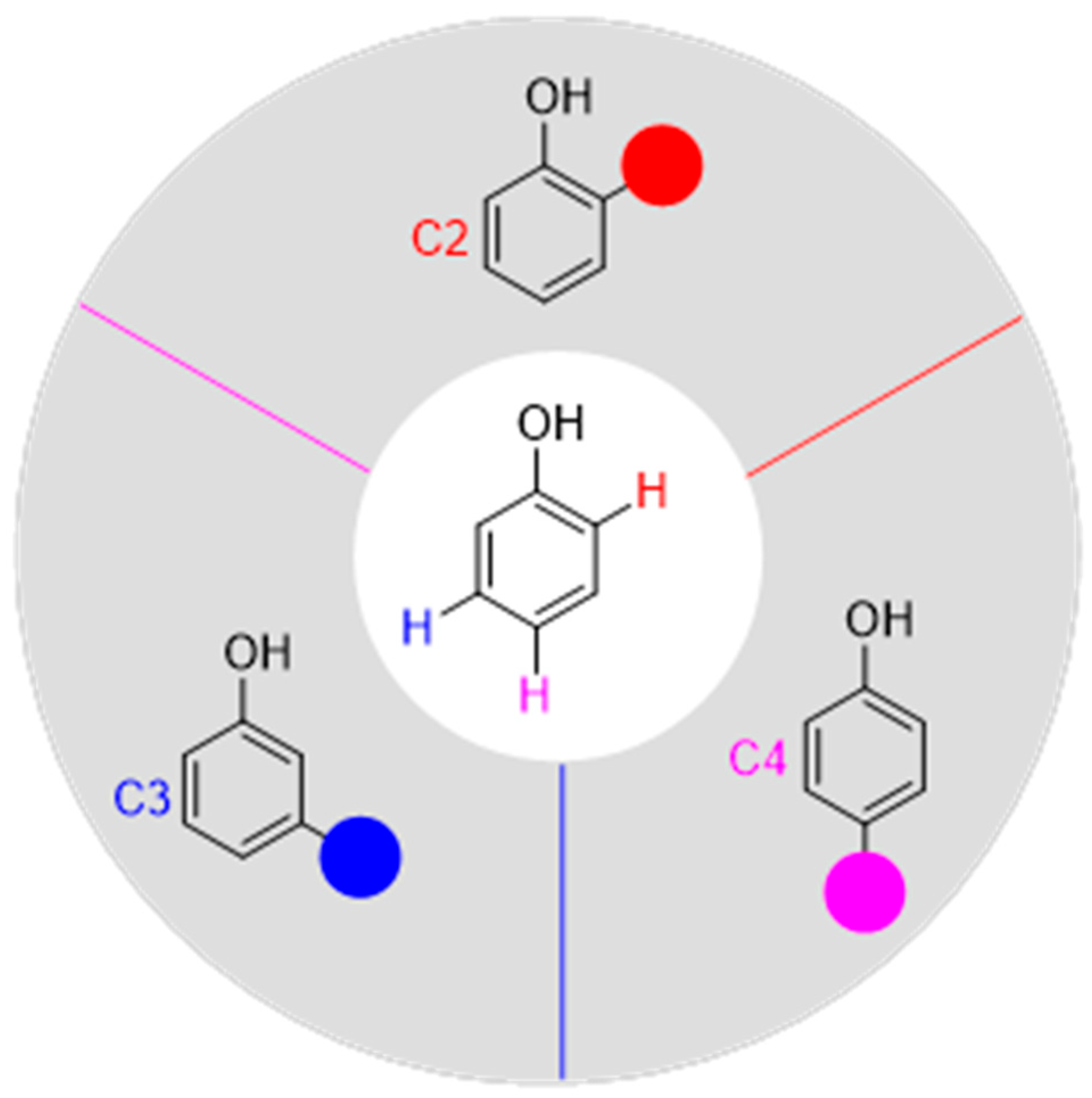
1. Introduction
2. C2-Functionalization
2.1. Metal-Catalyzed C–H Bond Functionalization of Free Phenol
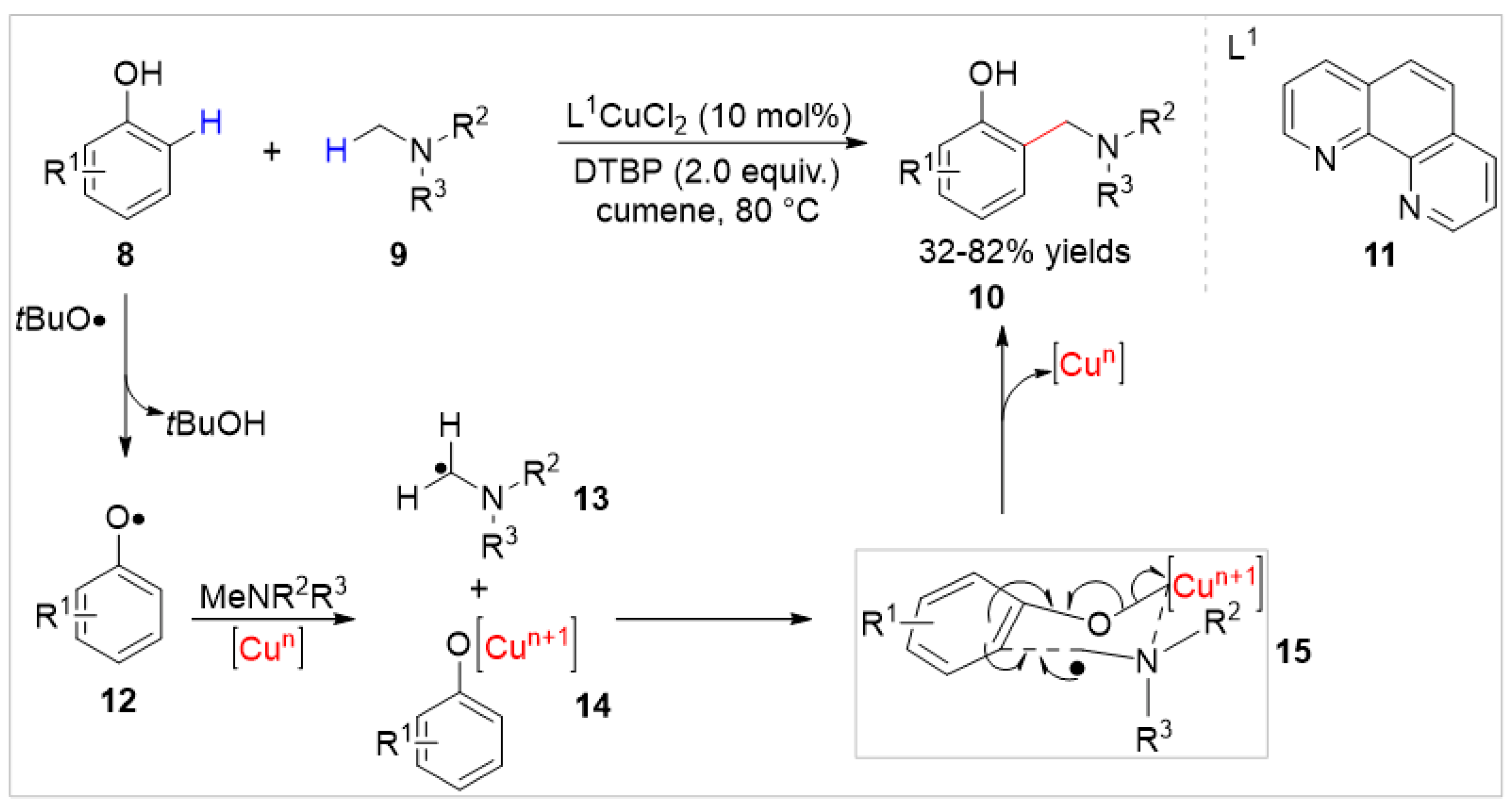
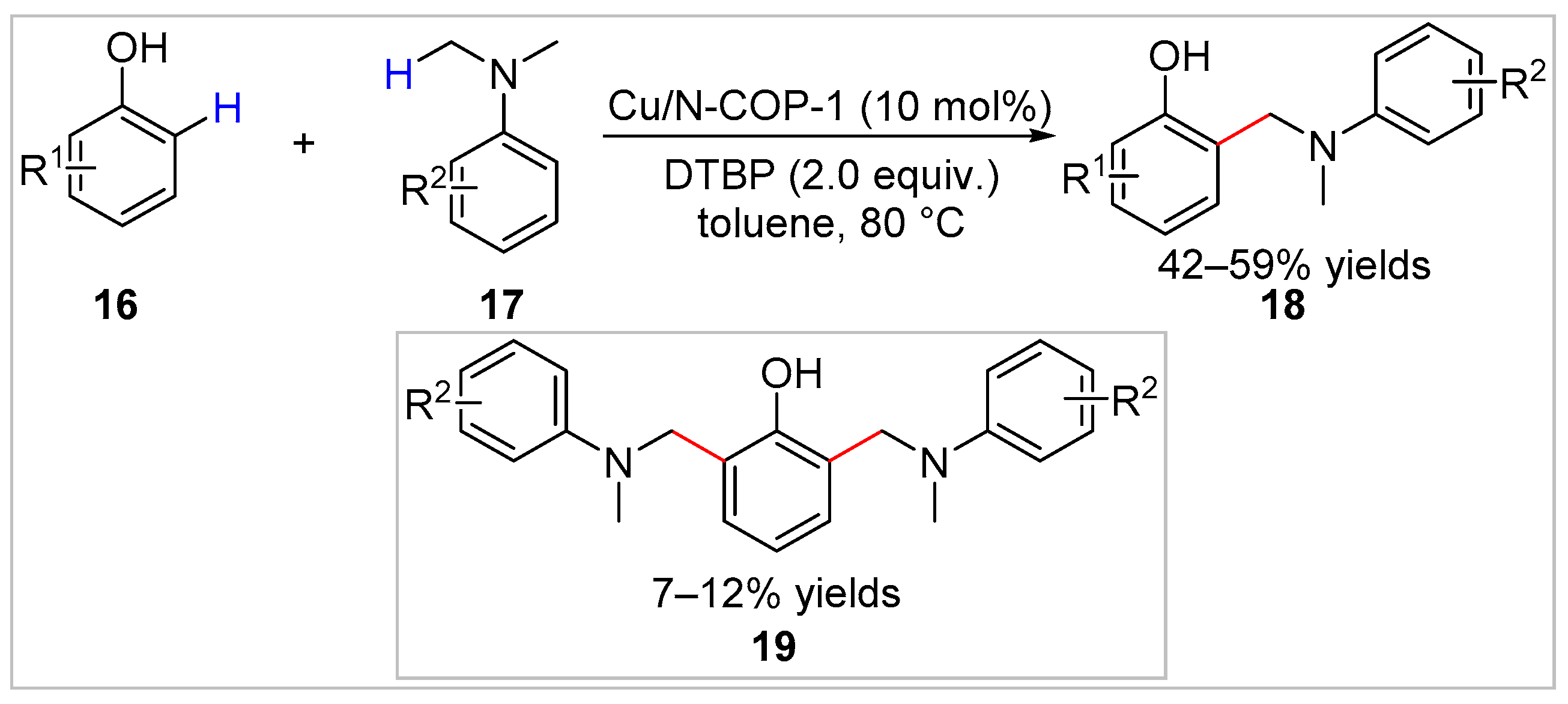
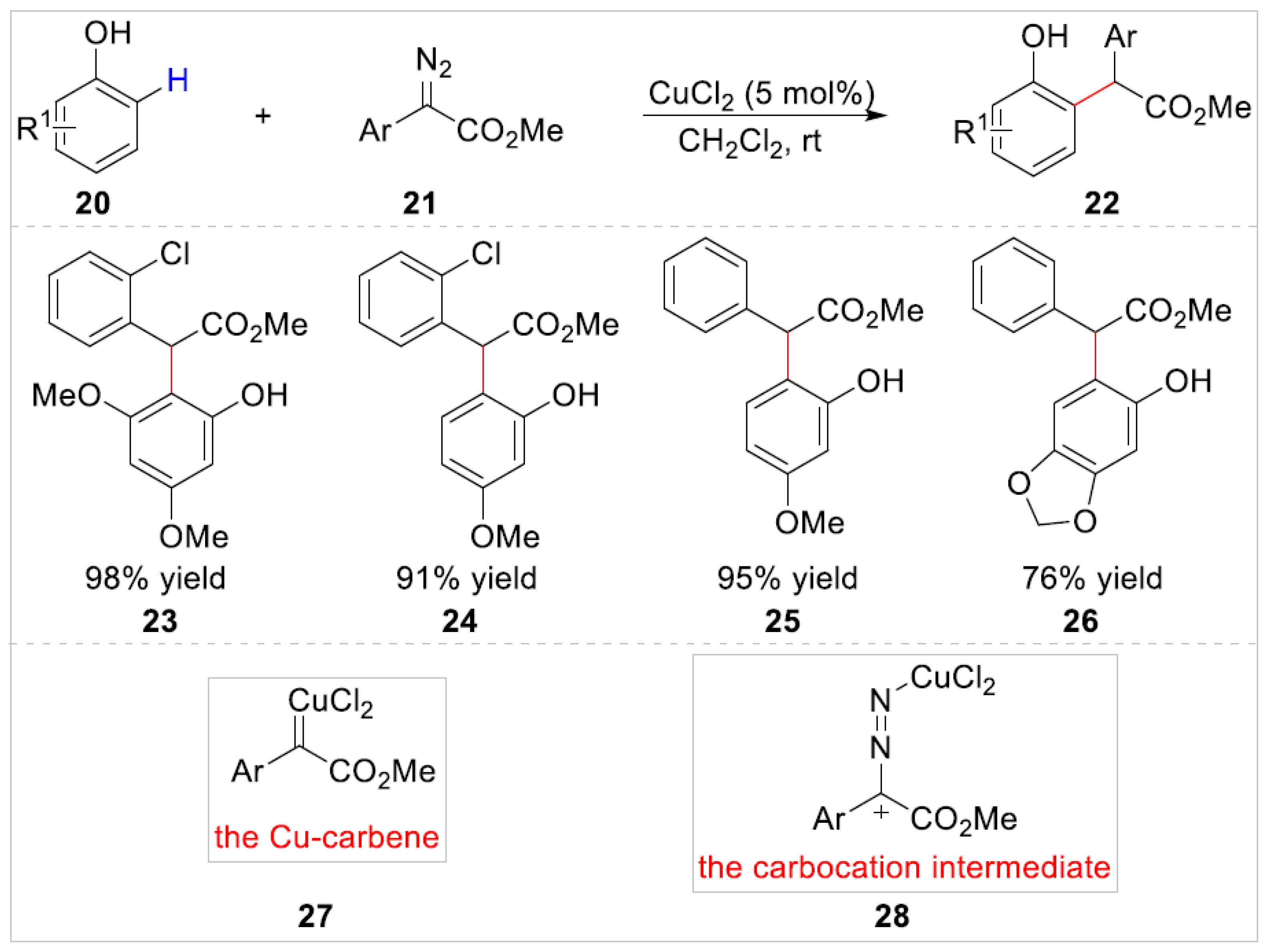
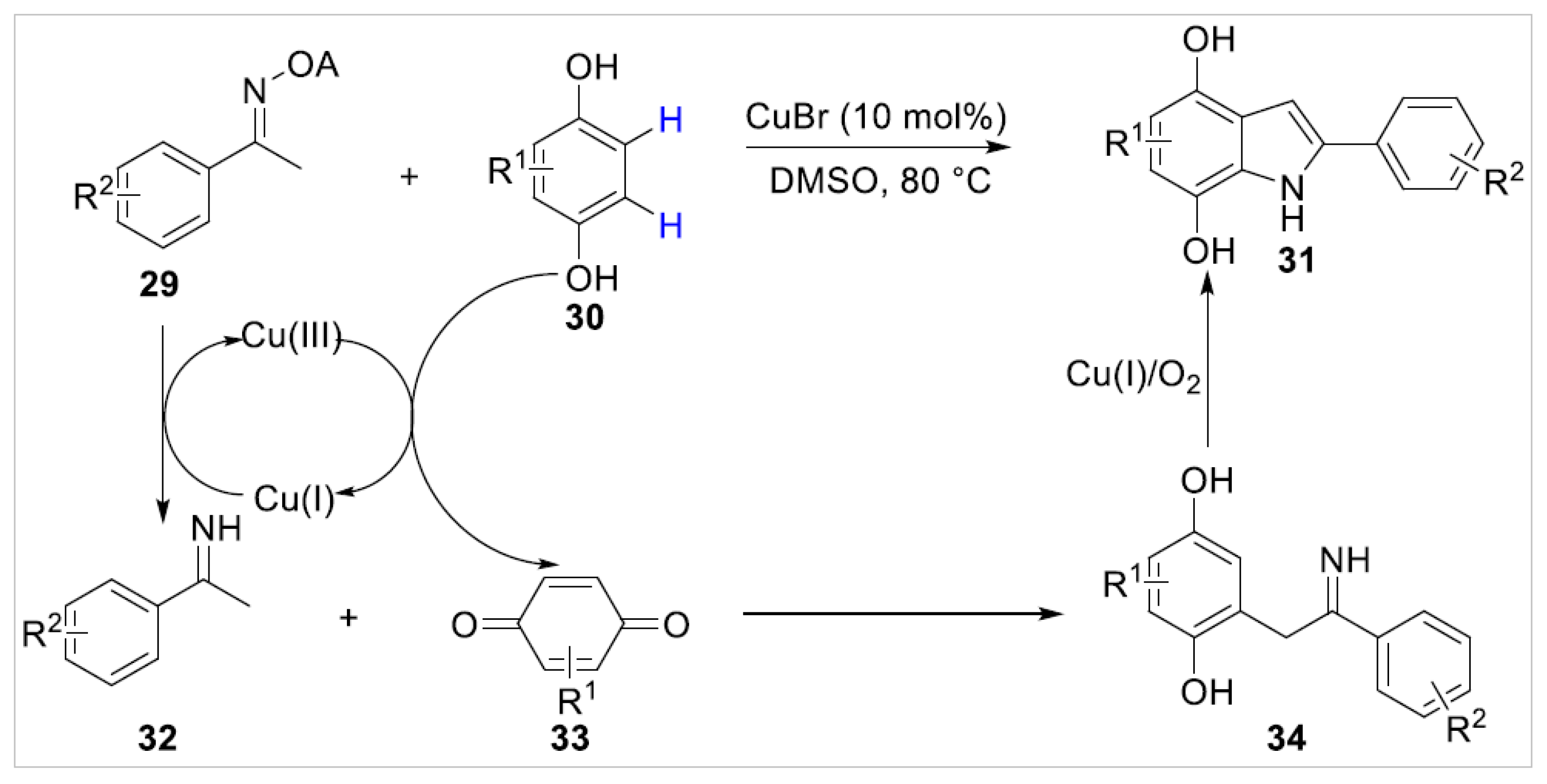
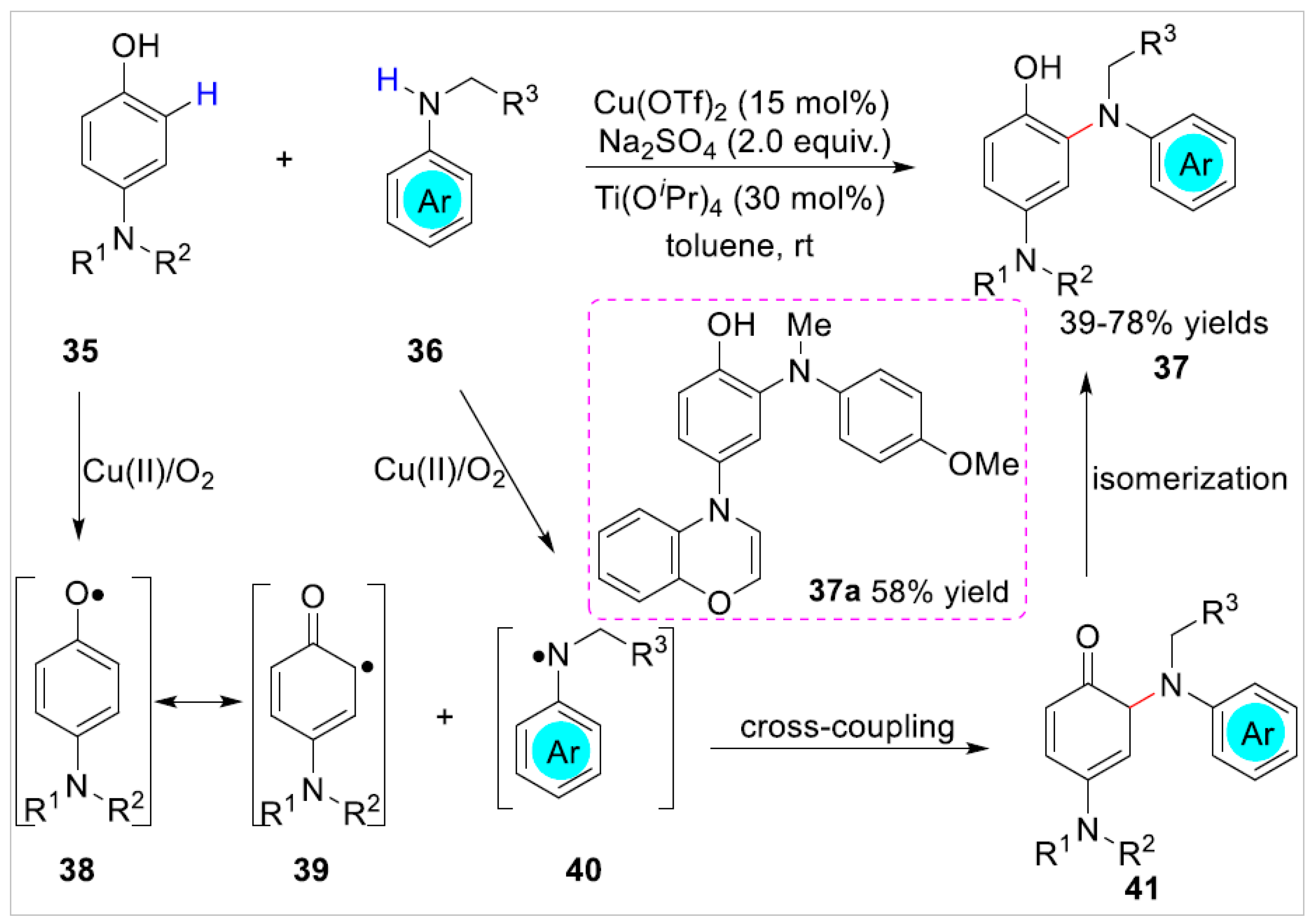
2.1.1. Cu-Catalyzed C–H Bond Functionalization
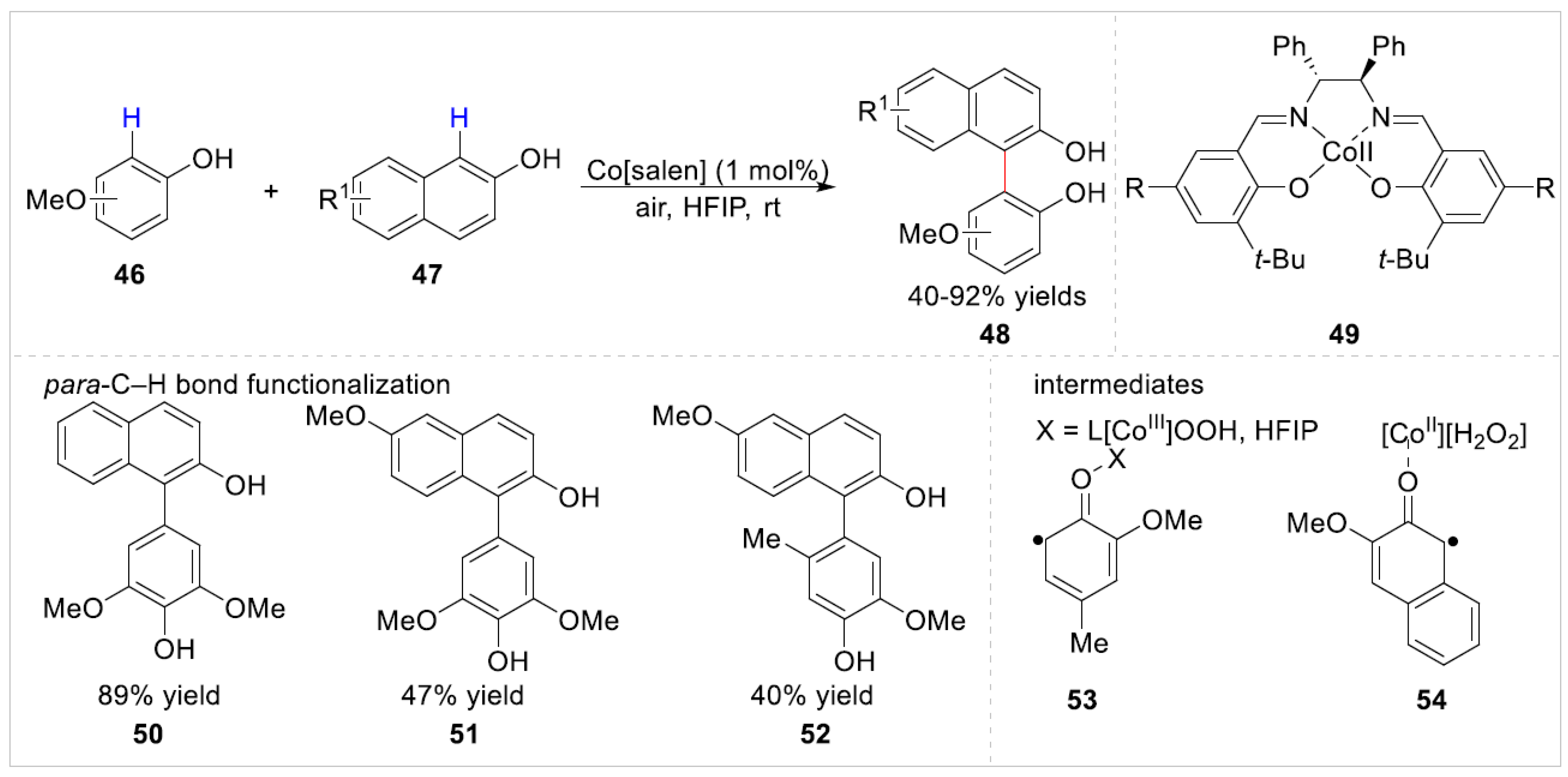
2.1.2. Co-Catalyzed C–H Bond Functionalization
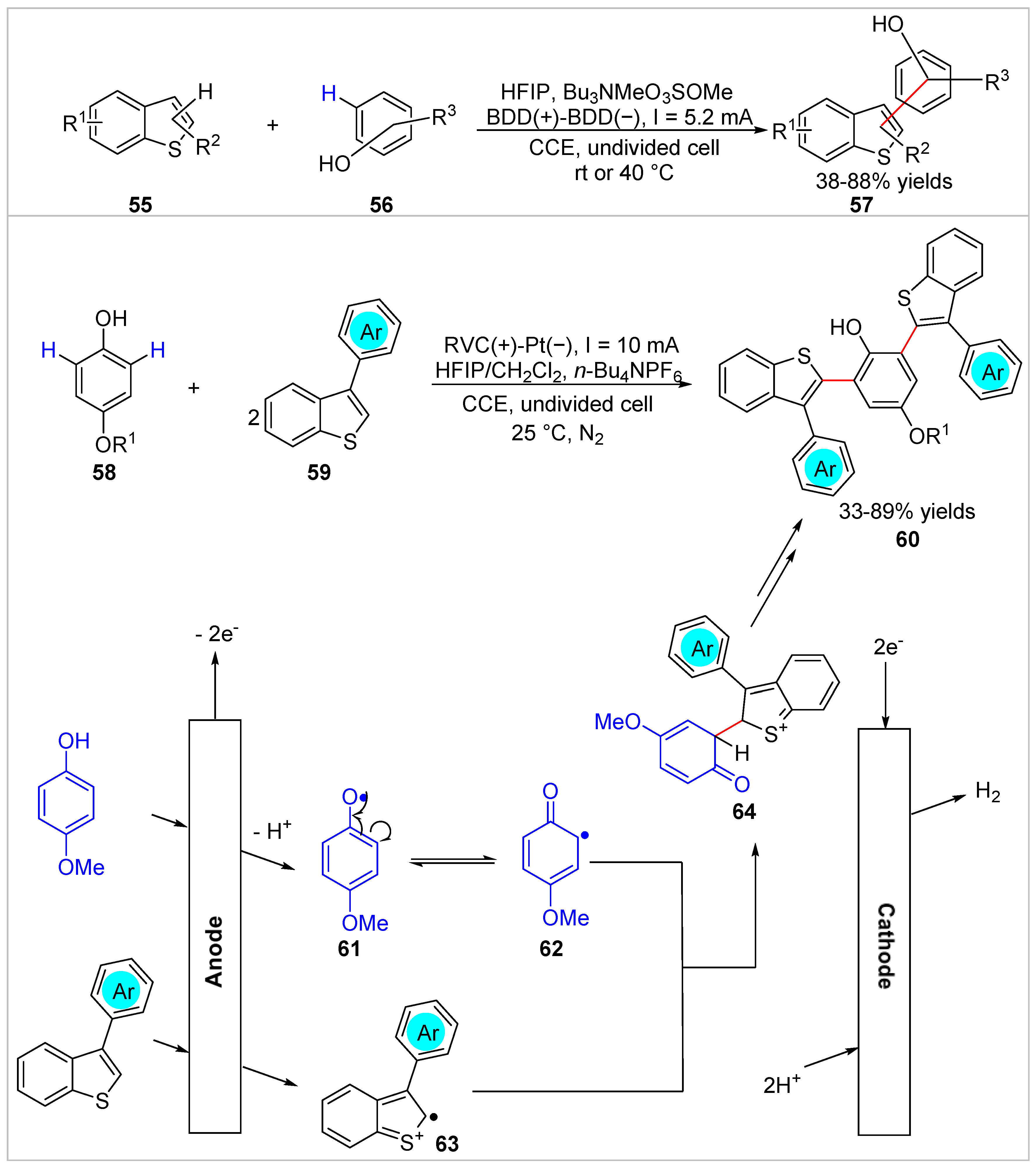
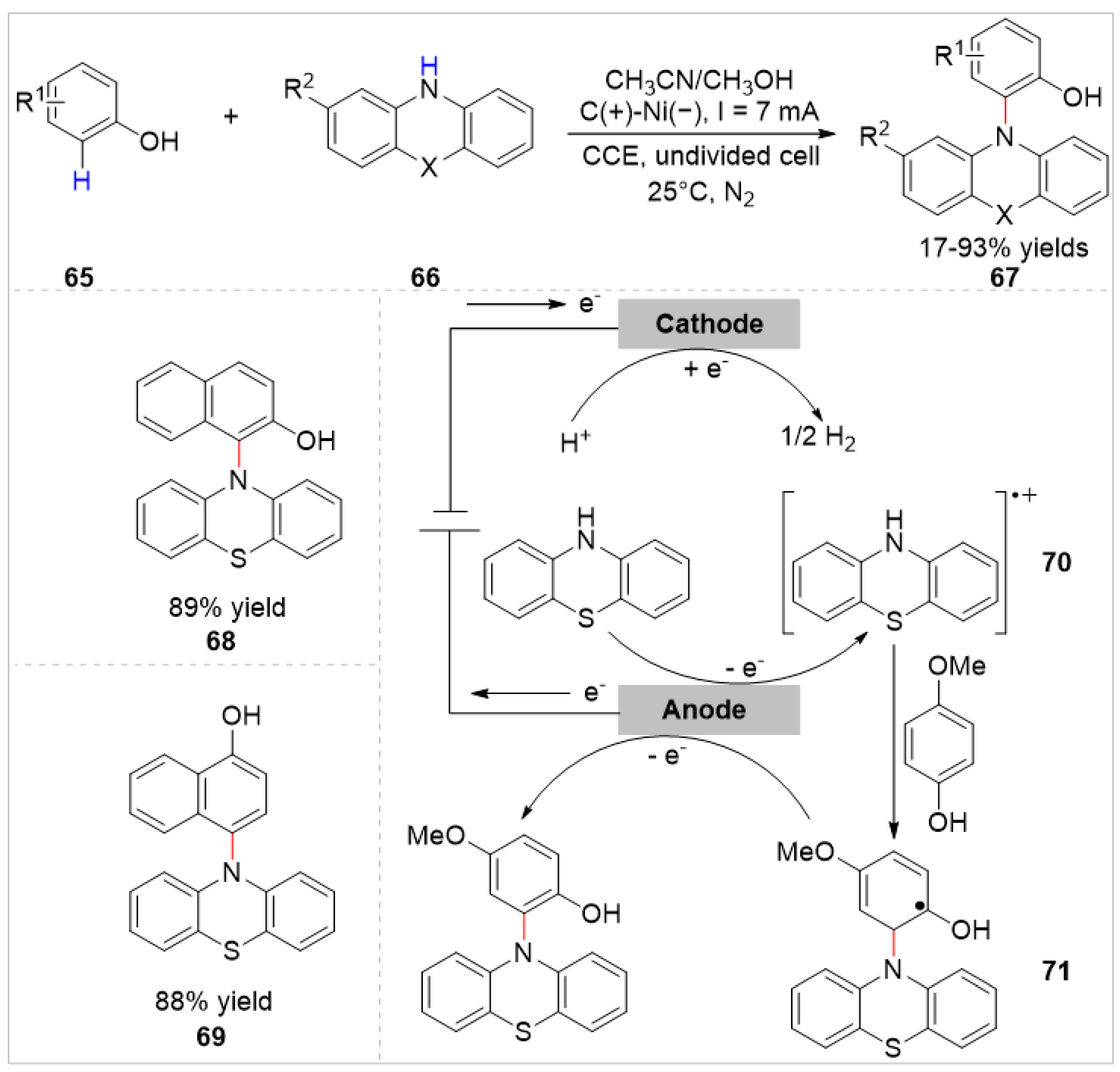
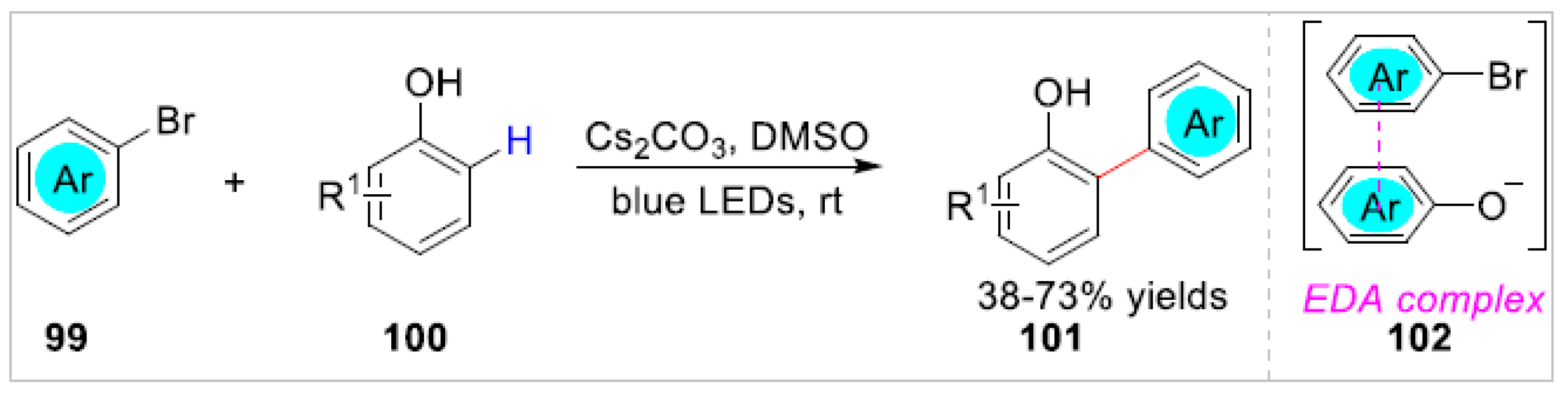
2.2. Electrochemical/Photochemical C–H Bond Functionalization
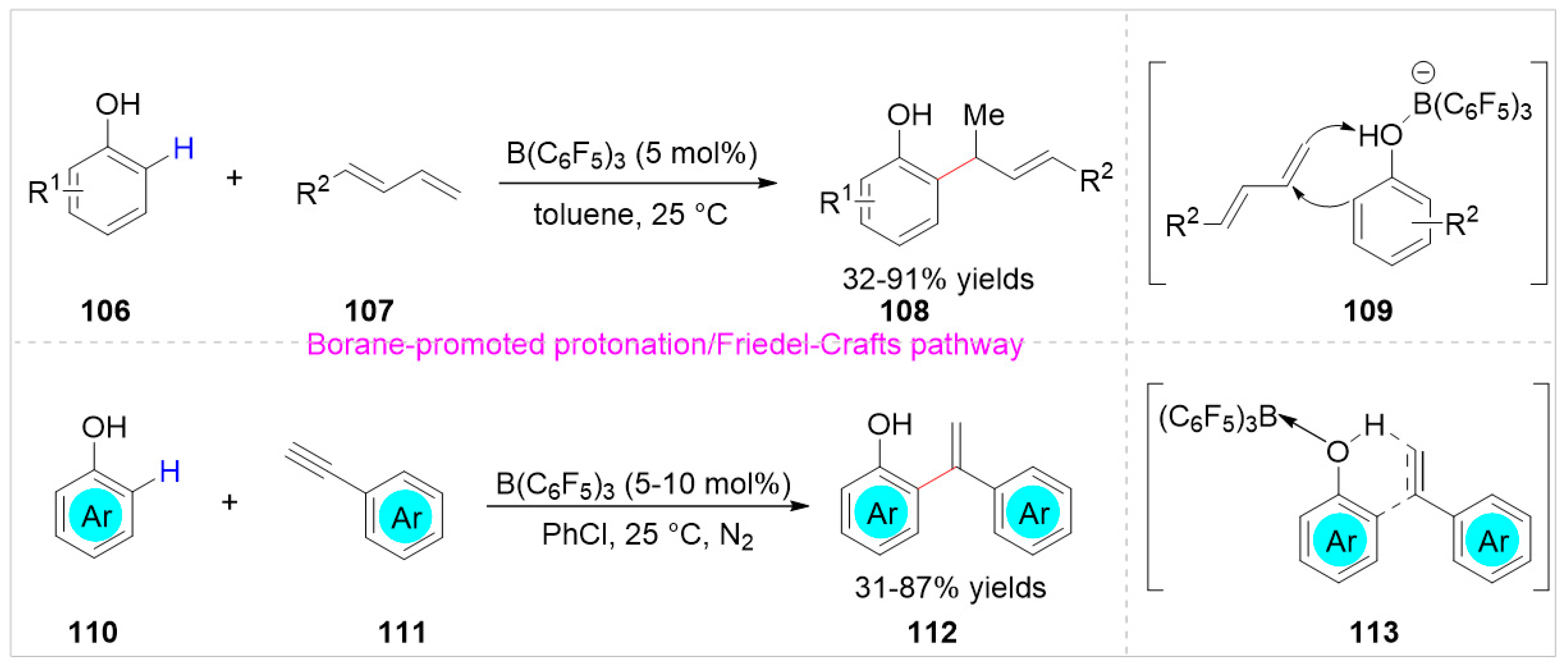
2.3. Others
3. C3-Functionalization
4. C4-Functionalization
4.1. Metal-Catalyzed C–H Bond Functionalization of Free Phenol
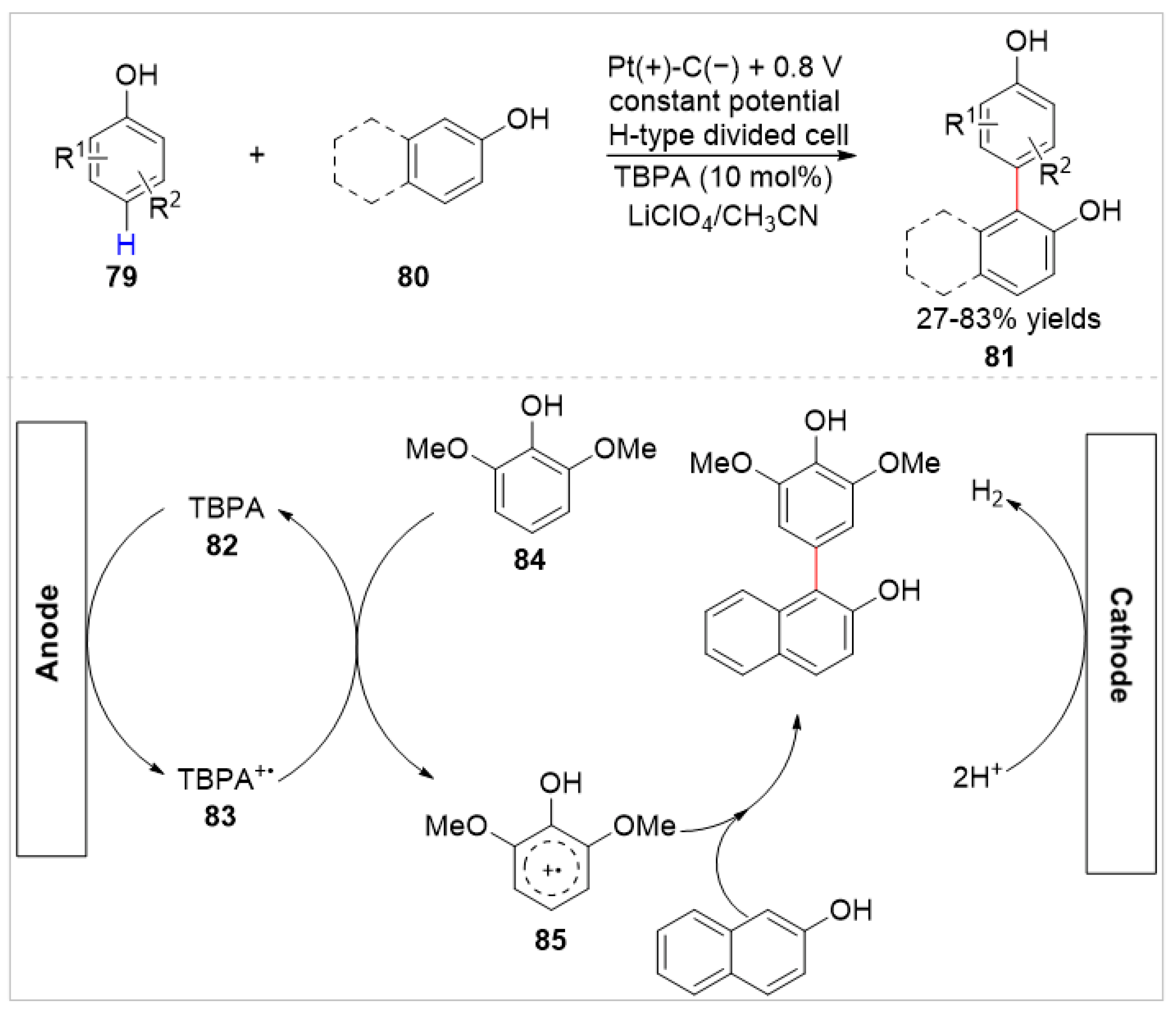
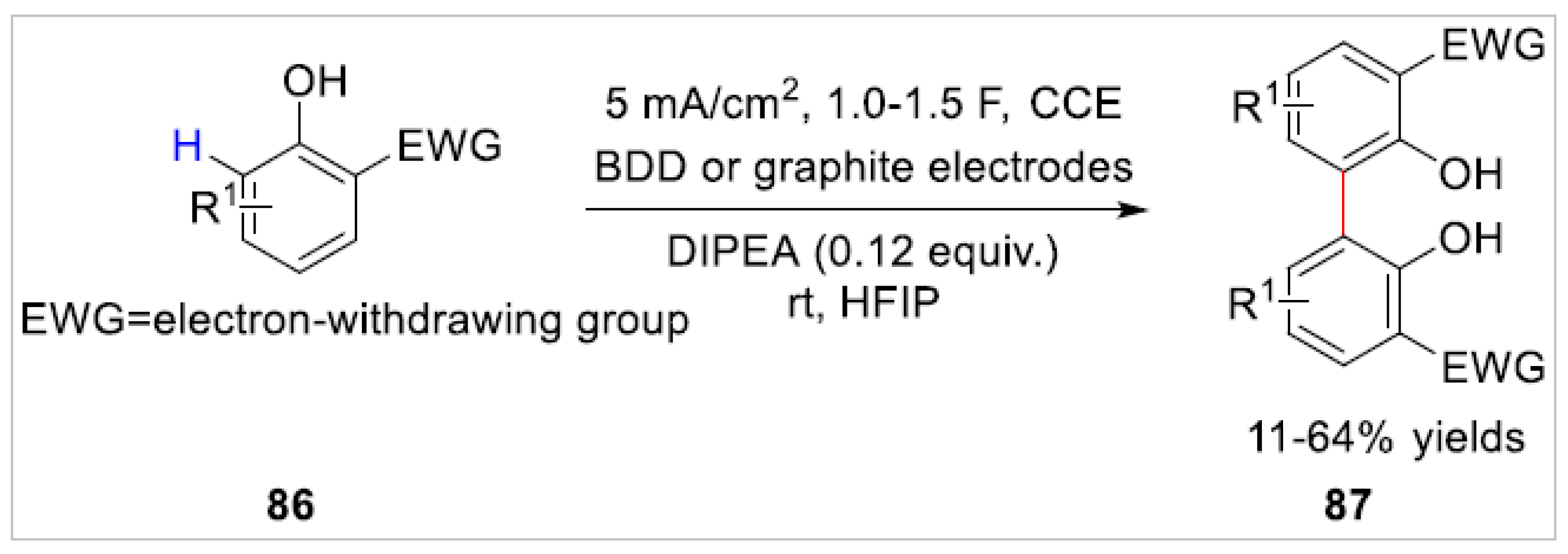
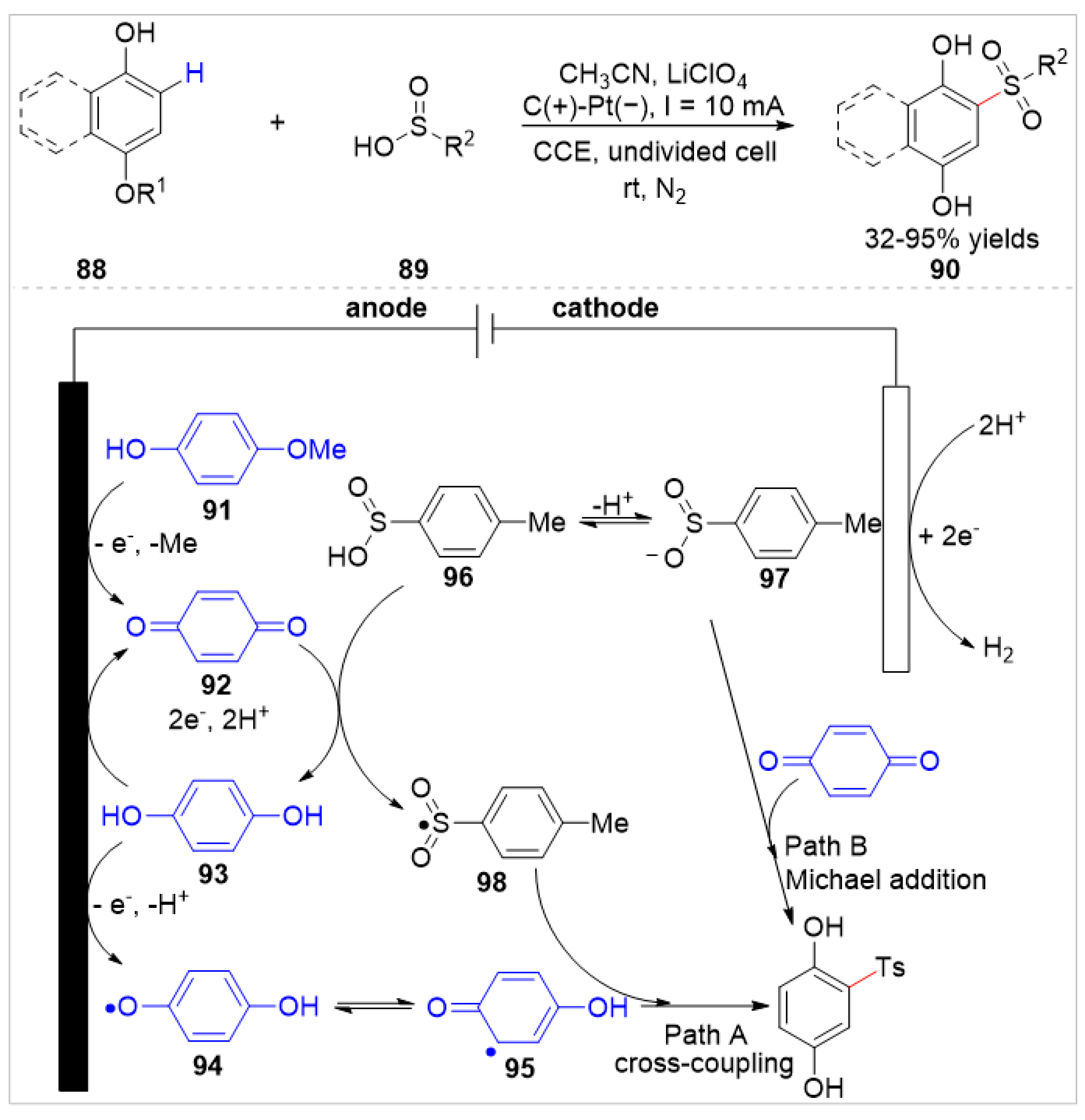
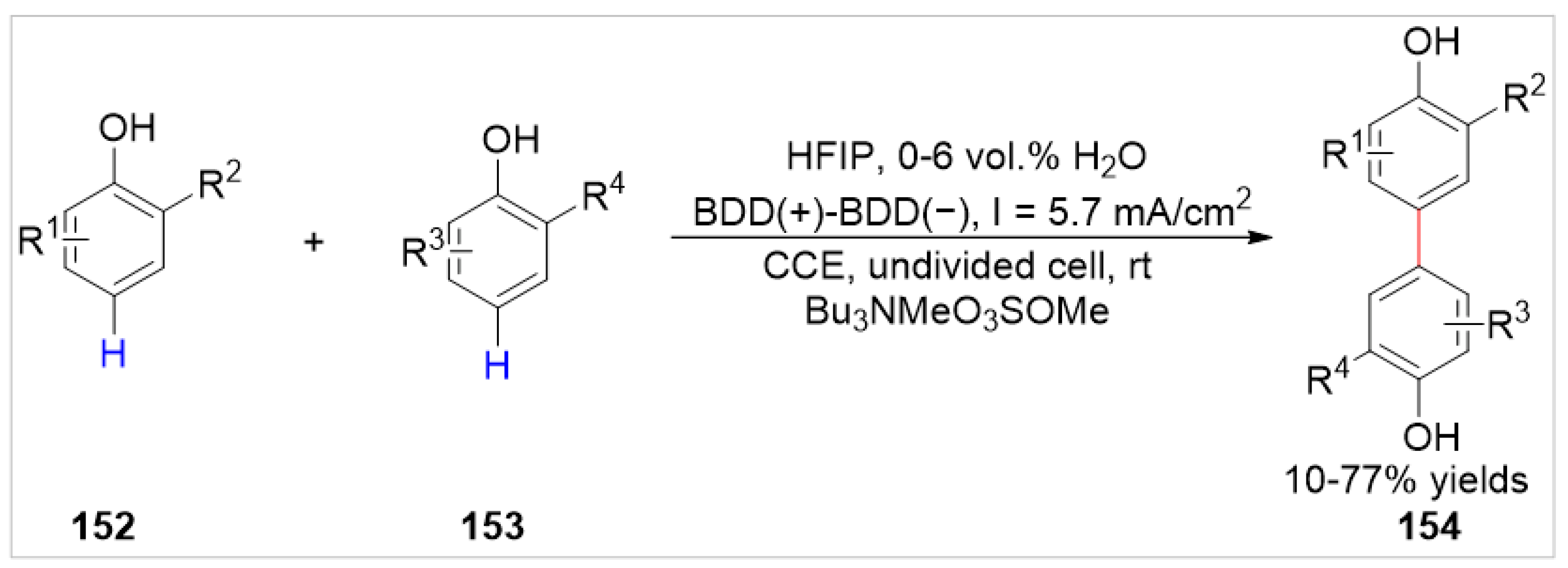
4.2. Electrochemical C–H Bond Functionalization
4.3. Others
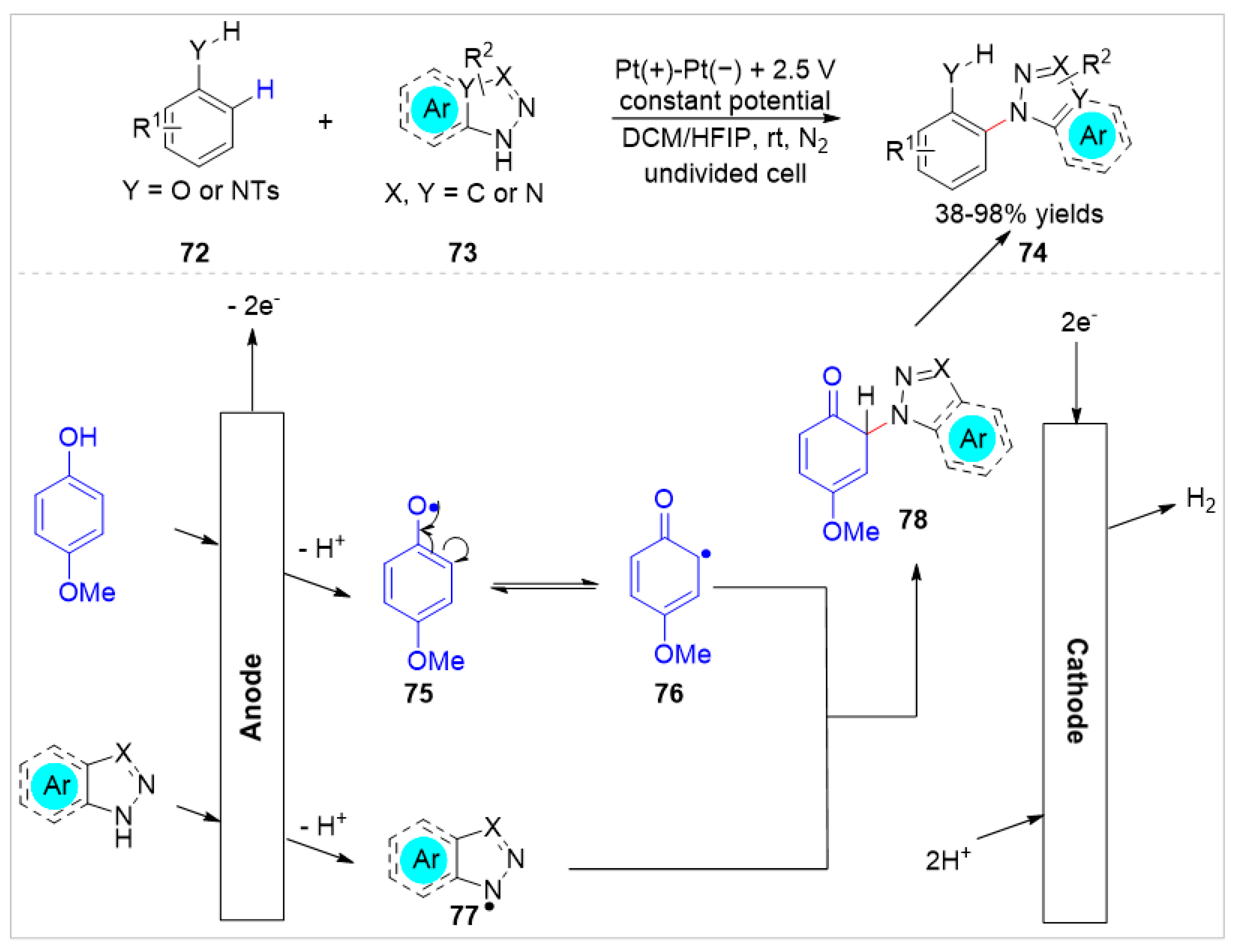
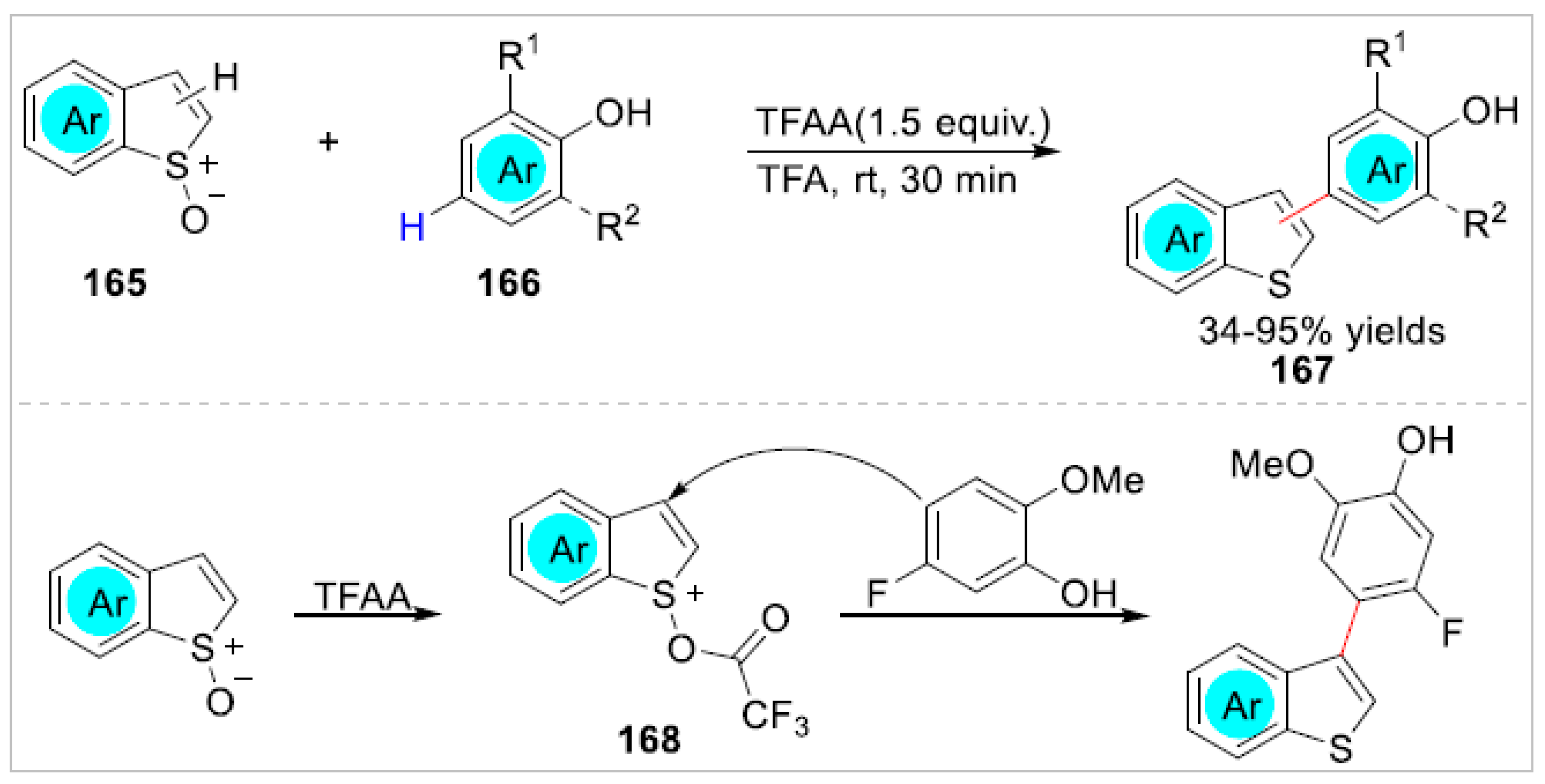
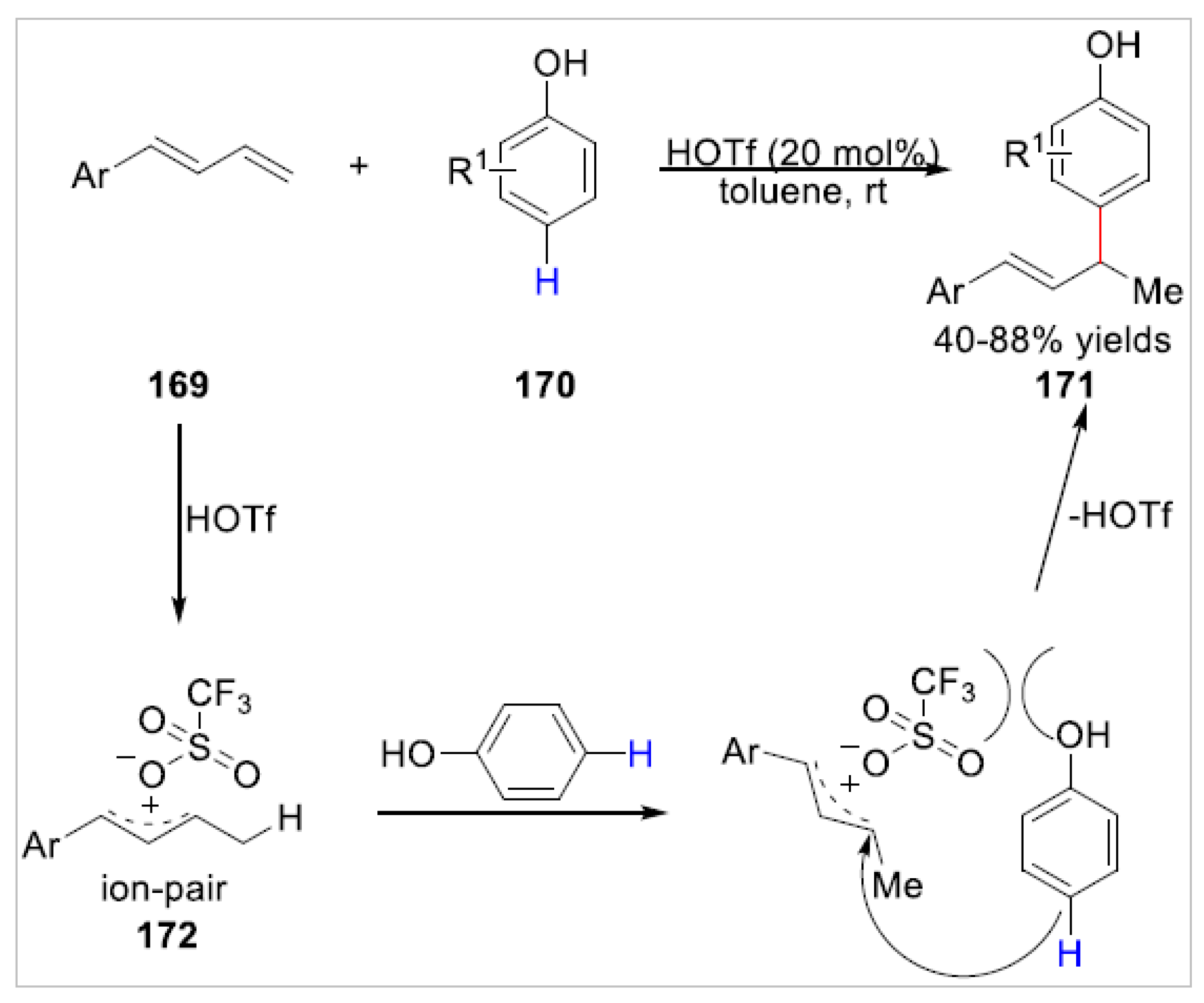
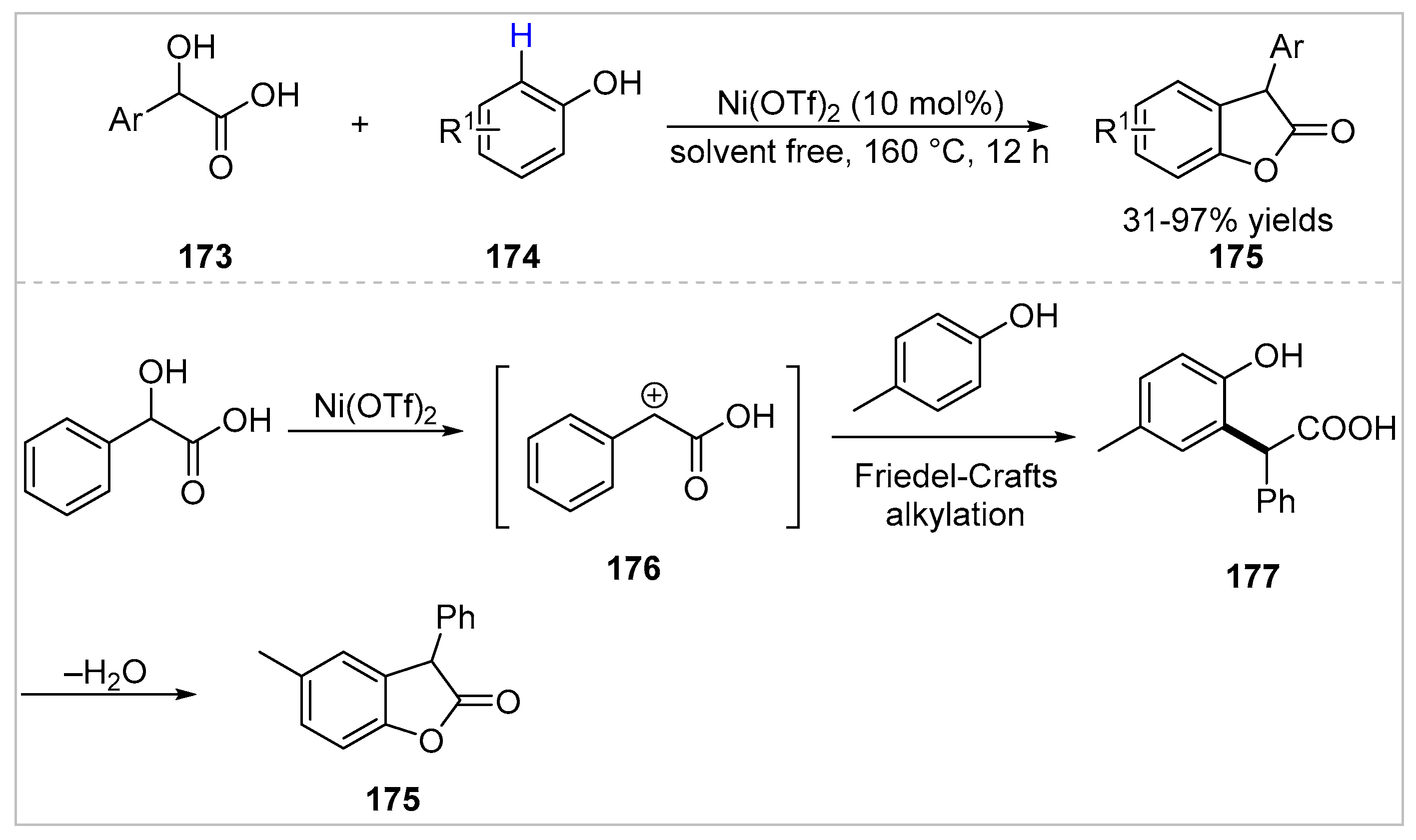
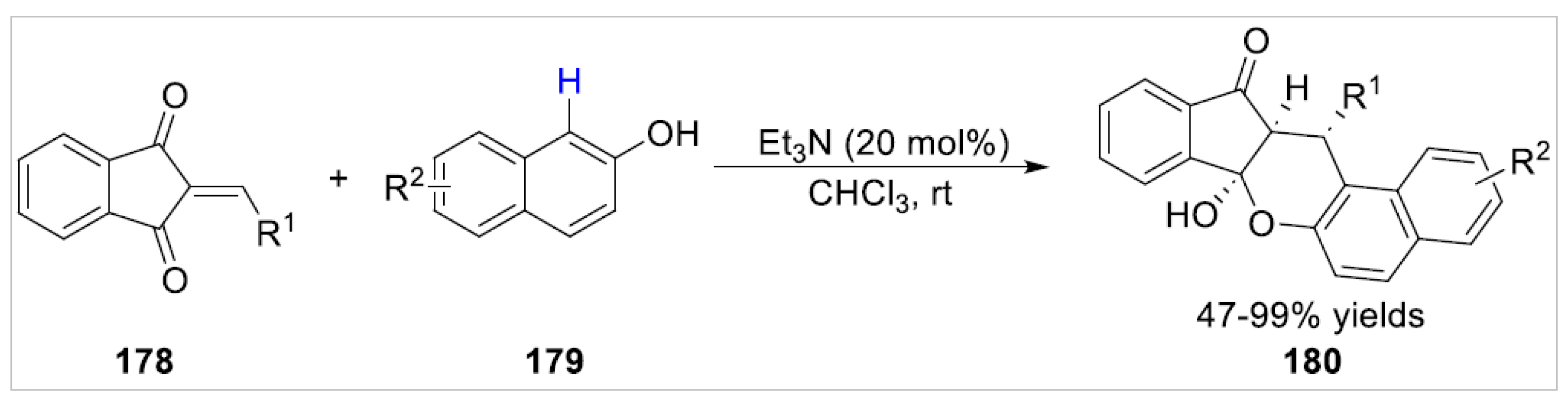
5. Ortho-Functionalization-Cyclization Process
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ackermann, L. Cobalt-Catalyzed Direct Arylation and Benzylation by C–H/C–O Cleavage with Sulfamates, Carbamates, and Phosphates. Angew. Chem. Int. Ed. 2012, 51, 8251–8254. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Rio, D.D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Tyman, J.H.P. Synthetic and Natural Phenols; Studies in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; Volume 52. [Google Scholar]
- Demchenko, A.P.; Tang, K.-C.; Chou, P.-T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, K.-H.; Yi, C.S. Dehydrative C–H Alkylation and Alkenylation of Phenols with Alcohols: Expedient Synthesis for Substituted Phenols and Benzofurans. J. Am. Chem. Soc. 2012, 134, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-J.; Li, C.-J.; Zeng, H. -Y. Dearomatization-Rearomatization Strategy for ortho-Selective Alkylation of Phenols with Primary Alcohols. Angew. Chem. Int. Ed. 2021, 60, 4043–4048. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Naveen, T.; Maji, A.; Manna, S.; Maiti, D. Palladium-Catalyzed Synthesis of Benzofurans and Coumarins from Phenols and Olefins. Angew. Chem. Int. Ed. 2013, 52, 12669–12673. [Google Scholar] [CrossRef]
- Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Significant Enhancement in the Efficiency and Selectivity of Iron-Catalyzed Oxidative Cross-Coupling of Phenols by Fluoroalcohols. Angew. Chem. Int. Ed. 2015, 54, 4198–4202. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lumb, J.-P. Phenol-Directed C-H Functionalization. ACS Catal. 2019, 9, 521–555. [Google Scholar] [CrossRef]
- Xu, X.; Luo, J.F. Transition Metal-Catalyzed Directing-Group-Assisted C–H Activation of Phenols. Chem. Sus. Chem. 2019, 12, 4601–4616. [Google Scholar] [CrossRef]
- Mamari, H.H.; Štefane, B.; Žugelj, H.B. Metal-Catalyzed C–H Bond Functionalization of Phenol Derivatives. Tetrahedron 2020, 76, 130925. [Google Scholar] [CrossRef]
- Youn, S.W.; Cho, C.-G. Transition-metal-catalyzed ortho-selective C–H functionalization reactions of free phenols. Org. Biomol. Chem. 2021, 19, 5028–5047. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Wei, J.; Wang, H.F.; Zhong, F.R.; Zhai, H.B. Recent advances in catalytic oxidative reactions of phenols and naphthalenols. Org. Chem. Front. 2022, 9, 5395–5413. [Google Scholar] [CrossRef]
- Truong, T.; Daugulis, O. Divergent reaction pathways for phenol arylation by arynes: Synthesis of helicenes and 2-arylphenols. Chem. Sci. 2013, 4, 531–535. [Google Scholar] [CrossRef]
- Yang, J.-F.; Wang, R.-H.; Wang, Y.-X.; Yao, W.-W.; Liu, Q.-S.; Ye, M. LigandAccelerated Direct C−H Arylation of BINOL: A Rapid One-Step Synthesis of Racemic 3,3′ -Diaryl BINOLs. Angew. Chem. Int. Ed. 2016, 55, 14116–14120. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-L.; Shao, N.-Q.; Zhang, J.; Jia, R.-P.; Wang, D.-H. Cu(II)-Catalyzed ortho-Selective Aminomethylation of Phenols. J. Am. Chem. Soc. 2017, 139, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chandna, N.; Dubey, P.; Singh, A.K.; Jain, N. GO–Cu7S4 catalyzed ortho-aminomethylation of phenol derivatives with N,N-dimethylbenzylamines: Site-selective oxidative CDC. Chem. Commun. 2018, 54, 7511–7514. [Google Scholar] [CrossRef]
- Yu, C.J.; Patureau, F.W. Cu-catalyzed cross-dehydrogenative ortho-aminomethylation of Phenols. Angew. Chem. Int. Ed. 2018, 57, 11807–11811. [Google Scholar] [CrossRef]
- Xie, J.; Chen, M.; Peng, L.-L.; Wu, J.Q.; Zhou, Q.; Zhou, C.S.; Xiong, B.Q.; Liu, Y. Facile preparation of Cu(II)-modified nitrogen-rich covalent organic polymer for cross-dehydrogenative ortho-aminomethylation of phenols. Catal. Commun. 2021, 159, 106348. [Google Scholar] [CrossRef]
- Ma, B.; Tang, Z.Q.; Zhang, J.L.; Liu, L. Copper-catalysed ortho-selective C–H bond functionalization of phenols and naphthols with a-aryl-a-diazoesters. Chem. Commun. 2020, 56, 9485–9488. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Li, Y.L.; Yang, Y.; Long, J.L.; Zhang, H. Copper-Catalyzed Tandem Cross-Coupling/Annulation of Phenols with Ketoximes through Dual C H Functionalization: Synthesis of Substituted 2-Aryl-1H-indoles. Asian J. Org. Chem. 2021, 10, 1382–1385. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Wang, L.-L.; Guo, J.; Xu, Y.-Q.; Chen, Z.-S.; Ji, K.G. Cu(II)-Catalyzed C2-site functionalization of p-aminophenols: An approach for selective cross-dehydrogenative aminations. Org. Chem. Front. 2022, 9, 1010–1015. [Google Scholar] [CrossRef]
- Rostami, A.; Khakyzadeh, V.; Zolfigol, M.A.; Rostami, A. Co(II)-catalyzed regioselective clean and smooth synthesis of 2-(aryl/alkylthio)phenols via sp2 C-H bond activation. Mol. Catal. 2018, 452, 260–263. [Google Scholar] [CrossRef]
- Khaef, S.; Rostami, A.; Khakyzadeh, V.; Zolfigol, M.A.; Taherpour, A.A.; Yarie, M. Regioselective Ortho-C-H sulfenylation of free phenols catalyzed by Co(II)-immobilized on silica-coated magnetic nanoparticles. Mol. Catal. 2020, 484, 110772. [Google Scholar] [CrossRef]
- Reiss, H.; Shalit, H.; Vershinin, V.; More, N.Y.; Forckosh, H.; Pappo, D. Cobalt(II)[salen]-Catalyzed Selective Aerobic Oxidative CrossCoupling between Electron-Rich Phenols and 2-Naphthols. J. Org. Chem. 2019, 84, 7950–7960. [Google Scholar] [CrossRef]
- Gao, H.H.; Zha, Z.G.; Zhang, Z.L.; Ma, H.Y.; Wang, Z.Y. A simple and efficient approach to realize difunctionalization of arylketones with malonate esters via an electrochemical oxidation. Chem. Commun. 2014, 50, 5034–5036. [Google Scholar] [CrossRef]
- Li, Y.N.; Gao, H.H.; Zhang, Z.L.; Qian, P.; Bi, M.X.; Zha, Z.G.; Wang, Z.Y. Electrochemical synthesis of α-enaminones from aryl ketones. Chem. Commun. 2016, 52, 8600–8603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Wang, B.; Huang, Y.-K.; Hu, J.-S.; Sun, J.-N. Recent advances in metal catalyst- and oxidant-free electrochemical C-H bond functionalization of nitrogen-containing heterocycles. Front. Chem. 2022, 10, 967501. [Google Scholar] [CrossRef]
- Lian, F.; Xu, K.; Zeng, C.-C. The Synergism of Sequential Paired Electrosynthesis with Halogen Bonding Activation for the Cyclization of Organochlorides with Olefins. Sci. China Chem. 2023, 66, 540–547. [Google Scholar] [CrossRef]
- Wang, H.M.; Gao, X.L.; Lv, Z.C.; Abdelilah, T.; Lei, A.W. Recent Advances in Oxidative R1-H/R2-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.A.; Niu, L.B.; Wang, S.C.; Liu, J.M.; Lei, A.W. Visible Light-Induced C(sp3)-H Oxidative Arylation with Heteroarenes. Org. Lett. 2019, 21, 2441–2444. [Google Scholar] [CrossRef]
- Lips, S.; Schollmeyer, D.; Franke, R.; Waldvogel, S.R. Regioselective Metal- and Reagent-Free Arylation of Benzothiophenes by Dehydrogenative Electrosynthesis. Angew. Chem. Int. Ed. 2018, 130, 13509–13513. [Google Scholar] [CrossRef]
- Yue, Y.Y.; Chao, J.L.; Wang, Z.Y.; Yang, Y.; Ye, Y.Q.; Sun, C.Y.; Guo, X.H.; Liu, J.M. Electrooxidative double C–H/C–H coupling of phenols with 3-phenylbenzothiophenes: Facile access to benzothiophene derivatives. Org. Biomol. Chem. 2021, 19, 7156–7160. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, S.Y.; Liu, Y.C.; Cong, H.J.; Lei, A.W. Electrochemical Oxidative C-H Amination of Phenols: Access to Triarylamine Derivatives. Angew. Chem. Int. Ed. 2018, 57, 4737–4741. [Google Scholar] [CrossRef]
- Feng, P.J.; Ma, G.J.; Chen, X.G.; Wu, X.; Lin, L.; Liu, P.; Chen, T.F. Electro-oxidative and Regioselective C-H Azolation of Phenol and Aniline Drivatives. Angew. Chem. Int. Ed. 2019, 58, 8400–8404. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Jiang, Y.-Y.; Zeng, C.C.; Sun, B.G. Electrocatalytic Synthesis of Non-Symmetric Biphenols Mediated by Tri(p-bromophenyl)amine: Selective Oxidative Cross-Coupling of Different Phenols and Naphthols. Chin. J. Chem. 2019, 37, 352–358. [Google Scholar] [CrossRef]
- Röckl, J.L.; Schollmeyer, D.; Franke, R.; Waldvogel, S.R. Dehydrogenative Anodic C-C Coupling of Phenols Bearing ElectronWithdrawing Groups. Angew. Chem. Int. Ed. 2020, 59, 315–319. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.J.; Yan, K.L.; Feng, W.F.; Sun, X.J.; Yang, J.J.; Wen, J.W. Electrochemical-In-Situ-Oxidative Sulfonylation of Phenols with Sulfinic Acids as an Access to Sulfonylated Hydroquinones. Adv. Synth. Catal. 2021, 363, 3485–3490. [Google Scholar] [CrossRef]
- Zhu, D.-L.; Jiang, S.; Young, D.J.; Wu, Q.; Li, H.-Y.; Li, H.-X. Visible-light-driven C(sp2)–H arylation of phenols with arylbromides enabled by electron donor–acceptor excitation. Chem. Commun. 2022, 58, 3637–3640. [Google Scholar] [CrossRef]
- Wu, J.Z.; Kozlowski, M.C. Visible-Light-Induced Oxidative Coupling of Phenols and Alkenylphenols with a Recyclable, Solid Photocatalyst. Org. Lett. 2023, 25, 907–911. [Google Scholar] [CrossRef]
- Wang, G.Q.; Gao, L.Z.; Chen, H.; Liu, X.T.; Cao, J.; Chen, S.D.; Cheng, X.; Li, S.H. Chemoselective Borane-Catalyzed Hydroarylation of 1,3-Dienes with Phenols. Angew. Chem. Int. Ed. 2019, 58, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Huang, J.; Lu, C.H.; Jiang, H.F.; Huang, L.B. B(C6F5)3-Catalyzed Hydroarylation of Terminal Alkynes with Phenols. Adv. Synth. Catal. 2021, 363, 3962–3967. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, S.C.; Gao, Y.M.; Sun, H.; Liang, X.; Bu, F.X.; Abdelilah, T.; Lei, A.W. Oxidant-Induced Azolation of Electron-Rich Phenol Derivatives. Org. Lett. 2020, 22, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Q.; Li, D.D.; Yue, Y.D.; Peng, D.; Liu, L. Brønsted acid catalysed chemo- and ortho-selective aminomethylation of phenol. Org. Biomol. Chem. 2021, 19, 5777–5781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Wang, B.; Ding, Y.; Loh, T.-K.; Tian, J.-S. Aqueous C–H aminomethylation of phenols by iodine catalysis. Chem. Commun. 2023, 59, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.B.; Limmert, M.E. Catalytic Intermolecular Ortho-Arylation of Phenols. J. Org. Chem. 2003, 68, 8669–8682. [Google Scholar] [CrossRef]
- Huang, C.H.; Chattopadhyay, B.; Gevorgyan, V. Silanol: A Traceless Directing Group for Pd-Catalyzed o-Alkenylation of Phenols. J. Am. Chem. Soc. 2011, 133, 12406–12409. [Google Scholar] [CrossRef]
- Cazorla, C.; De Vries, T.S.; Vedejs, E. P-Directed Borylation of Phenols. Org. Lett. 2013, 15, 984–987. [Google Scholar] [CrossRef]
- Dai, H.-X.; Li, G.; Zhang, X.-G.; Stepan, A.F.; Yu, J.-Q. Pd(II)-Catalyzed ortho- or meta-C–H Olefination of Phenol Derivatives. J. Am. Chem. Soc. 2013, 135, 7567–7571. [Google Scholar] [CrossRef]
- Hua, Y.D.; Asgari, P.; Avullala, T.; Jeon, J. Catalytic Reductive ortho-C–H Silylation of Phenols with Traceless, Versatile Acetal Directing Groups and Synthetic Applications of Dioxasilines. J. Am. Chem. Soc. 2016, 138, 7982–7991. [Google Scholar] [CrossRef]
- Senior, A.; Ruffell, K.; Ball, L.T. meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution. Nat. Chem. 2023, 15, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.V.; Bhanage, B.M. Palladium-Catalyzed Aerobic Oxidative Carbonylation of C-H Bond of Phenol for the Synthesis p-hydroxybenzoate. Eur. J. Org. Chem. 2018, 22, 2877–2881. [Google Scholar] [CrossRef]
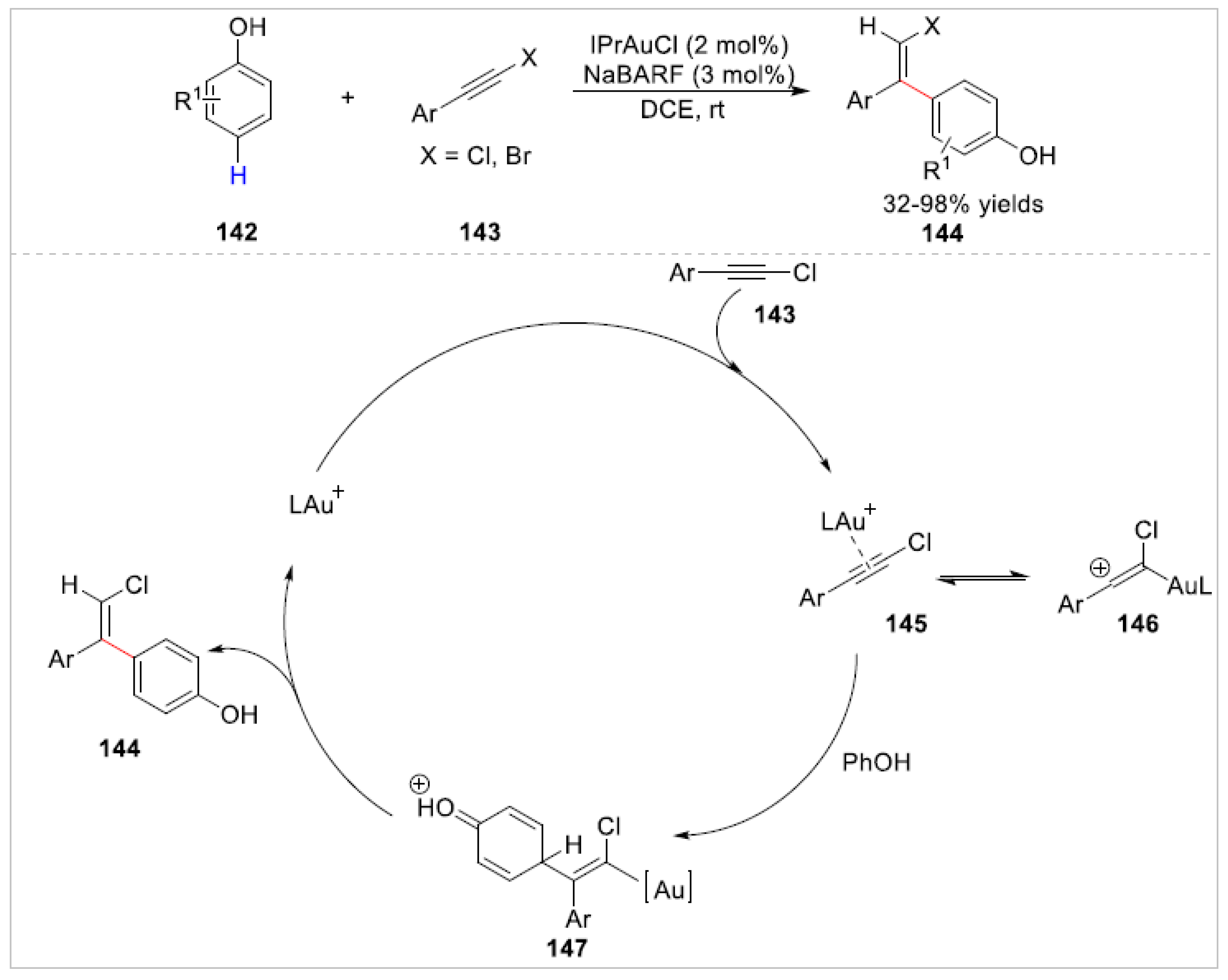
- Adak, T.; Schulmeister, J.; Dietl, M.C.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Highly Chemo- and Regioselective C-H Bond Functionalization of Phenols with Haloalkynes. Eur. J. Org. Chem. 2019, 24, 3867–3876. [Google Scholar] [CrossRef]
- Zhao, D.; Luo, J.Y.; Liu, L.; Liu, Y.Y. Regiospecific and site-selective C–H allylation of phenols with vinyldiazo compounds catalyzed by In(III). Org. Chem. Front. 2021, 8, 6252–6258. [Google Scholar] [CrossRef]
- Dahms, B.; Kohlpaintner, P.J.; Wiebe, A.; Breinbauer, R.; Schollmeyer, D.; Waldvogel, S.R. Selective Formation of 4,4’-Biphenols by Anodic Dehydrogenative Cross- and Homo-Coupling Reaction. Chem. Eur. J. 2019, 25, 2713–2716. [Google Scholar] [CrossRef]
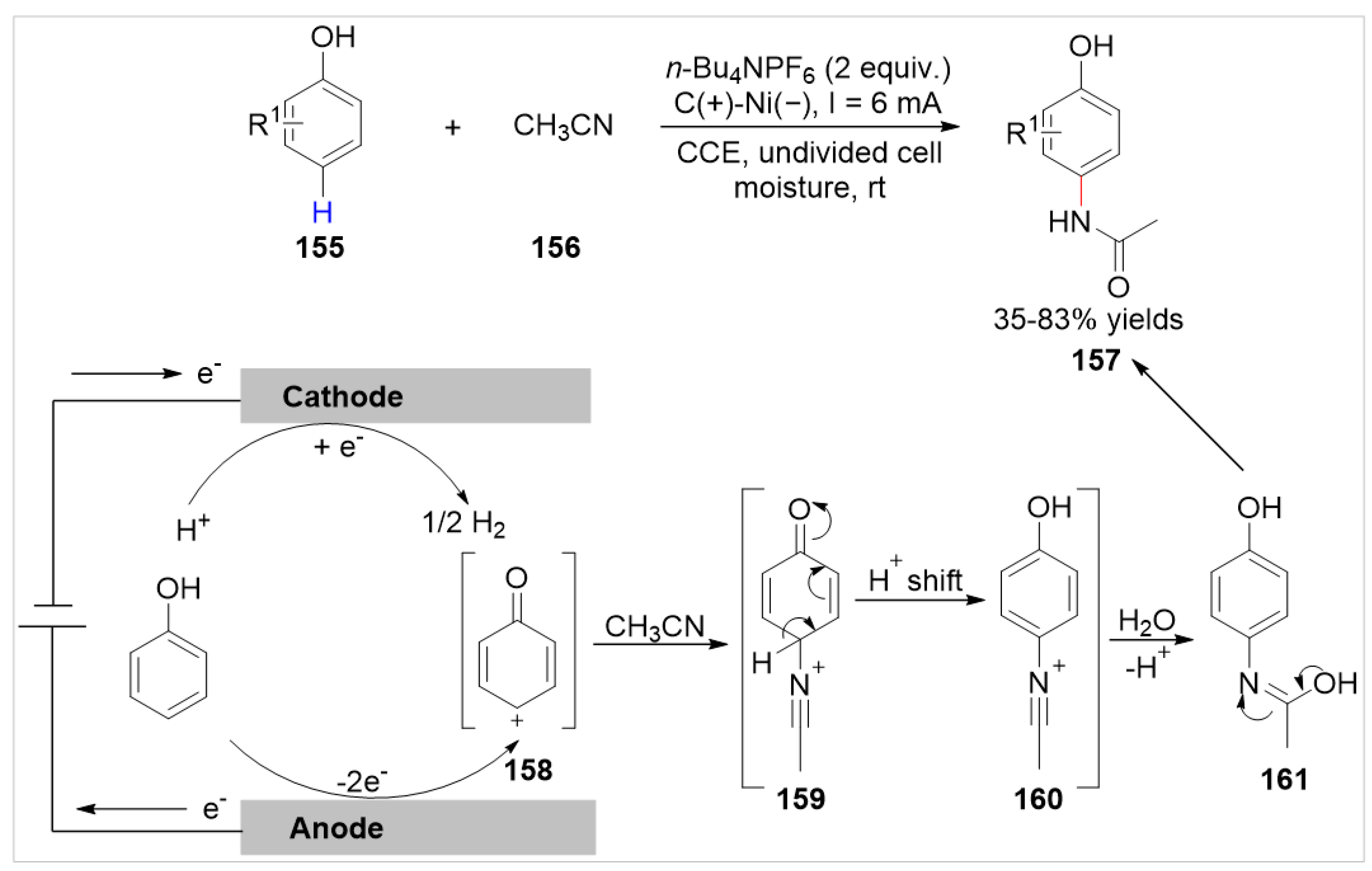
- Taily, I.M.; Saha, D.; Banerjee, P. Direct Synthesis of Paracetamol via Site-Selective Electrochemical Ritter-type C−H Amination of Phenol. Org. Lett. 2022, 24, 2310–2314. [Google Scholar] [CrossRef]
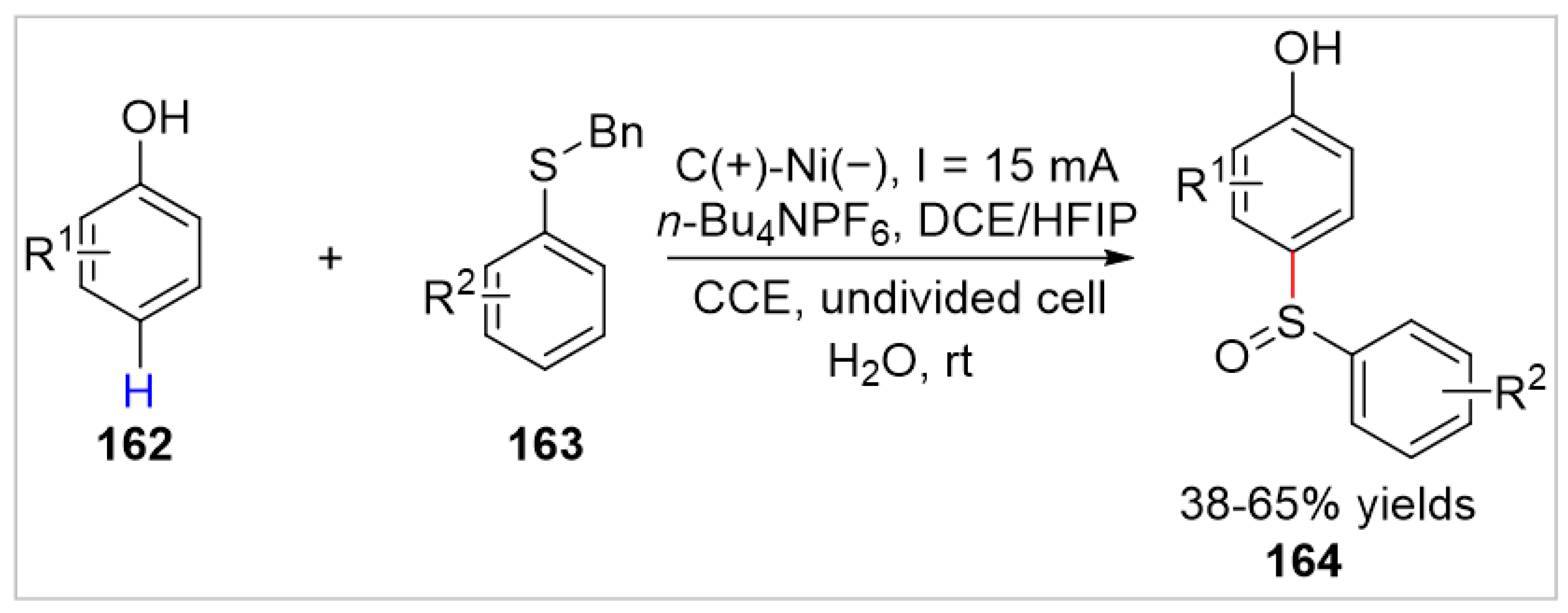
- Kumar, R.; Taily, I.M.; Banerjee, P. Electrochemical sulfinylation of phenols with sulfides: A metal- and oxidant-free cross-coupling for the synthesis of aromatic sulfoxides. Chem. Commun 2023, 59, 310–313. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Biremond, T.; Perry, G.J.P.; Procter, D.J. Para-coupling of phenols with C2/C3-substituted benzothiophene S-oxides. Tetrahedron 2020, 76, 131315. [Google Scholar] [CrossRef]
- Liu, Z.L.; Li, G.H.; Yao, T.F.; Zhang, J.L.; Liu, L. Triflic Acid-Catalyzed Chemo- and Site-Selective C H Bond Functionalization of Phenols with 1,3-Dienes. Adv. Synth. Catal. 2021, 363, 2740–2745. [Google Scholar] [CrossRef]
- Li, G.T.; Li, Z.K.; Gu, Q.; You, S.L. Asymmetric Synthesis of 4-Aryl-3,4-dihydrocoumarins by N-Heterocyclic Carbene Catalyzed Annulation of Phenols with Enals. Org. Lett. 2017, 19, 1318–1321. [Google Scholar] [CrossRef]
- Narute, S.; Pappo, D. Iron Phosphate Catalyzed Asymmetric Cross-Dehydrogenative Coupling of 2-Naphthols with β-Ketoesters. Org. Lett. 2017, 19, 2917–2920. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Srivastava, A.; Yadav, D.; Singh, M.S. Copper-Catalyzed One-Pot Cross-Dehydrogenative Thienannulation: Chemoselective Access to Naphtho[2,1-b]thiophene-4,5-diones and Subsequent Transformation to Benzo[a]thieno[3,2-c]phenazines. J. Org. Chem. 2018, 83, 2173–2181. [Google Scholar] [CrossRef]
- Tang, Z.; Tong, Z.; Xu, Z.H.; Au, C.T.; Qiu, R.H.; Yin, S.F. Recyclable nickel-catalyzed C–H/O–H dual functionalization of phenols with mandelic acids for the synthesis of 3-aryl benzofuran-2(3H)-ones under solvent-free conditions. Green Chem. 2019, 21, 2015–2022. [Google Scholar] [CrossRef]
- Li, N.; Tu, L.; Cheng, G.G.; Sa, H.L.; Li, Z.H.; Feng, T.; Zheng, Y.S.; Liu, J.K. Diastereoselective [3 + 3] cycloaddition reaction of 2-arylideneindan-1,3-diones with β-naphthols: Efficient assemble of immunosuppressive pentacyclic chromanes. Tetrahedron Lett. 2020, 61, 151579–151582. [Google Scholar] [CrossRef]
- Li, Z.H.; Peng, J.Y.; He, C.L.; Xu, J.F.; Ren, H.J. Silver(I)-mediated cascade reaction of 2-(1-alkynyl)-2-alken-1-ones with 2-naphthols. Org. Lett. 2020, 22, 5768–5772. [Google Scholar] [CrossRef]
- Han, C.H.; Fu, Z.Y.; Guo, S.J.; Fang, X.X.; Lin, A.J.; Yao, H.Q. Palladium-catalyzed remote 1,n-arylamination of unactivated terminal alkenes. ACS Catal. 2019, 9, 4196–4202. [Google Scholar] [CrossRef]
- Tang, B.C.; Lin, W.X.; Chen, X.L.; He, C.; Ma, J.T.; Wu, Y.D.; Lan, Y.; Wu, A.X. Quadruple C-H activation coupled to hydrofunctionalization and C-H silylation/borylation enabled by weakly coordinated palladium catalyst. Nat. Commun. 2020, 11, 5662–5673. [Google Scholar] [CrossRef]



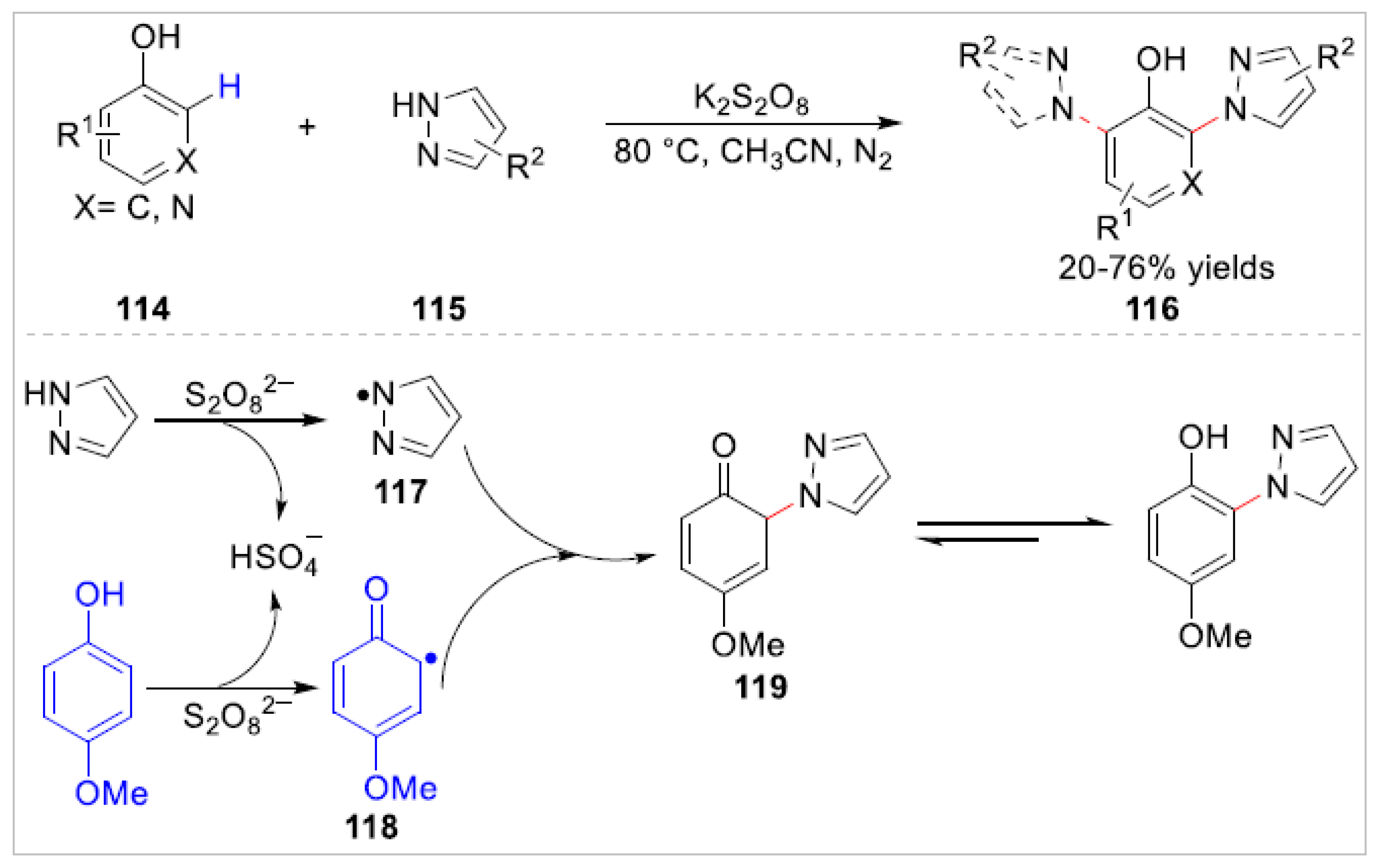
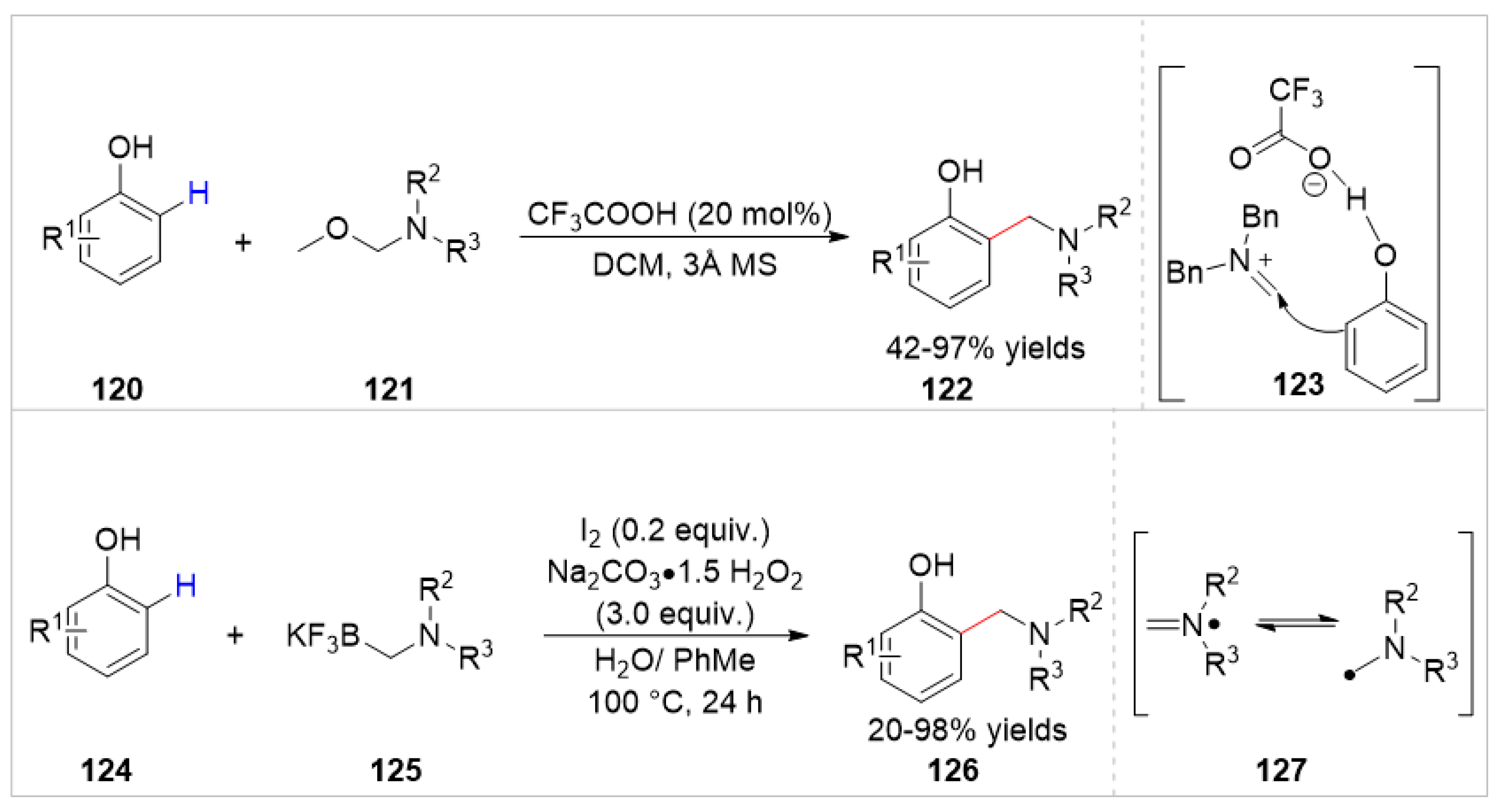
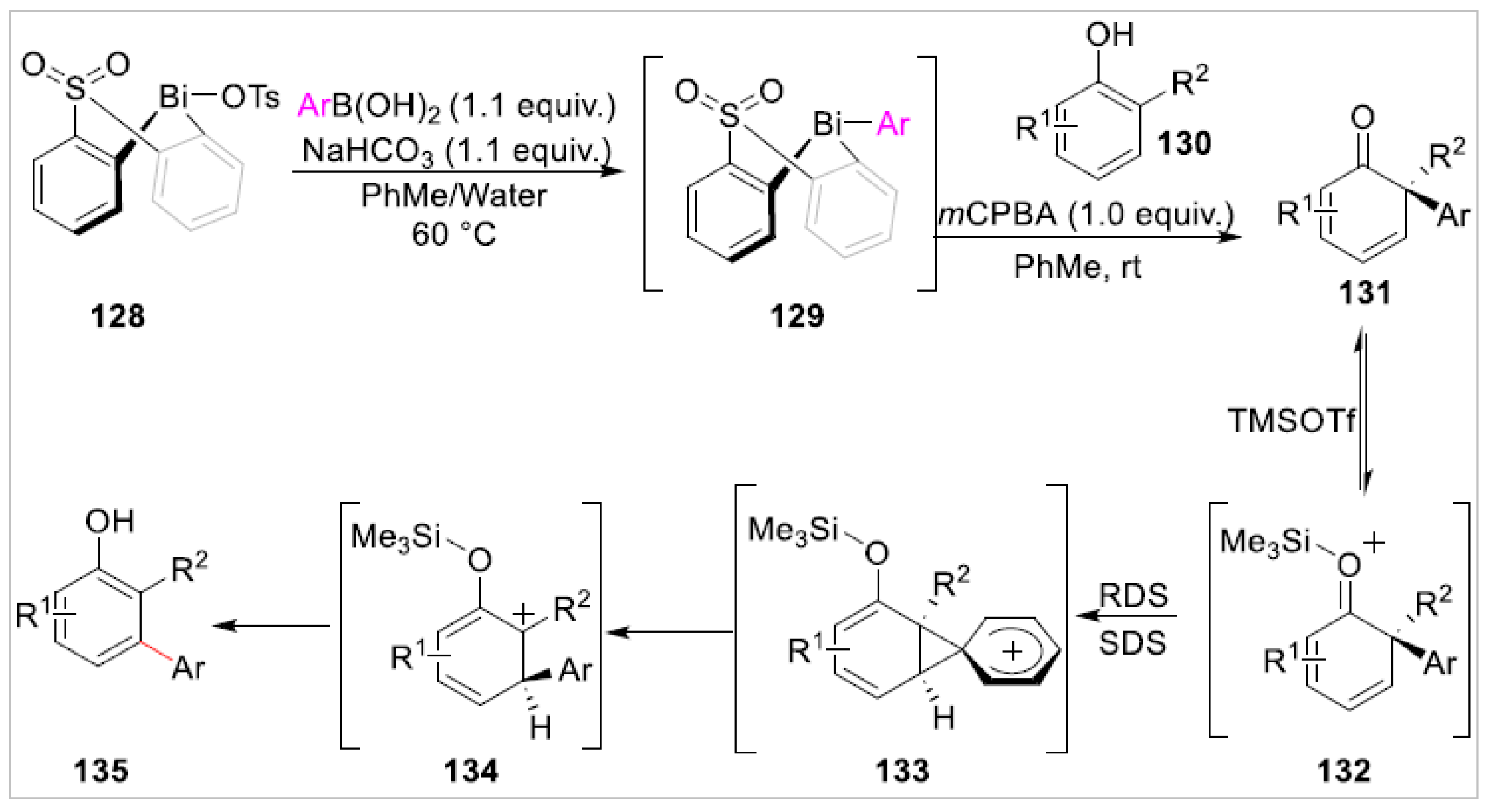
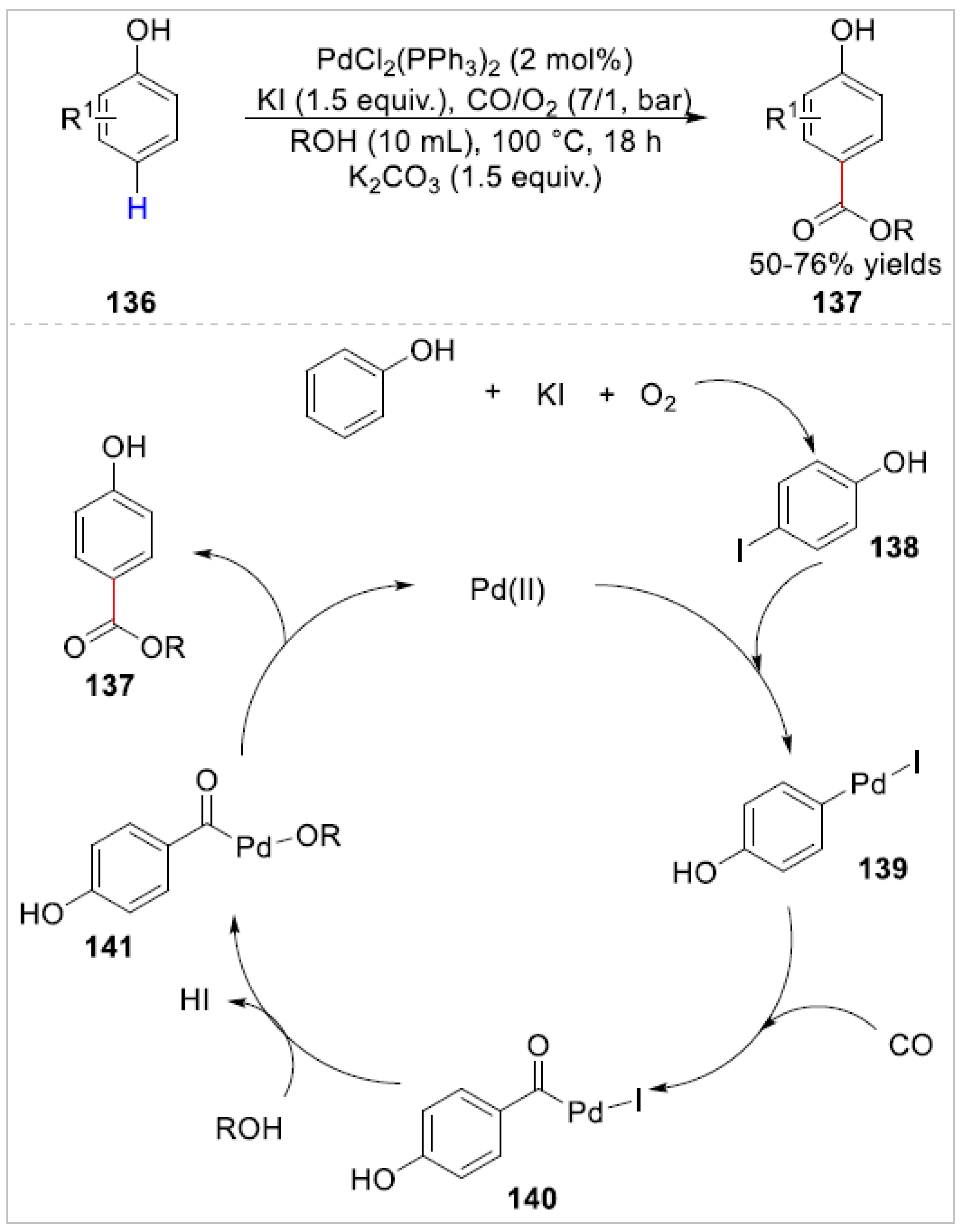


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, Y.; Li, Z.; Sun, J. Recent Advances in Regioselective C–H Bond Functionalization of Free Phenols. Molecules 2023, 28, 3397. https://doi.org/10.3390/molecules28083397
Li Y, Huang Y, Li Z, Sun J. Recent Advances in Regioselective C–H Bond Functionalization of Free Phenols. Molecules. 2023; 28(8):3397. https://doi.org/10.3390/molecules28083397
Chicago/Turabian StyleLi, Yanan, Yekai Huang, Zhi Li, and Jianan Sun. 2023. "Recent Advances in Regioselective C–H Bond Functionalization of Free Phenols" Molecules 28, no. 8: 3397. https://doi.org/10.3390/molecules28083397
APA StyleLi, Y., Huang, Y., Li, Z., & Sun, J. (2023). Recent Advances in Regioselective C–H Bond Functionalization of Free Phenols. Molecules, 28(8), 3397. https://doi.org/10.3390/molecules28083397






