Effects of Synthetic Ligustrazine-Based Chalcone Derivatives on Trypanosoma brucei brucei and Leishmania spp. Promastigotes
Abstract
1. Introduction
2. Results and Discussion
2.1. Antitrypanosomal Activity of Tetramethylpyrazine Containing Chalcone Derivatives
2.2. Antileishmanial Activity of Synthesized Chalcone Derivatives
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.2.1. Trypanosoma brucei brucei s427-WT
3.2.2. Leishmania spp. Promastigotes
3.3. Alamar Blue Assay
3.4. Drug Sensitivity
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Rycker, M.; Baragaña, B.; Duce, S.L.; Gilbert, I.H. Challenges and recent progress in drug discovery for tropical diseases. Nature 2018, 559, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; Seoud, O.E.; Ferreira, E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: A review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of African trypanosomiasis. Parasites Vectors 2008, 1, 3. [Google Scholar] [CrossRef]
- Barrett, M.P.; Burchmore, R.J.; Stich, A.; Lazzari, J.O.; Frasch, A.C.; Cazzulo, J.J.; Krishna, S. The trypanosomiases. Lancet 2003, 362, 1469–1480. [Google Scholar] [CrossRef]
- Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 2 March 2023).
- Dize, D.; Tata, R.B.; Keumoe, R.; Kouipou Toghueo, R.M.; Tchatat, M.B.; Njanpa, C.N.; Tchuenguia, V.C.; Yamthe, L.T.; Fokou, P.V.T.; Laleu, B.; et al. Preliminary Structure—Activity Relationship Study of the MMV Pathogen Box Compound MMV675968 (2,4-Diaminoquinazoline) Unveils Novel Inhibitors of Trypanosoma brucei brucei. Molecules 2022, 27, 6574. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Tihon, E.; Imamura, H.; Van den Broeck, F.; Vermeiren, L.; Dujardin, J.-C.; Van Den Abbeele, J. Genomic analysis of Isometamidium Chloride resistance in Trypanosoma congolense. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 350–361. [Google Scholar] [CrossRef]
- Black, S.J.; Mansfield, J.M. Prospects for vaccination against pathogenic African trypanosomes. Parasite Immunol. 2016, 38, 735–743. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Ferreira, C.S.; Branquinha, M.H.; Santos, A.L.; Chaud, M.V.; Jain, S.; Cardoso, J.C.; Kovačević, A.B.; Souto, E.B.; Severino, P. Overcoming multi-resistant leishmania treatment by nanoencapsulation of potent antimicrobials. J. Chem. Technol. Biotechnol. 2021, 96, 2123–2140. [Google Scholar] [CrossRef]
- Rasheed, Z.; Ahmed, A.A.; Salem, T.; Al-Dhubaibi, M.S.; Al Robaee, A.A.; Alzolibani, A.A. Prevalence of Leishmania species among patients with cutaneous leishmaniasis in Qassim province of Saudi Arabia. BMC Public Health 2019, 19, 384. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Mnasri, A.; Al Nasr, I.S.; Özdemir, I.; Gürbüzd, N.; Hamdi, N.; Koko, W. Activity of benzimidazole derivatives and their N-heterocyclic carbene silver complexes against Leishmania major promastigotes and amastigotes. Biointerface Res. Appl. Chem. 2022, 13, 135. [Google Scholar]
- Khan, M.A.A.; Faisal, K.; Chowdhury, R.; Nath, R.; Ghosh, P.; Ghosh, D.; Hossain, F.; Abd El Wahed, A.; Mondal, D. Evaluation of molecular assays to detect Leishmania donovani in Phlebotomus argentipes fed on post-kala-azar dermal leishmaniasis patients. Parasites Vectors 2021, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Ghatee, M.A.; Taylor, W.R.; Karamian, M. The Geographical Distribution of Cutaneous Leishmaniasis Causative Agents in Iran and Its Neighboring Countries, A Review. Front. Public Health 2020, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.D.; Alouffi, A.S.; Alyousif, M.S.; Rahi, A.A.; Ali, M.A.; Abdullah, H.H.A.M.; Brayner, F.A.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; Otranto, D. Molecular characterization of Leishmania species from stray dogs and human patients in Saudi Arabia. Parasitol. Res. 2021, 120, 4241–4246. [Google Scholar] [CrossRef]
- Abuzaid, A.A.; Aldahan, M.A.; Helal, M.A.A.; Assiri, A.M.; Alzahrani, M.H. Visceral leishmaniasis in Saudi Arabia: From hundreds of cases to zero. Acta Trop. 2020, 212, 105707. [Google Scholar] [CrossRef]
- Rivas, F.; Del Mármol, C.; Scalese, G.; Pérez-Díaz, L.; Machado, I.; Blacque, O.; Medeiros, A.; Comini, M.; Gambino, D. New multifunctional Ru(II) organometallic compounds show activity against Trypanosoma brucei and Leishmania infantum. J. Inorg. Biochem. 2022, 237, 112016. [Google Scholar] [CrossRef]
- Alkhaldi, A.A.; Koning, H.P.d.; Bukhari, S.N.A. Synthetic ligustrazine based cyclohexanone and oxime analogs as Anti-Trypanosoma and Anti-Leishmanial agentes. Braz. J. Pharm. Sci. 2021, 57, e18997. [Google Scholar] [CrossRef]
- Wang, M.; Qin, H.L.; Leng, J.; Ameeduzzafar; Amjad, M.W.; Raja, M.A.G.; Hussain, M.A.; Bukhari, S.N.A. Synthesis and biological evaluation of new tetramethylpyrazine-based chalcone derivatives as potential anti-Alzheimer agents. Chem. Biol. Drug Des. 2018, 92, 1859–1866. [Google Scholar] [CrossRef]
- Espinoza-Hicks, J.C.; Chacón-Vargas, K.F.; Hernández-Rivera, J.L.; Nogueda-Torres, B.; Tamariz, J.; Sánchez-Torres, L.E.; Camacho-Dávila, A. Novel prenyloxy chalcones as potential leishmanicidal and trypanocidal agents: Design, synthesis and evaluation. Eur. J. Med. Chem. 2019, 167, 402–413. [Google Scholar] [CrossRef]
- Hernández-Rivera, J.L.; Espinoza-Hicks, J.C.; Chacón-Vargas, K.F.; Carrillo-Campos, J.; Sánchez-Torres, L.E.; Camacho-Dávila, A.A. Synthesis, characterization and evaluation of prenylated chalcones ethers as promising antileishmanial compounds. Mol. Divers. 2022. ahead of print. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, L.; Spinelli, R.; Brandonisio, O. In vitro assays for evaluation of drug activity against Leishmania spp. Res. Microbiol. 2004, 155, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Raz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Al-Salabi, M.I.; Wallace, L.J.M.; de Koning, H.P. A Leishmania major Nucleobase Transporter Responsible for Allopurinol Uptake Is a Functional Homolog of the Trypanosoma brucei H2 Transporter. Mol. Pharmacol. 2003, 63, 814–820. [Google Scholar] [CrossRef] [PubMed]
| No. | Compounds | Trypanosoma brucei brucei EC50 µM | Leishmania spp. Promastigotes EC50 µM | |
|---|---|---|---|---|
| (WT) | L. major | L. mexicana | ||
| 1. | 1a | 65.24 ± 1.82 | >100 | 92.70 ± 1.320 |
| 2. | 1b | 65.24 ± 1.82 | >100 | 49.27 ± 3.893 |
| 3. | 1c | 23.32 ± 0.80 | 15.78 ± 2.29 | 36.41 ± 1.315 |
| 4. | 2a | 30.53 ± 1.10 | 65.97 ± 2.93 | 67.03 ± 2.823 |
| 5. | 2b | 30.59 ± 2.31 | 21.75 ± 0.94 | 33.60 ± 1.457 |
| 6. | 2c | 2.59 ± 0.11 | 18.74 ± 1.27 | 27.70 ± 4.390 |
| 7. | 3a | 25.31 ± 2.65 | >100 | >100 |
| 8. | 3b | >100 | >100 | >100 |
| 9. | 3c | 46.07 ± 3.66 | >100 | >100 |
| 10. | 4a | >100 | >100 | >100 |
| 11. | 4b | 22.87 ± 2.30 | 59.73 ± 4.58 | 66.99 ± 7.19 |
| 12. | 5a | >100 | >100 | >100 |
| 13. | 5b | 41.44 ± 8.49 | 87.01 ± 5.08 | 86.19 ± 2.18 |
| Eflornithine | 23.14 ± 3.05 | |||
| Pentamidine | 0.005 ± 0.00 | 4.34 ± 0.17 | 1.24 ± 0.11 | |
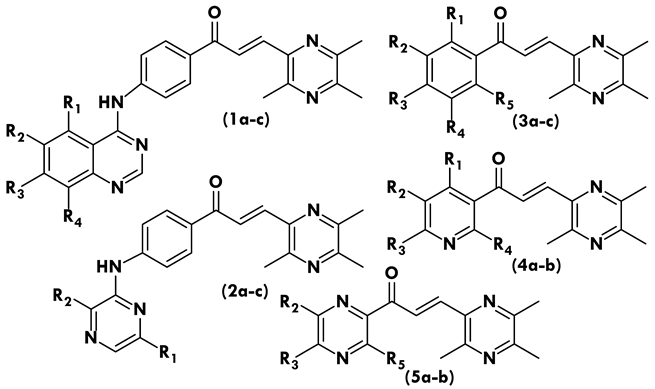 | |||||
| Compound | R1 | R2 | R3 | R4 | R5 |
| 1a | - | - | - | - | - |
| 1b | - | Cl | - | - | - |
| 1c | - | OCH3 | OCH3 | OCH3 | - |
| 2a | - | - | - | - | - |
| 2b | CH3 | - | - | - | - |
| 2c | - | CH3 | - | - | - |
| 3a | - | - | CH3 | - | - |
| 3b | - | - | CH3 | - | CH3 |
| 3c | - | CH3 | CH3 | - | CH3 |
| 4a | - | - | OCH3 | - | - |
| 4b | - | - | OCH3 | OCH3 | - |
| 5a | - | - | - | - | - |
| 5b | - | - | - | - | CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhaldi, A.A.M. Effects of Synthetic Ligustrazine-Based Chalcone Derivatives on Trypanosoma brucei brucei and Leishmania spp. Promastigotes. Molecules 2023, 28, 4652. https://doi.org/10.3390/molecules28124652
Alkhaldi AAM. Effects of Synthetic Ligustrazine-Based Chalcone Derivatives on Trypanosoma brucei brucei and Leishmania spp. Promastigotes. Molecules. 2023; 28(12):4652. https://doi.org/10.3390/molecules28124652
Chicago/Turabian StyleAlkhaldi, Abdulsalam A. M. 2023. "Effects of Synthetic Ligustrazine-Based Chalcone Derivatives on Trypanosoma brucei brucei and Leishmania spp. Promastigotes" Molecules 28, no. 12: 4652. https://doi.org/10.3390/molecules28124652
APA StyleAlkhaldi, A. A. M. (2023). Effects of Synthetic Ligustrazine-Based Chalcone Derivatives on Trypanosoma brucei brucei and Leishmania spp. Promastigotes. Molecules, 28(12), 4652. https://doi.org/10.3390/molecules28124652






