Zinc-Mediated Template Synthesis of Hierarchical Porous N-Doped Carbon Electrocatalysts for Efficient Oxygen Reduction
Abstract
1. Introduction
2. Results
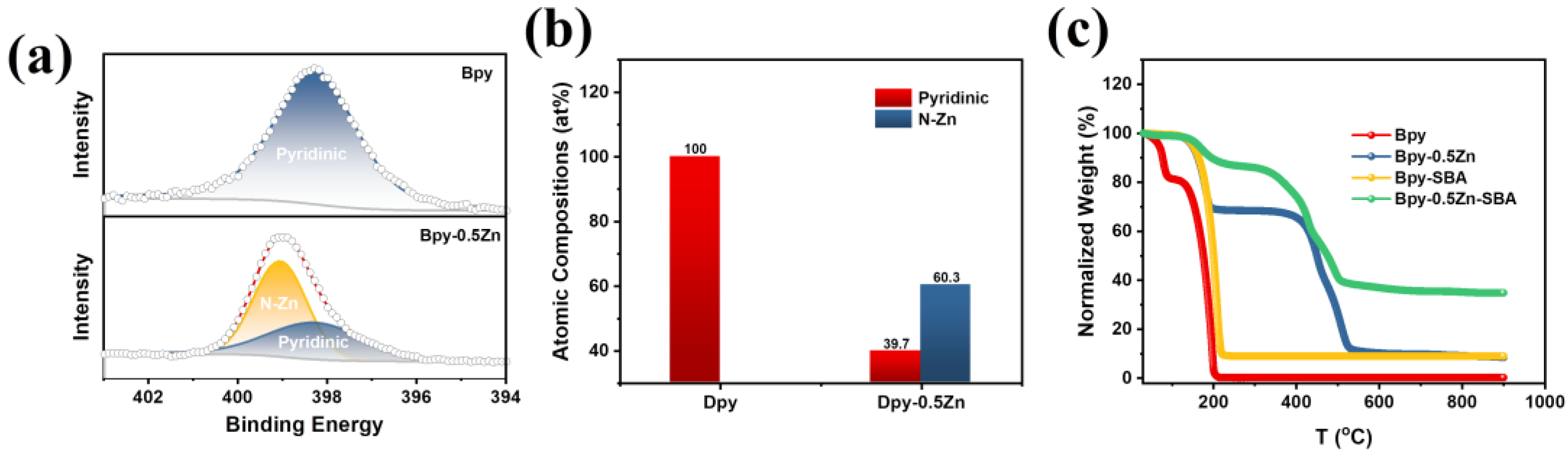
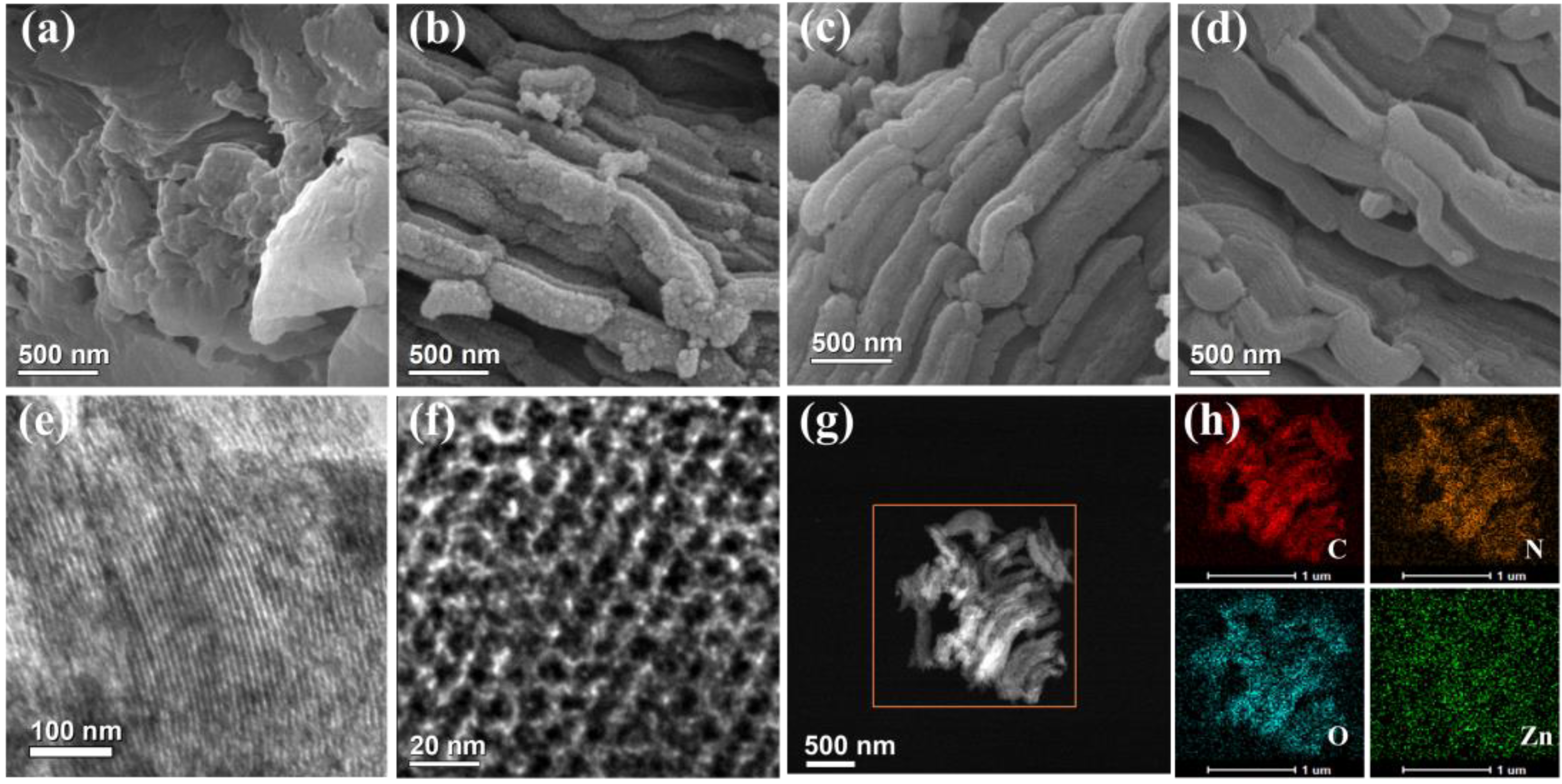
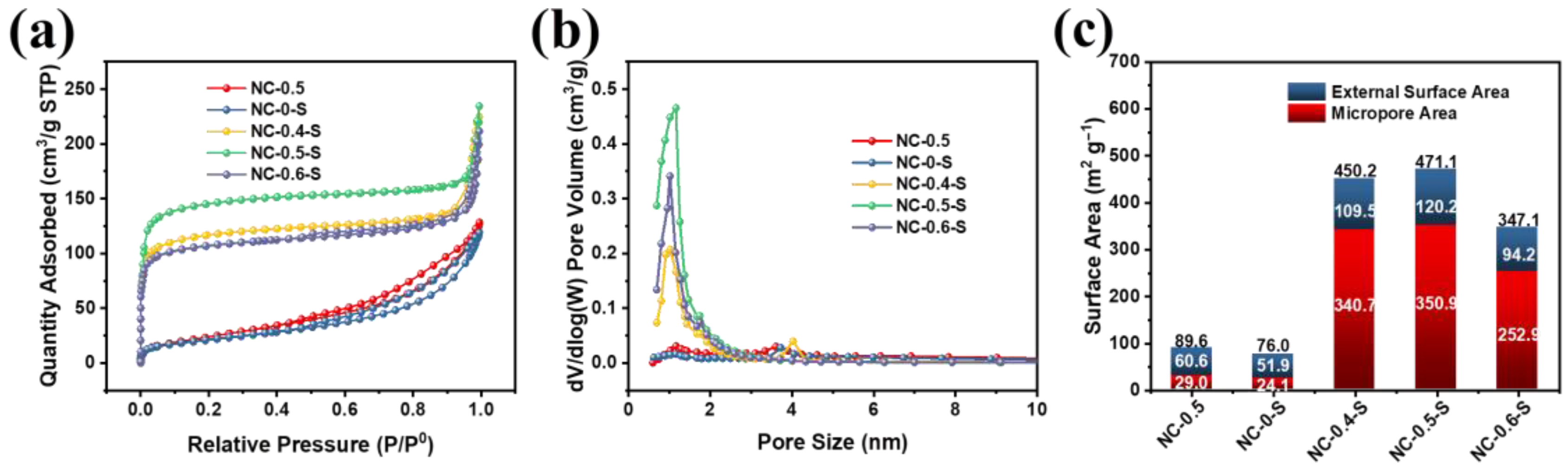
2.1. Physicochemical Characterization
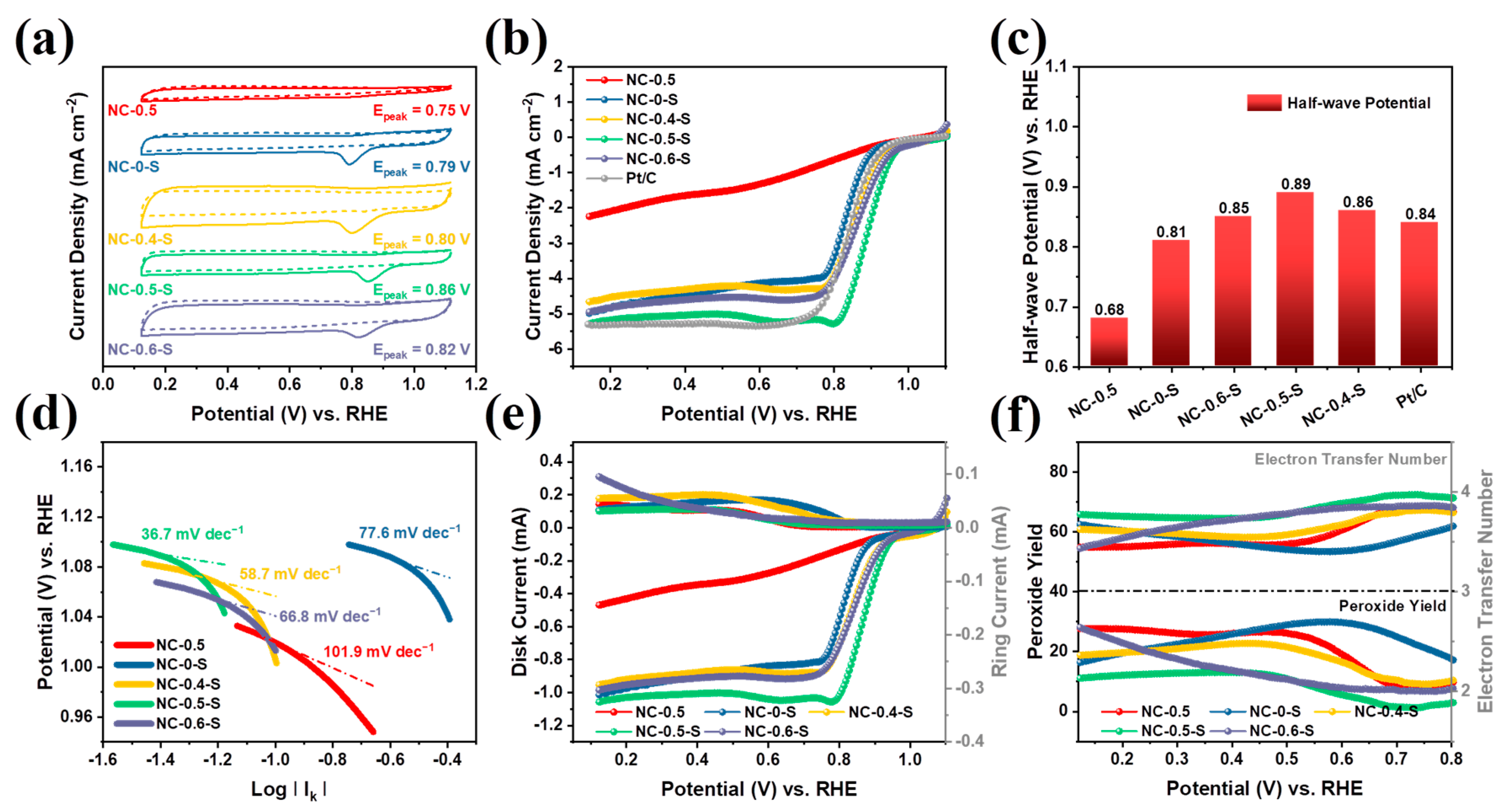
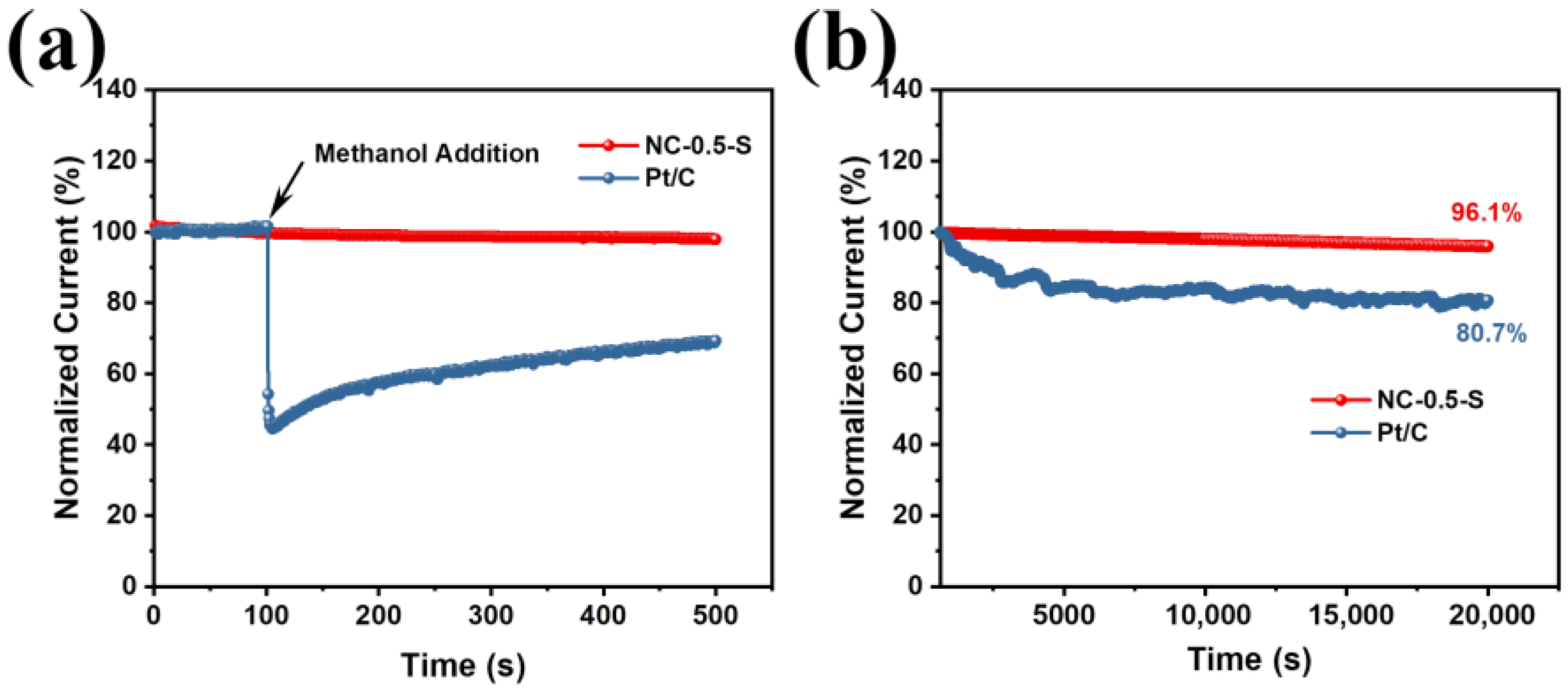
2.2. Electrochemical Characterization
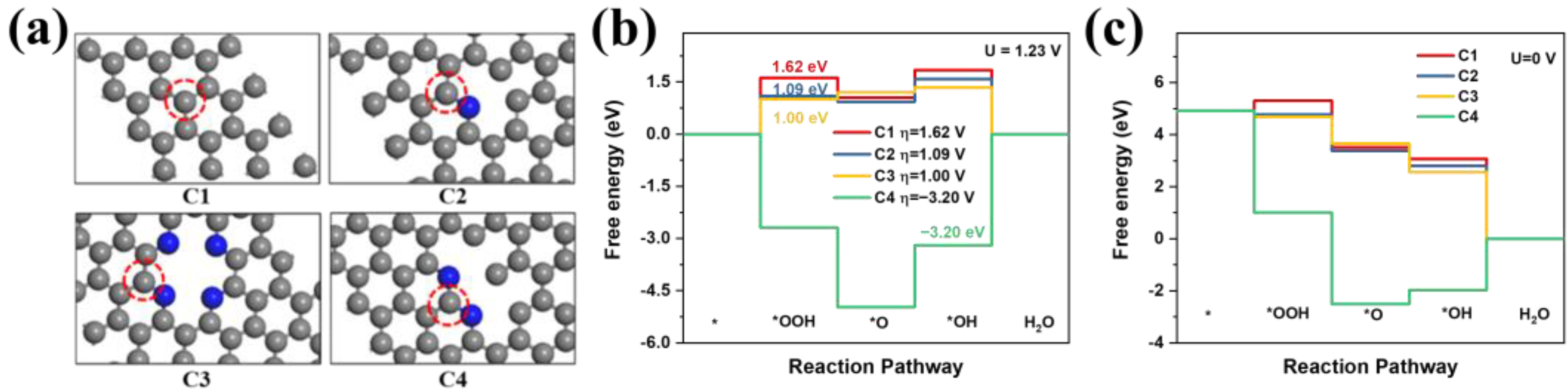
2.3. Theoretical Calculation
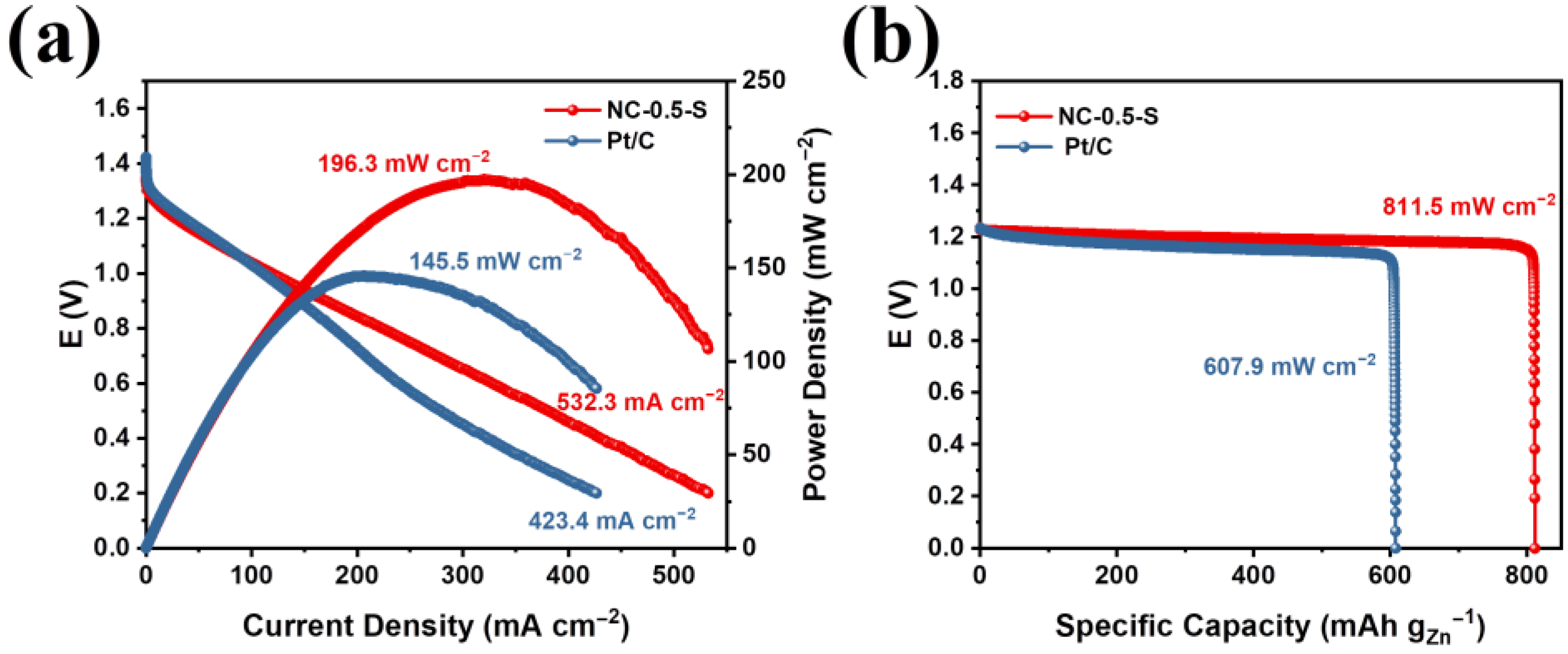
2.4. Battery Test
3. Materials and Methods
3.1. Materials
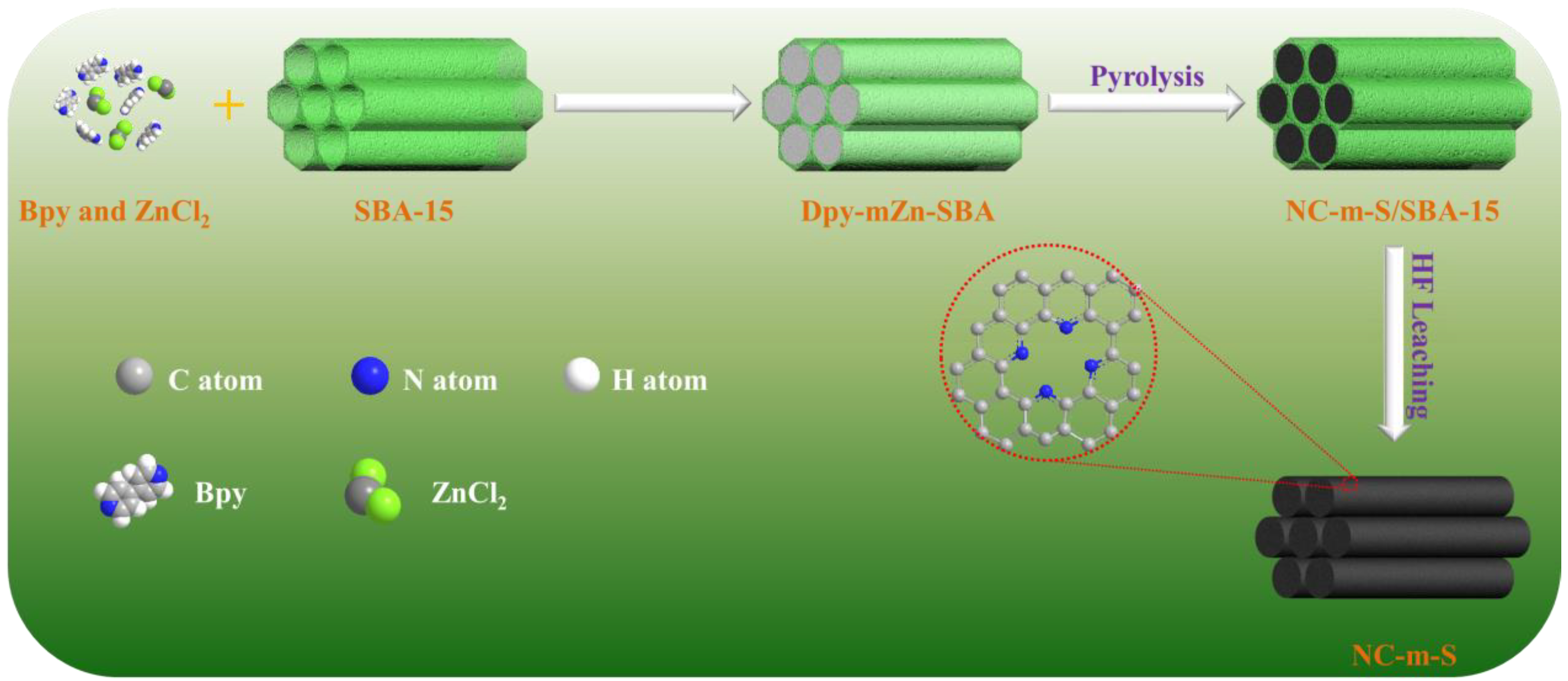
3.2. Preparation of Catalysts
3.3. Preparation of Working Electrodes
3.4. Preparation of Air Electrode for ZAB
3.5. Characterization
3.6. Electrochemical Measurements
3.7. Battery Tests
3.8. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, L.; Fu, C.; Luo, L.; You, J.; An, L.; Yan, X.; Shen, S.; Zhang, J. Electrochemical synthesis of monodispersed and highly alloyed PtCo nanoparticles with a remarkable durability towards oxygen reduction reaction. Appl. Catal. B 2022, 318, 121831. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Yu, L.; Liu, J.-N.; Wang, J.; Yao, N.; Li, X.-Y.; Chen, X.; Li, B.-Q.; Zhang, Q. Working Zinc–Air Batteries at 80 °C. Angew. Chem. Int. Ed. 2022, 61, e202208042. [Google Scholar]
- Guo, Y.; Zhang, R.; Zhang, S.; Hong, H.; Zhao, Y.; Huang, Z.; Han, C.; Li, H.; Zhi, C. Ultrahigh Oxygen-doped Carbon Quantum Dots for Highly Efficient H2O2 Production via Two-Electron Electrochemical Oxygen Reduction. Energy Environ. Sci. 2022, 15, 4167–4174. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Wang, Q.; You, C.; Tian, X.; Wang, C.; Hua, Y.; Liao, S. A mesoporous carbon derived from 4,4′-dipyridyl iron as an efficient catalyst for oxygen reduction. J. Mater. Chem. A 2020, 8, 2439–2444. [Google Scholar] [CrossRef]
- You, C.; Gao, X.; Wang, Q.; Li, X.; Tan, S.; Xu, P.; Cai, D.; Weng, Y.; Wang, C.; Tian, X.; et al. Rechargeable Zinc–Air Battery with Ultrahigh Power Density Based on Uniform N, Co Codoped Carbon Nanospheres. ACS Appl. Mater. Interfaces 2019, 11, 44153–44160. [Google Scholar] [CrossRef]
- Wu, Z.; Su, Y.-Q.; Hensen, E.J.M.; Tian, X.; You, C.; Xu, Q. Highly stable Pt3Ni nanowires tailored with trace Au for the oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 26402–26409. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Xu, P.; You, C.; Sun, W.; Wang, X.; Lin, Q.; Liao, S. Uniform Nitrogen and Sulfur Co-doped Carbon Bowls for the Electrocatalyzation of Oxygen Reduction. ACS Sustain. Chem. Eng. 2019, 7, 7148–7154. [Google Scholar] [CrossRef]
- You, C.; Wang, L.; Huang, Y.; Jiang, X.; Li, X.; Wang, C.; Hua, Y.; Wang, X.; Liao, S. High porosity nitrogen and phosphorous Co-doped carbon nanosheets as an efficient catalyst for oxygen reduction. Int. J. Hydrogen Energy 2018, 43, 9749–9756. [Google Scholar] [CrossRef]
- You, C.; Jiang, X.; Wang, X.; Hua, Y.; Wang, C.; Lin, Q.; Liao, S. Nitrogen, Sulfur Co-doped Carbon Derived from Naphthalene-Based Covalent Organic Framework as an Efficient Catalyst for Oxygen Reduction. ACS Appl. Energy Mater. 2018, 1, 161–166. [Google Scholar] [CrossRef]
- You, C.; Jiang, X.; Han, L.; Wang, X.; Lin, Q.; Hua, Y.; Wang, C.; Liu, X.; Liao, S. Uniform nitrogen and sulphur co-doped hollow carbon nanospheres as efficient metal-free electrocatalysts for oxygen reduction. J. Mater. Chem. A 2017, 5, 1742–1748. [Google Scholar] [CrossRef]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.-H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, 2006328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-X.; Liu, J.-N.; Wang, J.; Ren, D.; Li, B.-Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Wang, Q.; Liao, Z.; Zhang, N.; Guo, Y.; Xiang, Z. Hierarchically porous metal-free carbon with record high mass activity for oxygen reduction and Zn-air batteries. J. Mater. Chem. A 2019, 7, 9831–9836. [Google Scholar] [CrossRef]
- Ren, G.; Chen, S.; Zhang, J.; Zhang, N.; Jiao, C.; Qiu, H.; Liu, C.; Wang, H.-L. N-doped porous carbon spheres as metal-free electrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 5751–5758. [Google Scholar] [CrossRef]
- Tang, T.; Duan, Z.; Baimanov, D.; Bai, X.; Liu, X.; Wang, L.; Wang, Z.; Guan, J. Synergy between isolated Fe and Co sites accelerates oxygen evolution. Nano Res. 2023, 16, 2218–2223. [Google Scholar] [CrossRef]
- He, S.; Wu, M.; Li, S.; Jiang, Z.; Hong, H.; Cloutier, S.G.; Yang, H.; Omanovic, S.; Sun, S.; Zhang, G. Research Progress on Graphite-Derived Materials for Electrocatalysis in Energy Conversion and Storage. Molecules 2022, 27, 8644. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wang, Y.; Han, J.; Zhang, Q.; Bai, X.; Niu, X.; Wang, Z.; Guan, J. Dual-atom Co-Fe catalysts for oxygen reduction reaction. Chin. J. Catal. 2023, 46, 48–55. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Pak, D. Heteroatom-Doped Metal-Free Carbon Nanomaterials as Potential Electrocatalysts. Molecules 2022, 27, 670. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Sun-Waterhouse, D.; Zhang, T.; Waterhouse, G. Engineering local coordination environments and site densities for high-performance Fe-N-C oxygen reduction reaction electrocatalysis. SmartMat 2021, 2, 154–175. [Google Scholar] [CrossRef]
- Tao, D.-J.; Zhao, X.; Wang, Y.; Liu, X.; Li, H.-P.; Li, Z.-M.; Zhou, Y.; Yuan, Z.; Zhang, Z. Vitamin B9 derived nitrogen-doped graphene for metal-free aerobic oxidation of biomass-derived chemicals. Green Energy Environ. 2022, 7, 1084–1092. [Google Scholar] [CrossRef]
- Behan, J.A.; Mates-Torres, E.; Stamatin, S.N.; Dominguez, C.; Iannaci, A.; Fleischer, K.; Hoque, M.K.; Perova, T.S.; Garcia-Melchor, M.; Colavita, P.E. Untangling Cooperative Effects of Pyridinic and Graphitic Nitrogen Sites at Metal-Free N-Doped Carbon Electrocatalysts for the Oxygen Reduction Reaction. Small 2019, 15, 1902081. [Google Scholar] [CrossRef]
- Duan, Z.; Han, G.; Huo, H.; Lin, Z.; Ge, L.; Du, C.; Gao, Y.; Yin, G. Monovacancy Coupled Pyridinic N Site Enables Surging Oxygen Reduction Activity of Metal-Free CNx Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 1264–1271. [Google Scholar] [CrossRef]
- Takeyasu, K.; Furukawa, M.; Shimoyama, Y.; Singh, S.K.; Nakamura, J. Role of Pyridinic Nitrogen in the Mechanism of the Oxygen Reduction Reaction on Carbon Electrocatalysts. Angew. Chem. Int. Ed. 2021, 60, 5121–5124. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, Q.; Zhao, Y.; Wang, Z.; Wang, A.; Liu, Y.; Sun, K.; Wu, J.; Wang, L.; Jiang, J. A Facile “Double-Catalysts” Approach to Directionally Fabricate Pyridinic N-B-Pair-Doped Crystal Graphene Nanoribbons/Amorphous Carbon Hybrid Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2022, 34, 2107040. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wang, N.; Si, W.; Hou, Z.; Li, X.; Wang, X.; Zhao, F.; Yang, Z.; Zhang, Y.; Huang, C. Pyridinic nitrogen exclusively doped carbon materials as efficient oxygen reduction electrocatalysts for Zn-air batteries. Appl. Catal. B 2020, 261, 118234. [Google Scholar] [CrossRef]
- You, C.; Dang, D.; Qiao, X.; Wang, G.; Fan, W.; Chen, R.; Li, Y.; Li, X.; Liao, S. An ultra high performance multi-element doped mesoporous carbon catalyst derived from poly(4-vinylpyridine). J. Mater. Chem. A 2015, 3, 23512–23519. [Google Scholar] [CrossRef]
- Ingavale, S.; Marbaniang, P.; Kakade, B.; Swami, A. Starbon with Zn-N and Zn-O active sites: An efficient electrocatalyst for oxygen reduction reaction in energy conversion devices. Catal. Today 2021, 370, 55–65. [Google Scholar] [CrossRef]
- Xu, H.; Wang, D.; Yang, P.; Du, L.; Lu, X.; Li, R.; Liu, L.; Zhang, J.; An, M. A hierarchically porous Fe-N-C synthesized by dual melt-salt-mediated template as advanced electrocatalyst for efficient oxygen reduction in zinc-air battery. Appl. Catal. B 2022, 305, 121040. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, C.; Yan, B.; Chen, Q.; Feng, H.; Mao, X.; Cen, J.; Tian, Z.Q.; Tsiakaras, P.; Shen, P.K. ZIF-Mg(OH)2 Dual Template Assisted Self-Confinement of Small PtCo NPs as Promising Oxygen Reduction Reaction in PEM Fuel Cell. Adv. Energy Mater. 2022, 12, 2201600. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, W.; Li, J.; Luo, J.; Chen, S. In Situ Generated Dual-Template Method for Fe/N/S Co-Doped Hierarchically Porous Honeycomb Carbon for High-Performance Oxygen Reduction. ACS Appl. Mater. Interfaces 2018, 10, 8721–8729. [Google Scholar] [CrossRef]
- Shi, J.; Shao, H.; Yang, F.; Li, J.; Fan, L.; Cai, W. Dual-template induced multi-scale porous Fe@FeNC oxygen reduction catalyst for high-performance electrochemical devices. Chem. Eng. J. 2022, 445, 136628. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, R.-R.; Bi, H.-H.; Lu, Y.-H.; Ma, L.-B.; He, X.-J. A review of porous carbons produced by template methods for supercapacitor applications. New Carbon Mater. 2021, 36, 69–81. [Google Scholar] [CrossRef]
- Shakeri, M.; Khatami Shal, Z.; Van Der Voort, P. An Overview of the Challenges and Progress of Synthesis, Characterization and Applications of Plugged SBA-15 Materials for Heterogeneous Catalysis. Materials 2021, 14, 5082. [Google Scholar] [CrossRef]
- Qiao, X.; Peng, H.; You, C.; Liu, F.; Zheng, R.; Xu, D.; Li, X.; Liao, S. Nitrogen, phosphorus and iron doped carbon nanospheres with high surface area and hierarchical porous structure for oxygen reduction. J. Power Sources 2015, 288, 253–260. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yang, N.; Deng, M.; Ibraheem, S.; Deng, J.; Li, J.; Li, L.; Wei, Z. Ultrahigh-Loading Zinc Single-Atom Catalyst for Highly Efficient Oxygen Reduction in Both Acidic and Alkaline Media. Angew. Chem. Int. Ed. 2019, 58, 7035–7039. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Liu, X.; Wang, X.; Tian, H.; Waterhouse, G.I.; Kruger, P.E.; Telfer, S.G.; Ma, S. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience 2022, 2, 227–234. [Google Scholar] [CrossRef]
- Xiao, Y.; Pei, Y.; Hu, Y.; Ma, R.; Wang, D.; Wang, J. Co2P@P-Doped 3D Porous Carbon for Bifunctional Oxygen Electrocatalysis. Acta Phys. Chim. Sin. 2021, 37, 2009051. [Google Scholar]
- Wang, Y.; Sun, T.; Mostaghimi, A.H.B.; Goncalves, T.J.; Liang, Z.; Zhou, Y.; Zhang, W.; Huang, Z.; Ma, Y.; Cao, R.; et al. Two-Dimensional Metal–Organic Frameworks with Unique Oriented Layers for Oxygen Reduction Reaction: Tailoring the Activity through Exposed Crystal Facets. CCS Chem. 2022, 4, 1633–1642. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Cao, X.; Abbott, J.; Jin, C.; Wang, H.; Strasser, P.; Yang, R.; Chen, X.; Wu, G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl. Catal. B 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Kumar, Y.; Mooste, M.; Tammeveski, K. Recent progress of transition metal-based bifunctional electrocatalysts for rechargeable zinc–air battery application. Curr. Opin. Electrochem. 2023, 38, 101229. [Google Scholar] [CrossRef]
- You, C.H.; Zeng, X.Y.; Qiao, X.C.; Liu, F.F.; Shu, T.; Zeng, J.H.; Du, L.; Liao, S.J. Fog-like fluffy structured N-doped carbon with superior oxygen reduction reaction performance to commercial Pt/C catalyst. Nanoscale 2015, 7, 3780–3785. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Xi, S.; Zhang, L.; Chen, Z.; Zeng, Z.; Huang, M.; Yang, H.; Liu, B.; Pennycook, S.J. Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn–air battery and self-driven water splitting. Adv. Energy Mater. 2020, 10, 2002896. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Balamurugan, J.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Yoo, D.J. Nitrogen-doped graphene encapsulated FeCoMoS nanoparticles as advanced trifunctional catalyst for water splitting devices and zinc–air batteries. Appl. Catal. B 2020, 279, 119381. [Google Scholar] [CrossRef]
- Wagh, N.K.; Shinde, S.S.; Lee, C.H.; Jung, J.-Y.; Kim, D.-H.; Kim, S.-H.; Lin, C.; Lee, S.U.; Lee, J.-H. Densely colonized isolated Cu-N single sites for efficient bifunctional electrocatalysts and rechargeable advanced Zn-air batteries. Appl. Catal. B 2020, 268, 118746. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Velusamy, D.B.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc–air battery. Appl. Catal. B 2022, 300, 120752. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Balamurugan, J.; Lau, K.-T.; Kim, N.H.; Lee, J.H. Novel cobalt-doped molybdenum oxynitride quantum dot@N-doped carbon nanosheets with abundant oxygen vacancies for long-life rechargeable zinc–air batteries. J. Mater. Chem. A 2021, 9, 9092–9104. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.-P.; Liang, R.; Chen, N.; King, G.; Yu, A.; Chen, Z. Linker-compensated metal–organic framework with electron delocalized metal sites for bifunctional oxygen electrocatalysis. JACS 2022, 144, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Surendran, S.; Choi, H.; Han, M.K.; Sim, U. In Situ Grown CoMn2O4 3D-Tetragons on Carbon Cloth: Flexible Electrodes for Efficient Rechargeable Zinc–Air Battery Powered Water Splitting Systems. Small 2021, 17, 2103613. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.; Min, K.; An, H.; Kim, K.; Shim, S.E.; Baeck, S.-H. Oxygen-vacancy-rich CoFe/CoFe2O4 embedded in N-doped hollow carbon spheres as a highly efficient bifunctional electrocatalyst for Zn–air batteries. Chem. Eng. J. 2022, 448, 137665. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Hu, W.; Ning, J.; Zhong, Y.; Hu, Y. Formation of sandwiched leaf-like CNTs-Co/ZnCo2O4@ NC-CNTs nanohybrids for high-power-density rechargeable Zn-air batteries. Nano Energy 2021, 82, 105710. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, G.D.; Kang, Y.C. Aerosol-assisted synthesis of bimetallic nanoparticle-loaded bamboo-like N-doped carbon nanotubes as an efficient bifunctional oxygen catalyst for Zn-air batteries. Int. J. Energy Res. 2022, 46, 5215–5225. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.; Shen, J.; Ning, J.; Zhong, Y.; Hu, Y. Formation of mesoporous Co/CoS/Metal-NC@ S, N-codoped hairy carbon polyhedrons as an efficient trifunctional electrocatalyst for Zn-air batteries and water splitting. Chem. Eng. J. 2021, 403, 126385. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Yu, M.; Wang, W. Trifunctional Co nanoparticle confined in defect-rich nitrogen-doped graphene for rechargeable Zn-air battery with a long lifetime. Appl. Catal. B 2021, 281, 119514. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, J.; Ma, S.; Li, M.; Xu, R.; Zhang, Q.; Yang, Z.; Qu, K.; Cai, W.; Chen, Z. Regulated coordination environment of Ni single atom catalyst toward high-efficiency oxygen electrocatalysis for rechargeable Zinc-air batteries. Energy Storage Mater. 2021, 35, 723–730. [Google Scholar] [CrossRef]
- Yu, N.-F.; Wu, C.; Huang, W.; Chen, Y.-H.; Ruan, D.-Q.; Bao, K.-L.; Chen, H.; Zhang, Y.; Zhu, Y.; Huang, Q.-H. Highly efficient Co3O4/Co@ NCs bifunctional oxygen electrocatalysts for long life rechargeable Zn-air batteries. Nano Energy 2020, 77, 105200. [Google Scholar] [CrossRef]
- Lei, Z.; Tan, Y.; Zhang, Z.; Wu, W.; Cheng, N.; Chen, R.; Mu, S.; Sun, X. Defects enriched hollow porous Co-N-doped carbons embedded with ultrafine CoFe/Co nanoparticles as bifunctional oxygen electrocatalyst for rechargeable flexible solid zinc-air batteries. Nano Res. 2021, 14, 868–878. [Google Scholar] [CrossRef]
- Liu, T.; Mou, J.; Wu, Z.; Lv, C.; Huang, J.; Liu, M. A Facile and Scalable Strategy for Fabrication of Superior Bifunctional Freestanding Air Electrodes for Flexible Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003407. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.-A.; Xue, X.; Yin, H.; Cheng, W.; Cheng, J. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-NC catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B 2020, 263, 118344. [Google Scholar] [CrossRef]
- Pei, Z.; Yuan, Z.; Wang, C.; Zhao, S.; Fei, J.; Wei, L.; Chen, J.; Wang, C.; Qi, R.; Liu, Z.; et al. A Flexible Rechargeable Zinc–Air Battery with Excellent Low-Temperature Adaptability. Angew. Chem. Int. Ed. 2020, 59, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Lei, Z.; Yu, L.; Wu, W.; Wang, Z.; Cheng, N. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis. Appl. Catal. B 2022, 304, 121006. [Google Scholar] [CrossRef]
- Zhu, P.; Gao, J.; Liu, S. Facile in situ coupling CoFe/Co nanoparticles and N-doped carbon nanotubes/graphitic nanosheets as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. J. Power Sources 2020, 449, 227512. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Mooste, M.; Kozlova, J.; Kikas, A.; Aruväli, J.; Käärik, M.; Kisand, V.; Leis, J.; Tamm, A. Iron and Nickel Phthalocyanine-Modified Nanocarbon Materials as Cathode Catalysts for Anion-Exchange Membrane Fuel Cells and Zinc-Air Batteries. ChemElectroChem 2022, 9, e202200717. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Long, G.; Tang, X.; Li, X.; Wang, X.; You, C.; Fan, W.; Wang, Q. Zinc-Mediated Template Synthesis of Hierarchical Porous N-Doped Carbon Electrocatalysts for Efficient Oxygen Reduction. Molecules 2023, 28, 4257. https://doi.org/10.3390/molecules28114257
Ma Q, Long G, Tang X, Li X, Wang X, You C, Fan W, Wang Q. Zinc-Mediated Template Synthesis of Hierarchical Porous N-Doped Carbon Electrocatalysts for Efficient Oxygen Reduction. Molecules. 2023; 28(11):4257. https://doi.org/10.3390/molecules28114257
Chicago/Turabian StyleMa, Qianhui, Guifa Long, Xulei Tang, Xiaobao Li, Xianghui Wang, Chenghang You, Wenjun Fan, and Qingqing Wang. 2023. "Zinc-Mediated Template Synthesis of Hierarchical Porous N-Doped Carbon Electrocatalysts for Efficient Oxygen Reduction" Molecules 28, no. 11: 4257. https://doi.org/10.3390/molecules28114257
APA StyleMa, Q., Long, G., Tang, X., Li, X., Wang, X., You, C., Fan, W., & Wang, Q. (2023). Zinc-Mediated Template Synthesis of Hierarchical Porous N-Doped Carbon Electrocatalysts for Efficient Oxygen Reduction. Molecules, 28(11), 4257. https://doi.org/10.3390/molecules28114257





