Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methodology
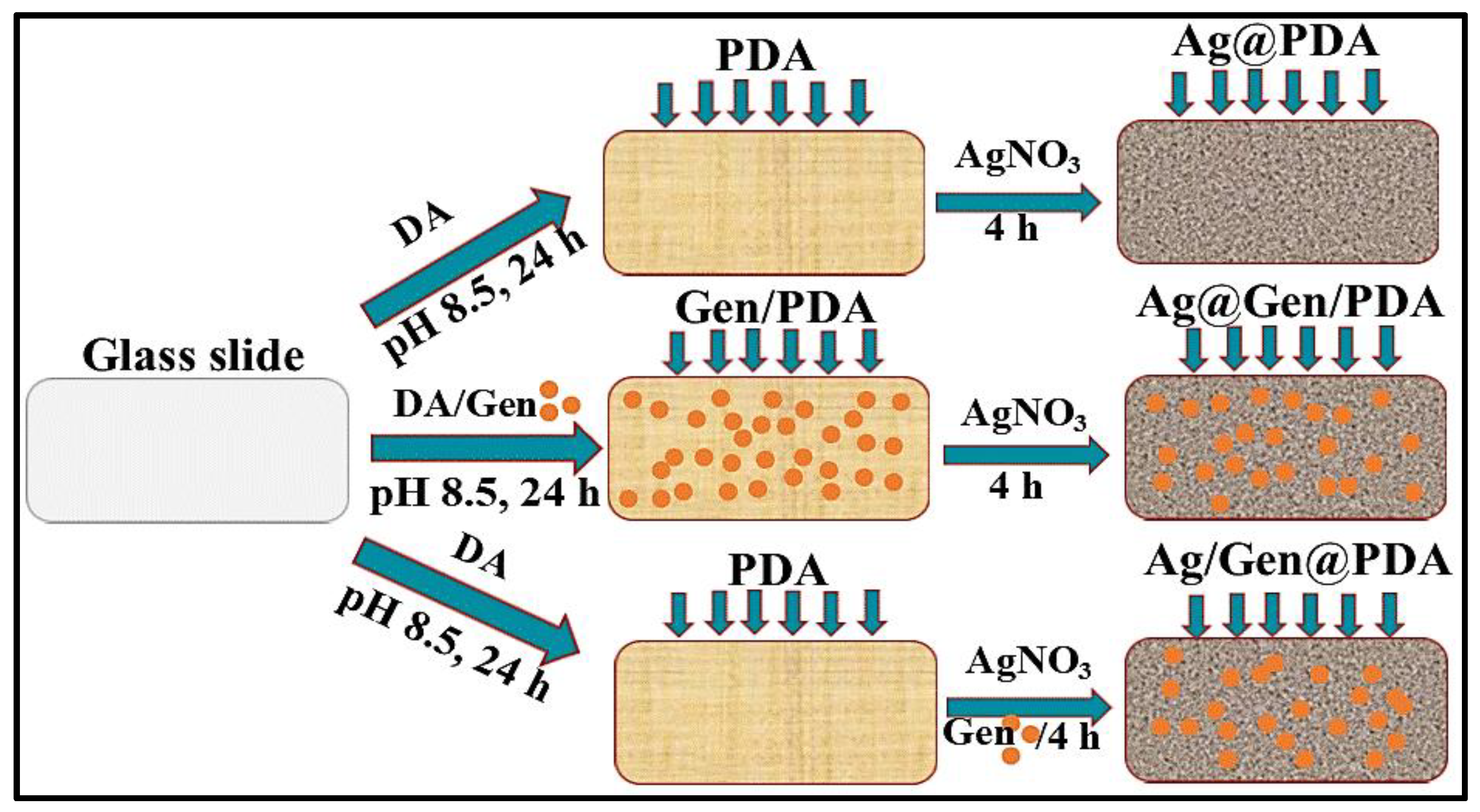
2.2.1. Preparation of PDA-Coated Glass Slides (PDA@glass)
2.2.2. Deposition of Silver NPs on PDA Coatings (Ag@PDA)
2.2.3. Deposition of Gentamicin and Ag NPs on PDA Coatings (Ag/Gen@PDA)
2.2.4. Deposition of Silver on Gentamicin Loaded PDA Coatings (Ag@Gen/PDA)
2.3. Characterization of PDA Coatings
2.4. Quantification of Silver Release from Glass Slides
2.5. Quantification of Gentamicin Release from Glass Slides
2.6. Antimicrobial Experiments for Glass Slides
2.7. Zone of Inhibition (ZOI)
2.8. Measurements of Optical Densities (OD600)
2.9. Spread Plate Method
3. Results and Discussion
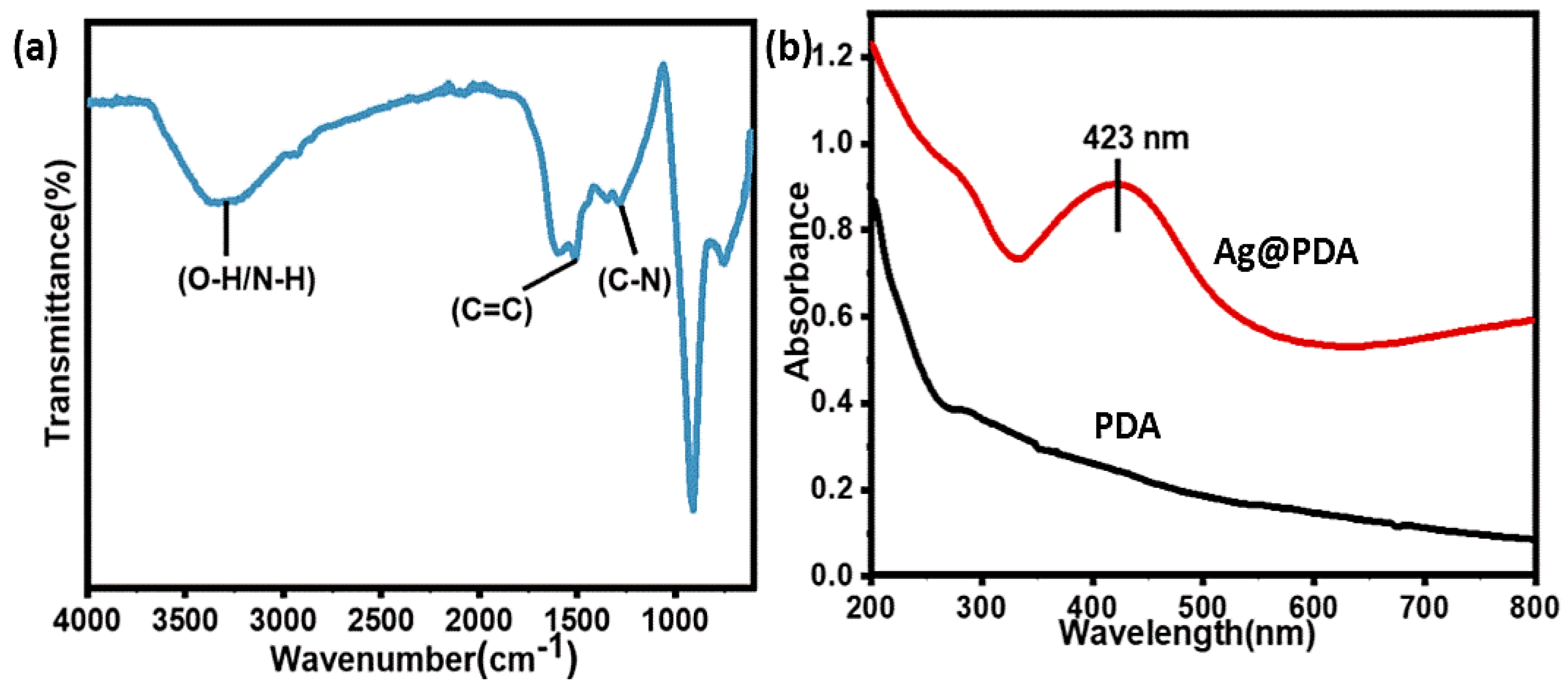
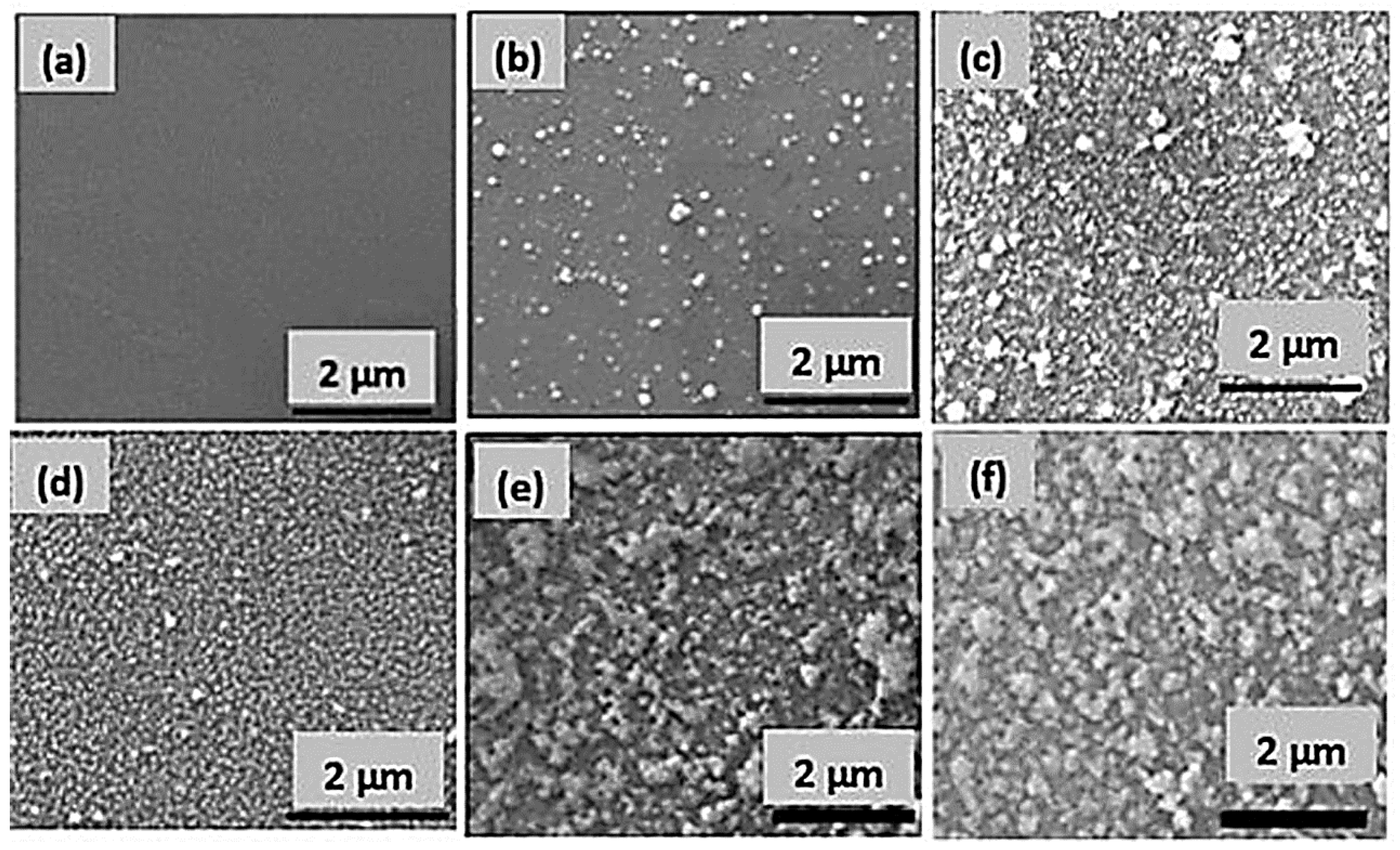
3.1. Preparation and Characterization of PDA-Based Antibacterial Coatings
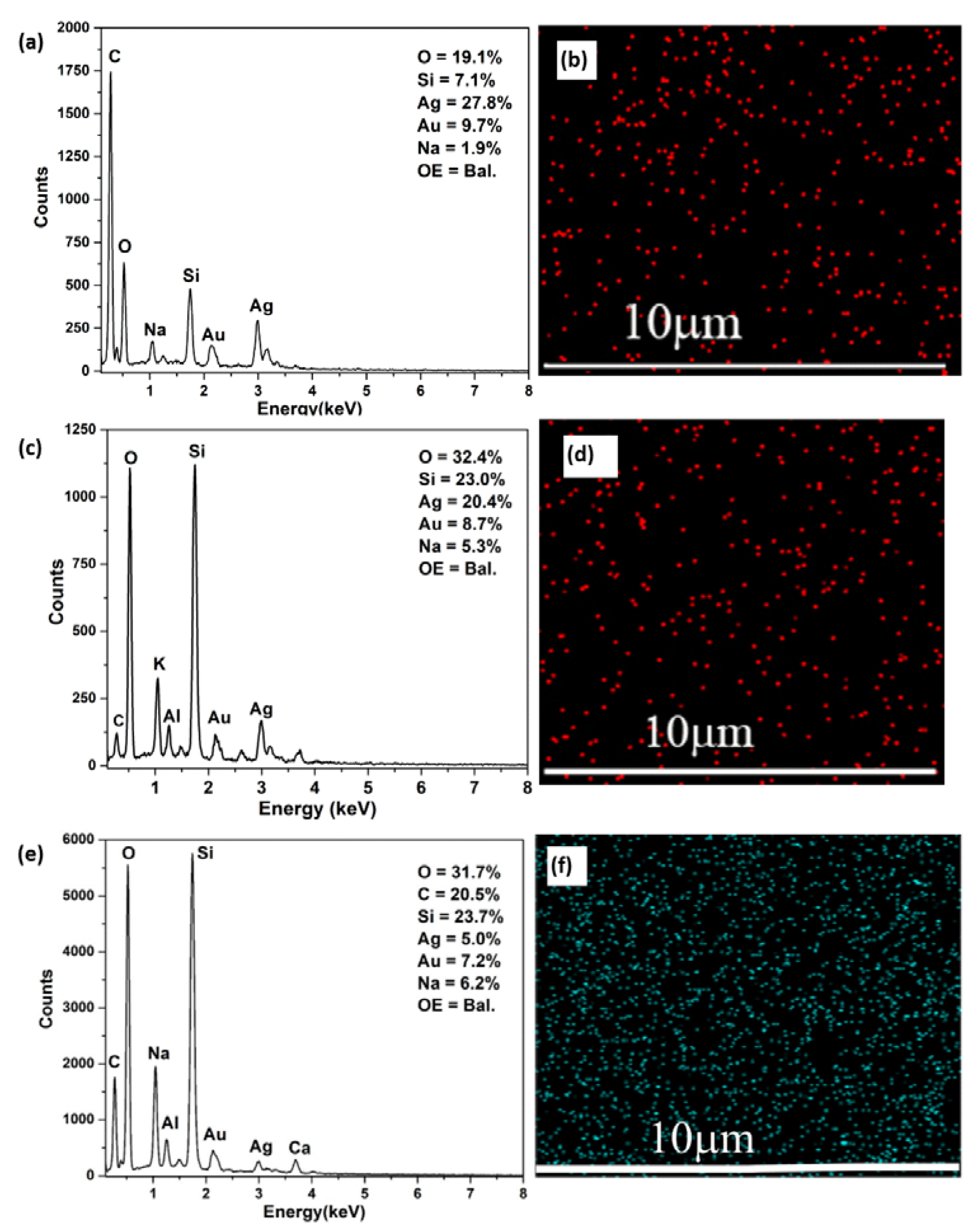
3.2. Deposition of Silver NPs on PDA (Ag@PDA) Coatings
3.3. Quantification of Sliver Loading and Release from PDA Coatings
3.3.1. Quantification of Silver Release from Ag@PDA
3.3.2. Quantification of Silver Release from Ag/Gen@PDA
3.3.3. Quantification of Silver Release from Ag@Gen/PDA
3.4. Quantification of Gentamicin Release from PDA Coatings
3.5. Evaluation of Synergistic Antimicrobial Properties of Silver and Gentamicin Loaded PDA Coatings
3.5.1. Zone of Inhibition
3.5.2. Optical Density Measurements
3.5.3. Spread Plate Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gaynes, R. The Discovery of Penicillin—New Insights after More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- He, X.; Obeng, E.; Sun, X.; Kwon, N.; Shen, J.; Yoon, J. Polydopamine, harness of the antibacterial potentials—A review. Mater. Today Bio 2022, 15, 100329. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Yu, Q.; Chen, H. Dual-function antibacterial surfaces to resist and kill bacteria: Painting a picture with two brushes simultaneously. J. Mater. Sci. Technol. 2021, 70, 24–38. [Google Scholar] [CrossRef]
- Qiao, Z.; Yao, Y.; Song, S.; Yin, M.; Luo, J. Silver nanoparticles with pH induced surface charge switchable properties for antibacterial and antibiofilm applications. J. Mater. Chem. B 2019, 7, 830–840. [Google Scholar] [CrossRef]
- Shakya, S.; He, Y.; Ren, X.; Guo, T.; Maharjan, A.; Luo, T.; Wang, T.; Dhakhwa, R.; Regmi, B.; Li, H.; et al. Ultrafine Silver Nanoparticles Embedded in Cyclodextrin Metal-Organic Frameworks with GRGDS Functionalization to Promote Antibacterial and Wound Healing Application. Small 2019, 15, 1901065. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Akter, N.; Rahman, M.M.; Shi, C.; Islam, M.T.; Zeng, H.; Azam, M.S. Mussel-Inspired Immobilization of Silver Nanoparticles toward Antimicrobial Cellulose Paper. ACS Sustain. Chem. Eng. 2018, 6, 9178–9188. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Su, H.L.; Chou, C.C.; Hung, D.J.; Lin, S.H.; Pao, I.C.; Lin, J.H.; Huang, F.L.; Dong, R.X.; Lin, J.J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, H.; Peng, B.; Chen, H.; Sun, Y.; Gang, X.; Jin, P.; Wang, J. Synthesis of PS/Ag Nanocomposite Spheres with Catalytic and Antibacterial Activities. ACS Appl. Mater. Interfaces 2012, 4, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, A.S.; Chumanov, G. Encapsulation of Silver Nanoparticles into Polystyrene Microspheres. Chem. Mater. 2009, 21, 2835–2839. [Google Scholar] [CrossRef]
- Necula, B.S.; van Leeuwen, J.P.; Fratila-Apachitei, L.E.; Zaat, S.A.; Apachitei, I.; Duszczyk, J. In vitro cytotoxicity evaluation of porous TiO₂-Ag antibacterial coatings for human fetal osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Tucek, J.; Jancik, D.; Kolar, M.; Panacek, A.; Zboril, R. Magnetically Controllable Silver Nanocomposite with Multifunctional Phosphotriazine Matrix and High Antimicrobial Activity. Adv. Funct. Mater. 2010, 20, 2347–2354. [Google Scholar] [CrossRef]
- Tran, H.Q.; Batul, R.; Bhave, M.; Yu, A. Current Advances in the Utilization of Polydopamine Nanostructures in Biomedical Therapy. Biotechnol. J. 2019, 14, 1900080. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, Y.; Lin, K.; Zhu, M. Research Progress on Polydopamine Nanoparticles for Tissue Engineering. Front. Chem. 2021, 9, 727123. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Liu, J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. Int. J. Mol. Sci. 2022, 23, 7278. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Hauser, D.; Septiadi, D.; Turner, J.; Petri-Fink, A.; Rothen-Rutishauser, B. From Bioinspired Glue to Medicine: Polydopamine as a Biomedical Material. Materials 2020, 13, 1730. [Google Scholar] [CrossRef]
- Wang, T.; Wusigale; Kuttappan, D.; Amalaradjou, M.A.; Luo, Y.; Luo, Y. Polydopamine-coated chitosan hydrogel beads for synthesis and immobilization of silver nanoparticles to simultaneously enhance antimicrobial activity and adsorption kinetics. Adv. Compos. Hybrid Mater. 2021, 4, 696–706. [Google Scholar] [CrossRef]
- Tan, Y.; Tan, G.-X.; Ning, C.-Y.; Rong, X.-C.; Zhang, Y.; Zhou, L. Bioinspired Polydopamine Functionalization of Titanium Surface for Silver Nanoparticles Immobilization with Antibacterial Property. J. Inorg. Mater. 2014, 29, 1320–1326. [Google Scholar]
- Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; He, H. Polydopamine-Assisted Silver Nanoparticle Self-Assembly on Sericin/Agar Film for Potential Wound Dressing Application. Int. J. Mol. Sci. 2018, 19, 2875. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiang, J.; Xia, Q.; Li, K.; Yan, H.; Yu, L. Fabrication of Durably Antibacterial Cotton Fabrics by Robust and Uniform Immobilization of Silver Nanoparticles via Mussel-Inspired Polydopamine/Polyethyleneimine Coating. Ind. Eng. Chem. Res. 2020, 59, 9666–9678. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Xia, T.; Li, Z.; Zhao, K.; Deng, Z.; Guo, D.; Peng, B. Bioinspired synthesis of polydopamine/Ag nanocomposite particles with antibacterial activities. Mater. Sci. Eng. C 2015, 55, 155–165. [Google Scholar] [CrossRef]
- Lu, Z.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef]
- Wang, D.; Bao, L.; Li, H.; Guo, X.; Liu, W.; Wang, X.; Hou, X.; He, B. Polydopamine stabilizes silver nanoparticles as a SERS substrate for efficient detection of myocardial infarction. Nanoscale 2022, 14, 6212–6219. [Google Scholar] [CrossRef]
- Song, G.J.; Choi, Y.S.; Hwang, H.S.; Lee, C.S. Silver-Composited Polydopamine Nanoparticles: Antibacterial and Antioxidant Potential in Nanocomposite Hydrogels. Gels 2023, 9, 183. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Zhou, L.; Cheng, C.; Hu, Z.; Peng, Z. In-situ synthesis of silver nanoparticles on cellulose and its catalytic performance. J. Macromol. Sci. Part A 2022, 59, 605–612. [Google Scholar] [CrossRef]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Nair, A.P.; Kr, S.; Mathew, J.; Ek, R. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 2014, 173, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Anima, N.; Bhat, M.A.; Nayak, B.K. Enhanced antibacterial activity of commercial antibiotics using AgNPs synthesized from Aspergillus niger. Der Pharm. Lett. 2015, 7, 281–285. [Google Scholar]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, Y.; Li, M.; Cheng, Y.; Zheng, Y. Bioinspired and Biomimetic AgNPs/Gentamicin-Embedded Silk Fibroin Coatings for Robust Antibacterial and Osteogenetic Applications. ACS Appl. Mater. Interfaces 2017, 9, 25830–25846. [Google Scholar] [CrossRef]
- Katva, S.; Das, S.; Moti, H.S.; Jyoti, A.; Kaushik, S. Antibacterial Synergy of Silver Nanoparticles with Gentamicin and Chloramphenicol against Enterococcus faecalis. Pharmacogn. Mag. 2018, 13 (Suppl. 4), S828–S833. [Google Scholar]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine Surface Coating Synergizes the Antimicrobial Activity of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 40067–40077. [Google Scholar] [CrossRef]
- Batul, R.; Bhave, M.; Mahon, P.J.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef]
- Batul, R.; Khaliq, A.; Alafnan, A.; Bhave, M.; Yu, A. Investigation of Gentamicin Release from Polydopamine Nanoparticles. Appl. Sci. 2022, 12, 6319. [Google Scholar] [CrossRef]
- Chen, C.Y.; Nace, G.W.; Irwin, P.L. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, R.; Tian, M.; Liu, L.; Zou, H.; Zhao, X.; Zhang, L. Surface silverized meta-aramid fibers prepared by bio-inspired poly(dopamine) functionalization. ACS Appl. Mater. Interfaces 2013, 5, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef]
- Mollick, M.; Bhowmick, B.; Maity, D.; Mondal, D.; Roy, I.; Sarkar, J.; Rana, D.; Acharya, K.; Chattopadhyay, S.; Chattopadhyay, D. Green synthesis of silver nanoparticles-based nanofluids and investigation of their antimicrobial activities. Microfluid. Nanofluid. 2014, 16, 541–551. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Tang, H.; Wu, D.; Liu, D.; Liu, Y.; Cao, A.; Wang, H. Enhanced bactericidal toxicity of silver nanoparticles by the antibiotic gentamicin. Environ. Sci. Nano 2016, 3, 788–798. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Batarseh, K.I. Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J. Antimicrob. Chemother. 2004, 54, 546–548. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with antibiotic resistance: Combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti-Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Jamaran, S.; Zarif, B.R. Synergistic Effect of Silver Nanoparticles with Neomycin or Gentamicin Antibiotics on Mastitis-Causing Staphylococcus aureus. Open J. Ecol. 2016, 6, 452–459. [Google Scholar] [CrossRef]

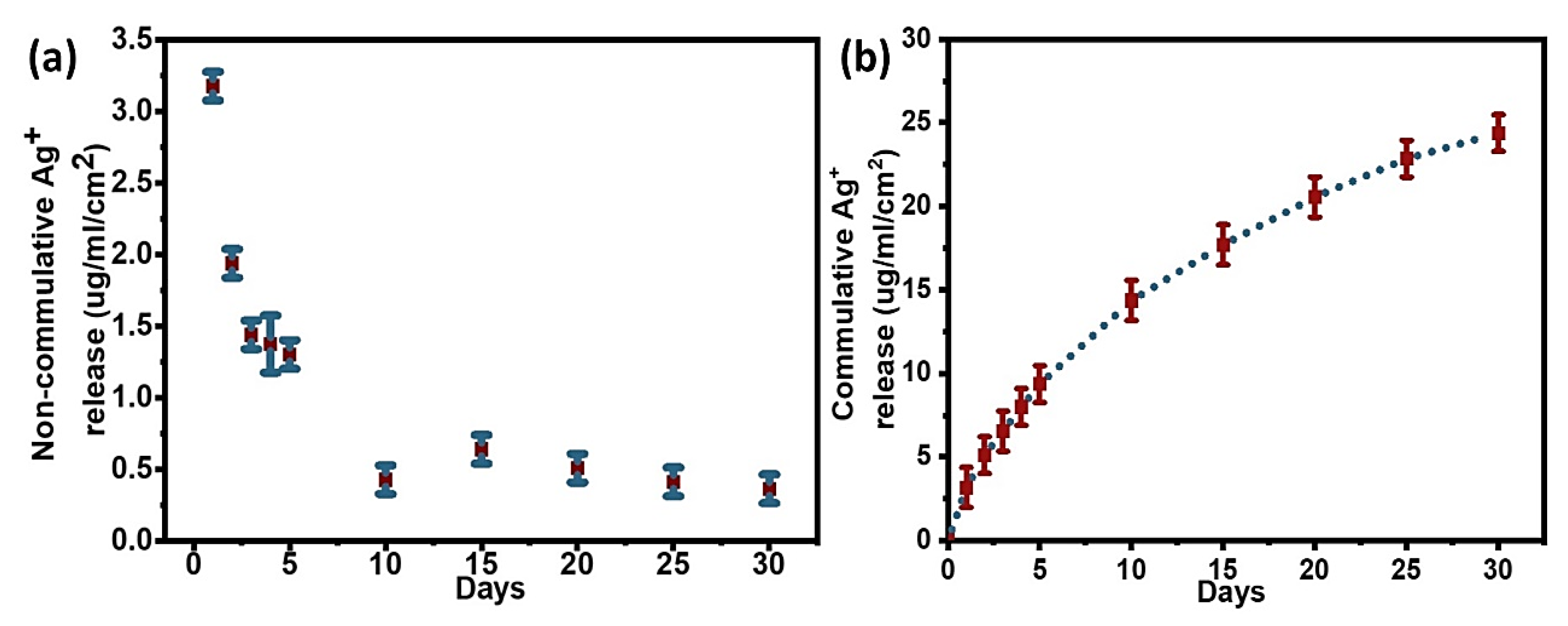
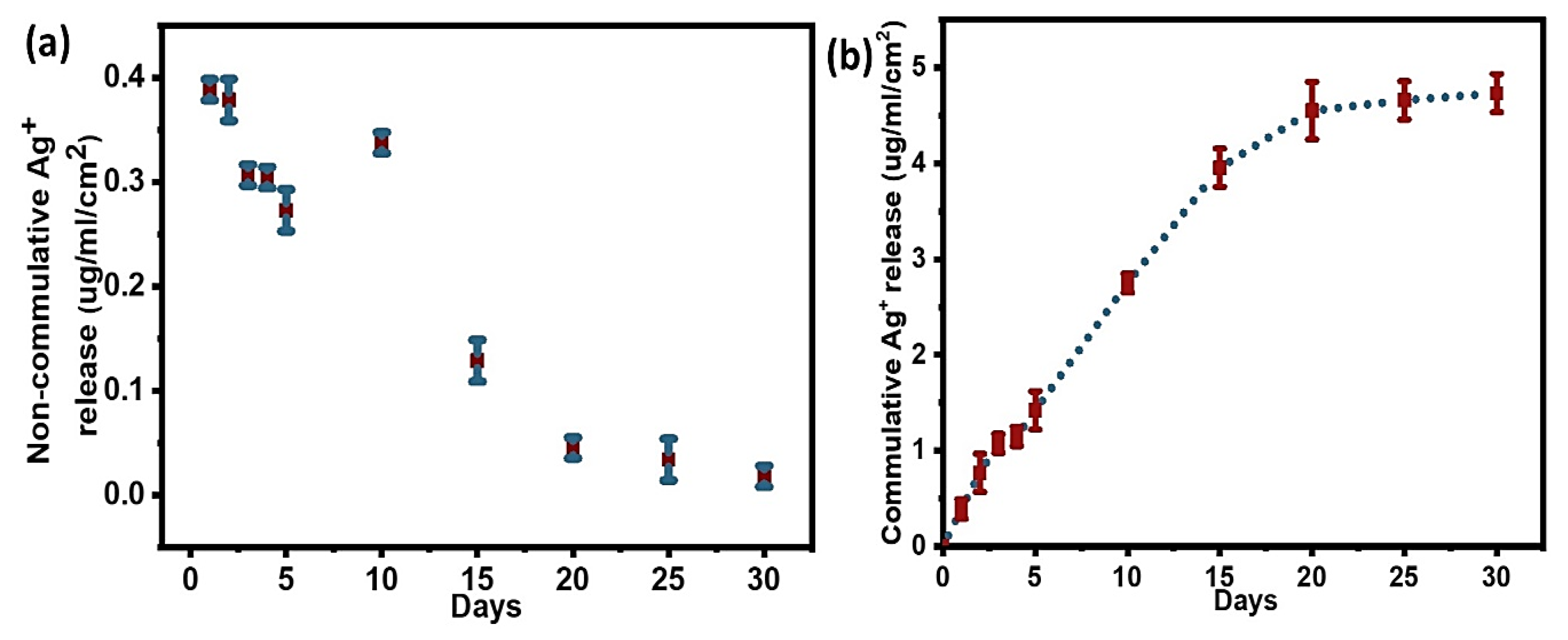
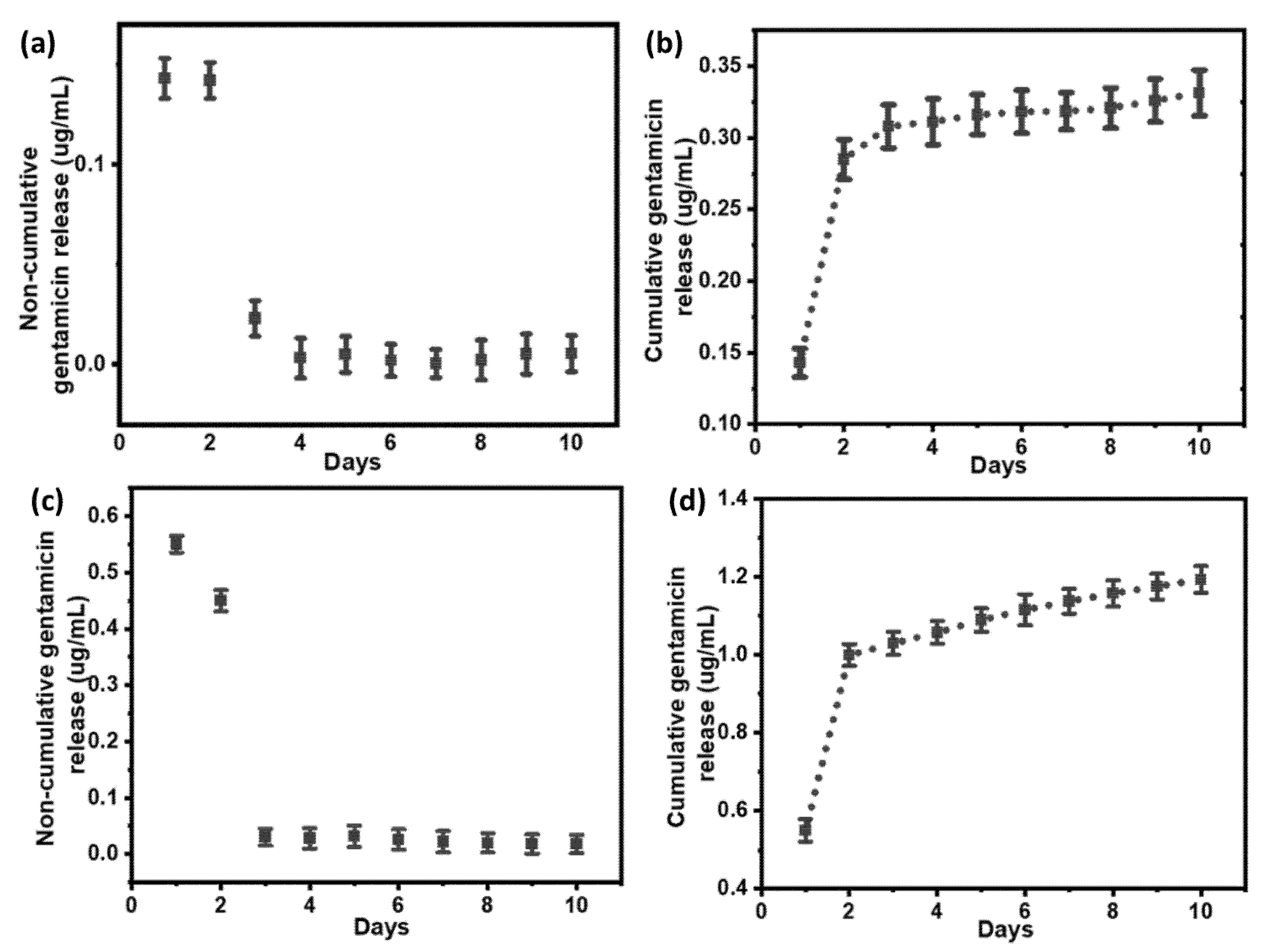
| Samples | Total Ag (µg/cm2) | Ag Released in 30 Days (%) |
|---|---|---|
| Ag@PDA | 10.4 ± 2.2 | 39.5 ± 1.4 |
| Ag@Gen/PDA | 10.3 ± 3.2 | 45.8 ± 1.6 |
| Ag/Gen@PDA | 26.4 ± 4.3 | 92.2 ± 0.9 |
| Samples | ZOI against S. aureus (mm) | ZOI against E. coli (mm) |
|---|---|---|
| PDA | No ZOI formed | No ZOI formed |
| Ag@PDA | 16.1 ± 1.1 | 14.6 ± 1.5 |
| Gen/PDA | 15.5 ± 1.5 | 15.9 ± 0.8 |
| Ag@Gen/PDA | 17.6 ± 0.8 | 16.8 ± 1.3 |
| Ag/Gen@PDA | 19.1 ± 1.0 | 17.8 ± 0.9 |
| Samples | OD600 against S. aureus | OD600 against E. coli |
|---|---|---|
| Glass | 0.393 ± 0.002 | 0.767 ± 0.003 |
| PDA | 0.314 ± 0.017 | 0.754 ± 0.009 |
| Ag@PDA | 0.054 ± 0.007 | 0.067 ± 0.002 |
| Gen/PDA | 0.072 ± 0.021 | 0.072 ± 0.008 |
| Ag/Gen@PDA | 0.003 ± 0.001 | 0.003 ± 0.001 |
| Ag@Gen/PDA | 0.021 ± 0.002 | 0.014 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batul, R.; Bhave, M.; Yu, A. Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings. Molecules 2023, 28, 4258. https://doi.org/10.3390/molecules28114258
Batul R, Bhave M, Yu A. Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings. Molecules. 2023; 28(11):4258. https://doi.org/10.3390/molecules28114258
Chicago/Turabian StyleBatul, Rahila, Mrinal Bhave, and Aimin Yu. 2023. "Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings" Molecules 28, no. 11: 4258. https://doi.org/10.3390/molecules28114258
APA StyleBatul, R., Bhave, M., & Yu, A. (2023). Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings. Molecules, 28(11), 4258. https://doi.org/10.3390/molecules28114258







