The Solid-State Synthesis of BiOIO3 Nanoplates with Boosted Photocatalytic Degradation Ability for Organic Contaminants
Abstract
1. Introduction
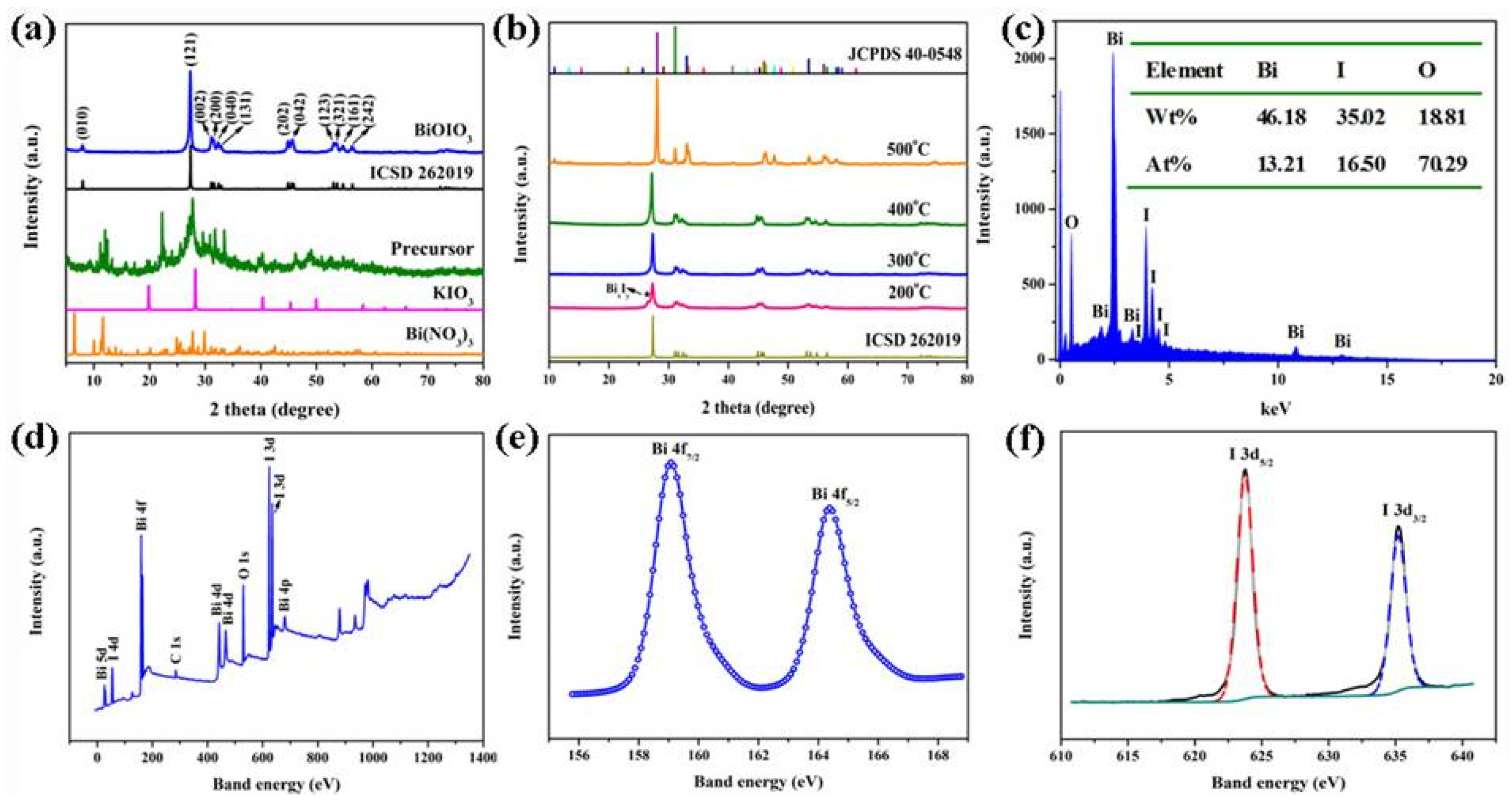
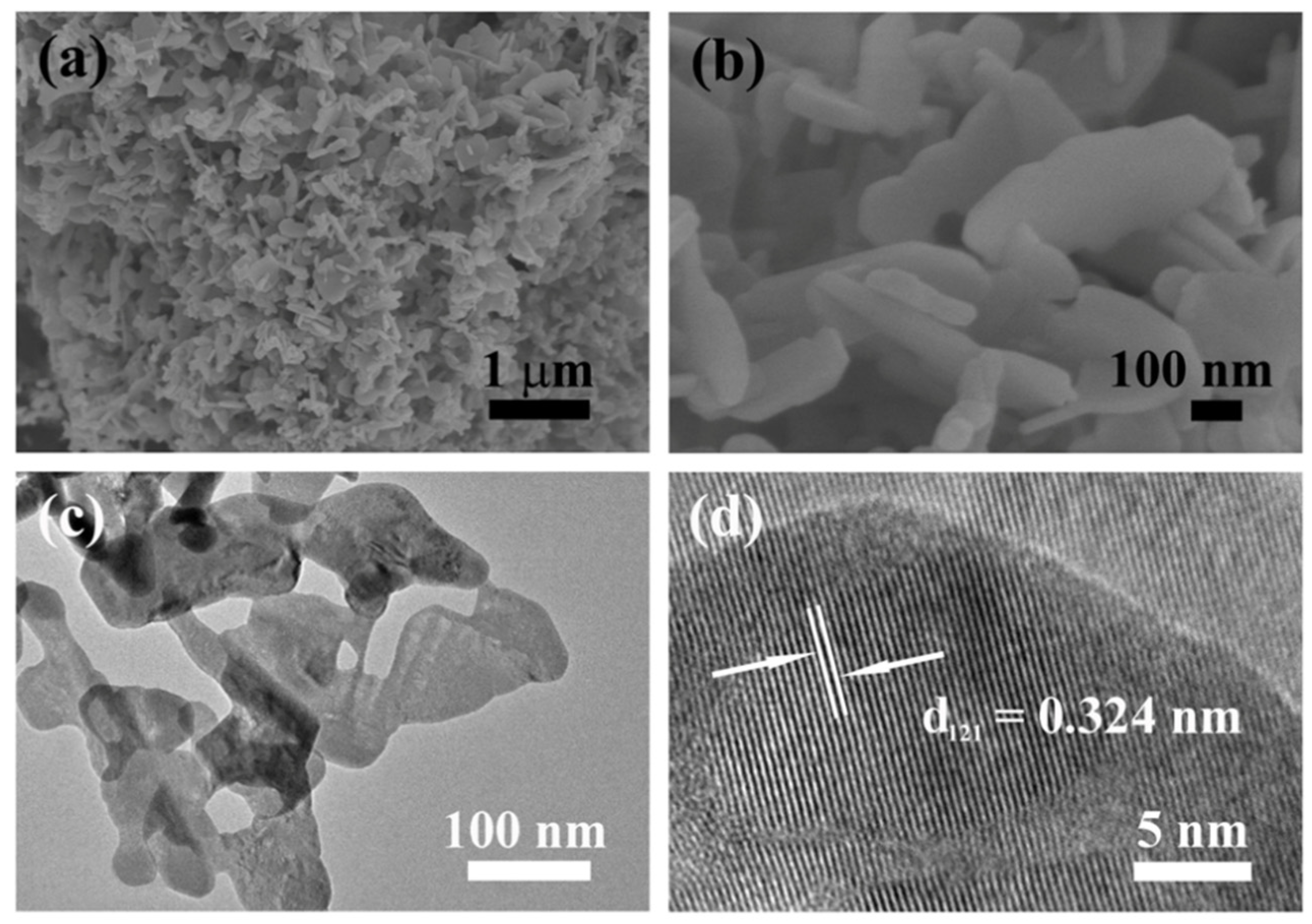
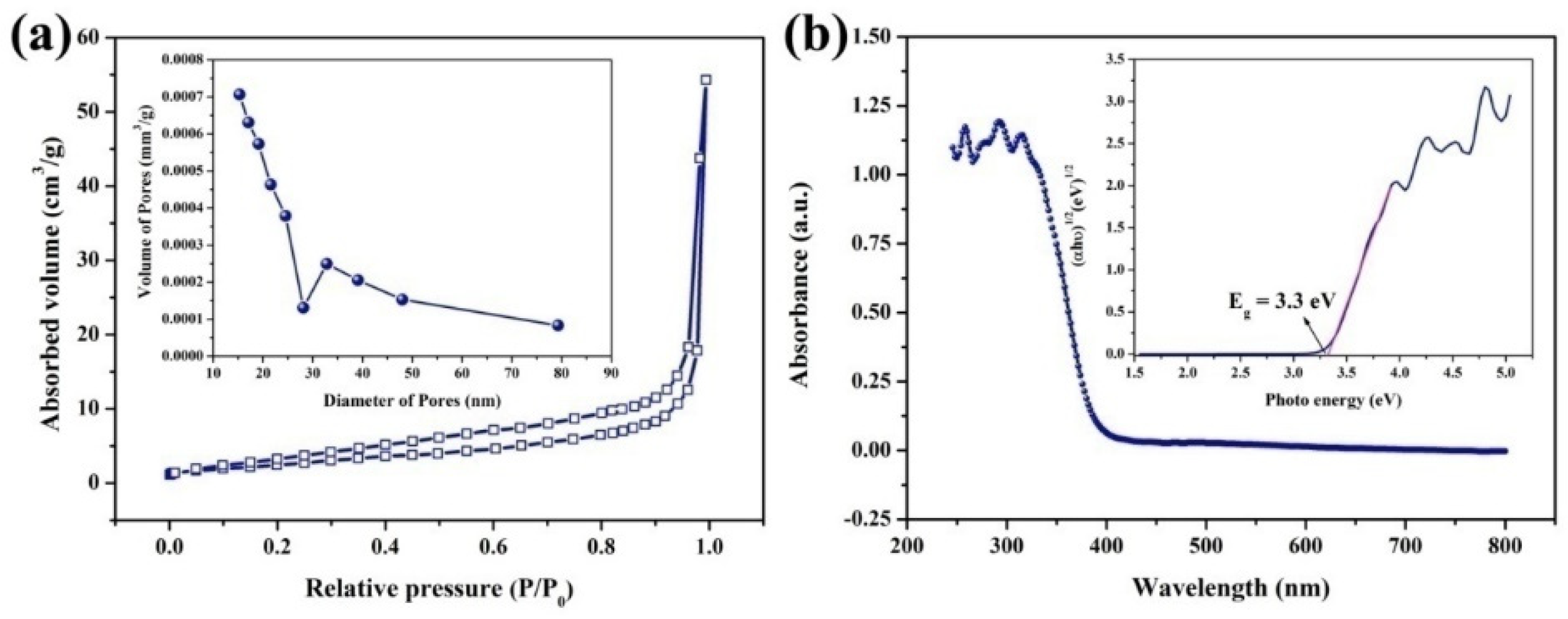
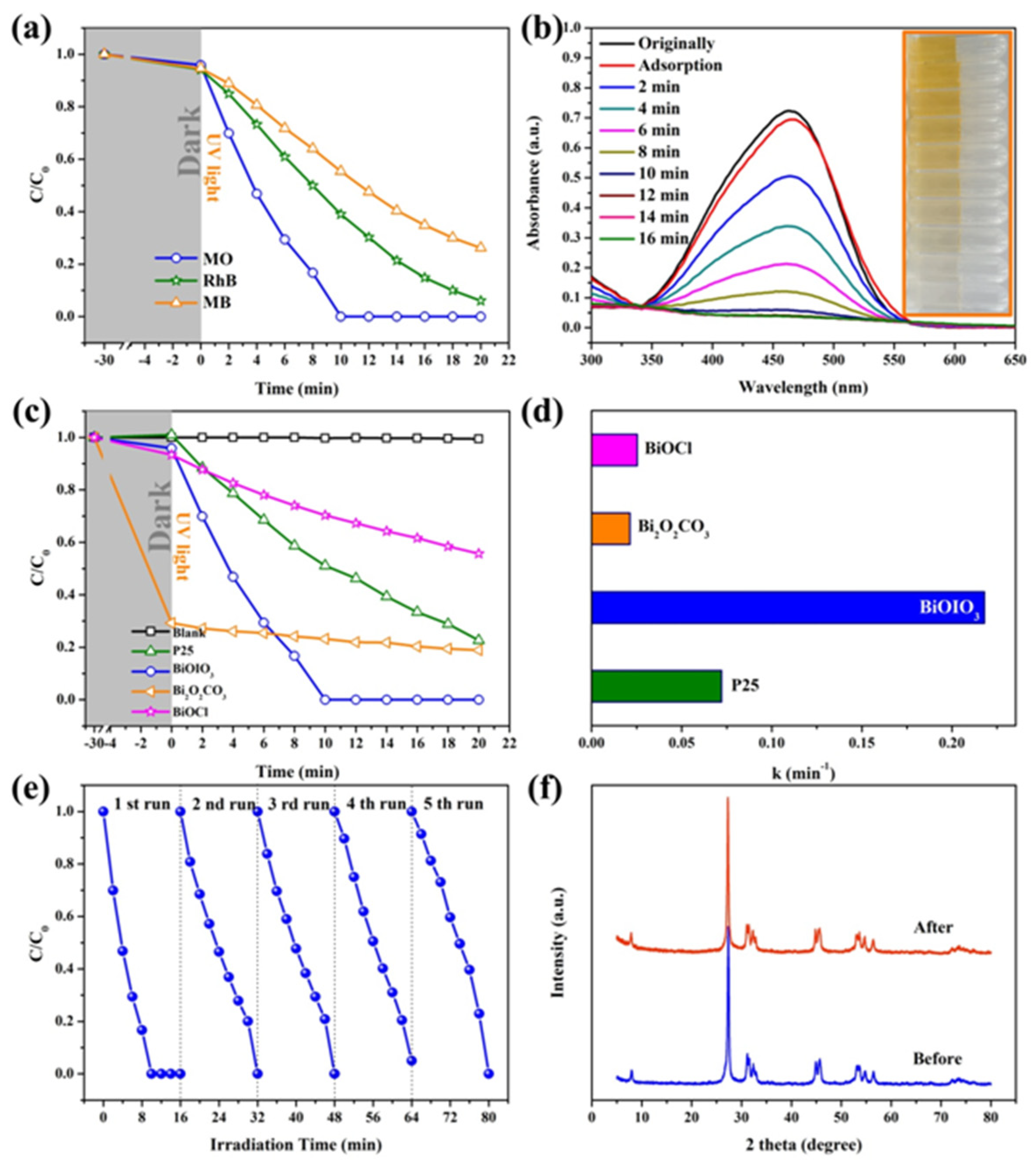
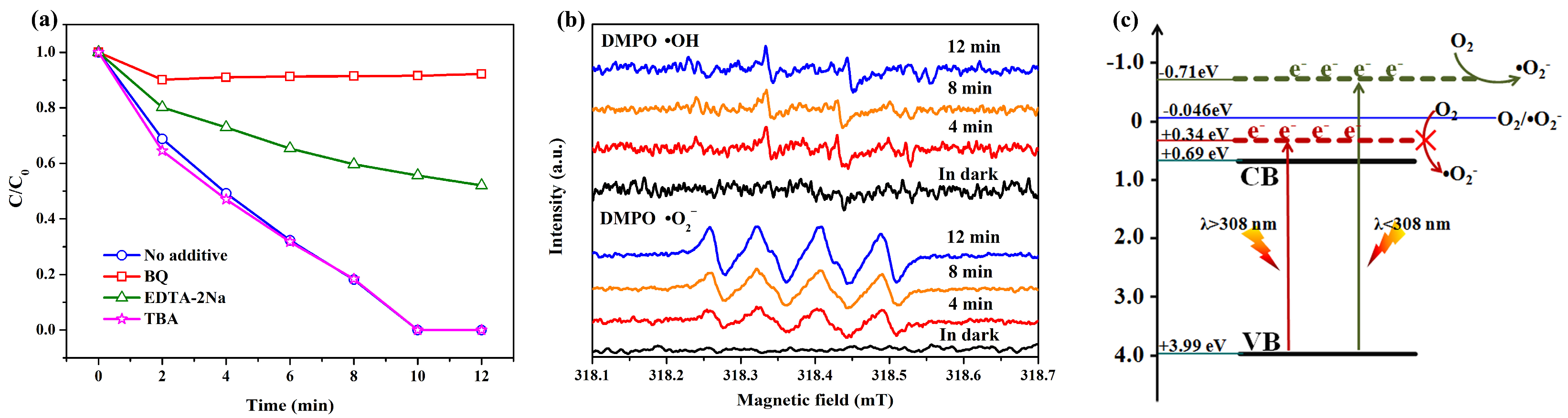
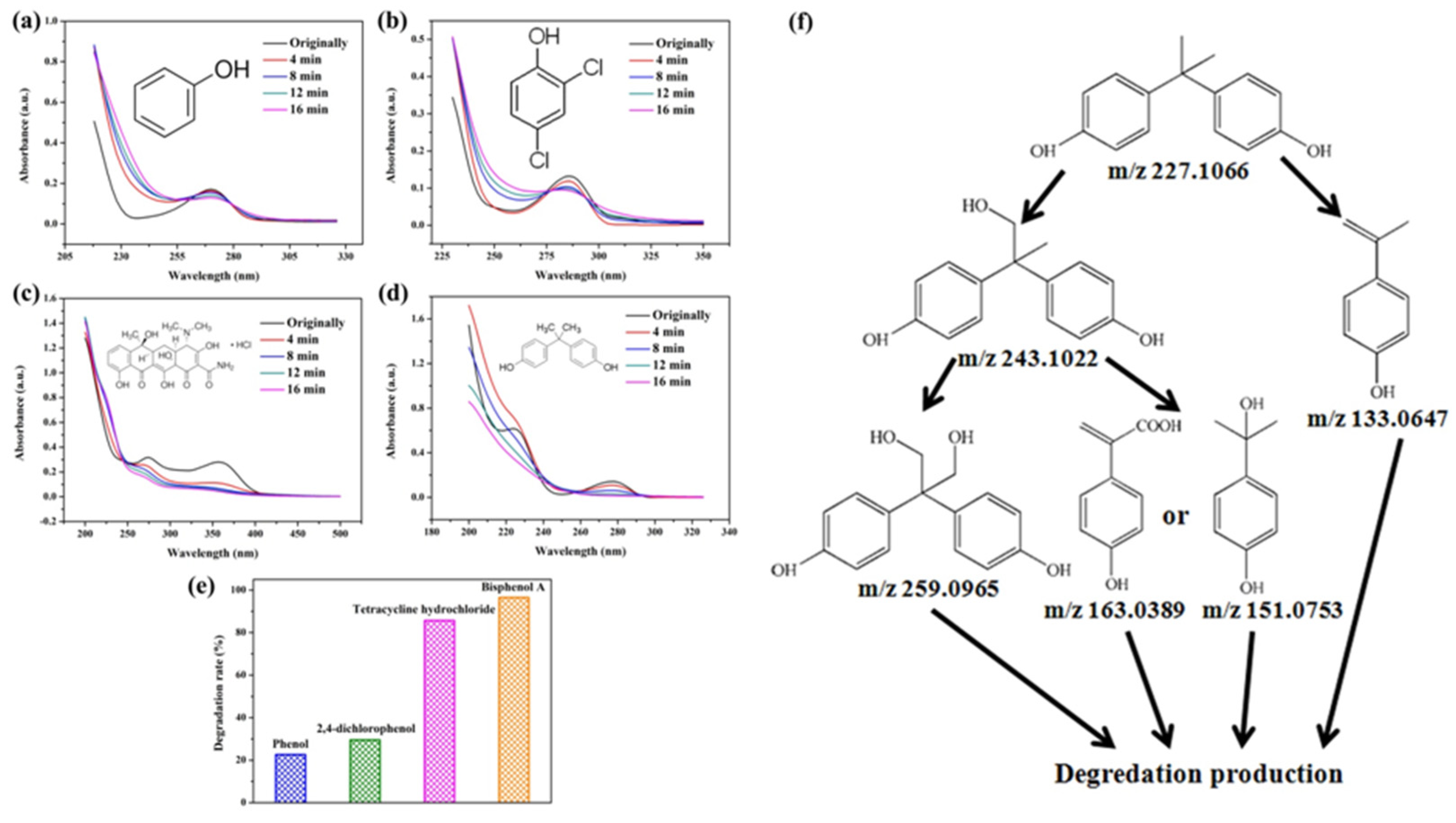
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of BiOIO3
3.2. Characterization
3.3. The Degradation Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Z.Q.; Liang, J.L.; Zhang, W.; Zhou, M.; Zhu, X.L.; Liu, Z.Y.; Li, Y.; Guan, Z.Q.; Lee, C.S.; Wong, P.K.; et al. Modified-pollen confined hybrid system: A promising union for visible-light-driven photocatalytic antibiotic degradation. Appl. Catal. B Environ. 2023, 330, 122621. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Christopher, W.K.; Chow, C.; Chris, S.C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Yang, L.L.; Pamela, C.A.; Li, Y.N.; Zhang, X.H.; El-Din, M.G. Enhanced photocatalytic degradation of organic contaminants in water by highly tunable surface microlenses. Chem. Eng. J. 2023, 463, 142345. [Google Scholar] [CrossRef]
- Swaraj, R.P.; Marta, P.G.; Dariusz, Ł.; Dmytro, L.; Juan, C.C. Bimetallic TiO2 nanoparticles for lignin-based model compounds valorization by integrating an optocatalytic flow-microreactor. Molecules 2022, 27, 8731. [Google Scholar]
- Karolina, S.; Monika, S.M.; Kinga, P.; Magdalena, G.; Grzegorz, D.S. Photoelectrochemical properties of annealed anodic TiO2 layers covered with CuOx. Molecules 2022, 27, 4789. [Google Scholar]
- Gao, P.; Yang, Y.N.; Yin, Z.; Kang, F.X.; Fan, W.E.; Sheng, J.Y.; Feng, L.; Liu, Y.Z.; Du, Z.W.; Zhang, L.Q. A critical review on bismuth oxyhalide based photocatalysis for pharmaceutical active compounds degradation: Modifications, reactive sites, and challenges. J. Hazard. Mater. 2021, 412, 125186–125216. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Jiang, X.J.; Li, J.R.; Wang, F.J.; Li, K.; Cheng, X. Self-assembly of a 3D hollow BiOBr@Bi-MOF heterostructure with enhanced photocatalytic degradation of dyes. ACS Appl. Mater. Interfaces 2021, 13, 56171–56180. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, T.D.; Cui, Y.; Wang, Z.Y.; Dong, R.Y.; Wu, Y.H.; Du, C.W.; Chen, R.Y.; Yu, C.F.; Feng, J.L.; et al. A bifunctional BiOBr/ZIF-8/ZnO photocatalyst with rich oxygen vacancy for enhanced wastewater treatment and H2O2 generation. Molecules 2023, 28, 2422. [Google Scholar] [CrossRef]
- Jiang, D.Q.; Song, H.M.; Wen, T.; Jiang, Z.M.; Li, C.; Liu, K.; Yang, W.G.; Huang, H.W.; Wang, Y.G. Pressure-driven two-step second-harmonic-generation switching in BiOIO3. Angew. Chem. Int. Edit. 2022, 61, 16656–16663. [Google Scholar]
- Lai, J.H.; Jiang, X.Y.; Zhao, M.; Cui, S.H.; Yang, J.; Li, Y.F. Thickness-dependent layered BiOIO3 modified with carbon quantum dots for photodegradation of bisphenol A: Mechanism, pathways and DFT calculation. Appl. Catal. B Environ. 2021, 298, 120622–120635. [Google Scholar] [CrossRef]
- Wang, W.J.; Huang, B.B.; Ma, X.C.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Whangbo, M.H. Efficient separation of photogenerated electron-hole pairs by the combination of a heterolayered structure and internal polar field in pyroelectric BiOIO3 nanoplates. Chem. Eur. J. 2013, 19, 14777–14780. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.W.; Zeng, C.; Du, X.; Zhang, Y.H. Achieving enhanced UV and visible light photocatalytic activity for ternary Ag/AgBr/BiOIO3: Decomposition for diverse industrial contaminants with distinct mechanisms and complete mineralization ability. ACS Sustain. Chem. Eng. 2017, 5, 7777–7791. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Zhang, Q.; Xiao, X.; Sun, F.Q.; Zuo, X.X.; Nan, J.M. Dual-iodine-doped BiOIO3: Bulk and surface co-modification for enhanced visible-light photocatalytic removal of bisphenol AF. Chem. Eng. J. 2021, 404, 126543–126556. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, B.; Wu, S.K.; Liu, Y.S.; Yoriya, S.; Hu, C.; Zhang, H.; Wu, J.; Ling, Y.; He, P.; et al. Ion doping engineering of nickel-doped BiOIO3 nanosheets for gaining oxygen vacancy to enhance light absorption. Fuel Process. Technol. 2023, 241, 170626. [Google Scholar] [CrossRef]
- Huang, H.; Ou, H.; Feng, J.; Du, X.; Zhang, Y. Achieving highly promoted visible-light sensitive photocatalyticactivity on BiOIO3 via facile iodine doping. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 158–165. [Google Scholar] [CrossRef]
- Xiong, T.; Dong, F.; Zhou, Y.; Fu, M.; Ho, W.K. New insights into how RGO influences the photocatalytic performance of BiOIO3/RGO nanocomposites under visible and UV irradiation. J. Colloid Interface Sci. 2015, 447, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Cheng, H.F.; Huang, B.B.; Liu, X.L.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Hydrothermal synthesis of C3N4/BiOIO3 heterostructures with enhanced photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, A.B.; Saha, N.; Srivastava, D.N.; Paul, P.; Adhikary, B. Enhanced photocatalytic performance of morphologically tuned Bi2S3 NPs in the degradation of organic pollutants under visible light irradiation. J. Colloid Interface Sci. 2016, 483, 49–59. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Kim, D.H.; Franco, C.; Terzopoulou, A.; Veciana, A.; Josep, P.L.; Chen, X.Z.; Nelson, B.J.; Pané, S. Enhanced piezocatalytic performance of BaTiO3 nanosheets with highly exposed {001} facets. Adv. Funct. Mater. 2022, 32, 2202180. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Zeng, G.M. An in depth mechanism insight of the degradation of multiple refractory pollutants via a novel SrTiO3/BiOI heterojunction photocatalysts. J. Catal. 2017, 356, 283–299. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.G.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Ohko, Y.; Ando, I.; Niwa, C.; Tatsuma, T.; Yamamura, T.; Nakashima, T.; Kubota, Y.; Fujishima, A. Degradation of bisphenol A in water by TiO2 photocatalyst. Environ. Sci. Technol. 2001, 35, 2365–2368. [Google Scholar] [CrossRef]
- Pan, M.L.; Zhang, H.J.; Gao, G.D.; Liu, L.; Chen, W. Facet-dependent catalytic activity of nanosheet-assembled bismuth oxyiodide microspheres in degradation of bisphenol A. Environ. Sci. Technol. 2015, 49, 6240–6248. [Google Scholar] [CrossRef]
- Tan, X.Q.; Wan, Y.F.; Huang, Y.J.; He, C.; Zhang, Z.L.; He, Z.Y.; Hu, L.L.; Zeng, J.W.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Qi, X.M.; Gu, M.L.; Zhu, X.Y.; Wu, J.; Long, H.M.; He, K.; Wu, Q. Fabrication of BiOIO3 nanosheets with remarkable photocatalytic oxidation removal for gaseous elemental mercury. Chem. Eng. J. 2016, 285, 11–19. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and characterization of Co-ZnO and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Wang, R.H.; Wang, Y.N.; Zhang, N.N.; Lin, S.; He, Y.J.; Yan, Y.J.; Hu, K.; Sun, H.J.; Liu, X.F. Synergetic piezo-photocatalytic effect in SbSI for highly efficient degradation of methyl orange. Ceram. Int. 2022, 48, 31818–31826. [Google Scholar] [CrossRef]
- Yang, J.; Du, J.; Li, X.; Liu, Y.; Jiang, C.; Qi, W.; Zhang, K.; Gong, C.; Li, R.; Luo, M.; et al. Highly hydrophilic TiO2 nanotubes network by alkaline hydrothermal method for photocatalysis degradation of methyl orange. Nanomaterials 2019, 9, 526. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.F.; Li, J.L.; Yang, J.; Ren, S.; Liu, W.C.; Liu, Q.C. Photocatalytic degradation of methyl orange by Ca doped β-In2S3 with varying Ca concentration. Res. Chem. Intermediatr. 2022, 48, 1813–1829. [Google Scholar] [CrossRef]
- Sun, H.; Lee, S.Y.; Park, S.J. Bimetallic CuPd alloy nanoparticles decorated ZnO nanosheets with enhanced photocatalytic degradation of methyl orange dye. J. Colloid Interface Sci. 2022, 629, 87–96. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Chen, J.; Huang, Y.; Zhang, H.; Qiu, H. Enhanced photocatalytic degradation of methyl orange by porous graphene/ZnO nanocomposite. Environ. Pollut. 2019, 249, 801–811. [Google Scholar] [CrossRef] [PubMed]
| Peak NO. | Retention Time (min) | Detected Ions m/z | Molecular Structure |
|---|---|---|---|
| 1 | 1.28 | 163.0389 | 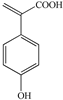 |
| 2 | 1.92 | 259.0965 |  |
| 3 | 1.99 | 133.0647 | 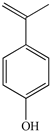 |
| 4 | 1.99 | 151.0753 |  |
| 5 | 2.90 | 243.1022 |  |
| 6 | 4.00 | 227.1066 | 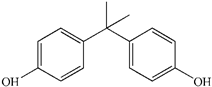 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, J.; Zhang, X.; Lu, E.; Cao, Y. The Solid-State Synthesis of BiOIO3 Nanoplates with Boosted Photocatalytic Degradation Ability for Organic Contaminants. Molecules 2023, 28, 3681. https://doi.org/10.3390/molecules28093681
Li J, Xie J, Zhang X, Lu E, Cao Y. The Solid-State Synthesis of BiOIO3 Nanoplates with Boosted Photocatalytic Degradation Ability for Organic Contaminants. Molecules. 2023; 28(9):3681. https://doi.org/10.3390/molecules28093681
Chicago/Turabian StyleLi, Jia, Jing Xie, Xiaojing Zhang, Enhui Lu, and Yali Cao. 2023. "The Solid-State Synthesis of BiOIO3 Nanoplates with Boosted Photocatalytic Degradation Ability for Organic Contaminants" Molecules 28, no. 9: 3681. https://doi.org/10.3390/molecules28093681
APA StyleLi, J., Xie, J., Zhang, X., Lu, E., & Cao, Y. (2023). The Solid-State Synthesis of BiOIO3 Nanoplates with Boosted Photocatalytic Degradation Ability for Organic Contaminants. Molecules, 28(9), 3681. https://doi.org/10.3390/molecules28093681





