Variation in the Main Health-Promoting Compounds and Antioxidant Capacity of Three Leafy Vegetables in Southwest China
Abstract
1. Introduction
2. Results
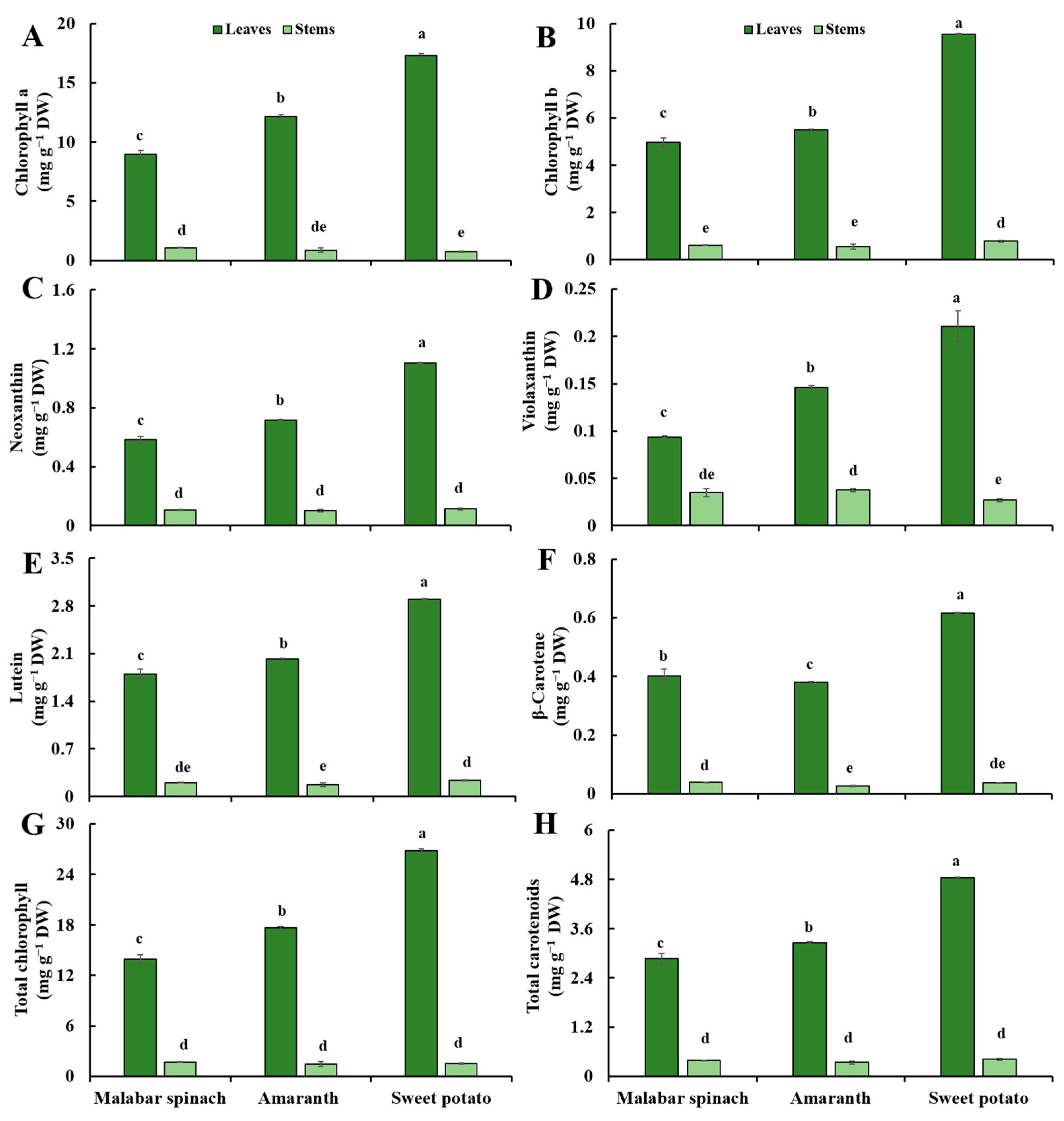
2.1. Chlorophyll
2.2. Carotenoids
2.3. Ascorbic Acid
2.4. Total Flavonoids
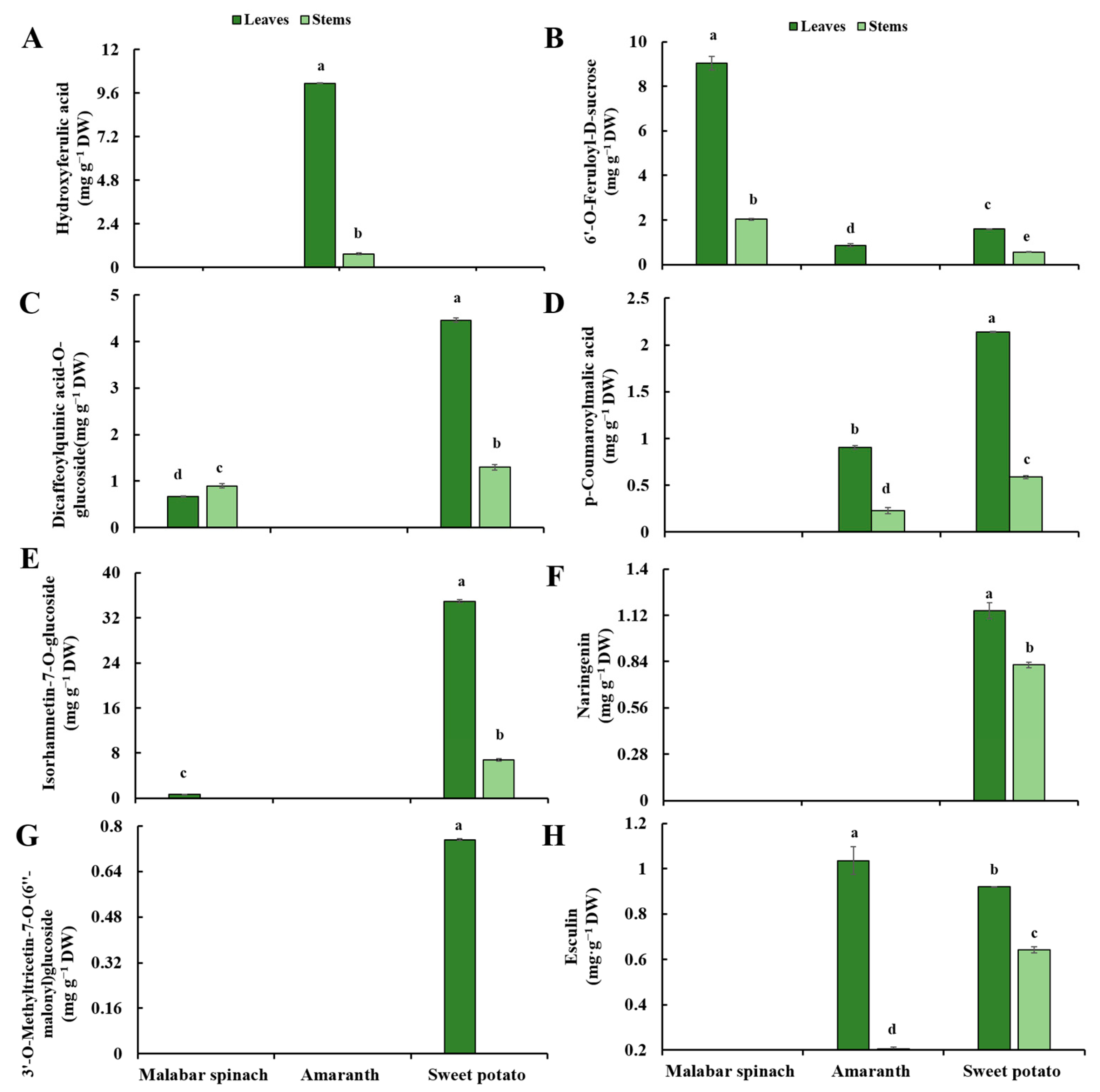
2.5. Phenolic Compounds
2.5.1. Phenolic Acids
2.5.2. Flavonoids
2.5.3. Coumarins
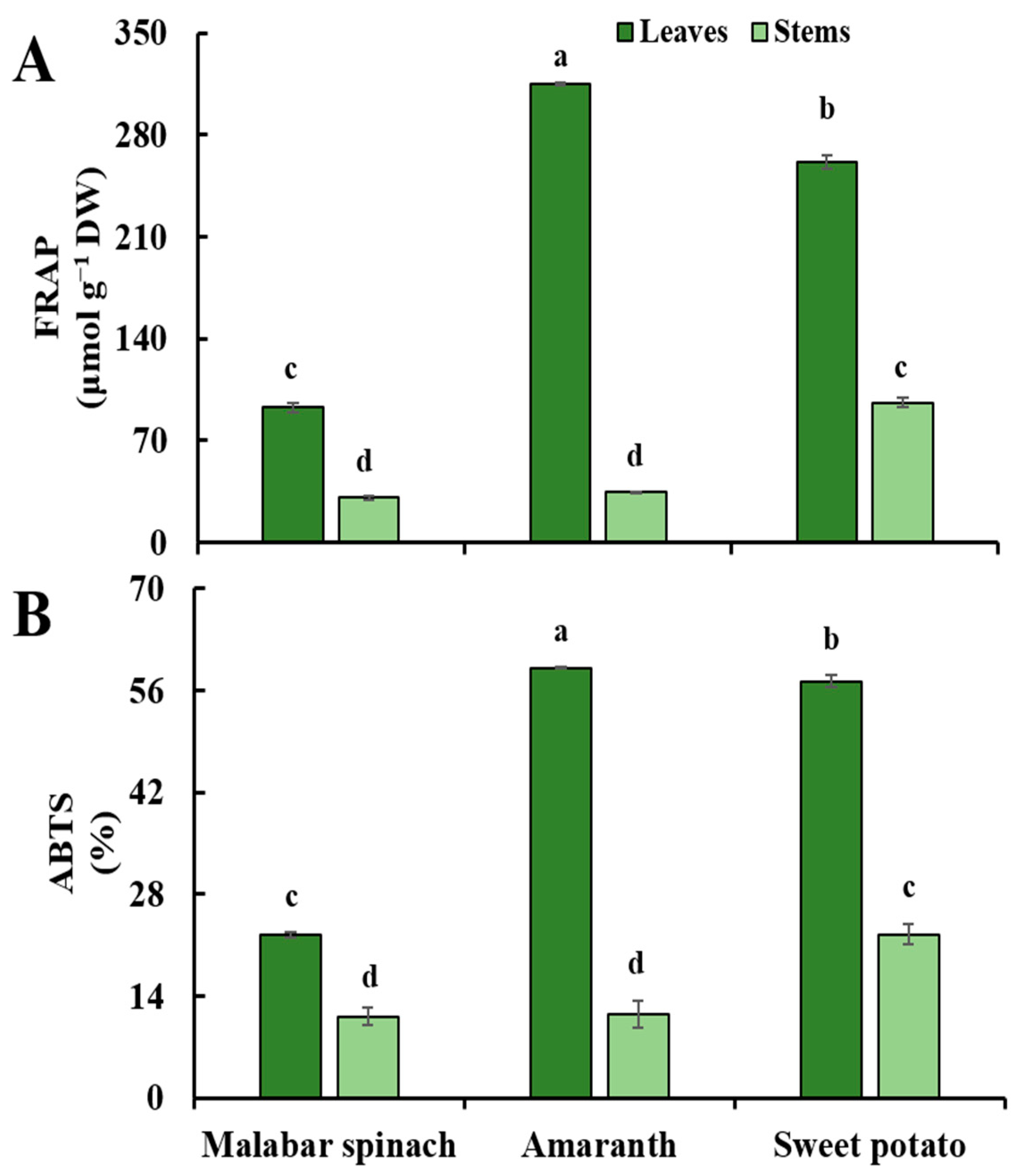
2.6. Antioxidant Capacity
2.7. Principal Components Analysis (PCA)
2.8. Correlation Analysis
2.9. Variance Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals, Regents, and Instrumentation
4.2. Plant Materials
4.3. Determination of Chlorophyll and Carotenoids Content
4.4. Determination of Ascorbic Acid
4.5. Determination of Total Flavonoids Content
4.6. Determination of Individual Phenolic Compounds
4.7. Ferric Reducing Antioxidant Power (FRAP)
4.8. 2,2-Azinobis (3-Ethyl-benzothiazoline-6-sulfonic Acid) (ABTS) Assay
4.9. Statistical Analysis
5. Limitations and Future Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Li, X.; Song, J. Vegetable genetic resources in China. Hortic. Plant J. 2018, 4, 83–88. [Google Scholar] [CrossRef]
- Sutor-Świeży, K.; Antonik, M.; Dziedzic, E.; Bieniasz, M.; Mielczarek, P.; Popenda, Ł.; Pasternak, K.; Tyszka-Czochara, M.; Wybraniec, S. Structural studies on diverse betacyanin classes in matured pigment-rich fruits of Basella alba L. and Basella alba L. var. ‘Rubra’ (Malabar Spinach). Int. J. Mol. Sci. 2022, 23, 11243. [Google Scholar] [CrossRef]
- Chaurasiya, A.; Pal, R.K.; Verma, P.K.; Katiyar, A.; Razauddin; Kumar, N. An updated review on Malabar spinach (Basella alba and Basella rubra) and their importance. J. Pharmacogn. Phytochem. 2021, 10, 1201–1207. [Google Scholar] [CrossRef]
- Seran, T.H.; Karunarathna, B. Biological and economic efficiency of radish (Raphanus Sativus L.) intercropped with vegetable Amaranthus (Amaranthus Tricolor L.). Open Hortic. J. 2009, 2, 17–21. [Google Scholar] [CrossRef]
- Guo, S.H.; Hu, N.; Li, Q.S.; Yang, P.; Wang, L.L.; Xu, Z.M.; Chen, H.J.; He, B.Y.; Zeng, E.Y. Response of edible amaranth cultivar to salt stress led to Cd mobilization in rhizosphere soil: A metabolomic analysis. Environ. Pollut. 2018, 241, 422–431. [Google Scholar] [CrossRef]
- Cui, L.; Liu, C.Q.; Li, D.J.; Song, J.F. Effect of processing on taste quality and health-relevant functionality of sweet potato tips. Agric. Sci. China 2011, 10, 456–462. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of sweet potato (Ipomoea batatas L.) leaves flavonoid. Food Bioprod. Process. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Yang, Q.H.; Zhang, Z.F.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X. Pigments in Fruits and Vegetables: Genomics and Dietetics; Springer: New York, NY, USA, 2015; pp. 11–12. [Google Scholar]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid. Med. Cell. Longev. 2014, 2, 99. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-Inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, D.; Sileoni, V.; Marconi, O.; Francesco, G.D.; Lee, E.G.; Perretti, G. Specialty rice malt optimization and improvement of rice malt beer aspect and aroma. LWT-Food Sci. Technol. 2019, 99, 299–305. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef]
- Diana, S.; Almeida, L.M.; Dinis, T.C. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar]
- Sun, B.; Tian, Y.X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Mareczek, A.; Starzyńska, A.; Rożek, S. Antioxidant ability of broccoli flower buds during short-term storage. Food Chem. 2001, 72, 219–222. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin, vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Liu, S.C.; Lin, J.T.; Hu, C.C.; Shen, B.Y.; Chen, T.Y.; Chang, Y.L.; Shih, C.H.; Yang, D.J. Phenolic compositions and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvested at various times. Food Chem. 2017, 215, 284–291. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Liu, H.Y.; Guo, H.; He, X.Q.; Liu, Y.; Wu, D.T.; Mai, Y.H.; Li, H.B.; Zou, L.; et al. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Gil-Izquierdo, Á.; Medina, S.; Ferreres, F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MSn. Food Res. Int. 2017, 100, 494–500. [Google Scholar] [CrossRef]
- Wang, Y.T.; Di, H.; Cheng, W.; Ren, G.; Luo, S.; Ma, J.; Ma, W.; Lian, H.; Li, X.; Huang, Z.; et al. Variation in the main health-promoting compounds and antioxidant activity of different edible parts of purple flowering stalks (Brassica campestris var. purpuraria) and green flowering stalks (Brassica campestris var. campestris). Plants 2022, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Deng, Z.Y.; Liu, R.H.; Zhu, H.H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.L.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef]
- Li, J.B.; Kim, Y.J.; Zhang, D.B. Source-To-Sink transport of sugar and its role in male reproductive development. Genes 2022, 13, 1323. [Google Scholar] [CrossRef]
- Mann, J. Secondary Metabolism, 2nd ed.; Clarendon Press: Oxford, UK, 1987; pp. 1–20, 79–152. [Google Scholar]
- Luckner, M. Secondary Metabolism in Micro Organisms, Plants and Animals; Springer: Berlin, Germany, 1984; pp. 15–61. [Google Scholar]
- Picchi, V.; Migliori, C.A.; Lo Scalzo, R.; Campanelli, G.; Ferrari, V.; Di Cesare, L.F. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chem. 2012, 130, 501–509. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xu, T.; Tian, Y.; Zhong, Y.H.; Ali, F.A.Z.; Yang, X.; Lu, B.Y. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine 2021, 93, 153771. [Google Scholar] [CrossRef] [PubMed]
- House, N.C.; Puthenparampil, D.; Malayil, D.; Narayanankutty, A. Variation in the polyphenol composition, antioxidant, and anticancer activity among different Amaranthus species. S. Afr. J. Bot. 2020, 135, 408–412. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, l-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yan, H.; Liu, N.; Wei, J.; Wang, Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Zhang, F.; Wang, Q. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Res. Int. 2012, 48, 359–366. [Google Scholar] [CrossRef]
- Costa, L.; Andreazza, N.L.; Correa, W.R.; Cunha, I.; Ruiz, A.; Carvalho, J.; Salvador, M.J.; Schinor, E.C.; Dias, D.A.; Salvador, M.J. Antiproliferative activity, antioxidant capacity and chemical composition of extracts from the leaves and stem of Chresta sphaerocephala. Rev. Bras. Farmacogn. 2015, 25, 369–374. [Google Scholar] [CrossRef]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int. J. Food Prop. 2012, 16, 45–60. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Sancho, G.G.; Yahia, E.M.; Gonzalez-Aguilar, G.A. Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L. cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of the major phenolic constituents in Juglans regia L. peeled kernels and pellicles, using HPLC–MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and anti-inflammatory capacity of ferulic acid released from wheat bran by solid-state fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [PubMed]
- Felipe, M.R.I.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Fabiola, E.E.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras. Farm. 2018, 28, 339–343. [Google Scholar]
- de Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef]
- El-Askary, H.; Salem, H.H.; Abdel Motaal, A. Potential mechanisms involved in the protective effect of dicaffeoylquinic acids from Artemisia annua L. Leaves against diabetes and its complications. Molecules 2022, 27, 857. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, M.J.; Park, H.J.; Chung, H.Y.; Kang, S.S. Further isolation of peroxynitrite and 1,1-diphenyl-2-picrylhy-drazyl radical scavenging isorhamnetin 7-O-glucoside from the leaves of Brassica juncea L. Arch. Pharmacal. Res. 2002, 25, 625–627. [Google Scholar] [CrossRef]
- Bahorun, T.; Soobrattee, M.A.; Luximon-Ramma, V.; Aruoma, O.I. Free radicals and antioxidants in cardiovascular health and disease. Aruoma O.I. Free radicals and antioxidants in cardiovascular health and disease. Internet J. Med. Update 2006, 1, 25–41. [Google Scholar]
- Ceriello, A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008, 31, S181–S184. [Google Scholar] [CrossRef]
- Chen, J.; Feng, T.; Wang, B.; He, R.; Xu, Y.; Gao, P.; Zhang, Z.; Zhang, L.; Fu, J.; Liu, Z.; et al. Enhancing organic selenium content and antioxidant activities of soy sauce using nano-selenium during soybean soaking. Front. Nutr. 2022, 9, 970206. [Google Scholar] [CrossRef]
- Pang, Y.J.; Jun, L.; Yu, C.Q.; Guo, Y.; Lee, L. Risk factors for cardiovascular disease in the Chinese population: Recent progress and implications. J. Glob. Health 2020, 4, 65–71. [Google Scholar] [CrossRef]
- Hu, S.S.; Kong, L.Z.; Gao, R.L.; Zhu, M.L.; Wang, W.; Wang, Y.J.; Wu, Z.S.; Chen, W.W.; Liu, M.B. Editorial Board. 2010. Biomed. Environ. Sci. 2012, 25, 251–256. [Google Scholar] [PubMed]
- Gawron-Skarbek, A.; Chrzczanowicz, J.; Kostka, J.; Nowak, D.; Drygas, W.; Jegier, A.; Kostka, T. Cardiovascular risk factors and total serum antioxidant capacity in healthy men and in men with coronary heart disease. Biomed. Res. Int. 2014, 2014, 216964. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, Z.; Li, L.; Nie, X.; Yu, C.; Shan, G.; Zhou, X.; Qin, R.; Chen, A.; et al. Prevalence of tobacco dependence and associated factors in China: Findings from nationwide China Health Literacy Survey during 2018–19. Lancet Reg. Health West. Pac. 2022, 24, 100464. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 2010, 118, 774–781. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C.J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Shen, D.; Lu, X.; Chang, Y.; Zhang, J.; Zhao, Y.; Wang, Y.; Xu, G.; Zhao, C. Determination of phenolic compounds in fresh tobacco leaves by high performance liquid chromatography-ultraviolet/mass spectrometry. Chin. J. Chromatogr. 2014, 32, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Sun, B.; Di, H.; Zhang, J.; Xia, P.; Huang, W.; Jian, Y.; Zhang, C.; Zhang, F. Effect of light on sensory quality, health-promoting phytochemicals and antioxidant capacity in post-harvest baby mustard. Food Chem. 2021, 339, 128057. [Google Scholar] [CrossRef] [PubMed]





| Classified | Phenolic | MW | Negative Ionization Model MS (m/z) | Positive Ionization Model MS (m/z) |
|---|---|---|---|---|
| Phenolic acids | Hydroxyferulic acid | 209.9 | 208.9 [M − H]− | 249 [M + K]+ |
| Phenolic acids | p-Coumaroylmalic acid | 280.2 | 279.2 [M − H]−/ 393.2 [M + CF3COO]− | 298.1 [M + NH4]+ |
| Phenolic acids | 6′-O-Feruloyl-d-sucrose | 518 | 517 [M − H]−/ 555 [M + Cl]− | 519 [M + H]+ |
| Phenolic acids | Dicaffeoylquinic acid-O-glucoside | 678.4 | 713.4 [M + Cl]−/ 737.4 [M + CH3COO]− | 701.4 [M + Na]+/ 679.4 [M + H]+ |
| Flavonoids (Flavonols) | Isorhamnetin-7-O-glucoside | 478 | 515 [M + Cl]−/ 537 [M + CH3COO]− | 538.9 [M + Na + CH3CN]+/ 499 [M + Na]+ |
| Flavonoids (Flvanones) | Naringenin | 272 | 385 [M + CF3COO]− | 294.7 [M + Na]+/ 272.7 [M + H]+ |
| Flavonoids (Flavones) | 3′-O-Methyltricetin-7-O-(6″-malnyl) glucoside | 564.1 | 563.1 [M − H]−/ 599.1 [M + Cl]− | 585 [M + Na]+ |
| Coumarins | Esculin | 339.9 | 384.9 [M + HCOO]− | 362.2 [M + Na]+/ 340.2 [M + H]+ |
| Parameter | VS/VP | VE/VP | VSE/VP |
|---|---|---|---|
| Chlorophyll a | 0.065 ** | 0.857 ** | 0.077 ** |
| Chlorophyll b | 0.102 ** | 0.812 ** | 0.086 ** |
| Neoxanthin | 0.087 ** | 0.830 ** | 0.082 ** |
| Violaxanthin | 0.107 ** | 0.743 ** | 0.142 ** |
| Lutein | 0.053 ** | 0.900 ** | 0.046 ** |
| β-Carotene | 0.057 ** | 0.890 ** | 0.052 ** |
| Total chlorophyll | 0.076 ** | 0.845 ** | 0.079 ** |
| Total carotenoids | 0.061 ** | 0.882 ** | 0.056 ** |
| Ascorbic acid | 0.472 ** | 0.355 ** | 0.161 ** |
| Total flavonoids | 0.156 ** | 0.695 ** | 0.131 ** |
| FRAP | 0.243 ** | 0.592 ** | 0.164 ** |
| ABTS | 0.249 ** | 0.607 ** | 0.142 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, W.; Zhang, C.; Huang, H.; Yang, S.; Wang, Y.; Huang, Z.; Tang, Y.; Li, X.; Lian, H.; et al. Variation in the Main Health-Promoting Compounds and Antioxidant Capacity of Three Leafy Vegetables in Southwest China. Molecules 2023, 28, 4780. https://doi.org/10.3390/molecules28124780
Zhang Y, Huang W, Zhang C, Huang H, Yang S, Wang Y, Huang Z, Tang Y, Li X, Lian H, et al. Variation in the Main Health-Promoting Compounds and Antioxidant Capacity of Three Leafy Vegetables in Southwest China. Molecules. 2023; 28(12):4780. https://doi.org/10.3390/molecules28124780
Chicago/Turabian StyleZhang, Yi, Wenli Huang, Chenlu Zhang, Huanhuan Huang, Shihan Yang, Yiqing Wang, Zhi Huang, Yi Tang, Xiaomei Li, Huashan Lian, and et al. 2023. "Variation in the Main Health-Promoting Compounds and Antioxidant Capacity of Three Leafy Vegetables in Southwest China" Molecules 28, no. 12: 4780. https://doi.org/10.3390/molecules28124780
APA StyleZhang, Y., Huang, W., Zhang, C., Huang, H., Yang, S., Wang, Y., Huang, Z., Tang, Y., Li, X., Lian, H., Li, H., Zhang, F., & Sun, B. (2023). Variation in the Main Health-Promoting Compounds and Antioxidant Capacity of Three Leafy Vegetables in Southwest China. Molecules, 28(12), 4780. https://doi.org/10.3390/molecules28124780







