Novel Thiazolylketenyl Quinazolinones as Potential Anti-MRSA Agents and Allosteric Modulator for PBP2a
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antibacterial Activity
2.3. Hemolytic Assay
2.4. Resistance Study
2.5. ADMET Study
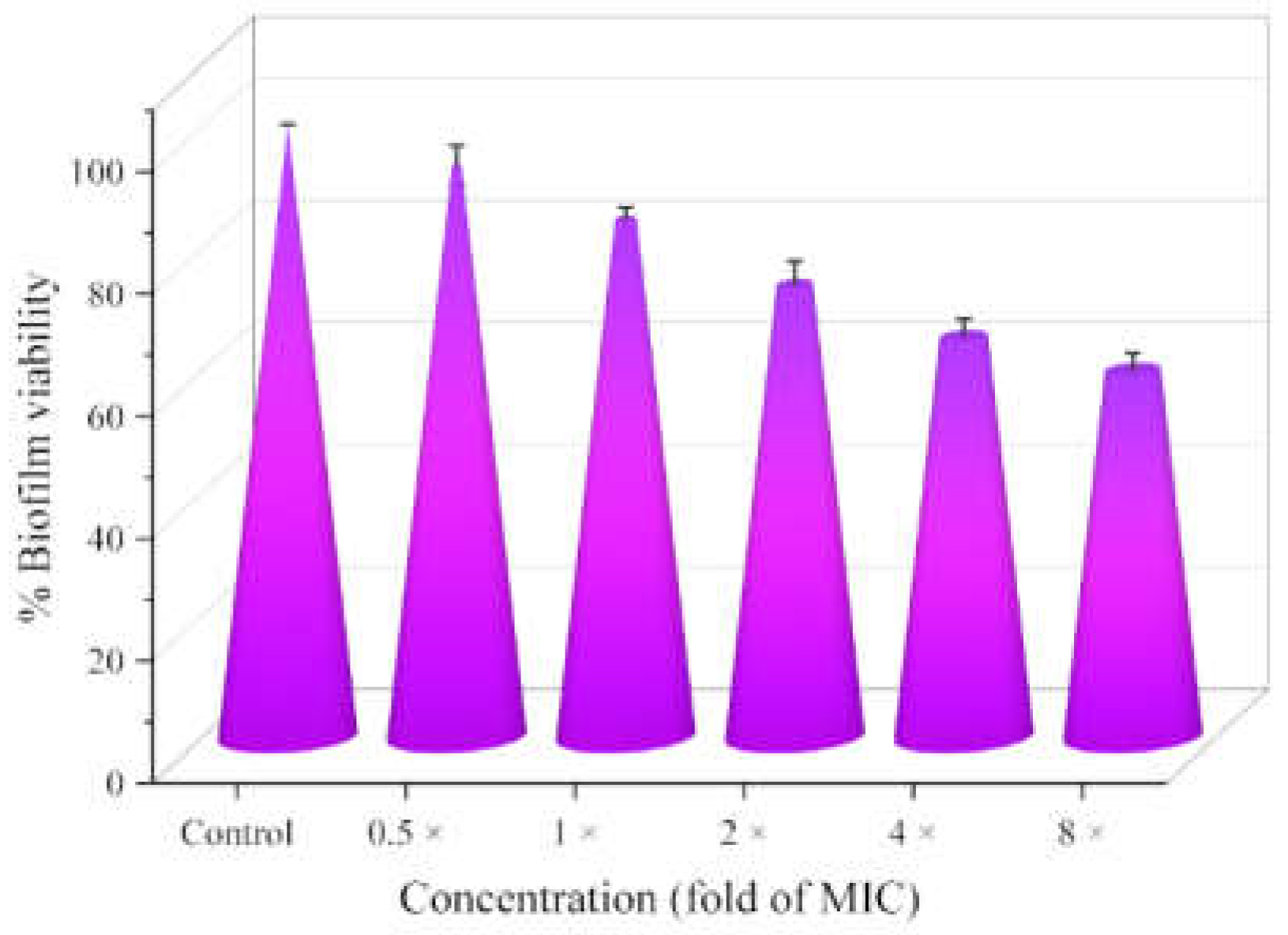
2.6. Antibiofilm Activity
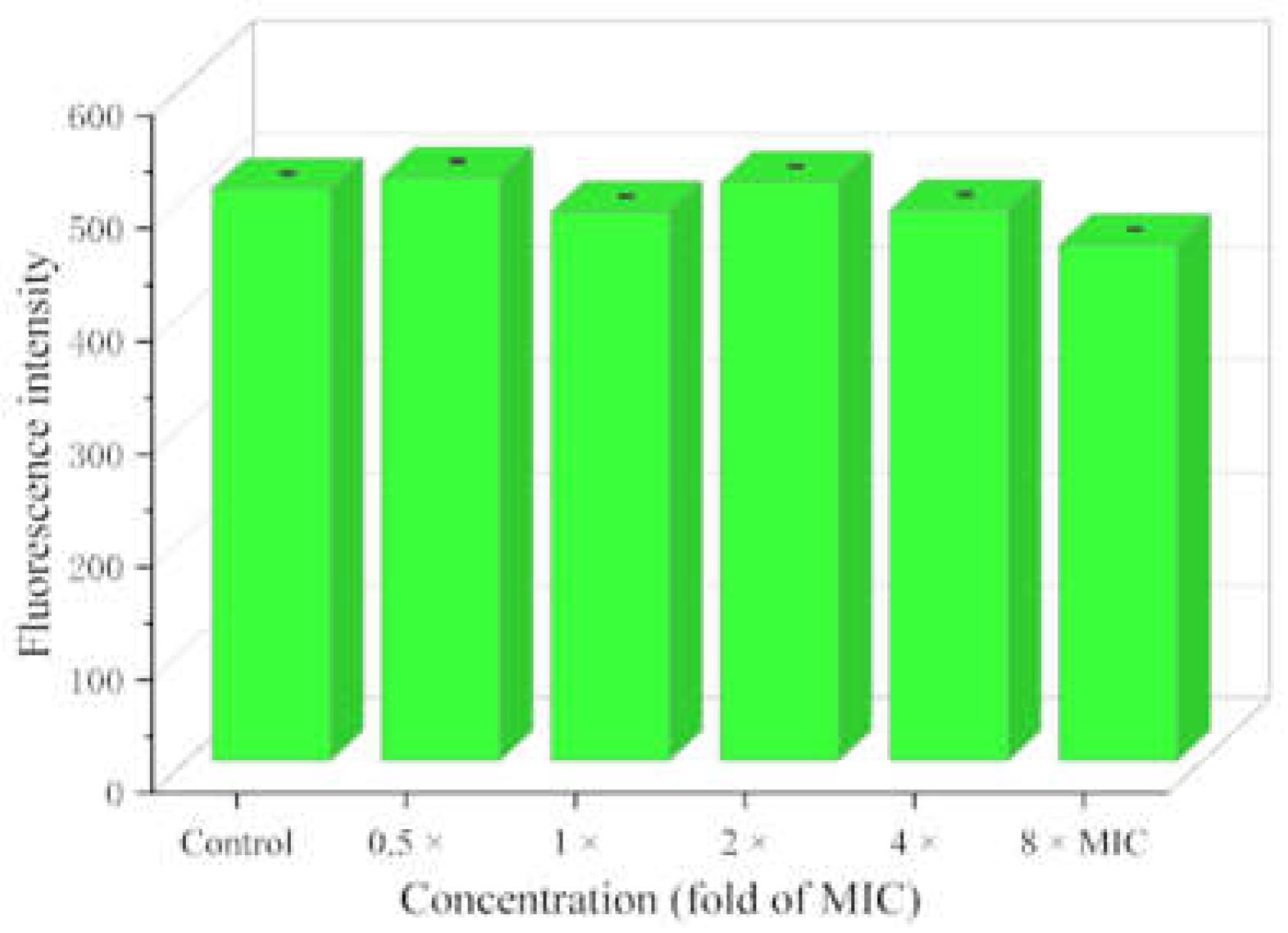
2.7. Metabolic Activity
2.8. Membrane Damage Assay
2.8.1. Membrane Depolarization Assay
2.8.2. Study of Inner Membrane Permeabilization
2.9. The Leakage of Intracellular Protein
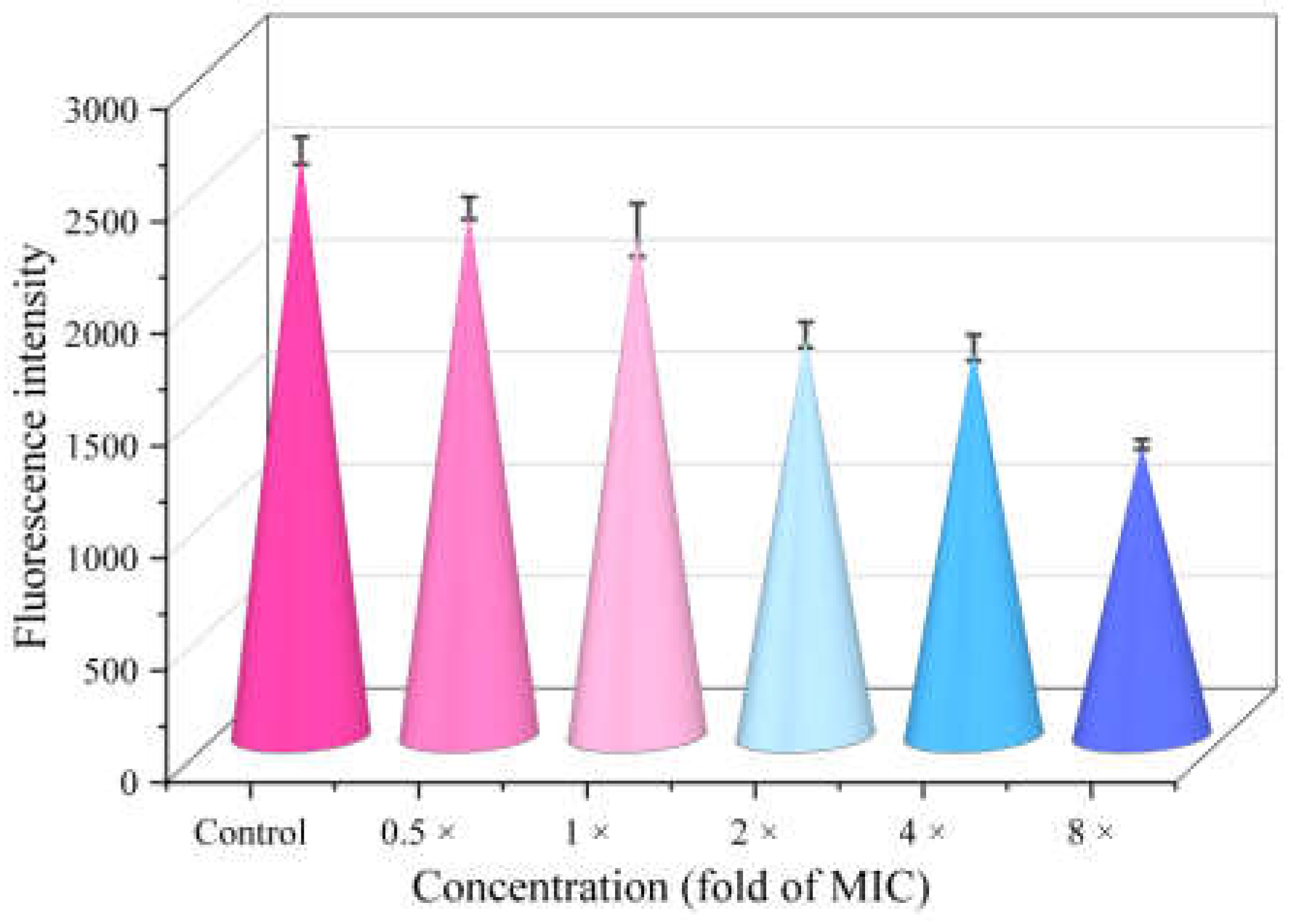
2.10. Intracellular ROS Accumulation
2.11. Interaction between Compound 4 and DNA
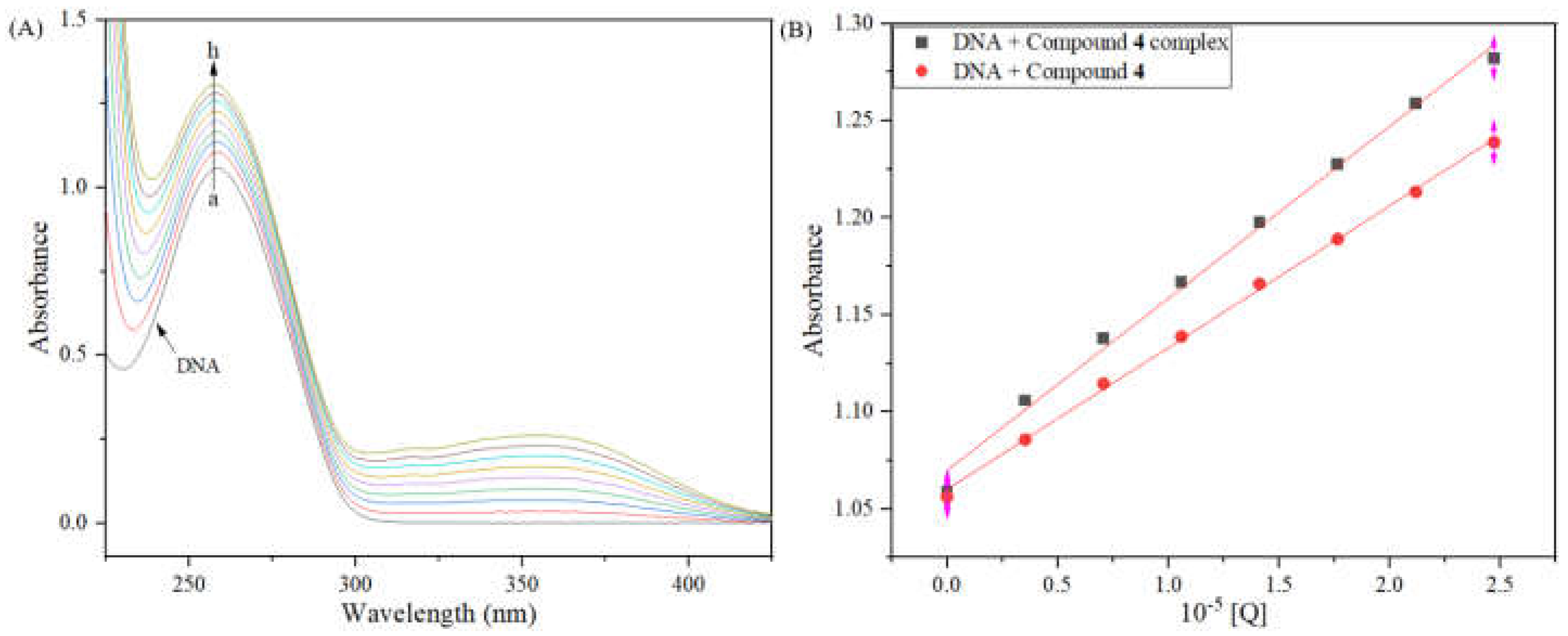
2.11.1. DNA Binding Study
2.11.2. Competitive Binding Study
2.12. Allosteric Modulation of Compound 4 with PBP2a
2.12.1. Contents of PBP2a and Drug Combination
2.12.2. Allosteric Site Binding Affinity of PBP2a
2.12.3. Molecular Docking
2.13. Summary of Anti-MRSA Behavior of Thiazolylketenyl Quinazolinones
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Synthesis of Intermediates and Thiazolylketenyl Quinazolinones and Analogs
3.2.1. Synthesis of Intermediates 2–3 and 15–16
3.2.2. Synthesis of Intermediate 14
3.2.3. Synthesis of (E)-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (4)
3.2.4. Synthesis of (E)-2-(3-(thiazol-5-yl)acryloyl)quinazolin-4(3H)-one (5a)
3.2.5. Synthesis of (E)-2-(3-(2-phenylthiazol-5-yl)acryloyl)quinazolin-4(3H)-one (5b)
3.2.6. Synthesis of (E)-2-(3-(furan-2-yl)acryloyl)quinazolin-4(3H)-one (6a)
3.2.7. Synthesis of (E)-2-(3-(5-methylfuran-2-yl)acryloyl)quinazolin-4(3H)-one (6b)
3.2.8. Synthesis of (E)-2-(3-(5-(hydroxymethyl)furan-2-yl)acryloyl)quinazolin-4(3H)-one (6c)
3.2.9. Synthesis of (E)-2-(3-(thiophen-2-yl)acryloyl)quinazolin-4(3H)-one (7a)
3.2.10. Synthesis of (E)-2-(3-(3-methylthiophen-2-yl)acryloyl)quinazolin-4(3H)-one (7b)
3.2.11. Synthesis of (E)-2-(3-(benzofuran-3-yl)acryloyl)quinazolin-4(3H)-one (8a)
3.2.12. Synthesis of (E)-2-(3-(benzo[b]thiophen-3-yl)acryloyl)quinazolin-4(3H)-one (8b)
3.2.13. Synthesis of (E)-2-(3-(1H-indol-3-yl)acryloyl)quinazolin-4(3H)-one (8c)
3.2.14. Synthesis of (E)-7-fluoro-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (17a)
3.2.15. Synthesis of (E)-7-chloro-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (17b)
3.2.16. Synthesis of (E)-6,8-dichloro-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (17c)
3.2.17. Synthesis of (E)-6-methyl-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (17d)
3.2.18. Synthesis of (E)-8-methyl-2-(3-(thiazol-2-yl)acryloyl)quinazolin-4(3H)-one (17e)
3.3. Biological Assay
3.3.1. Antibacterial Activity
3.3.2. Hemolytic Assay
3.3.3. Resistance Study
3.3.4. ADMET Study
3.3.5. Inhibition of Biofilm
3.3.6. Metabolic Activity
3.3.7. Membrane Depolarization Assay
3.3.8. Inner Membrane Permeability
3.3.9. Leakage of Cellular Protein
3.3.10. Reactive Oxygen Species (ROS) Production
3.3.11. Interactions of Compound 4 with Calf Thymus DNA
3.3.12. Measurement of PBP2a Contents
3.3.13. Drug Combination
3.3.14. Determination of Allosteric Site Binding Affinity by Fluorescent Quenching
3.3.15. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Robert, G.; Imai, Y.; Bolla, J.R.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Brady, S.F. Bioinformatic prospecting and synthesis of a bifunctional lipopeptide antibiotic that evades resistance. Science 2022, 376, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, D.; Guo, Y.L.; De Castro, C.; Kim, S.H.; Schlatterer, K.; Xu, F.F.; Pereira, C.; Seeberger, P.H.; Ali, S.; Codée, J.; et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 2018, 563, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, P.; Woischnig, A.K.; Hamri, G.C.; Ye, H.F.; Folcher, M.; Xie, M.Q.; Khanna, N.; Fussenegger, M. Immunomimetic designer cells protect mice from MRSA infection. Cell 2018, 174, 259–270. [Google Scholar] [CrossRef]
- Speri, E.; Kim, C.; De Benedetti, S.; Qian, Y.Y.; Lastochkin, E.; Fishovitz, J.; Fisher, J.F.; Mobashery, S. Cinnamonitrile adjuvants restore susceptibility to β-Lactams against methicillin-resistant Staphylococcus aureus. ACS Med. Chem. Lett. 2019, 10, 1148–1153. [Google Scholar] [CrossRef]
- Janardhanan, J.; Meisel, J.E.; Ding, D.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; Chang, M. In Vitro and In Vivo synergy of the oxadiazole class of antibacterials with β-lactams. Antimicrob. Agents Chemother. 2016, 60, 5581–5588. [Google Scholar] [CrossRef]
- Müller, S.; Wolf, A.J.; Iliev, I.D.; Berg, B.L.; Underhill, D.M.; Liu, G.Y. Poorly cross-linked peptidoglycan in MRSA due to mecA induction activates the inflammasome and exacerbates immunopathology. Cell Host. Microb. 2015, 18, 604–612. [Google Scholar] [CrossRef]
- Shalaby, M.A.W.; Dokla, E.M.E.; Serya, R.A.T.; Abouzid, K.A.M. Penicillin binding protein 2a: An overview and a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef]
- Bouley, R.; Ding, D.; Peng, Z.H.; Bastian, M.; Lastochkin, E.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; et al. Structure-activity relationship for the 4(3H)-quinazolinone antibacterials. J. Med. Chem. 2016, 59, 5011–5021. [Google Scholar] [CrossRef]
- Chang, M.; Mahasenan, K.V.; Hermoso, J.A.; Mobashery, S. Unconventional antibacterials and adjuvants. Acc. Chem. Res. 2021, 54, 917–929. [Google Scholar] [CrossRef]
- Zhang, P.L.; Gopal, L.; Zhan, S.L.; Cai, G.X.; Zhou, C.H. An unanticipated discovery towards novel naphthalimide corbelled aminothiazoximes as potential anti-MRSA agents and allosteric modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wang, M.X.; Yao, X.; Li, Y.; Tan, J.; Wang, L.Z.; Qiao, W.T.; Geng, Y.Q.; Liu, Y.X.; Wang, Q.M. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Fu, G.H.; Zhong, W.H. Natural quinazolinones: From a treasure house to promising anticancer leads. Eur. J. Med. Chem. 2023, 245, 114915. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Kamel, M.M.; Kassem, E.M.M.; Abotaleb, N.; El-moez, S.I.A.; Ahmed, M.F. Novel 6,8-dibromo-4(3H)quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur. J. Med. Chem. 2010, 45, 3311–3319. [Google Scholar] [CrossRef]
- Plescia, F.; Maggio, B.; Daidone, G.; Raffa, D. 4-(3H)-quinazolinones N-3 substituted with a five membered heterocycle: A promising scaffold towards bioactive molecules. Eur. J. Med. Chem. 2021, 213, 113070. [Google Scholar] [CrossRef]
- Zhou, X.M.; Hu, Y.Y.; Fang, B.; Zhou, C.H. Benzenesulfonyl thiazoloimines as unique multitargeting antibacterial agents towards Enterococcus faecalis. Eur. J. Med. Chem. 2023, 248, 115088. [Google Scholar] [CrossRef]
- Zhao, W.H.; Xu, J.H.; Tangadanchu, V.K.R.; Zhou, C.H. Thiazolyl hydrazineylidenyl indolones as unique potential multitargeting broad-spectrum antimicrobial agents. Eur. J. Med. Chem. 2023, 256, 115452. [Google Scholar] [CrossRef]
- Peng, X.M.; Cai, G.X.; Zhou, C.H. Recent developments in azole compounds as antibacterial and antifungal agents. Curr. Top. Med. Chem. 2013, 13, 1963–2010. [Google Scholar] [CrossRef]
- Li, S.R.; Tan, Y.M.; Zhang, L.; Zhou, C.H. Comprehensive insights into medicinal research on imidazole-based supramolecular complexes. Pharmaceutics 2023, 15, 1348. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Identification of unique quinazolinone thiazoles as novel structural scaffolds for potential gram-negative bacterial conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef]
- Wang, J.; Battini, N.; Ansari, M.F.; Zhou, C.H. Synthesis and biological evaluation of quinazolinonethiazoles as new potential conquerors towards Pseudomonas aeruginosa. Chin. J. Chem. 2021, 39, 1093–1103. [Google Scholar] [CrossRef]
- Boibessot, T.; Zschiedrich, C.P.; Lebeau, A.; Bénimèlis, D.; Dunyach-Rémy, C.; Lavigne, J.P.; Szurmant, H.; Benfodda, Z.; Meffre, P. The rational design, synthesis, and antimicrobial properties of thiophene derivatives that inhibit bacterial histidine kinases. J. Med. Chem. 2016, 59, 8830–8847. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Barlocco, D.; Beretta, G.; Gelain, A.; Pini, E.; Porcino, M.; Mori, G.; Stelitano, G.; Costantino, L.; et al. Discovery and development of novel salicylate synthase (MbtI) furanic inhibitors as antitubercular agents. Eur. J. Med. Chem. 2018, 155, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Dan, W.J.; Dai, J.K. Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem. 2020, 187, 111980. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Yadav, P.; Lal, K.; Kumar, L.; Kumar, A.; Kumar, A.; Paul, A.K.; Kumar, R. Synthesis, crystal structure and antimicrobial potential of some fluorinated chalcone-1, 2, 3-triazole conjugates. Eur. J. Med. Chem. 2018, 155, 263–274. [Google Scholar] [CrossRef]
- Liu, H.B.; Gopala, L.; Avula, S.R.; Jeyakkumar, P.; Peng, X.M.; Zhou, C.H.; Geng, R.X. Chalcone-benzotriazole conjugates as new potential antimicrobial agents: Design, synthesis, biological evaluation and synergism with clinical drugs. Chin. J. Chem. 2017, 35, 483–496. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Allegretta, G.; Janardhanan, J.; Peng, Z.H.; Mahasenan, K.V.; Lastochkin, E.; Gozun, M.M.N.; Tejera, S.; Schroeder, V.A.; Wolter, W.R.; et al. Exploration of the structural space in 4(3H)-quinazolinone antibacterials. J. Med. Chem. 2020, 63, 5287–5296. [Google Scholar] [CrossRef]
- Srisuknimit, V.; Qiao, Y.; Schaefer, K.; Kahne, D.; Walker, S. Peptidoglycan crosslinking preferences of Staphylococcus aureus penicillin-binding proteins have implications for treating MRSA infections. J. Am. Chem. Soc. 2017, 139, 9791–9794. [Google Scholar] [CrossRef]
- Sun, H.; Ansari, M.F.; Fang, B.; Zhou, C.H. Natural berberine-hybridized benzimidazoles as novel unique bactericides against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 7831–7840. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Sun, H.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Natural aloe emodin-hybridized sulfonamide aminophosphates as novel potential membrane-perturbing and DNA-intercalating agents against Enterococcus faecalis. Bioorg. Med. Chem. Lett. 2022, 64, 128695. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.F.; Ansari, M.F.; Fang, B.; Zhang, S.L.; Zhou, C.H. Discovery of novel purinylthiazolylethanone derivatives as anti-Candida albicans agents through possible multifaceted mechanisms. Eur. J. Med. Chem. 2021, 221, 113557. [Google Scholar] [CrossRef]
- Yang, X.C.; Hu, C.F.; Zhang, P.L.; Li, S.; Hu, C.S.; Geng, R.X.; Zhou, C.H. Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bioorg. Chem. 2022, 124, 105855. [Google Scholar] [CrossRef] [PubMed]
- Rasapalli, S.; Murphy, Z.F.; Sammeta, V.R.; Golen, J.A.; Weig, A.W.; Melander, R.J.; Melander, C.; Macha, P.; Vasudev, M.C. Synthesis and biofilm inhibition studies of 2-(2-amino-6-arylpyrimidin-4-yl) quinazolin-4(3H)-ones. Bioorg. Med. Chem. Lett. 2020, 30, 127550. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.S.; Sun, D.J.; Shi, D.F.; Wang, G.; Chen, Y.M.; Zhang, K.; Tan, H.D.; Liu, J.; Liu, B.; Ouyang, L. Design, synthesis, and biological evaluation of quinazolin-4(3H)-one derivatives co-targeting poly(ADP-ribose) polymerase-1 and bromodomain containing protein 4 for breast cancer therapy. Acta Pharm. Sin. B 2021, 11, 156–180. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Zhao, W.H.; Tangadanch, V.K.R.; Meng, J.P.; Zhou, C.H. Discovery of novel phenylhydrazone-based oxindole-thiolazoles as potent antibacterial agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Sangaraiah, N.; Meng, J.P.; Zhou, C.H. Unique carbazole-oxadiazole derivatives as new potential antibiotics for combating gram-positive and -negative bacteria. J. Med. Chem. 2022, 65, 6171–6190. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, B.B.; Yang, Y.Y.; Ba, Z.F.; Zhang, J.Y.; Zhang, T.Y.; Chang, L.L.; Zhang, F.Y.; Zhang, Y.; Liu, H.; et al. Improving the antimicrobial performance of amphiphilic cationic antimicrobial peptides using glutamic acid full-scan and positive charge compensation strategies. J. Med. Chem. 2022, 65, 13833–13851. [Google Scholar] [CrossRef]
- Ansari, M.F.; Tan, Y.M.; Sun, H.; Li, S.; Zhou, C.H. Unique iminotetrahydroberberine-corbelled metronidazoles as potential membrane active broad-spectrum antibacterial agents. Bioorg. Med. Chem. Lett. 2022, 76, 129012. [Google Scholar] [CrossRef]
- Cai, Q.N.; Yu, Q.; Liang, W.X.; Li, H.Z.; Liu, J.Y.; Li, H.X.; Chen, Y.Z.; Fang, S.F.; Zhong, R.C.; Liu, S.P.; et al. Membrane-active nonivamide derivatives as effective broad-spectrum antimicrobials: Rational design, synthesis, and biological evaluation. J. Med. Chem. 2022, 65, 16754–16773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Lv, J.S.; Ansari, M.F.; Battini, N.; Cai, G.X.; Zhou, C.H. Synthesis of a novel structural framework of naphthalimide triazoles and specific anti-Aspergillus fumigatus effects. Sci. Sin. Chim. 2021, 51, 1094–1103. [Google Scholar] [CrossRef]
- Legru, A.; Verdirosa, F.; Vo-Hoang, Y.; Tassone, G.; Vascon, F.; Thomas, C.A.; Sannio, F.; Corsica, G.; Benvenuti, M.; Feller, G.; et al. Optimization of 1,2,4-triazole-3-thiones toward broad-spectrum metallo-β-lactamase inhibitors showing potent synergistic activity on VIM- and NDM-1-producing clinical isolates. J. Med. Chem. 2022, 65, 16392–16419. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.M.; Li, D.; Li, F.F.; Ansari, M.F.; Fang, B.; Zhou, C.H. Pyrimidine-conjugated fluoroquinolones as new potential broad-spectrum antibacterial agents. Bioorg. Med. Chem. Lett. 2022, 73, 128885. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Unique para-aminobenzenesulfonyl oxadiazoles as novel structural potential membrane active antibacterial agents towards drug-resistant methicillin resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2021, 41, 127995. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Maddili, S.K.; Li, S.; Yang, R.G.; Zhou, C.H. Dihydropyrimidinone imidazoles as unique structural antibacterial agents for drug-resistant Gram-negative pathogens. Eur. J. Med. Chem. 2022, 232, 114188. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Lin, J.M.; Zhou, C.H. Design and synthesis of sulfanilamide aminophosphonates as novel antibacterial agents towards Escherichia coli. Chin. J. Chem. 2021, 39, 2251–2263. [Google Scholar] [CrossRef]
- Yang, X.C.; Zeng, C.M.; Avula, S.R.; Peng, X.M.; Geng, R.X.; Zhou, C.H. Novel coumarin aminophosphonates as potential multitargeting antibacterial agents against Staphylococcus aureus. Eur. J. Med. Chem. 2023, 245, 114891. [Google Scholar] [CrossRef]
- Zeng, C.M.; Srinivasa, R.A.; Meng, J.P.; Zhou, C.H. Synthesis and biological evaluation of piperazine hybridized coumarin indolylcyanoenones with antibacterial potential. Molecules 2023, 28, 2511. [Google Scholar] [CrossRef]
- Zhang, P.L.; Tangadanchu, V.K.R.; Zhou, C.H. Identification of novel antifungal skeleton of hydroxyethyl naphthalimides with synergistic potential for chemical and dynamic treatments. Molecules 2022, 27, 8453. [Google Scholar] [CrossRef]
- Liang, W.X.; Yu, Q.; Zheng, Z.X.; Liu, J.Y.; Cai, Q.M.; Liu, S.P.; Lin, S.M. Design and synthesis of phenyl sulfide-based cationic amphiphiles as membrane-targeting antimicrobial agents against Gram-positive pathogens. J. Med. Chem. 2022, 65, 14221–14236. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Koh, J.J.; Aung, T.T.; Sin, W.L.W.; Lim, F.H.; Wang, L.; Lakshminarayanan, R.; Zhou, L.; Tan, D.T.H.; Cao, D.R.; et al. Semisynthetic flavone-derived antimicrobials with therapeutic potential against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2017, 60, 6152–6165. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.R.; Billings, G.; Odermatt, P.D.; Auer, G.K.; Zhu, L.; Miguel, A.; Chang, F.; Weibel, D.B.; Theriot, J.A.; Huang, K.C. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Gopala, L.; Yu, Y.; Fang, B.; Zhou, C.H. Identification of a novel antifungal backbone of naphthalimide thiazoles with synergistic potential for chemical and dynamic treatment. Future Med. Chem. 2021, 13, 2047–2067. [Google Scholar] [CrossRef]
- Su, M.; Xia, D.L.; Teng, P.; Nimmagadda, A.; Zhang, C.; Odom, T.; Cao, A.; Hu, Y.; Cai, J.F. Membrane-active hydantoin derivatives as antibiotic agents. J. Med. Chem. 2017, 60, 8456–8465. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.Z.; Jeyakkumar, P.; Zang, Z.L.; Fang, B.; Zhou, C.H. A new discovery of unique 13-(benzimidazolylmethyl)berberines as promising broad-spectrum antibacterial agents. J. Agric. Food Chem. 2022, 70, 12320–12329. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Zhang, P.L.; Laiche, M.H.; Li, Y.L.; Gao, W.W.; Lin, J.M.; Zhou, C.H. An unanticipated discovery of novel naphthalimidopropanediols as potential broad-spectrum antibacterial members. Eur. J. Med. Chem. 2022, 241, 114657. [Google Scholar] [CrossRef]
- Hu, C.F.; Zhang, P.L.; Sui, Y.F.; Lv, J.S.; Ansari, M.F.; Battini, N.; Li, S.; Zhou, C.H.; Geng, R.X. Ethylenic conjugated coumarin thiazolidinediones as new efficient antimicrobial modulators against clinical methicillin-resistant Staphylococcus aureus. Bioorg. Chem. 2020, 94, 103434. [Google Scholar] [CrossRef]
- Li, F.F.; Zhang, P.L.; Tangadanchu, V.K.R.; Li, S.; Zhou, C.H. Novel metronidazole-derived three-component hybrids as promising broad-spectrum agents to combat oppressive bacterial resistance. Bioorg. Chem. 2022, 122, 105718. [Google Scholar] [CrossRef]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M.; et al. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, S.Y.; Jeyakkumar, P.; Cai, G.X.; Fang, B.; Zhou, C.H. Natural berberine-derived azolyl ethanols as new structural antibacterial agents against drug-resistant Escherichia coli. J. Med. Chem. 2022, 65, 436–459. [Google Scholar] [CrossRef]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New efforts toward aminothiazolylquinolones with multitargeting antibacterial potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.L.; Wang, J.; Gao, Y.; Ren, X.Y.; Rottenberg, M.E.; Lu, J.; Holmgren, A. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Sci. Rep. 2018, 8, 11131. [Google Scholar] [CrossRef]
- Deng, Z.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Aloe emodin-conjugated sulfonyl hydrazones as novel type of antibacterial modulators against S. aureus 25923 through multifaceted synergistic effects. Bioorg. Chem. 2022, 127, 106035. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, J.B.; Xie, J.W.; Chen, L.P.; Wei, X.Y.; Jiang, X.; Bao, M.; Qiu, Y.Y.; Chen, Q.; Li, W.L.; et al. Design, synthesis, and evaluation of chalcone analogues incorporate α,β-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. Eur. J. Med. Chem. 2018, 157, 1395–1405. [Google Scholar] [CrossRef]
- Yang, X.C.; Zhang, P.L.; Kumar, K.V.; Li, S.; Geng, R.X.; Zhou, C.H. Discovery of unique thiazolidinone-conjugated coumarins as novel broad spectrum antibacterial agents. Eur. J. Med. Chem. 2022, 232, 114192. [Google Scholar] [CrossRef]
- Farag, N.A.H.; El-Tayeb, W. Design, synthesis and docking studies of new furobenzopyranones and pyranobenzopyranones as photoreagent towards DNA and as antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 317–325. [Google Scholar] [CrossRef]
- Tan, Y.M.; Wang, Y.; Li, S.; Zhang, S.L.; Zhou, C.H. Azolylpyrimidinediols as novel structural scaffolds of DNA-groove binders against intractable Acinetobacter baumannii. J. Med. Chem. 2023, 66, 4910–4931. [Google Scholar] [CrossRef]
- Xie, Y.P.; Ansari, M.F.; Zhang, S.L.; Zhou, C.H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pestic. Biochem. Physiol. 2021, 175, 104849. [Google Scholar] [CrossRef]
- Chen, J.P.; Battini, N.; Ansari, M.F.; Zhou, C.H. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent anti-methicillin-resistant Staphylococcus aureus activity. Eur. J. Med. Chem. 2021, 217, 113340. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Tangadanchu, V.K.R.; Battini, N.; Bheemanaboina, R.R.Y.; Zang, Z.L.; Zhang, S.L.; Zhou, C.H. Indole-nitroimidazole conjugates as efficient manipulators to decrease the genes expression of methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2019, 179, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.F.; Ansari, M.F.; Zhou, C.H. Pyrimidinetrione-imidazoles as a unique structural type of potential agents towards Candida albicans: Design, synthesis and biological evaluation. Chem. Asian J. 2021, 16, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Janardhanan, J.; Bouley, R.; Martinez-Caballero, S.; Peng, Z.H.; Batuecas-Mordillo, M.; Meisel, J.E.; Ding, D.; Schroeder, V.A.; Wolter, W.R.; Mahasenan, K.V.; et al. The quinazolinone allosteric inhibitor of PBP 2a synergizes with piperacillin and tazobactam against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02637-18. [Google Scholar] [CrossRef]
- Wang, L.L.; Battini, N.; Bheemanaboina, R.R.Y.; Zhang, S.L.; Zhou, C.H. Design and synthesis of aminothiazolyl norfloxacin analogues as potential antimicrobial agents and their biological evaluation. Eur. J. Med. Chem. 2019, 167, 105–123. [Google Scholar] [CrossRef]
- Sui, Y.F.; Li, D.; Wang, J.; Bheemanaboina, R.R.Y.; Ansari, M.F.; Gan, L.L.; Zhou, C.H. Design and biological evaluation of a novel type of potential multi-targeting antimicrobial sulfanilamide hybrids in combination of pyrimidine and azoles. Bioorg. Med. Chem. Lett. 2020, 30, 126982. [Google Scholar] [CrossRef]














 | |||||
|---|---|---|---|---|---|
| Number | Solvent | Base | Temperature (°C) | Time (h) | Yields (%) |
| 1 | CH3CN | K2CO3 | 80 | 8 | - |
| 2 | CH3CN | Cs2CO3 | 80 | 8 | - |
| 3 | DMF | K2CO3 | 80 | 8 | - |
| 4 | DMF | K2CO3 | 120 | 8 | - |
| 5 | DMF | Cs2CO3 | 80 | 8 | - |
| 6 | DMF | NaH | 25 | 8 | - |
| 7 | DMF | NaH | 80 | 8 | - |
| Compds. | Gram-Positive Bacteria a | Gram-Negative Bacteria b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | S. a. | S. a. 25923 | S. a. 29213 | E. f. | K. p. | E. c. | E. c. 25922 | P. a. | P. a. 27853 | A. b. | |
| 4 | 0.5 | 4 | 8 | 1 | 2 | 2 | 2 | 0.5 | 4 | 2 | 64 |
| 5a | 16 | 32 | 16 | 1 | 32 | 16 | 32 | 8 | 16 | 8 | 2 |
| 5b | 64 | 64 | 128 | 32 | 128 | 256 | 128 | 32 | 128 | 128 | 256 |
| 6a | 4 | 16 | 16 | 32 | 16 | 2 | 4 | 32 | 8 | 64 | 16 |
| 6b | 8 | 8 | 8 | 16 | 16 | 16 | 8 | 64 | 16 | 128 | 16 |
| 6c | 128 | 32 | 64 | 8 | 128 | 128 | 128 | 64 | 8 | 128 | 32 |
| 7a | 8 | 32 | 64 | 128 | 256 | 64 | 4 | 32 | 16 | 16 | 2 |
| 7b | 8 | 8 | 4 | 16 | 4 | 8 | 4 | 8 | 8 | 0.5 | 4 |
| 8a | 16 | 32 | 8 | 8 | 32 | 2 | 32 | 16 | 4 | 64 | 4 |
| 8b | 8 | 4 | 8 | 16 | 32 | 2 | 16 | 16 | 32 | 32 | 1 |
| 8c | 64 | 8 | 4 | 8 | 16 | 128 | 32 | 32 | 64 | 16 | 32 |
| 17a | 32 | 32 | 16 | 32 | 4 | 8 | 64 | 4 | 4 | 32 | 16 |
| 17b | 128 | 8 | 16 | 8 | 8 | 4 | 32 | 4 | 4 | 2 | 8 |
| 17c | 4 | 16 | 8 | 32 | 1 | 16 | 8 | 8 | 2 | 2 | 2 |
| 17d | 8 | 16 | 32 | 64 | 2 | 4 | 16 | 64 | 2 | 4 | 4 |
| 17e | 8 | 32 | 16 | 32 | 1 | 16 | 32 | 8 | 4 | 32 | 4 |
| Norfloxacin | 4 | 8 | 4 | 1 | 2 | 4 | 4 | 2 | 4 | 1 | 2 |
| Properties | 4 | Norfloxacin |
|---|---|---|
| Molecular weight (g/mol) (<500) | 283.31 | 319.33 |
| MLOGP (≤4.15) | 0.84 | 1.04 |
| H-bond acceptors (<10) | 4 | 5 |
| H-bond donors (<5) | 1 | 2 |
| rotatable bonds (<10) | 3 | 3 |
| Lipinski’s Rule | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 |
| GI absorption | High | High |
| BBB permean | No | No |
| Compounds | PBP2a Contents in Treated MRSA (pg/mL) | MIC (Alone or Combination) |
|---|---|---|
| Control | 405.94 ± 21.19 | – |
| Compound 4 | 345.16 ± 2.80 | 0.5 (alone) |
| Cefdinir | 189.00 ± 11.04 | 1 (alone) |
| Compound 4 + Cefdinir | 127.47 ± 14.94 | 0.125/0.125 (combination) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Battini, N.; Zang, Z.; Luo, Y.; Zhou, C. Novel Thiazolylketenyl Quinazolinones as Potential Anti-MRSA Agents and Allosteric Modulator for PBP2a. Molecules 2023, 28, 4240. https://doi.org/10.3390/molecules28104240
Dai J, Battini N, Zang Z, Luo Y, Zhou C. Novel Thiazolylketenyl Quinazolinones as Potential Anti-MRSA Agents and Allosteric Modulator for PBP2a. Molecules. 2023; 28(10):4240. https://doi.org/10.3390/molecules28104240
Chicago/Turabian StyleDai, Jie, Narsaiah Battini, Zhonglin Zang, Yan Luo, and Chenghe Zhou. 2023. "Novel Thiazolylketenyl Quinazolinones as Potential Anti-MRSA Agents and Allosteric Modulator for PBP2a" Molecules 28, no. 10: 4240. https://doi.org/10.3390/molecules28104240
APA StyleDai, J., Battini, N., Zang, Z., Luo, Y., & Zhou, C. (2023). Novel Thiazolylketenyl Quinazolinones as Potential Anti-MRSA Agents and Allosteric Modulator for PBP2a. Molecules, 28(10), 4240. https://doi.org/10.3390/molecules28104240





