Abstract
The emergence of the antimicrobial resistance phenomena on and the harmful consequences of the use of antibiotics motivate the necessity of innovative antimicrobial therapies, while natural substances are considered a promising alternative. Rosmarin is an original plant compound listed among the hydroxycinnamic acids. This substance has been widely used to fight microbial pathology and chronic infections from microorganisms like bacteria, fungi and viruses. Also, various derivatives of rosmarinic acid, such as the propyl ester of rosmarinic acid, rosmarinic acid methyl ester or the hexyl ester of rosmarinic acid, have been synthesized chemically, which have been isolated as natural antimicrobial agents. Rosmarinic acid and its derivatives were combined with antibiotics to obtain a synergistic effect. This review reports on the antimicrobial effects of rosmarinic acid and its associated derivatives, both in their free form and in combination with other microbial pathogens, and mechanisms of action.
1. Introduction
Antimicrobial resistance (AMR) is a significant public health problem. According to ECDC and WHO estimates, more than 670,000 illnesses per year in the European area are caused by antibiotic-resistant bacteria, and over 33,000 human deaths are directly caused by these infections [1]. Antimicrobial resistance is directly related to the indiscriminate use of antibiotics and is developing at extremely high rates worldwide, including African countries like Cameroon [2]. Despite inadequate laboratory capacity to monitor AMR, the African continent shows evidence of the global trend of increasing drug resistance.
Some germs can not only spread in hospitals, but also in the great outdoors, where a significant resistance has been observed [3]. With increased drug consumption, increased hospital admissions, and increases in deaths, the financial consequences of AMR are financially devastating, including astronomical medical costs [4].
Antibiotic-resistant pathogenic bacteria include P. aeruginosa, S. aureus, Enterobacteriaceae and Enterococcus spp., while infections caused by nonresistant germs are significantly more difficult to treat [5].
In addition, the current overuse of conventional antibiotics and their improper use have reduced their efficacy, creating a serious problem for world health as well as development, and as such, the antimicrobial drug range is rapidly expanding compared to the available therapeutic treatments, making the latter ineffective or even inefficient [6]. The demand for new antimicrobial products is high, and products derived from natural sources are seen as a promising solution, including plant polyphenols, such as rosmarinic acid, which is a natural substance with antimicrobial activity.
2. Derivates of Rosmarinic Acid
Both 3,4-dihydroxyphenyllactic acid (DHPL) and caffeic acid (3,4- dihydroxycinnamic acid) are esterified to form rosmarinic acid (RA) (Figure 1). Scarpati and Oriente [7] were the first to determine the chemical structure of RA. They extracted rosemary RA from Rosmarinus officinalis from Lamiceae, and assigned the same name to rosmarinic acid. The tannin-like chemicals of the Lamiaceae have been called “Labiatengerbstoff” for a long time. However, not all species of Lamiaceae have RA, and “Labiatengerbstoff” may also have some other phenolic compounds. The plant family Boraginaceae also consistently contains RA. Other related caffeic acid esters as similar derivatives are in addition to RAs which are esters of caffeine and quinic acid including caffeoylshikimic and chlorogenic acids.
Helicteresisora has isolated isorinic acid (caffeoyl-4′-hydroxyphenyllactate), 4-O-glucosylated RA and 4/4′-O-diglucosylated RA [8], and also the cis-isomer of RA produced by Salvia nemorosa [9]. Many substances allegedly producing RA are reported, such as lithospermic acid from Lycopus europaeus, consisting of RA and caffeic acid [10] or Lithospermum ruderale [11], slithospermic acid B from Salvia miltiorrhiza (likewise called salvianolic acid B), which is composed of two RA molecules, and a number of other salvianolic acids [12,13], rabdosiin from Rabdosia japonica [14,15], sagecoumarin, melitric acid and sagerinic acid from Salvia officinalis [16,17], or yunnanic acids from Salvia yunnanensis [18] and several others reported in Bulgakov et al. [19]. RA methyl ethers and their derivatives are often reported, such as sage methylmelitric acid [16] and Clerodendranthusspicatus clerodendranoic acid [20].
The biosynthetic process of these more complex chemicals has not yet been studied, but their structures allow us to deduce that RA or derivatives of RA and other phenylpropanoids can be used for their production. There are more taxa with species that contain RA and related compounds in addition to the families Lamiaceae and Boraginaceae. The plants now recognized as having the “lowest” levels of RA are hornworts (Anthocerotaceae). In addition to RA, hornworts also contain lignan-like compounds that are associated with RA (such as anthocerotonic acid, megacerotonic acid, and anthocerodizonin) [21].
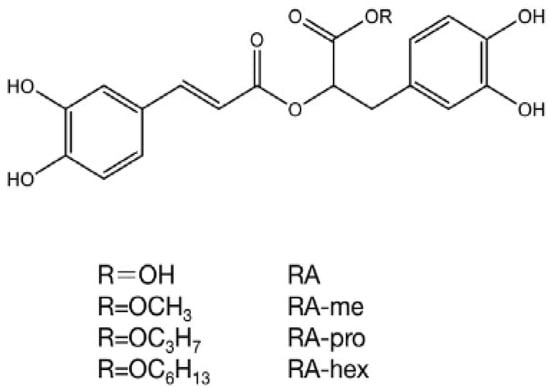
Figure 1.
Variations in the structure of the acid rosmarinic and their derivatives. RA: rosmarinic acid, RA-hex: RA hexyl ester, RA-pro: RA propyl ester, RA-me: RA methyl ester [22].
The RA has been identified and isolated in 162 plants to date as a monomeric component, which are Adenium obesum [23], Alkanna sfikasiana Tan [24], Anchusa azurea [25], Anchusa italica [26], Anchusa strigosa, Anthoceros punctatus [27], Apeiba tibourbou [28], Arctopus monacanthus [29], Arnebia purpurea [30], Baccharis chilco [31], Barbarea integrifolia [32], Bellis sylvestris [33], Blechnum Brasiliense [34], Canna edulis [35], Celastrus hindsii [36], Centella asiática [37], Chloranthus fortune [38], Chloranthus multistachys [39], Clerodendranthus spicatus [40], Clinopodium chinense var. Parviflorum [41], Clinopodium tomentosum [42], Clinopodium urticifolium [43], Coleus aromaticus [44], Coleus forskohlii [45], Coleus parvifolius [46], Colocasia esculenta [47], Cordia alliodora [48], Cordia bicolor [49], Cordia boissieri [50], Cordia dentata [49], Cordia latifolia [51], Cordia megalantha [49], Cordia morelosana Standley [52], Cordia sinensis [53], Cordia verbenácea [54], Cynoglossum columnae [55], Dracocephalum fruticulosum [56], Dracocephalum heterophyllum [57], Dracocephalum nutans [56], Dracocephalum palmatum [58], Dracocephalum tanguticum [59], Ehretia asperula [60], Ehretia obtusifolia [61], Ehretia philippinensis [62], Ehretia thyrsiflora [63], Elsholtiza bodinieri [64], Elsholtzia rugulosa [65], Elsholtzia splendens [66], Farfugium japonicum [67], Foeniculum vulgare [68], Forsythia koreana [69], Gastrocotyle hispida [70], Glechoma longituba [71], Hamelia patens [72], Hedera hélix [73], Helicteres angustifolia [74], Helicteres hirsuta [75], Helicteres isora [8], Hypenia salzmannii [76], Hyptis atrorubens Poit. [77], Hyptis capitata [78], Hyptis pectinata [79], Hyptis suaveolens [80], Hyptis verticillata [81], Hyssopus cuspidatus [82], Ipomoea turpethum [83], Isodon eriocalyx [84], Isodon flexicaulis [85], Isodon lophanthoides var. graciliflorus [86], Isodon oresbius [87], Isodon rubescens [88], Isodon rugosus [89], Isodon sculponeata [90], Keiskea japónica [91], Lallemantia iberica [92], Lavandula angustifolia [93], Lepechinia graveolens [94], Lepechinia meyenii [95], Lepechinia speciosa [96], Lycopus europaeus [97], Lycopus lucidus [98], Marrubium vulgare [99], Meehania urticifolia [100], Melissa officinalis [101], Mentha dumetorum [102], Mentha haplocalyx [103], Mentha longifolia [104], Mentha piperita [105], Mentha spicata [106], Mesona chinensis [107], Micromeria myrtifolia [108], Microsorum fortune [109], Momordica balsamina [110], Nepeta asterotricha [111], Nepeta cadmea [112], Nepeta curviflora [113], Ocimum campechianum [114], Ocimum sanctum [115], Origanum dictamnus [116], Origanum glandulosum [117], Origanum majorana [118], Origanum minutiflorum [119], Origanum rotundifolium [120], Origanum vulgare [121], Paris veriticillata [122], Perilla frutescens [123,124], Perilla frutescens var. acuta [125], Perovskia atriplicifolia [126], Plectranthus forsteri [127], Plectranthus hadiensis var. Tomentosus [128], Plectranthus madagascariensis [129], Plectranthus scutellarioides [130], Polygomun aviculane [131], Prunella vulgaris [132], Prunella vulgaris var. Lilacina [133,134], Quercus serrata [135], Rosmarinus officinalis [136], Salvia absconditiflora [137], Salvia castanea [138], Salvia cavaleriei [139], Salvia cerino-pruinosa [140], Salvia chinensis [141,142], Salvia deserta Schang [143], Salvia flava Forrest [144], Salvia grandifolia [145], Salvia kiaometiensis Lévl. [146], Salvia limbata [147], Salvia miltiorrhiza [148], Salvia officinalis [16], Salvia palaestina [149], Salvia plebeian [150], Salvia przewalskii [151], Salvia sonchifolia [152], Salvia splendens Sellow [153], Salvia trichoclada [154], Salvia viridis [155], Salvia trichoclada [154], Salvia viridis [155], Salvia yunaansis [156], Sanicula europaea [157], Sanicula lamelligera [158], Sarcandra glabra [159], Sideritis albiflora, Sideritis leptoclada [160], Solanum betaceum [161], Solenostemon monostachys [162], Symphytum officinale [163], Thunbergia laurifolia [164], Thymus alternans [165], Thymus atlanticus [166], Thymus praecox sub spgrossheimii [167], Thymus praecox sub spgrossheimii [168], Thymus quinquecostatus var. japonica [169], Thymus serpyllum [170], Thymus sibthorpii Bentham [171], Thymus sipyleus subsp. Sipyleus var. sipyleus [172], Thymus vulgaris [173], Tournefortia sarmentosa [174], Veronica sibirica L. [175], Ziziphora clinopodioides [176], Zostera marina [177], and Zostera noltii [178].
3. Antimicrobial Activity
There have been hundreds of research studies conducted on the antimicrobial activity of AR (Table 1). In this article, we will mention the most recent studies conducted, specifically, RA is used as a natural phytogenic additive in animal and poultry nutrition to improve their overall health, performance measures, the digestive system’s structure and function and its potential to modify the intestinal microbiota and decrease the number of disease-causing bacteria such as Salmonella spp, E. coli, and several other species of harmful bacteria [179,180]. Rosemary extracts may contain RA as the primary bioactive antimicrobial agent. However, using the methanol extract containing about 30% carnosic acid, with 16% carnosol and 5% RA, Gram-positive and Gram-negative bacteria were shown to be sensitive to rosemary, making it an excellent antibacterial, in contrast to an aqueous extract containing 15% RA which had more limited effects [181].
Due to their intense antimicrobial properties, medicinal plants, herbs and their oils are attracting great interest as innovative and alternative drugs, such as RA [182].
According to Benedec et al. [183], RA showed better antioxidant activity in vitro (DPPH technique) as well as significant action towards the Gram-positive bacteria. In addition, Rosmarinus officinalis extract showed even greater inhibition of the growth of Gram-positive bacteria than the Gentamicin control (Candida albicans). On the other hand, these researchers noted that this extract was without effect toward Gram-negative bacteria such as S. typhimurium, L. monocytogenes, E. coli, S. aureus, and C. albicans were found to be resistant to RAs derived from: Hyssopus officinalis L., M. officinalis L., O. vulgare L. [183]. RA addition decreases the rate of mortality in Japanese encephalitis virus-infected mice. Compared to animals infected with no RA treatment, the viral load was greatly reduced (p < 0.001) in RA-treated infected rats 8–9 days after infection [184].
The antibacterial properties of tannic acid have long been recognized as effective against both methicillin-resistant Staphylococcus aureus and other microorganisms [185]. Currently, one of the molecules used as a target for antibacterial polymer applications is the tannic acid–polymer metal complex [186]. Hospitalized patients are severely harmed by S. aureus, and tannic acid is known to be an inhibitor of various resistance phenotypes of S. aureus [187]. Furthermore, by reducing cell counts and numbers, RA inhibits the development of S. carnosus LTH1502 and E. coli K-12 [188].
Moreno et al. [181] examined extracts of Rosmarinus officinalis through a combination of biological tests. Antimicrobial activities were analyzed by both disk diffusion and dilution broth techniques. Gram-positive bacteria, including S. aureus, B. megaterium, B. subtilis, and E. faecalis, were more sensitive to the methanolic extract, which contains 30% carnosic acid, 16% carnosol, and 5% rosmarinic acid (minimum inhibition concentration (MIC), 2 to 15 mg/mL). Gram-negative bacteria such as K. pneumoniae, E. coli, X. campestris pv. campestris, and P. mirabilis were also treated with MIC 2 to 60 mg/mL, as well as yeasts such as S. cerevisiae, C. albicans, and P. pastoris (MIC of 4 mg/mL). However, the aqueous extract with a 15% rosmarinic acid content only exhibited a narrow spectrum of activity. The MICs for methanolic and water extracts correlated significantly with the values for pure carnosic acid and rosmarinic acid. So, these results indicated a good performance in relation to the antimicrobial efficacy with rosemary extracts combined with the relevant phenolic extracts. The principal antimicrobial bioactive agents in rosemary extracts were suggested to be carnosic acid or rosmarinic acid. From the point of view of practicality, it could be considered as a good nutritional supplement and herbal pharmaceutical product.
Rosmarinic acid has antibacterial properties against Staphylococcus aureus, E. coli, B. subtilis, and Salmonella. Hayriye [189] tested the effect of natural phenolic compounds extracted from vegetables, fruits, herbs and spices against these pathogens and E. coli had minimum bactericidal concentrations (MBC) of 0.9 mg/mL and minimum inhibitory concentrations (MIC) of 0.8 mg/mL. Salmonella had MIC and MBC of 0.9 and 1.0 mg/mL, respectively. Staphylococcus aureus and B. subtilis had MIC and MBC values of 1.0 and 1.1 mg/mL [189], respectively.
The strains LM1, LM2, and LM3 of L. monocytogenes were analyzed, and the presence of rosmarinic acid was shown to have no antibacterial effect over the incubation period of 60 h [190]. Previously, rosmarinic acid has been shown to exhibit high susceptibility to Gram-negative bacteria when exposed to rosmarinic acid, after 60 h of incubation, Salmonella species showed substantial levels of antimicrobial resistance, and the MICs of rosmarinic acid for S. enteridis, S. choleraesuis subsp., and S. paratyphi were less than 20 ppm [190].
Furthermore, rosmarinic acid had previously been recognized as an anti-HIV drug capable of inhibiting HIV replication [191]. The discovery of nitro and dinitro-rosmarinic acids, which inhibit viral replication by blocking HIV-I integrase, has significantly enhanced the anti-HIV efficacy of rosmarinic acid [192].

Table 1.
Rosmarinic acid and its derivatives are used as antibiotics against several pathogenic microorganisms.
Table 1.
Rosmarinic acid and its derivatives are used as antibiotics against several pathogenic microorganisms.
| Pathogenic Microorganisms | Active Concentrations | References | Pathogenic Microorganisms | Active Concentrations | References |
|---|---|---|---|---|---|
| Staphylococcus epidermidis 5001 Stenotrophomonas maltophilia Enterococcus faecalis C159-6 Staphylococcus lugdunensis T26A3 Pseudomonas aeruginosa ATCC 27583 | MIC (0.3 mg/mL of RA) MIC (0.3 mg/mL of RA) MIC (0.3 mg/mL of RA) MIC (0.6 mg/mL of RA) MIC (2.5 mg/mL of RA) | [77] | Escherichia coli | MIC 0.8 mg/mL of RA; MBC 0.9 mg/mL of RA | [189] |
| Staphylococcus aureus | MIC 1.0 mg/mL of RA; MBC 1.1 mg/mL of RA | ||||
| Salmonella | MIC 0.9 mg/mL of RA; MBC 1.0 mg/mL of RA | ||||
| Bacillus subtilis | MIC 1.0 mg/mL of RA; MBC 1.1 mg/mL of RA | ||||
| Corynebacterium T25-17 Mycobacterium smegmatis 5003 Staphylococcus warneri T12A12 | MIC (2.5 mg/mL of RA) MIC (1.2 mg/mL of RA) MIC (1.2 mg/mL of RA) | Micrococcus luteus | MIC 0.1 mg/mL; MBC 0.2 mg/mL | [193] | |
| Rothia mucilagenosa | MIC 0.1 mg/mL; MBC 0.2 mg/mL | ||||
| Klebsiella sp. | IZ 28 mm at 1 mg/mL of RA | [177] | Streptococcus agalactiae | MIC 0.05 mg/mL; MBC 0.1 mg/mL | |
| Stenotrophomonas maltophela | IZ 19 mm at 1 mg/mL of RA | Streptococcus angiosus | MIC 0.05 mg/mL; MBC 0.1 mg/mL | ||
| Streptomyces sp. | IZ 26 mm at 1 mg/mL of RA | Streptococcus dysgalactie | MIC 0.05 mg/mL; MBC 0.1 mg/mL | ||
| Pantoea agglomerans | IZ 18 mm at 1 mg/mL of RA | Streptococcus oralis | MIC 0.05 mg/mL; MBC 0.1 mg/mL | ||
| Paenibacillus chibensis | IZ < 1 mm at RA-methyl ester IZ 4.4 mm at tannic acid IZ > 2 mm at RA-hexyl ester IZ between 3 mm and 4 mm at RA-propyl ester | [194] | Streptococcus parasanquinis | MIC 0.05 mg/mL; MBC 0.1 mg/mL | |
| Streptococcus pyogenes | MIC 0.1 mg/mL; MBC 0.2 mg/mL | ||||
| Streptococcus salivarius | MIC 0.002 mg/mL; MBC 0.004 mg/mL | ||||
| Staphylococcus waeneri | IZ < 1 mm at RA-methyl ester IZ 5 mm at tannic acid IZ ˃ 2 mm at RA-hexyl ester IZ between 2 mm and 3 mm at RA-propyl ester | Staphylococcus aureus | MIC ˃ 0.8 mg/mL; MBC ˃ 0.8 mg/mL | ||
| Staphylococcus hominis | MIC 0.4 mg/mL; MBC 0.8 mg/mL | ||||
| Bacillus cereus | IZ > 3 mm at RA-methyl ester IZ 6 mm at tannic acid IZ 7.7 mm at RA-hexyl ester IZ 9 mm at RA-propyl ester | Enterobacter cloacae | MIC 0.1 mg/mL; MBC 0.2 mg/mL | ||
| Strenotrophomonas maltophila | MIC0.4 mg/mL; MBC 0.8 mg/mL | ||||
| Bacillus subtilis | MICs 5 ppm of AR | [181] | Candida albicans 475/15 | MIC 0.1 mg/mL of RA MFC 0.2 mg/mL of RA | |
| Bacillus cereus | MICs 10 ppm of AR | Candida albicans 13/15 | MIC 0.1 mg/mL of RA; MFC 0.2 mg/mL of RA | ||
| Bacillus polymyxa | MICs 15 ppm of AR | Candida albicans 17/15 | MIC 0.1 mg/mL of RA; MFC 0.2 mg/mL of RA | ||
| C. butyricum: C. sporogenes | MICs of <20 ppm of RA | [190] | Candida albicans 527/14 | MIC 0.15 mg/mL of RA; MFC 0.3 mg/mL of RA | |
| SARS-CoV-2 | IC50 at 25.47 ng μL−1 of RA | [195] | Candida albicans 10/15 | MIC 0.15 mg/mL of RA; MFC 0.3 mg/mL of RA | |
| Enterovirus A71 (EV-A71) | In vivo 100 mg/kg/day of RA | [196] | Candida albicans 532 | MIC 0.1 mg/mL of RA; MFC 0.2 mg/mL of RA | |
| S. aureus | IZ 22 ± 1.00 mm at 1.33 ± 0.01 mg/g of RA | [183] | Candida albicans ATCC 10231 | MIC 0.2 mg/mL of RA; MFC 0.4 mg/mL of RA | |
| L. monocytogenes | IZ 20 ± 2.00 mm at 1.33 ± 0.01 mg/g of RA | Candia krusei H1/16 | MIC 0.2 mg/mL of RA; MFC 0.4 mg/mL of RA | ||
| E. coli | IZ 8 ± 0.50 mm at 1.33 ± 0.01 mg/g of RA | Candida glabrata 4/6/15 | MIC 0.1 mg/mL of RA; MFC 0.2 mg/mL of RA | ||
| S. typhimurium | IZ 10 ± 0.00 mm at 1.33 ± 0.01 mg/g of RA | Candida tropicalis ATCC 750 | MIC at 0.2 mg/mL of RA MFC at 0.4 mg/mL of RA | ||
| C. albicans | IZ 28 ± 3.00 mm at 1.33 ± 0.01 mg/g of RA | Candida parapsilosis ATCC 22019 | MIC at 0.1 mg/mL of RA MFC at 0.2 mg/mL of RA |
IZ: Inhibition Zone, MIC:minimal inhibitory concentration. MFC: minimal fungicidal concentration.
4. Antibiofilm Activity
The production of biofilms is one of the main processes responsible for antibiotic resistance. Recent research has revealed that natural substances based on secondary metabolites from plants can prevent the development of biofilms, which are responsible for about 80% of bacterial diseases [197,198]. Biofilms, which are bacterial colonies adhering to the surface and enveloped in a protective extracellular matrix, make bacteria up to 1000 times less susceptible to antibiotics and represent a real health problem [197]. The most common opportunistic fungal diseases in the world are Candida species, which form highly structured biofilms, which are collections of cells of different natures surrounded by an extracellular matrix. Furthermore, the current standard treatment for these infections is to seek innovative treatments for biofilm-related disorders, as these fungal biofilms are typically resistant to conventional antifungal drugs [198].
The quorum sensing inhibition (QSI) potential of rosmarinic acid (RA) towards Aeromonas hydrophila strains MTCC 1739, AH 1, and AH 12 was examined. The A. hydrophila pathogenic strains were isolated from infectious zebrafish species as well as an RA biofilm inhibitory concentration (BIC) versus A. hydrophila strains that was found as 750 μg mL−1. RA at this concentration decreased QS-induced production of hemolysin, elastase, and lipase from A. hydrophila. However, in FT-IR analysis, AR-treated A. hydrophila cells exhibited a reduction in cellular components, and the analysis of gene expression affirmed the negative regulation of virulence genes such as aerA, ahh1, ahyB, and lip. Zebrafish contaminated with A. hydrophila and given RA showed increased survival. Therefore, a study demonstrated the use of RA as an herbal compound to control biofilm formation by QS as well as virulence factor generation in A. hydrophila [199].
Biofilms of C. krusei H1/16 showed the highest resistance against rosmarinic acid treatment; MBEC > 1.6 mg/mL was the minimum biofilm eradication concentration, and biofilms of both C. albicans 475/15 and C. albicans ATCC 10,231 and eradicated with 0.4 mg/mL of rosmarinic acid. In contrast to cell attachment, biofilm formation was more strongly affected for C. albicans strains than for non-C. albicans [193].
RA consumption affected the formation of biofilms at a concentration- and the time-dependent manner, further implying for RA as an effective antimicrobial agent as well as for destroying the activity of planktonic cells and reducing the formation of biofilms at the earlier time stage to their development [200]. RA also inhibits the growth of E. coli K-12 and S. carnosus LTH1502, reducing the density and number of cells [188]. In acidic medium, RA was found to react chemically to nitrite ions to generate 6,6-nitro and 6-dinitrorosmarinic acids, the latter were active at submolecular levels as HIV-1 integrase inhibitors and inhibited viral replication in MT-4 cells, and antiviral effects [192]. RA nitration significantly increased integrase inhibition and antiviral effects without increasing the levels of cellular toxicity. In addition, RA also possesses antimicrobial effects against lactic acid bacteria, yeasts, molds, Enterobacteriaceae spp, and Pseudomonas spp, as well as against psychotropic drugs and L. monocytogenes from chicken meat [201]. In addition, RA exhibits inhibitory effects on the S. aureus cocktail by intimating morphological changes, decreasing and reducing all viable cells, and inducing morphological alterations into cheese and meat samples, from cell shrinkage to the formation of burr-like structures on the cell surface [202,203,204].
The antibacterial effects of rosmarinic acid (RA) against clinical strains of S. aureus from catheter infections were tested by Slobodníková et al. [200]. The regeneration method detected 24 h biofilm eradication activity on microtiter plates. The microtiter plate approach permitted the quantification for biofilm formation activity following application of RA to bacterial samples at 0, 1, 3, and 6 h post biofilm formation, with RA exhibiting antimicrobial activity at concentrations ranging from 625 to 1250 g·mL−1 (MICs equal to MBCs). In the concentration of the 156 to 5000 g·mL−1 evaluated range, there were no biofilm eradicating actions on the 24 h biofilm. When processed at the beginning of biofilm formation, RA subinhibitory doses inhibited the synthesis of biofilm; in concentrations less than the subinhibitory level, the formation of biofilm mass was increased in a time- and concentration-dependent manner. This evidence indicates the potential for RA to be an effective topical antimicrobial agent for treating catheter-related infections, with activity against both planktonic forms of bacteria and inhibitory activity during the early stages of biofilm development. However, it is not practical to use RA as the only agent to treat catheter-related infections [205].
5. Modes of Action
RA action modes are numerous (Figure 2), including immunomodulatory, analgesic, antimicrobial, neuroprotective, anticancer, anti-inflammatory, antioxidant, and anti-Alzheimer effects, and fertility stimulators [179,206,207,208,209,210,211,212,213,214,215].
The mode of action of RA may involve inactivation of cellular enzymes and changes in membrane permeability [216]. Zhu et al. [194] examined the reaction of RA-hex to glucosidase using the MB docking technique as a model study to explain the mechanism related to the inhibitory activity of S. cerevisiae glucosidase. In general, the authors found that the dihydroxyphenyl and hexyl groups are required for interaction with the active site (Figure 3).
In addition, RA allows depolymerization of the cell membrane [217], and proteomics of the bacterial membrane will reveal a modification after contact with RA [77]. RA has a bacteriostatic effect, capable of destroying bacterial cells and their proteins as well as blocking Na+/K+-ATPase activity in the cell [189]. In addition, rosmarinic acid specifically inhibits the P-protein of hepatitis B virus [218], RA could influence the early state of viral infection and directly affect viral particles by affecting virus-P-selectin glycoprotein ligand-1 (PSGL1) interactions with heparan sulfate substance without affecting virus–scavenger receptor B2 (SCARB2) interactions [196].
Among the antifungal mechanisms of rosmarinic acid, there was a decrease in mitochondrial activity, deterioration of membrane integrity, and mild inhibition of virus activity, integrity, and a slight suppression in proteases production except ergosterol binding. Antibiofilmic activity was associated to a limited extent with a decrease in the production of exopolysaccharides [193].
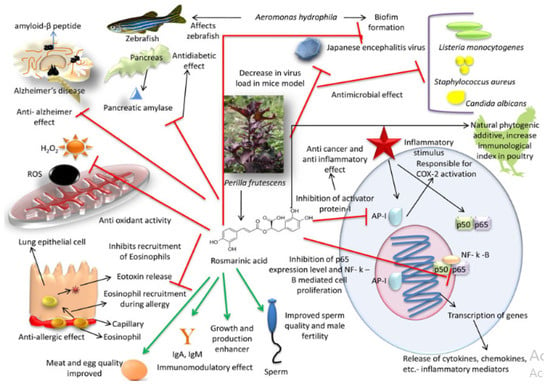
Figure 2.
Mode of action with a positive impact of rosmarinic acid [219].
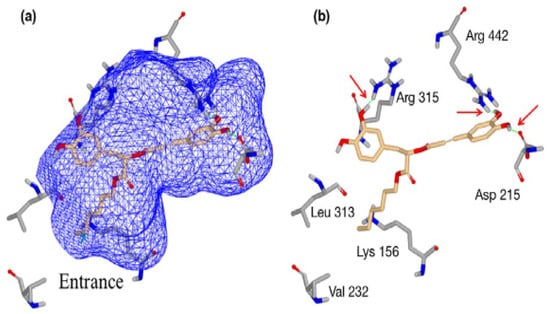
Figure 3.
A molecular docking system using rosmarinic acid hexyl ester (RA-hex) to the α-glucosidase active site from Saccharomyces cerevisiae, (a) RA-hex incorporated into the enzymatic pocket; (b) RA-hex interactions with key amino acid residues [194].
6. Combined Application of Rosmarinic Acid and Derivatives with Other Antimicrobial Agents
The main issue facing contemporary medicine: microbial resistance is being addressed from several angles, one of these approaches involves the use of new drugs in synergistic combination with conventional antibiotics that are already administered in treatment and to which microbial resistance has already evolved (Table 2). With penicillin, Polyalthia longifolia extracts have demonstrated a synergistic antibacterial action against a clinical isolate of MRSA, blocking the formation of biofilms, and the way that a substance works is connected to a phenomenon of cell agglutination and an alteration of the integrity of the cell membrane, which leads to cell lysis. These discoveries all indicate that extracts of P. longifolia leaves are a valuable source of trustworthy chemicals for the creation of new antimicrobials to combat antibiotic resistance [220].
S. aureus strains were discovered to be sensitive to RA’s antibacterial properties, with minimum inhibitory concentrations for methicillin-resistant S. aureus (MRSA) and S. aureus found to be 10 mg/mL and 0.8, respectively. Furthermore, RA showed synergistic benefits against S. aureus with amoxicillin, ofloxacin, and vancomycin drugs, but only with vancomycin against MRSA. In treatment with RA in conjunction with antibiotics, effectiveness is more than the use of individual antibiotics, according to a time-kill study. In addition, when RA and vancomycin are used together, the expression of the adhesion protein MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules) in MRSA and S. aureus is significantly reduced compared to RA alone [182].
The study’s findings by Coiai et al. [221] indicate that the antimicrobial powers of RA and ulvane, as well as their environmental impact, could be used in many products, especially since the COVID-19 pandemic made this need widespread. This would meet both the health and environmental needs of environmentalists.
Under in vitro conditions, RA has synergistic efficacy with antibiotics such as vancomycin to destroy methicillin-resistant S. aureus (MRSA) [182].
In this regard, it has been recommended to further exploit the antimicrobial characteristics of RA to discover useful applications for sustainable development. According to the research of Lu et al. [222], RA binds to VmFbpA, the FbpA of V. metschnikovii, both more strongly and competitively than Fe3+, with a KD value of 8 M vs. 17 M. Moreover, at a concentration of 1000 M, RA was able to reduce the growth of V. metschnikovii by up to 1/3 compared to controls. It was interesting to note that sodium citrate (SC), although not in turn a growth inhibitor, enhanced the impact of RA on growth. In addition to complete inhibition of V. metschnikovii growth at 100/100 M, the RA/SC mixture completely inhibits the growth of V. vulnificus and V. parahaemolyticus, at concentrations of 100/100 and 1000/100 M, respectively. In contrast, the growth of E. coli is not affected by RA/SC. Therefore, RA/SC is a potentially bacteriostatic drug active to Vibrio species while doing low damage to natural bacteria in the gastrointestinal tract.

Table 2.
Combined application of rosmarinic acid and derivatives with other antimicrobial agents.
Table 2.
Combined application of rosmarinic acid and derivatives with other antimicrobial agents.
| Rosmarinic Acid with | Microorganisms | Synergy | References |
|---|---|---|---|
| Vancomycin | Staphylococcus aureus | + | [182] |
| Ofloxacin | Staphylococcus aureus | + | |
| Amoxicillin | Staphylococcus aureus | + | |
| Vancomycin | MRSA | + | |
| Ofloxacin | MRSA | − | |
| Amoxicillin | MRSA | − | |
| Penicillin | MRSA | + | [220] |
| Methyl rosmarinate | Staphylococcus epidermidis 5001 | − | [77] |
| Stenotrophomonas maltophilia | − | ||
| Enterococcus faecalis C159-6 | − | ||
| Staphylococcus lugdunensis T26A3 | − | ||
| Pseudomonas aeruginosa ATCC 27583 | + | ||
| Corynebacterium T25-17 | − | ||
| Mycobacterium smegmatis 5003 | − | ||
| Staphylococcus warneri T12A12 | − | ||
| Isoquercetin | Staphylococcus epidermidis 5001 | − | |
| Stenotrophomonas maltophilia | − | ||
| Enterococcus faecalis C159-6 | − | ||
| Staphylococcus lugdunensis T26A3 | − | ||
| Pseudomonas aeruginosa ATCC 27583 | − | ||
| Corynebacterium T25-17 | − | ||
| Mycobacterium smegmatis 5003 | − | ||
| Staphylococcus warneri T12A12 | − | ||
| Hyperoside | Staphylococcus epidermidis 5001 | − | |
| Stenotrophomonas maltophilia | − | ||
| Enterococcus faecalis C159-6 | − | ||
| Staphylococcus lugdunensis T26A3 | − | ||
| Pseudomonas aeruginosa ATCC 27583 | − | ||
| Corynebacterium T25-17 | − | ||
| Mycobacterium smegmatis 5003 | + | ||
| Staphylococcus warneri T12A12 | + | ||
| Ulvan | COVID-19 | + | [221] |
| Chitosan | Escherichia coli | + | [223] |
| Polyvlactic acid/layered double hydroxides-Rosmarinic acid | Escherichia coli | + | [224] |
| Staphylococcus aureus | + | ||
| FeIII/MoO42/PO43 | herpes simplex virus | + | [225] |
| VSV-Ebola pseudotypes | + |
An isoblot analysis revealed an antioxidant synergistic activity of rosemary extract in methanol and BHA, and it is found that rosemary extract in methanol and BHA interaction synergistically inhibit the growth of S. aureus and E. coli. As a result, rosemary extract increases the antioxidant effectiveness of BHA and BHT as well as the antibacterial impact of BHA, allowing for a 4.4- to 17-fold reduction in the amount of synthetic chemicals used [226].
There is a chance that bacterial dietary pathogens will induce intestinal diseases, but Madureira et al. [227] proved that, with a zeta potential of 20 to 30 mV, RA-loaded nanoparticles can stick to the intestinal epithelium and release the antimicrobial agent into the gut (against L. innocua, B. cereus, E. coli O157, S. aureus, Y. enterocolitica, and S. typhimurium).
In addition, a combination of rosmarinic acid, chicoric acid, and caffeic acid with metal (FeIII) as well as inorganic (MoO42 and Po43) ions was shown to be antiviral towards VSV-Ebola, herpes simplex virus, vaccinia viruses, and pseudotypes [225]. These combinations have antiviral activity, and their mode of action occurs at a very preliminary level of viral replication with limited cellular toxicity.
The antibacterial efficacy on two pathogens, E. coli (Gram-negative) and Staphylococcus (Gram-positive), was tested on polylactic acid/double-laminated hydroxides/rosmarinic acid (PLA/LDH-RA) films. Cicogna et al. [224] adopted the recommended ISO 22196:2011 procedure for this test, and a percentage of the index (antibacterial activity R), which allows the evaluation of the effectiveness of an antibacterial agent or therapy, is obtained by comparing the amount of bacteria present in the tested sample immediately after their inoculation and after a certain period of time, and the strains of E. coli and S. aureus had an antibacterial activity R of 2.56 log CFU/cm2 and 2.74 log CFU/cm2, respectively.
7. Cytotoxic Effect of RA
The demand for new antimicrobial products is high, and products derived from natural sources are seen as a promising solution including plant polyphenols, such as rosmarinic acid, which is a natural substance with antimicrobial activity [226,227].
The cytotoxic effect of free RA on the viability of HeLa and MCF-7 cells was evaluated by Fuster et al. [228], and the results show that the cytotoxicity of free RA was much weaker against both cell lines, These two cell lines are of human origin and have been widely used in cytotoxicity studies.
Kolettas et al. [229] have reported that RA failed to suppress hydrogen peroxide-induced apoptosis and did not possess antioxidant properties on Jurkat cells. RA has been reported to induce apoptosis and cause cytotoxicity in HepG2 cells [230,231].
On the other hand, Huang et al. [232] found that RA combined with adriamycin induced apoptosis in HepG2 cells, and the cytotoxic concentration of RA (1000 mM) increased MG132-induced apoptosis.
For example, Murakami et al. [233] indicated that the cytotoxicity of RA can be related to its pro-oxidant action, while Hur et al. [234] reported that RA increases reactive oxygen species and induces apoptosis in Jurkat cells and peripheral T cells via the mitochondrial pathway. In accordance with these publications, there are increases in protein oxidation, apoptosis, and cytotoxicity in MG132-treated HepG2 cells when the cytotoxic concentration of RA was applied [235].
8. Conclusions
Rosmarinic acid has been shown to be a strong antibacterial agent that may be able to stop the growth of a wide range of bacterial and fungal infections. It has been shown that it can stop a wide range of microbial species from making biofilm. Some ways that antifungals might work are by breaking down membranes and changing how mitochondria work. Because of its wide antibacterial range as well as its existence in naturally occurring bioactive compounds, rosmarinic acid needs to be investigated further in the quest to discover novel antibiotics. In addition, the increasing number of pharmacological research projects reveals the strong interest in the biological activities of RA, which are extremely diverse. Rosmarinic acid can be considered a rich source of potential candidates to be included in the food system with promising effects at predetermined concentrations, avoiding toxicity.
Author Contributions
The authors (O.-N.K., Z.A., K.M. and P.R.) have equally contributed to this work. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their acknowledgment to Algeria’s Ministry of Higher Education, and This project was financed by the Foundation for Science and Technology (FCT, Portugal) by grants UIDB/04567/2020 and UIDP/04567/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simonsen, G.S. Antimicrobial resistance surveillance in Europe and beyond. Eurosurveillance 2018, 23, 1800560. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Drug Resist. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Scarpati, M.; Oriente, G. Chicoric acid (dicaffeyltartic acid): Its isolation from chicory (Chicorium intybus) and synthesis. Tetrahedron Lett. 1958, 4, 43–48. [Google Scholar] [CrossRef]
- Satake, T.; Kamiya, K.; Saiki, Y.; Hama, T.; Fujimoto, Y.; Kitanaka, S.; Kimura, Y.; Uzawa, J.; Endang, H.; Umar, M. Studies on the constituents of fruits of Helicteres isora L. Chem. Pharm. Bull. 1999, 47, 1444–1447. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Wysokińska, H. Production of secondary metabolites in shoots of Salvia nemorosa L. cultured in vitro. Biotechnologia 2003, 63, 154–159. [Google Scholar]
- Hörhammer, L.; Wagner, H.; Schilcher, H. On the knowledge of the constituents of Lycopus europaeus. 1. On the constituents of medicinal plants with hormone and antihormone-like action. Arzneim. Forsch. 1962, 12, 1–7. [Google Scholar]
- Kelley, C.J.; Harruff, R.C.; Carmack, M. Polyphenolic acids of Lithospermum ruderale. II. Carbon-13 nuclear magnetic resonance of lithospermic and rosmarinic acids. J. Org. Chem. 1976, 41, 449–455. [Google Scholar] [CrossRef]
- Tanaka, T.; Morimoto, S.; Nonaka, G.-I.; Nishioka, I.; Yokozawa, T.; Chung, H.Y.; Oura, H. Magnesium and ammonium-potassium lithospermates B, the active principles having a uremia-preventive effect from Salvia miltiorrhiza. Chem. Pharm. Bull. 1989, 37, 340–344. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Agata, I.; Hatano, T.; Nishibe, S.; Okuda, T. A tetrameric derivative of caffeic acid from Rabdosia japonica. Phytochem. Lett. 1989, 28, 2447–2450. [Google Scholar] [CrossRef]
- Nishizawa, M.; Tsuda, M.; Hayashi, K. Two caffeic acid tetramers having enantiomeric phenyldihydronaphthalene moieties from Macrotomia euchroma. Phytochem. Lett. 1990, 29, 2645–2649. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochem. Lett. 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y.; Wong, H. Sagecoumarin, a novel caffeic acid trimer from Salvia officinalis. Phytochem. Lett. 1999, 52, 1149–1152. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimura, A.; Kouno, I.; Nonaka, G.-i.; Young, T.-J. Isolation and characterization of yunnaneic acids a–d, four novel caffeic acid metabolites from Salvia yunnanensis. J. Nat. Prod. 1996, 59, 843–849. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Zheng, Q.; Sun, Z.; Zhang, X.; Yuan, J.; Wu, H.; Yang, J.; Xu, X. Clerodendranoic acid, a new phenolic acid from Clerodendranthus spicatus. Molecules 2012, 17, 13656–13661. [Google Scholar] [CrossRef]
- Trennheuser, F.; Burkhard, G.; Becker, H. Anthocerodiazonin an alkaloid from Anthoceros agrestis. Phytochem. Lett. 1994, 37, 899–903. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Hossain, M.A.; Said, S.A. Isolation and characterization of antimicrobial compound from the stem-bark of the traditionally used medicinal plant Adenium obesum. J. Tradit. Complement. Med. 2017, 7, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Tufa, T.; Damianakos, H.; Zengin, G.; Graikou, K.; Chinou, I. Antioxidant and enzyme inhibitory activities of disodium rabdosiin isolated from Alkanna sfikasiana Tan, Vold and Strid. S. Afr. J. Bot. 2019, 120, 157–162. [Google Scholar] [CrossRef]
- Kuruuzum-Uz, A.; Suleyman, H.; Cadirci, E.; Guvenalp, Z.; Demirezer, L.O. Investigation on anti-inflammatory and antiulcer activities of Anchusa azurea extracts and their major constituent rosmarinic acid. Z. Für Nat. C 2012, 67, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.-C.; Liu, Y.; Zheng, M.-Z.; Zhang, R.-Y.; Li, M.-X.; Bao, F.-Y.; Li, H.; Chen, L.-X. Triterpenoids from Anchusa italica and their protective effects on hypoxia/reoxygenation induced cardiomyocytes injury. Bioorg. Chem. 2020, 97, 103714. [Google Scholar] [CrossRef]
- Takeda, R.; Hasegawa, J.; Shinozaki, M. The first isolation of lignans, megacerotonic acid and anthocerotonic acid, from non-vascular plants, Anthocerotae (hornworts). Tetrahedron Lett. 1990, 31, 4159–4162. [Google Scholar] [CrossRef]
- Lasure, A.; Van Poel, B.; Pieters, L.; Claeys, M.; Gupta, M.; Berghe, D.V.; Vlietinck, A. Complement-inhibiting properties of Apeiba tibourbou. Planta Med. 1994, 60, 276–277. [Google Scholar] [CrossRef]
- Olivier, D.K.; van Wyk, B.-E.; van Heerden, F.R. The chemotaxonomic and medicinal significance of phenolic acids in Arctopus and Alepidea (Apiaceae subfamily Saniculoideae). Biochem. Syst. Ecol. 2008, 36, 724–729. [Google Scholar] [CrossRef]
- Yuzbasioglu, M.; Kuruuzum-Uz, A.; Guvenalp, Z.; Simon, A.; Tóth, G.; Harput, U.S.; Kazaz, C.; Bilgili, B.; Duman, H.; Saracoglu, I. Cytotoxic compounds from endemic Arnebia purpurea. Nat. Prod. Commun. 2015, 10, 1934578X1501000415. [Google Scholar] [CrossRef]
- Argoti, J.C.; Linares-Palomino, P.J.; Salido, S.; Ramírez, B.; Insuasty, B.; Altarejos, J. On-line activity screening for radical scavengers from Baccharis chilco. Chem. Biodivers. 2013, 10, 189–197. [Google Scholar] [CrossRef]
- Badem, M.; Sener, S.O.; Kanbolat, S.; Korkmaz, N.; Yildirmiş, S.; Ozgen, U.; Aliyazicioglu, R.; Salva, E.; Kaban, K.; Kandemir, A. Evaluation of biological activities of Barbarea integrifolia and isolation of a new glucosinolate derivated compound. Z. Für Nat. C 2021, 76, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, M.; Buommino, E.; Coretti, L.; Graziani, V.; Russo, R.; Caputo, P.; Donnarumma, G.; Fiorentino, A. Phytochemical investigation and antimicrobial assessment of Bellis sylvestris leaves. Phytochem. Lett. 2016, 17, 6–13. [Google Scholar] [CrossRef]
- de Mello Andrade, J.M.; dos Santos Passos, C.; Rubio, M.A.K.; Mendonça, J.N.; Lopes, N.P.; Henriques, A.T. Combining in vitro and in silico approaches to evaluate the multifunctional profile of rosmarinic acid from Blechnum brasiliense on targets related to neurodegeneration. Chem.-Biol. Interact. 2016, 254, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.-W.; Mi, Q. Phenolic compounds from Canna edulis Ker residue and their antioxidant activity. LWT-Food Sci. Technol. 2011, 44, 2091–2096. [Google Scholar] [CrossRef]
- Ly, T.N.; Shimoyamada, M.; Yamauchi, R. Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activity. J. Agric. Food Chem. 2006, 54, 3786–3793. [Google Scholar] [CrossRef]
- Yoshida, M.; Fuchigami, M.; Nagao, T.; Okabe, H.; Matsunaga, K.; Takata, J.; Karube, Y.; Tsuchihashi, R.; Kinjo, J.; Mihashi, K. Antiproliferative constituents from Umbelliferae plants VII. Active triterpenes and rosmarinic acid from Centella asiatica. Biol. Pharm. Bull. 2005, 28, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-Y. Studies on chemical constituents of Chloranthus fortunei. Chin. Tradit. Herb. Drugs 2020, 24, 1485–1490. [Google Scholar]
- Ma, X.; Huang, M.; Deng, S.; Yang, J.; Ke, R.; Song, P.; Yang, X. Chemical constituents and bioactivity of Chloranthus multistachys Pei. Yunnan Univ. 2017, 39, 124–129. [Google Scholar]
- Sun, Z.; Zheng, Q.; Ma, G.; Zhang, X.; Yuan, J.; Wu, H.; Liu, H.; Yang, J.; Xu, X. Four new phenolic acids from Clerodendranthus spicatus. Phytochem. Lett. 2014, 8, 16–21. [Google Scholar] [CrossRef]
- Murata, T.; Sasaki, K.; Sato, K.; Yoshizaki, F.; Yamada, H.; Mutoh, H.; Umehara, K.; Miyase, T.; Warashina, T.; Aoshima, H. Matrix metalloproteinase-2 inhibitors from Clinopodium chinense var. parviflorum. J. Nat. Prod. 2009, 72, 1379–1384. [Google Scholar] [CrossRef]
- Saltos, M.B.V.; Puente, B.F.N.; Malafronte, N.; Braca, A. Phenolic compounds from clinopodium tomentosum (Kunth) govaerts (Lamiaceae). J. Braz. Chem. Soc. 2014, 25, 2121–2124. [Google Scholar] [CrossRef]
- Wei, X.M.; Cheng, J.K.; Cheng, D.L.; Gao, L.M. Chemical constituents from Clinopodium urticifolium. J. Chin. Chem. Soc. 2004, 51, 1043–1049. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chem. 2007, 100, 356–361. [Google Scholar] [CrossRef]
- Petersen, M. Coleus spp.: In vitro culture and the production of forskolin and rosmarinic acid. In Medicinal and Aromatic Plants VI; Springer: Berlin/Heidelberg, Germany, 1994; pp. 69–92. [Google Scholar]
- Tewtrakul, S.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Yoshinaga, T.; Fujiwara, T.; Supavita, T.; Yuenyongsawad, S. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother. Res. 2003, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Hwang, S.H.; Kang, B.G.; Hong, J.S.; Lim, S.S. Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules 2014, 19, 13212–13224. [Google Scholar] [CrossRef] [PubMed]
- Fouseki, M.M.; Damianakos, H.; Karikas, G.A.; Roussakis, C.; Gupta, M.P.; Chinou, I. Chemical constituents from Cordia alliodora and C. colloccoca (Boraginaceae) and their biological activities. Fitoterapia 2016, 115, 9–14. [Google Scholar] [CrossRef]
- Marini, G.; Graikou, K.; Zengin, G.; Karikas, G.A.; Gupta, M.P.; Chinou, I. Phytochemical analysis and biological evaluation of three selected Cordia species from Panama. Ind. Crops Prod. 2018, 120, 84–89. [Google Scholar] [CrossRef]
- Owis, A.I.; Abo-Youssef, A.M.; Osman, A.H. Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats. Z. Für Nat. C 2017, 72, 107–118. [Google Scholar] [CrossRef]
- Fatima, M.; Siddiqui, B.; Begum, S. New neolignan glucoside and new biphenyl ether lignan from the fruits of Cordia latifolia. Chem. Nat. Compd. 2017, 53, 432–435. [Google Scholar] [CrossRef]
- Giles-Rivas, D.; Estrada-Soto, S.; Aguilar-Guadarrama, A.B.; Almanza-Pérez, J.; García-Jiménez, S.; Colín-Lozano, B.; Navarrete-Vázquez, G.; Villalobos-Molina, R. Antidiabetic effect of Cordia morelosana, chemical and pharmacological studies. J. Ethnopharmacol. 2020, 251, 112543. [Google Scholar] [CrossRef]
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef]
- Ticli, F.K.; Hage, L.I.; Cambraia, R.S.; Pereira, P.S.; Magro, Â.J.; Fontes, M.R.; Stábeli, R.G.; Giglio, J.R.; França, S.C.; Soares, A.M. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): Antiserum action potentiation and molecular interaction. Toxicon 2005, 46, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Damianakos, H.; Jeziorek, M.; Sykłowska-Baranek, K.; Buchwald, W.; Pietrosiuk, A.; Chinou, I. Pyrrolizidine alkaloids from Cynoglossum columnae ten.(Boraginaceae). Phytochem. Lett. 2016, 15, 234–237. [Google Scholar] [CrossRef]
- Sabrin, M.S.; Selenge, E.; Takeda, Y.; Batkhuu, J.; Ogawa, H.; Jamsransuren, D.; Suganuma, K.; Murata, T. Isolation and evaluation of virucidal activities of flavanone glycosides and rosmarinic acid derivatives from Dracocephalum spp. against feline calicivirus. Phytochem. Lett. 2021, 191, 112896. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-Q.; Dang, J.; Wen, H.-X.; Yuan, X.; Tao, Y.-D.; Wang, Q.-L. Anti-hepatitis, antioxidant activities and bioactive compounds of Dracocephalum heterophyllum extracts. Bot. Stud. 2016, 57, 16. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Yang, C.; Tian, Q.; Luo, Y.; Yang, C.; Liu, J. Chemical constituents of Dracoce-phalumtanguticum Maxim of genus Dracocephalum. Yunnan Minzu Univ. 2015, 24, 101–103. [Google Scholar]
- Le, T.T.; Kang, T.K.; Do, H.T.; Nghiem, T.D.; Lee, W.-B.; Jung, S.H. Protection against oxidative stress-induced retinal cell death by compounds isolated from Ehretia asperula. Nat. Prod. Commun. 2021, 16, 1934578X211067986. [Google Scholar] [CrossRef]
- Iqbal, K.; Nawaz, S.A.; Malik, A.; Riaz, N.; Mukhtar, N.; Mohammad, P.; Choudhary, M.I. Isolation and lipoxygenase-inhibition studies of phenolic constituents from Ehretia obtusifolia. Chem. Biodivers. 2005, 2, 104–111. [Google Scholar] [CrossRef]
- Simpol, L.R.; Otsuka, H.; Ohtani, K.; Kasai, R.; Yamasaki, K. Nitrile glucosides and rosmarinic acid, the histamine inhibitor from Ehretia philippinensis. Phytochem. Lett. 1994, 36, 91–95. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Xu, L.-J.; Li, M.-H.; Xiao, P.-G. Flavonoid glycosides and phenolic acids from Ehretia thyrsiflora. Biochem. Syst. Ecol. 2008, 36, 915–918. [Google Scholar] [CrossRef]
- Zhong, J.; Feng, F.; Li, H.; Li, H.; Li, R. Chemical constituents from Elsholtiza bodinieri Vaniot. Kunming Univ. Sci. Technol. 2013, 38, 75–79. [Google Scholar]
- Li, H.; Nakashima, T.; Tanaka, T.; Zhang, Y.-J.; Yang, C.-R.; Kouno, I. Two new maltol glycosides and cyanogenic glycosides from Elsholtzia rugulosa Hemsl. J. Nat. Med. 2008, 62, 75–78. [Google Scholar] [CrossRef]
- Peng, H.; Xing, Y.; Gao, L.; Zhang, L.; Zhang, G. Simultaneous separation of apigenin, luteolin and rosmarinic acid from the aerial parts of the copper-tolerant plant Elsholtzia splendens. Environ. Sci. Pollut. Res. 2014, 21, 8124–8132. [Google Scholar] [CrossRef] [PubMed]
- Devkota, H.P.; Tsushiro, K.; Watanabe, T. Bioactive phenolic compounds from the flowers of Farfugium japonicum (L.) Kitam. var. giganteum (Siebold et Zucc.) Kitam.(Asteraceae). Nat. Prod. Res. 2022, 36, 4036–4039. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Viladomat, F.; Bastida, J.; Schmeda-Hirschmann, G.; Burillo, J.; Codina, C. Bioguided isolation and identification of the nonvolatile antioxidant compounds from fennel (Foeniculum vulgare Mill.) waste. J. Agric. Food Chem. 2004, 52, 1890–1897. [Google Scholar] [CrossRef]
- Hawas, U.W.; Gamal-Eldeen, A.M.; El-Desouky, S.K.; Kim, Y.-K.; Huefner, A.; Saf, R. Induction of caspase-8 and death receptors by a new dammarane skeleton from the dried fruits of Forsythia koreana. Z. Für Nat. C 2013, 68, 29–38. [Google Scholar] [CrossRef]
- Shahat, A.A.; Hidayathulla, S.; Khan, A.A.; Alanazi, A.M.; Al Meanazel, O.T.; Alqahtani, A.S.; Alsaid, M.S.; Hussein, A.A. Phytochemical profiling, antioxidant and anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Trop. 2019, 191, 243–247. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Duan, J.-A.; Li, P.; Qian, S.-H. Chemical constituents of Glechoma longituba. Acta Pharm. Sin. 2006, 41, 431–434. [Google Scholar]
- Aquino, R.; Ciavatta, M.L.; De Simone, F.; Pizza, C. A flavanone glycoside from Hamelia patens. Phytochem. Lett. 1990, 29, 2359–2360. [Google Scholar] [CrossRef]
- Trute, A.; Nahrstedt, A. Identification and quantitative analysis of phenolic compounds from the dry extract of Hedera helix. Planta Med. 1997, 63, 177–179. [Google Scholar] [CrossRef]
- Yang, X.; Lei, Z.; Yu, Y.; Xiao, L.; Cheng, D.; Zhang, Z. Phytochemical characteristics of callus suspension culture of Helicteres angustifolia L. and its in vitro antioxidant, antidiabetic and immunomodulatory activities. S. Afr. J. Bot. 2019, 121, 178–185. [Google Scholar] [CrossRef]
- Tra, N.T.; Ha, N.T.T.; Cham, B.T.; Anh, L.T.T.; Yen, L.T.H.; Giang, B.L.; Anh, D.T.T.; Tuyen, N.V.; Kiem, P.V. A new benzofuran derivative from the stems of Helicteres hirsuta. Nat. Prod. Commun. 2019, 14, 1934578X19858814. [Google Scholar] [CrossRef]
- Sousa de Lucena, H.F.; Madeiro, S.A.L.; Siqueira, C.D.; Filho, J.M.B.; de Fátima Agra, M.; da Silva, M.S.; Fechine Tavares, J. Hypenol, a new lignan from Hypenia salzmannii. Helv. Chim. Acta 2013, 96, 1121–1125. [Google Scholar] [CrossRef]
- Abedini, A.; Roumy, V.; Mahieux, S.; Biabiany, M.; Standaert-Vitse, A.; Rivière, C.; Sahpaz, S.; Bailleul, F.; Neut, C.; Hennebelle, T. Rosmarinic acid and its methyl ester as antimicrobial components of the hydromethanolic extract of Hyptis atrorubens Poit.(Lamiaceae). Evid.-Based Complement. Altern. Med. 2013, 2013, 604536. [Google Scholar] [CrossRef] [PubMed]
- Almtorp, G.T.; Hazell, A.C.; Torssell, K.B. A lignan and pyrone and other constituents from Hyptis capitata. Phytochem. Lett. 1991, 30, 2753–2756. [Google Scholar] [CrossRef]
- Falcao, R.A.; do Nascimento, P.L.; de Souza, S.A.; da Silva, T.M.; de Queiroz, A.C.; da Matta, C.B.; Moreira, M.S.; Camara, C.A.; Silva, T. Antileishmanial phenylpropanoids from the leaves of Hyptis pectinata (L.) Poit. Evid.-Based Complement. Altern. Med. 2013, 2013, 460613. [Google Scholar] [CrossRef]
- Tang, G.; Liu, X.; Gong, X.; Lin, X.; Lai, X.; Wang, D.; Ji, S. Studies on the chemical compositions of Hyptis suaveolens (L.) Poit. J. Serb. Chem. Soc. 2019, 84, 245–252. [Google Scholar] [CrossRef]
- Kuhnt, M.; Rimpler, H.; Heinrich, M. Lignans and other compounds from the Mixe Indian medicinal plant Hyptis verticillata. Phytochem. Lett. 1994, 36, 485–489. [Google Scholar] [CrossRef]
- Furukawa, M.; Makino, M.; Ohkoshi, E.; Uchiyama, T.; Fujimoto, Y. Terpenoids and phenethyl glucosides from Hyssopus cuspidatus (Labiatae). Phytochem. Lett. 2011, 72, 2244–2252. [Google Scholar] [CrossRef]
- Arif, Z.; Khan, S.; Farheen, S.; Kazmi, M.H.; Fatima, I.; Malik, A.; Ali, M.S.; Inamullah, F.; Afaq, S.; Shaikh, S.A. Turpesteryl ester, a new antibacterial steroid from Ipomoea turpethum. Chem. Nat. Compd. 2020, 56, 270–273. [Google Scholar] [CrossRef]
- Niu, X.-M.; Li, S.-H.; Na, Z.; Mei, S.-X.; Zhao, Q.-S.; Sun, H.-D. Studies on chemical constituents of Isodon eriocalyx var. laxiflora. Chin. Tradit. Herb. Drugs 2003, 34, 300–303. [Google Scholar]
- LI, L.-J. Chemical constituents in ethyl acetate extract from Rabdosia flexicaulis. Chin. Tradit. Herb. Drugs 2015, 46, 339–343. [Google Scholar]
- Zhou, W.; Xie, H.; Xu, X.; Liang, Y.; Wei, X. Phenolic constituents from Isodon lophanthoides var. graciliflorus and their antioxidant and antibacterial activities. J. Funct. Foods 2014, 6, 492–498. [Google Scholar] [CrossRef]
- Huang, H.; Chao, Q.-R.; Tan, R.; Sun, H.-D.; Wang, D.-C.; Ma, J.; Zhao, S.-X. A new rosmarinic acid derivative from Isodon oresbius. Planta Med. 1999, 65, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Xiaoke, Z.; Qin, L.; Weisheng, F. Studies on chemical constituents of phenolic acids in Rabdosia rubescens. Zhongguo Yao Xue Za Zhi 2004, 39, 335–336. [Google Scholar]
- Khan, S.; Taning, C.N.T.; Bonneure, E.; Mangelinckx, S.; Smagghe, G.; Ahmad, R.; Fatima, N.; Asif, M.; Shah, M.M. Bioactivity-guided isolation of rosmarinic acid as the principle bioactive compound from the butanol extract of Isodon rugosus against the pea aphid, Acyrthosiphon pisum. PLoS ONE 2019, 14, e0215048. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, A.-J.; Li, M.-L.; Li, S.-H.; Han, Q.-B.; Wang, S.-J.; Lin, Z.-W.; Sun, H.-D. Cytotoxic ent-kaurane diterpenoids from Isodon sculponeata. Planta Med. 2002, 68, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Miyase, T.; Yoshizaki, F. Hyaluronidase inhibitors from Keiskea japonica. Chem. Pharm. Bull. 2012, 60, 121–128. [Google Scholar] [CrossRef]
- Dehaghi, N.K.; Gohari, A.; Sadat-Ebrahimi, S.; Badi, H.N.; Amanzadeh, Y. Phytochemistry and antioxidant activity of Lallemantia iberica aerial parts. Res. J. Pharmacogn. 2016, 3, 27–34. [Google Scholar]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Parejo, I.; Caprai, E.; Bastida, J.; Viladomat, F.; Jáuregui, O.; Codina, C. Investigation of Lepechinia graveolens for its antioxidant activity and phenolic composition. J. Ethnopharmacol. 2004, 94, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.I.; Chabán, M.F.; Lanza, P.A.; Joray, M.B.; Palacios, S.M.; Vera, D.M.A.; Carpinella, M.C. Inhibitory effects of compounds isolated from Lepechinia meyenii on tyrosinase. Food Chem. Toxicol. 2019, 125, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Esteves, P.F.; Kuster, R.M.; Barbi, N.d.S.; Menezes, F.d.S. Chemical composition and cytotoxic activity of Lepechinia speciosa (St. Hill) Epling. Lat. Am. J. Pharm 2010, 29, 38–44. [Google Scholar]
- Revoltella, S.; Baraldo, G.; Waltenberger, B.; Schwaiger, S.; Kofler, P.; Moesslacher, J.; Huber-Seidel, A.; Pagitz, K.; Kohl, R.; Jansen-Duerr, P. Identification of the NADPH oxidase 4 inhibiting principle of Lycopus europaeus. Molecules 2018, 23, 653. [Google Scholar] [CrossRef]
- Woo, E.-R.; Piao, M.S. Antioxidative constituents fromlycopus lucidus. Arch. Pharmacal Res. 2004, 27, 173–176. [Google Scholar] [CrossRef]
- Neamah, S.; Sarhan, I.A.; Al-Shaye’a, O.N. Extraction and evaluation of the anti-inflammatory activity of six compounds of Marrubium vulgare L. Biosci. Res. 2018, 15, 2393–2400. [Google Scholar]
- Murata, T.; Miyase, T.; Yoshizaki, F. Hyaluronidase inhibitory rosmarinic acid derivatives from Meehania urticifolia. Chem. Pharm. Bull. 2011, 59, 88–95. [Google Scholar] [CrossRef]
- Tagashira, M.; Ohtake, Y. A new antioxidative 1,3-benzodioxole from Melissa officinalis. Planta Med. 1998, 64, 555–558. [Google Scholar] [CrossRef]
- Aksit, H.; Çelik, S.M.; Sen, Ö.; Erenler, R.; Demirtas, I.; Telci, İ.; Elmastas, M. Complete isolation and characterization of polar portion of Mentha dumetorum water extract. Rec. Nat. Prod. 2014, 8, 277. [Google Scholar]
- She, G.M.; Xu, C.; Liu, B.; Shi, R.B. Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq.) with DPPH radical scavenging activity. J. Food Sci. 2010, 75, C359–C362. [Google Scholar] [CrossRef]
- Guvenalp, Z.; Ozbek, H.; Karadayi, M.; Gulluce, M.; Kuruuzum-Uz, A.; Salih, B.; Demirezer, O. Two antigenotoxic chalcone glycosides from Mentha longifolia subsp. longifolia. Pharm. Biol. 2015, 53, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sugimoto, Y.; Masuda, H.; Kamei, C. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol. Pharm. Bull. 2002, 25, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gao, H.; Chen, G.; Yang, X.; Wu, B.; Wu, L. Chemical constituents of the active parts of Mentha spicata L.(II). Shenyang Pharm. Univ 2006, 23, 212–216. [Google Scholar]
- Wang, F.; Xiang, R.; Lin, C.; Zhu, C. Chemical constituents from Mesona chinensis. Chin. Med. Mater. 2017, 40, 2839–2843. [Google Scholar]
- Küpeli Akkol, E.; Gürağaç Dereli, F.T.; Ilhan, M. Assessment of antidepressant effect of the aerial parts of micromeria myrtifolia Boiss. & Hohen on mice. Molecules 2019, 24, 1869. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, X.; Wang, P.; Tan, X.; Luo, Q.; Chen, X.; Pan, Z. Chemical constituents from stems and leaves of Microsorium fortunei. Chin. Med. Mater. 2017, 40, 2089–2092. [Google Scholar]
- De Tommasi, N.; De Simone, F.; De Feo, V.; Pizza, C. Phenylpropanoid glycosides and rosmarinic acid from Momordica balsamina. Planta Med. 1991, 57, 201. [Google Scholar] [CrossRef]
- Goldansaz, S.M.; Festa, C.; Pagano, E.; De Marino, S.; Finamore, C.; Parisi, O.A.; Borrelli, F.; Sonboli, A.; D’Auria, M.V. Phytochemical and biological studies of Nepeta asterotricha Rech. f.(Lamiaceae): Isolation of nepetamoside. Molecules 2019, 24, 1684. [Google Scholar] [CrossRef]
- Takeda, Y.; Ooiso, Y.; Masuda, T.; Honda, G.; Otsuka, H.; Sezik, E.; Yesilada, E. Iridoid and eugenol glycosides from Nepeta cadmea. Phytochem. Lett. 1998, 49, 787–791. [Google Scholar] [CrossRef]
- Rabee, M.; Andersen, Ø.M.; Fossen, T.; Enerstvedt, K.H.; Abu Ali, H.; Rayyan, S. Acylated flavone O-glucuronides from the aerial parts of Nepeta curviflora. Molecules 2020, 25, 3782. [Google Scholar] [CrossRef]
- Ruiz-Vargas, J.A.; Morales-Ferra, D.L.; Ramírez-Ávila, G.; Zamilpa, A.; Negrete-León, E.; Acevedo-Fernández, J.J.; Peña-Rodríguez, L.M. α-Glucosidase inhibitory activity and in vivo antihyperglycemic effect of secondary metabolites from the leaf infusion of Ocimum campechianum mill. J. Ethnopharmacol. 2019, 243, 112081. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Nair, M.; Strasburg, G.; DeWitt, D. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 2000, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and other polar constituents from Origanum dictamnus L. and their in vitro antimicrobial activity in clinical strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Delaunay, J.-C.; Pedrot, E.; Bernillon, S.; Madani, K.; Monti, J.-P.; Mérillon, J.-M.; Chibane, M.; Richard, T. New cyclolignans from Origanum glandulosum active against beta-amyloid aggregation. Rec. Nat. Prod. 2014, 8, 208–216. [Google Scholar]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef]
- Elmastas, M.; Celik, S.M.; Genc, N.; Aksit, H.; Erenler, R.; Gulcin, İ. Antioxidant activity of an Anatolian herbal tea—Origanum minutiflorum: Isolation and characterization of its secondary metabolites. Int. J. Food Prop. 2018, 21, 374–384. [Google Scholar] [CrossRef]
- Erenler, R.; Meral, B.; Sen, O.; Elmastas, M.; Aydin, A.; Eminagaoglu, O.; Topcu, G. Bioassay-guided isolation, identification of compounds from Origanum rotundifolium and investigation of their antiproliferative and antioxidant activities. Pharm. Biol. 2017, 55, 1646–1653. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Karioti, A.; Bergonzi, M.C.; Pescitelli, G.; Di Bari, L.; Skaltsa, H. Polar constituents from the aerial parts of Origanum vulgare L. ssp. hirtum growing wild in Greece. J. Agric. Food Chem. 2006, 54, 5388–5392. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, M.C.; Kim, K.H.; Kwon, H.C.; Choi, S.U.; Lee, K.R. A new phenolic amide from the roots of Paris verticillata. Molecules 2008, 13, 41–45. [Google Scholar] [CrossRef]
- Lim, H.J.; Woo, K.W.; Lee, K.R.; Lee, S.K.; Kim, H.P. Inhibition of proinflammatory cytokine generation in lung inflammation by the leaves of Perilla frutescens and its constituents. Biomol. Ther. 2014, 22, 62. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, J.H.; Lee, M.-H.; Lee, B.W.; Kwon, H.S.; Park, C.-H.; Shim, K.-B.; Kim, H.-T.; Baek, I.-Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wu, T.; Wang, Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT-Food Sci. Technol. 2009, 42, 131–136. [Google Scholar] [CrossRef]
- Senol, F.S.; Ślusarczyk, S.; Matkowski, A.; Pérez-Garrido, A.; Girón-Rodríguez, F.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Domaradzki, K. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L. Phytochem. Lett. 2017, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kubínová, R.; Švajdlenka, E.; Schneiderová, K.; Hanáková, Z.; Dall’Acqua, S.; Farsa, O. Polyphenols and diterpenoids from Plectranthus forsteri ‘Marginatus’. Biochem. Syst. Ecol. 2013, 49, 39–42. [Google Scholar] [CrossRef]
- Ji, H.-S.; Li, H.; Mo, E.-J.; Kim, U.-H.; Kim, Y.-H.; Park, H.-Y.; Jeong, T.-S. Low-density lipoprotein-antioxidant flavonoids and a phenolic ester from Plectranthus hadiensis var. tomentosus. Appl. Biol. Chem. 2019, 62, 58. [Google Scholar] [CrossRef]
- Kubínova, R.; Pořízková, R.; Navrátilová, A.; Farsa, O.; Hanáková, Z.; Bačinská, A.; Čížek, A.; Valentová, M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzym. Inhib. Med. Chem. 2014, 29, 749–752. [Google Scholar] [CrossRef]
- Kubínová, R.; Gazdová, M.; Hanáková, Z.; Jurkaninová, S.; Dall’Acqua, S.; Cvačka, J.; Humpa, O. New diterpenoid glucoside and flavonoids from Plectranthus scutellarioides (L.) R. Br. S. Afr. J. Bot. 2019, 120, 286–290. [Google Scholar] [CrossRef]
- Hu, H.; Wang, G.; Liu, J.; Cao, H.; Zheng, X. Studies on phenolic compounds from Polygonum aviculane. China J. Chin. Mater. Med. 2006, 31, 740–742. [Google Scholar]
- Zhu, J. Depsides from Prunella vulgaris. Chin. Chem. Lett. 2000, 11, 997–1000. [Google Scholar]
- Kim, H.-I.; Quan, F.-S.; Kim, J.-E.; Lee, N.-R.; Kim, H.J.; Jo, S.J.; Lee, C.-M.; Jang, D.S.; Inn, K.-S. Inhibition of estrogen signaling through depletion of estrogen receptor alpha by ursolic acid and betulinic acid from Prunella vulgaris var. lilacina. Biochem. Biophys. Res. Commun. 2014, 451, 282–287. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, D.H.; Lee, S.Y.; Kim, K.R.; Choi, S.U.; Hong, J.K.; Lee, J.H.; Park, Y.H.; Lee, K.R. Triterpenoic acids of Prunella vulgaris var. lilacina and their cytotoxic activities in vitro. Arch. Pharmacal Res. 2008, 31, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Chanu, M.B.; Labala, R.K.; Sheikh, Y.; Borah, J.C.; Ghosh, S.K.; Sahoo, D.; Singh, O.J.; Shakya, A.; Thongam, B. Bioassay guided isolation of alpha-glucosidase inhibitory compound, in vivo postprandial anti hyperglycemia and docking study of the isolated compound from the leaves of the methanolic extract of Quercus serrata. Biosci. Biotech. Res. Commun. 2018, 11, 647–657. [Google Scholar] [CrossRef]
- Hyun, H.B.; Shrestha, S.; Boo, K.H.; Cho, S.K. Evaluation of antioxidant potential of ethyl acetate fraction of Rosmarinus officinalis L. and its major components. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 715–722. [Google Scholar] [CrossRef]
- Koysu, P.; Genc, N.; Elmastas, M.; Aksit, H.; Erenler, R. Isolation, identification of secondary metabolites from Salvia absconditiflora and evaluation of their antioxidative properties. Nat. Prod. Res. 2019, 33, 3592–3595. [Google Scholar] [CrossRef]
- Qu, G.; Yue, X.; An, F.; Dai, S.; Li, G.; Li, B. Chemical constituents contained in Salvia castanea. China J. Chin. Mater. Med. 2012, 37, 1985–1989. [Google Scholar]
- Zhang, H.-J.; Li, L.-N. Salvianolic acid I: A new depside from Salvia cavaleriei. Planta Med. 1994, 60, 70–72. [Google Scholar] [CrossRef]
- Ertaş, A.; Çakırca, H.; Yener, I.; Akdeniz, M.; Fırat, M.; Topçu, G.; Kolak, U. Bioguided isolation of secondary metabolites from Salvia cerino-pruinosa Rech. f. var. cerino-pruinosa. Rec. Nat. Prod. 2021, 15, 585–592. [Google Scholar]
- Gao, J.-F.; Ding, L.; Zhang, P.; Liu, J.-X. Chemical constituents of Salvia chinensis. China J. Chin. Mater. Med. 2013, 38, 1556–1559. [Google Scholar]
- Qian, T.-X.; Li, L.-N. Isosalvianolic acid C, a depside possessing a dibenzooxepin skeleton. Phytochem. Lett. 1992, 31, 1068–1070. [Google Scholar] [CrossRef]
- Wang, X.; Kasimu, R.; Niyazi, Z. Studies on the chemical constituents of the flowers of salvia deserta schang. J. Xinjiang Med. Univ. 2003, 26, 583–585. [Google Scholar]
- Ai, C.-B.; Deng, Q.-H.; Song, W.-Z.; Li, L.-N. Salvianolic acid J, a depside from Salvia flava. Phytochem. Lett. 1994, 37, 907–908. [Google Scholar] [CrossRef]
- Kang, J.; Tang, Y.; Liu, Q.; Guo, N.; Zhang, J.; Xiao, Z.; Chen, R.; Shen, Z. Isolation, modification, and aldose reductase inhibitory activity of rosmarinic acid derivatives from the roots of Salvia grandifolia. Fitoterapia 2016, 112, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.-H. Triterpenes and phenolic acids from roots of Salvia kiaometiensis. Chin. Tradit. Herb. Drugs 2019, 24, 1043–1048. [Google Scholar]
- Gohari, A.R.; Saeidnia, S.; Malmir, M.; Hadjiakhoondi, A.; Ajani, Y. Flavones and rosmarinic acid from Salvia limbata. Nat. Prod. Res. 2010, 24, 1902–1906. [Google Scholar] [CrossRef]
- Tung, N.H.; Hung, L.Q.; Van Oanh, H.; Huong, D.T.L.; Thuong, P.T.; Long, D.D.; Hai, N.T. Bioactive phenolic compounds from the roots of Danshen (Salvia miltiorrhiza). Nat. Prod. Commun. 2018, 13, 1934578X1801301018. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, M.-H.; Choi, J.; Baek, N.-I.; Park, H.-J. In vivo sedative and gastroprotective activities of Salvia plebeia extract and its composition of polyphenols. Arch. Pharmacal Res. 2012, 35, 1403–1411. [Google Scholar] [CrossRef]
- Gong, X.; Yang, S. Isolation, identification and antioxidant properties of flavonoids from Salvia plebeia. Chin. Wild Plant. Resour. 2013, 32, 24–27. [Google Scholar]
- Yang, Y.; Bing, Z.; Sun, L.; Wu, Z.; Chen, W. Chemical constituents of Salvia przewalskii Maxim. Asian J. Chem. 2013, 25, 1747–1748. [Google Scholar]
- Wu, Z.-J.; Ouyang, M.-A.; Yang, C.-R. Polyphenolic constituents of Salvia sonchifolia. Acta Bot. Yunnanica 1999, 21, 393–398. [Google Scholar]
- Moharram, F.A.-e.; Marzouk, M.S.; El-Shenawy, S.M.; Gaara, A.H.; El Kady, W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012, 64, 1678–1687. [Google Scholar] [CrossRef]
- Çulhaoğlu, B.; Hatipoğlu, S.D.; Dönmez, A.A.; Topçu, G. Antioxidant and anticholinesterase activities of lupane triterpenoids and other constituents of Salvia trichoclada. Med. Chem. Res. 2015, 24, 3831–3837. [Google Scholar] [CrossRef]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochem. Lett. 2014, 108, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-F.; Chen, H.-s.; Li, J.-R.; Jiang, J.-D.; Li, Z.-R. Studies on polyphenolic chemical constitutents from root of Salvia yunnansis. China J. Chin. Mater. Med. 2007, 32, 1886–1890. [Google Scholar]
- Arda, N.; Gören, N.; Kuru, A.; Pengsuparp, T.; Pezzuto, J.M.; Qiu, S.-X.; Cordell, G.A. Saniculoside N from Sanicula europaea L. J. Nat. Prod. 1997, 60, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Liu, H.-Y.; Xie, B.-B.; Liu, Z.-H.; Chen, C.-X. Two new glycosides from Sanicula lamelligera. Z. Für Nat. B 2006, 61, 607–610. [Google Scholar] [CrossRef]
- Lee, I.-K.; Kim, M.-A.; Lee, S.-Y.; Hong, J.-K.; Lee, J.-H.; Lee, K.-R. Phytochemical constituents of Schizonepeta tenuifolia Briquet. Nat. Prod. Sci. 2008, 14, 100–106. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E.; Öztürk, M. Phytochemical contents, antioxidant effects, and inhibitory activities of key enzymes associated with Alzheimer’s disease, ulcer, and skin disorders of Sideritis albiflora and Sideritis leptoclada. J. Food Biochem. 2019, 43, e13078. [Google Scholar] [CrossRef]
- García, J.M.; Prieto, L.J.; Guevara, A.; Malagon, D.; Osorio, C. Chemical studies of yellow tamarillo (Solanum betaceum Cav.) fruit flavor by using a molecular sensory approach. Molecules 2016, 21, 1729. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Obuotor, E.; Onawunmi, G.O.; Ogundaini, A.O. Radical scavenging compounds from the aerial parts of Solenostemon monostachys briq (Lamiaceae). Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 140–144. [Google Scholar] [CrossRef]
- Trifan, A.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; Marcourt, L.; Wolfender, J.-L.; Wolfram, E.; Esslinger, N.; Grubelnik, A. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020, 262, 113169. [Google Scholar] [CrossRef]
- Boonyarikpunchai, W.; Sukrong, S.; Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 2014, 124, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Maggi, F. Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharm. Biol. 2017, 55, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Khouya, T.; Ramchoun, M.; Amrani, S.; Harnafi, H.; Rouis, M.; Couchie, D.; Simmet, T.; Alem, C. Anti-inflammatory and anticoagulant effects of polyphenol-rich extracts from Thymus atlanticus: An in vitro and in vivo study. J. Ethnopharmacol. 2020, 252, 112475. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Sen, O.; Yildiz, I.; Aydin, A. Antiproliferative activities of chemical constituents isolated from Thymus praecox subsp grossheimii (Ronniger) Jalas. Rec. Nat. Prod. 2016, 10, 766–770. [Google Scholar]
- Sevindik, H.G.; Ozgen, U.; Atila, A.; Er, H.O.; Kazaz, C.; Duman, H. Phtytochemical Studies and Quantitative HPLC Analysis of Rosmarinic Acid and Luteolin 5-O-β-D-Glucopyranoside on Thymus praecox subsp. grossheimii var. grossheimii. Chem. Pharm. Bull. 2015, 63, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Bae, J.-S.; Kim, T.; Kwon, O.J.; Kim, T.H. Polyphenolic constituents from the aerial parts of Thymus quinquecostatus var. japonica collected on Ulleung Island. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 811–816. [Google Scholar] [CrossRef]
- Aziz, S.; Irshad, M. Isolation of a new antibacterial polyphenol from Thymus serpyllum. Chem. Nat. Compd. 2014, 49, 1023–1027. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Ntella, M.; Mpompou, L.; Karallaki, F.; Athanasios, P.; Hadjipavlou-Litina, D.; Lazari, D. Study of the antioxidant activity of Thymus sibthorpii Bentham (Lamiaceae). J. Enzym. Inhib. Med. Chem. Res. 2016, 31, 154–159. [Google Scholar] [CrossRef]
- Ozgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Asci, A.; Kaya, Y.; Secen, H. Relationship between chemical structure and antioxidant activity of luteolin and its glycosides isolated from Thymus sipyleus subsp sipyleus var. sipyleus. Planta Med. 2010, 5, 12–21. [Google Scholar]
- Engelbertz, J.; Lechtenberg, M.; Studt, L.; Hensel, A.; Verspohl, E.J. Bioassay-guided fractionation of a thymol-deprived hydrophilic thyme extract and its antispasmodic effect. J. Ethnopharmacol. 2012, 141, 848–853. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Chang, Y.-Y.; Kuo, Y.-H.; Shiao, M.-S. Anti-Lipid-Peroxidative Principles from Tournefortia s armentosa. J. Nat. Prod. 2002, 65, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Shivaprasad Shetty, M.; Anil Kumar, N.V.; Živković, J.; Calina, D.; Oana Docea, A.; Emamzadeh-Yazdi, S.; Sibel Kılıç, C.; Goloshvili, T.; Nicola, S. Veronica plants—Drifting from farm to traditional healing, food application, and phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.-K.; Raita, O.; Bischin, C.; Silaghi-Dumitrescu, R.; Vlase, L. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak. J. Pharm. Sci 2015, 28, 2297–2303. [Google Scholar]
- Wang, J.; Pan, X.; Han, Y.; Guo, D.; Guo, Q.; Li, R. Rosmarinic acid from eelgrass shows nematicidal and antibacterial activities against pine wood nematode and its carrying bacteria. Mar. Drugs 2012, 10, 2729–2740. [Google Scholar] [CrossRef]
- Achamlale, S.; Rezzonico, B.; Grignon-Dubois, M. Rosmarinic acid from beach waste: Isolation and HPLC quantification in Zostera detritus from Arcachon lagoon. Food Chem. 2009, 113, 878–883. [Google Scholar] [CrossRef]
- Yesilbag, D.; Eren, M.; Agel, H.; Kovanlikaya, A.; Balci, F. Effects of dietary rosemary, rosemary volatile oil and vitamin E on broiler performance, meat quality and serum SOD activity. Br. Poult. Sci. 2011, 52, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Hsieh, F.-C.; Lin, Y.-J.; Wu, T.-Y.; Lin, C.-W.; Lin, C.-T.; Tang, N.-Y.; Jinn, T.-R. Magnesium lithospermate B and rosmarinic acid, two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Eur. J. Pharmacol. 2015, 755, 127–133. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358. [Google Scholar] [CrossRef]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar] [CrossRef]
- Myint, K.B.; Sing, L.C.; Wei, Z. Tannic acid as phytochemical potentiator for antibiotic resistance adaptation. APCBEE Procedia 2013, 7, 175–181. [Google Scholar] [CrossRef]
- Wang, T.; Ma, B.; Jin, A.; Li, X.; Zhang, X.; Wang, W.; Cai, Y. Facile loading of Ag nanoparticles onto magnetic microsphere by the aid of a tannic acid—Metal polymer layer to synthesize magnetic disinfectant with high antibacterial activity. J. Hazard. Mater. 2018, 342, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Costa, M.d.S.; Menezes, I.R.; de Matos, Y.M.L.; Calixto-Júnior, J.T.; Pereira, P.S.; Siqueira-Junior, J.P.; Leal-Balbino, T.C. Tannic acid affects the phenotype of Staphylococcus aureus resistant to tetracycline and erythromycin by inhibition of efflux pumps. Bioorganic Chem. 2017, 74, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Suriyarak, S.; Bayrasy, C.; Schmidt, H.; Villeneuve, P.; Weiss, J. Impact of fatty acid chain length of rosmarinate esters on their antimicrobial activity against Staphylococcus carnosus LTH1502 and Escherichia coli K-12 LTH4263. J. Food Prot. 2013, 76, 1539–1548. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Zhang, M.; Bai, B.; Yang, Y.; Fan, S. The antibacterial mechanism of perilla rosmarinic acid. Biotechnol. Appl. Biochem. 2022, 69, 1757–1764. [Google Scholar] [CrossRef]
- Cetin-Karaca, H. Evaluation of Natural Antimicrobial Phenolic Compounds against Foodborne Pathogens. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2011. [Google Scholar]
- Bailly, F.; Cotelle, P. Anti-HIV activities of natural antioxidant caffeic acid derivatives: Toward an antiviral supplementation diet. Curr. Med. Chem. 2005, 12, 1811–1818. [Google Scholar] [CrossRef]
- Dubois, M.; Bailly, F.; Mbemba, G.; Mouscadet, J.-F.; Debyser, Z.; Witvrouw, M.; Cotelle, P. Reaction of rosmarinic acid with nitrite ions in acidic conditions: Discovery of nitro-and dinitrorosmarinic acids as new anti-HIV-1 agents. J. Med. Chem. 2008, 51, 2575–2579. [Google Scholar] [CrossRef]
- Lindel, T. Chemistry and biology of the pyrrole–imidazole alkaloids. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 77, pp. 117–219. [Google Scholar]
- Zhu, F.; Wang, J.; Takano, H.; Xu, Z.; Nishiwaki, H.; Yonekura, L.; Yang, R.; Tamura, H. Rosmarinic acid and its ester derivatives for enhancing antibacterial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J. Food Biochem. 2019, 43, e12719. [Google Scholar] [CrossRef]
- Elebeedy, D.; Elkhatib, W.F.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Saleh, M.A.; Abd El Maksoud, A.I.; Badawy, I.; Al-Karmalawy, A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021, 11, 29267–29286. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Jheng, J.-R.; Lin, G.-H.; Chen, Y.-L.; Ho, J.-Y.; Liu, C.-J.; Hsu, K.-Y.; Chen, Y.-S.; Chan, Y.F.; Yu, H.-M. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg. Microbes Infect. 2020, 9, 1194–1205. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1934578X1300801223. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Rama Devi, K.; Srinivasan, R.; Kannappan, A.; Santhakumari, S.; Bhuvaneswari, M.; Rajasekar, P.; Prabhu, N.M.; Veera Ravi, A. In vitro and in vivo efficacy of rosmarinic acid on quorum sensing mediated biofilm formation and virulence factor production in Aeromonas hydrophila. Biofouling 2016, 32, 1171–1183. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–modes of antimicrobial and antibiofilm activities of a common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Honório, V.G.; Bezerra, J.; Souza, G.T.; Carvalho, R.J.; Gomes-Neto, N.J.; Figueiredo, R.C.; Melo, J.V.; Souza, E.L.; Magnani, M. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front. Microbiol. 2015, 6, 1223. [Google Scholar] [CrossRef]
- Zhuang, Y.; Jiang, J.; Bi, H.; Yin, H.; Liu, S.; Liu, T. Synthesis of rosmarinic acid analogues in Escherichia coli. Biotechnol. Lett. 2016, 38, 619–627. [Google Scholar] [CrossRef]
- Suriyarak, S.; Gibis, M.; Schmidt, H.; Villeneuve, P.; Weiss, J. Antimicrobial mechanism and activity of dodecyl rosmarinate against Staphylococcus carnosus LTH1502 as influenced by addition of salt and change in pH. J. Food Prot. 2014, 77, 444–452. [Google Scholar] [CrossRef]
- Simoes, M. Antimicrobial strategies effective against infectious bacterial biofilms. Curr. Med. Chem. 2011, 18, 2129–2145. [Google Scholar] [CrossRef]
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K.; Pauletti, P.M.; Januário, A.H.; Silva, M.L.A.E.; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm. Biol. 2013, 51, 1087–1090. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sehrawat, A.; Phour, M.; Kumar, R. Nutrient Acquisition and Soil Fertility: Contribution of Rhizosphere Microbiomes in Sustainable Agriculture. In Microbial BioTechnology for Sustainable Agriculture Volume 1; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–41. [Google Scholar]
- Yang, M.; Zhang, X.; Qiao, O.; Ji, H.; Zhang, Y.; Han, X.; Wang, W.; Li, X.; Wang, J.; Guo, L. Rosmarinic acid potentiates and detoxifies tacrine in combination for Alzheimer’s disease. Phytomedicine 2023, 109, 154600. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V. Multi-target neuroprotective effects of herbal medicines for Alzheimer’s disease. J. Ethnopharmacol. 2022, 290, 115107. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bispo, M.; Morocho-Jácome, A.L.; Escudeiro, C.C.; Martinez, R.M.; de Oliveira Pinto, C.A.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Photoprotective Efficacy of the Association of Rosmarinic Acid 0.1% with Ethylhexyl Methoxycinnamate and Avobenzone. Cosmetics 2023, 10, 11. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Yehya, A.H.; Asif, M.; Majid, A.M.A.; Oon, C.E. Polymolecular botanical drug of Orthosiphon stamineus extract (C5OSEW5050ESA) as a complementary therapy to overcome gemcitabine resistance in pancreatic cancer cells. J. Tradit. Complement. Med. 2023, 13, 39–50. [Google Scholar] [CrossRef]
- do Nascimento, R.F.; de Oliveira Formiga, R.; Machado, F.D.F.; de Sales, I.R.P.; de Lima, G.M.; Alves Júnior, E.B.; Vieira, G.C.; Pereira, R.F.; de Araújo, A.A.; de Araújo Junior, R.F. Rosmarinic acid prevents gastric ulcers via sulfhydryl groups reinforcement, antioxidant and immunomodulatory effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2265–2278. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Araniti, F.; Costas-Gil, A.; Cabeiras-Freijanes, L.; Lupini, A.; Sunseri, F.; Reigosa, M.J.; Abenavoli, M.R.; Sanchez-Moreiras, A.M. Rosmarinic acid induces programmed cell death in Arabidopsis seedlings through reactive oxygen species and mitochondrial dysfunction. PLoS ONE 2018, 13, e0208802. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Ikeda, S.; Uwai, K.; Taguchi, R.; Chayama, K.; Sakaguchi, T.; Narita, R.; Yao, W.-L.; Takeuchi, F.; Otakaki, Y. Rosmarinic acid is a novel inhibitor for Hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction. PLoS ONE 2018, 13, e0197664. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Savu, M.; Simo, M.K.; Fopokam, G.X.; Olaru, S.M.; Cioanca, O.; Boyom, F.F.; Stefan, M. New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus. Microorganisms 2022, 10, 1943. [Google Scholar] [CrossRef] [PubMed]
- Coiai, S.; Campanella, B.; Paulert, R.; Cicogna, F.; Bramanti, E.; Lazzeri, A.; Pistelli, L.; Coltelli, M.-B. Rosmarinic acid and Ulvan from terrestrial and marine sources in anti-microbial bionanosystems and biomaterials. Appl. Sci. 2021, 11, 9249. [Google Scholar] [CrossRef]
- Lu, P.; Sui, M.; Zhang, M.; Wang, M.; Kamiya, T.; Okamoto, K.; Itoh, H.; Okuda, S.; Suzuki, M.; Asakura, T. Rosmarinic acid and sodium citrate have a synergistic bacteriostatic effect against Vibrio species by inhibiting iron uptake. Int. J. Mol. Sci. 2021, 22, 13010. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan–Rosmarinic acid conjugates with antioxidant, anti-inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef] [PubMed]
- Cicogna, F.; Passaglia, E.; Benedettini, M.; Oberhauser, W.; Ishak, R.; Signori, F.; Coiai, S. Rosmarinic and Glycyrrhetinic Acid-Modified Layered Double Hydroxides as Functional Additives for Poly (Lactic Acid)/Poly (Butylene Succinate) Blends. Molecules 2023, 28, 347. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Romano, C.S.; Abadi, K.; Repetto, V.; Vojnov, A.A.; Moreno, S. Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 2009, 115, 456–461. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A.; Castro, P.M.; Pintado, M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015, 167, 210–216. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.; Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Abdelghany, T.M. Pharmacological Evaluation of Acacia nilotica Flower Extract against Helicobacter pylori and Human Hepatocellular Carcinoma In Vitro and In Silico. J. Funct. Biomater. 2023, 14, 237. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor activity of rosmarinic acid-loaded silk fibroin nanoparticles on HeLa and MCF-7 cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Kolettas, E.; Thomas, C.; Leneti, E.; Skoufos, I.; Mbatsi, C.; Sisoula, C.; Manos, G.; Evangelou, A. Rosmarinic acid failed to suppress hydrogen peroxide-mediated apoptosis but induced apoptosis of Jurkat cells which was suppressed by Bcl-2. Mol. Cell. Biochem. 2006, 285, 111–120. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Yan, H.; Wang, Y.-J.; Yang, Y.; Li, X.-B.; Shi, A.-C.; Xu, J.-W.; Lu, Y.-B.; Li, L.; Wang, X.-X. Proteomics analysis demonstrating rosmarinic acid suppresses cell growth by blocking the glycolytic pathway in human HepG2 cells. Biomed. Pharmacother. 2018, 105, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Elkhoely, A.A.; Eissa, A.A.; Youns, M.M. Rosmarinic acid enhances cisplatin cytotoxicity in hepg2 cell line and attenuates its nephrotoxicity in mice. Int. J. Pharm. Sci. Res. 2018, 9, 2731–2743. [Google Scholar]
- Huang, Y.; Cai, Y.; Huang, R.; Zheng, X. Rosmarinic acid combined with adriamycin induces apoptosis by triggering mitochondria-mediated signaling pathway in HepG2 and Bel-7402 Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Haneda, M.; Qiao, S.; Naruse, M.; Yoshino, M. Prooxidant action of rosmarinic acid: Transition metal-dependent generation of reactive oxygen species. Toxicol. Vitr. 2007, 21, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.-G.; Yun, Y.; Won, J. Rosmarinic acid induces p56lck-dependent apoptosis in Jurkat and peripheral T cells via mitochondrial pathway independent from Fas/Fas ligand interaction. J. Immunol. 2004, 172, 79–87. [Google Scholar] [CrossRef]
- Ozgun, G.; Ozgun, E. The cytotoxic concentration of rosmarinic acid increases MG132-induced cytotoxicity, proteasome inhibition, autophagy, cellular stresses, and apoptosis in HepG2 cells. Hum. Exp. Toxicol. 2020, 39, 514–523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).