Hydroxide-Mediated SNAr Rearrangement for Synthesis of Novel Depside Derivatives Containing Diaryl Ether Skeleton as Antitumor Agents
Abstract
1. Introduction
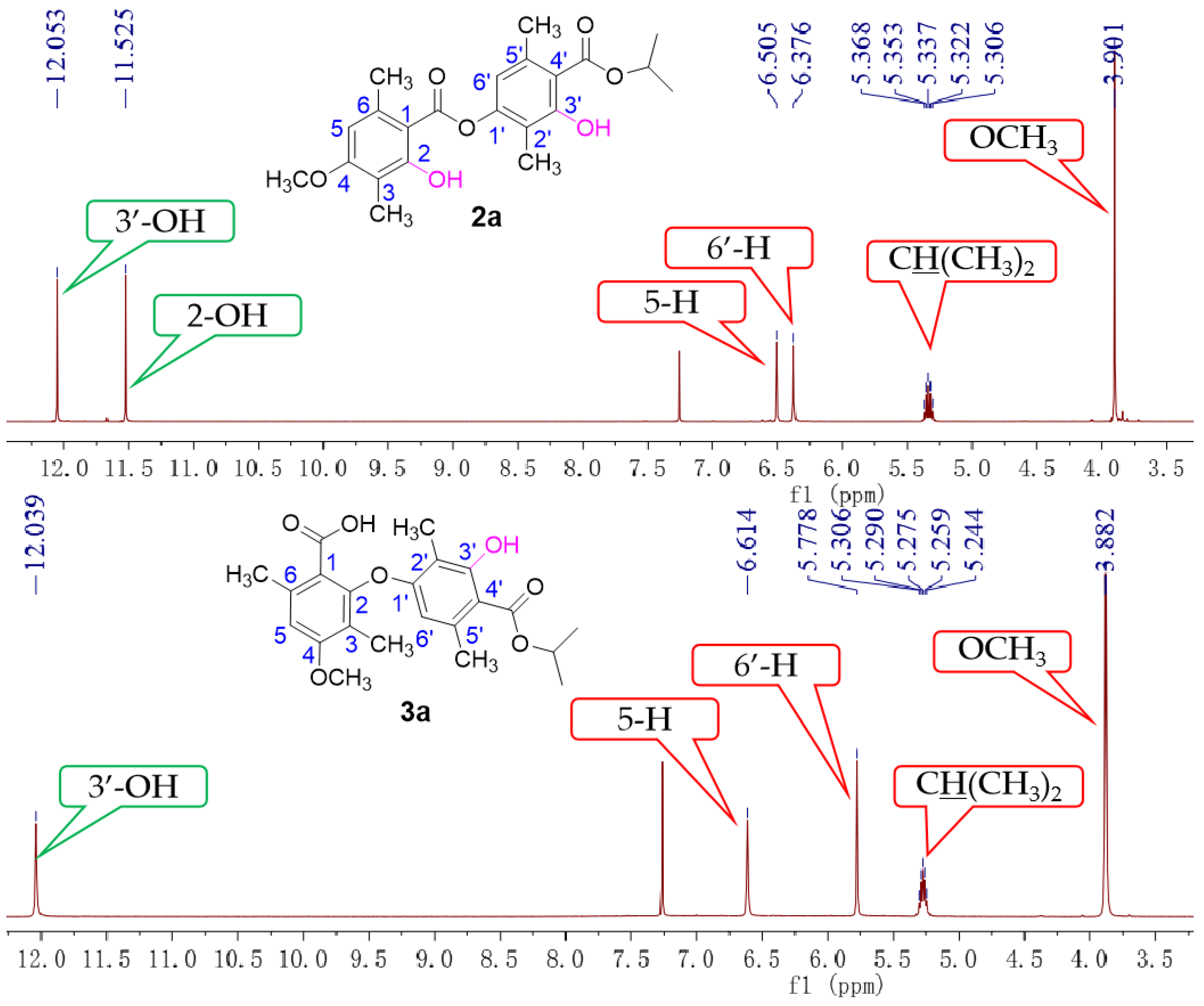
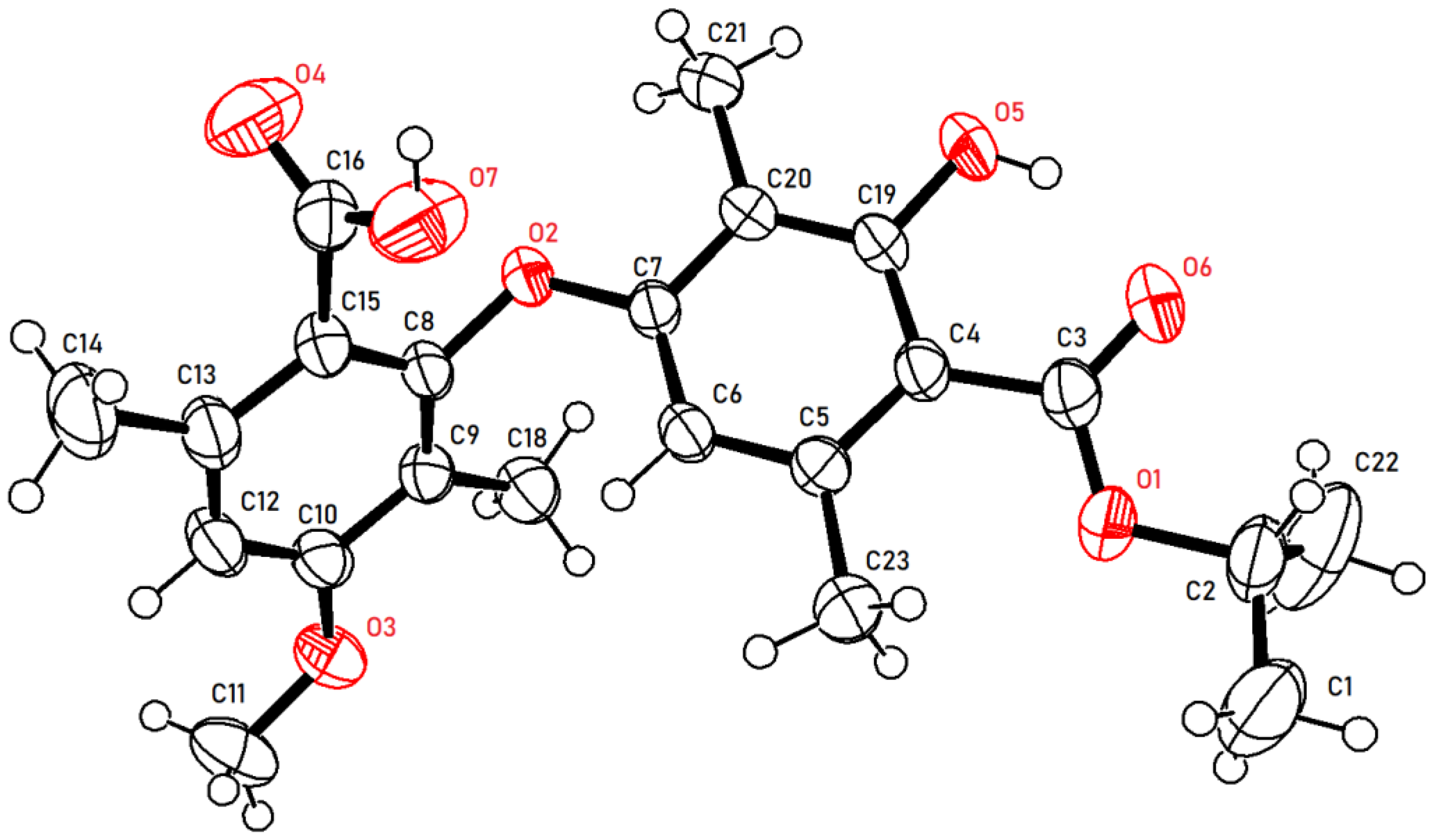
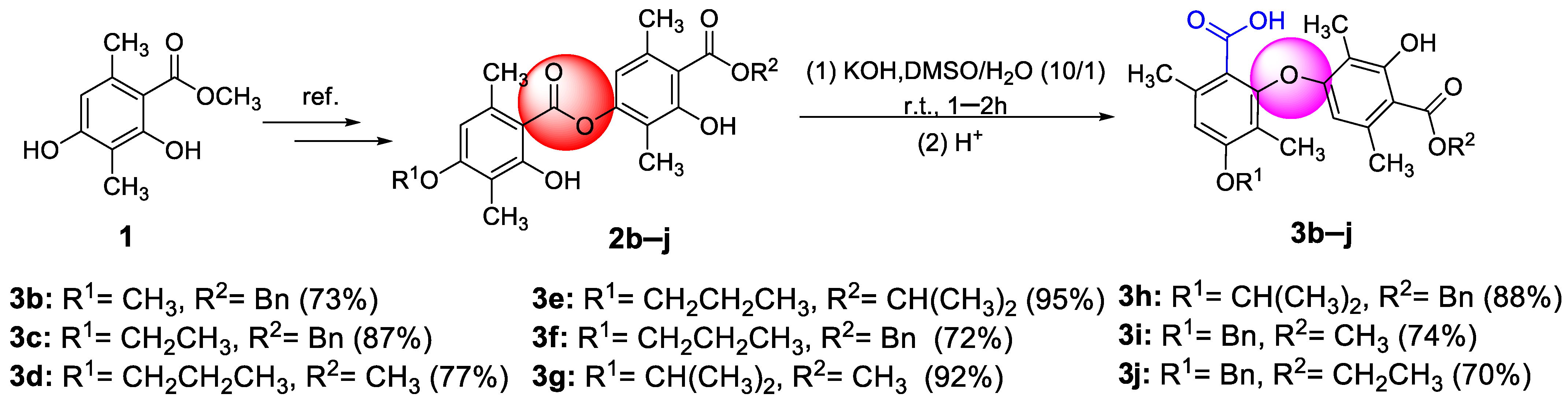
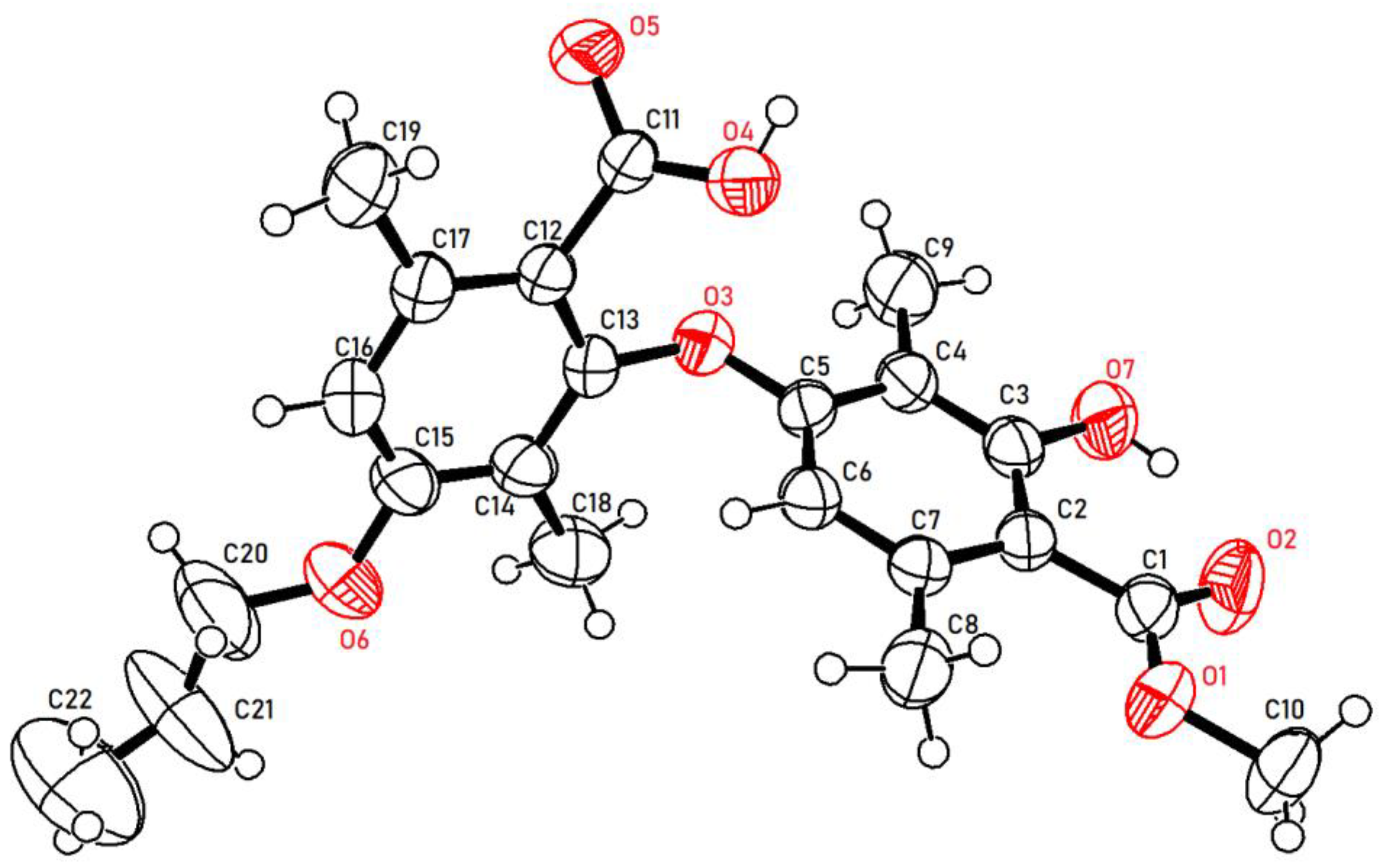
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Intermediates 2a–j
3.1.2. Synthesis of Target Compounds 3a–j
3.2. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ullah, M.; Uddin, Z.; Song, Y.H.; Li, Z.P.; Kim, J.Y.; Ban, Y.J.; Park, K.H. Bacterial neuraminidase inhibition by phenolic compounds from Usnea longissima. S. Afr. J. Bot. 2019, 120, 326–330. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Y.; Park, S.Y.; Nguyen, T.T.; Seo, Y.W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.S.; Kim, H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep. 2017, 7, 8136. [Google Scholar] [CrossRef] [PubMed]
- Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J.A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities. J. Nat. Prod. 2007, 70, 1218–1220. [Google Scholar] [CrossRef]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q. Antimycobacterial activity of lichen substances. Phytomedicine 2010, 17, 328–332. [Google Scholar] [CrossRef]
- Martins, M.C.; Silva, M.C.; Silva, H.A.; Silva, L.R.; Albuquerque, M.C.; Aires, A.L.; Falcão, E.P.; Pereira, E.C.; de Melo, A.M.; da Silva, N.H. Barbatic acid offers a new possibility for control of biomphalaria glabrata and schistosomiasis. Molecules 2017, 22, 568. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.D.; Siva, B.; Kumar, K.; Babu, V.S.P.; Sravanthi, V.; Boustie, J.; Nayak, V.L.; Tiwari, A.K.; Rao, C.H.V.; Sridhar, B.; et al. Comprehensive analysis of secondary metabolites in usnea longissima (lichenized ascomycetes, parmeliaceae) using UPLC-ESI-QTOF-MS/MS and pro-apoptotic activity of barbatic acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef]
- Silva, H.A.M.F.; Aires, A.L.; Soares, C.L.R.; Sá, J.L.F.; Martins, M.C.B.; Albuquerque, M.C.P.A.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Melo, A.M.M.A.; et al. Barbatic acid from Cladia aggregata (lichen): Cytotoxicity and in vitro schistosomicidal evaluation and ultrastructural analysis against adult worms of Schistosoma mansoni. Toxicol. In Vitro 2020, 65, 104771. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.; Brunauer, G.; Türk, R.; Stocker-Wörgötter, E. Production and bioactivity of common lichen metabolites as exemplified by Heterodea muelleri (Hampe) Nyl. J. Chem. Ecol. 2008, 34, 113–120. [Google Scholar] [CrossRef]
- Takahagi, T.; Ikezawa, N.; Endo, T.; Ifuku, K.; Yamamoto, Y.; Kinoshita, Y.; Takeshita, S.; Sato, F. Inhibition of PSII in atrazine-tolerant tobacco cells by barbatic acid, a lichen-derived depside. Biosci. Biotechnol. Biochem. 2006, 70, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xiong, H.; Yang, J.F.; Zhu, X.L.; Qu, R.Y.; Yang, G.F. Diaryl ether: A privileged scaffold for drug and agrochemical discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 239–266. [Google Scholar]
- Rainsford, K.D. Members of the Consensus Report Group on Nimesulide. Nimesulide—A multifactorial approach to inflammation and pain: Scientific and clinical consensus. Curr. Med. Res. Opin. 2006, 22, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, J.; Chankvetadze, B.; Cheng, Y.; Xu, J.; Liu, X.; Li, Y.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral triazole fungicide difenoconazole: Absolute stereochemistry, stereoselective bioactivity, aquatic toxicity, and environmental behavior in vegetables and soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, K.; Pan, L.; Xu, T.; Zhang, H.; Fantke, P. Measured and modeled residue dynamics of famoxadone and oxathiapiprolin in tomato fields. J. Agric. Food Chem. 2018, 66, 8489–8495. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, C.; Yi, H.; Meng, Q.; Bian, C.; Chen, H.; Jian, J.X.; Wu, L.Z.; Lei, A. External oxidant-free oxidative cross-coupling: A photoredox cobalt-catalyzed aromatic c-h thiolation for constructing c-s bonds. J. Am. Chem. Soc. 2015, 137, 9273–9280. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.R.; Giorgi, G.; Salas, C.O.; Tapia, R.A. New short strategy for the synthesis of the dibenz[b,f]oxepin scaffold. Molecules 2013, 18, 14797–14806. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, X.; Zhou, W.; Fan, M.; Lai, Y.; Ma, D. Copper-catalyzed diaryl ether formation from (hetero)aryl halides at low catalytic loadings. J. Org. Chem. 2017, 82, 4964–4969. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. N,N-dimethyl glycine-promoted Ullmann coupling reaction of phenols and aryl halides. Org. Lett. 2003, 5, 3799–37802. [Google Scholar] [CrossRef]
- Chen, G.; Chan, A.S.C.; Kwong, F.Y. Palladium-catalyzed C–O bond formation: Direct synthesis of phenols and aryl/alkyl ethers from activated aryl halides. Tetrahedron Lett. 2007, 48, 473–476. [Google Scholar] [CrossRef]
- Yu, X.; Xi, Y.K.; Luo, G.Y.; Long, Y.; Yang, W.D. Synthesis of barbacic acid. J. Asian Nat. Prod. Res. 2022, 24, 1150–1156. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, H.N.; Zhou, L.; Su, H.S.; Cheng, M.S.; Liu, Y. Synthesis and antitumor activity evaluation of oleanolic acid saponins bearing an acetylated l-arabinose moiety. Carbohydr. Res. 2021, 503, 108311. [Google Scholar] [CrossRef] [PubMed]




| Compound | A549 | HepG2 | 22RV1 |
|---|---|---|---|
| 3a | 2.61 | 0.48 | 32.84 |
| 3b | / a | 0.82 | / |
| 3c | 21.52 | 0.56 | / |
| 3d | / | 1.26 | 0.78 |
| 3e | 1.43 | / | / |
| 3f | 2.21 | / | 6.16 |
| 3g | 14.83 | / | / |
| 3h | 26.94 | / | 3.24 |
| 3i | / | 0.41 | / |
| 3j | / | 1.56 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xi, Y.; Sui, Y.; Liu, Y.; Chen, G.; Zhang, M.; Zhang, Y.; Luo, G.; Long, Y.; Yang, W. Hydroxide-Mediated SNAr Rearrangement for Synthesis of Novel Depside Derivatives Containing Diaryl Ether Skeleton as Antitumor Agents. Molecules 2023, 28, 4303. https://doi.org/10.3390/molecules28114303
Yu X, Xi Y, Sui Y, Liu Y, Chen G, Zhang M, Zhang Y, Luo G, Long Y, Yang W. Hydroxide-Mediated SNAr Rearrangement for Synthesis of Novel Depside Derivatives Containing Diaryl Ether Skeleton as Antitumor Agents. Molecules. 2023; 28(11):4303. https://doi.org/10.3390/molecules28114303
Chicago/Turabian StyleYu, Xiang, Yinkai Xi, Yi Sui, Yang Liu, Guifen Chen, Minjie Zhang, Yan Zhang, Guoyong Luo, Yi Long, and Wude Yang. 2023. "Hydroxide-Mediated SNAr Rearrangement for Synthesis of Novel Depside Derivatives Containing Diaryl Ether Skeleton as Antitumor Agents" Molecules 28, no. 11: 4303. https://doi.org/10.3390/molecules28114303
APA StyleYu, X., Xi, Y., Sui, Y., Liu, Y., Chen, G., Zhang, M., Zhang, Y., Luo, G., Long, Y., & Yang, W. (2023). Hydroxide-Mediated SNAr Rearrangement for Synthesis of Novel Depside Derivatives Containing Diaryl Ether Skeleton as Antitumor Agents. Molecules, 28(11), 4303. https://doi.org/10.3390/molecules28114303




