A Review of Research Progress on the Performance of Intelligent Polymer Gel
Abstract
1. Introduction
2. Single Response Intelligent Gel
2.1. Physical Stimulus Responsive Gel
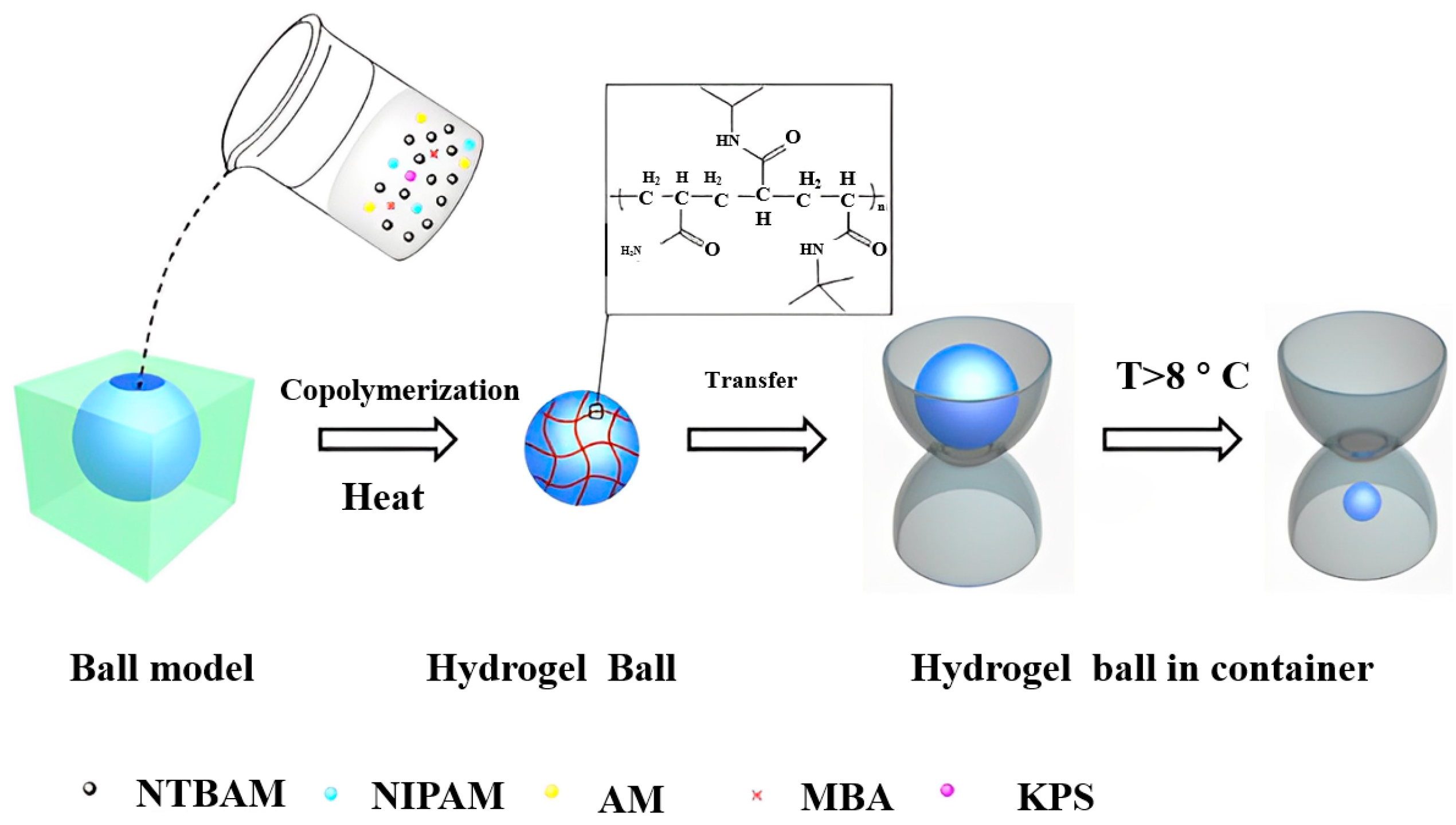
2.1.1. Temperature Response Type
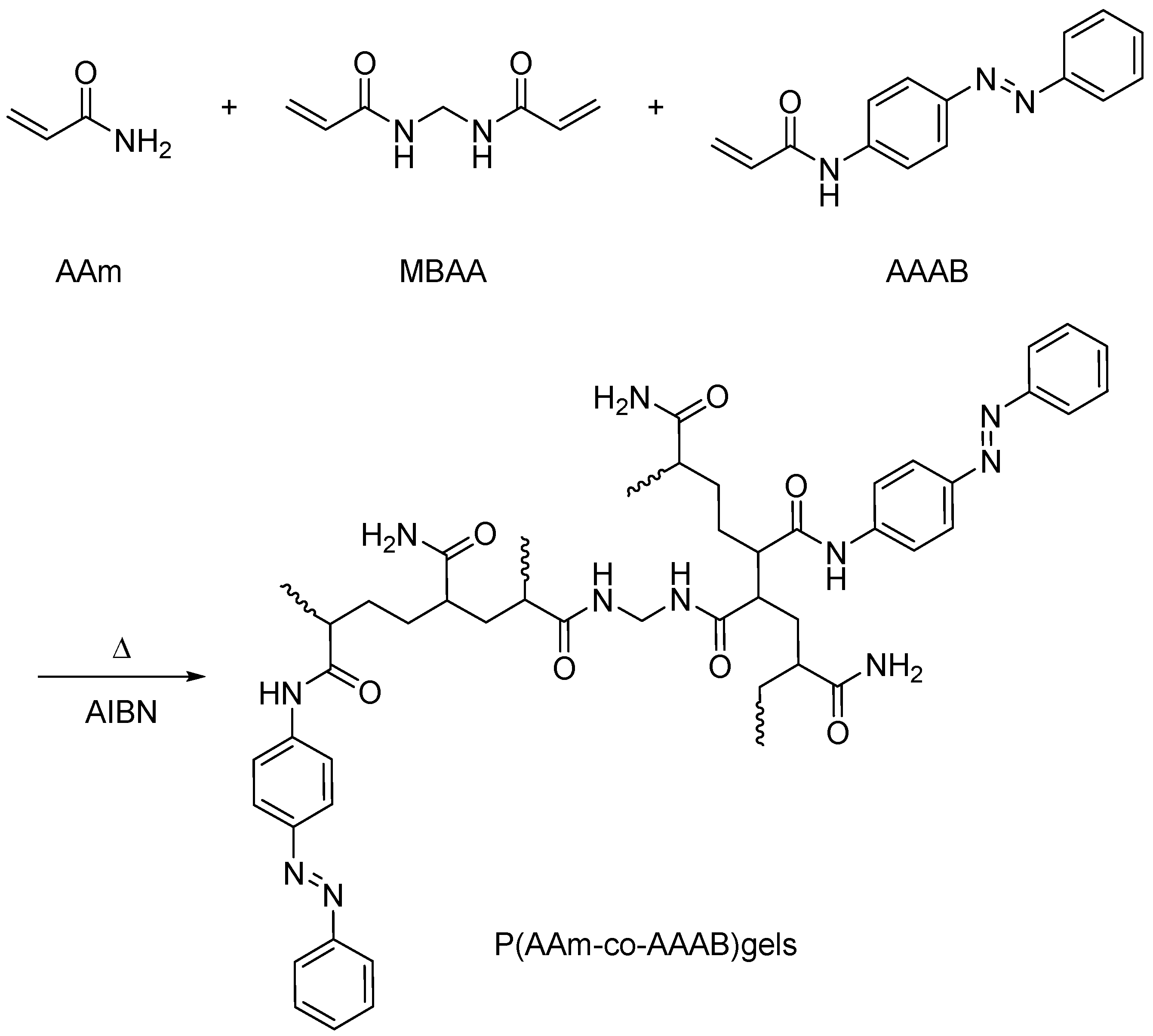
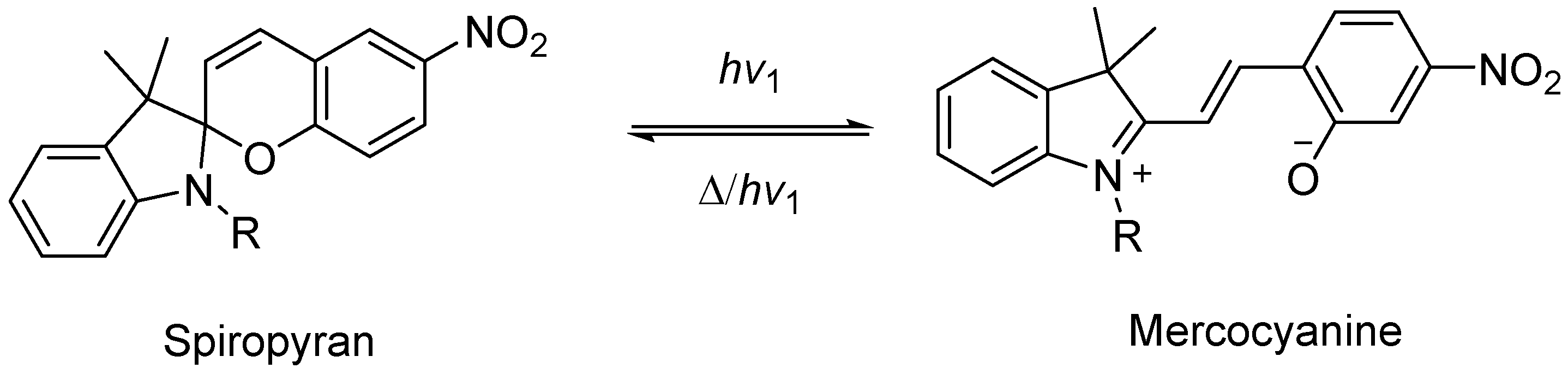
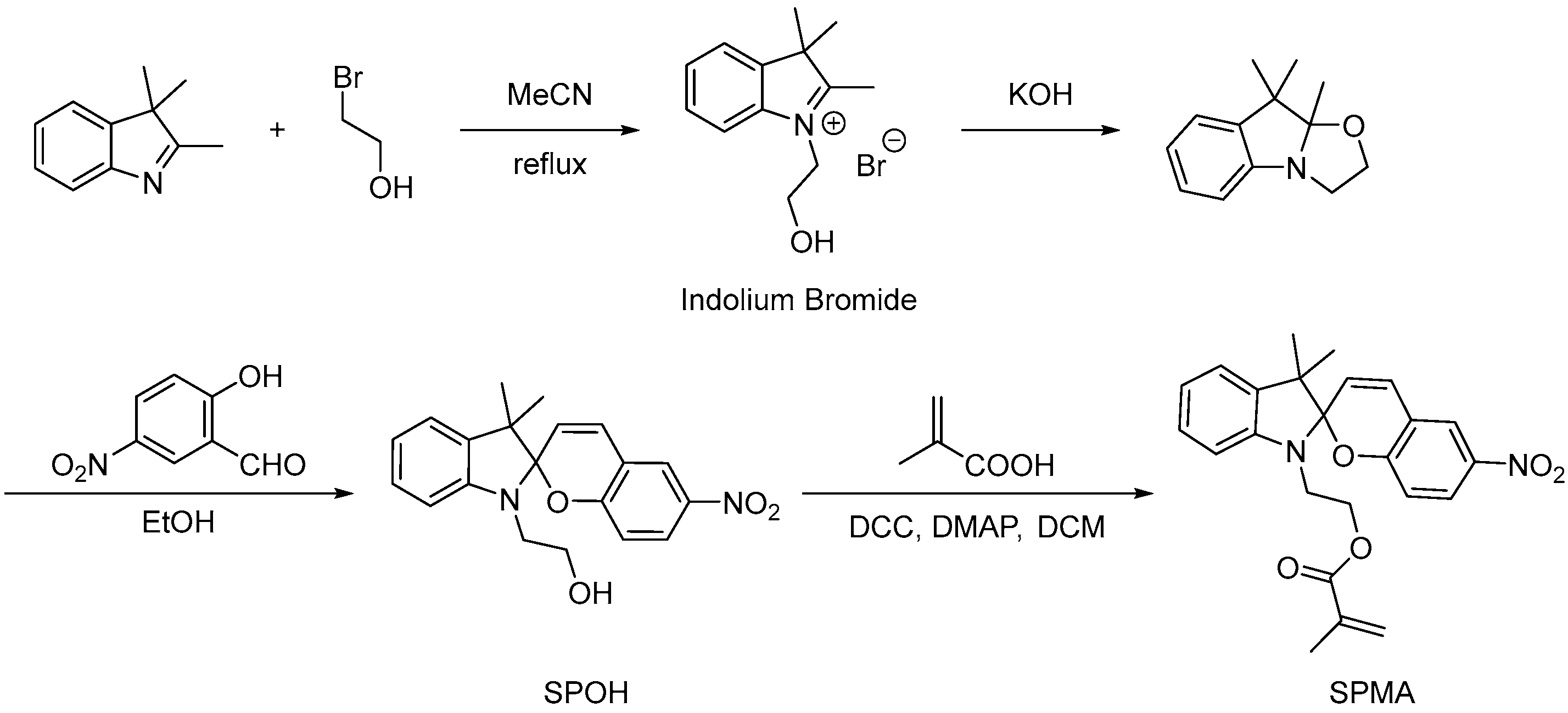
2.1.2. Light Response Type
Combining Photosensitive Compounds
Combining Photosensitive Group
React Oxide Particles with Hydrogel
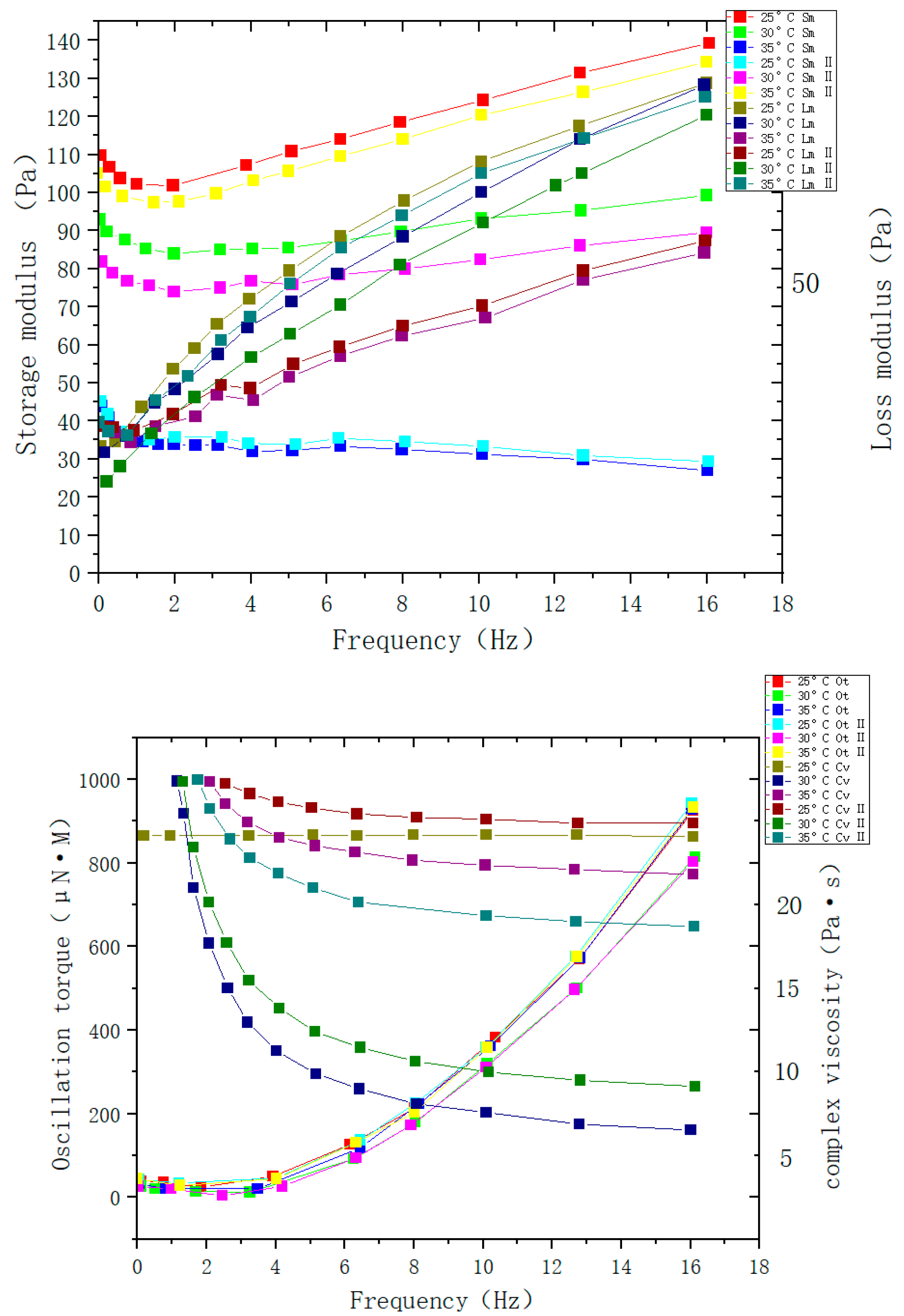
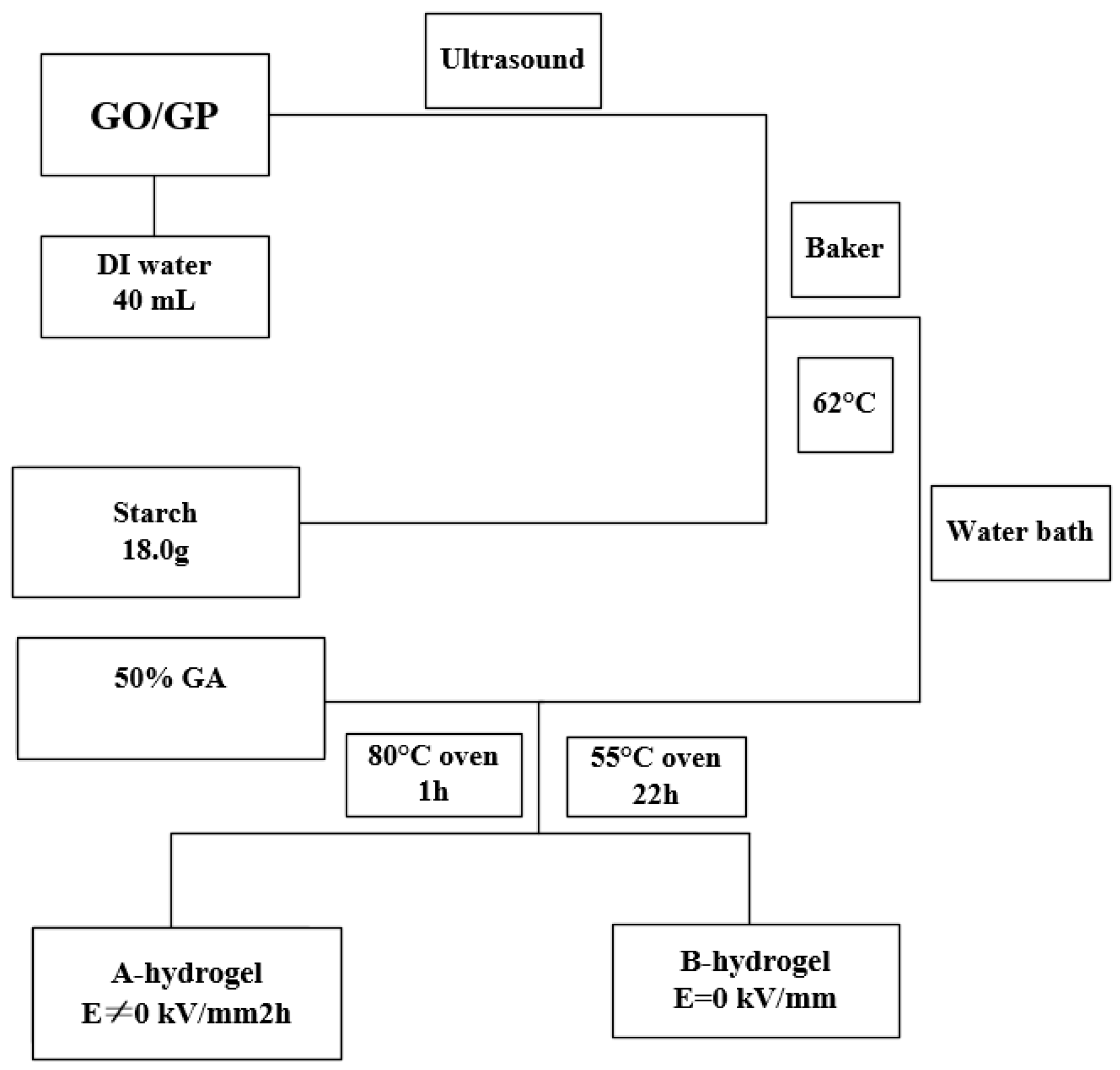
2.1.3. Electric Field Responsive Gel
2.2. Chemical Stimulus-Response Gel
2.2.1. pH-Responsive Type
2.2.2. Chemical Response Type
3. Multiple Stimulus-Response Type
4. Outlook
5. Conclusions
- (1)
- Single-response smart polymer gels have a single response and limited function, and generally have disadvantages such as poor biocompatibility and insufficient mechanical properties. They gradually cannot meet the needs of the development of various fields, and most of the current research is on physical response gels, with relatively little research on chemical response gels, and the research is still at the basic stage.
- (2)
- The multi-response intelligent polymer gel can respond to multiple external stimuli, and combines various single response characteristics. Yet these properties are not so directly connected, some of multi-response gels possess poor biocompatibility and no biodegradability, while gels with no responsive qualities may possess both of the abovementioned qualities. Some multi-response gels also have high mechanical strength and self-healing ability, which are more suitable for higher requirements in specific fields, but the diversification of functionality also means that their preparation methods are more complicated, and large-scale applications are not yet available, and still focus on a single response.
- (3)
- At present, most intelligent polymer gels have complex synthetic routes, poor biological compatibility, few response types, and poor mechanical properties. It is suggested to strengthen the development of these four characteristics to prepare new, environmentally, friendly, and excellent intelligent polymer gels, comparable to or even better than biological tissues. Temperature-sensitive smart hydrogels are commonly used as drug-delivery systems and show a great potential as responsive systems for cell detachment. Cytotoxicity is the main concern when using this type of stimuli-responsive hydrogel in the biomedical field. Magnetic hydrogels capable of changing their shape and properties in response to an external magnetic field have attracted high interest as a result of their numerous applications in the medical field. In the future, multi-responsive hydrogels will be the the next generation of smart hydrogels because they can respond to different stimuli and mimic biological processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIPAM-HA-MA | N-Isopropylacrylamide, hyaluronic acid (HA), Methacrylic anhydride (MA) |
| HDF | human dermal fibroblast |
| GO | graphite oxide |
| SP | Smart polymers |
| VPT | volumetric phase transition |
| ECM | extracellular matrix |
| PNIPAM | P-N-isopropylacrylamide |
| PEG-PCL-PEG, PECE | poly(ethylene glycol)-poly(-caprolactone)-poly(ethylene glycol) |
| FT-IR | Fourier transform infrared spectroscopy |
| 1H NMR | Nuclear magnetic resonance analysis, |
| GPC | Gel permeation chromatography |
| DSC | Differential scanning calorimetry |
| NIPAM | N-isopropylacrylamide |
| MSN | Mesoporous silica nanoparticles |
| LCST | Lower Critical Solution Temperature |
| KPS | Potassium persulfate |
| BSA | Bovine serum albumin |
| UV | Ultraviolet |
| MOAB | trans-4-methyl propenylhydr oxy azo benzene |
| AIBN | Azo-diisobutyronitrile(2, 2′-azo-bis(isobutyronitrile)) |
| AAAB | acrylamide azobenzene |
| AAm | acrylphthalamine |
| MBAA | methylene bisacrylamide |
| VIS | visible light |
| BIS | bisacrylamide |
| TEMED | tetramethylene diamine |
| PPy | nano-polypyrrole |
| CS | chitosan |
| AF | acid fuchsine |
| HEMA | hydroxyethyl methacrylate |
| AMPS | 2-acrylamide-2-methyl propane sulfonic acid |
| PANI/polyacrylamide | polyaniline/PAAM |
| ECH | Epichlorohydrin |
| SA | SODIUM ALGINATE |
| PGA | polyglutamic acid |
| Ty | tyramine |
| IPN | interpenetrating network |
| HRP | horseradish peroxidase |
| NHS | N-Hydroxysucci ni mide |
| EDC | 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide |
| CA | cysteamine |
| HA | hyaluronic acid |
| AAC | acrylicacid |
| PVP | polyvinyl pyrrolidone |
| EMK | 4,4′-Bis(diethylamino)benzophenone |
| TEA | triethanolamine |
| DPEPA | Dipentaerythritol Pentaacrylate |
| PLGA | polylactic acid-co-hydroxyacetic acid |
| BSA | bovine serum protein |
| HA-DOPA | dopamine modified hyaluronic acid |
| PVA | icrospheres/polyvinyl alcohol |
| PBA | Poly (butyl acrylate) |
| AAPBA | 3-Acrylamidobenzoic acid |
| DMAA | N,N-dimethylacrylamide |
| VPTT | volume phase transition temperature |
| FPBA | 4- formyl phenyl boronic acid |
| DPH | 3,3′–dithiobis(propionohydrazide) |
| NCA | nitrogen-carboxyl internal acid anhydride |
| BLG | Benzyl glutamate |
| PLG | γ-Acetylpropyl group-ʟ-Glutamic acid |
| UCST | upper critical solution temperature |
| RAFT | Reversible Addition-Fragmentation Chain Transfer Polymerization |
| NDA | PNIPAM-b-PDMA-b-PAA |
| NDD | PNIPAM-b-PDMA-b-PDMAEMA |
| ADAA | P(AM-co-AN)-b-PDMA-b-PAA |
| HMAM | Human mammaglobin copolymer |
| PMHD | P(MEO2MA-co-HMAM)-b-PDEAEMA |
| CP | chitosan grafted polyaniline |
| OD | Oxidized Dextran |
| DOX | doxorubicin |
References
- Kaplan, J.A.; Barthélémy, P.; Grinstaff, M.W. Self-assembled nanofiber hydrogels for mechanoresponsive therapeutic anti-TNFα antibody delivery. Chem. Commun. 2016, 52, 5860–5863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Weiss, R.G. Mechano-Responsive, Thermo-Reversible, Luminescent Organogels Derived from a Long-Chained, Naturally Occurring Fatty Acid. Chemistry 2016, 22, 8262–8272. [Google Scholar] [CrossRef] [PubMed]
- Manjua, A.C.; Alves, V.D.; Crespo, J.G.; Portugal, C. Magnetic Responsive PVA Hydrogels for Remote Modulation of Protein Sorption. ACS Appl. Mater. Interfaces 2019, 11, 21239–21249. [Google Scholar] [CrossRef]
- Sunakoda, K.; Yamamoto, N.; Nasuno, H.; Sakurai, H. Investigation on the Dynamic Shearing Characteristics of Magnetic Responsive Gel. ASME Digit. Collect. 2011, 54716, 17–23. [Google Scholar] [CrossRef]
- Yang, J.; He, X. A High-Strength, Salt-Responsive Dual-Network Hydrogel and Its Application. CN 110437472 A, 12 November 2019. [Google Scholar]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH-and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Ratner, B.; Hoffman, A.; Schoen, F.; Lemons, J. An Introduction to Materials in Medicine. Biomater. Sci. 2004, 26, 5093. [Google Scholar] [CrossRef]
- Carayon, I.; Gaubert, A.; Mousli, Y.; Philippe, B. Electro-responsive hydrogels: Macromolecular and supramolecular approaches in the biomedical field. Biomater. Sci. 2020, 8, 5589–5600. [Google Scholar] [CrossRef]
- Wang, K.; Dong, H.-Q.; Wen, H.-Y.; Xu, M.; Li, C.; Li, Y.-Y.; Jones, H.N.; Shi, D.-L.; Zhang, X.-Z. Novel Vesicles Self-Assembled from Amphiphilic Star-Armed PEG/Polypeptide Hybrid Copolymers for Drug Delivery. Macromol. Biosci. 2010, 11, 65–71. [Google Scholar] [CrossRef]
- Dang, V.L.; Rossbach, V. Mixed esters of chitin. J. Appl. Polym. Sci. 1995, 55, 679–685. [Google Scholar] [CrossRef]
- Pan, Y.; Cui, X.; Wang, H.; Lou, X.; Yang, S.; Oluwabusuyi, F.F. Research Progress of Intelligent Polymer Plugging Materials. Molecules 2023, 28, 2975. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 2015, 5, 159–164. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, J.; Zhang, X.; Shang, K. The Research Situation of Medicinal Gels. Chin. Med. Mod. Distance Educ. China 2016, 14, 150–152. [Google Scholar]
- Kollarigowda, R.H.; Mathews, A.S.; Abraham, S. Super Mechanical Stimuli Responsive Hydrogel: Dynamic Cues for Cell Applications. ACS Appl. Bio Mater. 2018, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly(N-Isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech 2019, 20, 119. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Mashimo, Y.; Mie, M.; Kobatake, E. Temperature-Responsive Multifunctional Protein Hydrogels with Elastin-like Polypeptides for 3D Angiogenesis. Biomacromolecules 2020, 21, 1126–1135. [Google Scholar] [CrossRef]
- Li, T.; Huang, F.; Diaz-Dussan, D.; Zhao, J.; Hao, X. Preparation and Characterization of Thermo-Responsive PEG Based Injectable Hydrogels and their Application for 3D Cell Culture. Biomacromolecules 2020, 21, 1254–1263. [Google Scholar] [CrossRef]
- Tu, X. Preparation of Graphite Oxide/Starch (Gelatin) Composite Hydrogel and Its Electric Field Response Properties, Shaanxi Normal University. 2018. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkWfZcByc-RON98J6vxPv10bWJ2_3EhnJD8Exaw6nxSFyRCaSJnr01ix30BGZTncts&uniplatform=NZKPT (accessed on 27 June 2018).
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Román, J.S. Chapter 1—Introduction to Smart Polymers and Their Applications, In Woodhead Publishing in Materials, Smart Polymers and their Applications, 2nd ed.; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing in Materials: Cambridge, UK, 2019; pp. 1–11. ISBN 9780081024164. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; San, R. Smart polymers and their applications as biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R.L., Chiellini, E., Eds.; 2007. [Google Scholar]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D.; Vo, V.T.; Lee, B.J. pH-sensitive polymeric systems for controlling drug release in nocturnal asthma treatment. In IFMBE Proceedings. 4th International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh City, Vietnam, 8–12 January 2012; Springer: Berlin/Heidelberg, Germany, 2013; Volume 40, pp. 304–308. [Google Scholar] [CrossRef]
- Arora, G.; Singh, I.; Nagpal, M.; Arora, S. Recent advances in stimuli induced pulsatile drug delivery system: A review. Res. J. Pharm. Technol. 2011, 4, 691–703. [Google Scholar]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Thermosensitive polymeric matrices for threedimensional cell culture strategies. Acta Biomater. 2011, 7, 526–529. [Google Scholar] [CrossRef]
- Piskin, E. Molecularly designed water soluble, intelligent, nanosize polymeric carriers. Int. J. Pharmaceut. 2004, 277, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S.; Stayton, P.S. Bioconjugates of smart polymers andproteins: Synthesis and applications. Macromol. Symp. 2004, 207, 139–151. [Google Scholar] [CrossRef]
- Maharjan, P.; Woonton, B.W.; Bennett, L.E.; Smithers, G.W.; DeSilva, K.; Hearn, M.T. Novel chromatographic separation—The potential of smart polymers. Innov. Food Sci. Emerg. Technol. 2008, 9, 232–242. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Kopeček, J. Polymer chemistry: Swell gels. Nature 2002, 417, 388–391. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for soft machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Bowser, B.H.; Craig, S.L. Empowering mechanochemistry with multi-mechanophore polymer architectures. Polym. Chem. 2018, 9, 3583–3593. [Google Scholar] [CrossRef]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef]
- Wang, Q.; Gossweiler, G.R.; Craig, S.L.; Zhao, X. Mechanics of mechanochemically responsive elastomers. J. Mech. Phys. Solids 2015, 82, 320–344. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef]
- King, W.J.; Murphy, W.L. Bioinspired conformational changes: An adaptable mechanism for bio-responsive protein delivery. Polym. Chem. 2011, 2, 476–491. [Google Scholar] [CrossRef]
- Kim, S.; Palomino, A.M.; Colina, C.M. Responsive polymer conformation and resulting permeability of clay–polymer nanocomposites. Mol. Simulat. 2012, 38, 723–734. [Google Scholar] [CrossRef]
- Brighenti, R.; Cosma, M.P. Swelling mechanism in smart polymers responsive to mechano-chemical stimuli. J. Mech. Phys. Solids 2020, 143, 104011. [Google Scholar] [CrossRef]
- Holtz, J.; Asher, S. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, F. Synthesis and properties of temperature-sensitive hydrogel based on hydroxyethyl cellulose. Int. J. Polym. Mater. 2010, 59, 450–461. [Google Scholar] [CrossRef]
- Sugiyama, I.; Ando, K.; Sadzuka, Y. The basic study of liposome in temperature-sensitive gel at body temperature for treatment of peritoneal dissemination. Gels 2022, 8, 252. [Google Scholar] [CrossRef]
- Kokufuta, E. Novel Applications for Stimulus-Sensitive Polymer Gels in the Preparation of Functional Immobilized Biocatalysts. In Responsive Gels: Volume Transitions II; Advances in Polymer Science, vol 110; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Argabright, P.A.; Luetzelschwab, W.E. Method of Diluting a Concentrated Polymer Solution for Use in a Hydrocarbon Recovery Process. U.S. Patent US4778607, 18 October 1988. [Google Scholar]
- Dubovik, A.S.; Kuznetsov, D.V.; Grinberg, N.V.; Grosberg, A.Y.; Tanaka, T. Studies of the Thermal Volume Transition of Poly(N-isopropylacrylamide) Hydrogels by High-Sensitivity Differential Scanning Microcalorimetry. 1. Dyn. Eff. 2000, 33, 8685–8692. [Google Scholar]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Kan, B.; Gou, M.L.; Wang, X.H.; Li, X.Y.; Luo, F.; Zhao, X.; Wei, Y.Q.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ning, L. Research on Biomimetic Structure Design and Preparation of Temperature-Sensitive Intelligent Hydrogel; Jilin University: Changchun, China, 2019. [Google Scholar]
- Zhang, Z. Preparation and Characterization of Temperature-Sensitive Intelligent Hydrogel Drug Delivery System Based on PNIPA; Shandong Agricultural University: Tai’an, China, 2020. [Google Scholar]
- Tang, L.; Gong, L.; Zhou, G.; Liu, L.; Zhang, D.; Tang, J.; Zheng, J. Design of low temperature-responsive hydrogels used as a temperature indicator. Polymer 2019, 173, 182–189. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, L.; Shen, L.; Xu, D. Selection of Phase-transition Temperature and Preparation of Polysaccharide Thermosensitive Gel for External Application. Chin. J. Mod. Appl. Pharm. 2009, 26, 294–297. [Google Scholar]
- Dong, Y.; Zhuang, H.; Hao, Y. Poly(N-Isopropyl- Acrylamide)/Poly(γ-Glutamic Acid) Thermo-Sensitive Hydrogels Loaded with Superoxide Dismutase for Wound Dressing Application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef]
- Schultz, J. Polymer materials science. Nature 1974. Available online: https://hdl.handle.net/2433/65404 (accessed on 20 February 2023).
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The light-controlling of temperature-responsivity in stimuli-responsive polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Atsushi, S.; Toyoichi, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Macromolecules 1990, 23, 1517–1519. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Sun, Z.; Fu, G. Preparation and performance study of intelligent photosensitive hydrogel. In Proceedings of the Abstracts of 2015 National Polymer Academic Papers Conference-Topic F-Biomedical Polymers, Nanjing China, 17–21 October 2015. [Google Scholar]
- Pourjavadi, A.; Mirjalili, B.F.; Entezami, A.A.; Zohuriaan-Mehr, M.J. Novel azo-containing polymethacrylates bearing spiroacetal-norbornene moiety and methylene spacers: Synthesis and characterization. Eur. Polym. J. 2001, 37, 2111–2121. [Google Scholar] [CrossRef]
- Cheng, H.; Tuo, X.; Gao, W.; Wang, X. Synthesis and properties of a novel side chain azo polyelectrolyte. Acta Polym. Sin. 2002, 1, 96–101. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Liu, D. Synthesis and light responsiveness of a water-soluble azo polyelectrolyte. J. Tsinghua Univ. Nat. Sci. Ed. 2002, 42, 622–624. [Google Scholar] [CrossRef]
- Kurihara, S.; Sakamoto, A.; Yoneyama, D.; Nonaka, T. Photochemical Switching Behavior of Liquid Crystalline Polymer Networks Containing Azobenzene Molecules. Macromolecules 1999, 32, 6493–6498. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Fernando, G.F.; Talbot, J.D.R. Synthesis and characterization of an azobenzene- and acrylamide-based photoresponsive copolymer and gel. J. Polym. Sci. Pol. Chem. 2004, 42, 2886–2896. [Google Scholar] [CrossRef]
- Zhao, Y. Synthesis and Properties of Light-Responsive Deformable Polymer Gel; Tianjin Polytechnic University: Tianjin, China, 2008. [Google Scholar]
- Liang, M.; Li, B.; Li, Q.; Chen, P.; Han, F.; Wang, Y. Research progress of intelligent polymer materials. Chem. Ind. Times 2002, 16, 16–19. [Google Scholar]
- Ma, T.; Walko, M.; Lepoitevin, M.; Janot, J.-M.; Balanzat, E.; Kocer, A.; Balme, S. Combining Light-Gated and pH-Responsive Nanopore Based on PEG-Spiropyran Functionalization. Adv. Mater. Interfaces 2018, 5, 1701051. [Google Scholar] [CrossRef]
- Yang, Q. Research on Mineralization and Light Response Properties of Dual-Network Hydrogels; Wuhan University: Wuhan, China, 2017. [Google Scholar]
- Feng, H.; Li, Y.; Li, J. Strong reduced graphene oxide–polymer composites: Hydrogels and wires. Rsc. Adv. 2012, 2, 6988–6993. [Google Scholar] [CrossRef]
- Huang, K. Preparation and Performance Study of Light-Responsive Intelligent Hydrogel; Nanjing University of Information Technology: Nanjing, China, 2019. [Google Scholar]
- Baillet, J.; Gaubert, A.; Bassani, D.M.; Verget, J.; Latxague, L.; Barthélémy, P. Supramolecular gels derived from nucleoside based bolaamphiphiles as a light-sensitive soft material. Chem. Commun. 2020, 56, 9569. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, N.; Peng, L.L.; Zhao, L.N.; Liu, M. A review of electro-stimulated gels and their applications: Present state and future perspectives. Mater. Sci. Eng. C 2019, 195, 104001. [Google Scholar] [CrossRef]
- Xing, J.; Yang, B.; Dang, W.; Li, J.; Bai, B. Preparation of Photo/Electro-Sensitive Hydrogel and Its Adsorption/Desorption Behavior to Acid Fuchsine. Water Air Soil Pollut. 2020, 231, 231. [Google Scholar] [CrossRef]
- Zhang, H. Preparation and Performance of P(HEMA-co-AMPS) Electric Field Response Gel and Photonic Crystal; Tianjin University: Tianjin, China, 2015. [Google Scholar]
- Han, X.; Dai, W.; Gao, M.; Xie, Z.; Gao, L. Preparation of sulfonated gelatin hydrogel and its electric field response properties. J. Funct. Polym. 2016, 29, 335–340. [Google Scholar]
- Wu, S.; Dong, H.; Li, Q.; Wang, G.; Cao, X. High strength, biocompatible hydrogels with designable shapes and special hollow-formed character using chitosan and gelatin. Carbohyd. Polym. 2017, 168, 147–152. [Google Scholar] [CrossRef]
- Dai, W. Preparation of Starch-Fe(III)/Al(III) Composite Hydrogel and Its Electric Field Response Properties; Shaanxi Normal University: Xi’an, China, 2017. [Google Scholar]
- Li, S.; Tao, Y.; Maryum, P.; Wang, Q.; Wang, C. Bifunctional polyaniline electroconductive hydrogels with applications in supercapacitor and wearable strain sensors. J. Biomater. Sci. Polym. Ed. 2020, 31, 938–953. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Biswanath, S.A. Electrically Responsive Intelligent Hydrogels in Drug Delivery: A Review. J. Appl. Biomater. Biomech. 2007, 5, 125–139. [Google Scholar]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohyd. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interfac Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Study on graphene-oxide-based polyacrylamide composite hydrogels. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1476–1481. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Li, L.; Gu, J.; Zhang, J.; Xie, Z.; Lu, Y.; Shen, L.; Dong, Q.; Wang, Y. Injectable and biodegradable pH-responsive hydrogels for localized and sustained treatment of human fibrosarcoma. ACS Appl. Mater. Interfaces 2015, 7, 8033–8040. [Google Scholar] [CrossRef]
- Morita-Imura, C.; Sakurai, Y.; Uchiumi, A.; Shindo, H. Ion-selective molecular inclusion of organic dyes into pH-responsive gel assemblies of zwitterionic surfactants. New J. Chem. 2019, 43, 8465–8471. [Google Scholar] [CrossRef]
- Castelletto, V.; Hamley, I.W.; Ma, Y. Microstructure and Physical Properties of a pH-Responsive Gel Based on a Novel Biocompatible ABA-Type Triblock Copolymer. Langmuir 2004, 20, 4306–4309. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, J.; Wang, Y.; Wu, J.; Meng, G.; Liu, Z. Preparation and adsorption and regeneration performance of pH-responsive cellulose-based aerogels. J. Shihezi Univ. Self Sci. Ed. 2017, 35, 287–292. [Google Scholar]
- Van Tran, V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, M.; Fu, L.; Yang, H. Preparation of alginic acid hydrogel based on reversible covalent acylhydrazone bond and its pH responsiveness. New Chem. Mater. 2019, 47, 180–184. [Google Scholar]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Cao, C.; Han, B.; Wang, X. Research progress of hydrogel for pulp regeneration. Int. J. Stomatol. 2021, 48, 192–197. [Google Scholar]
- Huang, Y.; He, K.; Wang, X. Rapid prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Ding, Z.; Zhao, Y.; Li, J.; Liu, M.; Prakash, S.; Zhang, D.; Wang, W.; Wang, Z.; et al. Preparation and Characterization of pH-Sensitive Poly(γ-glutamic acid)/Hyaluronic Acid Based Interpenetrating Network Medical Hydrogels. Chin. Polym. Bull. 2020, 2, 23–37. [Google Scholar]
- Nonoyama, T.; Gong, J.P. Double-network hydrogel and its potential biomedical application: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 853–863. [Google Scholar] [CrossRef]
- Hu, K. Processing and Application of Tunable Microfluidic Chip Based on pH-Responsive Hydrogel; University of Science and Technology of China: Hefei, China, 2020. [Google Scholar]
- Sattari, S.; Tehrani, A.D.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kuwata, H.; Matsumoto, H.; Ochi, K.; Suganami, T. Hollow fiber-combined glucose-responsive gel technology as an in vivo electronics-free insulin delivery system. Commun. Biol. 2020, 3, 313. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Siu, W.S.; Kan, C.-W.; Leung, P.-C.; Wanxue, C.; Chiou, J.-C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef]
- Shiino, D.; Murata, Y.; Kubo, A.; Kim, Y.J.; Kataoka, K.; Koyama, Y.; Kikuchi, A.; Yokoyama, M.; Sakurai, Y.; Okano, T. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J. Control Release 1995, 37, 269–276. [Google Scholar] [CrossRef]
- Matsumoto, A.; Ishii, T.; Kataoka, K.; Miyahara, Y. Glucose-Responsive Gel for Self-Regulated Insulin Delivery System. Drug Deliv. Syst. 2013, 28, 119–126. [Google Scholar] [CrossRef][Green Version]
- Lapeyre, V.; Ancla, C.; Catargi, B.; Ravaine, V. Glucose-responsive microgels with a core–shell structure. J. Colloid Interf. Sci. 2008, 327, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y. Drug Delivery System Based on Glucose-Sensitive Porous Microsphere/Polymer Composite Gel; Tianjin University: Tianjin, China, 2017. [Google Scholar]
- Tierney, S.; Volden, S.; Stokke, B.T. Glucose sensors based on a responsive gel incorporated as a Fabry-Perot cavity on a fiber-optic readout platform. Biosens. Bioelectron. 2009, 24, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yamamoto, K.; Yoshida, R.; Kataoka, K.; Aoyagi, T.; Miyahara, Y. A totally synthetic glucose responsive gel operating in physiological aqueous conditions. Chem. Commun. 2010, 46, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Preparation of poly(N-isopropylacrylamide-co-3-acrylamidophenylboronic acid) Glucose-Responsive Gel Fiber by Microfluidic Method; Donghua University: Shanghai, China, 2018. [Google Scholar]
- Wang, J. Preparation and Research of Glucose-Sensitive Hydrogel Based on PBA Microgel; Northwest University: Xi’an, China, 2019. [Google Scholar]
- Lan, R.; Zhu, L.; Wang, X.; Wu, W. Preparation of lectin-like microgel and its glucose response characteristics. Acta Polym. Sin. 2020, 51, 961–968. [Google Scholar]
- Tang, Z.; Guan, Y.; Zhang, Y. The synthesis of a contraction-type glucose-sensitive microgel working at physiological temperature guided by a new glucose-sensing mechanism. Polym. Chem. 2018, 9, 1012–1021. [Google Scholar] [CrossRef]
- Hisamitsu, I.; Kataoka, K.; Okano, T.; Sakurai, Y. Glucose-responsive gel from phenylborate polymer and poly(vinyl alcohol): Prompt response at physiological pH through the interaction of borate with amino group in the gel. Pharm. Res. 1997, 14, 289–293. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature-and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef]
- Mano, J.F. Stimuli-responsive polymeric systems for biomedical applications. Adv. Eng. Mater. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Ren, S.; Liang, H.; Sun, P.; Gao, Y.; Zheng, L. A tri-responsive and fast self-healing organogel with stretchability based on multiple dynamic covalent bonds. New J. Chem. 2020, 44, 1609–1614. [Google Scholar] [CrossRef]
- Meng, F. Preparation and Characterization of an Amino Acid-Based Temperature and pH Dual-Responsive Injectable Hydrogel; Qingdao University of Science and Technology: Qingdao, China, 2017. [Google Scholar]
- Chen, Y. Self-Assembly, Gelation and Drug Controlled Release Properties of Temperature/pH-Sensitive ABC Triblock Polymer; Shanghai University: Shanghai, China, 2019. [Google Scholar]
- Liu, S.; Tian, L.; Mao, H. Micellization and sol-gel transition of novel thermo- and pH-responsive ABC triblock copolymer synthesized by RAFT. J. Polym. Res. 2018, 25, 264. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “intelligent” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S. Study on the Biomimetic Preparation and Biocompatibility of Drug Intelligent Controlled-Release Hydrogel; Jilin University: Changchun, China, 2020. [Google Scholar]
- Zhao, S.; Zhu, H.; Shuai, S.; Chen, Z.; Hao, J. Preparation and properties of a temperature- and pH-responsive polypeptide hydrogel. Mater. Res. Express 2019, 6, 085711. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, G.; Cao, P.; Wang, Y.; Ge, Y.; Shuang, F. A Thin Film Pressure Sensor with Double Sensitive Units. In Proceedings of the 2015 IEEE International Conference on Information and Automation, Lijiang, China, 8–10 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 2947–2948. [Google Scholar] [CrossRef]
- Zhao, Y. Design Preparation and Biological Application of Biomimetic Intelligent Polymer Hydrogel Materials; Jilin University: Changchun, China, 2020. [Google Scholar]
- Shi, C.; Yao, Y.; Wu, G.; Sun, P.; Feng, J. The application progress of photopolymerization hydrogel in cartilage tissue engineering. Zhejiang Med. 2021, 43, 443–446+452. [Google Scholar]
- Biswas, S.; Yashin, V.V.; Balazs, A.C. Harnessing biomimetic cryptic bonds to form self-reinforcing gels. Soft Matter. 2020, 16, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hou, W.; Liang, Y.; Sun, D. Preparation and self-driving deformation of biomimetic magnetically responsive 4D intelligent hydrogel. Chin. J. Mech. Eng. 2020, 56, 90–96. [Google Scholar]




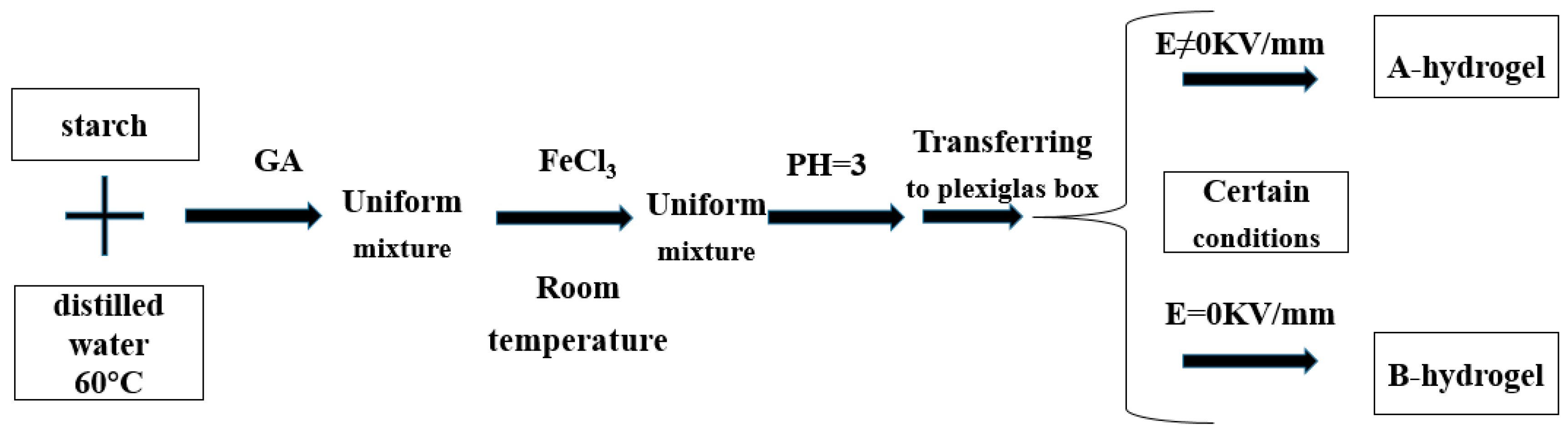
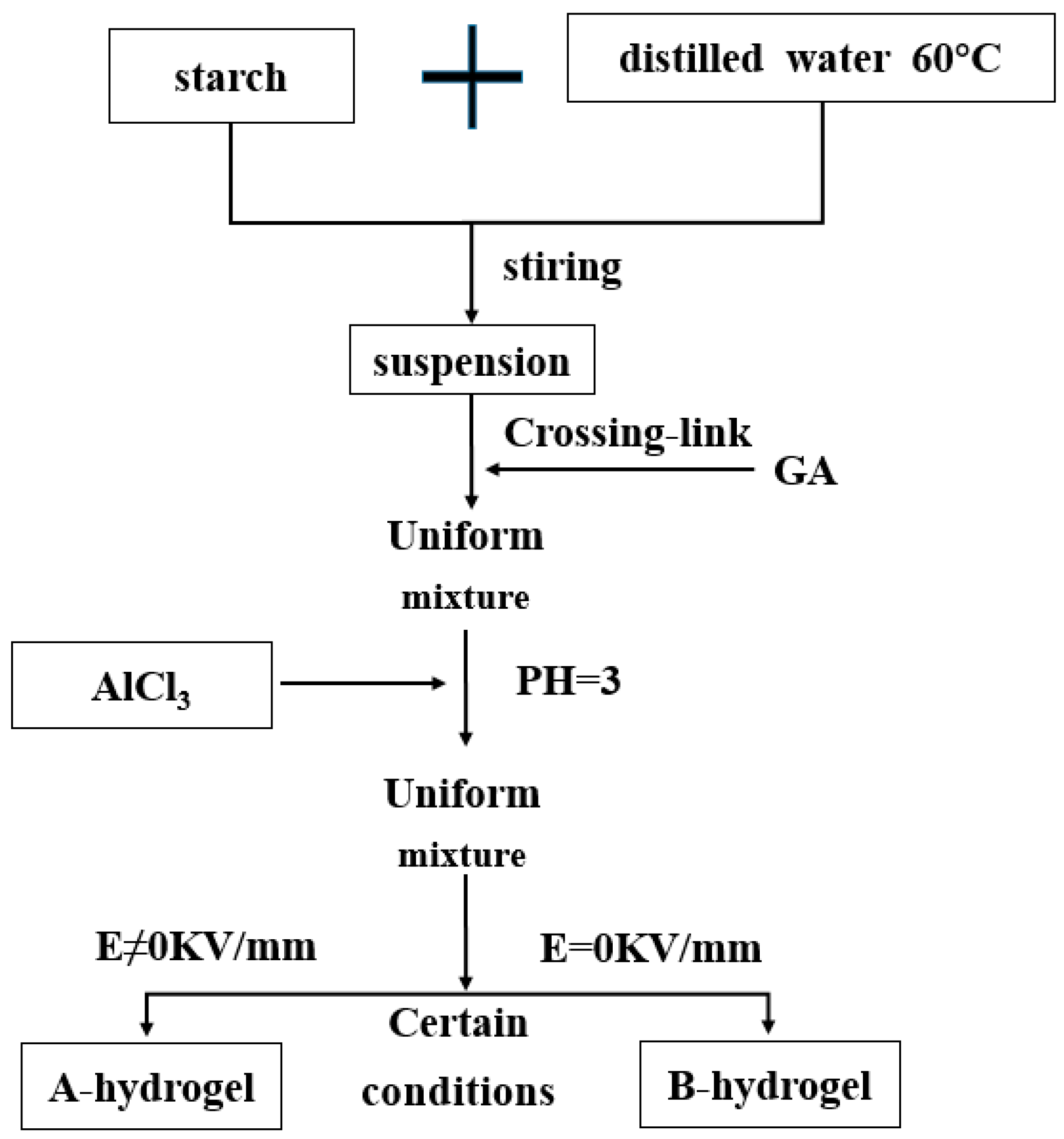

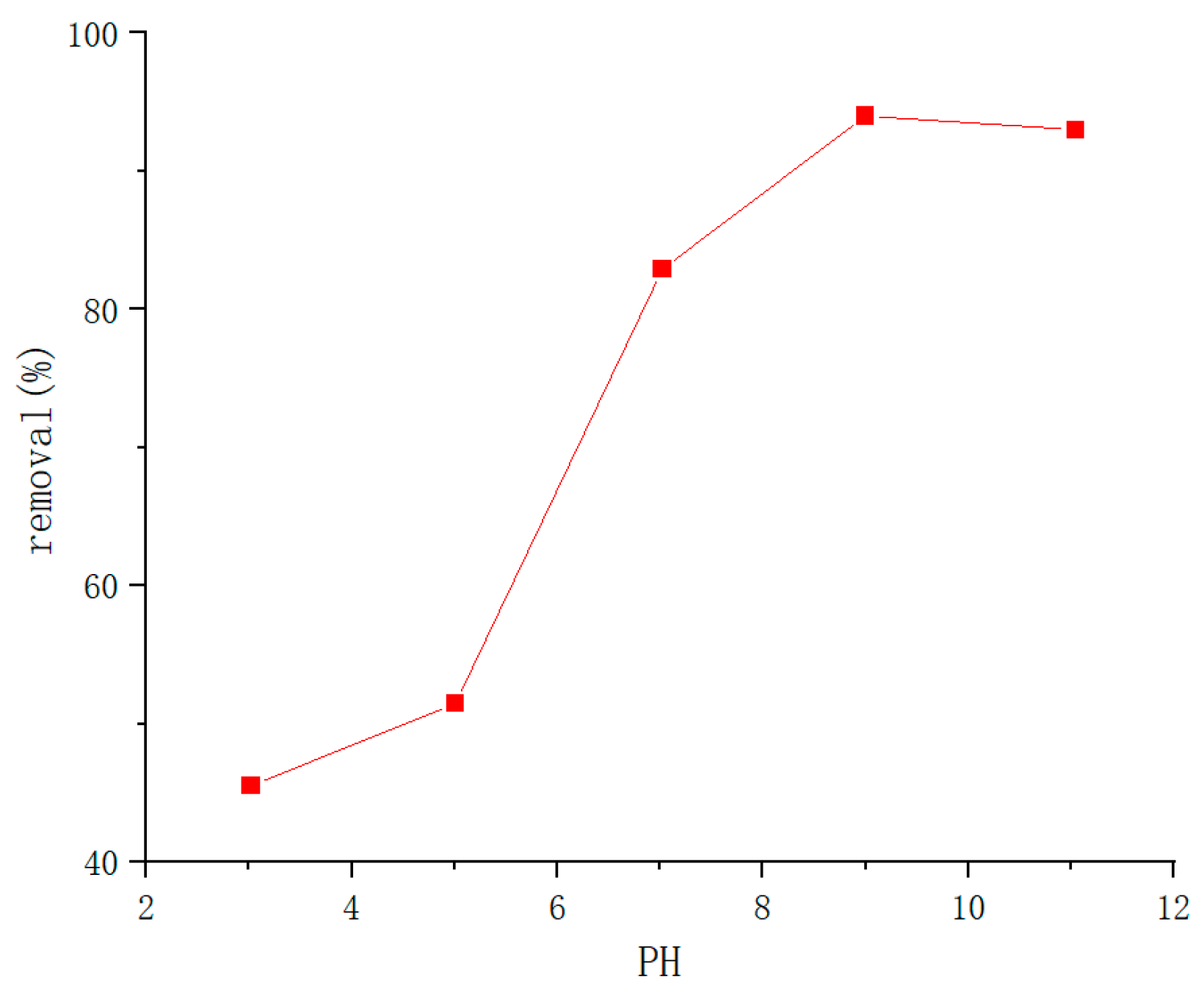
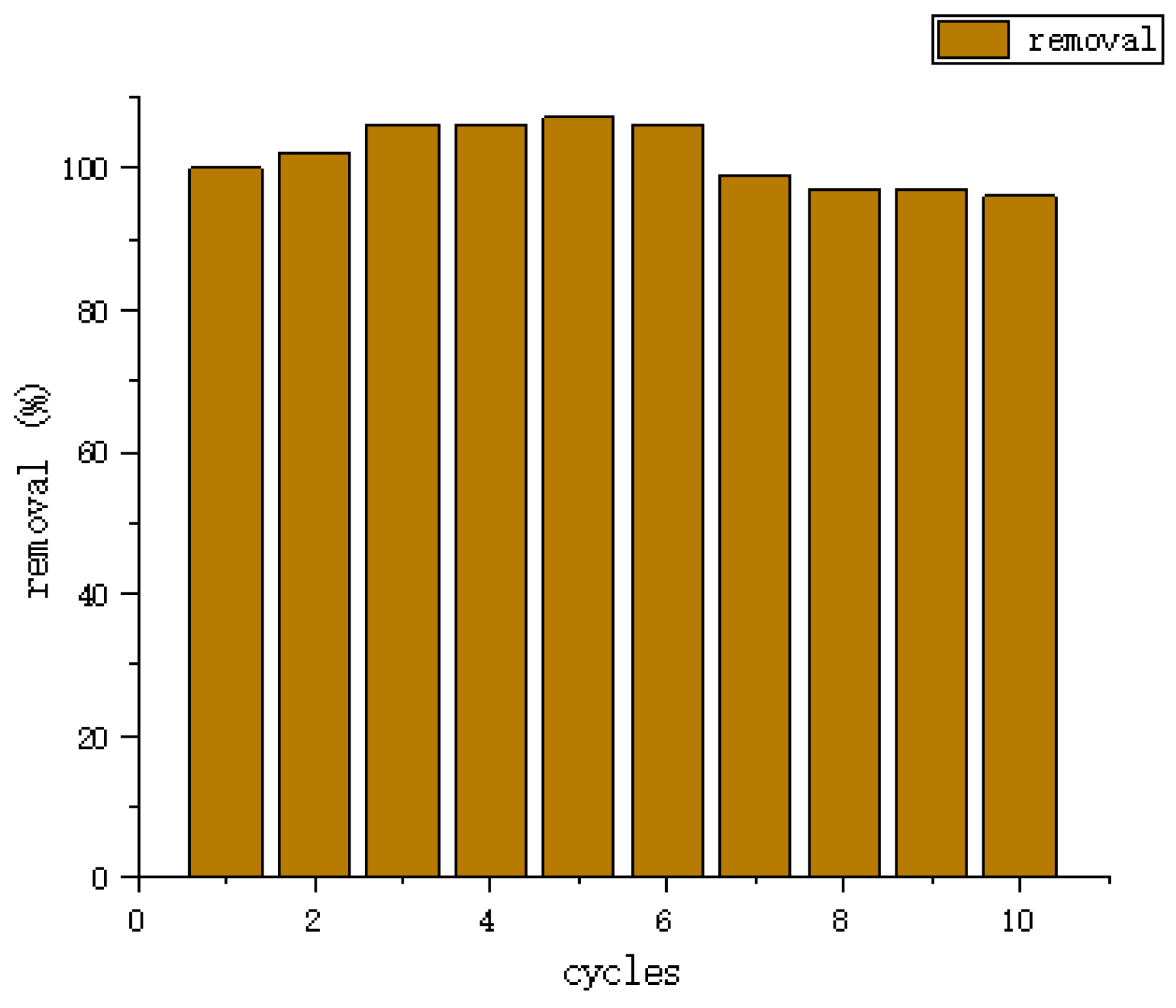
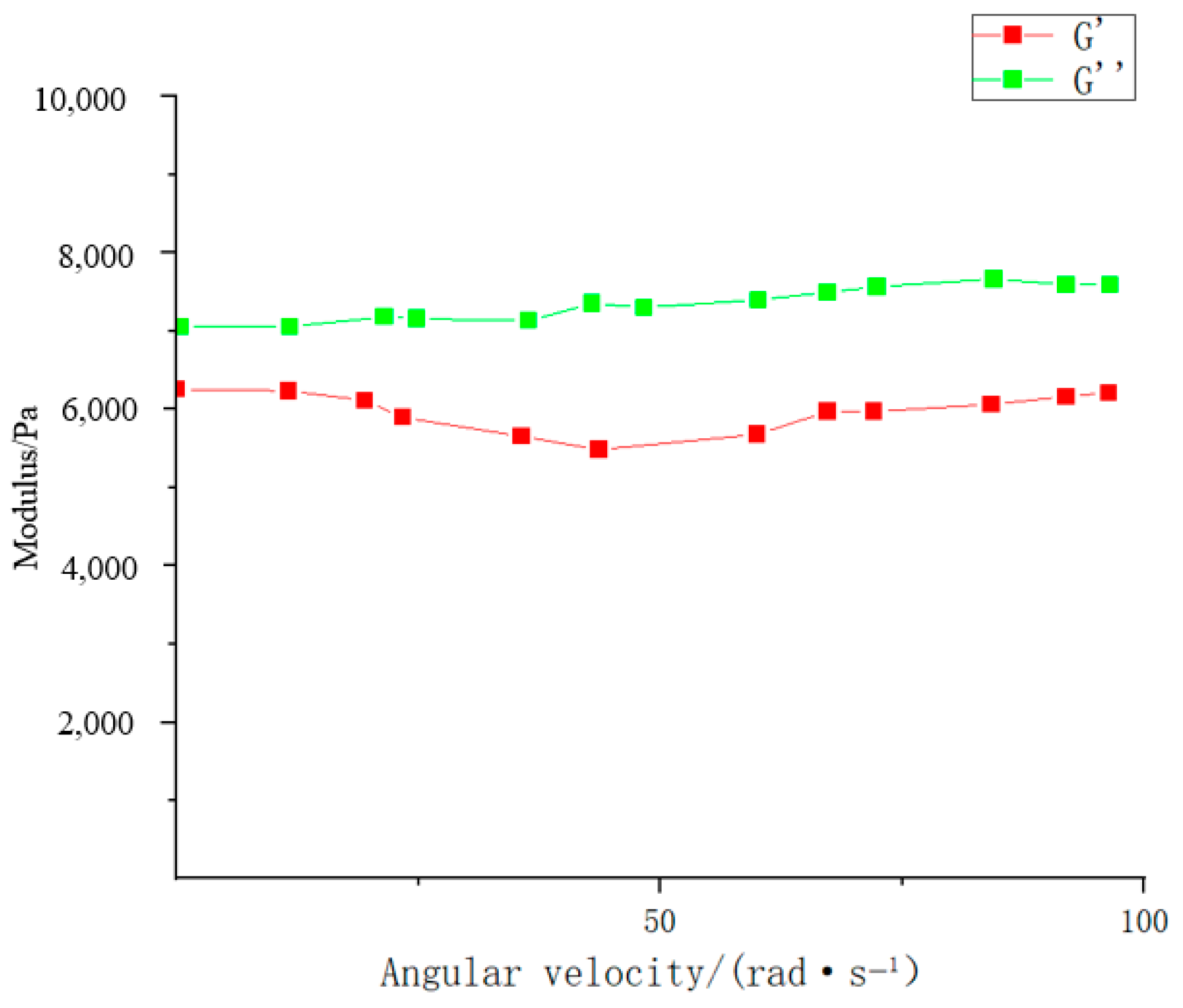
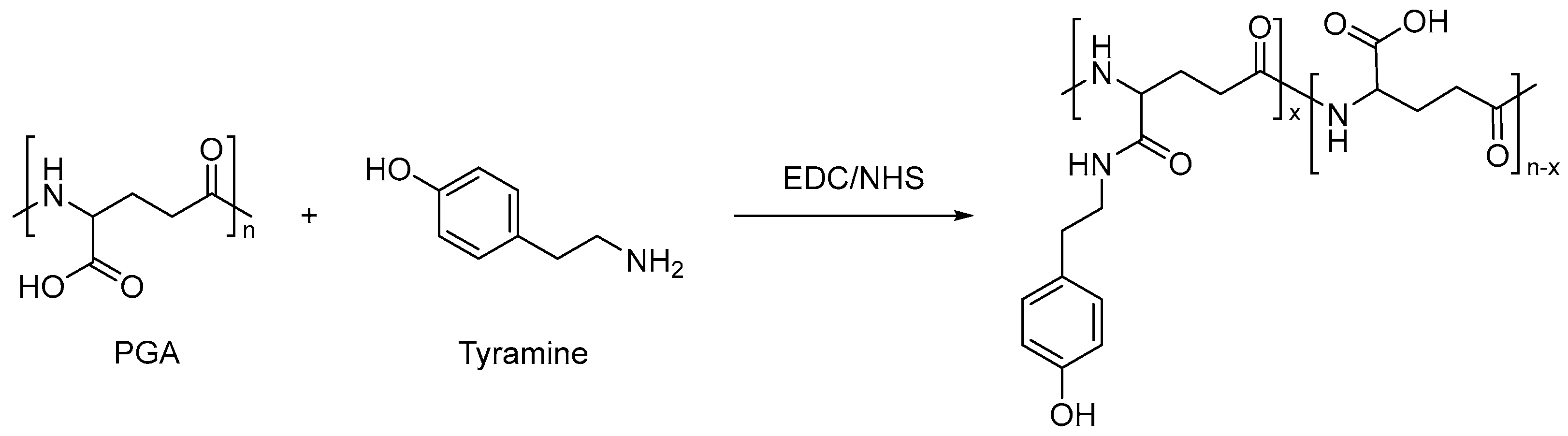
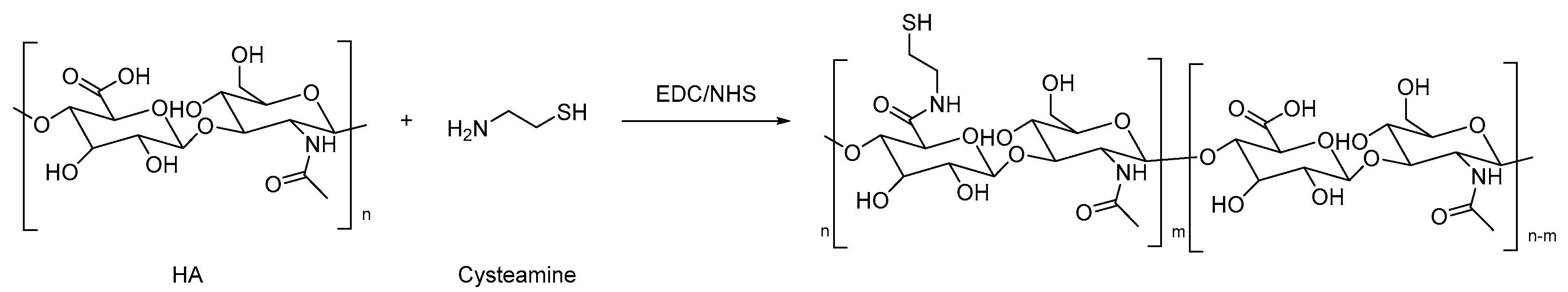
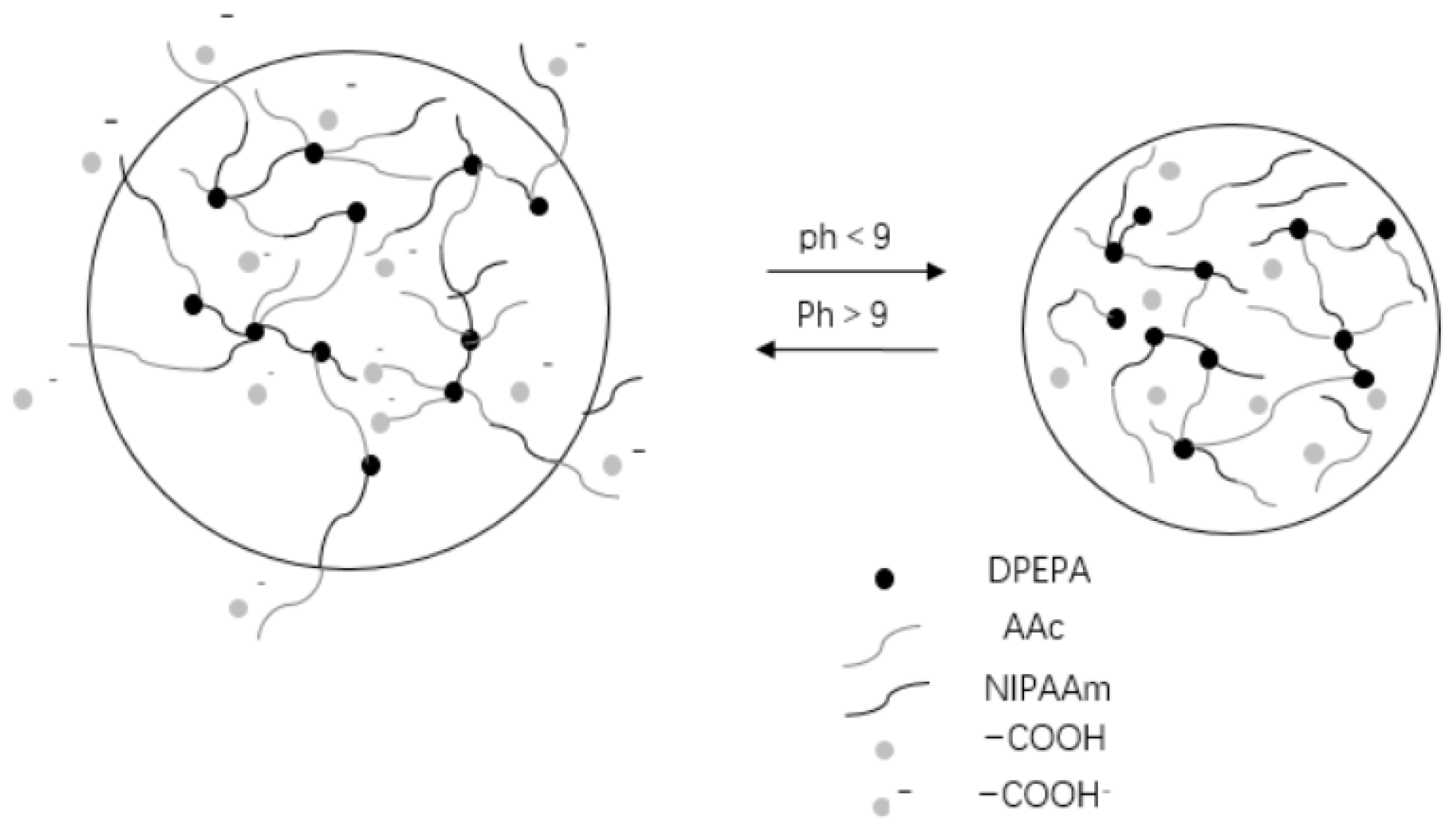
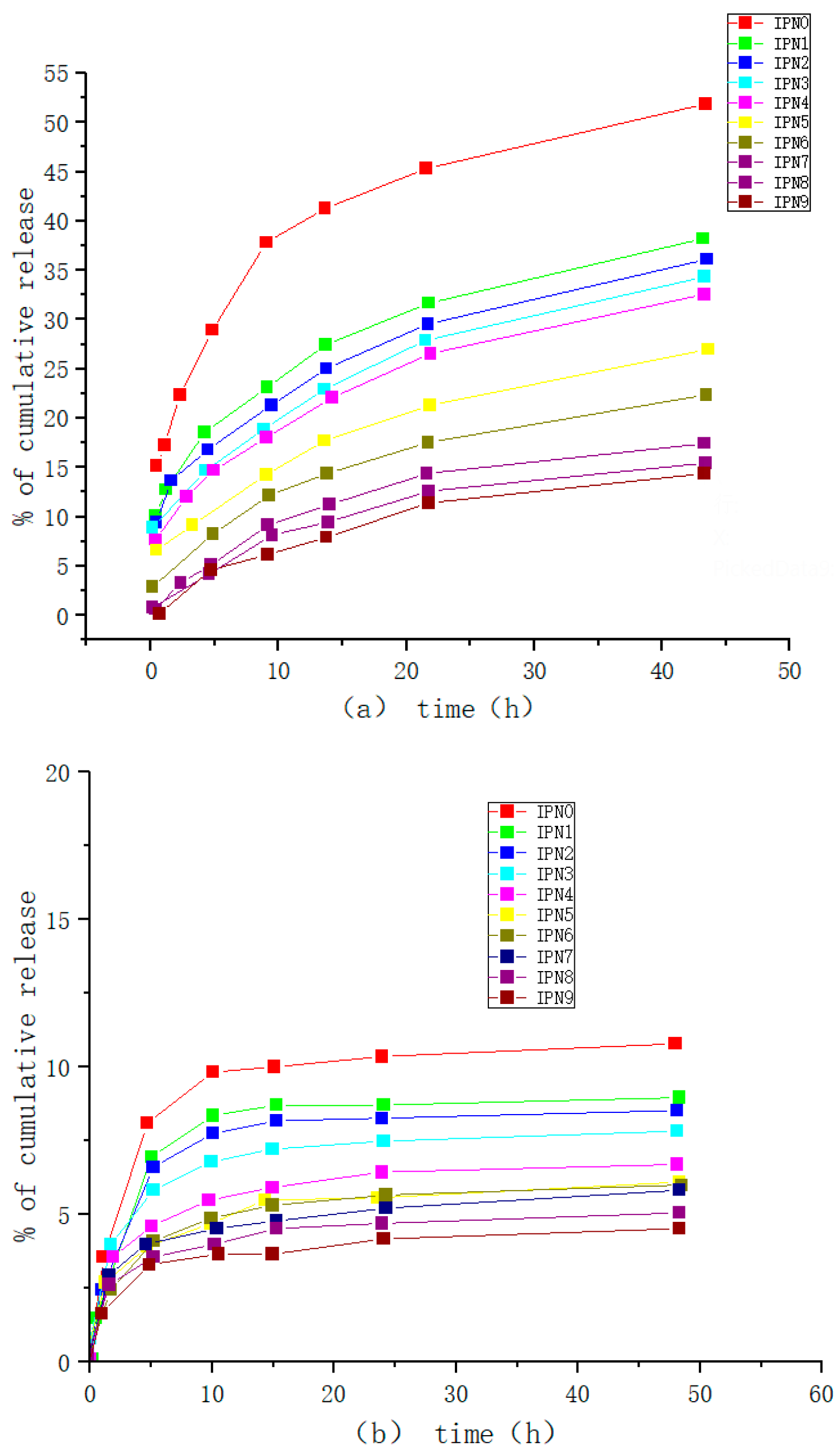
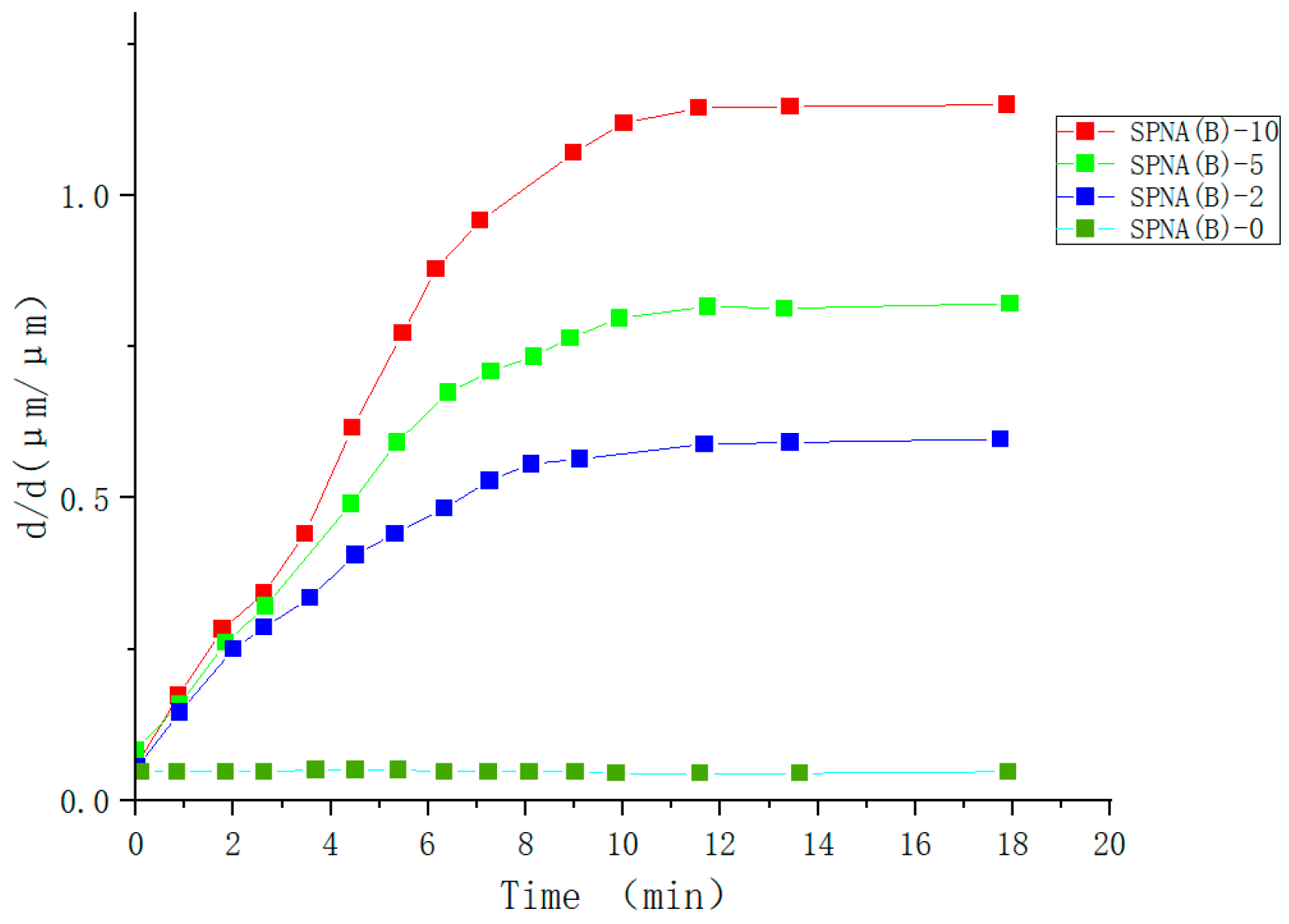
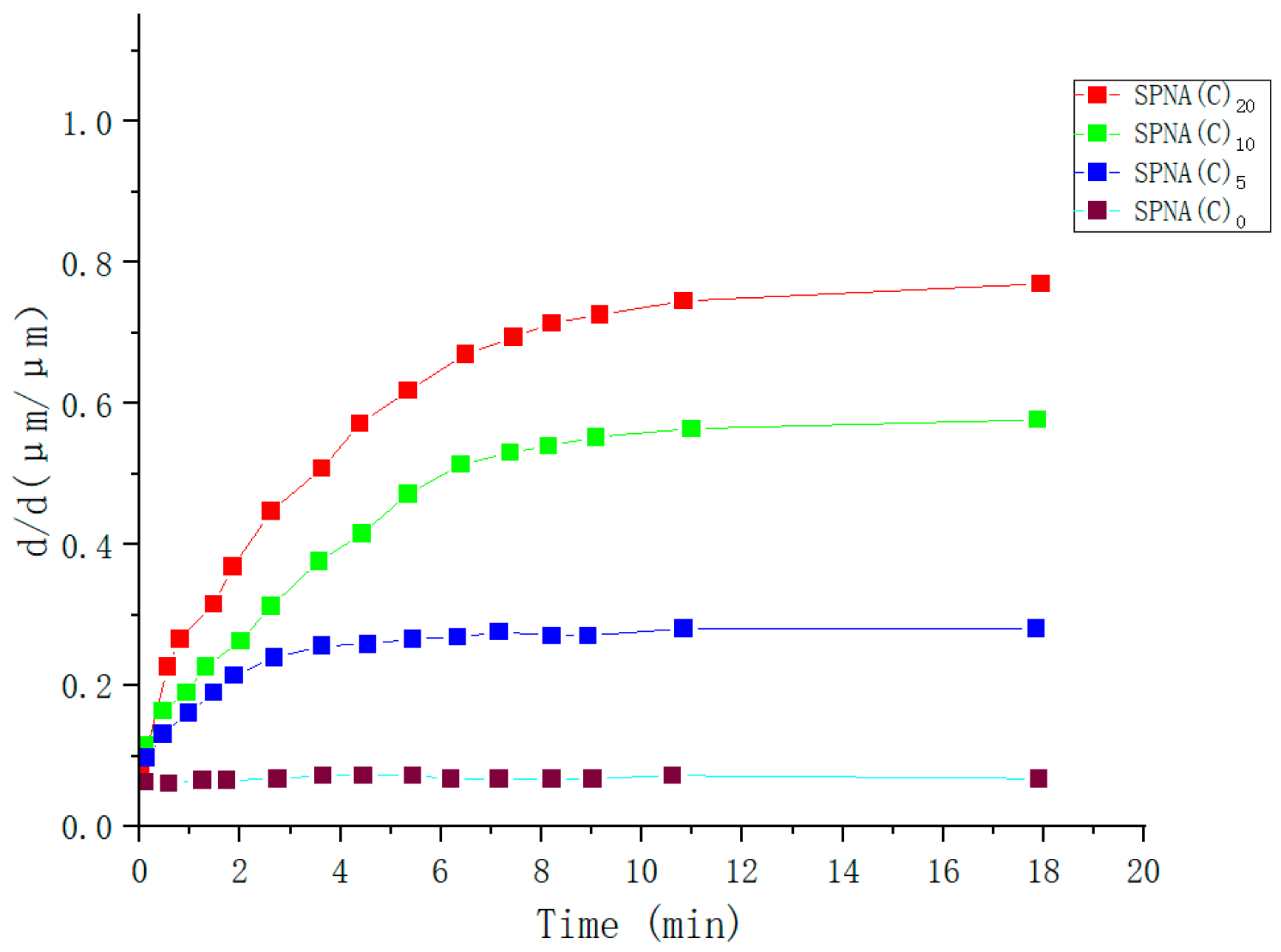
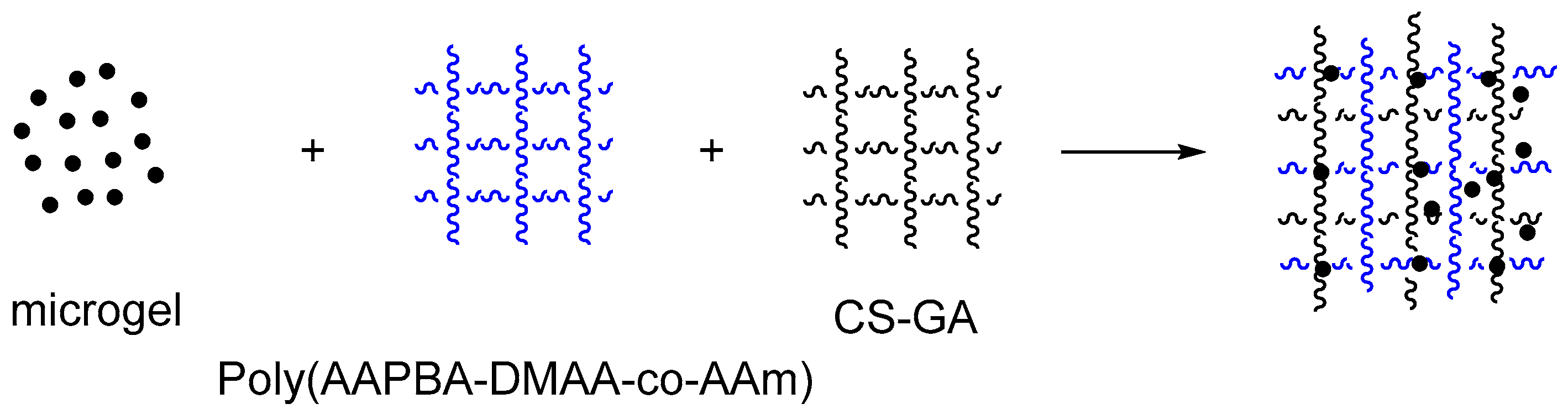
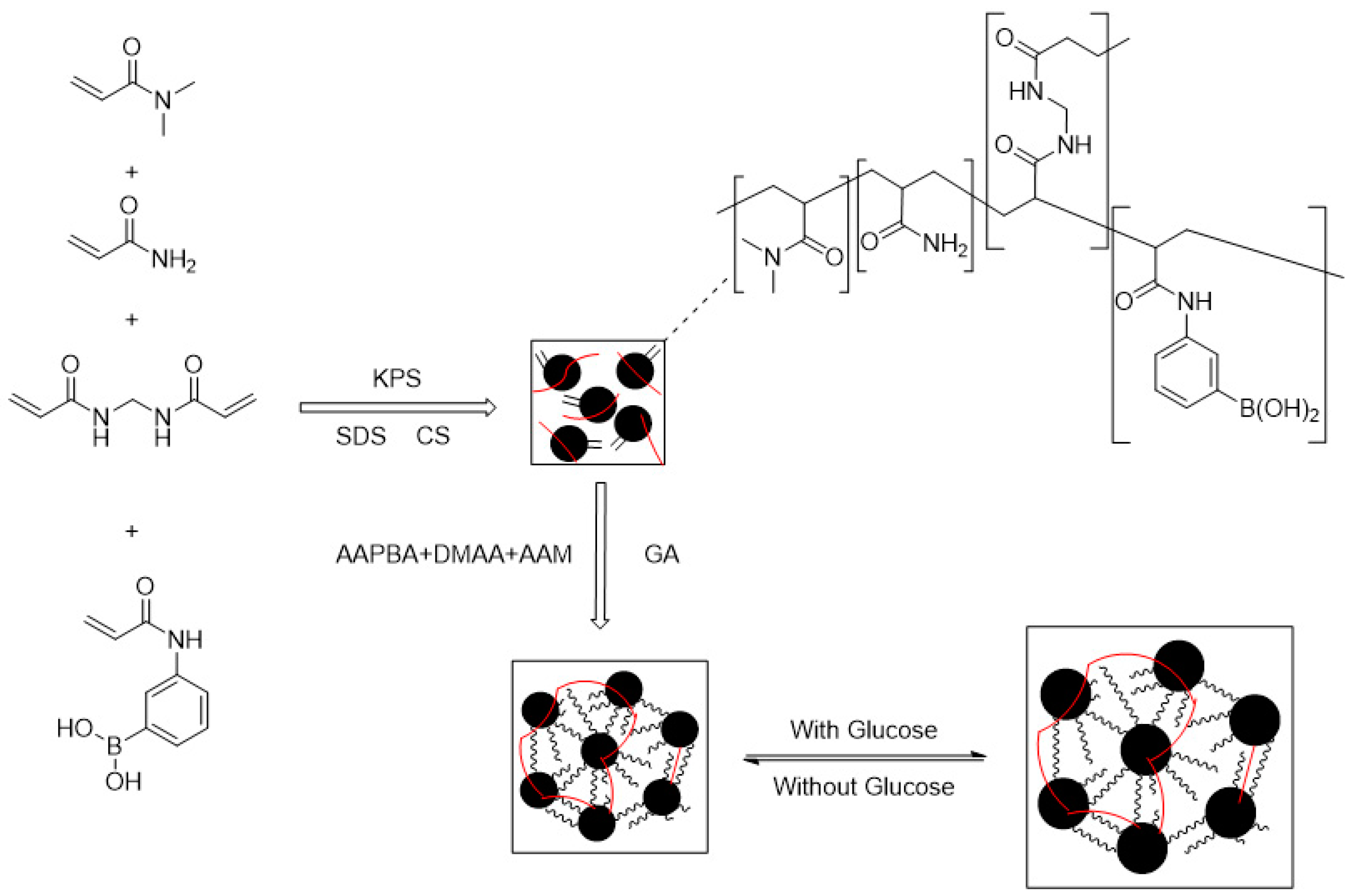
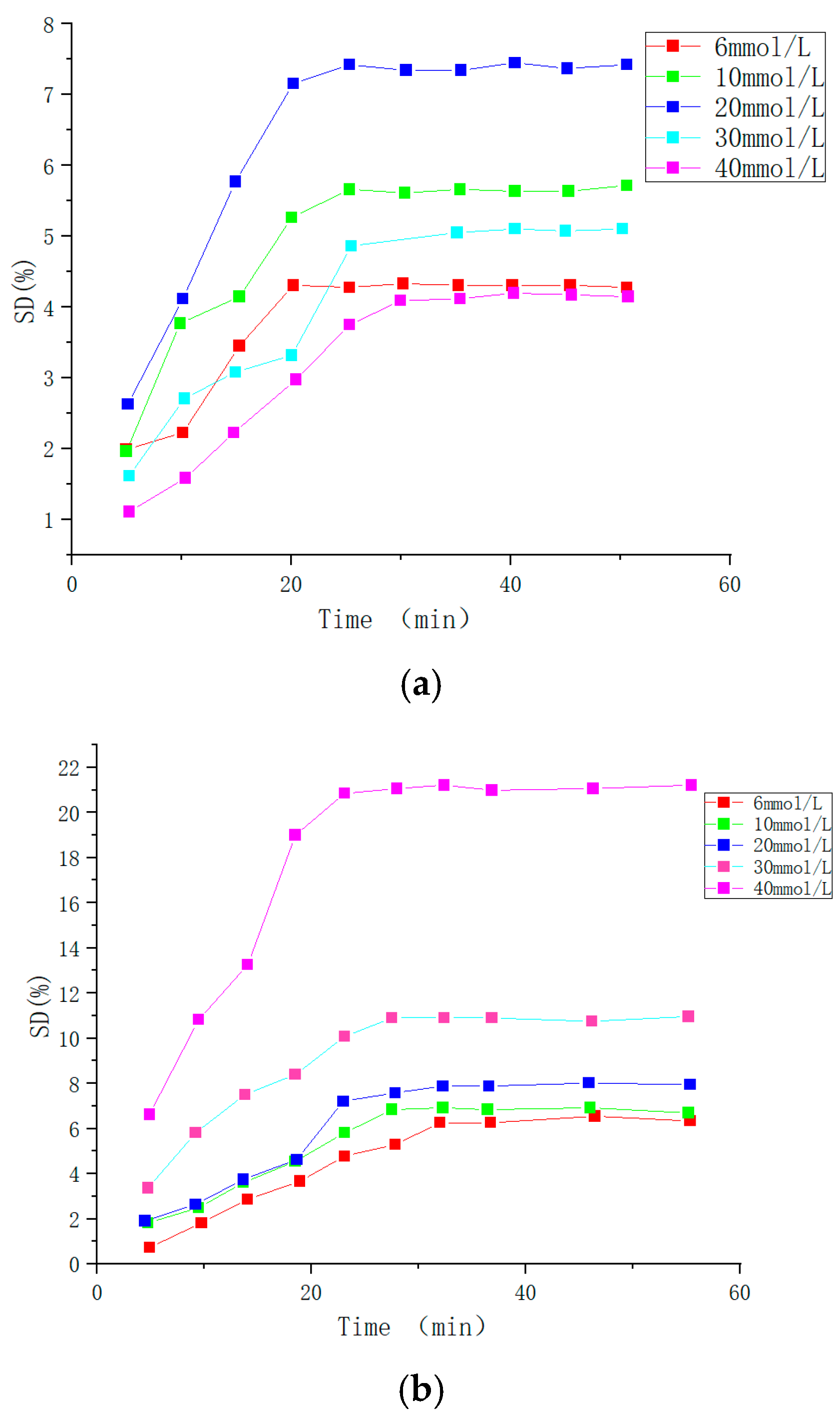

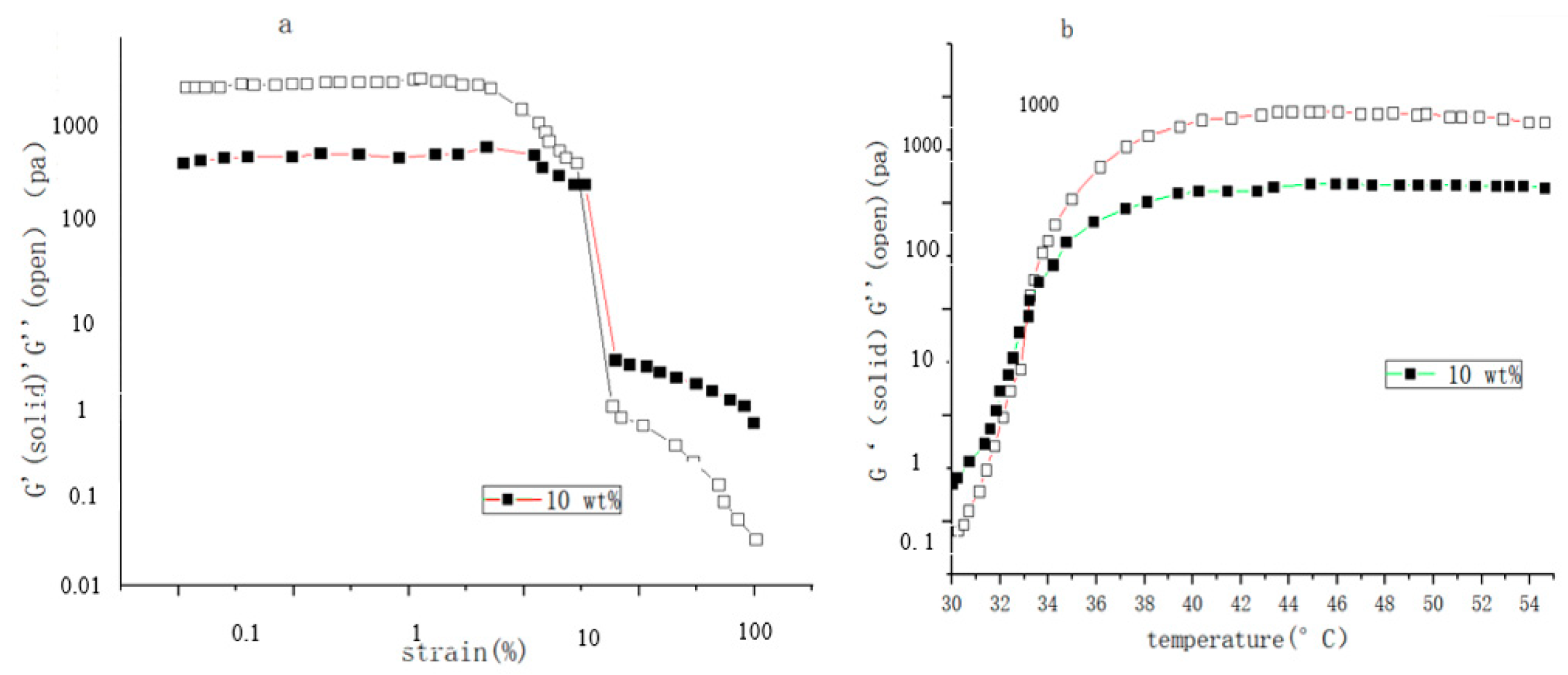
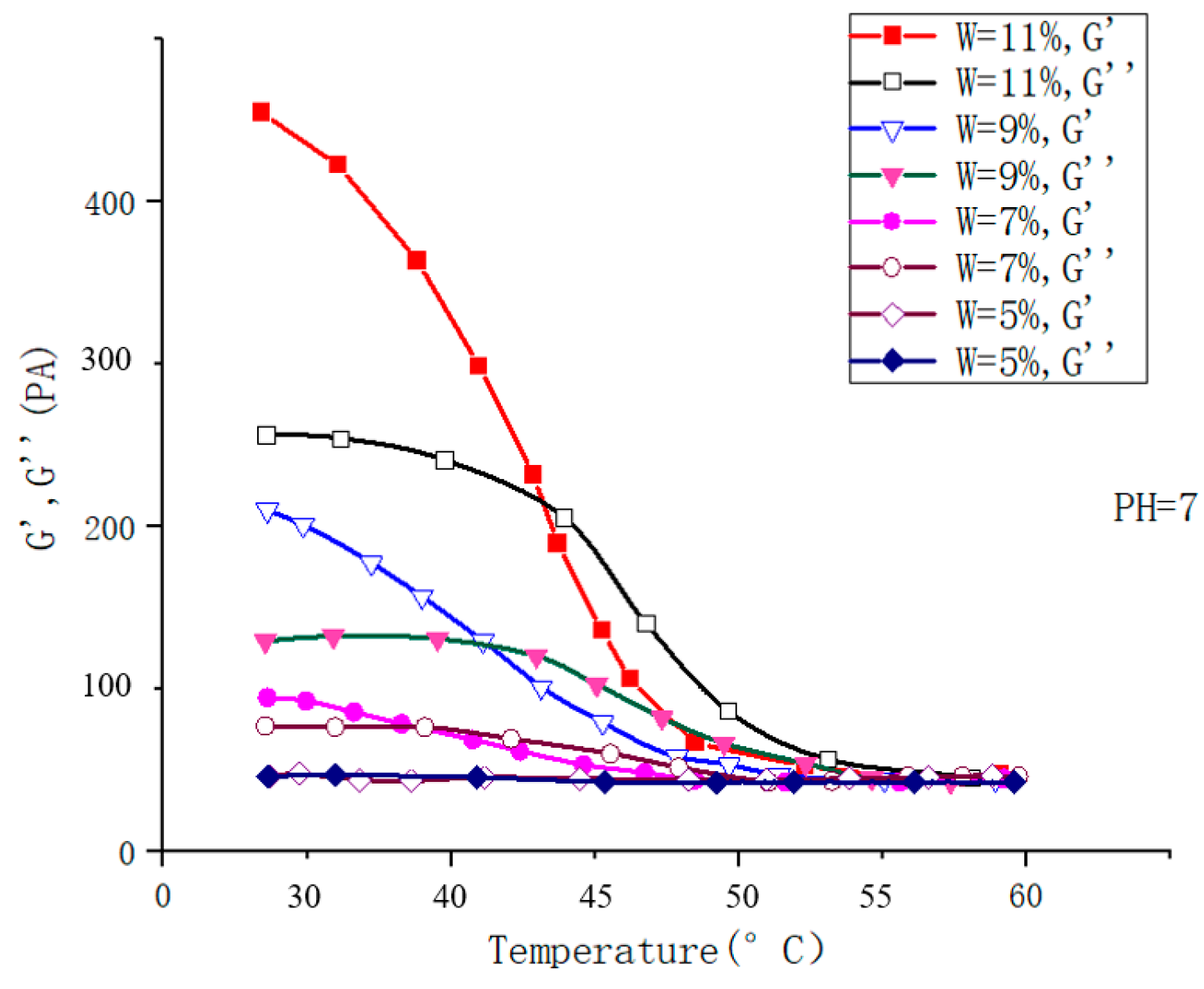

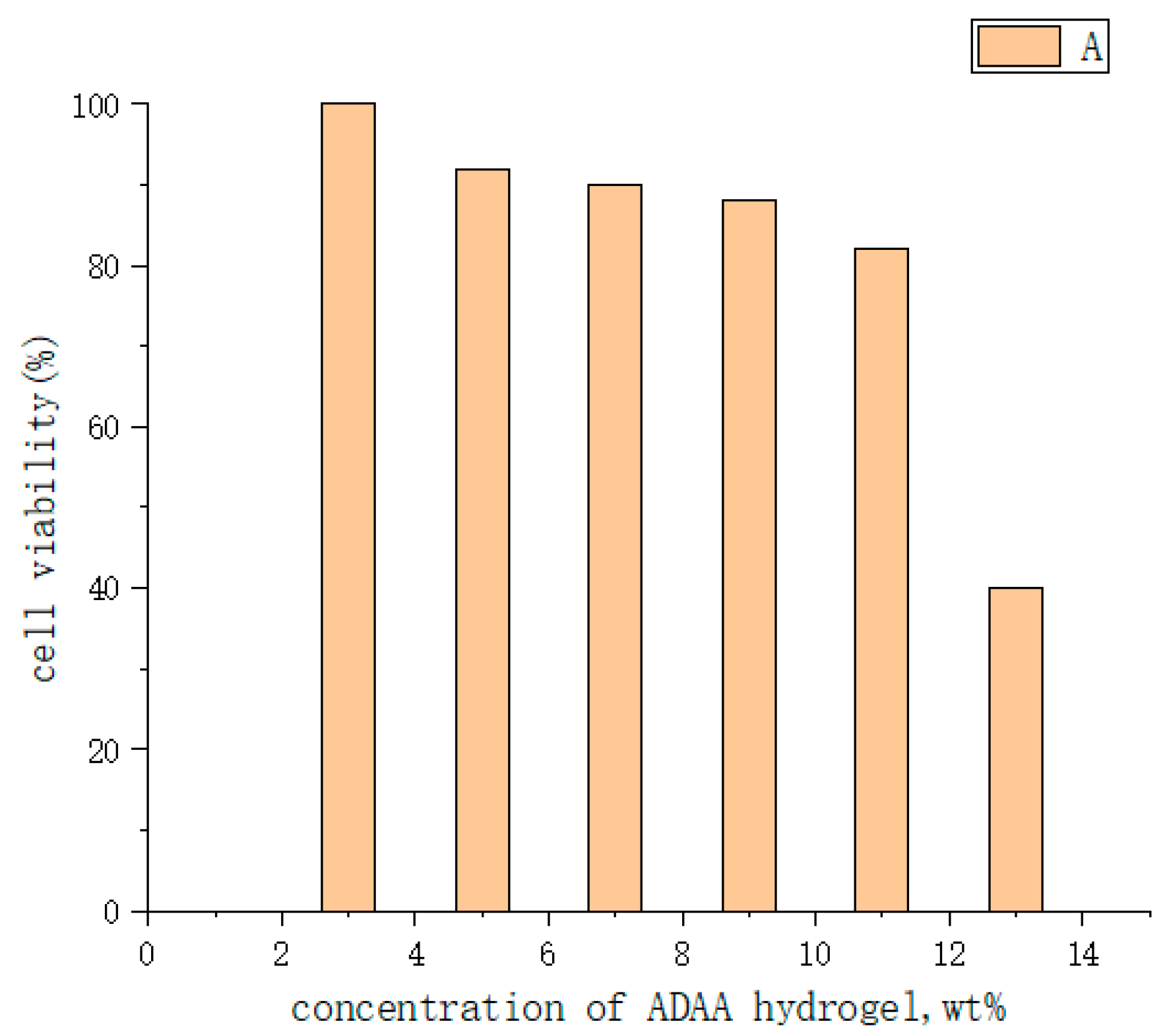
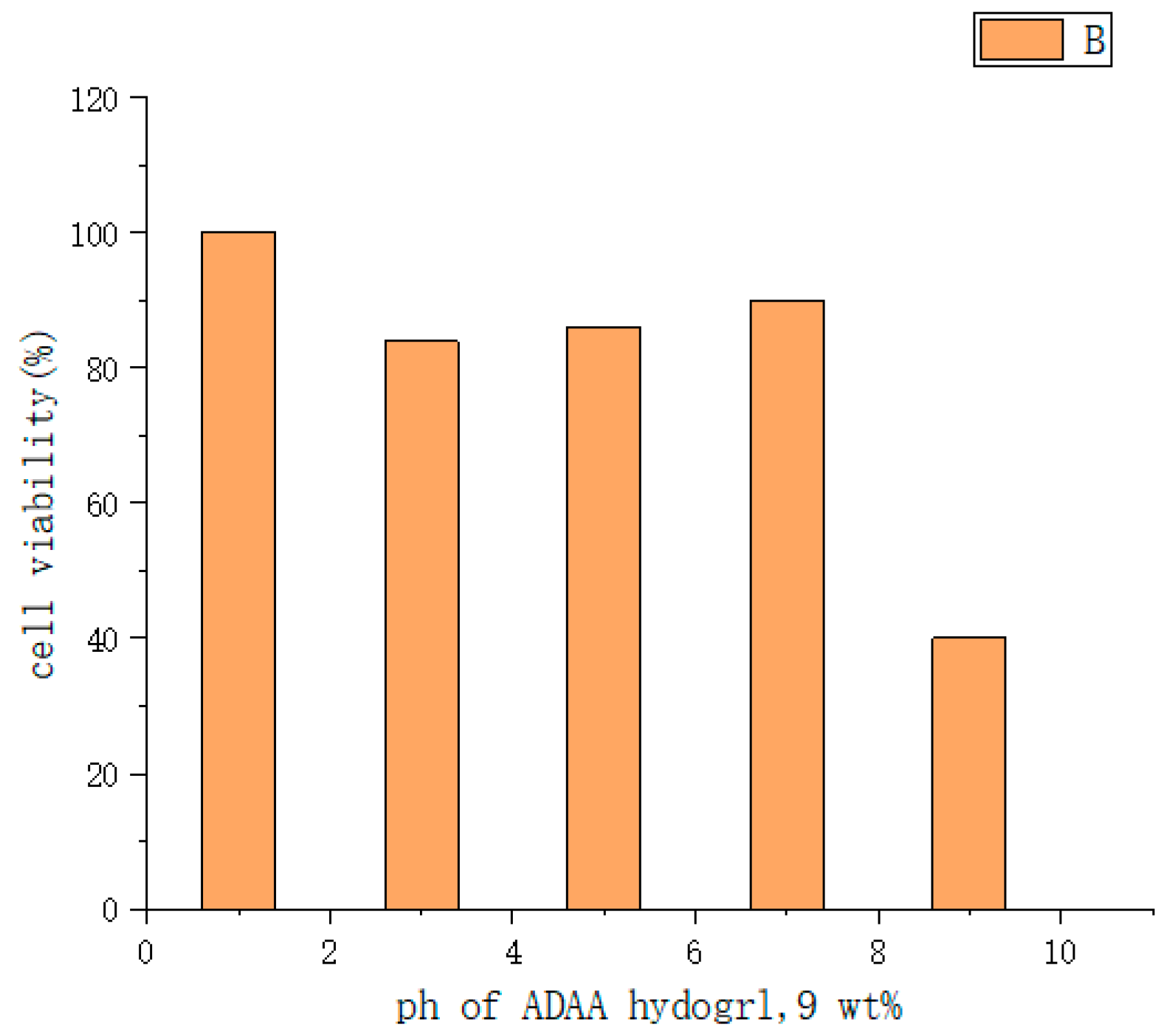
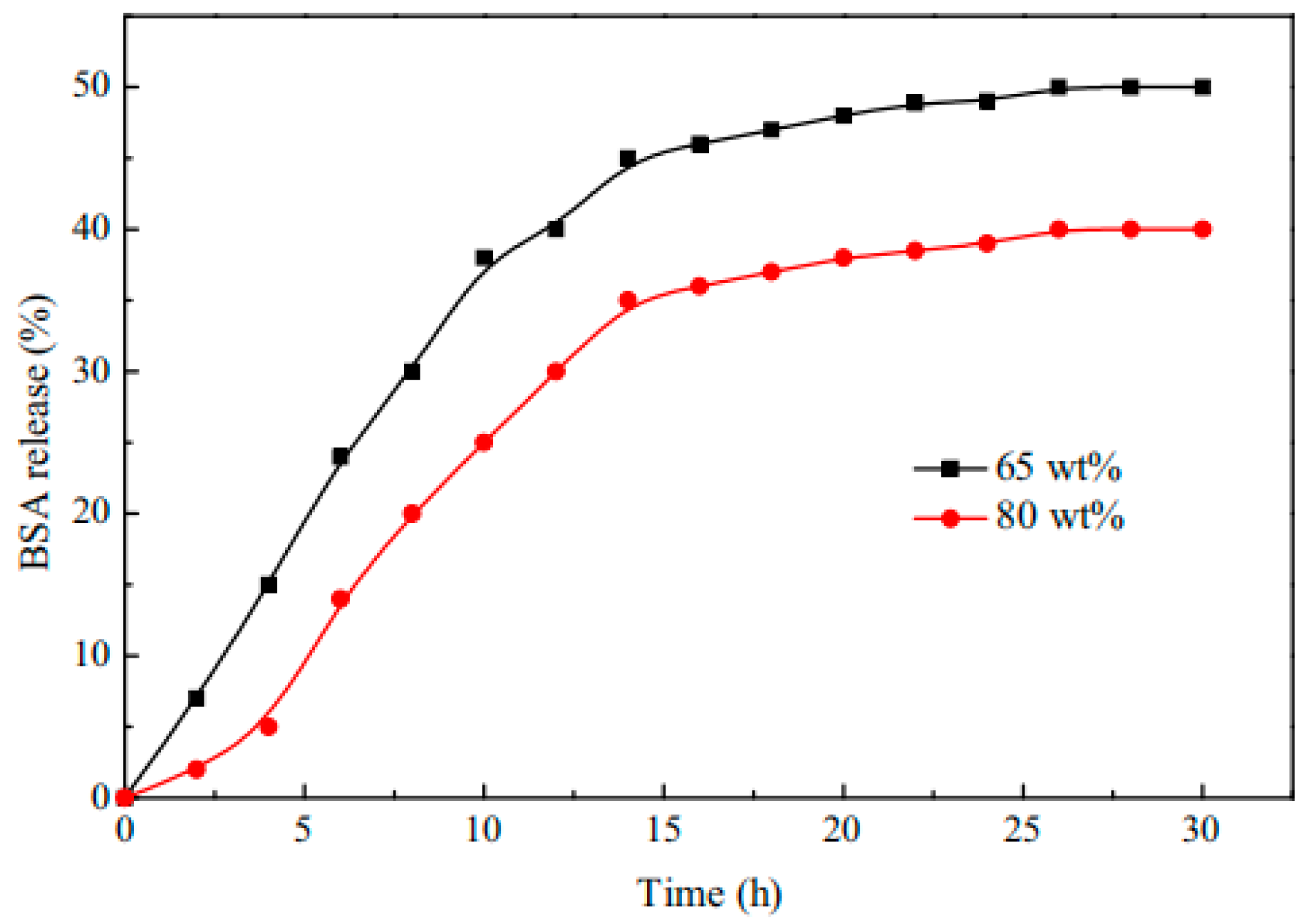
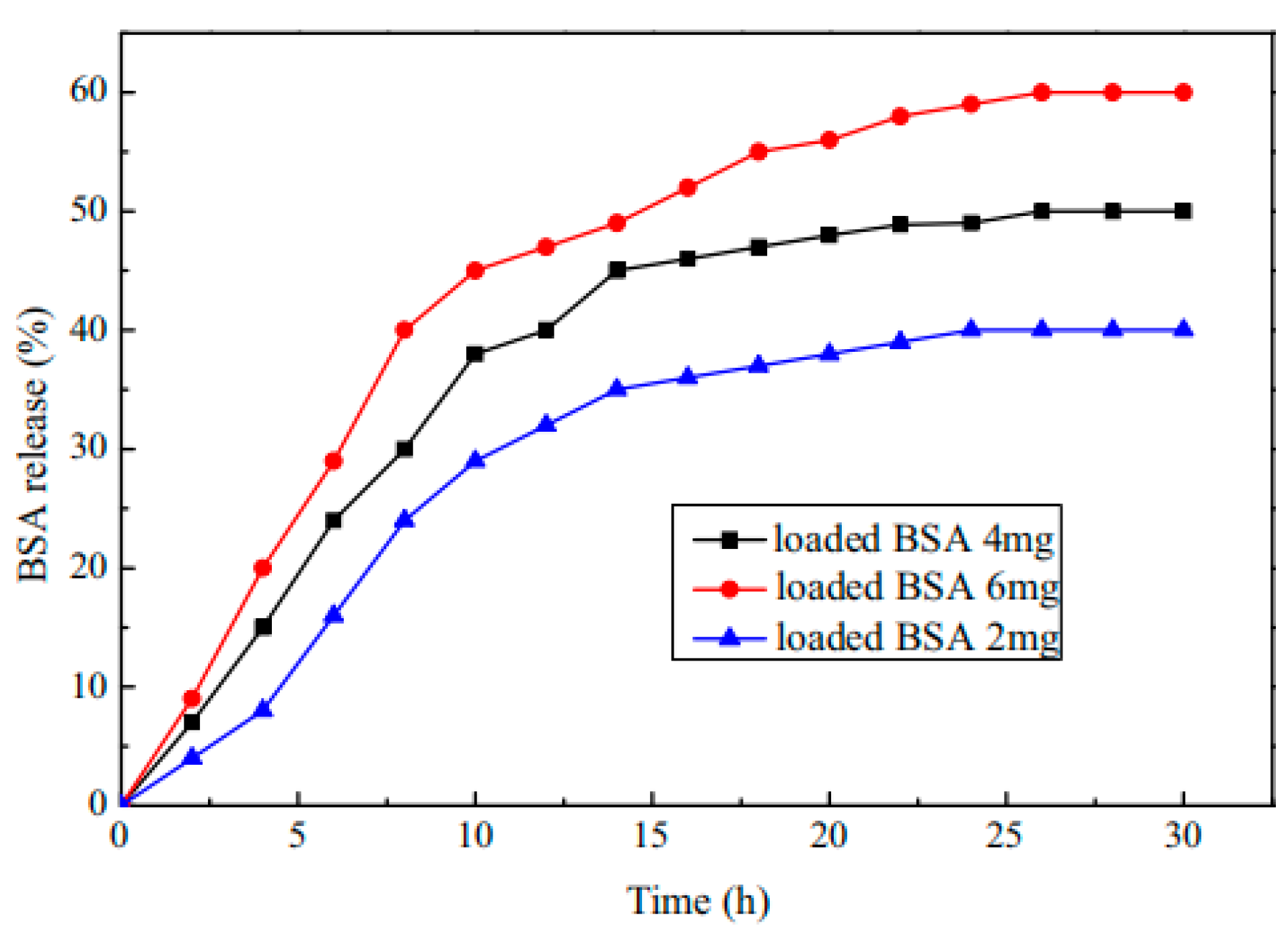
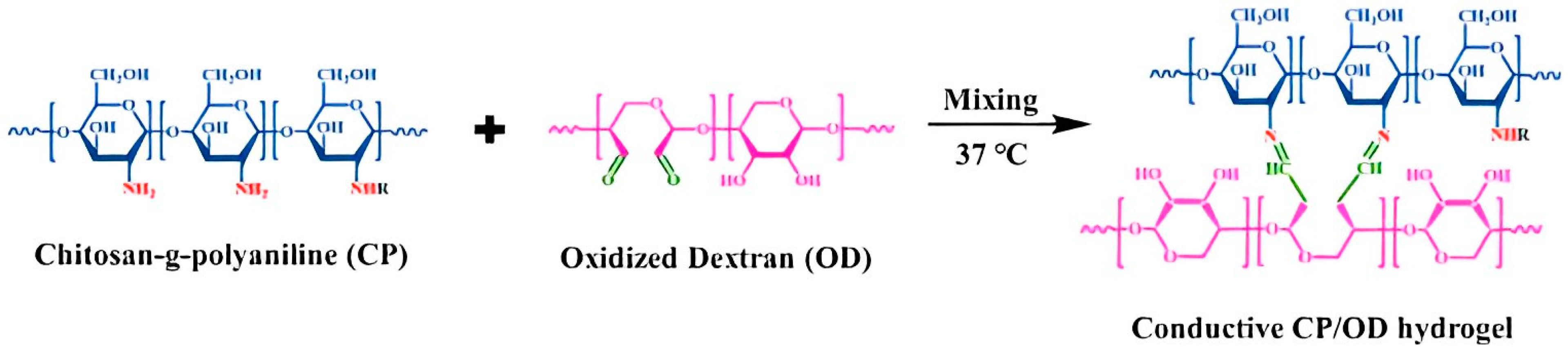
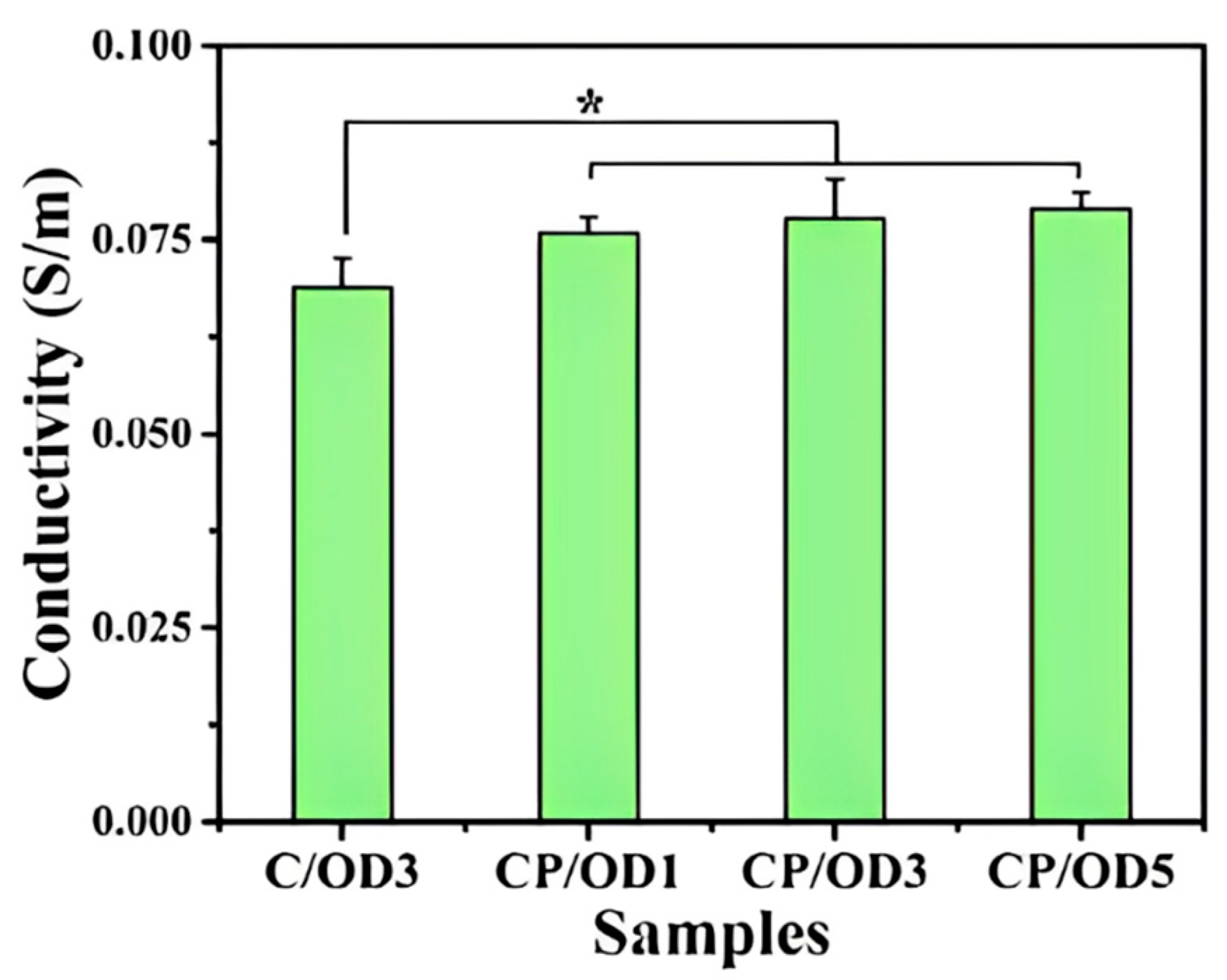
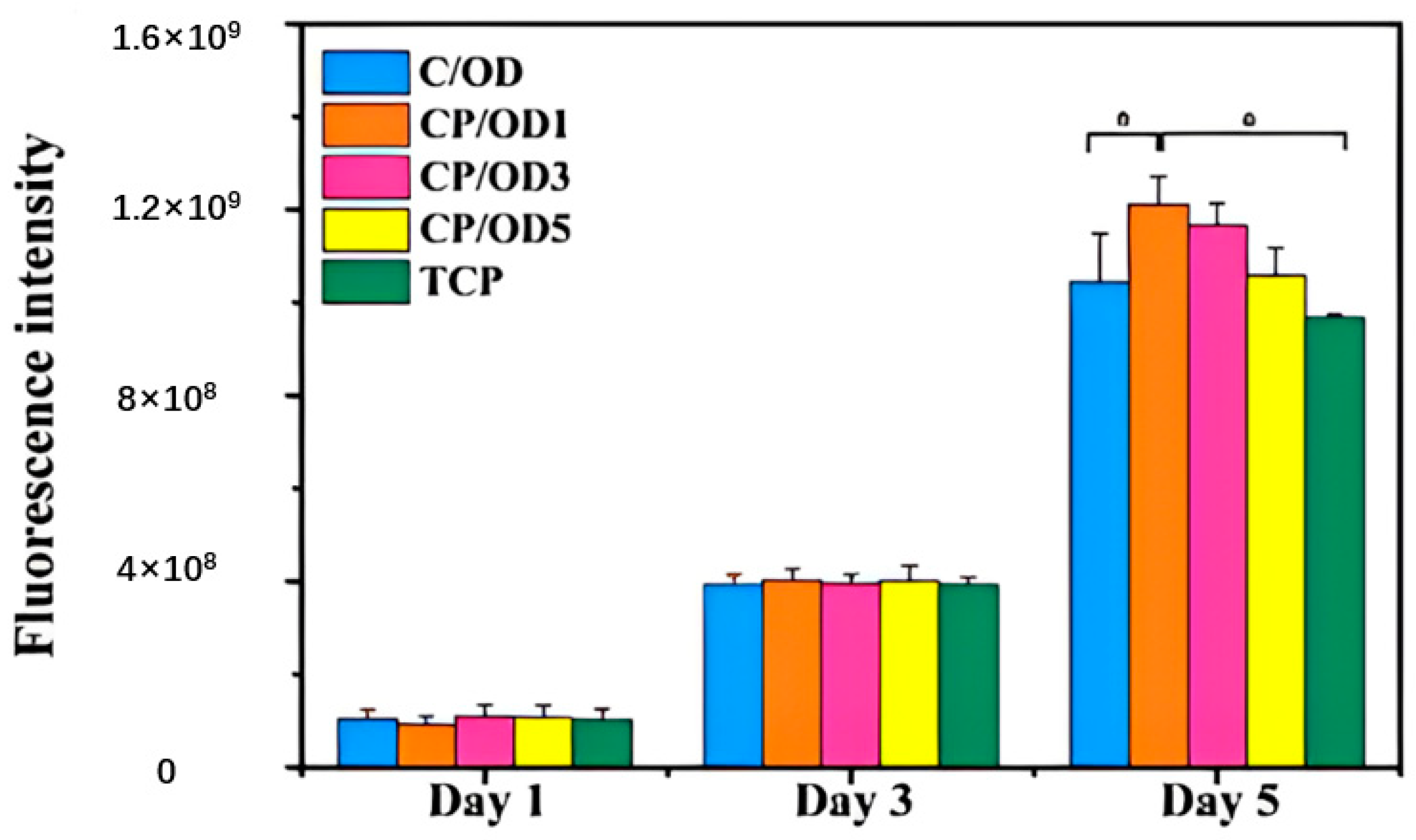

| Type | Performance and Features | Application Field | Proposed Development Direction |
|---|---|---|---|
| Temperature response type | It responds quickly to changes in external ambient temperature and changes in physical or chemical properties. | Transport and release, biological organs and other fields. | Due to its poor mechanical strength and biocompatibility, it is necessary to improve its mechanical properties, diversification and controllable self-driven deformation while maintaining high response rate [48,49,50,51,52,53,54,55,56,57,58]. |
| Light response type | It can respond quickly to chemical or physical changes under the action of light. | Industrial field and biomedical field, etc. | The research on photoresponsive gels is in its infancy, and its response mechanism needs to be further strengthened. Based on the understanding of intelligent light-responsive polymer gel materials, new light-sensitive materials can be synthesized by using the principles of polymer design and synthesis [59,60]. |
| Electric field response type | Under the action of electric field stimulation, the gel will change in volume or shape (mainly the swelling, deswelling and bending deformation of the gel), so as to realize the transformation from electrical energy to mechanical energy. | Biological engineering, electronic materials and other fields. | Electric-field-driven polymer gels have a wide application prospect in the field of bioengineering, but the theory in this field is not mature, and the electric field-response of polymer gels is not well understood. It is necessary to establish accurate mathematical model on the basis of a large number of experimental data, and carry out a lot of research to promote the indepth development of basic theory and synthesis technology [77,78,79,80,81,82,83,84,85,86,87,88]. |
| PH response type | Rapid response to changes in external pH, mutation. | Industrial field, biomedical field and so on. | Mechanical properties poor strength, unstable performance, poor biocompatibility, difficult to degrade. To study biodegradable gels, it is necessary to improve their mechanical properties while maintaining high response, and pay attention to the impact on the environment [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. |
| Chemical response type | The swelling behavior of chemical-influenced gels is mutated by the stimulation of specific chemicals, such as sugars. | Drug release, protein carrier, tissue engineering, biomedical fields, etc. | The mechanical properties are poor, the research is in the basic stage, the technology is not yet mature, and there are few kinds of chemical substances available. It is suggested to study these chemical substances more, prepare more functional intelligent polymer gels, and apply them in more fields [105,106,107,108,109,110,111,112,113,114,115]. |
| Multiple response type | With a variety of single response performance synthesis, can simultaneously respond to a variety of external environmental stimuli; With double or even triple response characteristics. | Drug control release system, memory switch, artificial muscle, chemical memory, material separation, biomedical field, etc. | It is suggested that the sensitive mechanism should be further elucidated in future studies. The mechanical strength and response rate of gel can be improved through the coordination of different technologies. An environmentally sensitive polymer gel with biocompatibility and biodegradability is constructed from the perspective of bionics. Vigorously promote the industrialization and scale of gel production [116,117,118,119,120,121,122,123,124,125,126,127,128]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, Z.; Pan, Y.; Guan, J.; Yang, P.; Asel, M. A Review of Research Progress on the Performance of Intelligent Polymer Gel. Molecules 2023, 28, 4246. https://doi.org/10.3390/molecules28104246
Yang S, Liu Z, Pan Y, Guan J, Yang P, Asel M. A Review of Research Progress on the Performance of Intelligent Polymer Gel. Molecules. 2023; 28(10):4246. https://doi.org/10.3390/molecules28104246
Chicago/Turabian StyleYang, Shuangchun, Zhenye Liu, Yi Pan, Jian Guan, Peng Yang, and Muratbekova Asel. 2023. "A Review of Research Progress on the Performance of Intelligent Polymer Gel" Molecules 28, no. 10: 4246. https://doi.org/10.3390/molecules28104246
APA StyleYang, S., Liu, Z., Pan, Y., Guan, J., Yang, P., & Asel, M. (2023). A Review of Research Progress on the Performance of Intelligent Polymer Gel. Molecules, 28(10), 4246. https://doi.org/10.3390/molecules28104246





