Trisubstituted 1,3,5-Triazines as Histamine H4 Receptor Antagonists with Promising Activity In Vivo
Abstract
1. Introduction
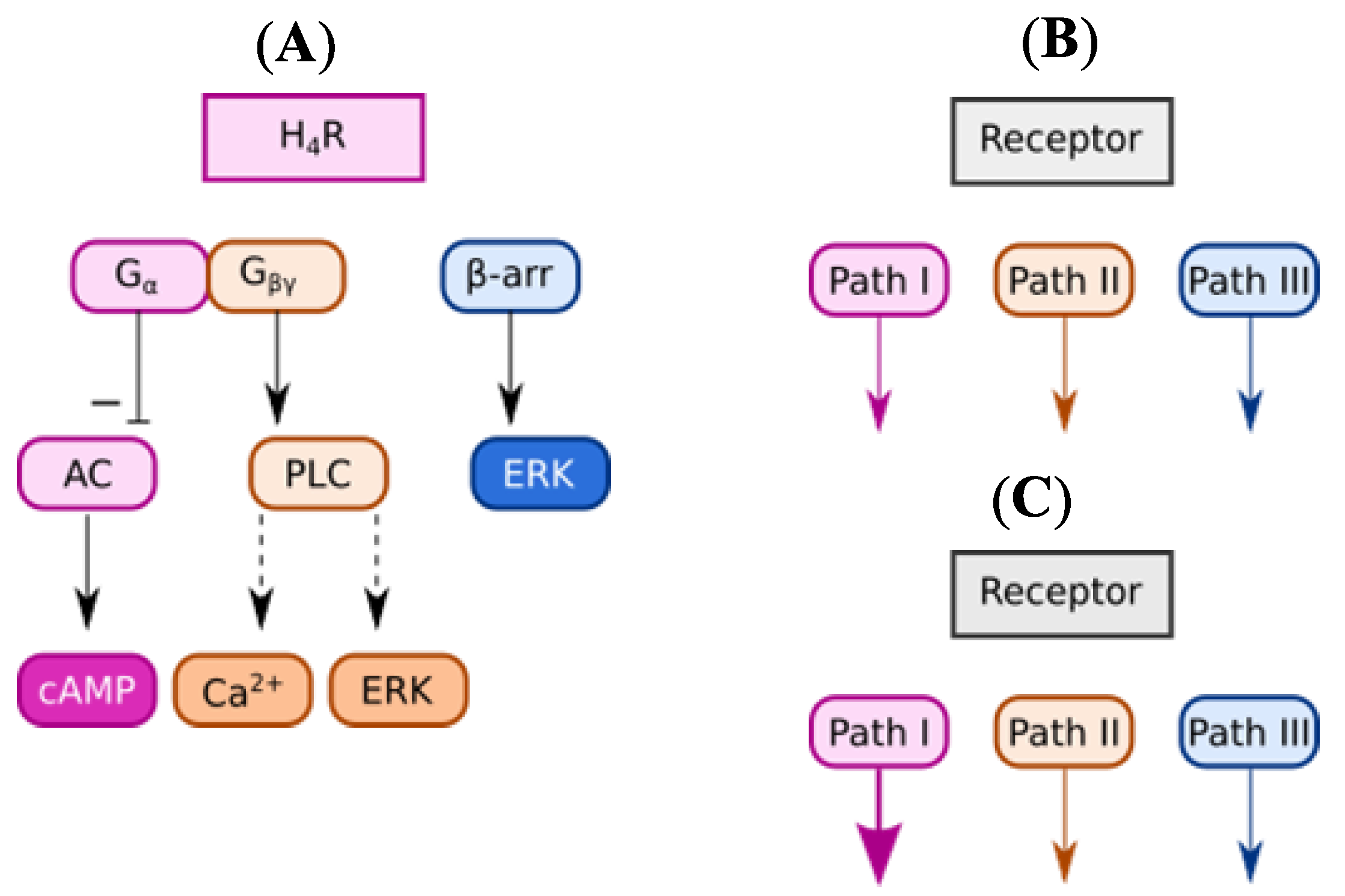
2. Results and Discussion
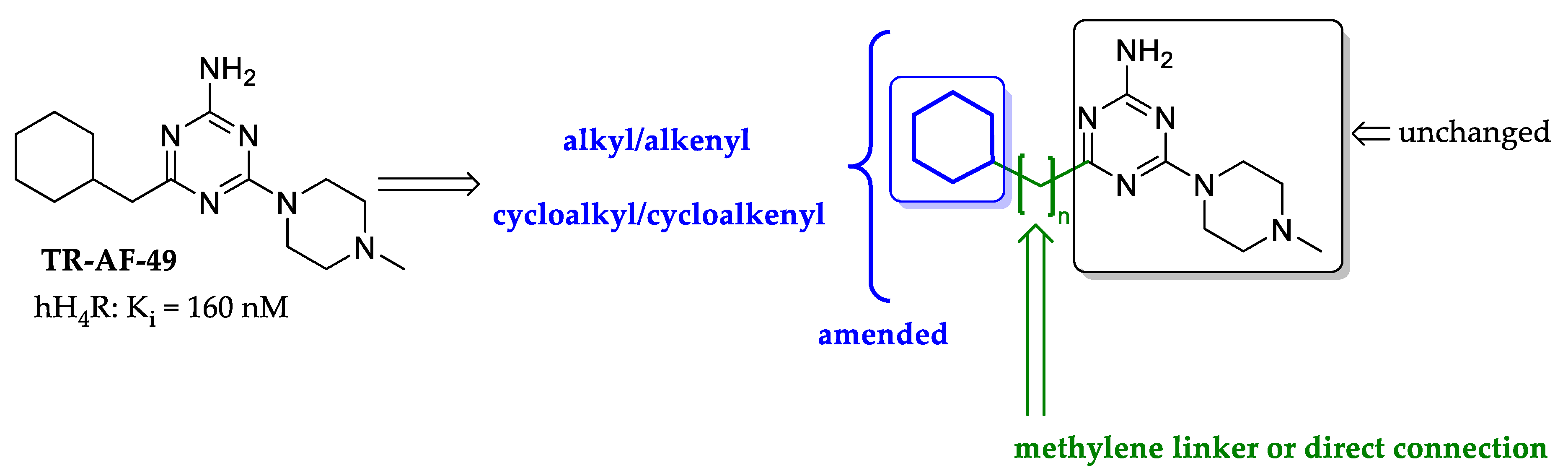
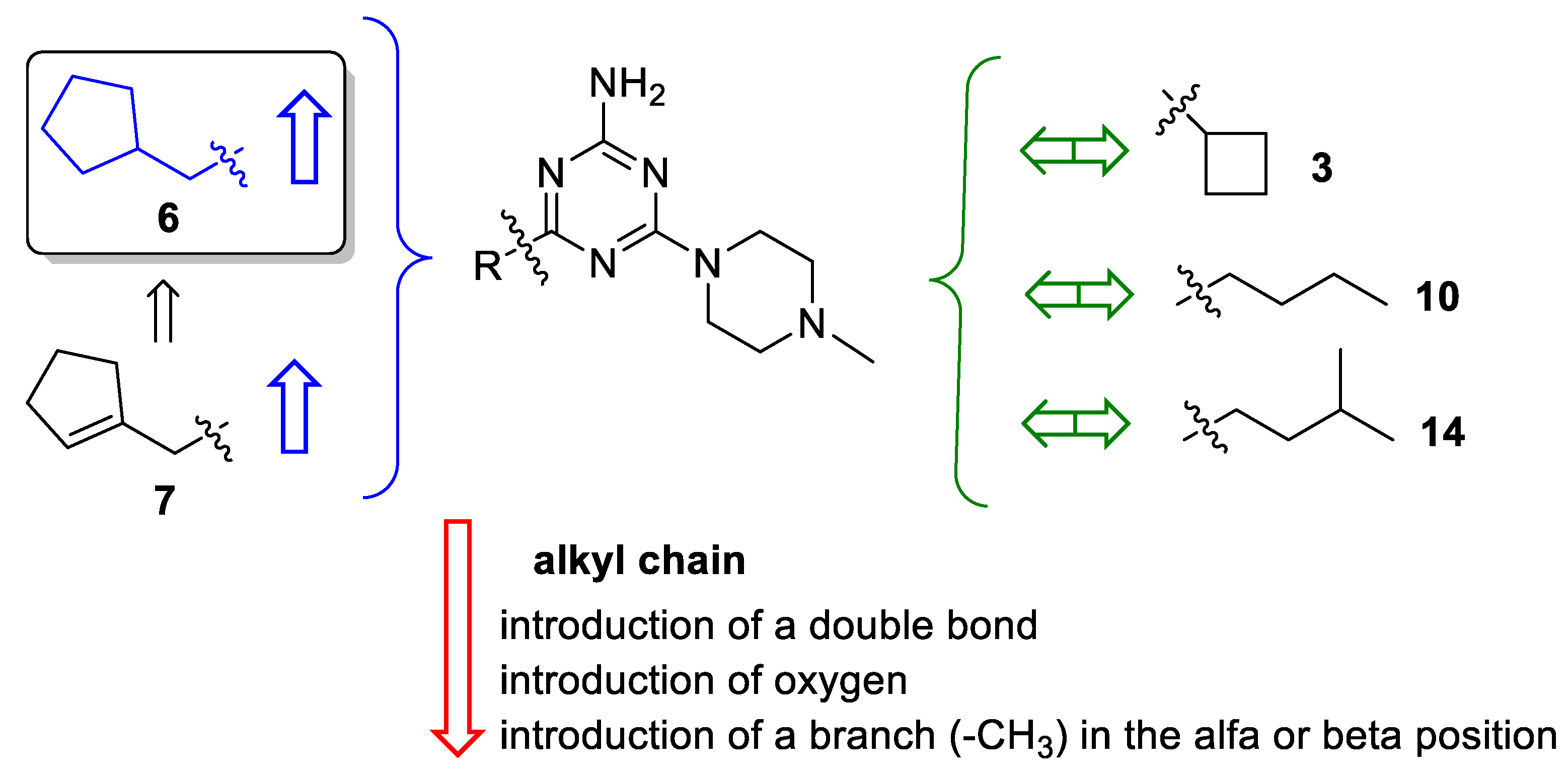
2.1. Design of Compounds
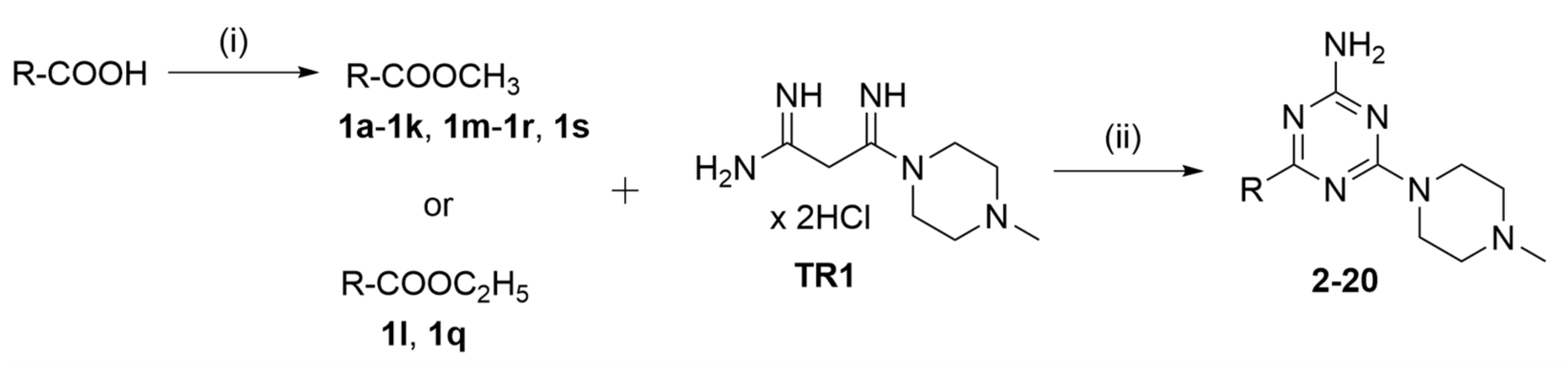
2.2. Synthesis of Compounds
| No | 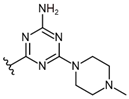 | hH4R a Ki [nM] [CI 95%] (n) b or (inh. at 1 μM) c | β-arrestin hH4R d IC50 ± SEM [nM] e (% of max. Antagonist Activity at 10 µM) | cAMP hH4R f IC50 ± SEM [nM] e (% of max. Antagonist Activity at 10 µM) |
|---|---|---|---|---|
| 2 |  | 574 [160;2058] (3) | 287 ± 30 (96) | 847 ± 53 (99) |
| 3 |  | 200 [156;257] (3) | 100 ± 15 (87) | 104 ± 17 (109) |
| 4 |  | 1237 [250;6115] (2) | 68.8 ± 2 (95) | 102 ± 2 (95) |
| 5 |  | 432 [128;1452] (3) | 101 ± 10 (92) | 219 ± 24 (98) |
| 6 | 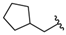 | 63 [18;214] (3) | 10 ± 1 (101) | 10 ± 2 (100) |
| 7 | 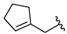 | 96 [20;450] (3) | 14 ± 1 (103) | 33 ± 2 (104) |
| TR-AF-49 (Lead) |  | 160 g [66.6;385] (4) | 68 ± 7 (99) | 271 ± 11 (98) |
| 8 |  | 4700 h (1) | 41 ± 6 (102) | 743 ± 30 (98) |
| 9 | 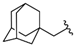 | (43%) (2) | nt i | nt i |
| JNJ7777120 | 32 j | 56 ± 8 (91) | 49 ± 2 (96) | |
| Thioperamide | 106 j | 209 ± 40 (102) | 453 ± 30 (111) | |
| No | 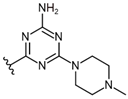 | hH4R Ki [nM] a [CI 95%] (n) b or (inh. at 1 μM) c | hH4R β-arrestin d IC50 ± SEM [nM] e (% of max. Antagonist Activity at 10 µM) | hH4R cAMP f IC50 ± SEM [nM] e (% of max. Antagonist Activity at 10 µM) |
|---|---|---|---|---|
| 10 | 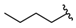 | 192 [42;874] (4) | 82 ± 5 (96) | 445 ± 10 (105) |
| 11 | 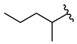 | 353 [275;454] (3) | 97 ± 16 (97) | 640 ± 49 (100) |
| 12 | 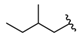 | 321 [127;814] (3) | 38 ± 7 (100) | 132 ± 9 (111) |
| 13 | 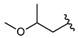 | 4264 [2074;8767] (2) | nt g | nt g |
| 14 | 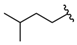 | 203 [69;601] (3) | 24 ± 3 (101) | 43 ± 17 (107) |
| 15 |  | 1490 [489;4538] (3) | nt g | nt g |
| 16 | 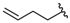 | 319 [223;458] (3) | 146 ± 38 (94) | 635 ± 123 (104) |
| 17 | 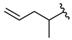 | 263 [112;617] (3) | 744 ± 139 (92) | 721 ± 70 (94) |
| 18 | 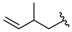 | 262 [91;756] (3) | 66.6 ± 3 (101) | 188 ± 45 (104) |
| 19 | 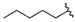 | 393 [224;688] (3) | 34.9 ± 7 (99) | 419 ± 77 (97) |
| 20 | 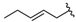 | (6%) (3) | 376 ± 9 (101) | nt g |
2.3. In Vitro Pharmacological Studies
2.3.1. Histamine H4 Receptor Affinity
2.3.2. Functional Characterization in β-Arrestin Recruitment Assay
2.3.3. Functional Characterization in cAMP Accumulation Assay
2.3.4. Comparison of Intrinsic Activities
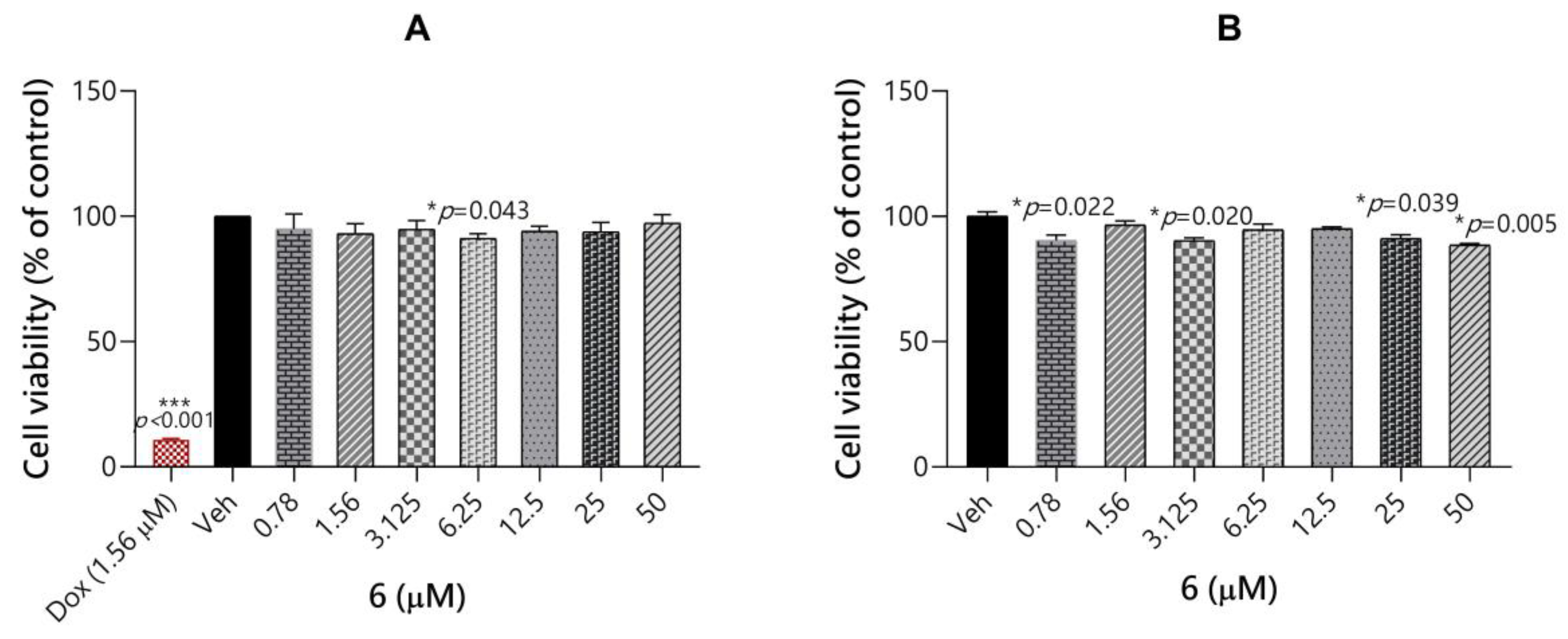
2.3.5. Toxicity Evaluation of Compound 6
2.3.6. Permeability of Compound 6 through Blood Brain Barrier
2.4. In Vivo Pharmacological Studies
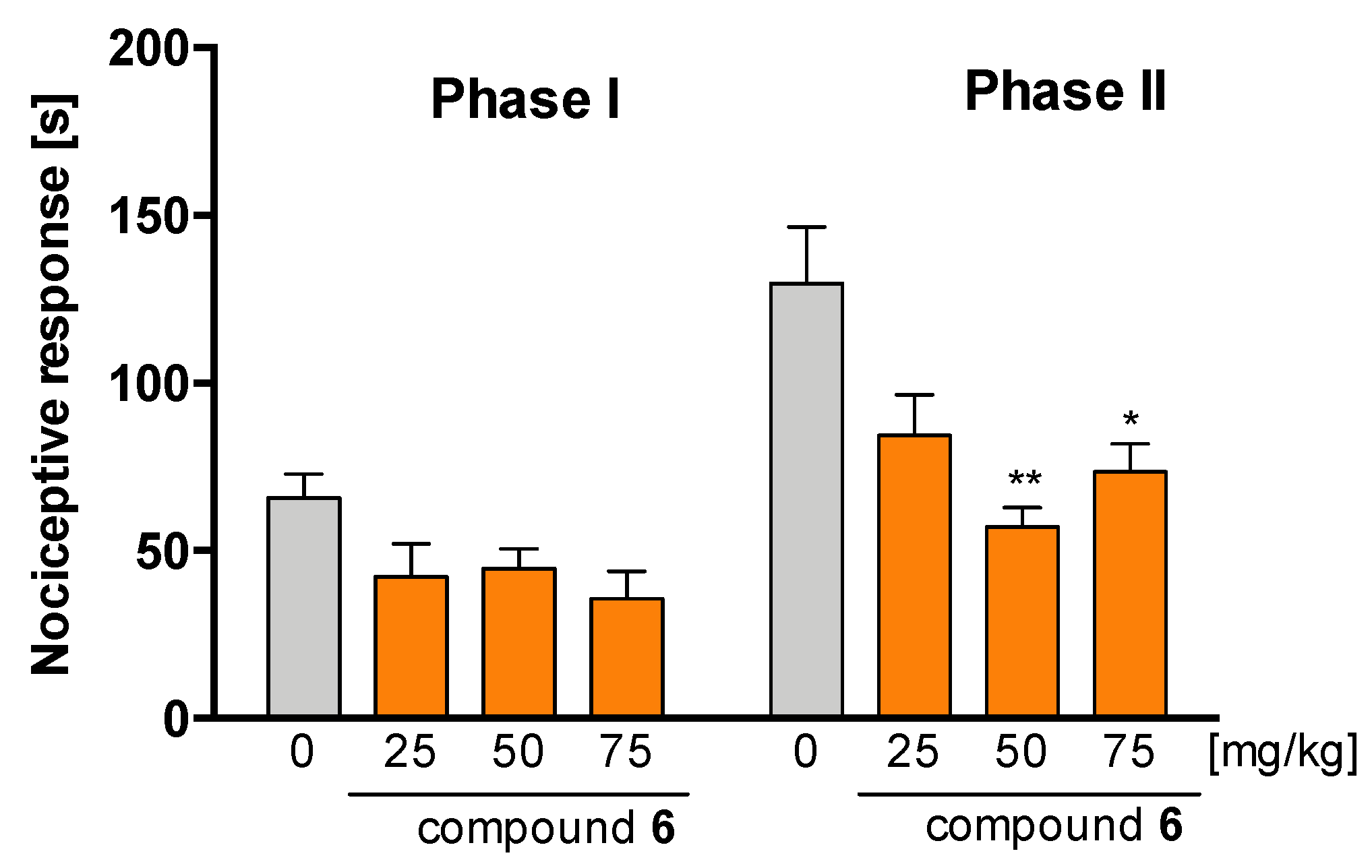
2.4.1. Antinociceptive Activity of Compound 6 in Formalin Test
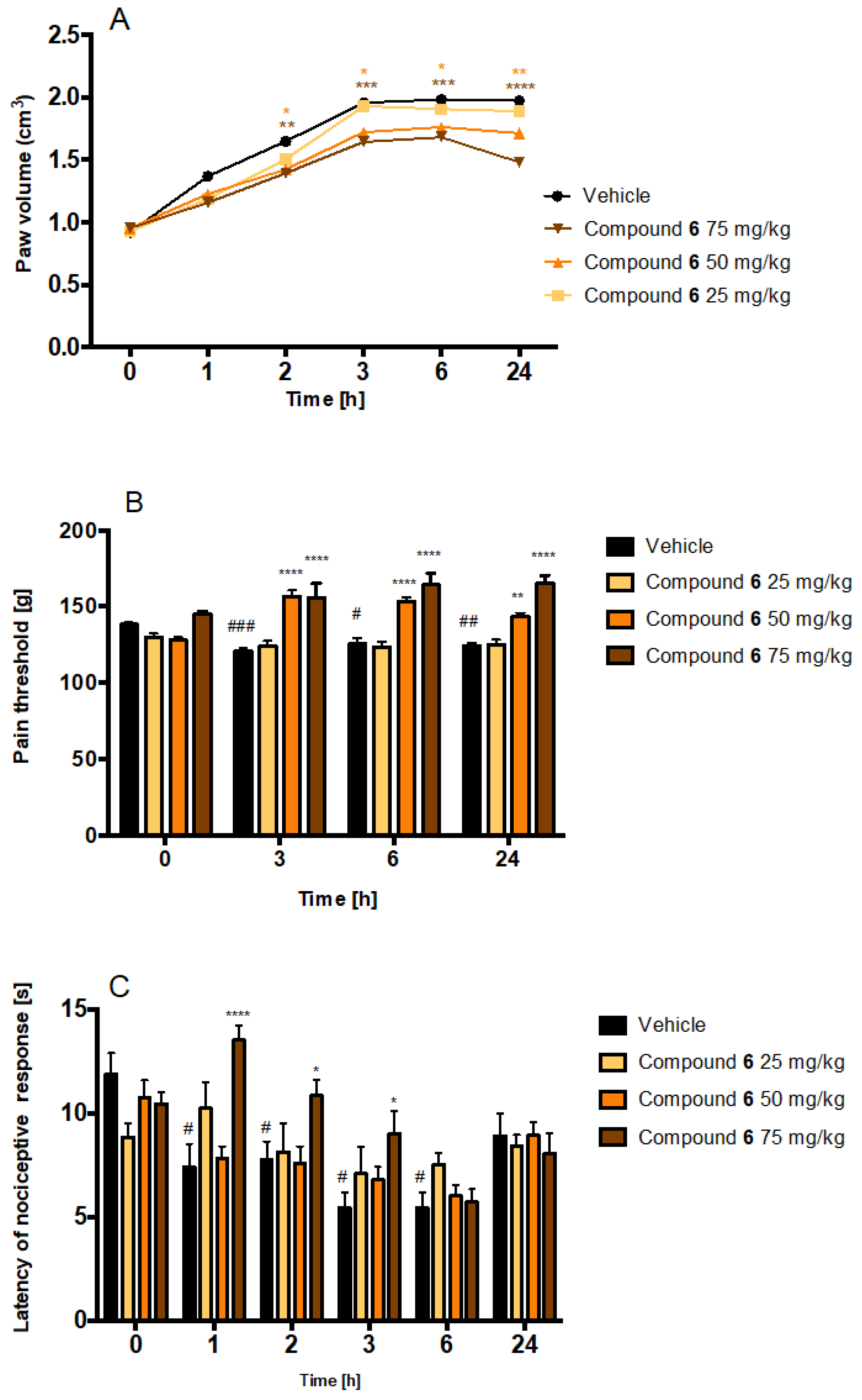
2.4.2. Antinociceptive and Anti-Inflammatory Activity of Compound 6 in Carrageenan-Induced Inflammatory Pain and Oedema
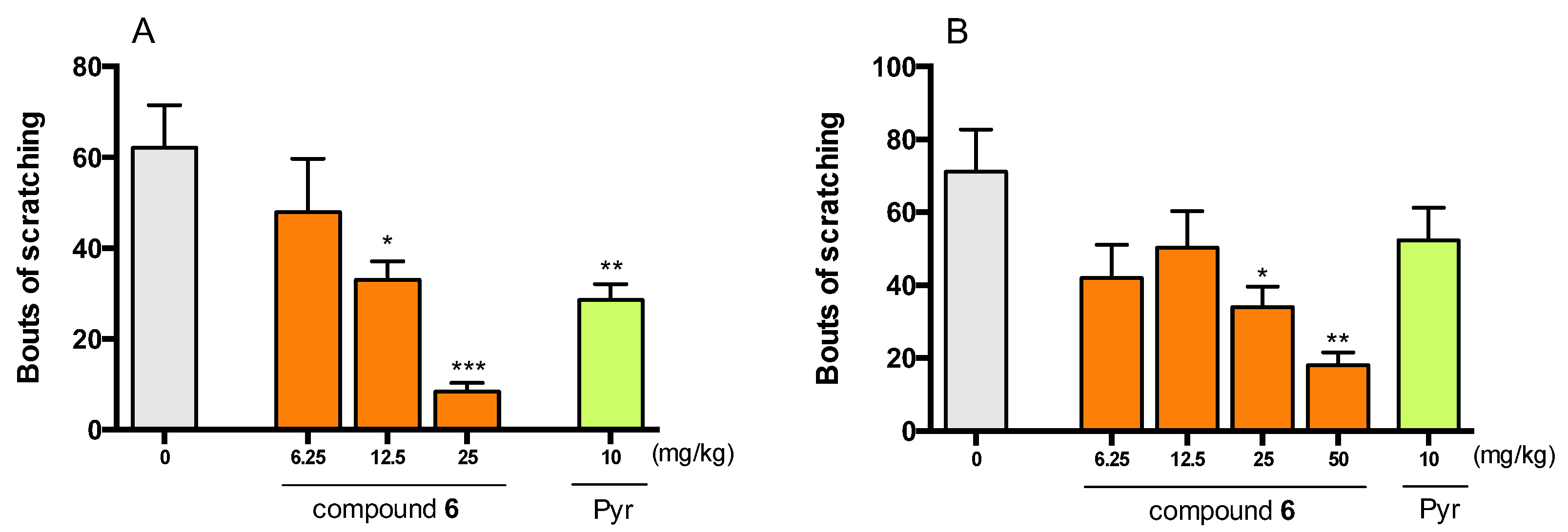
2.4.3. Antipruritic Effect of Compound 6 in Histamine- and Chloroquine-Induced Pruritus
3. Materials and Methods
3.1. Synthesis of Compounds
3.1.1. Synthesis of Esters
3.1.2. Synthesis of Triazines 2–20—General Procedure
3.2. In Vitro Biological Studies
3.2.1. Histamine H4 Receptor Affinity
3.2.2. β-Arrestin Recruitment Assay
3.2.3. cAMP Accumulation Assay
3.2.4. PAMPA Assay
3.2.5. Toxicity Evaluation
3.3. In Vivo Studies
3.3.1. Animals
3.3.2. Formalin Test
3.3.3. Carrageenan-Induced Inflammatory Pain and Oedema
3.3.4. Histamine- and Chloroquine-Induced Pruritus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tiligada, E.; Ennis, M. Histamine pharmacology: From Sir Henry Dale to the 21st century. Br. J. Pharmacol. 2020, 177, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2019, 177, 580–599. [Google Scholar] [CrossRef]
- Thurmond, R.L. The histamine H4 receptor: From orphan to the clinic. Front. Pharmacol. 2015, 6, 65. [Google Scholar] [CrossRef]
- Hofstra, C.L.; Desai, P.J.; Thurmond, R.L.; Fung-Leung, W.-P.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003, 305, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Ngo, K.; Nguyen, S.; Thurmond, R.L.; Edwards, J.P.; Karlsson, L.; Fung-Leung, W.-P. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br. J. Pharmacol. 2004, 142, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.K.; McAllister, B.; Cross, L.; Green, D.S.; Kornfeld, H.; Center, D.M.; Cruikshank, W.W. Histamine 4 Receptor Activation Induces Recruitment of FoxP3 + T Cells and Inhibits Allergic Asthma in a Murine Model. J. Immunol. 2007, 178, 8081–8089. [Google Scholar] [CrossRef]
- Ferreira, R.; Santos, T.; Gonçalves, J.; Baltazar, G.; Ferreira, L.; Agasse, F.; Bernardino, L. Histamine modulates microglia function. J. Neuroinflamm. 2012, 9, 90. [Google Scholar] [CrossRef]
- Jemima, E.A.; Prema, A.; Thangam, E.B. Functional characterization of histamine H4 receptor on human mast cells. Mol. Immunol. 2014, 62, 19–28. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H1R or H4R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zeng, X.; Hu, G.; Zhang, H.; He, S.; Zhang, S. Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Mol. Neurobiol. 2014, 49, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Gantner, F.; Sakai, K.; Tusche, M.W.; Cruikshank, W.W.; Center, D.M.; Bacon, K.B.; Timmerman, H.; Lovenberg, T.W.; Leurs, R. Histamine H4 and H2 Receptors Control Histamine-Induced Interleukin-16 Release from Human CD8 + T Cells. J. Pharmacol. Exp. Ther. 2002, 303, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Leite-de-Moraes, M.C.; Diem, S.; Michel, M.-L.; Ohtsu, H.; Thurmond, R.L.; Schneider, E.; Dy, M. Cutting Edge: Histamine Receptor H4 Activation Positively Regulates In Vivo IL-4 and IFN-γ Production by Invariant NKT Cells. J. Immunol. 2009, 182, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, D.; Leurs, R.; Chazot, P.; Shenton, F.C.; Elisa, C.C.L. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J. Allergy Clin. Immunol. 2007, 120, 300–307. [Google Scholar] [CrossRef]
- Gutzmer, R.; Mommert, S.; Gschwandtner, M.; Zwingmann, K.; Stark, H.; Werfel, T. The histamine H4 receptor is functionally expressed on TH2 cells. J. Allergy Clin. Immunol. 2009, 123, 619–625. [Google Scholar] [CrossRef]
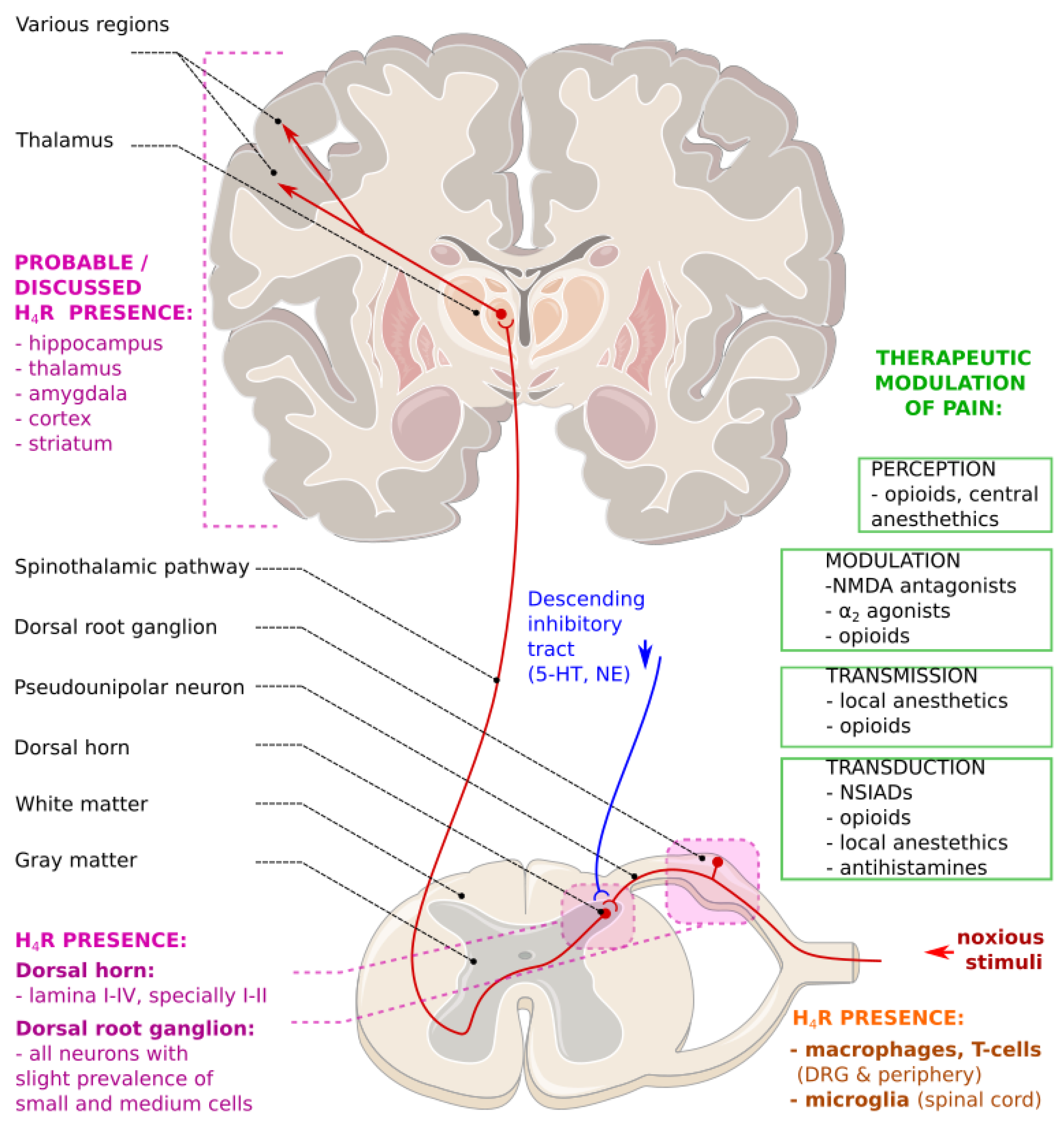
- Sanna, M.D.; Galeotti, N. Central neuronal functions of histamine H4 receptors. Oncotarget 2017, 8, 12556–12557. [Google Scholar] [CrossRef]
- Schneider, E.H.; Seifert, R. The histamine H4 receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology 2016, 106, 116–128. [Google Scholar] [CrossRef]
- Schneider, E.H.; Neumann, D.; Seifert, R. Histamine H4 receptor expression in the brain? Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 388, 5–9. [Google Scholar] [CrossRef]
- Sanna, M.D.; Ghelardini, C.; Thurmond, R.L.; Masini, E.; Galeotti, N. Behavioural phenotype of histamine H4 receptor knockout mice: Focus on central neuronal functions. Neuropharmacology 2017, 114, 48–57. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Strakhova, M.I.; Nikkel, A.L.; Manelli, A.M.; Hsieh, G.C.; Esbenshade, T.A.; Brioni, J.D.; Bitner, R.S. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009, 1250, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Stark, H.; Lucarini, L.; Ghelardini, C.; Masini, E.; Galeotti, N. Histamine H4 receptor activation alleviates neuropathic pain through differential regulation of ERK, JNK, and P38 MAPK phosphorylation. Pain 2015, 156, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Hudspith, M.J. Anatomy, physiology and pharmacology of pain. Anaesth. Intensive Care Med. 2019, 20, 419–425. [Google Scholar] [CrossRef]
- Dureja, G.P.; Iyer, R.N.; Das, G.; Ahdal, J.; Narang, P. Evidence and consensus recommendations for the pharmacological management of pain in India. J. Pain Res. 2017, 10, 709–736. [Google Scholar] [CrossRef]
- Connelly, W.M.; Shenton, F.C.; Lethbridge, N.; Leurs, R.; Waldvogel, H.J.; Faull, R.L.M.; Lees, G.; Chazot, P.L. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br. J. Pharmacol. 2009, 157, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Sanna, M.D.; Ghelardini, C. Pleiotropic effect of histamine H4 receptor modulation in the central nervous system. Neuropharmacology 2013, 71, 141–147. [Google Scholar] [CrossRef]
- Sanna, M.D.; Lucarini, L.; Durante, M.; Ghelardini, C.; Masini, E.; Galeotti, N. Histamine H4 receptor agonist-induced relief from painful peripheral neuropathy is mediated by inhibition of spinal neuroinflammation and oxidative stress. Br. J. Pharmacol. 2017, 174, 28–40. [Google Scholar] [CrossRef]
- Sanna, M.D.; Borgonetti, V.; Masini, E.; Galeotti, N. Histamine H4 receptor stimulation in the locus coeruleus attenuates neuropathic pain by promoting the coeruleospinal noradrenergic inhibitory pathway. Eur. J. Pharmacol. 2020, 868, 172859. [Google Scholar] [CrossRef]
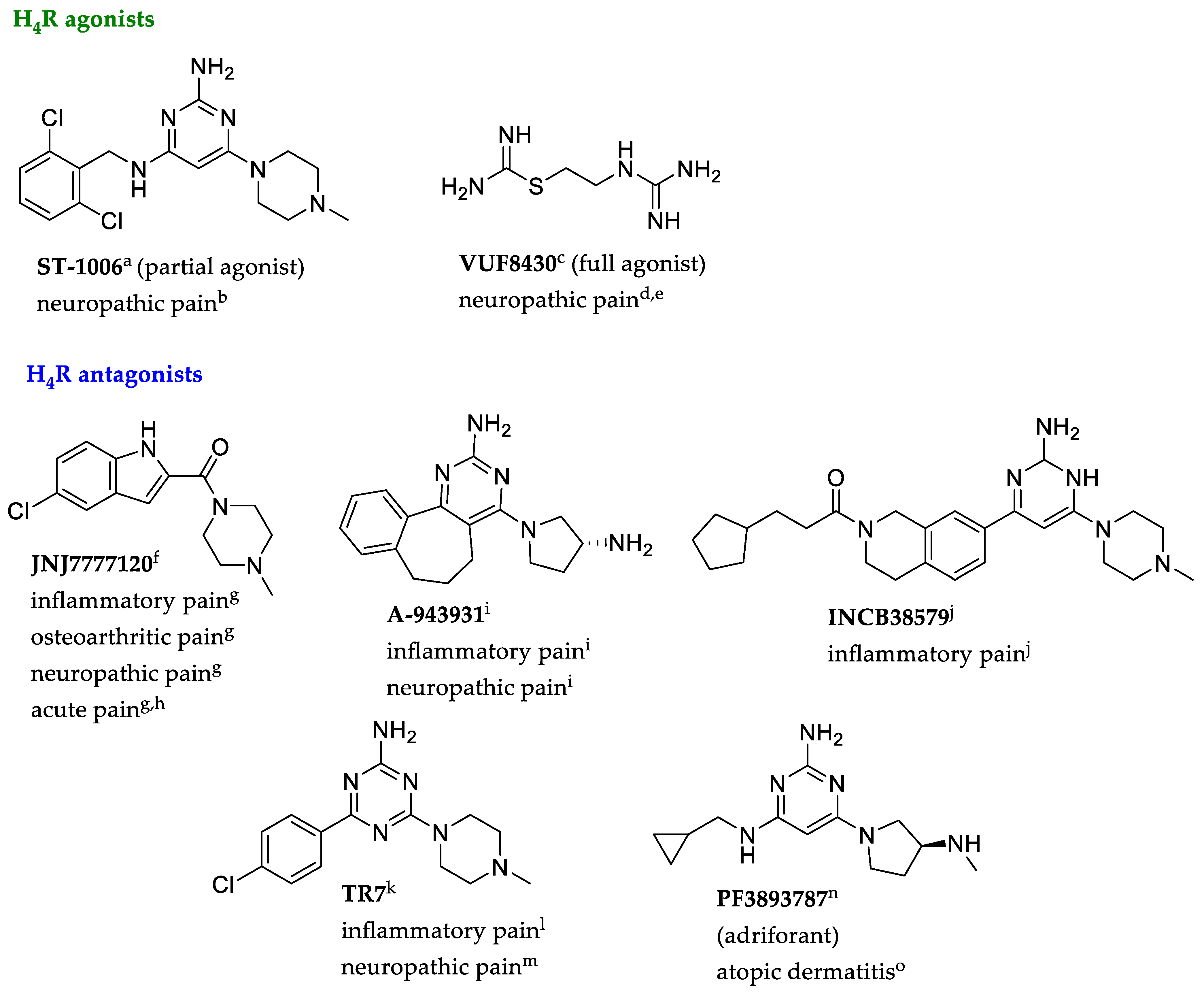
- Coruzzi, G.; Adami, M.; Guaita, E.; de Esch, I.J.P.; Leurs, R. Antiinflammatory and antinociceptive effects of the selective histamine H4 receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur. J. Pharmacol. 2007, 563, 240–244. [Google Scholar] [CrossRef]
- Hsieh, G.C.; Chandran, P.; Salyers, A.K.; Pai, M.; Zhu, C.Z.; Wensink, E.J.; Witte, D.G.; Miller, T.R.; Mikusa, J.P.; Baker, S.J.; et al. H4 receptor antagonism exhibits anti-nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol. Biochem. Behav. 2010, 95, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, R.; Leśniak, A.; Żochowska, M.; Sacharczuk, M.; Kieć-Kononowicz, K.; Bujalska-Zadrożny, M. Antinociceptive effect of co-administered NMDA and histamine H4 receptor antagonists in a rat model of acute pain. Pharmacol. Rep. 2017, 69, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cowart, M.D.; Altenbach, R.J.; Liu, H.; Hsieh, G.C.; Drizin, I.; Milicic, I.; Miller, T.R.; Witte, D.G.; Wishart, N.; Fix-Stenzel, S.R.; et al. Rotationally constrained 2,4-diamino-5,6-disubstituted pyrimidines: A new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models. J. Med. Chem. 2008, 51, 6547–6557. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Covington, M.; Bian, D.; Zhuo, J.; Bowman, K.; Li, Y.; Soloviev, M.; Qian, D.Q.; Feldman, P.; Leffet, L.; et al. INCB38579, a novel and potent histamine H₄ receptor small molecule antagonist with anti-inflammatory pain and anti-pruritic functions. Eur. J. Pharmacol. 2012, 675, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mogilski, S.; Kubacka, M.; Łażewska, D.; Więcek, M.; Głuch-Lutwin, M.; Tyszka-Czochara, M.; Bukowska-Strakova, K.; Filipek, B.; Kieć-Kononowicz, K. Aryl-1,3,5-triazine ligands of histamine H4 receptor attenuate inflammatory and nociceptive response to carrageen, zymosan and lipopolysaccharide. Inflamm. Res. 2017, 66, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Popiołek-Barczyk, K.; Łażewska, D.; Latacz, G.; Olejarz, A.; Makuch, W.; Stark, H.; Kieć-Kononowicz, K.; Mika, J. Antinociceptive effects of novel histamine H3 and H4 receptor antagonists and their influence on morphine analgesia of neuropathic pain in the mouse. Br. J. Pharmacol. 2018, 175, 2897–2910. [Google Scholar] [CrossRef]
- Altenbach, R.J.; Adair, R.M.; Bettencourt, B.M.; Black, L.A.; Fix-Stenzel, S.R.; Gopalakrishnan, S.M.; Hsieh, G.C.; Liu, H.; Marsh, K.C.; Mcpherson, M.J.; et al. Structure-Activity Studies on a Series of a 2-Aminopyrimidine-Containing Histamine H4 Receptor Ligands. J. Med. Chem. 2008, 51, 6571–6580. [Google Scholar] [CrossRef]
- Liu, H.; Altenbach, R.J.; Carr, T.L.; Chandran, P.; Hsieh, G.C.; Lewis, L.G.; Manelli, A.M.; Milicic, I.; Marsh, K.C.; Miller, T.R.; et al. Cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia. J. Med. Chem. 2008, 51, 7094–7098. [Google Scholar] [CrossRef]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837. [Google Scholar] [CrossRef]
- Thurmond, R.L.; Venable, J.; Savall, B.; La, D.; Snook, S.; Dunford, P.J.; Edwards, J.P. Clinical Development of Histamine H4 Receptor Antagonists. Handb. Exp. Pharmacol. 2017, 241, 301–320. [Google Scholar] [CrossRef]
- Sander, K.; Kottke, T.; Tanrikulu, Y.; Proschak, E.; Weizel, L.; Schneider, E.H.; Seifert, R.; Schneider, G.; Stark, H. 2,4-Diaminopyrimidines as histamine H4 receptor ligands-Scaffold optimization and pharmacological characterization. Bioorg. Med. Chem. 2009, 17, 7186–7196. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; Smits, R.A.; Bakker, R.A.; van Dam, C.M.; de Esch, I.J.; Leurs, R. Discovery of S-(2-guanidylethyl)-isothiourea (VUF 8430) as a potent nonimidazole histamine H4 receptor agonist. J. Med. Chem. 2006, 49, 6650–6651. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, J.A.; Grice, C.A.; Chai, W.; Dvorak, C.A.; Venable, J.D.; Kwok, A.K.; Ly, K.S.; Wei, J.; Baker, S.M.; Desai, P.J.; et al. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J. Med. Chem. 2003, 46, 3957–3960. [Google Scholar] [CrossRef] [PubMed]
- Łażewska, D.; Więcek, M.; Ner, J.; Kamińska, K.; Kottke, T.; Schwed, J.S.; Zygmunt, M.; Karcz, T.; Olejarz, A.; Kuder, K.; et al. Aryl-1,3,5-triazine derivatives as histamine H4 receptor ligands. Eur. J. Med. Chem. 2014, 83, 534–546. [Google Scholar] [CrossRef]
- Mowbray, C.E.; Bell, A.S.; Clarke, N.P.; Collins, M.; Jones, R.M.; Lane, C.A.; Liu, W.L.; Newman, S.D.; Paradowski, M.; Schenck, E.J.; et al. Challenges of drug discovery in novel target space. The discovery and evaluation of PF-3893787: A novel histamine H4 receptor antagonist. Bioorg. Med. Chem. Lett. 2011, 21, 6596–6602. [Google Scholar] [CrossRef]
- Rosethorne, E.M.; Charlton, S.J. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol. Pharmacol. 2011, 79, 749–757. [Google Scholar] [CrossRef]
- Terzioglu, N.; van Rijn, R.M.; Bakker, R.A.; De Esch, I.J.P.; Leurs, R. Synthesis and structure–activity relationships of indole and benzimidazole piperazines as histamine H4 receptor antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 5251–5256. [Google Scholar] [CrossRef]
- Nijmeijer, S.; Vischer, H.F.; Rosethorne, E.M.; Charlton, S.J.; Leurs, R. Analysis of Multiple Histamine H4 Receptor Compound Classes Uncovers Gα i Protein- and β-Arrestin2-Biased Ligands. Mol. Pharmacol. 2012, 82, 1174–1182. [Google Scholar] [CrossRef]
- Nijmeijer, S.; Vischer, H.F.; Sirci, F.; Schultes, S.; Engelhardt, H.; de Graaf, C.; Rosethorne, E.M.; Charlton, S.J.; Leurs, R. Detailed analysis of biased histamine H4 receptor signalling by JNJ 7777120 analogues. Br. J. Pharmacol. 2013, 170, 78–88. [Google Scholar] [CrossRef]
- Rajagopal, S.; Rajagopal, K.; Lefkowitz, R.J. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010, 9, 373–386. [Google Scholar] [CrossRef]
- Galandrin, S.; Oligny-Longpré, G.; Bouvier, M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol. Sci. 2007, 28, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Eiger, D.S.; Pham, U.; Gardner, J.; Hicks, C.; Rajagopal, S. GPCR systems pharmacology: A different perspective on the development of biased therapeutics. Am. J. Physiol. Physiol. 2022, 322, C887–C895. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Itadani, H.; Hidaka, Y.; Ohta, M.; Tanaka, K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem. Biophys. Res. Commun. 2000, 279, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Morikawa, N.; Saito, Y.; Masuho, Y.; Matsumoto, S.I. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 2000, 275, 36781–36786. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Kenakin, T.; Alexander, S.P.H.; Bermudez, M.; Bohn, L.M.; Breinholt, C.S.; Bouvier, M.; Hill, S.J.; Kostenis, E.; Martemyanov, K.A.; et al. Community guidelines for GPCR ligand bias: IUPHAR review 32. Br. J. Pharmacol. 2022, 179, 3651–3674. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, M.; Adami, M.; Micheloni, C.; Głuch-Lutwin, M.; Siwek, A.; Latacz, G.; Łażewska, D.; Więcek, M.; Reiner-Link, D.; Stark, H.; et al. Eosinophils adhesion assay as a tool for phenotypic drug screening-The pharmacology of 1,3,5–Triazine and 1H-indole like derivatives against the human histamine H4 receptor. Eur. J. Pharmacol. 2021, 890, 173611. [Google Scholar] [CrossRef]
- Zampeli, E.; Tiligada, E. The role of histamine H4 receptor in immune and inflammatory disorders. Br. J. Pharmacol. 2009, 157, 24–33. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef]
- Łażewska, D.; Mogilski, S.; Hagenow, S.; Kuder, K.; Głuch-Lutwin, M.; Siwek, A.; Więcek, M.; Kaleta, M.; Seibel, U.; Buschauer, A.; et al. Alkyl derivatives of 1,3,5-triazine as histamine H4 receptor ligands. Bioorg. Med. Chem. 2019, 27, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Schneider, E.H.; Dove, S.; Brunskole, I.; Neumann, D.; Strasser, A.; Buschauer, A. Paradoxical Stimulatory Effects of the “Standard” Histamine H4 receptor antagonist JNJ7777120: The H4 receptor joints the club of 7 transmembrane domain receptors exhibiting functional selectivity. Mol. Pharmacol. 2011, 79, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kisanga, P.B.; Ilankumaran, P.; Fetterly, B.M.; Verkade, J.G. P(RNCH(2)CH(2))(3)N: Efficient 1,4-addition catalysts. J. Org. Chem. 2002, 67, 3555–3560. [Google Scholar] [CrossRef]
- Schneider, E.H.; Schnell, D.; Papa, D.; Seifert, R. High constitutive activity and a G-protein-independent high-affinity state of the human histamine H4-receptor. Biochemistry 2009, 48, 1424–1438. [Google Scholar] [CrossRef]
- Leff, P.; Dougall, I.G. Further concerns over Cheng-Prusoff analysis. Trends Pharmacol. Sci. 1993, 14, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.F.; Martins, A.; Majolo, F.; Contini, V.; Laufer, S.; Goettert, M.I. Neural regeneration research model to be explored: SH-SY5Y human neuroblastoma cells. Neural Regen. Res. 2023, 18, 1265–1266. [Google Scholar] [CrossRef]
- Lubelska, A.; Latacz, G.; Jastrzębska-Więsek, M.; Kotańska, M.; Kurczab, R.; Partyka, A.; Marć, M.A.; Wilczyńska, D.; Doroz-Płonka, A.; Łażewska, D.; et al. Are the Hydantoin-1,3,5-triazine 5-HT6R Ligands a Hope to a Find New Procognitive and Anti-Obesity Drug? Considerations Based on Primary In Vivo Assays and ADME-Tox Profile In Vitro. Molecules 2019, 24, 4472. [Google Scholar] [CrossRef]
- Anzelc, M.; Burkhart, C.G. Pain and Pruritus: A study of their similarities and differences. Int. J. Dermatol. 2020, 59, 159–164. [Google Scholar] [CrossRef]
- Liu, T.; Ji, R.-R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflug. Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef]
- Dunford, P.J.; Williams, K.N.; Desai, P.J.; Karlsson, L.; McQueen, D.; Thurmond, R.L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007, 119, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Abarca, A.B.; Avila-Rojas, S.H.; Barragán-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Formalin injection produces long-lasting hypersensitivity with characteristics of neuropathic pain. Eur. J. Pharmacol. 2017, 797, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Mello, T.; Masini, E.; Galeotti, N. Activation of ERK/CREB pathway in noradrenergic neurons contributes to hypernociceptive phenotype in H4 receptor knockout mice after nerve injury. Neuropharmacology 2018, 128, 340–350. [Google Scholar] [CrossRef]
- Lay, M.; Dong, X. Neural Mechanisms of Itch. Annu. Rev. Neurosci. 2020, 43, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.L.; Balimane, P.V. A novel design of artificial membrane for improving the PAMPA model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef]
- Łażewska, D.; Kaleta, M.; Hagenow, S.; Mogilski, S.; Latacz, G.; Karcz, T.; Lubelska, A.; Honkisz, E.; Handzlik, J.; Reiner, D.; et al. Novel naphthyloxy derivatives-Potent histamine H3 receptor ligands. Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2018, 26, 2573–2585. [Google Scholar] [CrossRef]
- Bell, J.K.; McQueen, D.S.; Rees, J.L. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in BalbC mice. Br. J. Pharmacol. 2004, 142, 374–380. [Google Scholar] [CrossRef]
- Zhou, F.-M.; Cheng, R.-X.; Wang, S.; Huang, Y.; Gao, Y.-J.; Zhou, Y.; Liu, T.-T.; Wang, X.-L.; Chen, L.-H.; Liu, T. Antioxidants Attenuate Acute and Chronic Itch: Peripheral and Central Mechanisms of Oxidative Stress in Pruritus. Neurosci. Bull. 2016, 33, 423–435. [Google Scholar] [CrossRef]
- Coavoy-Sánchez, S.A.; Rodrigues, L.; Teixeira, S.A.; Soares, A.G.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Costa, S.K.P.; Muscará, M.N. Hydrogen sulfide donors alleviate itch secondary to the activation of type-2 protease activated receptors (PAR-2) in mice. Pharmacol. Res. 2016, 113, 686–694. [Google Scholar] [CrossRef]

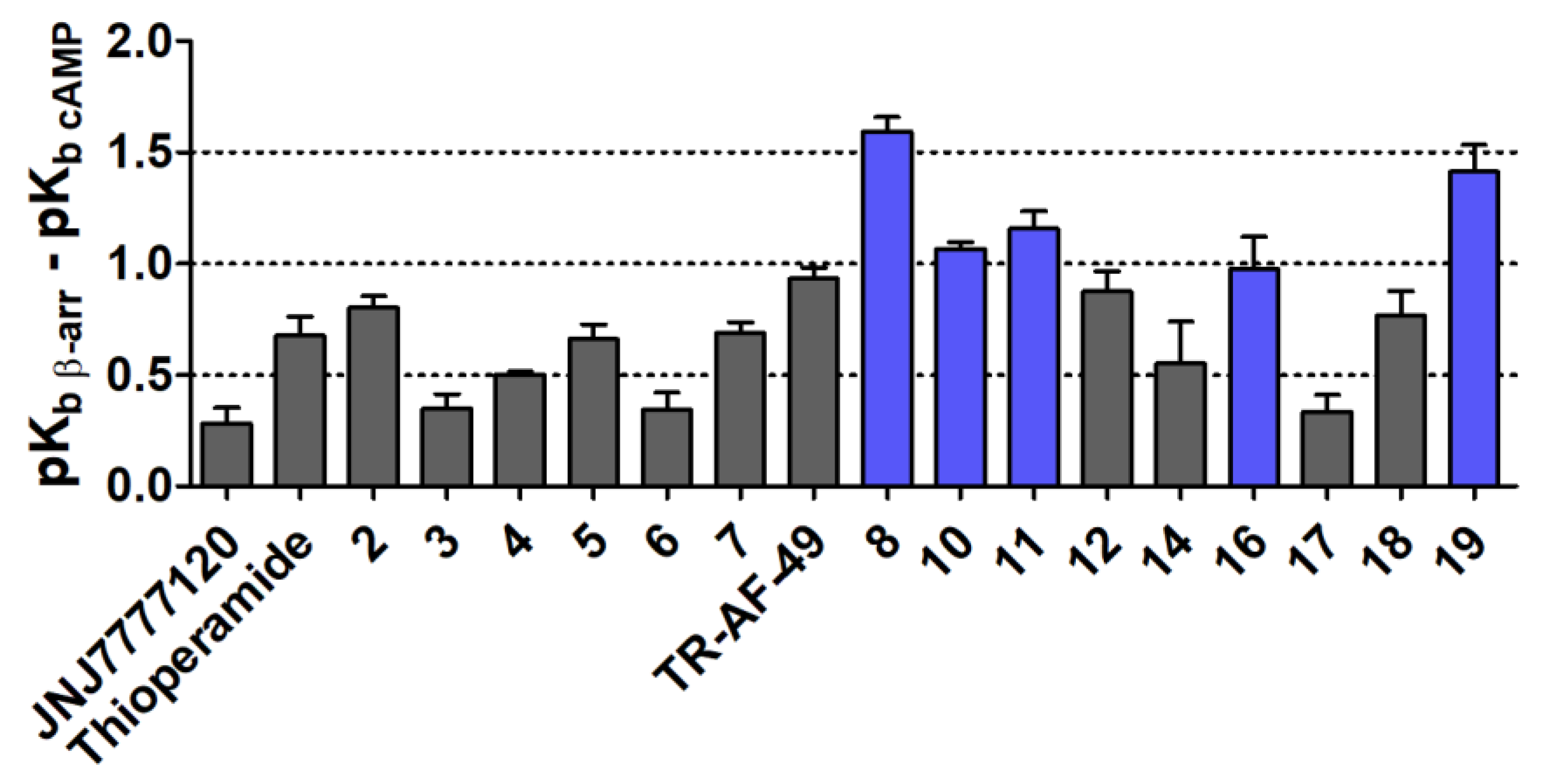
| No | pKb β-arrestin ± SEM | pKb cAMP ± SEM | Bias Factor (pKb β-arrestin–pKb cAMP) ± SEM |
|---|---|---|---|
| 2 | 7.32 ± 0.05 | 6.52 ± 0.03 | 0.8 ± 0.1 |
| 3 | 7.78 ± 0.06 | 7.43 ± 0.01 | 0.4 ± 0.1 |
| 4 | 7.94 ± 0.01 | 7.44 ± 0.01 | 0.5 ± 0.0 |
| 5 | 7.78 ± 0.04 | 7.12 ± 0.05 | 0.7 ± 0.1 |
| 6 | 8.79 ± 0.03 | 8.45 ± 0.07 | 0.3 ± 0.1 |
| 7 | 8.63 ± 0.04 | 7.94 ± 0.03 | 0.7 ± 0.1 |
| TR-AF-49 | 7.95 ± 0.04 | 7.02 ± 0.02 | 0.9 ± 0.0 |
| 8 | 8.17 ± 0.06 | 6.58 ± 0.02 | 1.6 ± 0.1 |
| 10 | 7.87 ± 0.03 | 6.8 ± 0.01 | 1.1 ± 0.0 |
| 11 | 7.8 ± 0.07 | 6.65 ± 0.03 | 1.2 ± 0.1 |
| 12 | 8.21 ± 0.08 | 7.33 ± 0.03 | 0.9 ± 0.1 |
| 14 | 8.41 ± 0.05 | 7.86 ± 0.18 | 0.6 ± 0.2 |
| 16 | 7.63 ± 0.12 | 6.66 ± 0.09 | 1.0 ± 0.1 |
| 17 | 6.93 ± 0.07 | 6.59 ± 0.04 | 0.3 ± 0.1 |
| 18 | 7.96 ± 0.02 | 7.19 ± 0.11 | 0.8 ± 0.1 |
| 19 | 8.25 ± 0.09 | 6.84 ± 0.08 | 1.4 ± 0.1 |
| JNJ7777120 | 8.04 ± 0.06 | 7.76 ± 0.02 | 0.3 ± 0.1 |
| Thioperamide | 7.47 ± 0.08 | 6.8 ± 0.03 | 0.7 ± 0.1 |
| Compound | Pe 1,2 [10−6 cm/s] ± SD | Mass Retention (R) |
|---|---|---|
| 6 | 12.26 ± 0.31 | 3.15% |
| Caffeine | 9.78 ± 1.75 | 1.54% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejarz-Maciej, A.; Mogilski, S.; Karcz, T.; Werner, T.; Kamińska, K.; Kupczyk, J.; Honkisz-Orzechowska, E.; Latacz, G.; Stark, H.; Kieć-Kononowicz, K.; et al. Trisubstituted 1,3,5-Triazines as Histamine H4 Receptor Antagonists with Promising Activity In Vivo. Molecules 2023, 28, 4199. https://doi.org/10.3390/molecules28104199
Olejarz-Maciej A, Mogilski S, Karcz T, Werner T, Kamińska K, Kupczyk J, Honkisz-Orzechowska E, Latacz G, Stark H, Kieć-Kononowicz K, et al. Trisubstituted 1,3,5-Triazines as Histamine H4 Receptor Antagonists with Promising Activity In Vivo. Molecules. 2023; 28(10):4199. https://doi.org/10.3390/molecules28104199
Chicago/Turabian StyleOlejarz-Maciej, Agnieszka, Szczepan Mogilski, Tadeusz Karcz, Tobias Werner, Katarzyna Kamińska, Jarosław Kupczyk, Ewelina Honkisz-Orzechowska, Gniewomir Latacz, Holger Stark, Katarzyna Kieć-Kononowicz, and et al. 2023. "Trisubstituted 1,3,5-Triazines as Histamine H4 Receptor Antagonists with Promising Activity In Vivo" Molecules 28, no. 10: 4199. https://doi.org/10.3390/molecules28104199
APA StyleOlejarz-Maciej, A., Mogilski, S., Karcz, T., Werner, T., Kamińska, K., Kupczyk, J., Honkisz-Orzechowska, E., Latacz, G., Stark, H., Kieć-Kononowicz, K., & Łażewska, D. (2023). Trisubstituted 1,3,5-Triazines as Histamine H4 Receptor Antagonists with Promising Activity In Vivo. Molecules, 28(10), 4199. https://doi.org/10.3390/molecules28104199






