The Reducing Agents in Sonochemical Reactions without Any Additives
Abstract
1. Introduction
2. Sonochemical Reduction without Any Additives
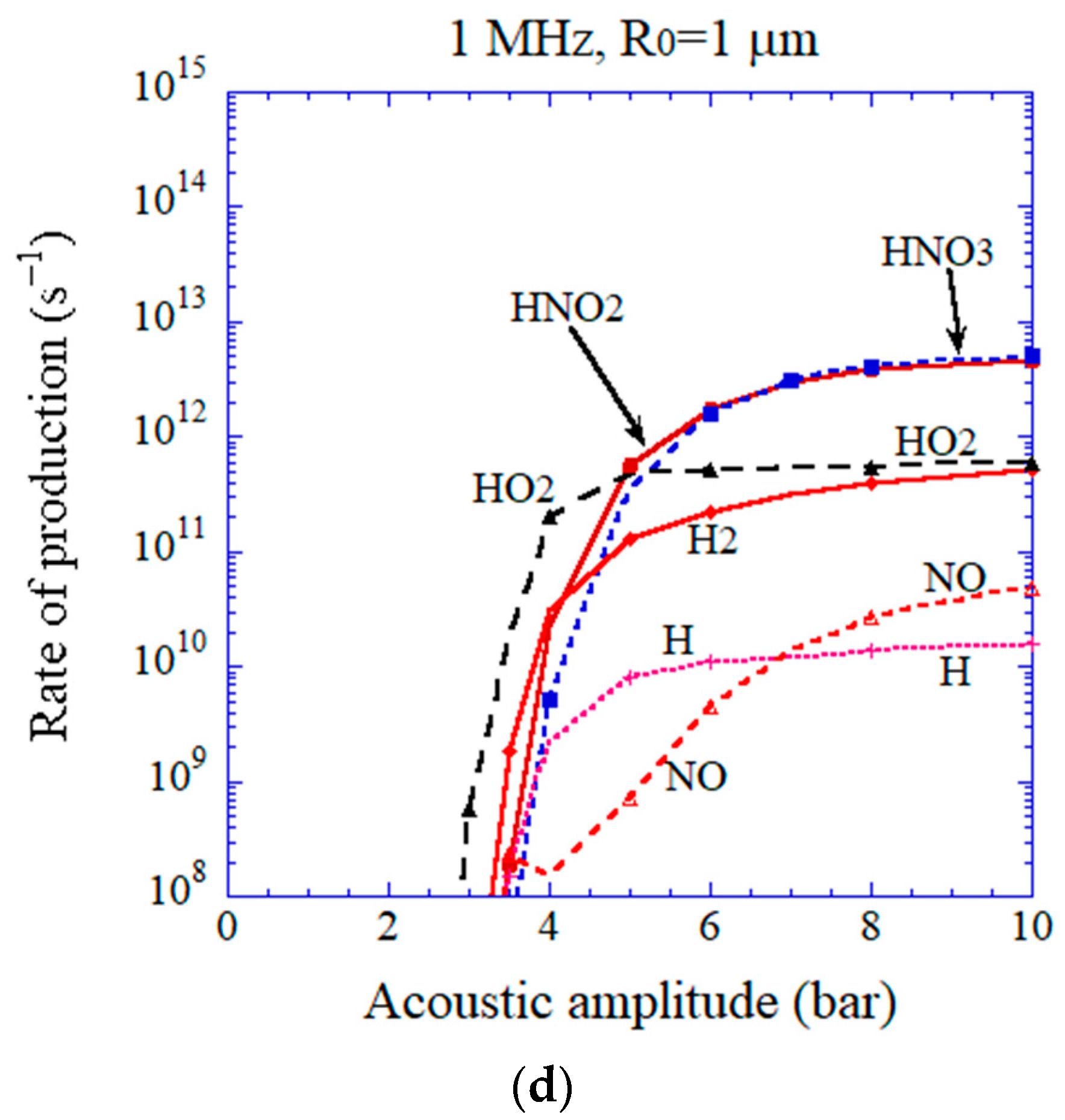
3. Results of Numerical Simulations and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, F.J. Ultrasonic cleaning and washing of surfaces. In Power Ultrasonics; Gallego-Juarez, J.A., Graff, K.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 577–609. [Google Scholar]
- Yasui, K. Acoustic Cavitation and Bubble Dynamics; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.M.; Afrand, M.; Żyła, G.; Ahn, H.S.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.E.; Sugden, M.; Litchfield, R.E.; Hutt, D.A.; Mason, T.J.; Cobley, A.J. Ultrasound assisted dispersal of a copper nanopowder for electroless copper activation. Ultrason. Sonochem. 2016, 29, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Z.; Li, C.; Sun, Q.; Huang, M.; Li, C.-R.; Qi, B. Effect of dispersion method on stability and dielectric strength of transformer oil-based TiO2 nanofluids. Nanoscale Res. Lett. 2016, 11, 515. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, J.-K.; Jeong, Y.-M.; Cheong, S.-I.; Ahn, Y.-C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Sato, K.; Li, J.-G.; Kamiya, H.; Ishigaki, T. Ultrasonic dispersion of TiO2 nanoparticles in aqueous suspension. J. Am. Ceram. Soc. 2008, 91, 2481–2487. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.A.; Schuchmann, H.P.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef]
- Yasui, K.; Towata, A.; Tuziuti, T.; Kozuka, T.; Kato, K. Effect of static pressure on acoustic energy radiated by cavitation bubbles in viscous liquids under ultrasound. J. Acoust. Soc. Am. 2011, 130, 3233–3242. [Google Scholar] [CrossRef]
- Yasui, K.; Kato, K. Numerical simulations of sonochemical production and oriented aggregation of BaTiO3 nanocrystals. Ultrason. Sonochem. 2017, 35, 673–680. [Google Scholar] [CrossRef]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Towata, A.; Kozuka, T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. Technol. 2008, 9, 140–146. [Google Scholar] [CrossRef]
- Yasui, K. Multibubble sonoluminescence from a theoretical perspective. Molecules 2021, 26, 4624. [Google Scholar] [CrossRef]
- Weninger, K.R.; Camara, C.G.; Putterman, S.J. Observation of bubble dynamics within luminescent cavitation clouds: Sonoluminescence at the nano-scale. Phys. Rev. E 2001, 63, 016310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ashokkumar, M.; Kentish, S.; Grieser, F. Determination of the size distribution of sonoluminescence bubbles in a pulsed acoustic field. J. Am. Chem. Soc. 2005, 127, 16810–16811. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Lee, J.; Kozuka, T.; Towata, A.; Iida, Y. The range of ambient radius for an active bubble in sonoluminescence and sonochemical reactions. J. Chem. Phys. 2008, 128, 184705. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.P.; Hilgenfeldt, S.; Lohse, D. Single-bubble sonoluminescence. Rev. Mod. Phys. 2002, 74, 425–484. [Google Scholar] [CrossRef]
- Yasui, K. Dynamics of acoustic bubbles. In Sonochemistry and the Acoustic Bubble; Grieser, F., Choi, P.-K., Enomoto, N., Harada, H., Okitsu, K., Yasui, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 41–83. [Google Scholar]
- Yasui, K. Alternative model of single-bubble sonoluminescence. Phys. Rev. E 1997, 56, 6750–6760. [Google Scholar] [CrossRef]
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. The chemical history of a bubble. Acc. Chem. Res. 2018, 51, 2169–2178. [Google Scholar] [CrossRef]
- Didenko, Y.T.; McNamara, W.B., III; Suslick, K.S. Temperature of multibubble sonoluminescence in water. J. Phys. Chem. A 1999, 103, 10783–10788. [Google Scholar] [CrossRef]
- McNamara, W.B., III; Didenko, Y.T.; Suslick, K.S. Pressure during sonoluminescence. J. Phys. Chem. B 2003, 107, 7303–7306. [Google Scholar] [CrossRef]
- Henglein, A. Contributions to various aspects of cavitation chemistry. In Advances in Sonochemistry; Mason, T.J., Ed.; JAI Press: London, UK, 1993; Volume 3, pp. 17–83. [Google Scholar]
- Yasui, K.; Tuziuti, T.; Sivakumar, M.; Iida, Y. Theoretical study of single-bubble sonochemistry. J. Chem. Phys. 2005, 122, 224706. [Google Scholar] [CrossRef]
- Yasui, K. Production of O radicals from cavitation bubbles under ultrasound. Molecules 2022, 27, 4788. [Google Scholar] [CrossRef] [PubMed]
- Choi, P. Sonoluminescence. In Sonochemistry and the Acoustic Bubble; Grieser, F., Choi, P.-K., Enomoto, N., Harada, H., Okitsu, K., Yasui, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–117. [Google Scholar]
- Young, F.R. Sonoluminescence; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nikitenko, S.I.; Pflieger, R. Toward a new paradigm for sonochemistry: Short review on nonequilibrium plasma observations by means of MBSL spectroscopy in aqueous solutions. Ultrason. Sonochem. 2017, 35, 623–630. [Google Scholar] [CrossRef]
- Sharipov, G.L.; Gareev, B.M.; Abdrakhmanov, A.M. Confirmation of hydrated electrons formation during the moving single-bubble sonolysis: Activation of Tb3+ ion sonoluminescence by acceptors in an aqueous solution. J. Photochem. Photobiol. A 2020, 402, 112800. [Google Scholar] [CrossRef]
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. Extreme conditions during multibubble cavitation: Sonoluminescence as a spectroscopic probe. Ultrason. Sonochem. 2011, 18, 842–846. [Google Scholar] [CrossRef]
- Choi, P.-K. Sonoluminescence and acoustic cavitation. Jpn. J. Appl. Phys. 2017, 56, 07JA01. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Sivakumar, M.; Iida, Y. Sonoluminescence. Appl. Spectrosc. Rev. 2004, 39, 399–436. [Google Scholar] [CrossRef]
- Grieser, F.; Choi, P.-K.; Enomoto, N.; Harada, H.; Okitsu, K.; Yasui, K. (Eds.) Sonochemistry and the Acoustic Bubble; Elsevier: Amsterdam, The Netherlands, The Netherlands, 2015. [Google Scholar]
- Chen, D.; Sharma, S.K.; Mudhoo, A. (Eds.) Handbook on Applications of Ultrasound Sonochemistry for Sustainability; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ashokkumar, M.; Cavalieri, F.; Chemat, F.; Okitsu, K.; Sambandam, A.; Yasui, K.; Zisu, B. (Eds.) Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; Volumes 1–2. [Google Scholar]
- Petrier, C. The use of power ultrasound for water treatment. In Power Ultrasonics; Gallego-Juarez, J.A., Graff, K.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 939–972. [Google Scholar]
- Mason, T.J.; Lorimer, J.P. Sonochemistry: Theory, Applications and Uses of Ultrasound in Chemistry; Ellis Horwood: Chichester, UK, 1988. [Google Scholar]
- Mason, T.J. Sonochemistry; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Serna-Galvis, E.A.; Porras, J.; Torres-Palma, R.A. A critical review on the sonochemical degradation of organic pollutants in urine, seawater, and mineral water. Ultrason. Sonochem. 2022, 82, 105861. [Google Scholar] [CrossRef]
- McMurray, H.N.; Wilson, B.P. Mechanistic and spatial study of ultrasonically induced luminol chemiluminescence. J. Phys. Chem. A 1999, 103, 3955–3962. [Google Scholar] [CrossRef]
- Hatanaka, S.; Yasui, K.; Tuziuti, T.; Mitome, H. Difference in threshold between sono- and sonochemical luminescence. Jpn. J. Appl. Phys. 2000, 39, 2962–2966. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Lee, J.; Iida, Y.; Yasui, K.; Kozuka, T.; Tuziuti, T.; Towata, A. Spatial distribution of acoustic cavitation bubbles at different ultrasound frequencies. ChemPhysChem 2010, 11, 1680–1684. [Google Scholar] [CrossRef]
- Rivas, D.F.; Ashokkumar, M.; Leong, T.; Yasui, K.; Tuziuti, T.; Kentish, S.; Lohse, D.; Gardeniers, H.J.G.E. Sonoluminescence and sonochemiluminescence from a microreactor. Ultrason. Sonochem. 2012, 19, 1252–1259. [Google Scholar] [CrossRef]
- Yasuda, K.; Torii, T.; Yasui, K.; Iida, Y.; Tuziuti, T.; Nakamura, M.; Asakura, Y. Enhancement of sonochemical reaction of terephthalate ion by superposition of ultrasonic fields of various frequencies. Ultrason. Sonochem. 2007, 14, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Sunartio, D.; Yasui, K.; Tuziuti, T.; Kozuka, T.; Iida, Y.; Ashokkumar, M.; Grieser, F. Correlation between Na* emission and “chemically active” acoustic cavitation bubbles. ChemPhysChem 2007, 8, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Tuziuti, T.; Yasui, K.; Lee, J.; Kozuka, T.; Towata, A.; Iida, Y. Mechanism of enhancement of sonochemical-reaction efficiency by pulsed ultrasound. J. Phys. Chem. A 2008, 112, 4875–4878. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Umemura, S.-I.; Yoshizawa, S. Effect of ultrasonic intensity and intervals of ultrasonic exposure on efficiency of sonochemiluminescence in gel phantom for sonodynamic therapy. Jpn. J. Appl. Phys. 2021, 60, SDDE12. [Google Scholar] [CrossRef]
- Choi, P.-K.; Akiu, T.; Minowa, S.; Kim, J.; Kim, M. Cavitation threshold pressure of focused ultrasound observed with sonochemiluminescence. Jpn. J. Appl. Phys. 2022, 61, SG1003. [Google Scholar] [CrossRef]
- Du, F.; Ma, X.; Yuan, F.; Wang, C.; Snizhko, D.; Guan, Y.; Xu, G. Sonochemiluminescence based on a small, cheap, and low-power USB mesh-type piezoelectric ultrasonic transducer. Anal. Chem. 2020, 92, 4755–4759. [Google Scholar] [CrossRef]
- Lee, J.; Hallez, L.; Touyeras, F.; Ashokkumar, M.; Hihn, J.-Y. Influence of frequency sweep on sonochemiluminescence and sonoluminescence. Ultrason. Sonochem. 2020, 64, 105047. [Google Scholar] [CrossRef]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Iida, Y.; Yasui, K.; Tuziuti, T.; Sivakumar, M. Sonochemistry and its dosimetry. Microchem. J. 2005, 80, 159–164. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kozuka, T.; Towata, A.; Iida, Y. Relationship between the bubble temperature and main oxidant created inside an air bubble under ultrasound. J. Chem. Phys. 2007, 127, 154502. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K.; Cavalieri, F. Sonochemical Production of Nanomaterials; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Enomoto, N.; Okitsu, K. Application of ultrasound in inorganic synthesis. In Sonochemistry and the Acoustic Bubble; Grieser, F., Choi, P.-K., Enomoto, N., Harada, H., Okitsu, K., Yasui, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–206. [Google Scholar]
- Okitsu, K. Sonochemical synthesis of metal nanoparticles. In Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Pankaj, A.M., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 131–150. [Google Scholar]
- Anandan, S.; Ashokkumar, M. Sonochemical preparation of monometallic, bimetallic and metal-loaded semiconductor nanoparticles. In Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Pankaj, A.M., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 151–169. [Google Scholar]
- Okitsu, K. Generation of size, structure, and shape-controlled metal nanoparticles using cavitation. In Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials; Manickam, S., Ashokkumar, M., Eds.; Pan Stanford: Singapore, 2014; pp. 29–54. [Google Scholar]
- Anandan, S.; Ashokkumar, M. Sonochemical synthesis of noble monometallic and bimetallic nanoparticles for catalytic applications. In Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials; Manickam, S., Ashokkumar, M., Eds.; Pan Stanford: Singapore, 2014; pp. 55–88. [Google Scholar]
- Okitsu, K.; Ashokkumar, M.; Grieser, F. Sonochemical synthesis of gold nanoparticles: Effects of ultrasound frequency. J. Phys. Chem. B 2005, 109, 20673–20675. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K.; Yue, A.; Tanabe, S.; Matsumoto, H. Sonochemical preparation and catalytic behavior of highly dispersed palladium nanoparticles on alumina. Chem. Mater. 2000, 12, 3006–3011. [Google Scholar] [CrossRef]
- Dhas, N.A.; Gedanken, A. Sonochemical preparation and properties of nanostructured palladium metallic clusters. J. Mater. Chem. 1998, 8, 445–450. [Google Scholar] [CrossRef]
- Qiu, L.; Pol, V.G.; Calderon-Moreno, J.; Gedanken, A. Synthesis of tin nanorods via a sonochemical method combined with a polyol process. Ultrason. Sonochem. 2005, 12, 243–247. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Takagi, E.; Okuno, H.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of platinum nanoparticles by sonochemical reduction of the Pt(IV) ions: Role of surfactants. Ultrason. Sonochem. 2001, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G. The use of ultrasound for the fabrication of fuel cell materials. Int. J. Hydrog. Energy 2010, 35, 11986–12004. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, characterization, and properties of metallic copper nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- Caruso, R.A.; Ashokkumar, M.; Grieser, F. Sonochemical formation of gold sols. Langmuir 2002, 18, 7831–7836. [Google Scholar] [CrossRef]
- Sakai, T.; Enomoto, H.; Torigoe, K.; Sakai, H.; Abe, M. Surfactant- and reducer-free synthesis of gold nanoparticles in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 18–26. [Google Scholar] [CrossRef]
- Sakai, T.; Enomoto, H.; Sakai, H.; Abe, M. Hydrogen-assisted fabrication of spherical gold nanoparticles through sonochemical reduction of tetrachloride gold(III) ions in water. Ultrason. Sonochem. 2014, 21, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.E.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Frequency controlled agglomeration of Pt-nanoparticles in sonochemical synthesis. Ultrason. Sonochem. 2022, 85, 105991. [Google Scholar] [CrossRef]
- Okitsu, K.; Semboshi, S. Synthesis of Au nanorods via autocatalytic growth of Au seeds formed by sonochemical reduction of Au(I): Relation between formation rate and characteristic of Au nanorods. Ultrason. Sonochem. 2020, 69, 105229. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.E.; Fakhri, D.Ø.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Sonochemical synthesis of Cu@Pt bimetallic nanoparticles. Molecules 2022, 27, 5281. [Google Scholar] [CrossRef]
- Meichtry, J.M.; Cancelada, L.; Destaillats, H.; Litter, M.I. Effect of different gases on the sonochemical Cr(VI) reduction in the presence of citric acid. Chemosphere 2020, 260, 127211. [Google Scholar] [CrossRef]
- Hoinkis, N.; Litter, M.I. Mechanisms of sonochemical transformation of nitrate and nitrite under different conditions: Influence of additives and pH. Ind. Eng. Chem. Res. 2022, 61, 16408–16417. [Google Scholar] [CrossRef]
- Okitsu, K.; Kurisaka, I.; Nanzai, B.; Takenaka, N.; Bandow, H. Mechanism for sonochemical reduction of Au(III) in aqueous butanol solution under Ar based on the analysis of gaseous and water-soluble products. Ultrason. Sonochem. 2020, 69, 105241. [Google Scholar] [CrossRef]
- Yasui, K. Effect of volatile solutes on sonoluminescence. J. Chem. Phys. 2002, 116, 2945–2954. [Google Scholar] [CrossRef]
- Kamali, M.; Davarazar, M.; Aminabhavi, T.M. Single precursor sonochemical synthesis of mesoporous hexagonal-shape zero-valent copper for effective nitrate reduction. Chem. Eng. J. 2020, 384, 123359. [Google Scholar] [CrossRef]
- Saleh, N.M.; Aziz, A.A. Simulation of surface plasmon resonance on different size of a single gold nanoparticle. J. Phys. Conf. Ser. 2018, 1083, 012041. [Google Scholar] [CrossRef]
- Yasui, K. Single-bubble sonoluminescence from hydrogen. J. Chem. Phys. 1999, 111, 5384–5389. [Google Scholar] [CrossRef]
- Gutierrez, M.; Henglein, A.; Dohrmann, J.K. H atom reactions in the sonolysis of aqueous solutions. J. Phys. Chem. 1987, 91, 6687–6690. [Google Scholar] [CrossRef]
- Yasui, K. Unsolved problems in acoustic cavitation. In Handbook of Ultrasonics and Sonochemistry; Ashokkumar, M., Cavalieri, F., Chemat, F., Okitsu, K., Sambandam, A., Yasui, K., Zisu, B., Eds.; Springer: Singapore, 2016; Volume 1, pp. 259–292. [Google Scholar]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of OH radical production from ozone bubbles in water after stopping cavitation. Ultrason. Sonochem. 2019, 58, 104707. [Google Scholar] [CrossRef]
- Yasuda, K.; Sato, T.; Asakura, Y. Size-controlled synthesis of gold nanoparticles by ultrafine bubbles and pulsed ultrasound. Chem. Eng. Sci. 2020, 217, 115527. [Google Scholar] [CrossRef]
- Terasaka, K. Introduction to experiments. In Ultrafine Bubbles; Terasaka, K., Yasui, K., Kanematsu, W., Aya, N., Eds.; Jenny Stanford: Singapore, 2022; pp. 17–72. [Google Scholar]
- Wang, W.; Fan, W.; Huo, M.; Zhao, H.; Lu, Y. Hydroxyl radical generation and contaminant removal from water by the collapse of microbubbles under different hydrochemical conditions. Water Air Soil Pollut. 2018, 229, 86. [Google Scholar] [CrossRef]
- Takahashi, M. Nanobubbles: An introduction. In Micro- and Nanobubbles; Tsuge, H., Ed.; Pan Stanford: Singapore, 2014; pp. 307–315. [Google Scholar]
- Kanematsu, W.; Tuziuti, T.; Yasui, K. The influence of storage conditions and container materials on the long term stability of bulk nanobubbles—Consideration from a perspective of interactions between bubbles and surroundings. Chem. Eng. Sci. 2020, 219, 115594. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Interaction of bulk nanobubbles (ultrafine bubbles) with a solid surface. Langmuir 2021, 37, 1674–1681. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W.; Kato, K. Dynamic equilibrium model for a bulk nanobubble and a microbubble partly covered with hydrophobic material. Langmuir 2016, 32, 11101–11110. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mysteries of bulk nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason. Sonochem. 2018, 48, 259–266. [Google Scholar] [CrossRef]
- Sugano, K.; Miyoshi, Y.; Inazato, S. Study of ultrafine bubble stabilization by organic material adhesion. Jpn. J. Multiph. Flow 2017, 31, 299–306. [Google Scholar] [CrossRef]
- Sugano, K.; Miyoshi, Y.; Inazato, S. Study of ultrafine bubbles stabilization by organic material adhesion. In Ultrafine Bubbles; Terasaka, K., Yasui, K., Kanematsu, W., Aya, N., Eds.; Jenny Stanford: Singapore, 2022; pp. 155–177. [Google Scholar]
- Yasui, K. Theory of ultrafine bubbles. In Ultrafine Bubbles; Terasaka, K., Yasui, K., Kanematsu, W., Aya, N., Eds.; Jenny Stanford: Singapore, 2022; pp. 109–153. [Google Scholar]
- Yasui, K. Critical roles of impurities and imperfections in various phases of materials. Materials 2023, 16, 1612. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. On some aspects of nanobubble-containing systems. Nanomaterials 2022, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y. Experimental methods in sonochemistry. In Sonochemistry and the Acoustic Bubble; Grieser, F., Choi, P.-K., Enomoto, N., Harada, H., Okitsu, K., Yasui, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 119–150. [Google Scholar]
- Sonoda, A. Real UFB sample measurements: A few cases. In Ultrafine Bubbles; Terasaka, K., Yasui, K., Kanematsu, W., Aya, N., Eds.; Jenny Stanford: Singapore, 2022; pp. 87–108. [Google Scholar]
- Tuziuti, T.; Yasui, K.; Kanematsu, W. Decrease in the surface tension of nanobubble dispersion in water: Results of surface excess of bulk nanobubbles at interfaces. Langmuir 2023, 39, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.; Prosperetti, A.; Egolfopoulos, F.N. A theoretical study of sonoluminescence. J. Acoust. Soc. Am. 1993, 94, 248–260. [Google Scholar] [CrossRef]
- Storey, B.D.; Szeri, A.J. Water vapour, sonoluminescence and sonochemistry. Proc. R. Soc. A 2000, 456, 1685–1709. [Google Scholar] [CrossRef]
- Storey, B.D.; Szeri, A.J. A reduced model of cavitation physics for use in sonochemistry. Proc. R. Soc. A 2001, 457, 1685–1700. [Google Scholar] [CrossRef]
- An, Y. Mechanism of single-bubble sonoluminescence. Phys. Rev. E 2006, 74, 026304. [Google Scholar] [CrossRef]
- Kalmár, C.; Klapcsik, K.; Hegedűs, F. Relationship between the radial dynamics and the chemical production of a harmonically driven spherical bubble. Ultrason. Sonochem. 2020, 64, 104989. [Google Scholar] [CrossRef]
- Kalmár, C.; Turányi, T.; Zsély, I.G.; Papp, M.; Hegedűs, F. The importance of chemical mechanisms in sonochemical modelling. Ultrason. Sonochem. 2022, 83, 105925. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Sensitivity of free radicals production in acoustically driven bubble to the ultrasonic frequency and nature of dissolved gases. Ultrason. Sonochem. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Kerboua, K.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Islam, M.H.; Hansen, H.E.; Pollet, B.G. How do dissolved gases affect the sonochemical process of hydrogen production? an overview of thermodynamic and mechanistic effects–on the “hot spot theory”. Ultrason. Sonochem. 2021, 72, 105422. [Google Scholar] [CrossRef]
- Hauke, G.; Fuster, D.; Dopazo, C. Dynamics of a single cavitating and reacting bubble. Phys. Rev. E 2007, 75, 066310. [Google Scholar] [CrossRef]
- Yuan, L. Sonochemical effects on single-bubble sonoluminescence. Phys. Rev. E 2005, 72, 046309. [Google Scholar] [CrossRef]
- Gong, C.; Hart, D.P. Ultrasound induced cavitation and sonochemical yields. J. Acoust. Soc. Am. 1998, 104, 2675–2682. [Google Scholar] [CrossRef]
- Yasui, K. Numerical simulations for sonochemistry. Ultrason. Sonochem. 2021, 78, 105728. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Variation of liquid temperature at bubble wall near the sonoluminescence threshold. J. Phys. Soc. Jpn. 1996, 65, 2830–2840. [Google Scholar] [CrossRef]
- Yasui, K. Effect of liquid temperature on sonoluminescence. Phys. Rev. E 2001, 64, 016310. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Iida, Y.; Mitome, H. Theoretical study of the ambient-pressure dependence of sonochemical reactions. J. Chem. Phys. 2003, 119, 346–356. [Google Scholar] [CrossRef]
- Yasui, K. Effect of non-equilibrium evaporation and condensation on bubble dynamics near the sonoluminescence threshold. Ultrasonics 1998, 36, 575–580. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Iida, Y. Optimum bubble temperature for the sonochemical production of oxidants. Ultrasonics 2004, 42, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Segregation of vapor and gas in a sonoluminescing bubble. Ultrasonics 2002, 40, 643–647. [Google Scholar] [CrossRef]
- Baulch, D.L.; Drysdale, D.D.; Horne, D.G.; Lloyd, A.C. Homogeneous Gas Phase Reactions of the H2-O2 System. In Evaluated Kinetic Data for High Temperature Reactions; Butterworths: London, UK, 1972; Volume 1. [Google Scholar]
- Baulch, D.L.; Drysdale, D.D.; Horne, D.G.; Lloyd, A.C. Homogeneous Gas Phase Reactions of the H2-N2-O2 System. In Evaluated Kinetic Data for High Temperature Reactions; Butterworths: London, UK, 1973; Volume 2. [Google Scholar]
- Baulch, D.L.; Drysdale, D.D.; Duxbury, J.; Grant, S.J. Homogeneous Gas Phase Reactions of the O2-O3 Systems, the CO-O2-H2 System, and of Sulphur-containing Species. In Evaluated Kinetic Data for High Temperature Reactions; Butterworths: London, UK, 1976; Volume 3. [Google Scholar]
- Matula, T.J.; Cordry, S.M.; Roy, R.A.; Crum, L.A. Bjerknes force and bubble levitation under single-bubble sonoluminescence conditions. J. Acoust. Soc. Am. 1997, 102, 1522–1527. [Google Scholar] [CrossRef]
- Barber, B.P.; Hiller, R.A.; Löfstedt, R.; Putterman, S.J.; Weninger, K.R. Defining the unknowns of sonoluminescence. Phys. Rep. 1997, 281, 65–143. [Google Scholar] [CrossRef]
- Barber, B.P.; Putterman, S.J. Observation of synchronous picosecond sonoluminescence. Nature 1991, 352, 318–320. [Google Scholar] [CrossRef]
- Didenko, Y.T.; Suslick, K.S. The energy efficiency of formation of photons, radicals and ions during single-bubble cavitation. Nature 2002, 418, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Lohse, D.; Brenner, M.P.; Dupont, T.F.; Hilgenfeldt, S.; Johnston, B. Sonoluminescing air bubbles rectify argon. Phys. Rev. Lett. 1997, 78, 1359–1362. [Google Scholar] [CrossRef]
- Matula, T.J.; Crum, L.A. Evidence for gas exchange in single-bubble sonoluminescence. Phys. Rev. Lett. 1998, 80, 865–868. [Google Scholar] [CrossRef]
- Yasui, K. Merits and demerits of ODE modeling of physicochemical systems for numerical simulations. Molecules 2022, 27, 5860. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. (Eds.) Standard Potentials in Aqueous Solutions; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Rao, P.S.; Hayon, E. Redox potentials of free radicals. I. simple organic radicals. J. Am. Chem. Soc. 1974, 96, 1287–1294. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms. J. Phys. Chem. 1983, 87, 1369–1377. [Google Scholar] [CrossRef]
- Carmichael, A.J.; Mossoba, M.M.; Riesz, P.; Christman, C.L. Free radical production in aqueous solutions exposed to simulated ultrasonic diagnostic conditions. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1986, 33, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Riesz, P.; Berdahl, D.; Christman, C.L. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ. Health Perspect. 1985, 64, 233–252. [Google Scholar] [CrossRef]
- Fischer, C.H.; Hart, E.J.; Henglein, A. H/D isotope exchange in the D2–H2O system under the influence of ultrasound. J. Phys. Chem. 1986, 90, 222–224. [Google Scholar] [CrossRef]
- Fischer, C.H.; Hart, E.J.; Henglein, A. H/D isotope exchange in the HD–H2O system under the influence of ultrasound. J. Phys. Chem. 1986, 90, 3059–3060. [Google Scholar] [CrossRef]
- Hart, E.J.; Henglein, A. Sonolytic decomposition of nitrous oxide in aqueous solution. J. Phys. Chem. 1986, 90, 5992–5995. [Google Scholar] [CrossRef]
- Kerboua, K.; Hamdaoui, O.; Islam, M.H.; Alghyamah, A.; Hansen, H.E.; Pollet, B.G. Low carbon ultrasonic production of alternate fuel: Operational and mechanistic concerns of the sonochemical process of hydrogen generation under various scenarios. Int. J. Hydrog. Energy 2021, 46, 26770–26787. [Google Scholar] [CrossRef]
- Rashwan, S.S.; Dincer, I.; Mohany, A. An investigation of ultrasonic based hydrogen production. Energy 2020, 205, 118006. [Google Scholar] [CrossRef]
- Rashwan, S.S.; Dincer, I.; Mohany, A. A unique study on the effect of dissolved gases and bubble temperatures on the ultrasonic hydrogen (sonohydrogen) production. Int. J. Hydrog. Energy 2020, 45, 20808–20819. [Google Scholar] [CrossRef]
- Rashwan, S.S.; Dincer, I.; Mohany, A. A review on the importance of operating conditions and process parameters in sonic hydrogen production. Int. J. Hydrog. Energy 2021, 46, 28418–28434. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Yasui, K.; Ashokkumar, M. A hydrogen-based technique for determining the number density of acoustic microreactors (actives bubbles) in sonicated solutions. Int. J. Hydrog. Energy 2023, 48, 13430–13441. [Google Scholar] [CrossRef]
- Mead, E.L.; Sutherland, R.G.; Verrall, R.E. The effect of ultrasound on water in the presence of dissolved gases. Can. J. Chem. 1976, 54, 1114–1120. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 75th ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Kwedi-Nsah, L.-M.; Kobayashi, T. Sonochemical nitrogen fixation for the generation of NO2− and NO3− ions under high-powered ultrasound in aqueous medium. Ultrason. Sonochem. 2020, 66, 105051. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Choi, J. Effects of gas saturation and sparging on sonochemical oxidation activity in open and closed systems, part II: NO2−/NO3− generation and a brief critical review. Ultrason. Sonochem. 2023, 92, 106250. [Google Scholar] [CrossRef]
- Yang, Y.; Cho, Y.I.; Fridman, R.S. Plasma Discharge in Liquid; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Okitsu, K.; Iwatani, M.; Nanzai, B.; Nishimura, R.; Maeda, Y. Sonochemical reduction of permanganate to manganese dioxide: The effects of H2O2 formed in the sonolysis of water on the rates of reduction. Ultrason. Sonochem. 2009, 16, 387–391. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Kondo, T.; Mišík, V.; Riesz, P. Sonochemistry of cytochrome c. evidence for superoxide formation by ultrasound in argon-saturated aqueous solution. Ultrason. Sonochem. 1996, 3, S193–S199. [Google Scholar] [CrossRef]
- Morrissey, J.M.; Taylor, K.D.; Gilman, S.D. Ultrasound-mediated release of iron from ferritin. Ultrasound Med. Biol. 2003, 29, 1799–1803. [Google Scholar] [CrossRef]
- Castellanos, M.M.; Reyman, D.; Sieiro, C.; Calle, P. ESR-spin trapping study on the sonochemistry of liquids in the presence of oxygen. Evidence for the superoxide radical anion formation. Ultrason. Sonochem. 2001, 8, 17–22. [Google Scholar] [CrossRef]
- Matheson, M.S.; Rabani, J. Pulse radiolysis of aqueous hydrogen solutions. I. Rate constants for reaction of eaq− with itself and other transients. II. The interconvertibility of eaq− and H1. J. Phys. Chem. 1965, 69, 1324–1335. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Suslick, K.S. Plasma formation and temperature measurement during single-bubble cavitation. Nature 2005, 434, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Kappus, B.; Bataller, A.; Putterman, S.J. Energy balance for a sonoluminescence bubble yields a measure of ionization potential lowering. Phys. Rev. Lett. 2013, 111, 234301. [Google Scholar] [CrossRef]
- Yasui, K.; Kato, K. Bubble dynamics and sonoluminescence from helium or xenon in mercury and water. Phys. Rev. E 2012, 86, 036320. [Google Scholar] [CrossRef] [PubMed]
- Sharipov, G.L.; Gareev, B.M.; Vasilyuk, K.S.; Galimov, D.I.; Abdrakhmanov, A.M. New sonochemiluminescence involving solvated electron in Ce(III)/Ce(IV) solutions. Ultrason. Sonochem. 2021, 70, 105313. [Google Scholar] [CrossRef]
- Mišík, V.; Riesz, P. Effect of Cd2+ on the •H atom yield in the sonolysis of water. Evidence against the formation of hydrated electrons. J. Phys. Chem. A 1997, 101, 1441–1444. [Google Scholar] [CrossRef]
- Yasui, K. Temperature in multibubble sonoluminescence. J. Chem. Phys. 2001, 115, 2893–2896. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Iida, Y. Dependence of the characteristics of bubbles on types of sonochemical reactors. Ultrason. Sonochem. 2005, 12, 43–51. [Google Scholar] [CrossRef]
- Yasui, K.; Kozuka, T.; Tuziuti, T.; Towata, A.; Iida, Y.; King, J.; Macey, P. FEM calculation of an acoustic field in a sonochemical reactor. Ultrason. Sonochem. 2007, 14, 605–614. [Google Scholar] [CrossRef]
- Yasui, K. Influence of ultrasonic frequency on multibubble sonoluminescence. J. Acoust. Soc. Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef]
- Mettin, R.; Luther, S.; Ohl, C.-D.; Lauterborn, W. Acoustic cavitation structures and simulations by a particle model. Ultrason. Sonochem. 1999, 6, 25–29. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T.; Mettin, R.; Ohl, C.D. Experimental and theoretical bubble dynamics. Adv. Chem. Phys. 1999, 110, 295–380. [Google Scholar] [CrossRef]
- Yasui, K.; Iida, Y.; Tuziuti, T.; Kozuka, T.; Towata, A. Strongly interacting bubbles under an ultrasonic horn. Phys. Rev. E 2008, 77, 016609. [Google Scholar] [CrossRef]
- Stricker, L.; Dollet, B.; Rivas, D.F.; Lohse, D. Interacting bubble clouds and their sonochemical production. J. Acoust. Soc. Am. 2013, 134, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S.; Chibani, A.; Hamdaoui, O.; Ashokkumar, M. Sonochemical and sono-assisted reduction of carbon dioxide: A critical review. Chem. Eng. Process. 2022, 179, 109075. [Google Scholar] [CrossRef]
- Yasuda, K. Sonochemical green technology using active bubbles: Degradation of organic substances in water. Curr. Opin. Green Sustain. Chem. 2021, 27, 100411. [Google Scholar] [CrossRef]
- Pham, T.M.H.; Vu, M.T.; Cong, T.D.; Nguyen, N.S.; Doan, T.A.; Truong, T.T.; Nguyen, T.H. Green sonochemical process for preparation of polyethylene glycol–Fe3O4/ZnO magnetic nanocomposite using rambutan peel extract as photocatalyst, for removal of methylene blue in solution. Bull. Mater. Sci. 2022, 45, 13. [Google Scholar] [CrossRef]
- Chen, T.-W.; Chinnapaiyan, S.; Chen, S.-M.; Mahmoud, A.H.; Elshikh, M.S.; Ebaid, H.; Yassin, M.T. Facile sonochemical synthesis of rutile-type titanium dioxide microspheres decorated graphene oxide composite for efficient electrochemical sensor. Ultrason. Sonochem. 2020, 62, 104872. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent developments in hydrodynamic cavitation reactors: Cavitation mechanism, reactor design, and applications. Engineering 2022, 19, 180–198. [Google Scholar] [CrossRef]
- Talabazar, F.R.; Aghdam, A.S.; Jafarpour, M.; Grishenkov, D.; Koşar, A.; Ghorbani, M. Chemical effects in “hydrodynamic cavitation on a chip”: The role of cavitating flow patterns. Chem. Eng. J. 2022, 445, 136734. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, J.; Lv, B.; Liu, W.; Shen, S.; Wang, D.; Ji, J. Chemical effects induced by gas-liquid jet flow. React. Chem. Eng. 2022, 7, 566–569. [Google Scholar] [CrossRef]
- Podbevšek, D.; Ledoux, G.; Dular, M. Investigation of hydrodynamic cavitation induced reactive oxygen species production in microchannels via chemiluminescent luminol oxidation reactions. Water Res. 2022, 220, 118628. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, J.; Ji, L.; Wang, G.; Zhao, S.; Yoon, J.Y.; Chen, S. A review on hydrodynamic cavitation disinfection: The current state of knowledge. Sci. Total Environ. 2020, 737, 139606. [Google Scholar] [CrossRef] [PubMed]
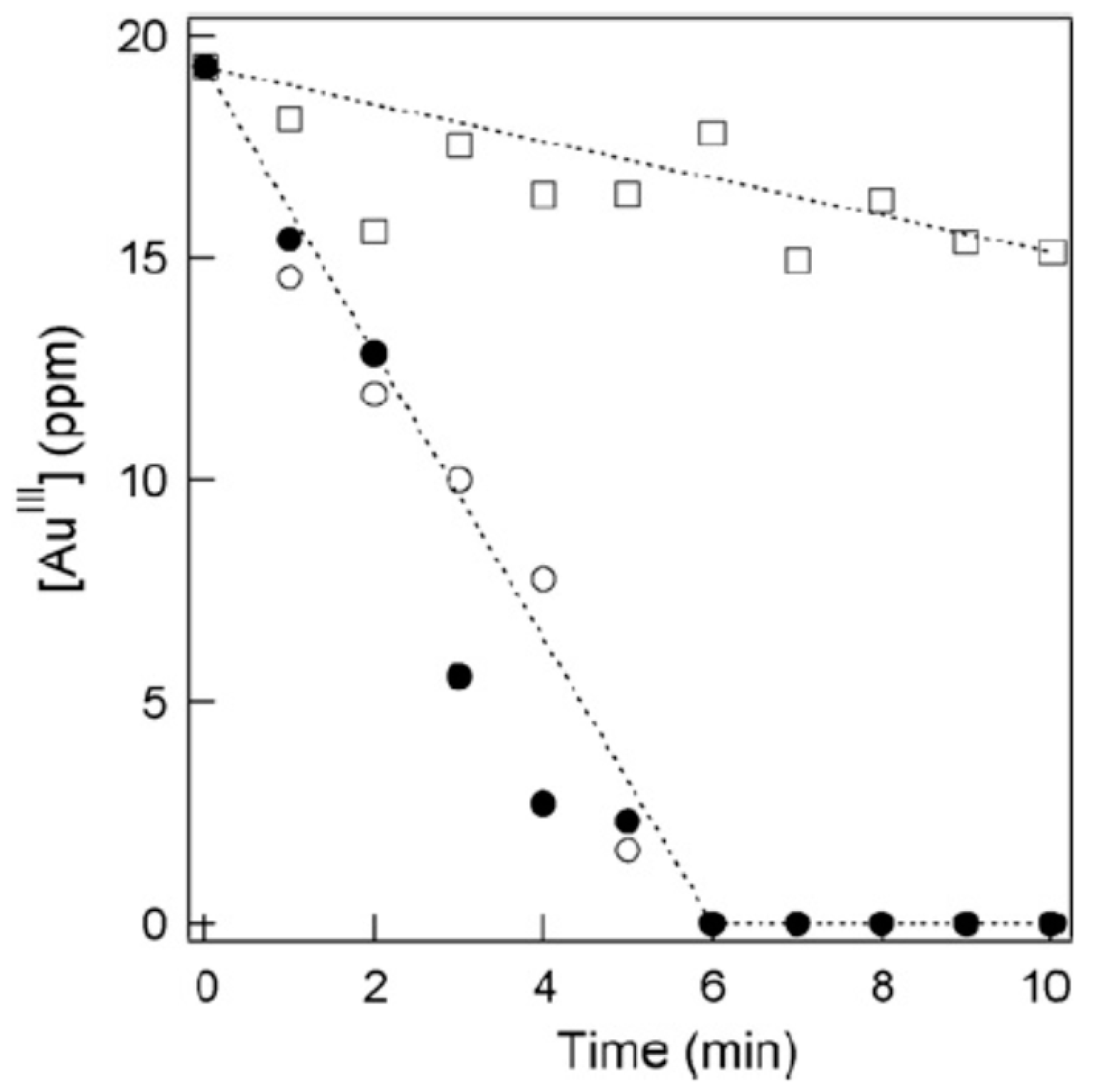
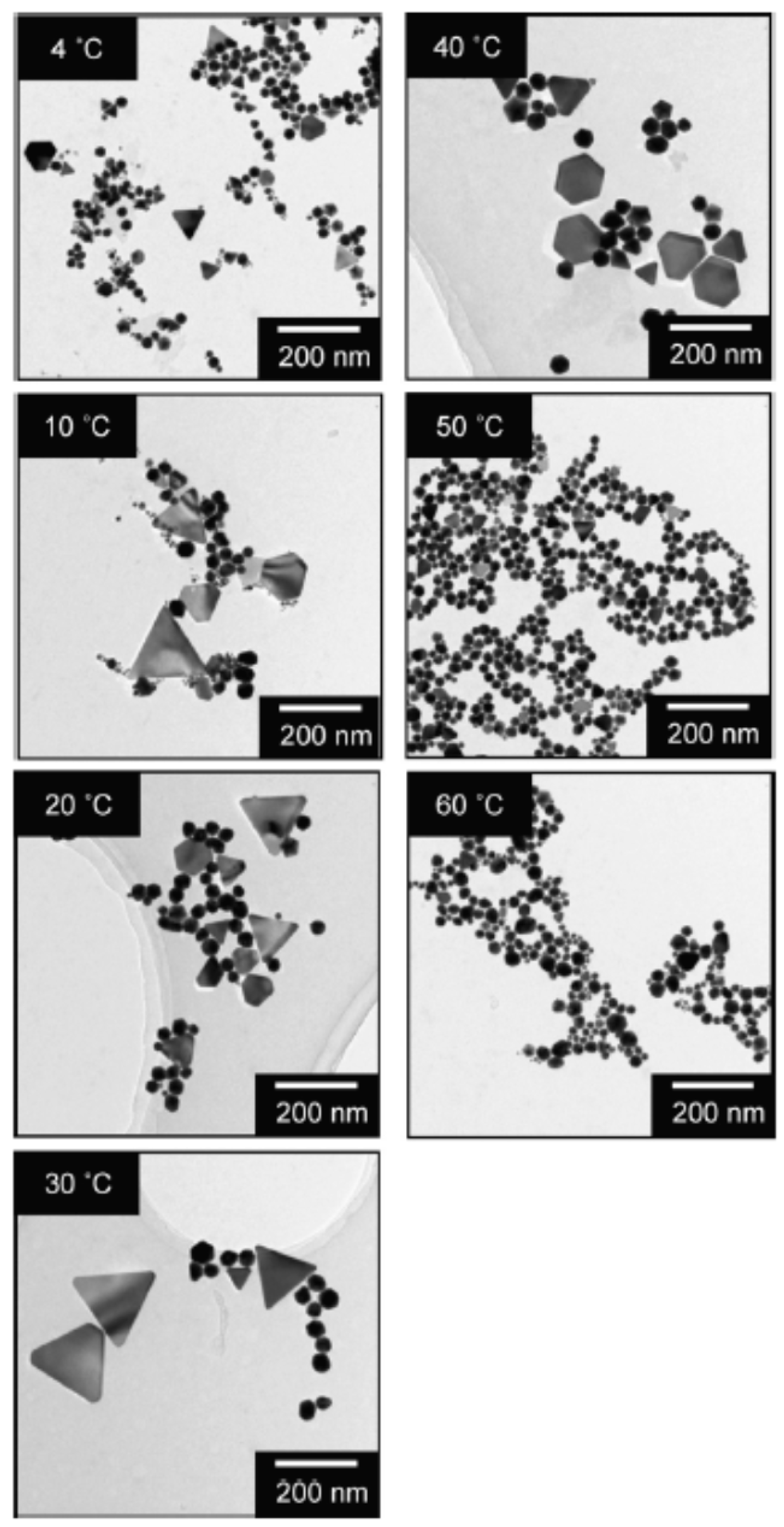
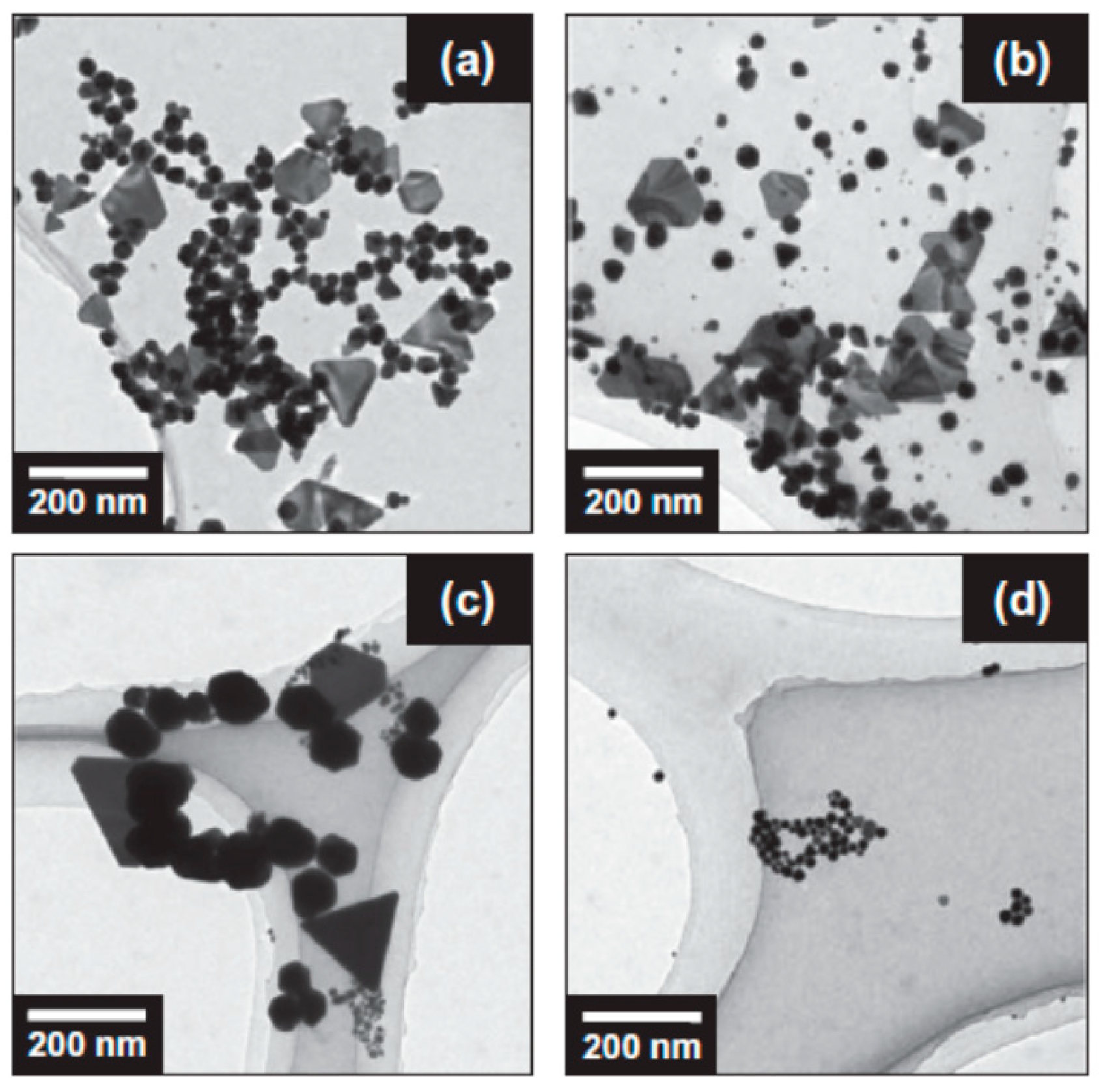

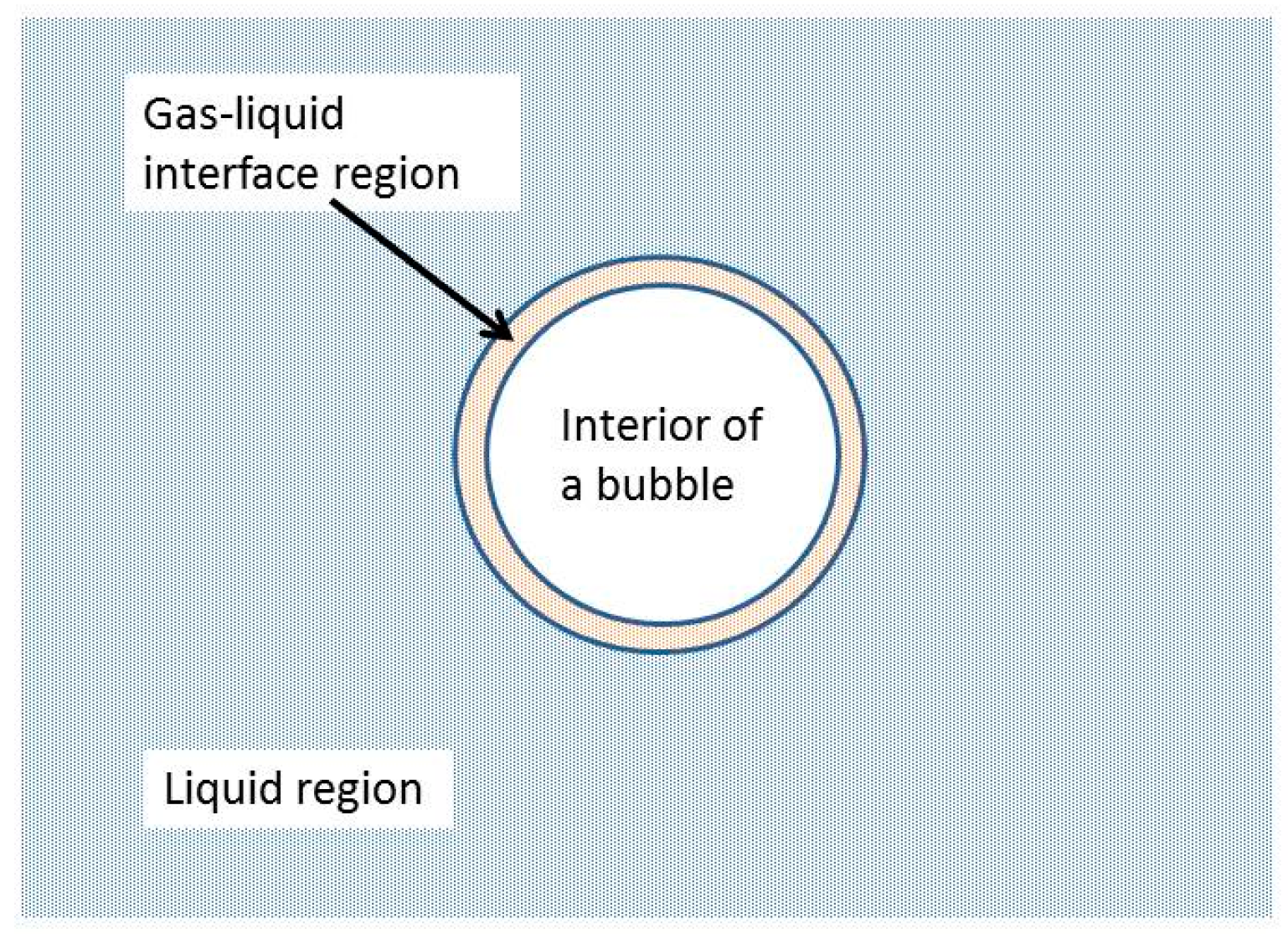
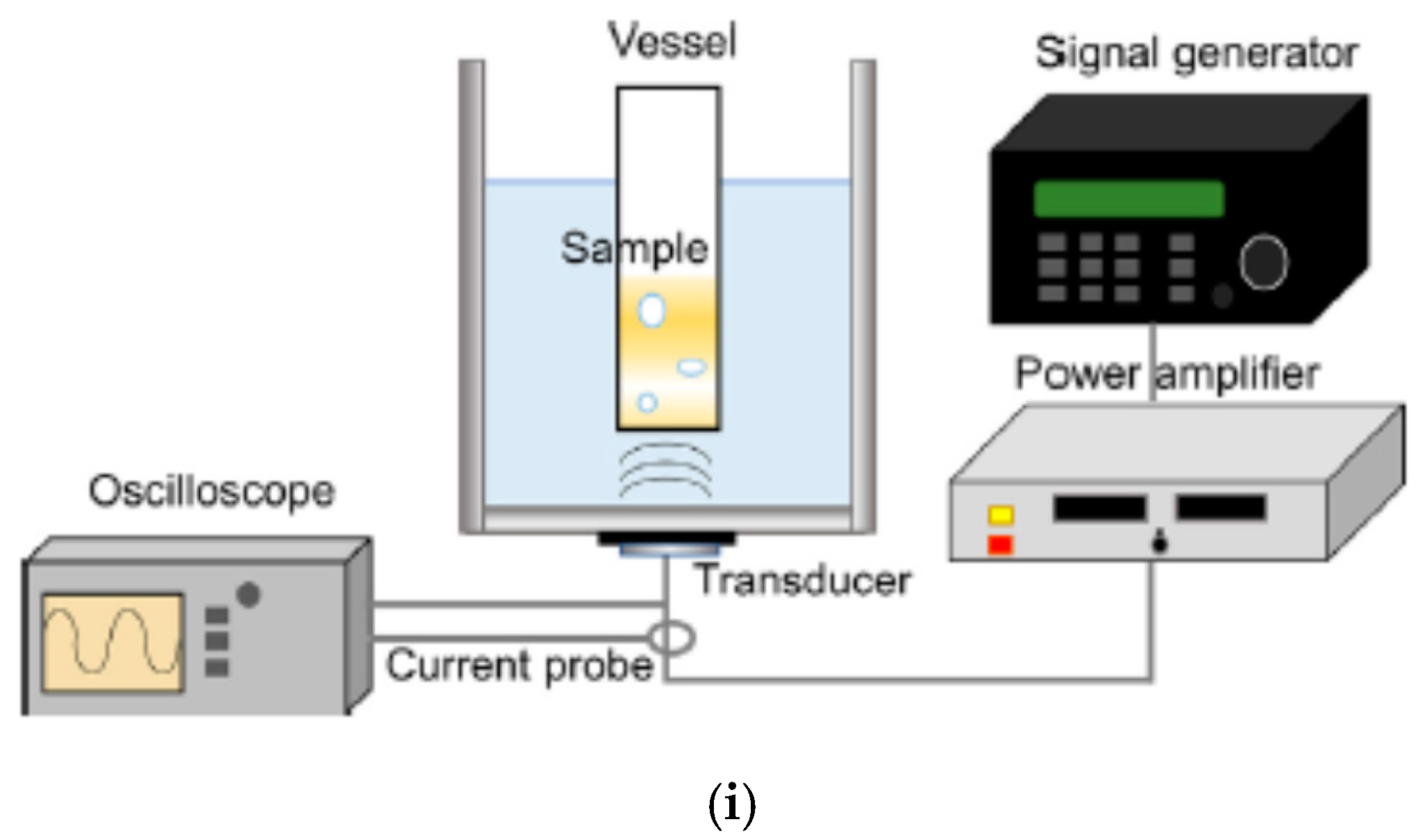
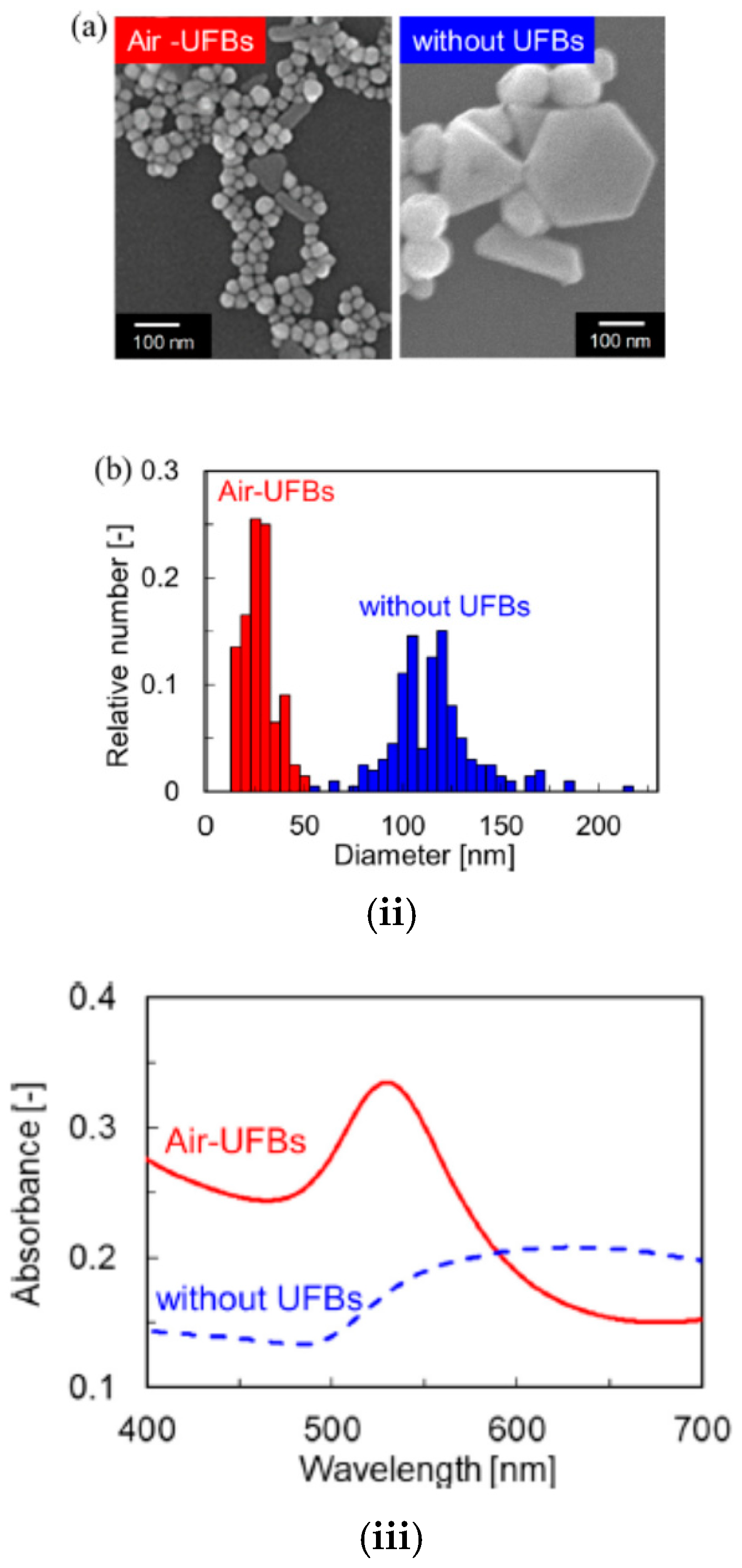
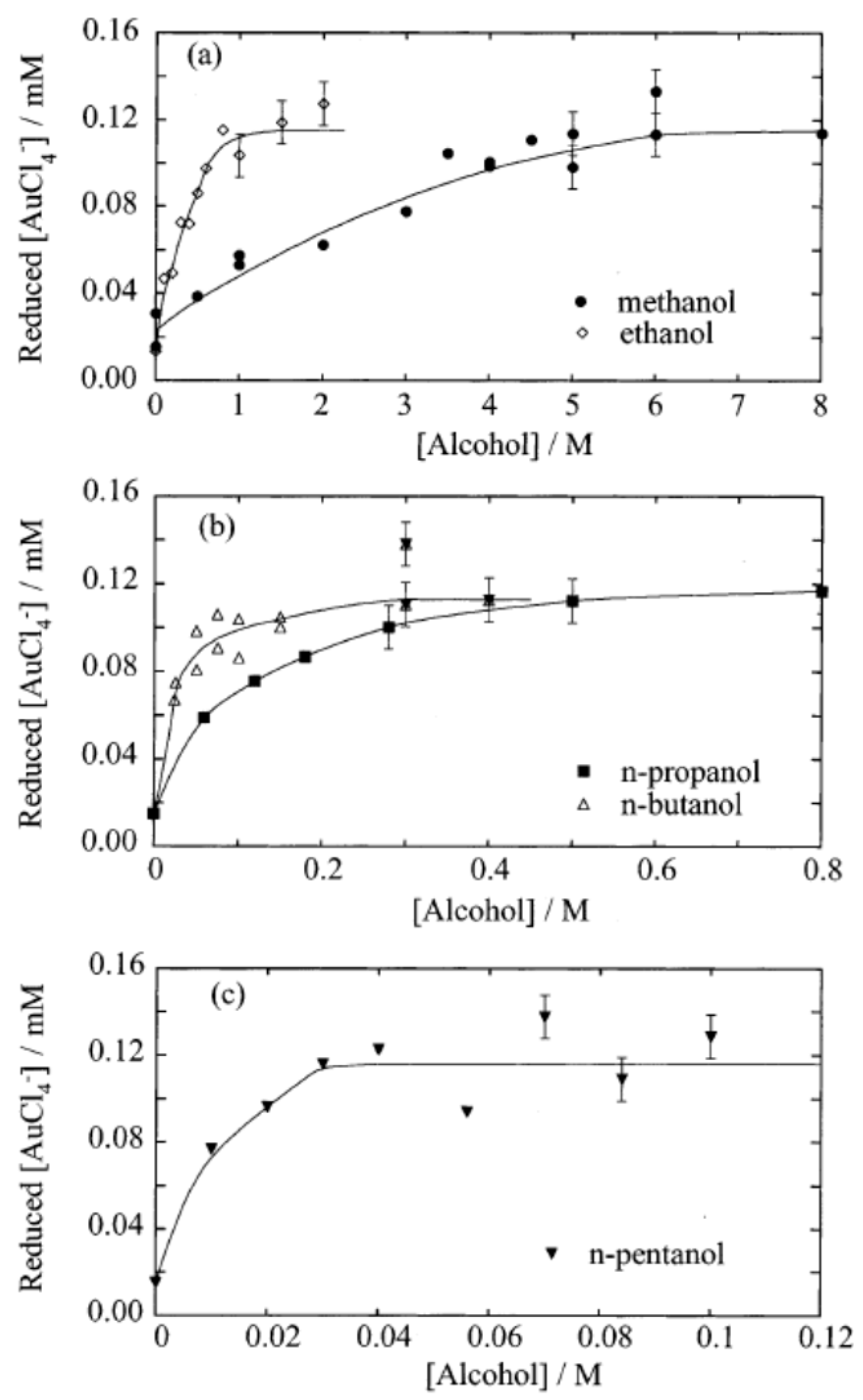
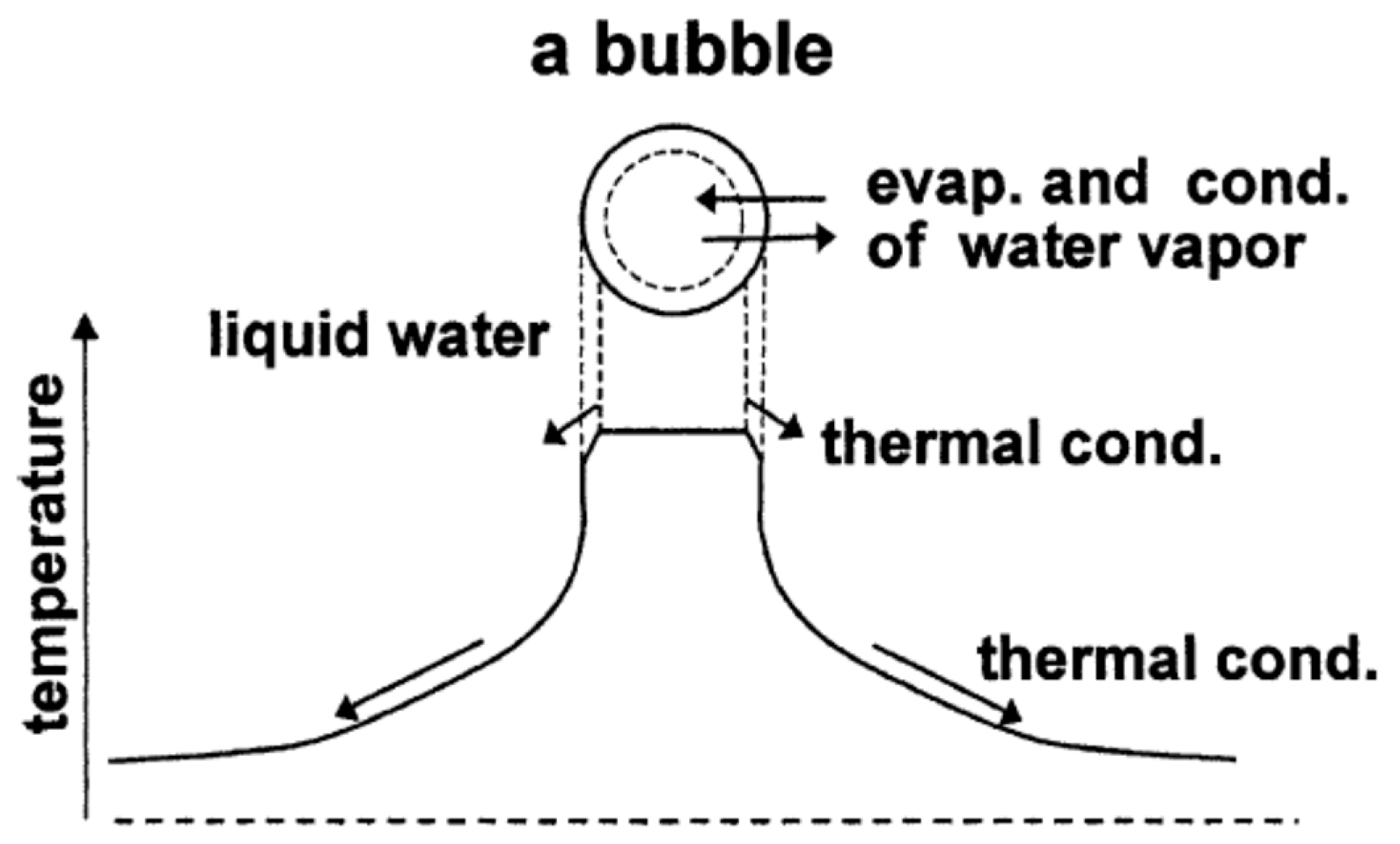
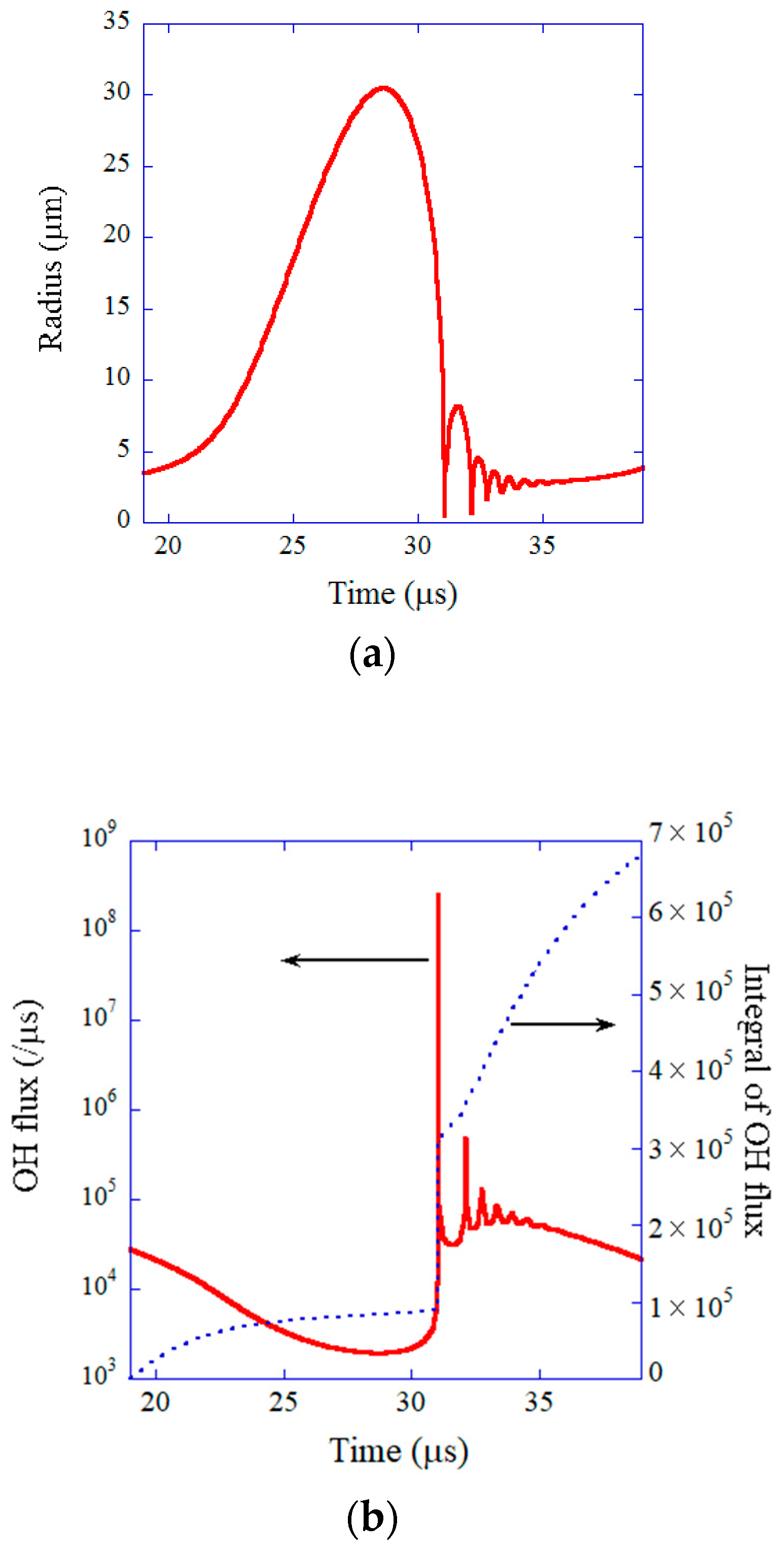

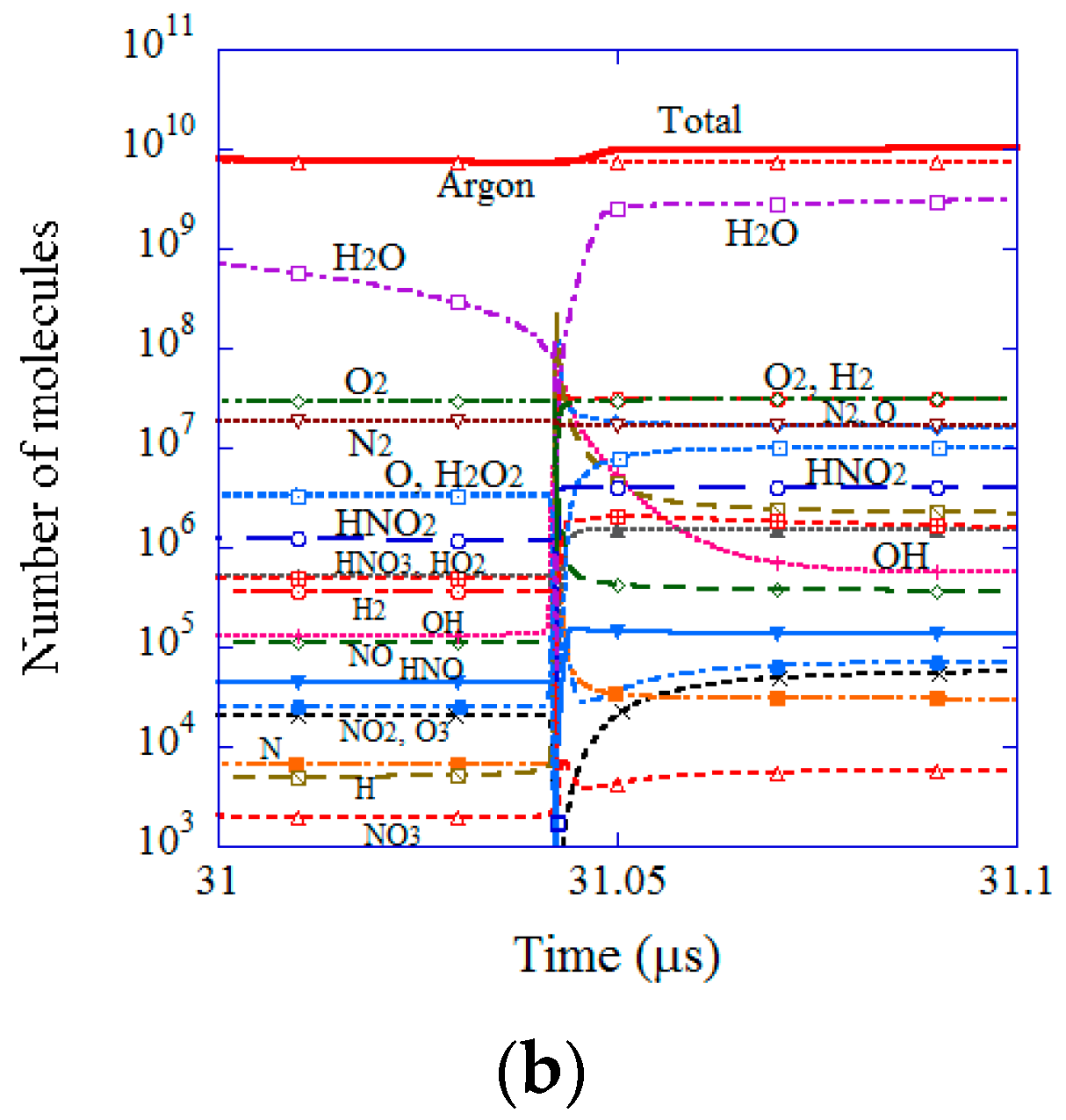
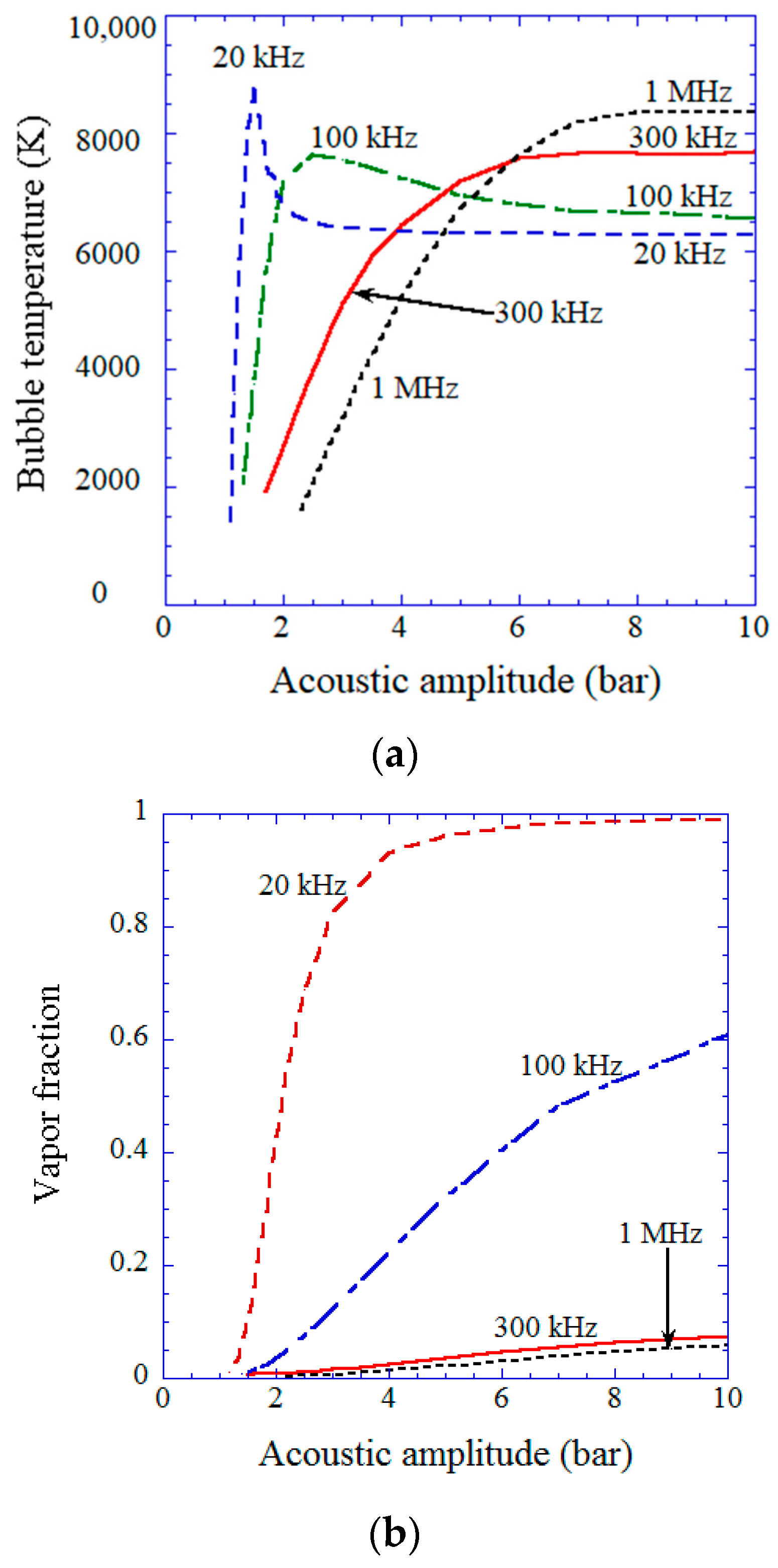
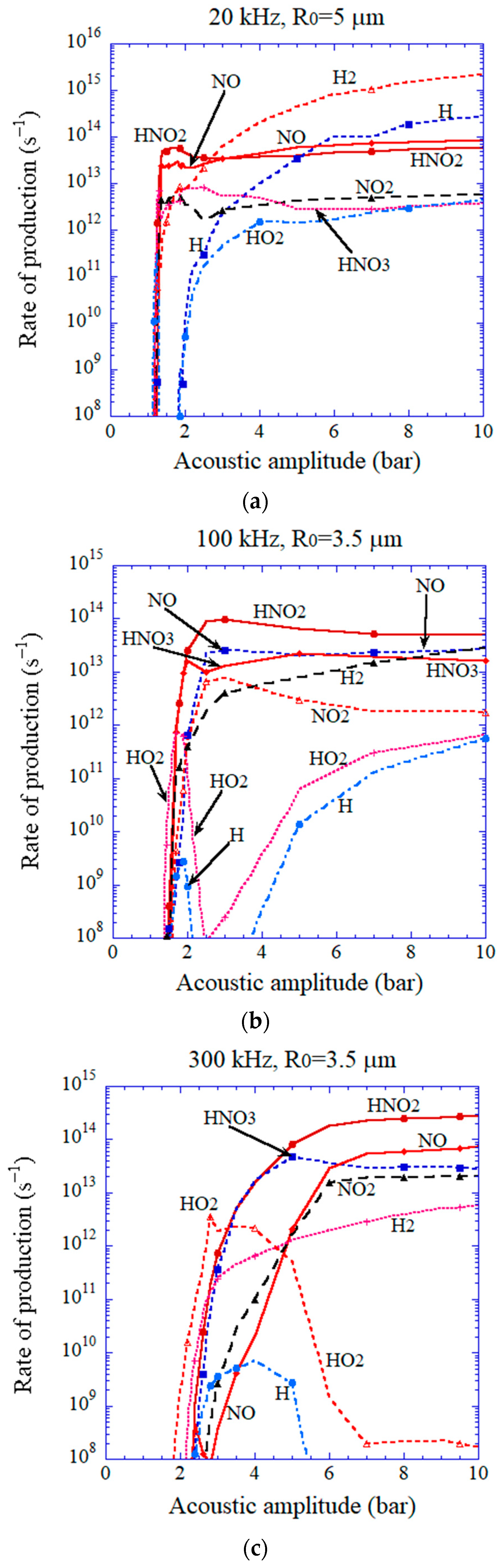
| Chemical Species | Number of Molecules per Acoustic Cycle |
|---|---|
| Reducing Agent | Reaction | Reduction Potential (V) |
|---|---|---|
| 2.88 [128] | ||
| 2.31 [128] | ||
| 0.83 [129] 1 | ||
| 0.73 [130] | ||
| 0.69 [130] | ||
| 0.18 [128] | ||
| 0.11 [129] | ||
| 0.05 [129] | ||
| 0.00 [129] | ||
| [129] 1 | ||
| [129] 1 | ||
| [129] | ||
| [129] | ||
| [129] | ||
| [129] |
| Chemical Species | Number of Molecules per Acoustic Cycle |
|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, K. The Reducing Agents in Sonochemical Reactions without Any Additives. Molecules 2023, 28, 4198. https://doi.org/10.3390/molecules28104198
Yasui K. The Reducing Agents in Sonochemical Reactions without Any Additives. Molecules. 2023; 28(10):4198. https://doi.org/10.3390/molecules28104198
Chicago/Turabian StyleYasui, Kyuichi. 2023. "The Reducing Agents in Sonochemical Reactions without Any Additives" Molecules 28, no. 10: 4198. https://doi.org/10.3390/molecules28104198
APA StyleYasui, K. (2023). The Reducing Agents in Sonochemical Reactions without Any Additives. Molecules, 28(10), 4198. https://doi.org/10.3390/molecules28104198






