Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey
Abstract
1. Introduction
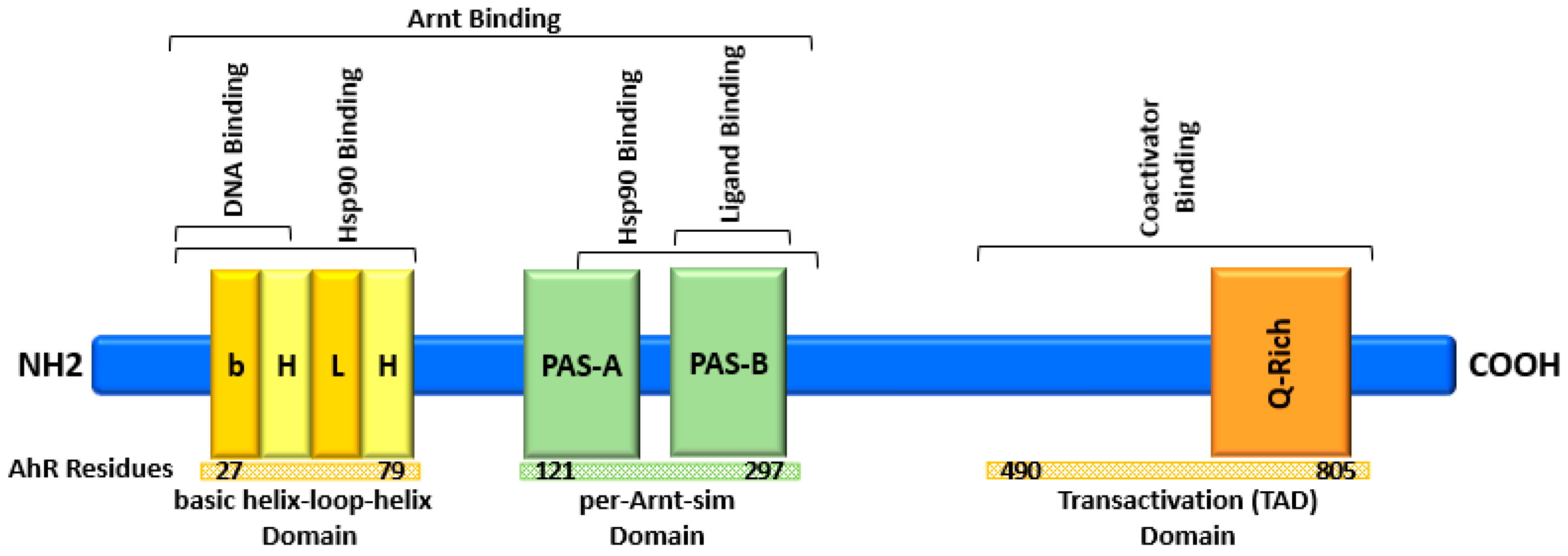
2. AhR Structure and Activation
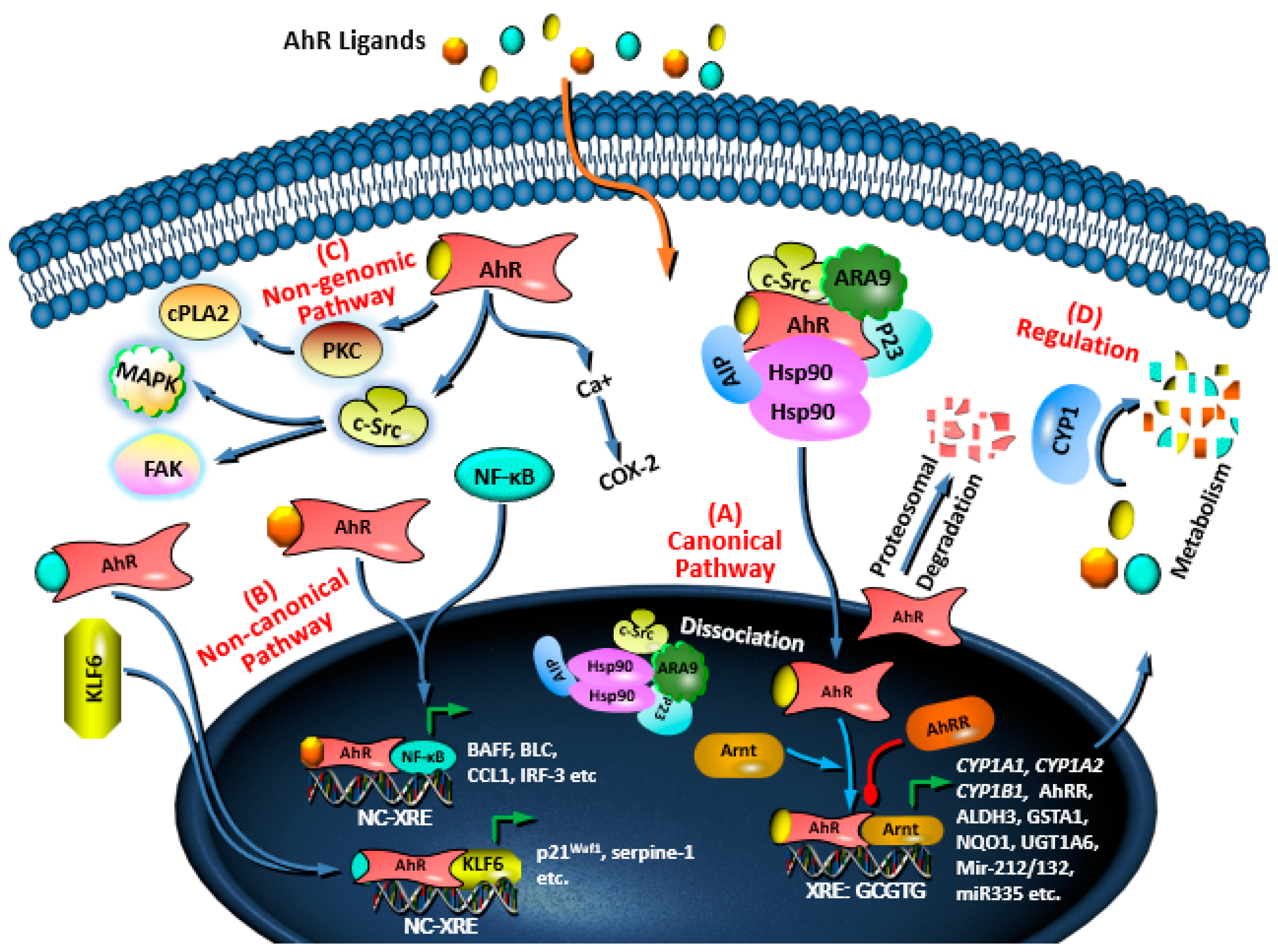
3. AhR Signaling Pathways and Regulation
4. Role of AhR in Cancer at Glance
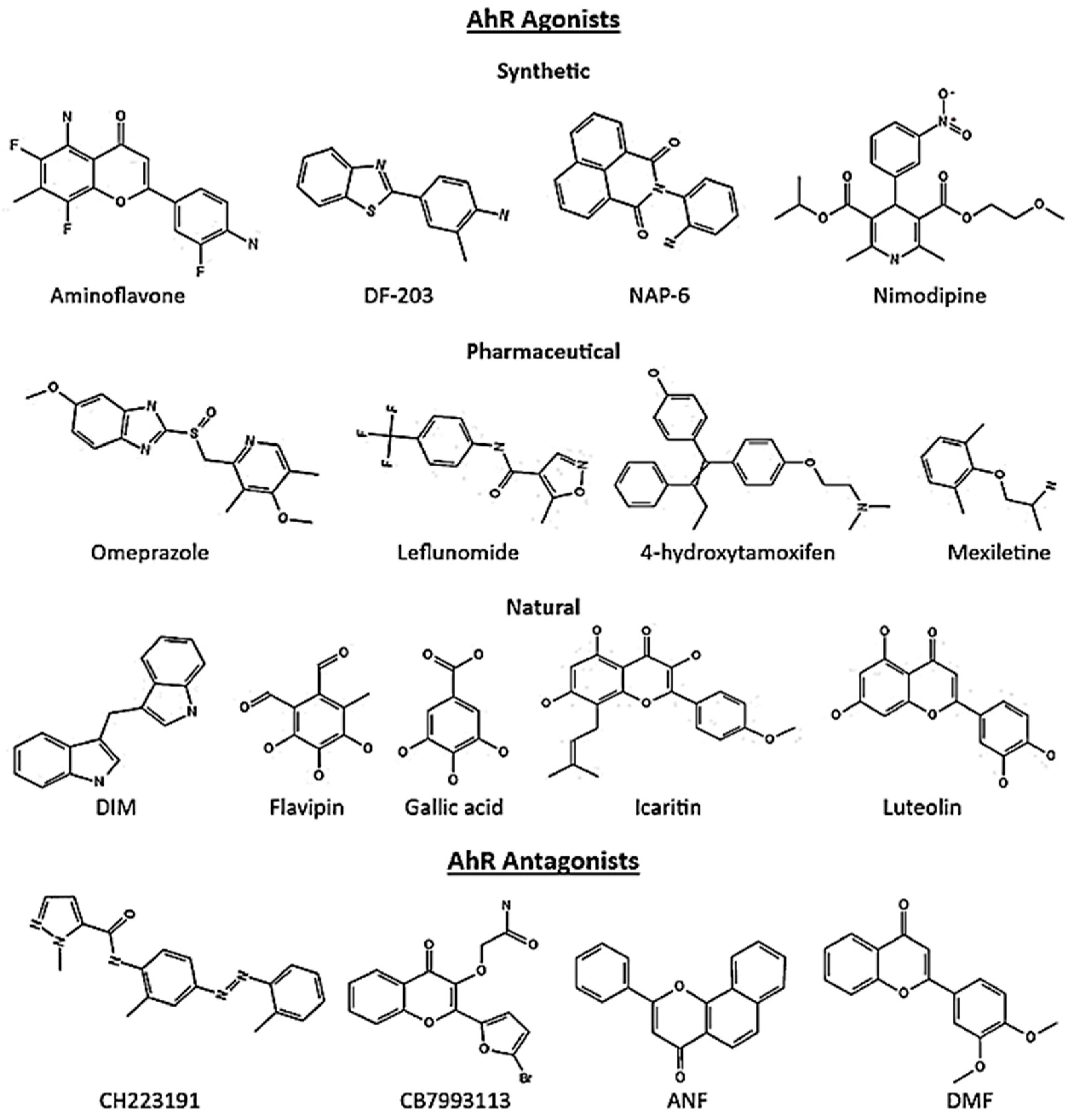
5. AhR Ligands in Cancer
5.1. Breast Cancer
| Compound | Ligand | Response | Cells | Refs. |
|---|---|---|---|---|
| TCDD | Agonist | Proliferation, Migration, Invasion Metastasis | MDA-MB-231, T47D | [58] |
| MCDF | Agonist | Invasion | MDA-MB-231, BT474 | [59] |
| NAP-6 | Agonist | Proliferation, Cell cycle, Checkpoint, DNA damage | MDA-MB-468, MDA-MB-231, ZR-75-1, SKBR3, T47D, MCF-7, BT474, BT20 | [100] |
| 10-Cl-BBQ | Agonist | Proliferation | MDA-MB-468, T47D, ZR-75-1, SKBR3 | [101] |
| CGS-15943 | Antagonist | Apoptosis | MDA-MB-486 | [103] |
| ANI-7 | Agonist | Proliferation, DNA damage, Cell cycle, Checkpoint | MDA-MB-468, MDA-MB-231, ZR-75-1, SKBR3, T47D, MCF-7, BT474 and BT20 | [28,101] |
| 13f (acrylonitrile) | Agonist | Proliferation | MCF-7 | [29] |
| Compound 12 (quinazoline) | Antagonist | Apoptosis, Cell cycle arrest, Growth | MCF-7 | [31] |
| 2-phenylacrylonitriles (analogues) | Agonist | Proliferation (Predicted) | MCF-7 | [102] |
| FDI-6 | Agonist | Tumorsphere formation | MCF-7 | [110] |
| CB7993113 | Antagonist | Migration, Invasion | BP1, Hs578T, and SUM149 | [32] |
| CH223191 | Antagonist | Growth, Migration | MDA-MB-231 | [111] |
| DMBA | Agonist | Migration, Invasion | BP1, Hs5787 | [32] |
| Bap, 3MC, | Agonist | Mammosphere | MCF-7 | [104] |
| 5F-203 | Agonist | DNA damage, Single strand breaks (SSBs) | MD-AMB-468 | [112] |
| Raloxifene | Agonist | Apoptosis | MDA-MB-231 | [103] |
| Omeprazole | Agonist | Invasion, Metastasis | MDA-MB-231 | [92] |
| Leflunomide | Agonist | Migration | MDA-MB-468 | [107] |
| Sulindac | Agonist | Migration | MDA-MB-468 | [107] |
| Nimodipine | Agonist | Migration | MDA-MB-468 | [107] |
| Flutamide | Agonist | Migration, Proliferation | MDA-MB-468, MCF-7 | [107,113] |
| Tranilast | Agonist | Migration | MDA-MB-468 | [107] |
| Flavipin | Agonist | Proliferation, Migration, Invasion | MDA-MB-231, T47D | [108] |
| Gallic acid | Agonist | Proliferation, Migration, Invasion, Growth | MDA-MB-231, T47D | [109] |
| Luteolin | Agonist | Migration, Growth, Metastasis | MDA-MB-231 | [114] |
| Icaritin | Agonist | Growth | MCF-7 | [115] |
| DIM | Agonist | Proliferation, Migration, Invasion, Growth | MDA-MB-231, T47D | [58,116] |
| Galangin | Antagonist | Proliferation, Apoptosis | MCF-7 | [117] |
5.2. Colon Cancer
5.3. Lung Cancer
5.4. Other Cancers
6. AhR: A Potential Target in Cancer Immunotherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Markham, A. Mobocertinib: First approval. Drugs 2021, 81, 2069–2074. [Google Scholar] [CrossRef]
- Passi, I.; Kumar, B. US-FDA Approved Drugs in 2020 and 2021: A Review. Mini Rev. Med. Chem. 2022, 23, 1–25. [Google Scholar] [CrossRef]
- Mak, G.; Soria, J.-C.; Blagden, S.P.; Plummer, R.; Fleming, R.A.; Nebot, N.; Zhang, J.; Mazumdar, J.; Rogan, D.; Gazzah, A. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br. J. Cancer 2019, 120, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.-C.; Suarez, C.; De Vos, F.; Steeghs, N.; Cassier, P.A. Results from a first-in-human phase I study of siremadlin (HDM201) in patients with advanced wild-type TP53 solid tumors and acute leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakatani, T.; Kamitani, T. Negative regulation of NEDD8 conjugation pathway by novel molecules and agents for anticancer therapy. Curr. Pharm. Des. 2013, 19, 4131–4139. [Google Scholar] [CrossRef]
- Puri, S.; Ahmad, I.; Patel, H.; Kumar, K.; Juvale, K. Evaluation of oxindole derivatives as a potential anticancer agent against breast carcinoma cells: In vitro, in silico, and molecular docking study. Toxicol. In Vitro 2023, 86, 105517. [Google Scholar] [CrossRef]
- Salem, M.G.; El-Maaty, D.M.A.; El-Deen, Y.I.M.; Elesawy, B.H.; Askary, A.E.; Saleh, A.; Saied, E.M.; Behery, M.E. Novel 1, 3-thiazole analogues with potent activity against breast cancer: A design, synthesis, in vitro, and in silico study. Molecules 2022, 27, 4898. [Google Scholar] [CrossRef] [PubMed]
- Yousef, R.G.; Elkady, H.; Elkaeed, E.B.; Gobaara, I.M.; Al-Ghulikah, H.A.; Husein, D.Z.; Ibrahim, I.M.; Metwaly, A.M.; Eissa, I.H. (E)-N-(3-(1-(2-(4-(2, 2, 2-Trifluoroacetamido) benzoyl) hydrazono) ethyl) phenyl) nicotinamide: A Novel Pyridine Derivative for Inhibiting Vascular Endothelial Growth Factor Receptor-2: Synthesis, Computational, and Anticancer Studies. Molecules 2022, 27, 7719. [Google Scholar] [CrossRef]
- Elmaaty, A.A.; Darwish, K.M.; Chrouda, A.; Boseila, A.A.; Tantawy, M.A.; Elhady, S.S.; Shaik, A.B.; Mustafa, M.; Al-Karmalawy, A.A. In silico and in vitro studies for benzimidazole anthelmintics repurposing as VEGFR-2 antagonists: Novel mebendazole-loaded mixed micelles with enhanced dissolution and anticancer activity. ACS Omega 2021, 7, 875–899. [Google Scholar] [CrossRef] [PubMed]
- Gunder, L.C.; Moyer, T.H.; Johnson, H.R.; Auyeung, A.S.; Leverson, G.E.; Zhang, W.; Matkowskyj, K.A.; Carchman, E.H. Anal Cancer Prevention Through the Topical Use of Single or Dual PI3K/mTOR Inhibitors. J. Surg. Res. 2023, 282, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shukla, A.; Kalani, K.; Dubey, V.; Luqman, S.; Srivastava, S.K.; Khan, F. In-silico & in-vitro identification of structure-activity relationship pattern of serpentine & gallic acid targeting PI3Kγ as potential anticancer target. Curr. Cancer Drug Targets 2017, 17, 722–734. [Google Scholar]
- Wu, X.; Xu, Y.; Liang, Q.; Yang, X.; Huang, J.; Wang, J.; Zhang, H.; Shi, J. Recent advances in dual PI3K/mTOR inhibitors for tumour treatment. Front. Pharmacol. 2022, 13, 875372. [Google Scholar] [CrossRef]
- Lee, J.B.; Jung, M.; Beom, S.H.; Kim, G.M.; Kim, H.R.; Choi, H.J.; Sohn, J.H.; Ahn, J.B.; Rha, S.Y.; Chung, H.C. Phase 2 study of TAS-117, an allosteric akt inhibitor in advanced solid tumors harboring phosphatidylinositol 3-kinase/v-akt murine thymoma viral oncogene homolog gene mutations. Investig. New Drugs 2021, 39, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Meglio, P.D.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Rejano-Gordillo, C.M.; Marín-Díaz, B.; Ordiales-Talavero, A.; Merino, J.M.; González-Rico, F.J.; Fernández-Salguero, P.M. From Nucleus to Organs: Insights of Aryl Hydrocarbon Receptor Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 14919. [Google Scholar] [CrossRef]
- Nakahama, T.; Hanieh, H.; Nguyen, N.T.; Chinen, I.; Ripley, B.; Millrine, D.; Lee, S.; Nyati, K.K.; Dubey, P.K.; Chowdhury, K. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17–producing T-helper cell differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 11964–11969. [Google Scholar] [CrossRef] [PubMed]
- Chinen, I.; Nakahama, T.; Kimura, A.; Nguyen, N.T.; Takemori, H.; Kumagai, A.; Kayama, H.; Takeda, K.; Lee, S.; Hanieh, H. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int. Immunol. 2015, 27, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Zhang, L. The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers 2022, 14, 5574. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2, 3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Nakahama, T.; Kimura, A.; Nguyen, N.T.; Chinen, I.; Hanieh, H.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 14222–14227. [Google Scholar] [CrossRef]
- Abdulla, O.A.; Neamah, W.; Sultan, M.; Chatterjee, S.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Ahr ligands differentially regulate Mirna-132 which targets Hmgb1 and to control the differentiation of Tregs and Th-17 cells during delayed-type hypersensitivity response. Front. Immunol. 2021, 12, 635903. [Google Scholar] [CrossRef]
- Masuda, K.; Kimura, A.; Hanieh, H.; Nguyen, N.T.; Nakahama, T.; Chinen, I.; Otoyo, Y.; Murotani, T.; Yamatodani, A.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. Int. Immunol. 2011, 23, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, L.; Qiu, M.; Feng, L.; Chen, L.; Liu, Z.; Duan, S.; Zhao, Y.; Wu, K.; Zhang, N. Dietary tryptophan-mediated aryl hydrocarbon receptor activation by the gut microbiota alleviates Escherichia coli-induced endometritis in mice. Microbiol. Spectr. 2022, 10, e00811–e00822. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Maged, M.; Hairul-Islam, M.I.; Osama, I.A.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019, 14, e0215981. [Google Scholar] [CrossRef]
- Alzahrani, A.; Hanieh, H. Differential modulation of Ahr and Arid5a: A promising therapeutic strategy for autoimmune encephalomyelitis. Saudi Pharm. J. 2020, 28, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.M.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.W.; Carney, P.R.; Bradfield, C.A.; Kennedy, G.D. The aryl hydrocarbon receptor is a repressor of inflammation-associated colorectal tumorigenesis in mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Hanieh, H.; Ibrahim, H.-I.M.; Mohafez, O.; Shehata, T.; Ismail, M.B.; Alfwuaires, M. Enhancing miR-132 expression by aryl hydrocarbon receptor attenuates tumorigenesis associated with chronic colitis. Int. Immunopharmacol. 2017, 52, 342–351. [Google Scholar] [CrossRef]
- Gilbert, J.; De Iuliis, G.N.; Tarleton, M.; McCluskey, A.; Sakoff, J.A. (Z)-2-(3, 4-Dichlorophenyl)-3-(1H-pyrrol-2-yl) acrylonitrile exhibits selective antitumor activity in breast cancer cell lines via the aryl hydrocarbon receptor pathway. Mol. Pharmacol. 2018, 93, 168–177. [Google Scholar] [CrossRef]
- Baker, J.R.; Russell, C.C.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. Amino alcohol acrylonitriles as broad spectrum and tumour selective cytotoxic agents. RSC Med. Chem. 2021, 12, 929–942. [Google Scholar] [CrossRef]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.R.; Schiering, C.; Stockinger, B. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 2019, 50, 1542. [Google Scholar] [CrossRef]
- Wang, K.; Zhong, H.; Li, N.; Yu, N.; Wang, Y.; Chen, L.; Sun, J. Discovery of novel anti-breast-cancer inhibitors by synergistically antagonizing microtubule polymerization and aryl hydrocarbon receptor expression. J. Med. Chem. 2021, 64, 12964–12977. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.J.; Pollastri, M.P.; Hahn, M.E.; Stanford, E.A.; Novikov, O.; Franks, D.G.; Haigh, S.E.; Narasimhan, S.; Ashton, T.D.; Hopper, T.G. In silico identification of an aryl hydrocarbon receptor antagonist with biological activity in vitro and in vivo. Mol. Pharmacol. 2014, 86, 593–608. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Vázquez-Gómez, G.; Rocha-Zavaleta, L.; Rodríguez-Sosa, M.; Petrosyan, P.; Rubio-Lightbourn, J. Benzo [a] pyrene activates an AhR/Src/ERK axis that contributes to CYP1A1 induction and stable DNA adducts formation in lung cells. Toxicol. Lett. 2018, 289, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy–challenges and opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Kubli, S.P.; Bassi, C.; Roux, C.; Wakeham, A.; Göbl, C.; Zhou, W.; Jafari, S.M.; Snow, B.; Jones, L.; Palomero, L. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Recent advances in the development of AHR antagonists in immuno-oncology. RSC Med. Chem. 2021, 12, 902–914. [Google Scholar] [CrossRef]
- Hahn, M.E.; Karchner, S.I.; Shapiro, M.A.; Perera, S.A. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc. Natl. Acad. Sci. USA 1997, 94, 13743–13748. [Google Scholar] [CrossRef]
- Hanieh, H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: Current progress and future trends. BioMed Res. Int. 2014, 2014, 520763. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Q.; Zhang, Z.; Yi, T.; He, Y.; Zheng, J.; Lei, W. Aryl hydrocarbon receptor pathway: Role, regulation and intervention in atherosclerosis therapy. Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Hanieh, H.; Nakahama, T.; Kishimoto, T. The roles of aryl hydrocarbon receptor in immune responses. Int. Immunol. 2013, 25, 335–343. [Google Scholar] [CrossRef]
- Lin, L.; Dai, Y.; Xia, Y. An overview of aryl hydrocarbon receptor ligands in the Last two decades (2002–2022): A medicinal chemistry perspective. Eur. J. Med. Chem. 2022, 244, 114845. [Google Scholar] [CrossRef] [PubMed]
- Soshilov, A.; Denison, M.S. Ligand displaces heat shock protein 90 from overlapping binding sites within the aryl hydrocarbon receptor ligand-binding domain. J. Biol. Chem. 2011, 286, 35275–35282. [Google Scholar] [CrossRef]
- Kumar, M.B.; Ramadoss, P.; Reen, R.K.; Heuvel, J.P.V.; Perdew, G.H. The Q-rich subdomain of the human AhReceptor transactivation domain is required for dioxin-mediated transcriptional activity. J. Biol. Chem. 2001, 276, 42302–42310. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Qu, L.; Li, J.; Zhang, Y.; Jiang, L.; Wei, H.; Guo, M.; Chen, X.; Chen, Y. Structural insight into the ligand binding mechanism of aryl hydrocarbon receptor. Nat. Commun. 2022, 13, 6234. [Google Scholar] [CrossRef]
- Gruszczyk, J.; Grandvuillemin, L.; Lai-Kee-Him, J.; Paloni, M.; Savva, C.G.; Germain, P.; Grimaldi, M.; Boulahtouf, A.; Kwong, H.-S.; Bous, J. Cryo-EM structure of the agonist-bound Hsp90-XAP2-AHR cytosolic complex. Nat. Commun. 2022, 13, 7010. [Google Scholar] [CrossRef]
- Ikuta, T.; Eguchi, H.; Tachibana, T.; Yoneda, Y.; Kawajiri, K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J. Biol. Chem. 1998, 273, 2895–2904. [Google Scholar] [CrossRef]
- Petrulis, J.R.; Kusnadi, A.; Ramadoss, P.; Hollingshead, B.; Perdew, G.H. The hsp90 co-chaperone XAP2 alters importin β recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J. Biol. Chem. 2003, 278, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Kudo, I.; Hosaka, M.; Haga, A.; Tsuji, N.; Nagata, Y.; Okada, H.; Fukuda, K.; Kakizaki, Y.; Okamoto, T.; Grave, E. The regulation mechanisms of AhR by molecular chaperone complex. J. Biochem. 2018, 163, 223–232. [Google Scholar] [CrossRef]
- Dolciami, D.; Ballarotto, M.; Gargaro, M.; López-Cara, L.C.; Fallarino, F.; Macchiarulo, A. Targeting Aryl hydrocarbon receptor for next-generation immunotherapies: Selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs). Eur. J. Med. Chem. 2020, 185, 111842. [Google Scholar] [CrossRef]
- Swanson, H.I.; Chan, W.K.; Bradfield, C.A. DNA Binding Specificities and Pairing Rules of the Ah Receptor, ARNT, and SIM Proteins. J. Biol. Chem. 1995, 270, 26292–26302. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.; Phelan, D.; Winter, G.; Ziccardi, M. Carbaryl, a carbamate insecticide, is a ligand for the hepatic Ah (dioxin) receptor. Toxicol. Appl. Pharmacol. 1998, 152, 406–414. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Friling, R.S.; Bensimon, A.; Tichauer, Y.; Daniel, V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc. Natl. Acad. Sci. USA 1990, 87, 6258–6262. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Kong, A.N. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm. Drug Dispos. 2009, 30, 345–355. [Google Scholar] [CrossRef]
- Hughes, T.; Becknell, B.; Freud, A.G.; McClory, S.; Briercheck, E.; Yu, J.; Mao, C.; Giovenzana, C.; Nuovo, G.; Wei, L. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 2010, 32, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Hamza, H.; Abdullah, A. MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: A new Ahr-based exploration. Eur. J. Immunol. 2013, 43, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, K.; Jin, U.H.; Pfent, C.; Cao, H.; Amendt, B.; Liu, X.; Wilson-Robles, H.; Safe, S. Aryl Hydrocarbon Receptor Agonists Induce MicroRNA-335 Expression and Inhibit Lung Metastasis of Estrogen Receptor Negative Breast Cancer CellsAHR-Dependent Inhibition of Breast Cancer Metastasis. Mol. Cancer Ther. 2012, 11, 108–118. [Google Scholar] [CrossRef]
- Neamah, W.H.; Singh, N.P.; Alghetaa, H.; Abdulla, O.A.; Chatterjee, S.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P. AhR activation leads to massive mobilization of myeloid-derived suppressor cells with immunosuppressive activity through regulation of CXCR2 and microRNA miR-150-5p and miR-543-3p that target anti-inflammatory genes. J. Immunol. 2019, 203, 1830–1844. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Y.; Wang, Y.; An, R. Aryl hydrocarbon receptor enhances the expression of miR-150-5p to suppress in prostate cancer progression by regulating MAP3K12. Arch. Biochem. Biophys. 2018, 654, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Li, W.; Wong, P.; Lazennec, G.; Matsumura, F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007, 21, 2941–2955. [Google Scholar] [CrossRef]
- Jackson, D.P.; Li, H.; Mitchell, K.A.; Joshi, A.D.; Elferink, C.J. Ah Receptor–Mediated Suppression of Liver Regeneration through NC-XRE–Driven p21Cip1 Expression. Mol. Pharmacol. 2014, 85, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Elferink, C.J. A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol. Pharmacol. 2012, 81, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz, C.; Herry, L.; Bui, L.; Metayer, C.; Bourdeloux, M.; Barouki, R.; Coumoul, X. The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene 2013, 32, 1811–1820. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Suzuki, T.; Aizawa, K.; Sawaki, D.; Munemasa, Y.; Ishida, J.; Nagai, R. Regulation of transforming growth factor-β-dependent cyclooxygenase-2 expression in fibroblasts. J. Biol. Chem. 2009, 284, 35861–35871. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Barouki, R.; Coumoul, X. Aryl hydrocarbon receptor and its diverse ligands and functions: An exposome receptor. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lei, P.; Liu, X.; Li, X.; Walker, K.; Kotha, L.; Rowlands, C.; Safe, S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer 2009, 16, 835. [Google Scholar] [CrossRef]
- Li, B.B.; Scott, E.Y.; Olafsen, N.E.; Matthews, J.; Wheeler, A.R. Analysis of the effects of aryl hydrocarbon receptor expression on cancer cell invasion via three-dimensional microfluidic invasion assays. Lab Chip 2022, 22, 313–325. [Google Scholar] [CrossRef]
- Karasová, M.; Procházková, J.; Tylichová, Z.; Fedr, R.; Ciganek, M.; Machala, M.; Dvořák, Z.; Vyhlídalová, B.; Zůvalová, I.; Ehrmann, J. Inhibition of Aryl Hydrocarbon Receptor (AhR) Expression Disrupts Cell Proliferation and Alters Energy Metabolism and Fatty Acid Synthesis in Colon Cancer Cells. Cancers 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, R.P.; Yen, A. Activation of the Aryl hydrocarbon receptor ahr promotes retinoic acid–induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011, 71, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kanno, Y.; Nakayama, M.; Makimura, M.; Ohara, S.; Inouye, Y. Activation of the aryl hydrocarbon receptor represses mammosphere formation in MCF-7 cells. Cancer Lett. 2012, 317, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.A.; Zhang, F.; Han, L.; Bao, G.; He, X.; Xu, Z. AhR expression is increased in hepatocellular carcinoma. J. Mol. Histol. 2013, 44, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chang, H.; Tsai, W.-T.; Wu, M.-H.; Liao, Y.-S.; Chen, J.-T.; Su, J.-M. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol. Pathol. 2003, 31, 22–30. [Google Scholar] [CrossRef]
- Sarić, N.; Selby, M.; Ramaswamy, V.; Kool, M.; Stockinger, B.; Hogstrand, C.; Williamson, D.; Marino, S.; Taylor, M.D.; Clifford, S.C. The AHR pathway represses TGFβ-SMAD3 signalling and has a potent tumour suppressive role in SHH medulloblastoma. Sci. Rep. 2020, 10, 148. [Google Scholar] [CrossRef]
- Mohamed, H.T.; Gadalla, R.; El-Husseiny, N.; Hassan, H.; Wang, Z.; Ibrahim, S.A.; El-Shinawi, M.; Sherr, D.H.; Mohamed, M.M. Inflammatory breast cancer: Activation of the aryl hydrocarbon receptor and its target CYP1B1 correlates closely with Wnt5a/b-β-catenin signalling, the stem cell phenotype and disease progression. J. Adv. Res. 2019, 16, 75–86. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.-X.; Bu, Y.; Bottum, K.M.; Tischkau, S.A. Beta-naphthoflavone (DB06732) mediates estrogen receptor-positive breast cancer cell cycle arrest through AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling. Carcinogenesis 2014, 35, 703–713. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Chang, H.; Lin, P.; Sun, S.-J.; Chen, P.-H.; Lin, Y.-Y.; Sheu, G.-T.; Ko, J.-L.; Hsu, S.-L.; Chang, J.T. Up-regulation of osteopontin expression by aryl hydrocarbon receptor via both ligand-dependent and ligand-independent pathways in lung cancer. Gene 2012, 492, 262–269. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.; Ramos, K. Aryl hydrocarbon receptor regulates LINE-1 expression through epigenetic mechanisms that are linked to the canonical TGF-β1 signaling pathway. Toxicol. Lett. 2016, 259, S54. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, H.; Zhou, K.; Bai, Y.; Qi, R.; Zhang, S. 3, 3′-Diindolylmethane modulates aryl hydrocarbon receptor of esophageal squamous cell carcinoma to reverse epithelial-mesenchymal transition through repressing RhoA/ROCK1-mediated COX2/PGE2 pathway. J. Exp. Clin. Cancer Res. 2020, 39, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafsson, J.-Å. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl. Recept. Signal. 2006, 4, nrs-04016. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Peng, Z.; Raufman, J.-P. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1006–G1015. [Google Scholar] [CrossRef]
- Yang, T.; Feng, Y.-L.; Chen, L.; Vaziri, N.D.; Zhao, Y.-Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef]
- Kronenberg, S.; Esser, C.; Carlberg, C. An aryl hydrocarbon receptor conformation acts as the functional core of nuclear dioxin signaling. Nucleic Acids Res. 2000, 28, 2286–2291. [Google Scholar] [CrossRef]
- Murray, I.A.; Morales, J.L.; Flaveny, C.A.; DiNatale, B.C.; Chiaro, C.; Gowdahalli, K.; Amin, S.; Perdew, G.H. Evidence for ligand-mediated selective modulation of aryl hydrocarbon receptor activity. Mol. Pharmacol. 2010, 77, 247–254. [Google Scholar] [CrossRef]
- Waller, C.L.; McKinney, J.D. Three-dimensional quantitative structure-activity relationships of dioxins and dioxin-like compounds: Model validation and Ah receptor characterization. Chem. Res. Toxicol. 1995, 8, 847–858. [Google Scholar] [CrossRef]
- Henry, E.C.; Kende, A.S.; Rucci, G.; Totleben, M.J.; Willey, J.J.; Dertinger, S.D.; Pollenz, R.S.; Jones, J.P.; Gasiewicz, T.A. Flavone antagonists bind competitively with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) to the aryl hydrocarbon receptor but inhibit nuclear uptake and transformation. Mol. Pharmacol. 1999, 55, 716–725. [Google Scholar]
- Turyanska, L.; Itkin, B.; Breen, A.; Loaiza-Perez, A.I.; Sandes, E.O.; Bradshaw, T.D. New Treatments in Renal Cancer: The AhR Ligands. Int. J. Mol. Sci. 2020, 21, 3551. [Google Scholar]
- Hu, W.; Sorrentino, C.; Denison, M.S.; Kolaja, K.; Fielden, M.R. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007, 71, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Lee, S.-O.; Pfent, C.; Safe, S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Jorge Rodríguez, M.E.; Veitía, M.S.-I.; Giner, R.M. Plant occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Burgoon, L.D.; Ding, Q.; N’jai, A.; Dere, E.; Burg, A.R.; Rowlands, J.C.; Budinsky, R.A.; Stebbins, K.E.; Zacharewski, T.R. Automated dose-response analysis of the relative hepatic gene expression potency of TCDF in C57BL/6 mice. Toxicol. Sci. 2009, 112, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.K.; Burgoon, L.D.; Ibrahim-Aibo, D.; Burg, A.R.; Lee, A.W.; Tashiro, C.; Potter, D.; Sharratt, B.; Harkema, J.R.; Rowlands, J.C. Automated dose-response analysis and comparative toxicogenomic evaluation of the hepatic effects elicited by TCDD, TCDF, and PCB126 in C57BL/6 mice. Toxicol. Sci. 2010, 118, 286–297. [Google Scholar] [CrossRef]
- Gouédard, C.; Barouki, R.; Morel, Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell. Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef]
- Giani Tagliabue, S.; Faber, S.C.; Motta, S.; Denison, M.S.; Bonati, L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Sci. Rep. 2019, 9, 10693. [Google Scholar] [CrossRef]
- Whelan, F.; Hao, N.; Furness, S.G.; Whitelaw, M.L.; Chapman-Smith, A. Amino acid substitutions in the aryl hydrocarbon receptor ligand binding domain reveal YH439 as an atypical AhR activator. Mol. Pharmacol. 2010, 77, 1037–1046. [Google Scholar] [CrossRef]
- Perkins, A.; Phillips, J.L.; Kerkvliet, N.I.; Tanguay, R.L.; Perdew, G.H.; Kolluri, S.K.; Bisson, W.H. A structural switch between agonist and antagonist bound conformations for a ligand-optimized model of the human aryl hydrocarbon receptor ligand binding domain. Biology 2014, 3, 645–669. [Google Scholar] [CrossRef]
- Gilbert, J.; De Iuliis, G.; McCluskey, A.; Sakoff, J. A novel naphthalimide that selectively targets breast cancer via the arylhydrocarbon receptor pathway. Sci. Rep. 2020, 10, 13978. [Google Scholar] [CrossRef]
- Baker, J.R.; Pollard, B.L.; Lin, A.J.; Gilbert, J.; Paula, S.; Zhu, X.; Sakoff, J.A.; McCluskey, A. Modelling and Phenotypic Screening of NAP-6 and 10-Cl-BBQ, AhR Ligands Displaying Selective Breast Cancer Cytotoxicity In Vitro. ChemMedChem 2021, 16, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.T.; Baker, J.R.; McCluskey, A.; Paula, S. Development and interpretation of a QSAR model for in vitro breast cancer (MCF-7) cytotoxicity of 2-phenylacrylonitriles. J. Comput. Aided Mol. Des. 2021, 35, 613–628. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.F., III.; Jang, H.S.; Liefwalker, D.F.; Kerkvliet, N.I.; Kolluri, S.K. Discovery and mechanistic characterization of a select modulator of AhR-regulated transcription (SMAhRT) with anti-cancer effects. Apoptosis 2021, 26, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kanno, Y.; Yamashita, N.; Degawa, M.; Yoshinari, K.; Nemoto, K. The differential selectivity of aryl hydrocarbon receptor (AHR) agonists towards AHR-dependent suppression of mammosphere formation and gene transcription in human breast cancer cells. Biol. Pharm. Bull. 2021, 44, 571–578. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, H.; Han, C.; Ma, J.; Zhao, J.; Tang, J. Circlular RNA BARD1 (Hsa_circ_0001098) overexpression in breast cancer cells with TCDD treatment could promote cell apoptosis via miR-3942/BARD1 axis. Cell Cycle 2018, 17, 2731–2744. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, E.; Koch, D.; Bisson, W.; Jang, H.; Kolluri, S. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis. 2014, 5, e1038. [Google Scholar] [CrossRef]
- Jin, U.-H.; Lee, S.-O.; Safe, S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J. Pharmacol. Exp. Ther. 2012, 343, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H.; Mohafez, O.; Hairul-Islam, V.I.; Alzahrani, A.; Bani Ismail, M.; Thirugnanasambantham, K. Novel aryl hydrocarbon receptor agonist suppresses migration and invasion of breast cancer cells. PLoS ONE 2016, 11, e0167650. [Google Scholar] [CrossRef]
- Hanieh, H.; Ibrahim, H.-I.M.; Mohammed, M.; Alwassil, O.I.; Abukhalil, M.H.; Farhan, M. Activation of aryl hydrocarbon receptor signaling by gallic acid suppresses progression of human breast cancer in vitro and in vivo. Phytomedicine 2022, 96, 153817. [Google Scholar] [CrossRef]
- Yamashita, N.; Kawai, K.; Yoshikawa, M.; Watabe, M.; Kanno, Y.; Sanada, N.; Kizu, R. FDI-6, a FOXM1 inhibitor, activates the aryl hydrocarbon receptor and suppresses tumorsphere formation. Biochem. Biophys. Res. Commun. 2023, 639, 29–35. [Google Scholar] [CrossRef]
- Dwyer, A.R.; Kerkvliet, C.P.; Krutilina, R.I.; Playa, H.C.; Parke, D.N.; Thomas, W.A.; Smeester, B.A.; Moriarity, B.S.; Seagroves, T.N.; Lange, C.A. Breast Tumor Kinase (Brk/PTK6) Mediates Advanced Cancer Phenotypes via SH2-Domain Dependent Activation of RhoA and Aryl Hydrocarbon Receptor (AhR) SignalingPTK6 Oncogenic Activity Is SH2 Domain-Dependent. Mol. Cancer Res. 2021, 19, 329–345. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.S.; Watkins, C.N.; Campbell, P.; Zylstra, D.; Rowland, L.; Amis, L.H.; Scott, L.; Babb, C.E.; Livingston, W.J.; Darwanto, A. Aryl hydrocarbon receptor ligand 5F 203 induces oxidative stress that triggers DNA damage in human breast cancer cells. Chem. Res. Toxicol. 2015, 28, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.; Jang, H.; O’donnell, E.; Punj, S.; Kopparapu, P.; Bisson, W.; Kerkvliet, N.; Kolluri, S. Anti-androgen flutamide suppresses hepatocellular carcinoma cell proliferation via the aryl hydrocarbon receptor mediated induction of transforming growth factor-β1. Oncogene 2015, 34, 6092–6104. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Tiong, C.T.; Chen, C.; Zhang, S.J.; Li, J.; Soshilov, A.; Denison, M.S.; Lee, L.S.-U.; Tam, V.H.; Wong, S.P.; Xu, H.E. A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERα protein. Carcinogenesis 2012, 33, 1089–1097. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Firestone, G.L.; Bjeldanes, L.F. 3, 3′-diindolylmethane rapidly and selectively inhibits hepatocyte growth factor/c-Met signaling in breast cancer cells. J. Nutr. Biochem. 2013, 24, 1882–1888. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and pinocembrin from propolis ameliorate insulin resistance in HepG2 cells via regulating Akt/mTOR signaling. Evid. Based Complement. Altern. Med. 2018, 2018, 7971842. [Google Scholar] [CrossRef]
- Pandurangan, A.; Ismail, S.; Saadatdoust, Z.; Esa, N. Allicin alleviates dextran sodium sulfate-(DSS-) induced ulcerative colitis in BALB/c mice. Oxid. Med. Cell. Longev. 2015, 2015, 605208. [Google Scholar] [CrossRef]
- Liu, D.; You, P.; Luo, Y.; Yang, M.; Liu, Y. Galangin induces apoptosis in MCF-7 human breast cancer cells through mitochondrial pathway and phosphatidylinositol 3-kinase/Akt inhibition. Pharmacology 2018, 102, 58–66. [Google Scholar] [CrossRef]
- Stone, E.L.; Citossi, F.; Singh, R.; Kaur, B.; Gaskell, M.; Farmer, P.B.; Monks, A.; Hose, C.; Stevens, M.F.; Leong, C.-O. Antitumour benzothiazoles. Part 32: DNA adducts and double strand breaks correlate with activity; synthesis of 5F203 hydrogels for local delivery. Bioorg. Med. Chem. 2015, 23, 6891–6899. [Google Scholar] [CrossRef]
- Bradshaw, T.D.; Stone, E.L.; Trapani, V.; Leong, C.-O.; Matthews, C.S.; Te Poele, R.; Stevens, M.F. Mechanisms of acquired resistance to 2-(4-Amino-3-methylphenyl) benzothiazole in breast cancer cell lines. Breast Cancer Res. Treat. 2008, 110, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.S.; Mavingire, N.; Khan, S.; Rowland, L.K.; Wooten, J.V.; Opoku-Agyeman, A.; Guevara, A.; Soto, U.; Cavalli, F.; Loaiza-Pérez, A.I. AhR ligand aminoflavone suppresses α6-integrin–Src–Akt signaling to attenuate tamoxifen resistance in breast cancer cells. J. Cell. Physiol. 2019, 234, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R.; Russell, C.C.; Gilbert, J.; Sakoff, J.A.; McCluskey, A. Amino alcohol acrylonitriles as activators of the aryl hydrocarbon receptor pathway: An unexpected MTT phenotypic screening outcome. ChemMedChem 2020, 15, 490–505. [Google Scholar] [CrossRef]
- Megna, B.W.; Carney, P.R.; Depke, M.G.; Nukaya, M.; McNally, J.; Larsen, L.; Rosengren, R.J.; Kennedy, G.D. The aryl hydrocarbon receptor as an antitumor target of synthetic curcuminoids in colorectal cancer. J. Surg. Res. 2017, 213, 16–24. [Google Scholar] [CrossRef]
- Kabátková, M.; Zapletal, O.; Tylichová, Z.; Neča, J.; Machala, M.; Milcová, A.; Topinka, J.; Kozubík, A.; Vondráček, J. Inhibition of β-catenin signalling promotes DNA damage elicited by benzo [a] pyrene in a model of human colon cancer cells via CYP1 deregulation. Mutagenesis 2015, 30, 565–576. [Google Scholar] [CrossRef]
- Ronnekleiv-Kelly, S.M.; Nukaya, M.; Díaz-Díaz, C.J.; Megna, B.W.; Carney, P.R.; Geiger, P.G.; Kennedy, G.D. Aryl hydrocarbon receptor-dependent apoptotic cell death induced by the flavonoid chrysin in human colorectal cancer cells. Cancer Lett. 2016, 370, 91–99. [Google Scholar] [CrossRef]
- Megna, B.W.; Carney, P.R.; Nukaya, M.; Geiger, P.; Kennedy, G.D. Indole-3-carbinol induces tumor cell death: Function follows form. J. Surg. Res. 2016, 204, 47–54. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Stevens, B.L.; Elson, D.J.; Finlay, D.; Gamble, J.T.; Kopparapu, P.R.; Tanguay, R.L.; Buermeyer, A.B.; Kerkvliet, N.I.; Kolluri, S.K. 11-Cl-BBQ, a select modulator of AhR-regulated transcription, suppresses lung cancer cell growth via activation of p53 and p27Kip1. FEBS J. 2022, 290, 2064–2084. [Google Scholar] [CrossRef]
- Nothdurft, S.; Thumser-Henner, C.; Breitenbücher, F.; Okimoto, R.A.; Dorsch, M.; Opitz, C.A.; Sadik, A.; Esser, C.; Hölzel, M.; Asthana, S. Functional screening identifies aryl hydrocarbon receptor as suppressor of lung cancer metastasis. Oncogenesis 2020, 9, 102. [Google Scholar] [CrossRef]
- Holbrook, M.B.; Schindler, R.M. Nostalgic bonding: Exploring the role of nostalgia in the consumption experience. J. Consum. Behav. Int. Res. Rev. 2003, 3, 107–127. [Google Scholar] [CrossRef]
- Cai, D.-J.; Zhang, Z.-Y.; Bu, Y.; Li, L.; Deng, Y.-Z.; Sun, L.-Q.; Hu, C.-P.; Li, M. Asparagine synthetase regulates lung-cancer metastasis by stabilizing the β-catenin complex and modulating mitochondrial response. Cell Death Dis. 2022, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Hýžďalová, M.; Procházková, J.; Strapáčová, S.; Svržková, L.; Vacek, O.; Fedr, R.; Andrysík, Z.; Hrubá, E.; Líbalová, H.; Kléma, J. A prolonged exposure of human lung carcinoma epithelial cells to benzo [a] pyrene induces p21-dependent epithelial-to-mesenchymal transition (EMT)-like phenotype. Chemosphere 2021, 263, 128126. [Google Scholar] [CrossRef] [PubMed]
- Zgarbová, E.; Vrzal, R. The Impact of Indoles Activating the Aryl Hydrocarbon Receptor on Androgen Receptor Activity in the 22Rv1 Prostate Cancer Cell Line. Int. J. Mol. Sci. 2023, 24, 502. [Google Scholar] [CrossRef] [PubMed]
- Arabnezhad, M.-R.; Montazeri-Najafabady, N.; Chatrabnous, N.; Bahreman, A.G.; Mohammadi-Bardbori, A. Anti-androgenic effect of 6-formylindolo [3,2-b] carbazole (FICZ) in LNCaP cells is mediated by the aryl hydrocarbon-androgen receptors cross-talk. Steroids 2020, 153, 108508. [Google Scholar] [CrossRef]
- Sun, F.; Indran, I.R.; Zhang, Z.W.; Tan, M.E.; Li, Y.; Lim, Z.R.; Hua, R.; Yang, C.; Soon, F.-F.; Li, J. A novel prostate cancer therapeutic strategy using icaritin-activated arylhydrocarbon-receptor to co-target androgen receptor and its splice variants. Carcinogenesis 2015, 36, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cai, A.; Zheng, H.; Huang, H.; Sun, R.; Cui, X.; Ye, W.; Yao, Q.; Chen, R.; Kou, L. Carbidopa suppresses prostate cancer via aryl hydrocarbon receptor-mediated ubiquitination and degradation of androgen receptor. Oncogenesis 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Kim, S.-B.; Safe, S. Omeprazole inhibits pancreatic cancer cell invasion through a nongenomic aryl hydrocarbon receptor pathway. Chem. Res. Toxicol. 2015, 28, 907–918. [Google Scholar] [CrossRef]
- Jin, U.-H.; Karki, K.; Kim, S.-B.; Safe, S. Inhibition of pancreatic cancer Panc1 cell migration by omeprazole is dependent on aryl hydrocarbon receptor activation of JNK. Biochem. Biophys. Res. Commun. 2018, 501, 751–757. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Korac, K.; Rajasekaran, D.; Sniegowski, T.; Schniers, B.K.; Ibrahim, A.F.; Bhutia, Y.D. Carbidopa, an activator of aryl hydrocarbon receptor, suppresses IDO1 expression in pancreatic cancer and decreases tumor growth. Biochem. J. 2022, 479, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front. Immunol. 2015, 5, 673. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, N.C.; Rogers, T.J.; Gordon, M.A.; Greene, L.I.; Cochrane, D.R.; Spoelstra, N.S.; Nemkov, T.G.; D’Alessandro, A.; Hansen, K.C.; Richer, J.K. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015, 75, 4651–4664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Stone, E.; Triplett, T.A.; Triplett, K.; Lamb, C.; Karamitros, C.S.; Blazek, J.; Georgiou, G.; Manfredi, M.G. A novel approach to targeting the IDO/TDO pathway through degradation of the immunosuppressive metabolite kynurenine. Cancer Res. 2017, 77, 5570. [Google Scholar] [CrossRef]
- Triplett, T.A.; Garrison, K.C.; Marshall, N.; Donkor, M.; Blazeck, J.; Lamb, C.; Qerqez, A.; Dekker, J.D.; Tanno, Y.; Lu, W.-C. Reversal of indoleamine 2, 3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol. 2018, 36, 758–764. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2, 3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid MalignanciesIDO1 Inhibitor in Advanced Solid Cancers. Clin. Cancer Res. 2017, 23, 3269–3276. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Li, W.; Wu, D.; Miller, J.K.; Sweeney, C.; Lazennec, G.; Fujisawa, Y.; Matsumura, F. Interaction of aryl hydrocarbon receptor and NF-κB subunit RelB in breast cancer is associated with interleukin-8 overexpression. Arch. Biochem. Biophys. 2011, 512, 78–86. [Google Scholar] [CrossRef]
- Sato, Y.; Fujimura, T.; Hidaka, T.; Lyu, C.; Tanita, K.; Matsushita, S.; Yamamoto, M.; Aiba, S. Possible roles of proinflammatory signaling in keratinocytes through aryl hydrocarbon receptor ligands for the development of squamous cell carcinoma. Front. Immunol. 2020, 11, 534323. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Zhou, L.; Wang, J.; Zhou, Y.; Zhang, T.; Fang, Y.; Deng, J.; Gao, Y.; Liang, X. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021, 22, 358–369. [Google Scholar] [CrossRef]
- Mo, Z.; Li, P.; Cao, Z.; Zhang, S. A comprehensive pan-cancer analysis of 33 human cancers reveals the immunotherapeutic value of aryl hydrocarbon receptor. Front. Immunol. 2021, 12, 564948. [Google Scholar] [CrossRef]
- Wang, G.-Z.; Zhang, L.; Zhao, X.-C.; Gao, S.-H.; Qu, L.-W.; Yu, H.; Fang, W.-F.; Zhou, Y.-C.; Liang, F.; Zhang, C. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
| Compound | Ligand | Response | Cells | Refs. |
|---|---|---|---|---|
| Colon Cancer | ||||
| Compound 12g (acetamide) | Agonist | Proliferation | HT29 | [124] |
| RL66 | Agonist | Apoptosis, Proliferation | DLD1, HCT116, LS513, RKO | [125] |
| RL118 | Agonist | Apoptosis, Proliferation | DLD1, HCT116, LS513, RKO | [125] |
| Bap | Agonist | DNA damage | HCT116, FHC, HT29 | [126] |
| Chrysin | Agonist | Apoptosis, Proliferation | HCT116, DLD-1, SW837 | [127] |
| I3C | Agonist | Apoptosis, Proliferation | DLD1, HCT116, HT-29, LS513, and RKO | [128] |
| Lung Cancer | ||||
| 11-Cl-BBQ | Agonist | Cell Cycle Arrest, Proliferation | H460 | [129] |
| Bap | Agonist | Proliferation, Migration, Invasion, OPN | H1355, A549 | [80,113] |
| Omeprazole | Agonist | ASNS, ATF4 | H1975, A549, H1299 | [130] |
| Compound | Ligand | Response | Cells | Refs. |
|---|---|---|---|---|
| Prostate | ||||
| 3MI | Agonist | Proliferation | 22Rv1 | [134] |
| 4MI | Agonist | Proliferation | 22Rv1 | [134] |
| 2,3,7TMI | Agonist | Proliferation | 22Rv1 | [134] |
| 7MeO4MI | Agonist | Proliferation | 22Rv1 | [134] |
| Flutamide | Agonist | Proliferation | LNCaP, PC3 | [113,135] |
| Carbidopa | Agonist | Proliferation, Migration, Growth | LNCaP | [137] |
| Icaritin | Agonist | Apoptosis, Proliferation, Growth | LNCaP, C4-2, 22Rv1 | [136] |
| Hepatocellular Carcinoma | ||||
| FDI-6 | Agonist | Tumorsphere formation | HepG2 | [110,140] |
| Flutamide | Agonist | Proliferation | HepG2, HuH-7, 5L | [113] |
| Pancreatic Cancer | ||||
| Omeprazole | Agonist | Invasion | Panc1, MiaPaCa2 | [138] |
| Tranilast | Agonist | Invasion | Panc1, MiaPaCa2 | [138] |
| Carbidopa | Agonist | Growth | PDAC | [141] |
| Ovarian | ||||
| Compound 12a (acrylonitrile) | Proliferation | A2780 | [29] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanieh, H.; Bani Ismail, M.; Alfwuaires, M.A.; Ibrahim, H.-I.M.; Farhan, M. Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey. Molecules 2023, 28, 3978. https://doi.org/10.3390/molecules28103978
Hanieh H, Bani Ismail M, Alfwuaires MA, Ibrahim H-IM, Farhan M. Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey. Molecules. 2023; 28(10):3978. https://doi.org/10.3390/molecules28103978
Chicago/Turabian StyleHanieh, Hamza, Mohammad Bani Ismail, Manal A. Alfwuaires, Hairul-Islam M. Ibrahim, and Mahdi Farhan. 2023. "Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey" Molecules 28, no. 10: 3978. https://doi.org/10.3390/molecules28103978
APA StyleHanieh, H., Bani Ismail, M., Alfwuaires, M. A., Ibrahim, H.-I. M., & Farhan, M. (2023). Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey. Molecules, 28(10), 3978. https://doi.org/10.3390/molecules28103978






