An Insight into the Degradation Processes of the Anti-Hypertensive Drug Furosemide
Abstract
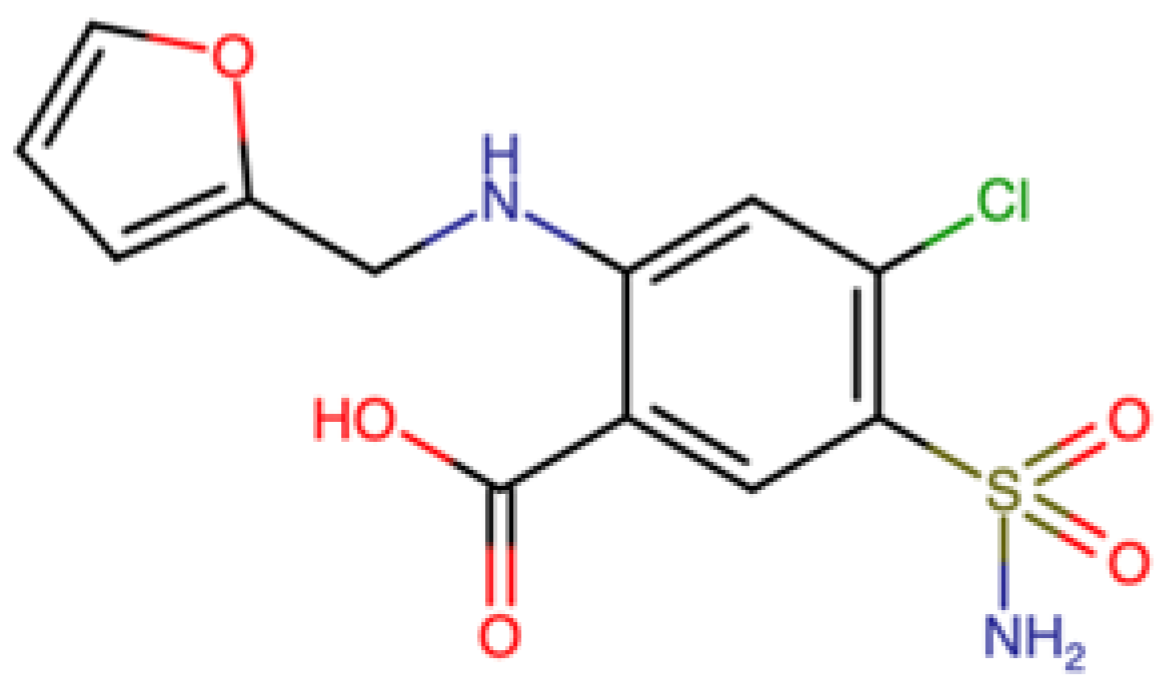
1. Introduction
2. Results and Discussion
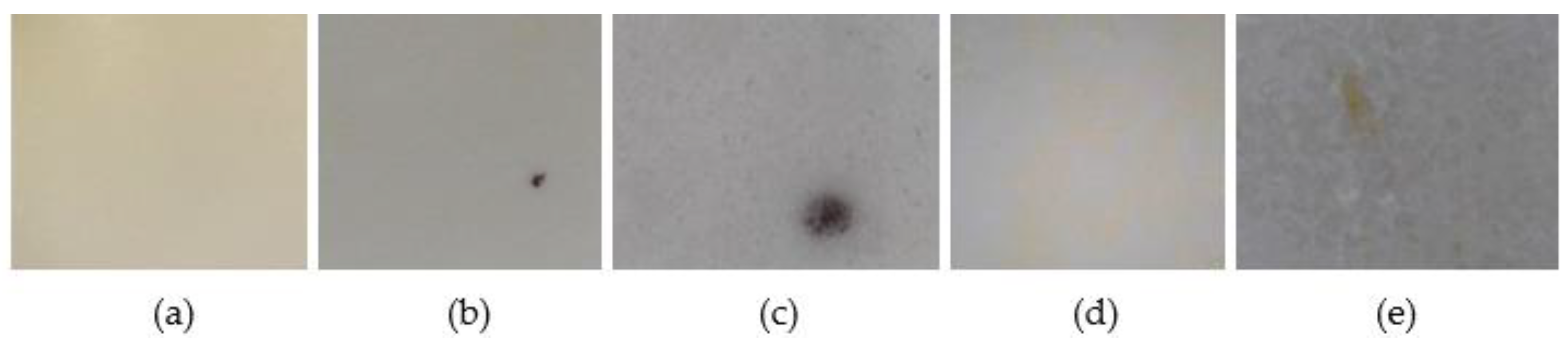
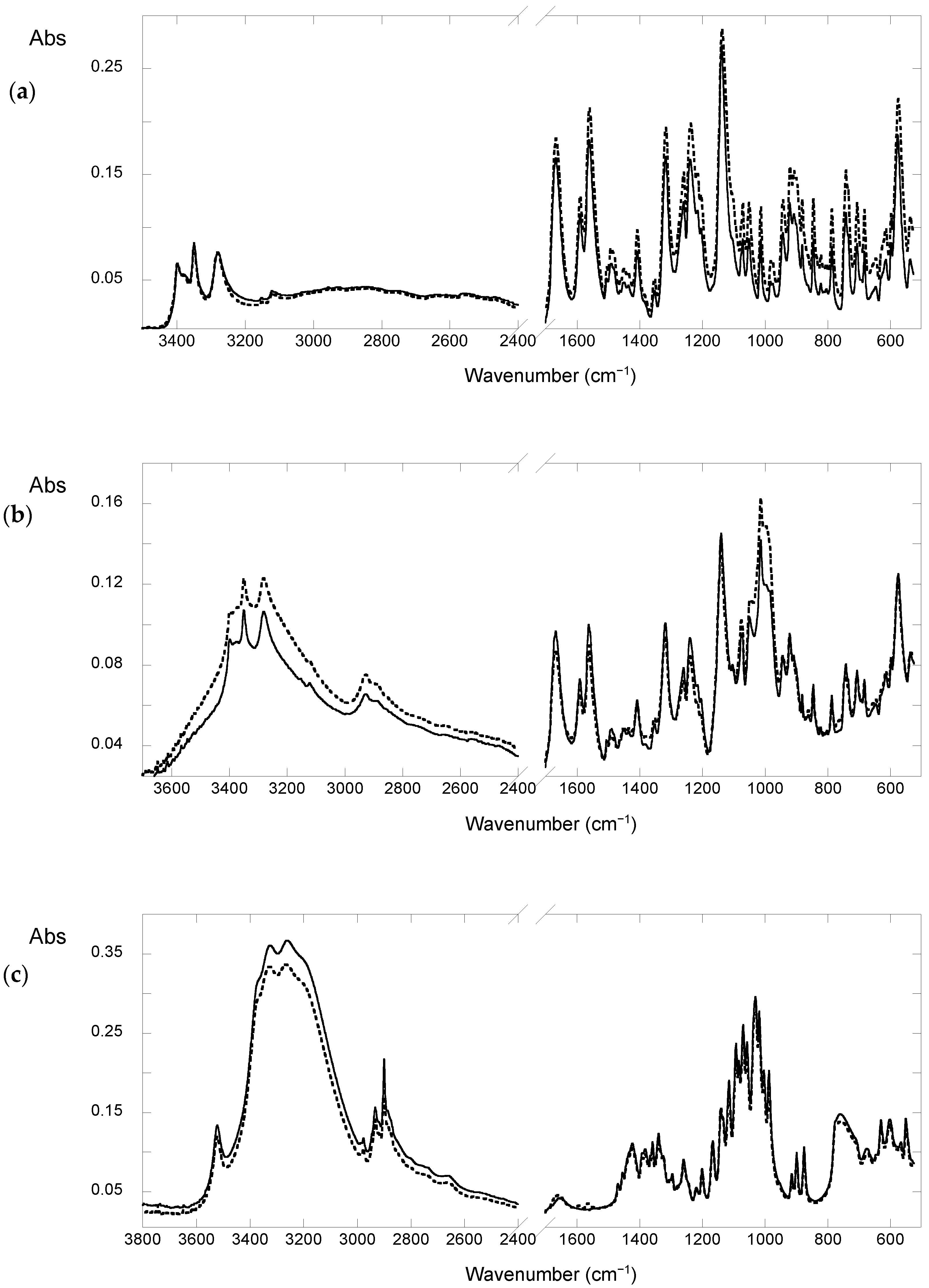
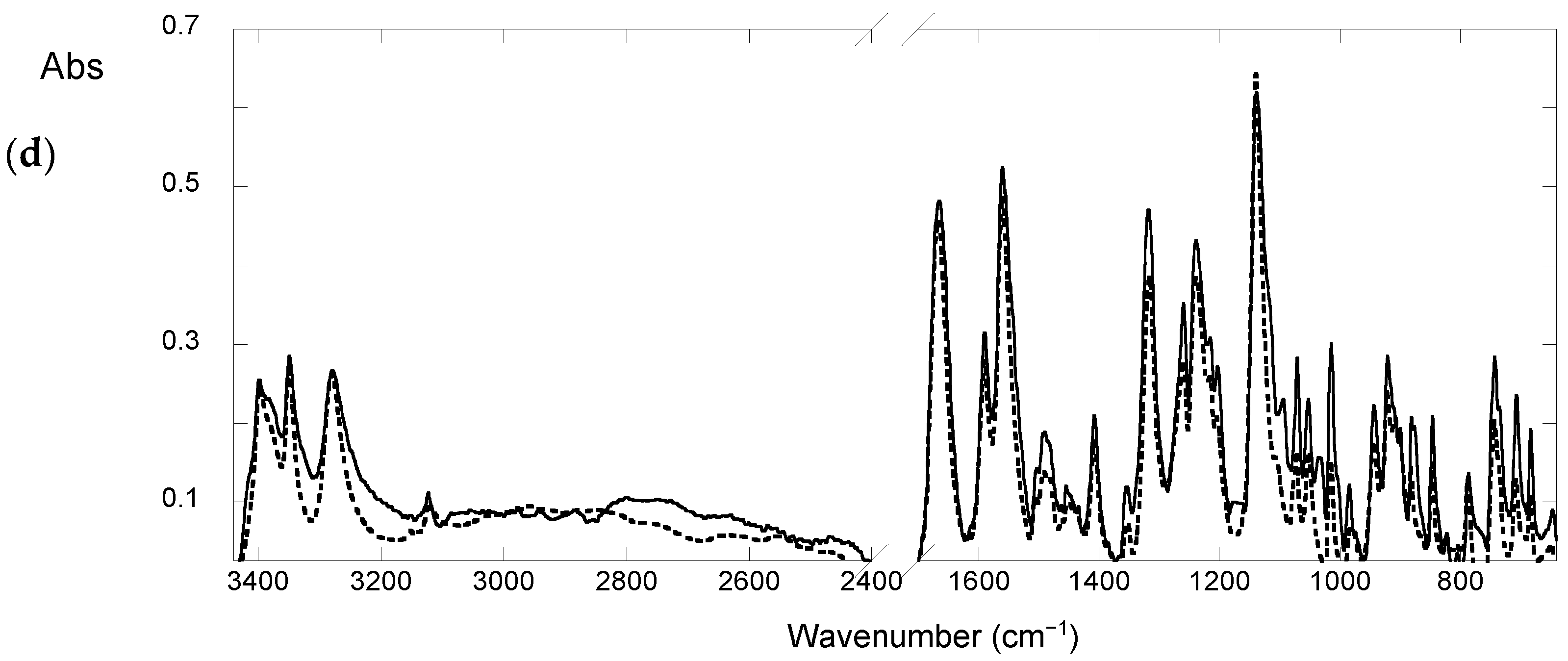
2.1. Studies in Solid State
Effect of Light
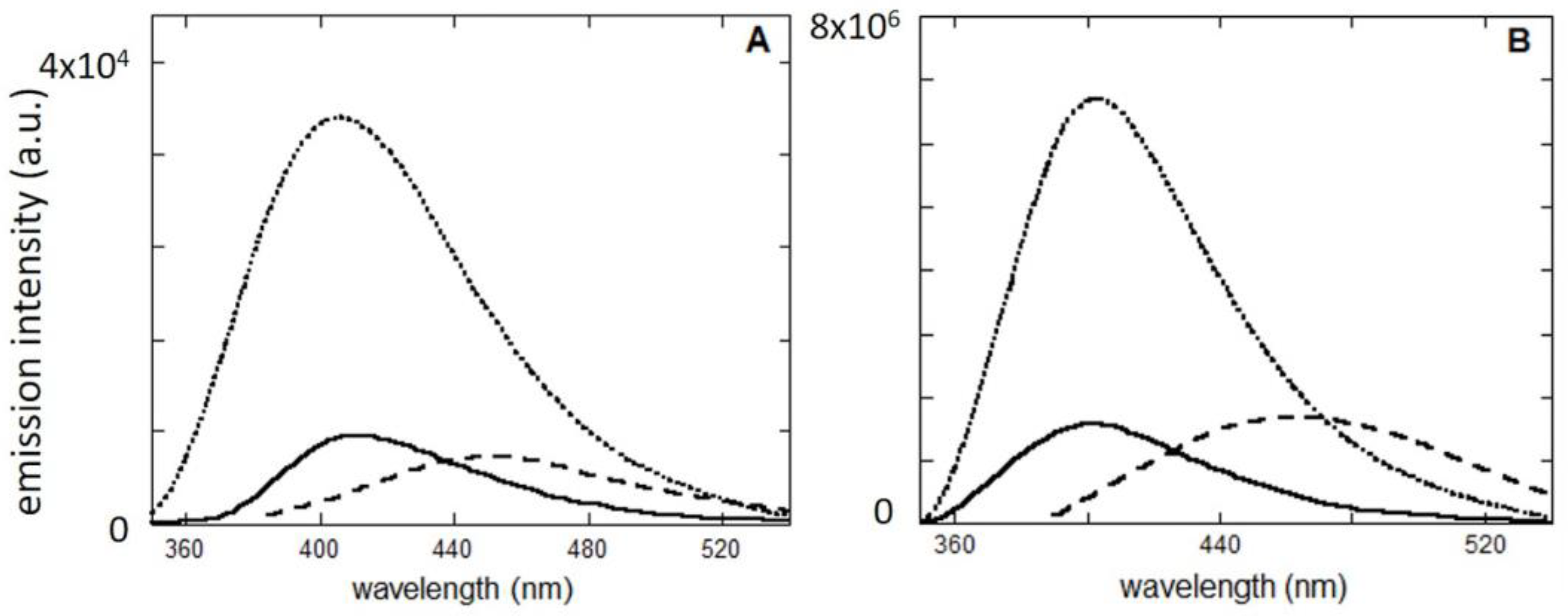
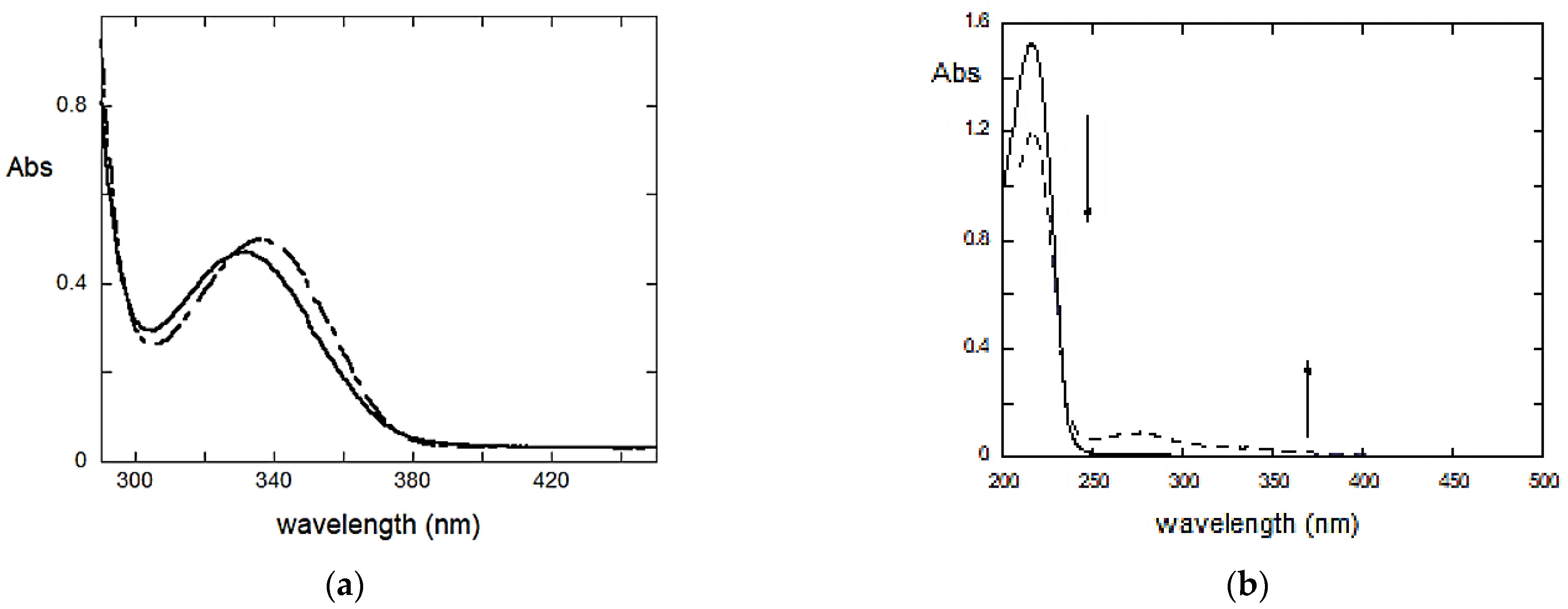
2.2. Studies in Solution
2.3. Effect of Yellowing on Sticking
3. Materials and Methods
3.1. Materials
3.2. Tablet Formation
3.3. Aging of Samples
3.4. Absorption and Emission UV-Vis Spectroscopy
3.5. FTIR Absorption Spectroscopy
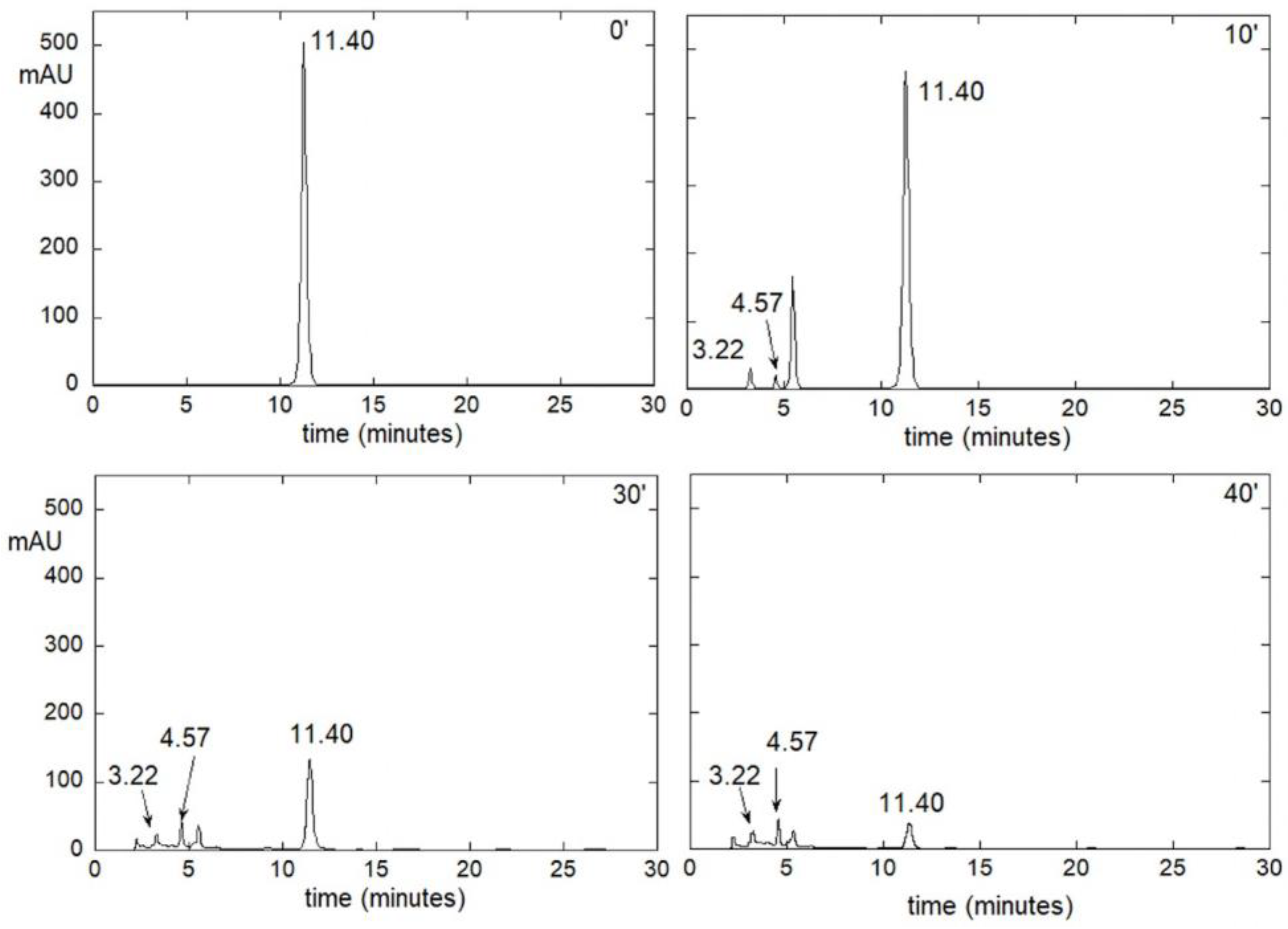
3.6. Chromatographic Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Andrisano, V.; Bertucci, C.; Battaglia, A.; Cavrini, V. Photostability of Drugs: Photodegradation of Melatonin and Its Determination in Commercial Formulations. J. Pharm. Biomed. Anal. 2000, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Grande, F.; De Luca, M.; Occhiuzzi, M.A.; Garofalo, A.; Ragno, G. Photodegradation of Anti-Inflammatory Drugs: Stability Tests and Lipid Nanocarriers for Their Photoprotection. Molecules 2021, 26, 5989. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E. Photosensitization by Drugs. J. Pharm. Sci. 1977, 66, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Trawiński, J.; Skibiński, R. Studies on Photodegradation Process of Psychotropic Drugs: A Review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-Scheme Photocatalyst TaON/Bi2WO6 Nanofibers with Oxygen Vacancies for Efficient Abatement of Antibiotics and Cr(VI): Intermediate Eco-Toxicity Analysis and Mechanistic Insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Shen, R.; Hao, L.; Ng, Y.H.; Zhang, P.; Arramel, A.; Li, Y.; Li, X. Heterogeneous N-Coordinated Single-Atom Photocatalysts and Electrocatalysts. Chin. J. Catal. 2022, 43, 2453–2483. [Google Scholar] [CrossRef]
- Gonzalo, C. Photodegradation and photosensitization in pharmaceutical products: Assessing drug phototoxicity. Pure Appl. Chem. 2004, 76, 263–275. [Google Scholar]
- Kochevar, I.E.; LAmola, A.A. Chlorpromazine And Protriptyline Phototoxicity: Photosensitized, Oxygen Independent Red Cell Hemolysis. Photochem. Photobiol. 1979, 29, 791–796. [Google Scholar] [CrossRef]
- Benet, L.Z. Pharmacokinetics/Pharmacodynamics of Furosemide in Man: A Review. J. Pharmacokinet. Biopharm. 1979, 7, 1–27. [Google Scholar] [CrossRef]
- Katsura, S.; Yamada, N.; Nakashima, A.; Shiraishi, S.; Gunji, M.; Furuishi, T.; Endo, T.; Ueda, H.; Yonemochi, E. Investigation of Discoloration of Furosemide Tablets in a Light-Shielded Environment. Chem. Pharm. Bull. 2017, 65, 373–380. [Google Scholar] [CrossRef]
- Della Greca, M.; Iesce, M.R.; Previtera, L.; Rubino, M.; Temussi, F. A New Photoproduct of the Drug Furosemide in Aqueous Media. Environ. Chem. Lett. 2004, 2, 155–158. [Google Scholar] [CrossRef]
- Moore, D.E.; Sithipitaks, V. Photolytic Degradation of Frusemide. J. Pharm. Pharmacol. 2011, 35, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Zanocco, A.L.; Fuente, J.R.; de la Fuente, J.R.; Pizarro, N.U. Kinetics and Mechanism of the Photosensitized Oxidation of Furosemide. Photochem. Photobiol. 1998, 68, 487–493. [Google Scholar] [CrossRef]
- Bundgaard, H.; Nørgaard, T.; Nielsen, N.M. Photodegradation and Hydrolysis of Furosemide and Furosemide Esters in Aqueous Solutions. Int. J. Pharm. 1988, 42, 217–224. [Google Scholar] [CrossRef]
- Carda-Broch, S.; Esteve-Romero, J.; García-Alvarez-Coque, M.C. Furosemide Assay in Pharmaceuticals by Micellar Liquid Chromatography: Study of the Stability of the Drug. J. Pharm. Biomed. Anal. 2000, 23, 803–817. [Google Scholar] [CrossRef]
- Matsuda, Y.; Tatsumi, E. Physicochemical Characterization of Furosemide Modifications. Int. J. Pharm. 1990, 60, 11–26. [Google Scholar] [CrossRef]
- Vargas, F.; Martinez Volkmar, I.; Sequera, J.; Mendez, H.; Rojas, J.; Fraile, G.; Velasquez, M.; Medina, R. Photodegradation and Phototoxicity Studies of Furosemide. Involvement of Singlet Oxygen in the Photoinduced Hemolysis and Lipid Peroxidation. J. Photochem. Photobiol. B Biol. 1998, 42, 219–225. [Google Scholar] [CrossRef]
- Rowbotham, P.C.; Stanford, J.B.; Sugden, J.L. Some aspects of the photochemical degradation of Furosemide. Pharma. Acta Helv. 1976, 51, 304–307. [Google Scholar]
- Cruz, J.E.; Maness, D.D.; Yakatan, G.J. Kinetics and Mechanism of Hydrolysis of Furosemide. Int. J. Pharm. 1979, 2, 275–281. [Google Scholar] [CrossRef]
- Zelkó, R.; Szakonyi, G. The Effect of Water on the Solid-State Characteristics of Pharmaceutical Excipients: Molecular Mechanisms, Measurement Techniques, and Quality Aspects of Final Dosage Form. Int. J. Pharm. Investig. 2012, 2, 18. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Koshy, K.T.; Duvall, R.N.; Troup, A.E.; Pyles, J.W. Factors Involved in the Browning of Spray-Dried Lactose. J. Pharm. Sci. 1965, 54, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Stowell, J.G.; Morris, K.R.; Byrn, S.R.; Pinal, R. Kinetic Study of the Maillard Reaction between Metoclopramide Hydrochloride and Lactose. Int. J. Pharm. 2005, 303, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wirth, D.D.; Baertschi, S.W.; Johnson, R.A.; Maple, S.R.; Miller, M.S.; Hallenbeck, D.K.; Gregg, S.M. Maillard Reaction of Lactose and Fluoxetine Hydrochloride, a Secondary Amine. J. Pharm. Sci. 1998, 87, 31–39. [Google Scholar] [CrossRef]
- Gumieniczek, A.; Trębacz, H.; Komsta, Ł.; Atras, A.; Jopa, B.; Popiołek, Ł. DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA. For determination of chemical stability of diuretic drugs: Impact of excipients. De Gruyter 2018, 16, 116–132. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and Incompatibilities of Pharmaceutical Excipients with Active Pharmaceutical Ingredients: A Comprehensive Review. J. Excip. Food Chem. 2016, 1, 1131. [Google Scholar]
- Teraoka, R.; Fukami, T.; Furuishi, T.; Nagase, H.; Ueda, H.; Tode, C.; Yutani, R.; Kitagawa, S.; Sakane, T. Improving the Solid-State Photostability of Furosemide by Its Cocrystal Formation. Chem. Pharm. Bull. 2019, 67, 940–944. [Google Scholar] [CrossRef]
- Grigg, R.; Knight, A.J.; Sargent, M.V. 1131. Studies in Furan Chemistry. Part I. The Infrared Spectra of 2,5-Disubstituted Furans. J. Chem. Soc. 1965, 6057–6060. [Google Scholar] [CrossRef]
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and Infrared Spectra of Starch Samples of Sweet Potato and Cassava. J. Chem. Sci. 1990, 102, 705–712. [Google Scholar] [CrossRef]
- Foti, M.C.; Amorati, R. Non-Phenolic Radical-Trapping Antioxidants. J. Pharm. Pharmacol. 2010, 61, 1435–1448. [Google Scholar] [CrossRef]
- Kim, T.; Assary, R.S.; Marshall, C.L.; Gosztola, D.J.; Curtiss, L.A.; Stair, P.C. Acid-Catalyzed Furfuryl Alcohol Polymerization: Characterizations of Molecular Structure and Thermodynamic Properties. ChemCatChem 2011, 3, 1451–1458. [Google Scholar] [CrossRef]
- Shibutani, K.; Nakai, J.; Aphichartsuphapkhajorn, K.; Kayaki, Y.; Kuwata, S.; Arao, Y.; Kubouchi, M. The Activation of Furfuryl Alcohol Polymerization by Oxygen and Its Enhanced Mechanical Properties. J. Appl. Polym. Sci. 2021, 138, 50311. [Google Scholar] [CrossRef]
- Yagi, N.; Kenmotsu, H.; Sekikawa, H.; Takada, M. Studies on the Photolysis and Hydrolysis of Furosemide in Aqueous Solution. Chem. Pharm. Bull. 1991, 39, 454–457. [Google Scholar] [CrossRef]
- Chattoraj, S.; Daugherity, P.; McDermott, T.; Olsofsky, A.; Roth, W.J.; Tobyn, M. Sticking and Picking in Pharmaceutical Tablet Compression: An IQ Consortium Review. J. Pharm. Sci. 2018, 107, 2267–2282. [Google Scholar] [CrossRef] [PubMed]



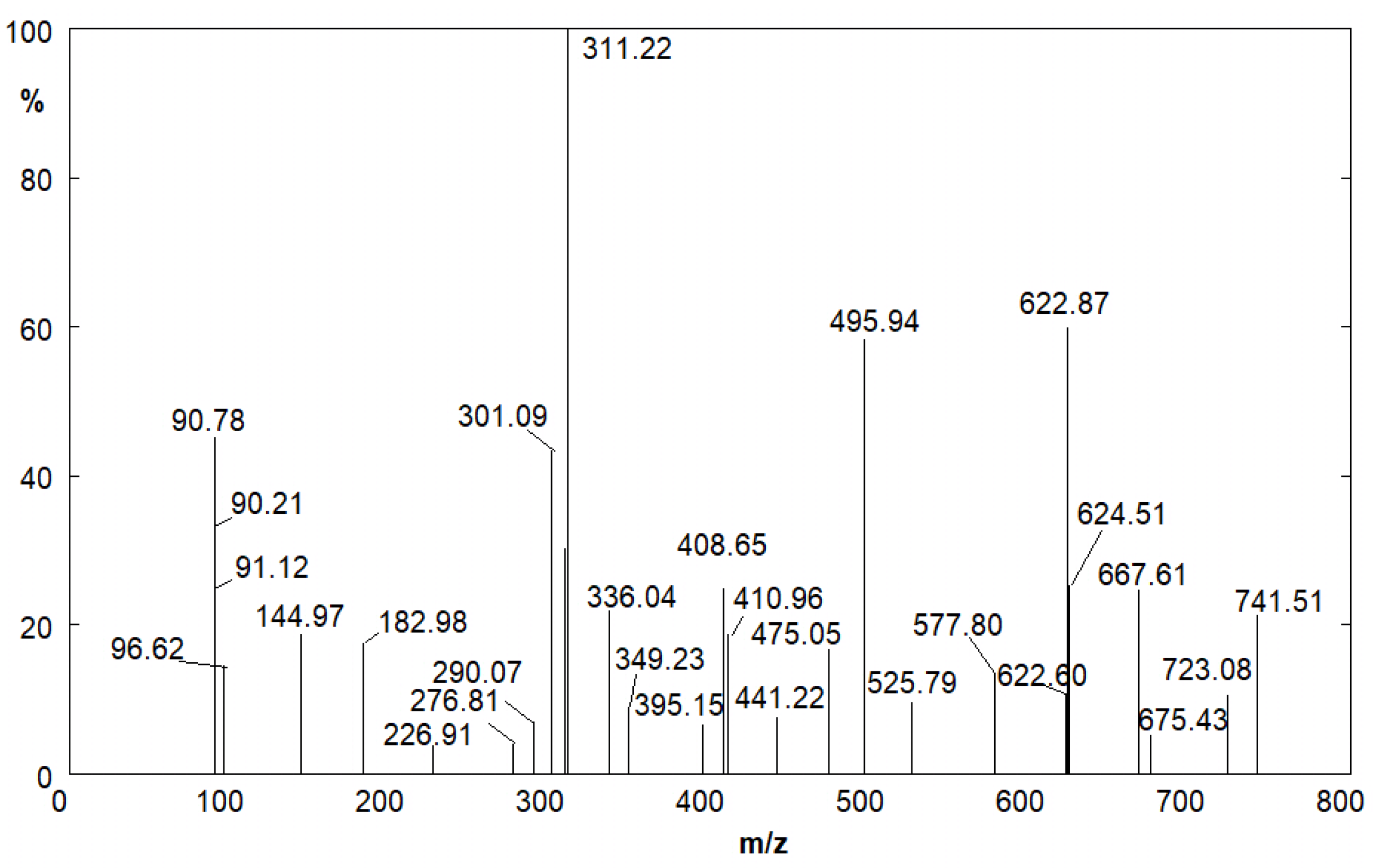

| m/z Value | Compound |
|---|---|
| 96.62 | Furfural |
| 226.91 | Furfuryl Alcohol trimer |
| 311.22 | 4-hydroxy-N-furfuryl-5-sulfamoilanthranilc acid |
| 495.94 | CSA deprotonated dimer |
| 525.79 | Furfuryl Alcohol eptamer |
| 622.84 | 4-hydroxy-N-furfuryl-5-sulfamoilanthranilc acid dimer |
| 741.51 | CSA deprotonated trimer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, M.; Canale, V.C.; Micheli, L.; Fiori, M.; Mazzuca, C.; Palleschi, A. An Insight into the Degradation Processes of the Anti-Hypertensive Drug Furosemide. Molecules 2023, 28, 381. https://doi.org/10.3390/molecules28010381
Giannetti M, Canale VC, Micheli L, Fiori M, Mazzuca C, Palleschi A. An Insight into the Degradation Processes of the Anti-Hypertensive Drug Furosemide. Molecules. 2023; 28(1):381. https://doi.org/10.3390/molecules28010381
Chicago/Turabian StyleGiannetti, Micaela, Viviana Claudia Canale, Laura Micheli, Maurizio Fiori, Claudia Mazzuca, and Antonio Palleschi. 2023. "An Insight into the Degradation Processes of the Anti-Hypertensive Drug Furosemide" Molecules 28, no. 1: 381. https://doi.org/10.3390/molecules28010381
APA StyleGiannetti, M., Canale, V. C., Micheli, L., Fiori, M., Mazzuca, C., & Palleschi, A. (2023). An Insight into the Degradation Processes of the Anti-Hypertensive Drug Furosemide. Molecules, 28(1), 381. https://doi.org/10.3390/molecules28010381







