Abstract
The positron-emitting radionuclide gallium-68 has become increasingly utilised in both preclinical and clinical settings with positron emission tomography (PET). The synthesis of radiochemically pure gallium-68 radiopharmaceuticals relies on careful consideration of the coordination chemistry. The short half-life of 68 min necessitates rapid quantitative radiolabelling (≤10 min). Desirable radiolabelling conditions include near-neutral pH, ambient temperatures, and low chelator concentrations to achieve the desired apparent molar activity. This review presents a broad overview of the requirements of an efficient bifunctional chelator in relation to the aqueous coordination chemistry of gallium. Developments in bifunctional chelator design and application are then presented and grouped according to eight categories of bifunctional chelator: the macrocyclic chelators DOTA and TACN; the acyclic HBED, pyridinecarboxylates, siderophores, tris(hydroxypyridinones), and DTPA; and the mesocyclic diazepines.
1. Introduction
Non-invasive molecular nuclear imaging has become a valuable tool to assist clinicians diagnosing and treating certain diseases. Single photon emission computed tomography (SPECT) is the most routinely used nuclear imaging technique. SPECT utilises radionuclides that emit γ radiation which penetrates the body for external detection [1]. The wide availability of technetium-99m (99mTc) (t1/2 = 6.01 h) from 99Mo/99mTc generators and the ideal single 140 keV γ emission has made 99mTc coordination complexes for SPECT the most common radiopharmaceuticals used in nuclear medicine [2]. Positron emission tomography (PET) utilises positron emitting radionuclides. As the radionuclide decays, the positrons (β+) annihilate with their antiparticles, electrons, up to a few millimetres away from the site of emission, causing two coincident 511 keV γ photons, approximately 180° apart. These photons are converted to a three-dimensional image by a circular ring of detectors [3].
PET offers higher sensitivity and resolution than SPECT, which means that the images generated are often of a higher quality at lower radiotracer concentration (10−10 M for PET cf. 10−6 M for SPECT) [4,5]. Both techniques are often combined with anatomical imaging techniques such as CT to correlate functional data with anatomical information. The availability of PET has historically been limited because of the need for cyclotron-produced radionuclides, such as fluorine-18 (18F) (t1/2 = 109.8 min), for use in imaging agents [6]. The growth in the use of PET has been stimulated by the introduction of 18F-fluorodeoxyglucose (18F-FDG), which is a glucose analogue that allows for the imaging of sites where there is a large cellular energy requirement (e.g., in primary tumour and metastatic cells) [7]. There are several other clinically approved radiopharmaceuticals containing 18F, ranging in function from brain imaging to prostate and breast cancers and associated metastases [8].
The high cost of running and maintaining cyclotron facilities, as well as the synthetically challenging formation of covalent C-F bonds for labelling biologically active targeting molecules, has encouraged the use of metallic radionuclides. The formation of coordination complexes using positron emitting radionuclides offers the opportunity for the simple preparation and straightforward incorporation of biologically active molecules to the ligand structure. There are several positron emitting radiometals with suitable half-lives that match the biological residence times of the targeting molecule. The success of the 99Mo/99mTc generator for SPECT imaging spurred interest in the development of generator-produced radiometals suitable for PET, such as gallium-68 (68Ga), which has since become a practical and successful alternative to cyclotron-produced radionuclides for PET imaging [9]. Compared to cyclotrons, generators do not require special premises with large radiation shielding equipment, specialist personnel for the maintenance of cyclotron equipment, nor a large consumption of energy.
To apply metallic radionuclides to specific biological applications, it is most often necessary to use chelators that form complexes with high thermodynamic stability and kinetic inertness to avoid transmetallation to competing proteins and hydrolysis of the radiometal. To influence the biodistribution and pharmacokinetics and improve selectivity to disease states in vivo, it is important that the chelators, termed ‘bifunctional chelators’ (BFC), feature a reactive functional group (primary amine, carboxylic acid, isothiocyanate, etc.) that can be tethered to a targeting vector biomolecule, such as a peptide or antibody.
2. Aqueous Gallium Chemistry and Gallium Radioisotopes
Gallium is a semi-metallic group 13 element. The chemistry of gallium in physiological systems is dominated by Ga3+. Lower oxidation states of gallium are known but are generally unstable in water [10,11,12]. Ga3+ is readily hydrolysed at pH > 3 forming insoluble Ga(OH)3. Radiolabelling at pH values between 3 and 7 is often difficult due to the low solubility of the Ga(OH)3 precipitate (Ksp = 7.28 × 10−36) [13,14]. The precipitate is amphoteric, forming [Ga(OH)4]− and re-dissolving at pH > 7, as demonstrated in the following equilibrium equations:
[Ga(OH2)6]3+ + H2O ⇌ [Ga(OH)(OH2)5]2+ + H3O+
[Ga(OH)(OH2)5]2+ + H2O ⇌ [Ga(OH)2(OH2)4]+ + H3O+
[Ga(OH)2(OH2)4]+ + H2O ⇌ [Ga(OH)3](s) + H3O+ + 3H2O
[Ga(OH3)](s) + OH− ⇌ [Ga(OH)4]−
Stabilising buffers are used to avoid precipitate formation, with citrate and particularly acetate common choices [7]. With its high charge and small octahedral ionic radius (0.62 Å), Ga3+ is classified as a hard Lewis acid [15,16]. Therefore, Ga3+ will generally coordinate preferentially to hard Lewis bases, such as those containing nitrogen and oxygen donor atoms. However, sulphur, selenium, and tellurium-containing coordinating systems are known [17,18,19]. Ga3+ is generally found to form 4- and 6-coordinate complexes [20,21,22]. The solution and coordination chemistries of Ga3+ are somewhat similar to Al3+ and In3+, and very similar to high-spin Fe3+ [7]. Ga3+ is expected to follow many of the same chemical pathways as Fe3+ in the body, having similar electronegativities (Ga3+ = 1.81 Pauling units; Fe3+ = 1.83 Pauling units), 4th ionisation potentials (Ga3+ = 64 eV; Fe3+ = 55 eV), and electron affinities (3rd ionisation potential Ga3+ = 30.71 eV; Fe3+ = 30.65 eV) [23]. Indeed, the biochemical chelation and protein binding similarities between Ga3+ and Fe3+ are likely causative of physiological Ga3+ activity and uptake. Tissue distribution studies have shown that the majority of administered Ga3+ ions bind to iron-transporting proteins, such as transferrin, lactoferrin, and ferritin, with some localising in osteoblasts [23,24]. However, differences in reduction potential (unlike Fe3+, Ga3+ will not be reduced to Ga2+ under physiological conditions) mean that Ga3+ does not compete with Fe2+ containing molecules, such as heme, in the body [23].
Currently, 30 different isotopes of gallium are known, of which 3 are medically relevant: 66Ga, 67Ga and 68Ga (Table 1) [25]. 66Ga and 67Ga are cyclotron produced, and 67Ga has been used for SPECT imaging. 66Ga and 68Ga are β+-emitters with half-lives of 9.5 h and 67.7 min, respectively. 68Ga is by far the most extensively studied for medical purposes, mostly due to the widespread availability of the 68Ge/68Ga generator, which can be stored on-site in hospitals [26]. In a typical 68Ge/68Ga generator, 68Ge4+ is immobilised on a column filled with metal oxide/hydroxide matrices where it spontaneously decays to 68Ga3+. Most 68Ge/68Ga generators use acidic eluent (0.1 M HCl) to elute a mixture of [68Ga][GaCl3], [68Ga][GaCl4]− or [68Ga][Ga(OH2)6]3+ [7]. The long half-life of the 68Ge parent isotope (t1/2 = 270.95 d) means that a 68Ge/68Ga generator has a working life of up to one year depending on the initial activity (cf. ~14 d for the 99Mo/99mTc generator) [9]. Additionally, 68Ga can be produced in a cyclotron in large amounts [27]. This second supply source could potentially be important to mitigate supply issues, as well as allowing for a centralised local site for radiopharmaceutical production. 68Ga decays to the stable isotope 68Zn via β+ emission and electron capture (EC). The 67.7-min half-life allows patients to be scanned in clinics quickly, minimising wait times and radiation exposure to both patients and personnel. 68Ga also allows for repetitive examinations due to relatively low radiation exposure. 68Ga has a maximum β+ energy of 1880 keV, an average β+ energy of 890 keV, and an annihilation radiation of 511 keV [26]. Although the higher β+ energy means a slightly lower resolution than 18F, this provides an adequate level of radioactivity for high quality PET images.

Table 1.
Decay properties of medically relevant gallium radioisotopes.
3. Bifunctional Chelator Design for Gallium Radiopharmaceuticals
There are currently four FDA-approved 67/68Ga radiopharmaceuticals in clinical use [8]. Of these, three 68Ga radiopharmaceuticals incorporate a BFC tethered to a targeting group via a covalent bond: [68Ga]Ga-DOTATATE, [68Ga]Ga-DOTATOC and [68Ga]Ga-HBED-CC-PSMA (Table 2) [28]. [68Ga]Ga-DOTATATE and [68Ga]Ga-DOTATOC are both comprised of the 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) macrocycle attached to either the D-Phe1-Tyr3-octreotate or D-Phe1-Tyr3-octreotide peptides, respectively, via amide bond formation between a carboxylic acid of the macrocycle and primary amine of the peptide. DOTA has historically been the “workhorse” chelator in metallic radiopharmaceutical development, due to its ability to form complexes with a large number of radiometals, excellent in vivo stability, and commercialisation of various bifunctional derivatives [25]. [68Ga]Ga-HBED-CC-PSMA, meanwhile, is comprised of the acyclic chelator, N,N′-bis [2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC), tethered to a prostate-specific membrane antigen (PSMA)-targeting small molecule motif, glutamate-urea-lysine (Glu-urea-Lys) (Figure 1). HBED-CC found success due to its ability to quantitatively coordinate 68Ga3+ quickly at room temperature, which was a marked advantage over DOTA [29].

Table 2.
FDA-approved radiopharmaceuticals containing 67/68Ga [8].
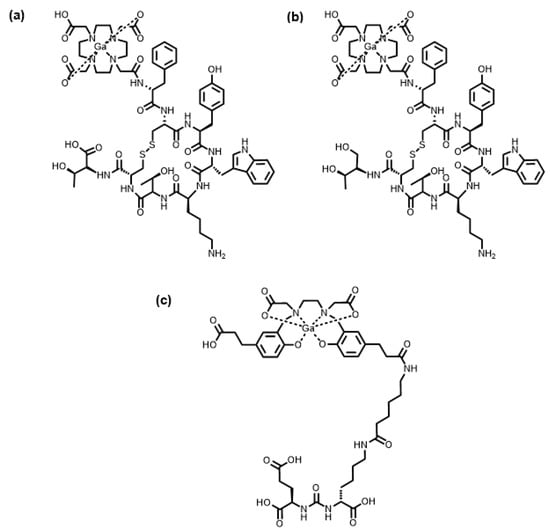
Figure 1.
FDA-approved radiopharmaceuticals containing 68Ga: (a) [68Ga]Ga-DOTATATE; (b) [68Ga]Ga-DOTATOC; (c) [68Ga]Ga-HBED-CC-PSMA.
Although [68Ga]Ga-DOTATATE, [68Ga]Ga-DOTATOC, and [68Ga]Ga-HBED-CC-PSMA are used clinically, their syntheses have drawbacks. The radiosynthesis of [68Ga]Ga-DOTATATE and [68Ga]Ga-DOTATOC requires heating to between 80 and 100 °C to ensure adequate chelation of 68Ga3+ within the timeframe allowed by the relatively short half-life of 67.7 min [30]. At room temperature, the synthesis of [68Ga]Ga-HBED-CC-PSMA produces multiple geometric isomers (in addition to optical isomers) of the [68Ga]Ga-HBED-CC complex [31].
Clinical radiosynthesis of the three radiopharmaceuticals takes 5–20 min at pH 3–5 with heating, followed by purification to remove by-products and unreacted 68Ga [29]. These conditions add process complexity, limit molar activity (the measured radioactivity per mole of radiopharmaceutical), and the heat and low pH may damage vector biomolecules. The ideal synthesis should be a one-step procedure, matching the simplicity of the long-established kit-based 99mTc radiolabelling protocols [29,32,33,34]. This would enable fast, simple, and reproducible formulations of the radiopharmaceutical in keeping with good manufacturing practice (GMP) standards [35,36]. The design of BFCs that can quantitatively coordinate metallic radioisotopes such as 68Ga, with the resulting complexes exhibiting high thermodynamic stability and kinetic inertness in vitro and in vivo, is now considered a mature field [37].
A plethora of bifunctional chelating systems have been developed, with varying stabilities, inertness, radiolabelling conditions, and biological conjugates, to circumvent some of the limitations of both DOTA and HBED-CC in biomolecule radiolabelling [38,39]. These include derivatives of 1,4,7-triazacyclononane (TACN)-based chelators (1,4,7-triazacyclononane-1,4,7-acetic acid, (NOTA), -phosphonic acid (NOTP), and -phosphinic acid (TRAP) and the mixed phosphonate/carboxylate NOA2P and NO2AP) [40]; tetraazamacrocycles (e.g., pyridyl-substituted PCTA) [41]; the acyclic siderophore desferrioxamine-B (DFO) and related siderophores [38]; substituted pyridine carboxylate-based acyclic chelators (e.g., dedpa) [42]; 6-amino-1,4-diazepanes with N-substituted pendant arms (e.g., AAZTA, DATAm and PIDAZTA) [43]; N-hydroxypyridinones (THP); and other acyclic chelators such as DTPA (Figure 2) [44].
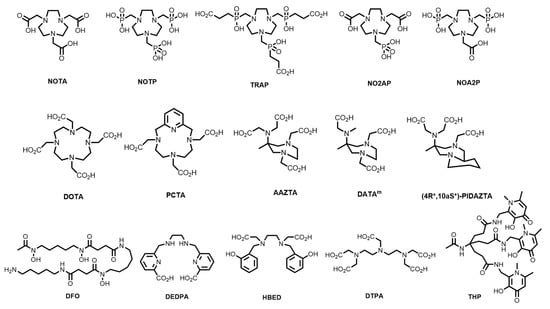
Figure 2.
Structures of some common macrocyclic and acyclic chelators for use in 68Ga radiopharmaceuticals.
The following sections will discuss the recent developments (2012–2022) of bifunctional chelators for radiolabelling with Ga radioisotopes and are divided by chelator type (beginning with developments in HBED and DOTA bifunctional chelators). A discussion of the acyclic families of chelators (DTPA, siderophores, pyridinecarboxylates, and hydroxypyridinones) is followed by the hybrid acyclic/macrocyclic diazepines, and finally, macrocyclic TACN. A discussion of the ligand design and the coordination chemistry with Ga3+, where applicable, is provided as well as progress that has been made to deliver the radiation to specific sites in vivo by tethering the complexes to targeting vectors, such as peptides, antibody fragments, and receptor-specific molecules. In this context, we hope to obtain a comprehensive understanding of the library of bifunctional chelating systems available for Ga radioisotopes, with a view to designing novel systems for specific applications.
4. DOTA and Other Tetraazamacrocyclic-Based Bifunctional Chelator Development
The coordination sphere of the Ga3+-DOTA complex is a N4O2 distorted octahedron (Figure 3), and it is well-understood that the large size of the macrocycle is not well-suited for Ga3+ complexation (logK1 = 26.05) [45] compared with more flexible acyclic chelators or smaller macrocycles [25]. As previously mentioned, DOTA and its associated bifunctional derivatives are often used as a motivating factor for researchers to develop better chelating systems for 68Ga radiolabelling. However, the fact remains that two of the four 68Ga radiopharmaceuticals currently approved by the FDA are based on the DOTA macrocycle. Consequently, effort has been made towards developing DOTA-based BFCs for radiopharmaceutical applications, as well as novel cyclen-based and other tetraazamacrocyclic non-bifunctional 68Ga chelators.
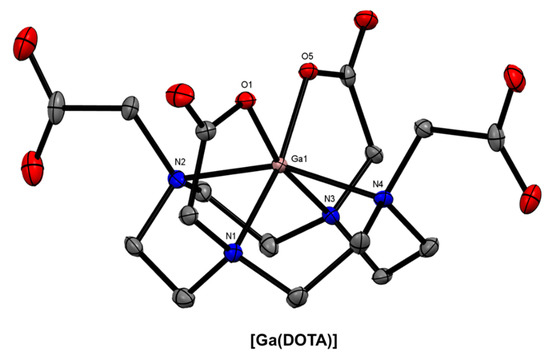
Figure 3.
ORTEP representation of [Ga(DOTA)] (Cambridge Structural Database Refcode (CSD)-DEVHIW) [46,47]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms and solvent molecules are omitted for clarity.
Edem and co-workers prepared a poly(ethylene glycol)-tetrazine DOTA conjugate (DOTA-PEG11-Tz, Figure 4) that was investigated as a pre-targeting probe for both the 68Ga radiolabelling of bone (via conjugation to a trans-cyclooctene (TCO)-derived bone-targeting bisphosphonate, alendronate), as well as the TCO-conjugated antibody, CC49 (which targets the tumour associated glycoprotein 72 antigen on colorectal cancer cells) [48]. The advantage of pre-targeting is that you can heat the complex without damaging the antibody. Additionally, pre-targeting provides an opportunity to use 68Ga, with its short half-life, with biomolecules that have long biological half-lives, such as antibodies [49,50,51]. The radiotracers showed target-specific uptake of [68Ga][Ga(DOTA-PEG11-Tz)] in the bone (3.7% injected dose/gram (%ID/g) in the knee) in mice pre-treated with TCO-alendronate, as well as tumour-specific uptake (5.8% ID/g) in mice containing LS174 xenografts that were pre-treated with TCO-CC49.
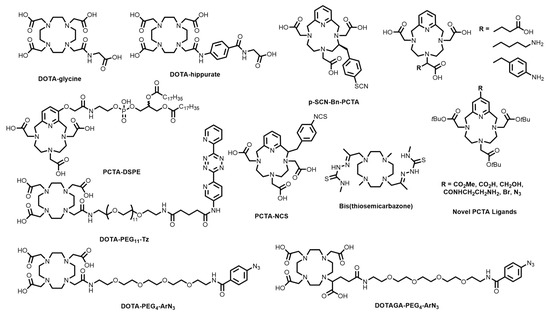
Figure 4.
Bifunctional chelators and conjugates based on tetraazamacrocyclic chelators.
Pathuri and co-workers reported DOTA-hippurate and DOTA-glycine conjugates (Figure 4) with the aim of developing alternative renographic agents to 99mTc-DTPA (used in SPECT) for PET [3]. Both 68Ga complexes demonstrated high radiochemical purity (RCP > 98%) at pH 4–5 within 10 min and cleared from circulation primarily through the kidneys (with <0.2% ID/g remaining in the blood at 1 h post-injection (p.i.)). DOTA-hippurate and DOTA-glycine both showed kidney-to-blood %ID/g ratios of ~3:1, which were deemed insufficient for further investigation.
DOTA conjugates containing octreotate [52], antibodies [53], peptides, including arginine-glycine-aspartic acid (RGD) [54,55], and PP-F11 (a target for cholecystokinin-2 receptors, which are overexpressed on small cell lung cancer among others) [56], drug-loading dendrimers [57], neoplastic tissue-targeting porphyrins [58,59], aptamers [60], Fibroblast Activation Protein (FAP) inhibitors [61], nitroimidazoles [62], and siderophores for bacterial imaging [63], have also been prepared and radiolabelled with 68Ga in recent years using previously-described amide-bond and thiourea coupling. The N4O3 DOTA analogue, 3,6,9,15-tetraazabicyclo-[9.3.1]-pentadeca-1(15),11,13-triene-3,6,9-triacetic acid (PCTA), containing a pyridine group in lieu of one of the macrocyclic secondary amines, has shown significant promise with many bifunctional variants reported [64,65,66,67]. Additionally, 67/68Ga complexes of PCTA have shown superior human serum stability compared to the DOTA complexes [68]. Bifunctional variants have found success in various studies, including the 68Ga radiolabelling of peptides (p-NO2-Bn-PCTA, Figure 4) [66], RGD radiolabelling (p-SCN-Bn-PCTA, Figure 4) [68], and visualising atherosclerotic plaques (PCTA-DSPE, Figure 4) [69], among other novel PCTA ligands (Figure 4) [68].
A novel bis(thiosemicarbazone)-dimethylcyclen ligand was recently reported and investigated as a chelator for 68Ga (Figure 4) [19]. The ligand quantitatively coordinated 68Ga3+ at 90 °C after 10 min at both pH 3.5 (radiochemical yield (RCY) = 95.1 ± 2.3%) and pH 6 (RCY = 89.0 ± 4.3%), which were similar to results reported for DOTA (pH 3.5 RCY = 95.3 ± 0.9%; pH 6 RCY = 97.2 ± 0.3%) at a chelator concentration of 50 μM. However, at lower temperatures (25 °C and 40 °C), the chelator performed much worse at both pH values. No bifunctional variants of the scaffold have been reported. 67/68Ga labelled porphyrins and tetrapyrroles have been reported, however the unfavourable radiolabelling efficiencies may not allow clinical translation [58,70,71,72,73,74].
5. HBED-Based Bifunctional Chelator Development
The acyclic chelator, HBED, based on an ethylenediaminetetraacetic acid (EDTA)-type framework with two pendant phenol arms, has several structural characteristics (N and O donor atoms, potential hexadentate coordination environment) that make it an ideal chelator for Ga3+. The original chelator synthesis was described in the 1960s [75]. The resulting Ga3+ complex is highly thermodynamically stable (logβ1 = 38.51) [76]. An X-ray crystal structure of HBED with Ga3+ revealed an N2O4 octahedral coordination sphere with the N2O3 pentadentate chelator and an apical water molecule (Figure 5). The development and characterisation of novel bifunctional variants of the dicarboxylate analogue, HBED-CC, has been growing over the past decade since Eder and co-workers’ reported the conjugation of the PSMA-targeting motif, Glu-urea-Lys, to HBED-CC for the imaging of prostate cancer ([68Ga]Ga-HBED-CC-PSMA) in 2012 [31,77].
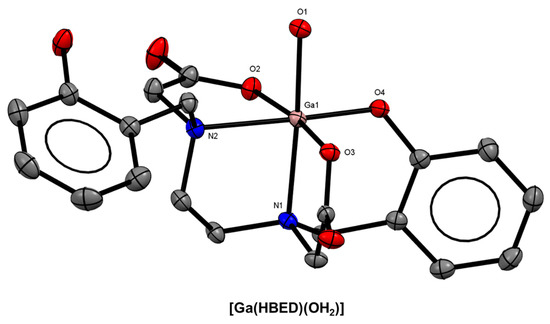
Figure 5.
ORTEP representation of [Ga(HBED)(OH2)] (CSD-PEGSIG) [[38]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms and solvent molecules are omitted for clarity.
In an effort to improve on the characteristics of Eder’s HBED-CC-PSMA platform, Zha and co-workers introduced an O-(carboxymethyl)-L-tyrosine linker group into the structure (termed HBED-PSMA-093, Figure 6), which demonstrated increased cell internalisation (12.5% ID/106 cells at 1 h) compared to HBED-CC-PSMA (7.4% ID/106 cells at 1 h), whilst also demonstrating comparable fast clearance from non-target organs [78]. The development of a robust kit-based synthesis followed, and direct comparison with [68Ga]Ga-PSMA-617 (a DOTA analogue) in PET/CT of patients with prostate cancers showed higher tumour uptake, less blood pooling, and reduced bladder accumulation [33,79]. Targeting the HBED-CC chelator for bone imaging was achieved by tethering a bisphosphonate (HBED-CC-BP, Figure 6) [80]. The 68Ga complex (termed [68Ga]Ga-P15-041) demonstrated excellent in vivo and in vitro stability, and compared favourably to the more widely used tracer, [18F]NaF. A kit-based formulation of the complex showed high RCP and radiochemical conversion (RCC) (>90%) within 10 min and required no further purification [81].
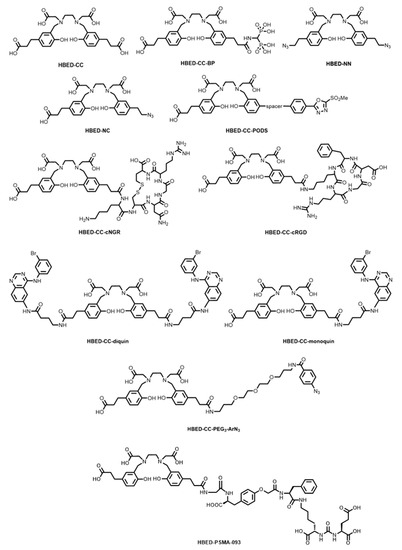
Figure 6.
Bifunctional chelators and conjugates based on the HBED platform.
Satpati and co-workers reported the conjugation of HBED-CC via amide bond formation to RGD (HBED-CC-cRGD, Figure 6) and asparagine-glycine-arginine (NGR)-containing peptides (HBED-CC-cNGR, Figure 6) for 68Ga radiolabelling and PET imaging of tumour vasculature markers, integrin αvβ3 and CD13/aminopeptidase N, respectively. They showed that whilst uptake was similar in HT-1080 human xenografts, the radiolabelled HBED-CC-c(RGD) conjugate displayed higher uptake in B16F10 tumours and higher specificity to αvβ3-positive cells than the NGR conjugate [82].
The conjugation of HBED-CC to monoclonal antibodies (mAbs) and antibody fragments for antibody-based PET imaging (immuno-PET) has also been explored. Fay and co-workers used photoactivatable aryl azides (HBED-CC-PEG3-ArN3, Figure 6) for the fast (~10 min) conjugation of the HBED-CC chelator to a model anti-c-MET antibody, onartuzumab, via the formation of an azepin group [83]. Klika and co-workers have recently reported a thiol-reactive HBED-CC derivative containing a phenyloxadiazolyl methylsulfone (PODS) group (HBED-CC-PODS, Figure 6) to mitigate the instability of the resulting succinimidyl linkage of the traditional maleimide coupling procedure (the reaction between a thiol of cysteine-containing biomolecules and a maleimide-containing chelator) [84,85].
Analogues of HBED-CC have recently been synthesised for unique targeting applications as well as direct click chemistry reactions. Liolios and co-workers functionalised the propionic acid groups of HBED-CC with the tyrosine kinase (TK) pharmacophore, 4-amino-N-(4-((3-bromophenyl)amino)quinazolin-6-yl)-butanamide, to form both the monomeric (HBED-CC-monoquin, Figure 6) and dimeric (HBED-CC-diquin, Figure 6) quinazoline derivatives [86]. TK receptors, including epidermal growth factor receptor (EGFR), are overexpressed in several tumours. Cytotoxicity studies of the natGa3+ complex in A431 cells (which overexpress EGFR) showed half maximal inhibitory concentration (IC50) values in the micromolar range (50–70 μM), the first Ga3+-quinazoline derivatives to show this. The 68Ga radiolabelled HBED-CC-monoquin complex displayed superior tumour uptake compared to HBED-CC-diquin.
Makarem and co-workers have published the syntheses of two novel azide-containing derivatives, namely the symmetric diazido HBED-NN (Figure 6) and asymmetric monoazido-monocarboxylate HBED-NC (Figure 6), for labelling via the Cu+-catalysed azide-alkyne cycloaddition (CuAAC) reaction [87,88]. HBED-NC, in particular, shows significant promise through the potential construction of heterodimeric architectures (i.e., for use in multi-modal imaging procedures such as PET/CT/fluorescence imaging, studies of which were recently reported) [89]. The synthesis of HBED-CC and derivatives has been adapted to solid-phase techniques, which could potentially ease the multistep synthetic protecting group strategies currently employed for the synthesis of HBED-CC derivatives [90].
The approval of [68Ga]Ga-HBED-CC-PSMA in December 2020 by the FDA for the PET imaging of prostate cancer has catalysed the developed of novel BFCs based on the HBED scaffold, with renewed interest in the once-overlooked chelating platform [25]. The successful clinical translation of other HBED bioconjugates has yet to be seen. However, it seems likely that the favourable 68Ga radiolabelling kinetics of HBED will lead to further radiopharmaceuticals with novel biological targets in future.
6. DTPA-Based Bifunctional Chelator Development
Another acyclic chelator that has been investigated as a 68Ga3+ chelator for radiopharmaceutical development is 2-(bis{2-[bis(carboxymethyl)amino]ethyl}-amino)acetic acid (DTPA) [25,91]. The X-ray crystal structure of the Ga3+ complex was reported by Wallin and co-workers and revealed a N2O4 coordination sphere comprising the 5-coordinate DTPA chelator and an apical water molecule (Figure 7) [92]. This is somewhat surprising given the eight potential donor atoms and is potentially due to the small ionic radius of the Ga3+ ion or the conditions of crystallisation. The analogous Fe3+ complex, [Fe(DTPA)]2−, is 7-coordinate [93,94].
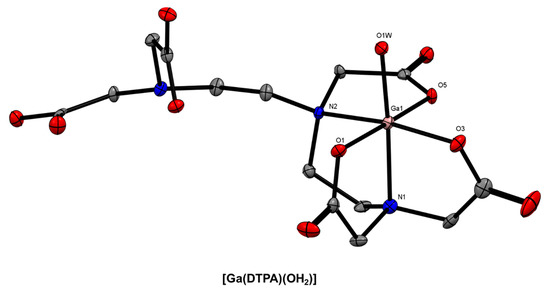
Figure 7.
ORTEP representation of [Ga(DTPA)(OH2)] (CSD-TICDUH01 [93] Ellipsoids are drawn at the 50% probability level. Hydrogen atoms and solvent molecules are omitted for clarity.
The synthetic development of DTPA BFCs has involved derivatisation of the carboxylic acids or the incorporation of a functional group to the diethylenetriamine backbone. Bis-amide derivatives (Figure 8) synthesised from DTPA bis-anhydride were shown to form complexes with Ga3+, In3+, and Lu3+ [95]. DTPA bis-anhydride reacts with amine-containing compounds, including biomolecules containing surface lysine residues, to generate the resulting DTPA bis-amide. However, radiolabelling studies with 68Ga were not performed. DTPA bis-anhydride was also used to label β-neurotoxins of Micrurus fulvius with 67Ga to track the biodistribution of the venoms via reaction with the proteins’ surface lysine residues [96].
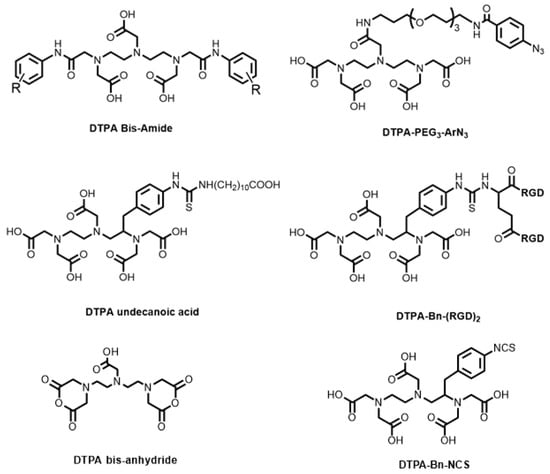
Figure 8.
Bifunctional chelators and conjugates based on the DTPA platform.
Gut and Holland utilised similar aryl azide chemistry to the HBED-CC-PEG3-ArN3 system to produce DTPA-PEG3-ArN3 (Figure 8) via amide coupling for radiolabelling of a model antibody, trastuzumab, with 68Ga [97]. In comparison to HBED-CC-PEG3-ArN3 (18.5 ± 0.5%, n = 2) [83], as well as the NOTA- (15.5 ± 1.5%, n = 3) [53], 1,4,7-triazacyclononane-1-glutaric acid-4,7-diacetic acid (NODAGA)- (22.0 ± 3.5%, n = 3) [98], DOTA- (12.7 ± 3.2%, n = 3, Figure 4) [53], DOTAGA- (11.1 ± 0.2, n = 3, Figure 4) [53], and DFO- (67–88%, n = 2) analogues [99], DTPA-PEG3-ArN3 showed the lowest RCC values of the antibody conjugates (3.9 ± 1.0%, n = 4), with the precise reasons currently unknown.
Another way to generate DTPA BFCs is via functionalisation of the alkyl backbone. This has the advantage of not interfering with the available donor atoms, allowing for a potential non-coordinating carboxylic acid to be derivatised further. An isothiocyanate (NCS) analogue of DTPA (DTPA-Bn-NCS, Figure 8) was derivatised from the diethylenetriamine backbone. The NCS group reacts with amines under mild conditions to form a thiourea bond.
Jain and co-workers compared the relative stability of various 68Ga-labelled bis-RGD peptide conjugates (utilising the DTPA-Bn-NCS, NOTA-Bn-NCS, and DOTA-Bn-NCS BFCs) and found that the DTPA analogue had by far the worst metabolic stability, as well as requiring high temperature radiolabelling conditions to achieve high RCY (along with the DOTA analogue) [100]. The attachment of DTPA-Bn-NCS to a short-chain fatty acid, undecanoic acid (DTPA undecanoic acid, Figure 8), was developed for imaging cardiac metabolic events [101]. However, the biodistribution of the 68Ga complex revealed lower myocardial uptake (1.3 ± 0.5% ID/g) compared to the TACN derivatives, NOTA undecanoic acid (3.8 ± 0.6% ID/g), and NODAGA undecanoic acid (3.8 ± 0.6% ID/g).
In recent years, DTPA and derivatives have also been used to decorate iron oxide nanoparticles for 68Ga radiolabelling studies, as well as for the molecular imaging of glomeruli using the targeting agent, tilmanocept (using a DTPA-mannosyl-dextran adduct) [102,103]. Taken together, these results seem to suggest that DTPA is not an optimal chelator platform for 68Ga, as it often shows relatively poor RCC results compared to other acyclic as well as macrocyclic chelators. With the success of HBED and promising results of other acyclic chelators (vide infra), DTPA has been supplanted.
7. Siderophore-Based Bifunctional Chelator Development
A promising class of acyclic chelators for 68Ga are the siderophores. Siderophores are most well-known as Fe3+ sequestration agents used by fungi, bacteria, and plants [104,105]. Due to the chemical similarities between Fe3+ and Ga3+ (vide supra), siderophores have been identified as potential 68Ga3+ chelating agents. Desferrioxamine-B (DFO) is a bacterial siderophore that was originally characterised in 1958 as a metabolite of Streptomyces pilosus [106]. DFO is a linear trihydroxamic acid that chelates Fe3+ to form ferrioxamine B ([Fe(DFO)]+ or FOB) (Figure 9). DFO was one of the first reported bifunctional siderophores to be radiolabelled with radioisotopes of Ga in high RCY [107]. The DFO-human serum albumin (HSA) conjugate was radiolabelled with 67Ga at pH 7–8, achieving a RCY of 99.8 ± 0.3%. Despite the fact that DFO conjugates have been seen to leach radioisotopes of Ga in vivo [108,109,110,111], several bifunctional chelating agents have been synthesised over the past decade based on the DFO scaffold, as well as other siderophore-based ligands that are structurally distinct from DFO [112].
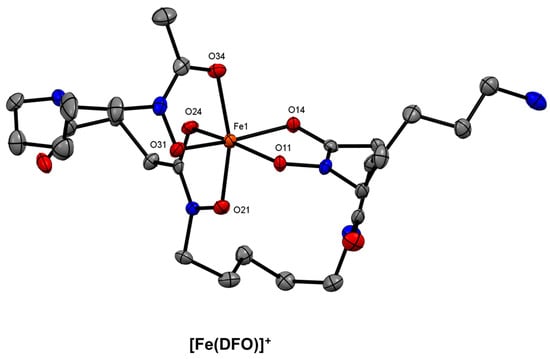
Figure 9.
ORTEP representation of the [Fe(DFO)]+ cation (CSD-OFUYET) [113]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms, counterions, and solvent molecules are omitted for clarity.
Gourni and co-workers reported succinic acid (DFO-Nsucc, Figure 10) and isothiocyanate (DFO-pNCS-Bn-NCS, Figure 10) derivatives of DFO and subsequent conjugation to PSMA for 68Ga preclinical imaging of prostate cancer in mice bearing subcutaneous LNCaP tumours [114]. Compared to HBED-CC-PSMA, however, both conjugates showed decreased tumour uptake. Ioppolo and co-workers produced a library of alkyl-substituted DFO carbamates (-Me, -Et, -nPr, -iPr, -nBu, -iBu, -nhexyl, -boc, -Bn, (CH2)6NHboc and (CH2)6NH2, Figure 10) to tune the uptake of the 67Ga-labelled complexes in bacteria (Staphylococcus aureus) and sites of bacterial infection [115].
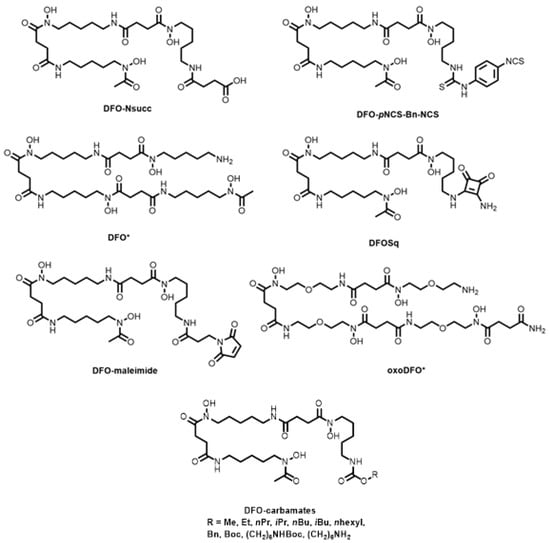
Figure 10.
Bifunctional chelators and conjugates based on desferrioxamine-B.
Octadentate derivatives of DFO (namely DFO *, Figure 10) were synthesised by incorporating a fourth hydroxamic acid and O atoms into the backbone structure for increased water solubility [116,117,118]. The resulting chelator, oxoDFO * (Figure 10), was more hydrophilic than DFO * and DFO, and was successfully radiolabelled with 68Ga at pH 4.5 and 95 °C, achieving >99% RCY.
The versatility of the DFO-squarate platform (DFOSq, Figure 10), where primary amine-containing compounds can be tethered together via the squaric acid group, was demonstrated with 68Ga radiolabelling and in vivo studies of octreotate, octreotide, and PSMA conjugates [119,120]. Similar derivatives of the free primary amine, including isothiocyanates and maleimides (DFO-maleimide, Figure 10), have been reported for the 68Ga PET imaging of tumour-induced angiogenesis and 66Ga radiolabelling of EGFR, respectively [121,122].
Bauman and co-workers and in a subsequent publication, Kaeppeli and co-workers, reported on DFO-Exendin 4 conjugates (using the bifunctional chelator, DFO-pNCS-Bn-NCS, Figure 10) for the radiolabelling of insulinomas and compared the radiolabelling as well as in vitro and in vivo stability to NODAGA and DTPA analogues [123,124]. Both studies demonstrated comparable tumour uptake with DFO outperforming NODAGA in terms of target-to-kidney ratio. DFO-pNCS-Bn-NCS was also used to radiolabel a short-chain variable fragment (scFv) targeting the human epidermal growth factor 2 (HER2) receptor [125]. The radiolabelled DFO-scFv conjugate showed high accumulation in HER2-positive xenograft-bearing mice and was used to monitor changes in HER2 expression following anti-HER2 therapy.
Siderophores not based on the DFO scaffold have also seen developments over the last decade (Figure 11). Pandey and co-workers reported the conjugation of the desferrichrome chelator to the fluoroquinolone, ciprofloxacin, for 68Ga monitoring of a potential therapeutic for bacterial infection [105]. The catecholamide, enterobactin, is produced by the Enterobacteriaceae family of bacteria and has been shown to have one of the highest pFe values (35.5) [126] for all Fe3+ complexes (where the higher the pFe value, the more stable the complex), where pFe is defined as:
pFe = −log[Fe]free when [enterobactin]total = 10−5 M and [Fe]total = 10−6 M
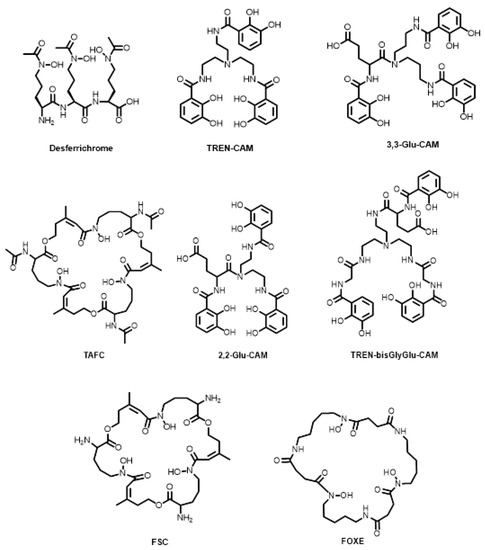
Figure 11.
Bifunctional chelators and conjugates based on non-DFO siderophores.
Joaqui-Joaqui and co-workers reported a class of enterobactin-based compounds, namely the catecholamides TREN-CAM, 2,2-Glu-CAM, 3,3-Glu-CAM, and TREN-bisGlyGly-CAM (Figure 11). The 68Ga complexes of TREN-CAM, 2,2-Glu-CAM, 3,3-Glu-CAM and TREN-bisGlyGly-CAM performed similarly to other acyclic ligands, including DFO, in terms of in vitro (human serum) and in vivo stability and in vivo renal clearance [108]. Petrik and co-workers reported the 68Ga radiolabelling of the siderophores, triacetylfusarinine C (TAFC, Figure 11) and ferrioxamine E (FOXE, Figure 11) for PET imaging of invasive pulmonary aspergillosis caused by the bacterium Aspergillus fumigatus [127]. TAFC was radiolabelled at room temperature for 15 min whereas FOXE required elevated temperature (80 °C) for 20 min for quantitative (>95% RCC) radiolabelling. Both hexadentate complexes were investigated in an in vitro model of A. fumigatus and showed rapid uptake in iron-deficient cultures. Follow-up studies were performed in vivo, showing vastly different organ uptake depending on the siderophore used, including DFO and fusarinine C (FSC, Figure 11) [128,129]. FSC is the deacetylated form of TAFC containing three primary amines that are separated from the hexadentate coordination sphere. Knetsch and co-workers followed by Zhai and co-workers demonstrated favourable 68Ga radiolabelling of FSC (>90% RCY after 5 min at room temperature) and conjugation to three RGD moieties through amide bond formation.
Given the similarity in coordination chemistry between Fe3+ and Ga3+, siderophores are a natural choice of chelator, and subsequent bifunctional chelator, for 68Ga radiopharmaceutical development. The resulting complexes are thermodynamically stable (e.g., logK1 ([Ga(DFO)] = 28.65) [130] but suffer from a lack of kinetic inertness, resulting in radiation leaching in vivo [108]. With the development of higher-denticity DFO ligands on the rise [116,117,118,131,132], these systems are seemingly better suited to other metallic radioisotopes, such as 89Zr4+. Exploring non-DFO siderophores may lead to better candidates for 68Ga radiolabelling, with the underexplored catechol- and catecholamide-based ligands a promising alternative.
8. Pyridinecarboxylate-Based Bifunctional Chelator Development
In 2010, Boros and Orvig reported the synthesis, 67/68Ga radiolabelling, bioconjugation, stability, and biodistribution of an acyclic pyridinecarboxylate (“pa”) ligand, H2dedpa, based on earlier synthetic work by Platas-Iglesias and co-workers [133,134]. The X-ray crystal structure of the monocationic complex shows an N2O4, pseudo-octahedral coordination environment (Figure 12). The resulting 68Ga3+ complex (log KML = 28.11(8)) demonstrated quantitative radiolabelling at ligand concentrations as low as 10−7 M and has inspired the design and synthesis of several derivatives (Figure 13). Follow-up studies demonstrated the versatility of the bifunctional platform via conjugation to RGD peptides, synthesis of lipophilic analogues for myocardial imaging, as well as translation to copper-64 (64Cu) radiolabelling and subsequent in vitro and in vivo studies [135,136,137].
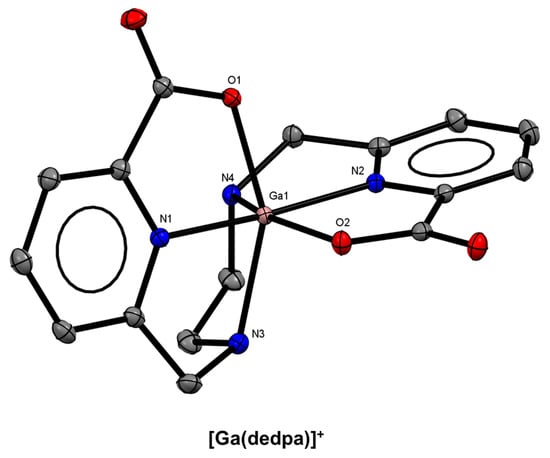
Figure 12.
ORTEP representation of the [Ga(dedpa)]+ cation (CSD-OSUDOW) [133]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms, counterions, and solvent molecules are omitted for clarity.
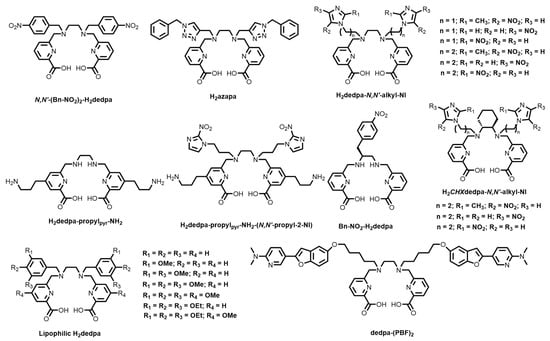
Figure 13.
Bifunctional chelators based on the pyridinecarboxylate family of chelators.
Bailey and co-workers reported a triazole dedpa derivative, H2azapa (Figure 13), that quantitatively radiolabelled 67Ga and 64Cu, as well as the large radiometal ions indium-111 (111In) and lutetium-177 (177Lu) [138]. Bis(propylamine) derivatives were also prepared (H2dedpa-propylpyr-NH2 and H2dedpa-propylpyr-NH2-(N,N’-propyl-2-NI, Figure 13) that show promise for bifunctionality via the reactive primary amine [139]. Bifunctional bi-modal fluorescent/nuclear imaging H2dedpa-fluorescein conjugates have also been reported, but showed that the bulky fluorescein moieties prevented effective 67Ga radiolabelling [140]. However, a recent report has shown that increasing the distance between the chelating unit (in this case, DOTA) and the fluorophore improves radiolabelling capabilities [89]. The analogous dedpa study has yet to be reported.
Ramogida and co-workers investigated the effect of rigidifying the ethylenediamine backbone of H2dedpa by incorporating a chiral trans-cyclohexane ring. The resulting chelator, H2CHXdedpa (Figure 13) [42] was shown to form a more stable 67Ga3+ complex in human serum, and showed similar thermodynamic stability (log KML = 27.61(8)) and solid-state structure to H2dedpa (Figure 14). Nitroimidazole (NI) derivatives of both H2dedpa (H2dedpa-N,N′-alkyl-NI, Figure 13) and H2CHXdedpa (H2CHXdedpa-N,N′-alkyl-NI, Figure 13) were investigated for PET imaging of hypoxia using both 68Ga and 64Cu [139,140,141] Three H2dedpa-NI derivatives and one H2CHXdedpa-NI derivative were initially investigated in three in vitro cancerous cell line models (HT-29 (colon), LCC6HER−2 (breast), and CHO (ovary)) and all were found to have preferential uptake into hypoxic (0.5% O2) cells compared to normoxic (21% O2) cells, with hypoxic/normoxic ratios as high as 7.9 ± 2.7 after 2 h. Lipophilic analogues of H2dedpa containing a range of methoxy-substituted aromatic substituents (Figure 13) have also been reported with a view to develop heart imaging agents [142].
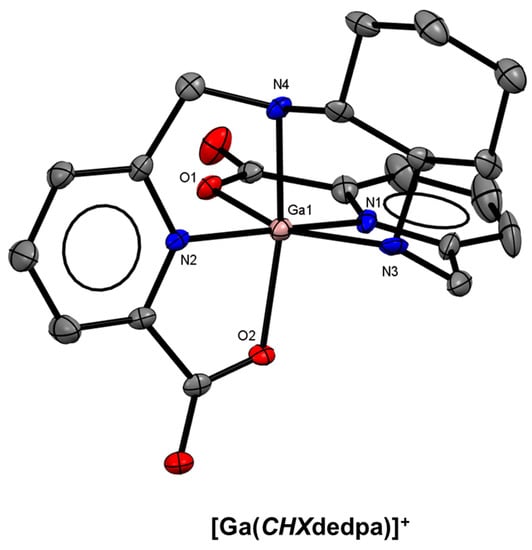
Figure 14.
ORTEP representation of the [Ga(CHXdedpa)]+ cation (CSD-POXQOK) [42]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms, counterions, and solvent molecules are omitted for clarity.
Saito and co-workers developed bifunctional H2dedpa derivatives bearing pyridylbenzofuran groups (dedpa-(PBF)2, Figure 13) for targeting islet amyloid deposition in pancreas islets, a key marker for Type 2 diabetes mellitus [143]. The authors were able to radiolabel the BFC with 67/68Ga but noted that improvement in non-target organ clearance was needed. Recently, Wang and co-workers reported 8-hydroxyquinoline (“hox”) analogues of both H2dedpa and H2CHXdedpa and investigated the preferential heart uptake of the Ga3+ complexes compared to the ‘pa’ family analogues [144,145]. Additionally, the inherent fluorescence of the ‘hox’ group shows promise for potential multi-modal PET/SPECT-fluorescence imaging in future.
Given the early promising results of the pyridinecarboxylate chelators, it is perhaps somewhat surprising that there have not been more pre-clinical and clinical studies reported. Moreover, the excellent kinetic inertness of the rigidified Ga3+ complexes make dedpa and its derivatives excellent choices for radiopharmaceutical development. Consequently, further exploration of their potential is warranted.
9. Hydroxypyridinone-Based Bifunctional Chelator Development
Hydroxypyridinones are a class of aromatic heterocycles that have been extensively studied for iron overload disease. They contain hydroxyl and ketone functional groups as donor atoms resulting in bidentate chelators [44,146,147]. Depending on the substitution pattern of the aromatic ring, three isomeric forms can exist: 1,2-hydroxypyridinones, 2,3-hydroxypyridinones, and 3,4-hydroxypyridinones. Binding to metal ions occurs with deprotonation of the acidic hydroxyl. The relative stability of the three Ga3+ complexes is known to follow the order 3,4-hydroxypyridinone > 2,3-hydroxypyridinone > 1,2-hydroxypyridinone, which reflects the charge density on the O atoms caused by the relative delocalisation of aromaticity around the ring [91,148,149,150]. 3,4-Hydroxypyridinones, such as deferiprone, form isostructural octahedral complexes with Fe3+ and Ga3+ (Figure 15).
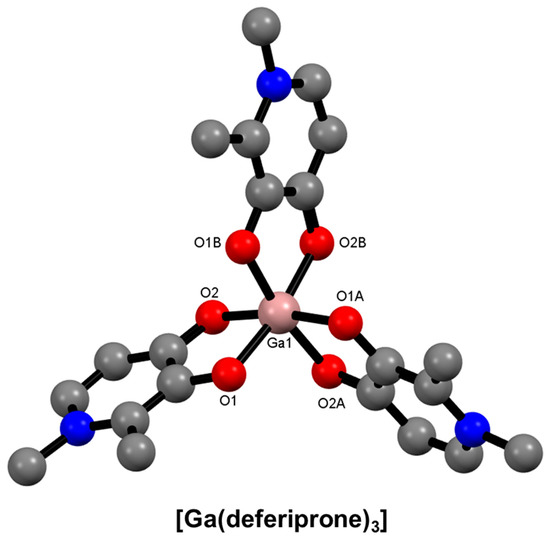
Figure 15.
Representation of the molecular structure of [Ga(deferiprone)3] (ORTEP not reported) (CSD-GAVZIM) [151]. Hydrogen atoms and solvent molecules are omitted for clarity.
Hexadentate tris(hydroxypyridinones) (THPs) have been of interest for Ga3+ complexation due to the 1:1 complexation stoichiometry compared to the 3:1 that would eventuate from tris(3,4-hydroxypyridinones). The first report of THP was in 2011 by Berry and Blower, where a radiolabelling study showed that THP could achieve higher RCY values than HBED, DOTA, and NOTA at pH 6.5 after 5 min at room temperature [146]. THPs have been synthesised from either tripodal polyamines or tripodal carboxylic acids, and dendritic constructs have also been reported [152].
Over the past decade, several bifunctional THP derivatives have been reported (Figure 16) with the accompanying 68Ga3+ radiolabelling studies. Ma and co-workers reported the kit-based synthesis of [68Ga][Ga(THP-Tyr3-octreotate)] ([68Ga][Ga(THP-TATE)]) using an isothiocyanate derivative of THP (THP-NCS, Figure 16), and compared it against the FDA-approved [68Ga][Ga(DOTATATE)] in terms of somatostatin-2 receptor (SSTR2)-positive cell uptake [153]. THP-TATE was able to be radiolabelled within 2 min at >95% RCY (pH 5–6.5) and molar activity of 60–80 MBq/nmol, and proved comparable in vitro SSTR2-positive A427-7 cell uptake compared to [68Ga][Ga(DOTATATE)]. The biodistribution of [68Ga][Ga(THP-TATE)] in Balb/c nu/nu mice bearing SSTR2-positive AR42J tumours showed a clear delineation of the tumour as well as radiation uptake in the kidneys.
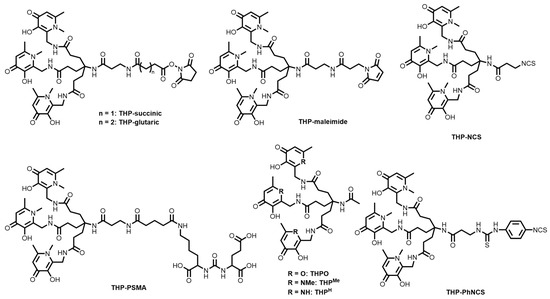
Figure 16.
Bifunctional chelators and conjugates based on the hydroxypyridinone/THP family of chelators.
The conjugation of THP-NCS (Figure 16) and THP-PhNCS (Figure 16) to RGD peptides showed that both THP-peptide conjugates could be radiolabelled with 68Ga in under 5 min at >95% RCY at the same specific activity (60–80 MBq/nmol) as [68Ga][Ga(THP-TATE)] [154]. The radiolabelled conjugates showed uptake in αvβ3 integrin-positive glioblastoma U87MG tumours in Balb/c mice, and cleared from circulation within 2 h. However, both [68Ga][Ga(THP-TATE)] and the RGD conjugates have demonstrated lower tumour/non-target organ ratios compared to DOTA-based systems [154,155].
Imberti and co-workers explored dendritic variants of THP and synthesised three phenyl-isothiocyanate constructs conjugated to the RGD peptide (HP9-RGD3, HP3-RGD and HP3-RGD3) [152]. The HP9-RGD3 bioconjugate, containing three hexadentate metal binding sites, was shown to radiolabel at three times the specific activity value as the other two (180–240 MBq/nmol). However, it was demonstrated to have large uptake in non-target organs that compared unfavourably to the HP3-RGD3 construct. Although each of the chelators could quantitatively radiolabel 68Ga to 97% RCY, studies indicated that the distance between the RGD units was not large enough to sufficiently bind more than one integrin receptor.
Efforts have also been made towards synthesising THP bioconjugates for the PET imaging of prostate cancer. Nawaz and co-workers reported the conjugation of THP-maleimide (Figure 16) to a C-terminal cysteine residue of the scFv of the monoclonal antibody J591 that specifically binds to an external epitope of PSMA [156]. Radiolabelling of the THP-scFv conjugate proceeded at room temperature and neutral pH in a one-step synthesis, and the resulting radiotracer showed high affinity for PSMA in vitro and demonstrated uptake in a xenograft model (DU145-PSMA) of prostate cancer in mice. Blower and co-workers had arguably more success with their THP-PSMA conjugates, which can be radiolabelled in a kit-based, one-step synthesis at room temperature and near-neutral pH [29,157,158,159]. Phase I clinical trials have been reported and, in 2020, a 118-patient study was reported demonstrating the effectiveness of [68Ga][Ga(THP-PSMA)] in influencing clinical management of prostate cancer before therapy by identifying metastases in bone [159].
Despite the promising results with [68Ga][Ga(THP-PSMA)], the unfavourable comparisons of [68Ga][Ga(THP-TATE)] and the RGD conjugates with DOTA have motivated further tuning of the THP ligand design. Imberti and co-workers and Floresta and co-workers developed novel bifunctional THP derivatives incorporating changes to the hydroxypyridinone groups (THPMe, THPH, THPO, Figure 16) to tune the hydrogen-bonding and lipophilicity of the resulting ligands, as well as bifunctional pendant groups (THP-NHS, THP-succinic, THP-glutaric, THPMe-NCS, Figure 16) [147,155,160]. Preliminary radiolabelling studies have been reported, and work is ongoing to form radiolabelled bioconjugates with these promising systems.
The favourable radiolabelling properties of the THP chelators (near neutral pH and room temperature) are ideal for large biomolecules, such as proteins and antibodies [37]. Work is ongoing to translate THP and various bioconjugates to simple, rapid kit-based systems for clinical use, with the most promising to date being [68Ga][Ga(THP-PSMA)].
10. Diazepine-Based Bifunctional Chelator Development
The family of chelators based on the 6-amino-6-methyl-perhydro-1,4-diazepine (diazepine) scaffold has attracted much attention over the last two decades for 68Ga radiopharmaceutical development. This has been primarily spurred on by studies investigating the coordination chemistry of the parent and derivatised ligands between 2004 and 2009 (including transition metals and Gd3+ for MRI contrast agents), which highlighted the similarities between the resultant complexes and those of TACN, a constitutional isomer of diazepine [161,162,163,164,165]. The ligands form hexadentate Ga3+ complexes, generally in a facial (fac) arrangement of donor atoms with the N3 plane remaining consistent among the various complexes (Figure 17).
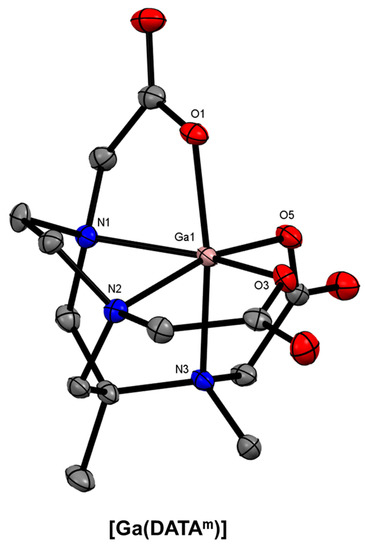
Figure 17.
ORTEP representation of [Ga(DATAm)] (CSD-XENPAJ) [166]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms and solvent molecules are omitted for clarity.
Waldron and Parker and co-workers reported three studies on the development of AAZTA (Figure 18) and various derivatives in 2013, showing hexadentate coordination of Ga3+, high kinetic inertness, and quantitative 68Ga radiolabelling at pH 4–7 [166,167,168,169]. Seeman and co-workers introduced the ‘DATA’ chelators, with the best performing, DATAm (Figure 18), demonstrating 97% RCY in under 1 min at room temperature (23 °C) at a chelator concentration of 3.6 μM [43]. Farkas and co-workers then showed that the structurally constrained, bicyclic PIDAZTA (Figure 18) chelators coordinate 68Ga3+ at pH 7.5 (RCC > 89%) at concentrations as low as 10 μM [170].
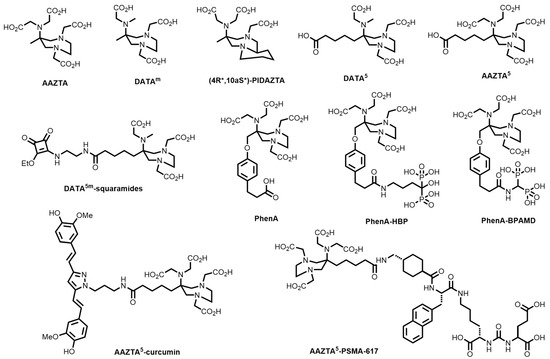
Figure 18.
Bifunctional chelators and conjugates based on the diazepine family of chelators.
Several groups have developed bifunctional variants of the diazepine chelators with various applications over the past decade. Wu and co-workers reported the synthesis of bisphosphonate AAZTA chelators (PhenA, PhenA-BPAMD, and PhenA-HBP, Figure 18) for the 68Ga PET imaging of bone [171]. The introduction of the bifunctional phenylcarboxylate pendant arm was enabled through tosylation of a methanol-diazepine derivative, originally reported in 2009 by Gugliotta and co-workers [172].
A pentanoic acid version of AAZTA, AAZTA5 (Figure 18) [173], was synthesised and conjugated to the cyclic peptide, Phe1-Tyr3-octreotide (TOC), via amide bond formation, and achieved quantitative radiolabelling (>98% RCC) at room temperature in less than 5 min at pH 4–5.5 at a ligand concentration of 10 nmol [174]. Hofstetter and co-workers tethered a Gastrin Releasing Peptide receptor (GRPr) antagonist, D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2, to AAZTA5 via a 4-amino-1-carboxylmethyl-piperidine (Pip) spacer, for diagnostic imaging of GRPr-positive cancers (tested in an epithelial human prostate cancer cell line, PC3) [175]. Squaramide coupling has been utilised with both AAZTA5 and DATAm (Figure 18) to conjugate to the fibroblast activation protein (FAP) inhibitor, UAMC-1110, and PSMA inhibitors [61,176,177,178]. DATA5m-squaramide (Figure 18) was tethered to the small molecule, UAMC-1110, and showed >97% RCY after 10 min and excellent stability in EtOH, HSA and saline over 2 h. An AAZTA5-squaramide was tethered to a Glu-urea-Lys motif for PSMA-targeting and radiolabelled successfully after 10 min at room temperature at pH 4–5.5.
AAZTA-curcumin conjugates (Figure 18) were reported by Orteca and co-workers for potential 68Ga PET imaging of colorectal cancer. The tetra-tert-butyl ester protected AAZTA was pre-activated with the coupling agent, HBTU, and reacted directly with curcumin to form the BFC [179]. Compared to the TACN derivative, NODAGA-curcumin, the AAZTA-curcumin conjugate showed greater stability in human blood over 2 h (with comparable stability in plasma and serum).
Yadav and co-workers were interested in comparing the biodistribution and gastroenteropancreatic neuroendocrine tumour (GEP-NET) uptake of [68Ga][Ga(DATA-TOC)], originally developed by Waldron and Parker, with [68Ga][Ga(DOTA-NOC)] (NOC = NaI3-octreotide), which required harsh (95 °C) radiolabelling conditions for quantitative coordination of 68Ga [180]. The authors showed that [68Ga][Ga(DATA-TOC)] demonstrated comparable PET/CT imaging of GEP-NET lesions (98.6% agreement with [68Ga][Ga(DOTA-NOC)], n = 235). This is promising, as DATA chelators can be more easily synthesised in a kit-based synthesis than DOTA-based systems [181,182].
The excellent radiolabelling conditions (very low ligand concentrations, neutral pH, and room temperature) make the diazepine family of chelators, particularly DATAm, a promising candidate for 68Ga radiopharmaceutical development. This facilitates preparation of instant kit-type preparations, which are crucial for successful clinical translation.
11. TACN-Based Bifunctional Chelator Development
Interest in TACN-based chelators has been spurred on by the particularly attractive coordination properties of triazamacrocyclic chelators with tricarboxylic acid (NOTA), triphosphonic acid (NOTP) and triphosphinic acid (TRAP) pendant groups for Ga3+. They are known to be highly rigid (due to the preformed geometry offered by the macrocycle), kinetically inert, and thermodynamically stable due in part to the macrocyclic effect (stability constants logK1 > 26), which encompasses entropic gain from a pre-organised structure around the metal ions (Table 3) [52,183,184].

Table 3.
Proton and Ga3+ affinity constants of example Ga3+ chelators. Adapted from Ref. [38] with permission from the Royal Society of Chemistry [38].
In the case of [Ga(NOTA)], the X-ray crystal structure of the complex indicates that the distorted octahedral coordination environment contains three deprotonated carboxyl groups [189]. [Ga(TRAP)] exhibits similar coordination environments to [Ga(NOTA)], with deprotonated phosphinic acids contributing the O3 donor atoms. The radiolabelling properties of these chelators with 68Ga3+ have also been investigated. The triphosphinic acid, TRAP, was shown to radiolabel with 68Ga3+ to >95% RCC at a reduced ligand concentration (3 μM) compared to DOTA (500 μM) and NOTA (100 μM) at 25 °C and pH 0.5–5 [38,184]. Phosphonate-containing ligands such as NOTP have been shown to chelate 68Ga3+ at room temperature (25 °C) and neutral pH (6.5) at higher RCYs than DOTA, NOTA, and TRAP chelators at chelator concentrations of 0.5 μM (Figure 19) [38]. The X-ray crystal structure of [Ga(NOTP)] shows a hexadentate N3O3 coordination environment that is closer to ideal octahedral than [Ga(NOTA)] [190].
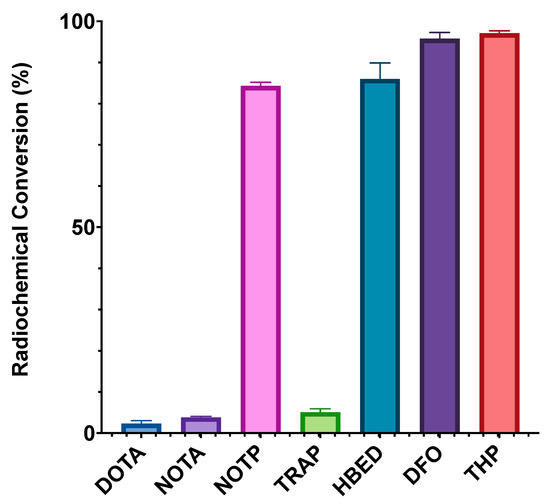
Figure 19.
A comparison of the 68Ga radiolabelling efficiencies of THP, DFO, HBED, TRAP, NOTP, NOTA, and DOTA at pH 6.5, 25 °C after 10 min and a chelator concentration of 0.5 μM. Adapted from Ref. [38] with permission from the Royal Society of Chemistry [38].
The simplest method of forming bifunctional variants of NOTA is through derivatisation of one carboxylic acid pendant arm (Figure 20). Coupling to an amine is achieved either through agents, such as HBTU or HATU or activated (sulfo)esters [191,192]. Less commonly used methods include the use of click compounds or thiol coupling [53,193,194]. However, these methods have a marked disadvantage, which is that the resulting amide is a worse coordinating group for Ga3+ than the carboxylic acid due to the lower thermodynamic stability of the resulting complexes [195,196].
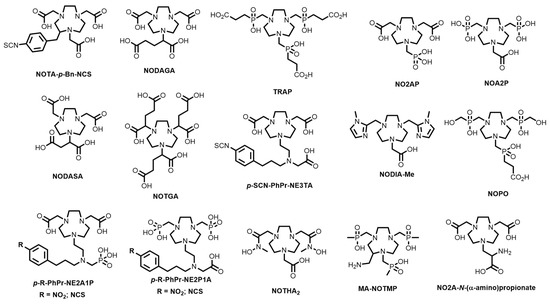
Figure 20.
Bifunctional chelators based on TACN.
Several well-known NOTA-based BFCs have been developed where an additional reactive functional group has been provided for conjugation reactions but does not interfere with the coordination environment. NODAGA (Figure 20), a glutaric acid analogue of NOTA, which was first reported in 2002 and has seen extensive conjugation and radiolabelling optimisation [55,56,62,124,197,198,199,200,201,202]. The succinic acid derivative, NODASA (Figure 20), was reported in 1998, but has seen less application than NODAGA, presumably due to their structural similarities and comparable radiolabelling efficiencies. The X-ray crystal structure of [Ga(NODASA)] shows a hexadentate N3O3 coordination environment that is typical of Ga3+ complexes of TACN and derivatives (Figure 21).
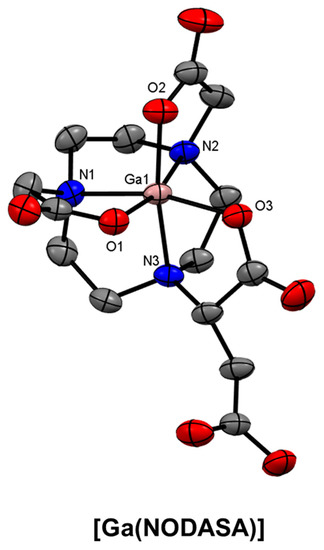
Figure 21.
ORTEP representation of [Ga(NODASA)] (CSD-NUHLOR) [203]. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms and solvent molecules are omitted for clarity.
NOTA-p-Bn-NCS (Figure 20) is one of the most widely-studied BFCs for 68Ga, with a plethora of studies reporting its conjugation to targeting vector biomolecules, such as small molecules, peptides, and antibodies [100,204,205,206,207,208,209]. Massa and co-workers reported a triglycine derivative of NOTA-p-Bn-NCS, GGGYK-NOTA, for use in enzyme-mediated site-specific labelling of camelid single-domain antibody fragments with 68Ga [210]. Bone-targeting bisphosphonate-NOTA conjugates have been reported by Holub and co-workers and Passah and co-workers, demonstrating improved bone uptake compared to commercially available bone imaging agents [211,212]. Recently, the imidazole-based BFC, NODIA-Me (Figure 20), was reported and tethered to a PSMA targeting vector [213,214,215,216]. The resulting 68Ga complex and conjugate was kinetically inert to transmetallation in vivo, which underlined its potential use as a radiopharmaceutical agent.
Bifunctional variants of the phosphinate-containing TACN chelators, TRAP and NOPO (Figure 20), have also been synthesised and extensively evaluated [54,187,217,218,219,220,221,222,223,224]. which has been motivated by the ease by which the phosphinate pendant arms can be synthetically modified without affecting the Ga3+ coordination sphere O donor. Additionally, the distal carboxylic acids can be modified using uronium coupling agents (e.g., HATU) without protecting the phosphinic acids [189]. Dendritic TRAP ligands, containing four metal binding sites, have been prepared by tethering the macrocycles together via CuAAC click chemistry (through modification of the carboxylic acids with azides) [223].
TRAP was tethered to an α5β1 integrin-targeted trimeric pseudopeptide via modification of the carboxylic acids with three azides and subsequent CuAAC [218]. The TRAP-peptide conjugate, 68Ga-aquibeprin, showed high selectivity for integrin α5β1 (IC50 = 0.09 nM) over integrin αvβ3 (IC50 = 620 nM), and the 68Ga radiolabelled bioconjugate demonstrated a good tumour-to-blood ratio (10.6 ± 2.5, 90 min p.i.) in an ex-vivo biodistribution mice model xenografted with M21 human melanoma.
NOPO was evaluated as an αvβ3 integrin-targeting BFC via conjugation to the cyclic pentapeptide, c(RGDfK) [219]. The Ga3+ complex showed high affinity to αvβ3 integrin (IC50 = 1.02 nM) and showed a tumour-to-blood ratio of 19.6 ± 6.8 at 60 min p.i. in the same M21 mice model. A monomeric TRAP-azide construct has also been radiolabelled and tethered to an αvβ8 integrin targeting cyclic peptide [225].
TRAP-(azide)3 was tethered to Glu-urea-Lys for PSMA-targeting using CuAAC [224]. The trimeric conjugate displayed high PSMA affinity (IC50 up to 1.5 nM). Interestingly, lowering the molar activity of the radiolabelled conjugate from 1200 MBq/nmol to 8 MBq/nmol resulted in better kidney-to-tumour ratios (11.4 compared to 1.4, respectively) in subcutaneous murine PSMA-positive human prostate carcinoma xenografts. The trimethylphosphinate, MA-NOTMP (Figure 20), contains a reactive primary amine and has been used to tether the octapeptide, bombesin(7-14), which shows high affinity for three G protein-coupled receptors (overexpressed on a wide range of cancer types) [221]. The chelator was radiolabelled at RCC > 90% at pH 1.5–3 at 95 °C within 5 min but could not be radiolabelled >pH 4.
The presence of phosphonate pendant groups increases the thermodynamic stability and rigidity of the Ga3+ TACN chelates. For example, [Ga(NOA2P)] (Figure 20) has a high thermodynamic stability constant (logKML = 34.44) [226] compared to logKML = 29.63 for [Ga(NOTA)], and greater stability against apo-transferrin than [Ga(DOTA)] and [Ga(NOTA)] in competition experiments. Unlike the phosphinate-containing chelators, however, minimal bifunctional TACN chelators have been reported containing phosphonates [38]. This may be due to perceived difficulties with designing and synthesising BFCs. To date, the only BFCs that have been derivatives of NO2AP and NOA2P (Figure 20) were achieved by Gai and co-workers, who reported synthesis of bisphosphonate and monophosphonate-containing TACN BFCs (p-R-PhPr-NE2A1P and p-R-PhPr-NE2P1A, where R = NO2 or NCS, Figure 20) [183,226,227,228]. The development of novel bifunctional variants of phosphonate-containing TACN ligands is timely because, despite the excellent radiolabelling conditions displayed by [68Ga][Ga(NOTP)], [68Ga][Ga(NOA2P)] and [68Ga][Ga(NO2AP)], bifunctional analogues are sorely lacking [38].
12. Conclusions
Proper chelator design and an understanding of Ga3+ aqueous coordination chemistry are essential for the successful development of 68Ga radiopharmaceuticals. The key components of this development process include the use of well-designed chelators, capable of forming thermodynamically stable and kinetically inert 68Ga3+ complexes using mild conditions (near-neutral pH, room temperature and low concentrations of ligand) in a short timeframe (<10 min), which are tethered to vector biomolecules, such as peptides and antibodies.
The most promising 68Ga radiotracers are those that can be streamlined to pre-clinical and clinical applications using robust, reliable, and cost-efficient radiopharmaceutical kits. These include derivatives of THP, DATA, and TRAP, which have been applied to clinically relevant targeting vectors, such as RGD, octreotate, NaI3-octreotide, and PSMA-targeting motifs. Newer generation BFCs, such as PIDAZTA, non-DFO siderophores, and TACN phosphonates, fulfil the requirements for effective nat/67/68Ga3+ complexation (N and O donor atoms, six-coordinate complexes, mild radiolabelling conditions), yet remain underexplored in terms of bifunctionality. Further developments in this area will continue to enable the effective and simple incorporation of 68Ga into new radiopharmaceuticals.
Finally, the choice of chelator has been shown to play a role in determining the biodistribution of small molecule and peptide-based radiopharmaceuticals. Therefore, assessing multiple BFCs to tune optimal biodistribution should be considered when utilising new targeting vectors.
Author Contributions
Conceptualization, P.R.W.J.D. and B.M.P.; writing—original draft preparation, P.R.W.J.D.; writing—review and editing, P.R.W.J.D. and B.M.P.; visualization, P.R.W.J.D.; supervision, B.M.P.; project administration, P.R.W.J.D.; funding acquisition, B.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Research Council (grant number DE1701005400), the National Imaging Facility, a Research Training Program Stipend (P.R.W.J.D.), and the Faculty of Science, Monash University (Monash Graduate Excellence Scholarship) (P.R.W.J.D.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Cormac A. A. Kelderman (School of Chemistry, Monash University) is kindly thanked for proof-reading the manuscript. Crystallographic data were accessed via the Cambridge Crystallographic Database.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Bartholomä, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Pathuri, G.; Hedrick, A.F.; January, S.E.; Galbraith, W.K.; Awasthi, V.; Arnold, C.D.; Cowley, B.D.; Gali, H. Synthesis and in vivo evaluation of gallium-68-labeled glycine and hippurate conjugates for positron emission tomography renography. J. Label. Compd. Radiopharm. 2015, 58, 14–19. [Google Scholar] [CrossRef]
- Smith, D.L.; Breeman, W.A.P.; Sims-Mourtada, J. The untapped potential of Gallium 68-PET: The next wave of ⁶⁸Ga-agents. Appl. Radiat. Isot. 2013, 76, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- Turlakow, A.; Yeung, H.W.; Pui, J.; Macapinlac, H.; Liebovitz, E.; Rusch, V.; Goy, A.; Larson, S.M. Fludeoxyglucose Positron Emission Tomography in the Diagnosis of Giant Cell Arteritis. Arch. Intern. Med. 2001, 161, 1003–1007. [Google Scholar] [CrossRef]
- McInnes, L.E.; Rudd, S.E.; Donnelly, P.S. Copper, gallium and zirconium positron emission tomography imaging agents: The importance of metal ion speciation. Coord. Chem. Rev. 2017, 352, 499–516. [Google Scholar] [CrossRef]
- FDA-Approved Radiopharmaceuticals. Cardinal Health, Denver, CO. 2022. Available online: https://www.cardinalhealth.com/content/dam/corp/web/documents/fact-sheet/cardinal-health-fda-approved-radiopharmaceuticals.pdf (accessed on 28 July 2022).
- Rösch, F. Theranostics, Gallium-68, and Other Radionuclides; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–16. [Google Scholar]
- Schnökel, H. Formation, structure and bonding of metalloid Al and Ga clusters. A challenge for chemical efforts in nanosciences. Dalton Trans. 2008, 4344–4362. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Stasch, A. The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fischer, R.C.; Power, P.P. π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef]
- Moerlein, S.M.; Welch, M.J. The Chemistry of Gallium and Indium as Related to Radiopharmaceutical Production. Int. J. Nucl. Med. Biol. 1981, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics (Internet Version 2016); CRC Press LLC/Taylor and Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pearson, R.G. Absolute Electronegativity and Hardness: Application to Inorganic Chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Peters, J.A.; Görls, H.; Platas-Iglesias, C. Structural Study of Ga(III), In(III), and Fe(III) Complexes of Triaza-Macrocycle Based Ligands with N3S3 Donor Set. Inorg. Chem. 2009, 48, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, C.; Levason, W.; Ratnani, R.; Reid, G.; Webster, M. Synthesis, characterisation and structures of thio-, seleno- and telluro-ether complexes of gallium(III). Dalton Trans. 2008, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M.L.; Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules 2021, 26, 3646. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Dolmella, A.; Tisato, F.; Porchia, M.; Refosco, F. Mononuclear six-coordinated Ga(III) complexes: A comprehensive survey. Coord. Chem. Rev. 2009, 253, 56–77. [Google Scholar] [CrossRef]
- Cutler, C.S.; Giron, M.C.; Reichert, D.E.; Snyder, A.Z.; Herrero, P.; Anderson, C.J.; Quarless, D.A.; Koch, S.A.; Welch, M.J. Evaluation of Gallium-68 Tris(2-Mercaptobenzyl)Amine: A Complex with Brain and Myocardial Uptake. Nucl. Med. Biol. 1999, 26, 305–316. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E.; Koch, S.A.; Hwang; Quarless, D.A.; Welch, M.J. The Gallium(III) and Indium(III) Complexes of Tris(2-mercaptobenzyl)amine and Tris(2-hydroxybenzyl)amine. Inorg. Chem. 1998, 37, 5902–5911. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Weiner, R.E.; Schreiber, G.J.; Hoffer, P.B.; Bushberg, J.T. Compounds Which Mediate Gallium-67 Transfer from Lactoferrin to Ferritin. J. Nucl. Med. 1985, 26, 908–916. [Google Scholar]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2013, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef]
- Bois, F.; Noirot, C.; Dietemann, S.; Mainta, I.C.; Zilli, T.; Garibotto, V.; Walter, M.A. [68Ga]Ga-PSMA-11 in prostate cancer: A comprehensive review. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 349–374. [Google Scholar] [PubMed]
- Young, J.D.; Abbate, V.; Imberti, C.; Meszaros, L.K.; Ma, M.T.; Terry, S.Y.; Hider, R.C.; Mullen, G.E.; Blower, P.J. 68Ga-THP-PSMA: A PET Imaging Agent for Prostate Cancer Offering Rapid, Room-Temperature, 1-Step Kit-Based Radiolabeling. J. Nucl. Med. 2017, 58, 1270–1277. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-Based Post-processing of Generator-Derived ⁶⁸Ga Toward Kit-Type Preparation of ⁶⁸Ga-Radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Alban, R.; François, H.; Sandrine, J.-K.; Andrea, S.; Catherine, R.; Marc, F. Feasibility and Evaluation of Automated Methods for Radiolabeling of Radiopharmaceutical Kits with Gallium-68. Curr. Radiopharm. 2019, 12, 229–237. [Google Scholar]
- Hong, H.; Wang, G.; Ploessl, K.; Zha, Z.; Zang, J.; Zhu, Z.; Zhu, L.; Kung, H.F. Kit-based preparation of [68Ga]Ga-P16-093 (PSMA-093) using different commercial 68Ge/68Ga generators. Nucl. Med. Biol. 2022, 106–107, 1–9. [Google Scholar] [CrossRef]
- Satpati, D. Recent Breakthrough in 68Ga-Radiopharmaceuticals Cold Kits for Convenient PET Radiopharmacy. Bioconjug. Chem. 2021, 32, 430–447. [Google Scholar] [CrossRef]
- Mishoe, A.; DeNoble, P. Setting Up a Successful Radiopharmaceutical Production Facility. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 627–633. [Google Scholar]
- Sequeira, S.; Lyashchenko, S.K. The Clinical Translation Process in the United States. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 619–625. [Google Scholar]
- Blower, P.J.; Cusnir, R.; Darwesh, A.; Long, N.J.; Ma, M.T.; Osborne, B.E.; Price, T.W.; Pellico, J.; Reid, G.; Southworth, R.; et al. Advances in Inorganic Chemistry; Hubbard, C.D., van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 78, pp. 1–35. [Google Scholar]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Kubeil, M.; Nsubuga, A.; Singh, G.; Gasser, G.; Stephan, H. Harnessing the Coordination Chemistry of 1,4,7-Triazacyclononane for Biomimicry and Radiopharmaceutical Applications. ChemPlusChem 2018, 83, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Kubíček, V.; Böhmová, Z.; Ševčíková, R.; Vaněk, J.; Lubal, P.; Poláková, Z.; Michalicová, R.; Kotek, J.; Hermann, P. NOTA Complexes with Copper(II) and Divalent Metal Ions: Kinetic and Thermodynamic Studies. Inorg. Chem. 2018, 57, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.L.; Yapp, D.T.T.; Mandel, D.; Gill, R.K.; Boros, E.; Wong, M.Q.; Jurek, P.; Kiefer, G.E. 68 Ga Small Peptide Imaging: Comparison of NOTA and PCTA. Bioconjug. Chem. 2012, 23, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Cawthray, J.F.; Boros, E.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. H2CHXdedpa and H4CHXoctapa—Chiral Acyclic Chelating Ligands for 67/68Ga and 111In Radiopharmaceuticals. Inorg. Chem. 2015, 54, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Seemann, J.; Waldron, B.P.; Roesch, F.; Parker, D. Approaching ‘Kit-Type’ Labelling with 68Ga: The DATA Chelators. ChemMedChem 2015, 10, 1019–1026. [Google Scholar] [CrossRef]
- Cusnir, R.; Imberti, C.; Hider, R.C.; Blower, P.J.; Ma, M.T. Hydroxypyridinone Chelators: From Iron Scavenging to Radiopharmaceuticals for PET Imaging with Gallium-68. Int. J. Mol. Sci. 2017, 18, 116. [Google Scholar] [CrossRef]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, E.; Lukes, I. Gallium(III) Complexes of DOTA and DOTA-Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef]
- Viola, N.A.; Rarig, R.S.; Ouellette, W.; Doyle, R.P. Synthesis, structure and thermal analysis of the gallium complex of 1,4,7,10-tetraazacyclo-dodecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA). Polyhedron 2006, 25, 3457–3462. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Edem, P.E.; Jørgensen, J.T.; Nørregaard, K.; Rossin, R.; Yazdani, A.; Valliant, J.F.; Robillard, M.; Herth, M.M.; Kjaer, A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 2020, 25, 463. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Chang, C.-H.; Rossi, E.A.; McBride, W.J.; Sharkey, R.M. Pretargeted Molecular Imaging and Radioimmunotherapy. Theranostics 2012, 2, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. A Revisit to the Pretargeting Concept—A Target Conversion. Front. Pharmacol. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef]
- Eidherr, H.; Girschele, F.; Mitterhauser, M.; Wadsak, W. Radiochemical Syntheses; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 321–334. [Google Scholar]
- Eichenberger, L.S.; Patra, M.; Holland, J.P. Photoactive chelates for radiolabelling proteins. Chem. Commun. 2019, 55, 2257–2260. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Wester, H.-J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: Practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012, 2, 28–32. [Google Scholar] [CrossRef]
- Vatsa, R.; Shukla, J.; Kumar, S.; Chakraboarty, S.; Dash, A.; Singh, G.; Mittal, B.R. Effect of Macro-Cyclic Bifunctional Chelators DOTA and NODAGA on Radiolabeling and In Vivo Biodistribution of Ga-68 Cyclic RGD Dimer. Cancer Biother. Radiopharm. 2019, 34, 427–435. [Google Scholar] [PubMed]
- Roosenburg, S.; Laverman, P.; Joosten, L.; Cooper, M.S.; Kolenc-Peitl, P.K.; Foster, J.M.; Hudson, C.; Leyton, J.; Burnet, J.; Oyen, W.J.G.; et al. PET and SPECT Imaging of a Radiolabeled Minigastrin Analogue Conjugated with DOTA, NOTA, and NODAGA and Labeled with 64Cu, 68Ga, and 111In. Mol. Pharm. 2014, 11, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Ghai, A.; Singh, B.; Hazari, P.P.; Schultz, M.K.; Parmar, A.; Kumar, P.; Sharma, S.; Dhawan, D.; Mishra, A.K. Radiolabeling optimization and characterization of 68Ga labeled DOTA–polyamido-amine dendrimer conjugate—Animal biodistribution and PET imaging results. Appl. Radiat. Isot. 2015, 105, 40–46. [Google Scholar] [CrossRef]
- Bhadwal, M.; Das, T.; Sarma, H.D.; Banerjee, S. Radiosynthesis and Bioevaluation of [68Ga]-Labeled 5,10,15,20-Tetra(4-methylpyridyl)-porphyrin for Possible Application as a PET Radiotracer for Tumor Imaging. Mol. Imaging Biol. 2015, 17, 111–118. [Google Scholar] [CrossRef]
- Guleria, M.; Das, T.; Amirdhanayagam, J.; Sarma, H.D.; Dash, A. Comparative Evaluation of Using NOTA and DOTA Derivatives as Bifunctional Chelating Agents in the Preparation of 68Ga-Labeled Porphyrin: Impact on Pharmacokinetics and Tumor Uptake in a Mouse Model. Cancer Biother. Radiopharm. 2018, 33, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sicco, E.; Báez, J.; Margenat, J.; García, F.; Ibarra, M.; Cabral, P.; Moreno, M.; Cerecetto, H.; Calzada, V. Derivatizations of Sgc8-c aptamer to prepare metallic radiopharmaceuticals as imaging diagnostic agents: Syntheses, isolations, and physicochemical characterizations. Chem. Biol. Drug Des. 2018, 91, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; de Lombaerde, S.; Vangestel, C.; de Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, R.; Mallia, M.B.; Sarma, H.D. 68Ga-labeled PET tracers for targeting tumor hypoxia: Role of bifunctional chelators on pharmacokinetics. Nucl. Med. Biol. 2021, 96–97, 61–67. [Google Scholar] [CrossRef]
- Peukert, C.; Langer, L.N.B.; Wegener, S.M.; Tutov, A.; Bankstahl, J.P.; Karge, B.; Bengel, F.M.; Ross, T.L.; Brönstrup, M. Optimization of Artificial Siderophores as 68Ga-Complexed PET Tracers for In Vivo Imaging of Bacterial Infections. J. Med. Chem. 2021, 64, 12359–12378. [Google Scholar] [CrossRef]
- Devreux, M.; Henoumont, C.; Dioury, F.; Stanicki, D.; Boutry, S.; Larbanoix, L.; Ferroud, C.; Muller, R.N.; Laurent, S. Bimodal Probe for Magnetic Resonance Imaging and Photoacoustic Imaging Based on a PCTA-Derived Gadolinium(III) Complex and ZW800–1. Eur. J. Inorg. Chem. 2019, 2019, 3354–3365. [Google Scholar] [CrossRef]
- Enel, M.; Leygue, N.; Saffon, N.; Galaup, C.; Picard, C. Facile Access to the 12-Membered Macrocyclic Ligand PCTA and Its Derivatives with Carboxylate, Amide, and Phosphinate Ligating Functionalities. Eur. J. Org. Chem. 2018, 2018, 1765–1773. [Google Scholar] [CrossRef]
- Leygue, N.; Enel, M.; Diallo, A.; Mestre-Voegtlé, B.; Galaup, C.; Picard, C. Efficient Synthesis of a Family of Bifunctional Chelators Based on the PCTA[12] Macrocycle Suitable for Bioconjugation. Eur. J. Org. Chem. 2019, 2019, 2899–2913. [Google Scholar] [CrossRef]
- Pandey, U.; Gamre, N.; Kumar, Y.; Shetty, P.; Sarma, H.D.; Dash, A. A systematic evaluation of the potential of PCTA-NCS ligand as a bifunctional chelating agent for design of 177Lu radiopharmaceuticals. J. Radioanal. Nucl. Chem. 2016, 307, 187–194. [Google Scholar] [CrossRef]
- Ferreira, C.; Lamsa, E.; Woods, M.; Duan, Y.; Fernando, P.; Bensimon, C.; Kordos, M.; Guenther, K.; Jurek, P.; Kiefer, G. Evaluation of Bifunctional Chelates for the Development of Gallium-Based Radiopharmaceuticals. Bioconjug. Chem. 2010, 21, 531–536. [Google Scholar] [CrossRef]
- Yong-Sang, J.; Dioury, F.; Meneyrol, V.; Ait-Arsa, I.; Idoumbin, J.-P.; Guibbal, F.; Patché, J.; Gimié, F.; Khantalin, I.; Couprie, J.; et al. Development, synthesis, and 68Ga-Labeling of a Lipophilic complexing agent for atherosclerosis PET imaging. Eur. J. Med. Chem. 2019, 176, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Azad, B.B.; Cho, C.-F.; Lewis, J.D.; Luyt, L.G. Synthesis, radiometal labeling and in vitro evaluation of a targeted PPIX derivative. Appl. Radiat. Isot. 2012, 70, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bryden, F.; Savoie, H.; Rosca, E.V.; Boyle, R.W. PET/PDT theranostics: Synthesis and biological evaluation of a peptide-targeted gallium porphyrin. Dalton Trans. 2015, 44, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Enakieva, Y.Y.; Volostnykh, M.V.; Nefedov, S.E.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y.; Bessmertnykh-Lemeune, A.G.; Stern, C.; Guilard, R. Gallium(III) and Indium(III) Complexes with meso-Monophosphorylated Porphyrins: Synthesis and Structure. A First Example of Dimers Formed by the Self-Assembly of meso-Porphyrinylphosphonic Acid Monoester. Inorg. Chem. 2017, 56, 3055–3070. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, Y.; Jalilian, A.R.; Amini, M.M.; Ardaneh, K.; Rahiminejad, A.; Bolourinovin, F.; Moradkhani, S.; Majdabadi, A. Development of a 68Ga-Fluorinated Porphyrin Complex as a Possible PET Imaging Agent. Nucl. Med. Mol. Imaging 2012, 46, 20–26. [Google Scholar] [CrossRef]
- Zoller, F.; Riss, P.J.; Montforts, F.-P.; Kelleher, D.K.; Eppard, E.; Rösch, F. Radiolabelling and preliminary evaluation of 68Ga-tetrapyrrole derivatives as potential tracers for PET. Nucl. Med. Biol. 2013, 40, 280–288. [Google Scholar] [CrossRef]
- L’Eplattenier, F.; Murase, I.; Martell, A. New multidentate ligands. VI. Chelating tendencies of N,N′-Di(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid. J. Am. Chem. Soc. 1967, 89, 837–843. [Google Scholar] [CrossRef]
- Bartholomä, M.D. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in Positron Emission Tomography. Inorg. Chim. Acta 2012, 389, 36–51. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Zha, Z.; Ploessl, K.; Choi, S.R.; Wu, Z.; Zhu, L.; Kung, H.F. Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor. Nucl. Med. Biol. 2018, 59, 36–47. [Google Scholar] [CrossRef]
- Wang, G.; Hong, H.; Zang, J.; Liu, Q.; Jiang, Y.; Fan, X.; Zhu, Z.; Zhu, L.; Kung, H.F. Head-to-head comparison of [68Ga]Ga-P16-093 and [68Ga]Ga-PSMA-617 in dynamic PET/CT evaluation of the same group of recurrent prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Wu, Z.; Choi, S.R.; Ploessl, K.; Smith, M.; Alexoff, D.; Zhu, L.; Kung, H.F. A New [68Ga]Ga-HBED-CC-Bisphosphonate as a Bone Imaging Agent. Mol. Pharm. 2020, 17, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Ploessl, K.; Zha, Z.; Wang, H.; Guo, R.; Xie, Q.; Zhu, H.; Yang, Z.; Zhu, L.; Kung, H.F. Development and validation of a kit formulation of [68Ga]Ga-P15-041 as a bone imaging agent. Appl. Radiat. Isot. 2021, 169, 109485. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Sharma, R.; Kumar, C.; Sarma, H.D.; Dash, A. 68Ga-Chelation and comparative evaluation of N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. MedChemComm 2017, 8, 673–679. [Google Scholar] [CrossRef]
- Fay, R.; Gut, M.; Holland, J.P. Photoradiosynthesis of 68Ga-Labeled HBED-CC-Azepin-MetMAb for Immuno-PET of c-MET Receptors. Bioconjug. Chem. 2019, 30, 1814–1820. [Google Scholar] [CrossRef]
- Klika, K.D.; da Pieve, C.; Kopka, K.; Smith, G.; Makarem, A. Synthesis and application of a thiol-reactive HBED-type chelator for development of easy-to-produce Ga-radiopharmaceutical kits and imaging probes. Org. Biomol. Chem. 2021, 19, 1722–1726. [Google Scholar] [CrossRef]
- Adumeau, P.; Davydova, M.; Zeglis, B.M. Thiol-Reactive Bifunctional Chelators for the Creation of Site-Selectively Modified Radioimmunoconjugates with Improved Stability. Bioconjug. Chem. 2018, 29, 1364–1372. [Google Scholar] [CrossRef]
- Liolios, C.; Shegani, A.; Roupa, I.; Kiritsis, C.; Makarem, A.; Paravatou-Petsotas, M.; Pelecanou, M.; Bouziotis, P.; Papadopoulos, M.; Kopka, K.; et al. Synthesis, characterization and evaluation of 68Ga labelled monomeric and dimeric quinazoline derivatives of the HBED-CC chelator targeting the epidermal growth factor receptor. Bioorg. Chem. 2020, 100, 103855. [Google Scholar] [CrossRef]
- Makarem, A.; Klika, K.D.; Litau, G.; Remde, Y.; Kopka, K. HBED-NN: A Bifunctional Chelator for Constructing Radiopharmaceuticals. J. Org. Chem. 2019, 84, 7501–7508. [Google Scholar] [CrossRef]
- Makarem, A.; Sarvestani, M.K.; Klika, K.D.; Kopka, K. A Multifunctional HBED-Type Chelator with Dual Conjugation Capabilities for Radiopharmaceutical Development. Synlett 2019, 30, 1795–1798. [Google Scholar] [CrossRef]
- McDonagh, A.W.; McNeil, B.L.; Rousseau, J.; Roberts, R.J.; Merkens, H.; Yang, H.; Bénard, F.; Ramogida, C.F. Development of a multi faceted platform containing a tetrazine, fluorophore and chelator: Synthesis, characterization, radiolabeling, and immuno-SPECT imaging. EJNMMI Radiopharm. Chem. 2022, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Jerzyk, K.; Kludkiewicz, D.; Pijarowska-Kruszyna, J.; Jaron, A.; Maurin, M.; Sikora, A.; Kordowski, L.; Garnuszek, P. Synthesis of HBED–CC–tris(tert-butyl ester) using a solid phase and a microwave reactor. Tetrahedron 2021, 84, 132018. [Google Scholar] [CrossRef]
- Hider, R.C.; Hall, A.D. Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 28, pp. 41–173. [Google Scholar]
- Wallin, M.; Turner, P.; Katsifis, A.; Yang, M.; Chan, H.-K. Crystal structure of aqua(2-{[2-({2-[bis(carboxylato-κO-methyl)amino-κN]ethyl}(carboxylato-κO-methyl)amino-κN)ethyl](carboxymethyl)azaniumyl}acetato)gallium(III) trihydrate. Acta Cryst. E 2018, 74, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.E.; Robb, B.H.; Marshak, M.P. Effect of Chelation on Iron–Chromium Redox Flow Batteries. ACS Energy Lett. 2020, 5, 1758–1762. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Greiser, J.; Hagemann, T.; Niksch, T.; Traber, P.; Kupfer, S.; Gräfe, S.; Görls, H.; Weigand, W.; Freesmeyer, M. Synthesis and Characterization of GaIII, InIII and LuIII Complexes of a Set of dtpa Bis-Amide Ligands. Eur. J. Inorg. Chem. 2015, 2015, 4125–4137. [Google Scholar] [CrossRef]
- Vergara, I.; Castillo, E.Y.; Romero-Piña, M.E.; Torres-Viquez, I.; Paniagua, D.; Boyer, L.V.; Alagón, A.; Medina, L.A. Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging. Toxins 2016, 8, 85. [Google Scholar] [CrossRef]
- Gut, M.; Holland, J.P. Synthesis and Photochemical Studies on Gallium and Indium Complexes of DTPA-PEG3-ArN3 for Radiolabeling Antibodies. Inorg. Chem. 2019, 58, 12302–12310. [Google Scholar] [CrossRef]
- Patra, M.; Eichenberger, L.S.; Fischer, G.; Holland, J.P. Photochemical Conjugation and One-Pot Radiolabelling of Antibodies for Immuno-PET. Angew. Chem. Int. Ed. 2019, 58, 1928–1933. [Google Scholar] [CrossRef]
- Patra, M.; Klingler, S.; Eichenberger, L.S.; Holland, J.P. Simultaneous Photoradiochemical Labeling of Antibodies for Immuno-Positron Emission Tomography. iScience 2019, 13, 416–431. [Google Scholar] [CrossRef]
- Jain, A.; Chakraborty, S.; Sarma, H.D.; Dash, A. A Systematic Comparative Evaluation of 68Ga-Labeled RGD Peptides Conjugated with Different Chelators. Nucl. Med. Mol. Imaging 2018, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mathur, A.; Pandey, U.; Sarma, H.D.; Dash, A. 68Ga labeled fatty acids for cardiac metabolic imaging: Influence of different bifunctional chelators. Bioorg. Med. Chem. Lett. 2016, 26, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Hoh, C.K.; Olson, E.S.; Jahromi, A.H.; Hall, D.J.; Barback, C.V.; You, Y.-H.; Yanagita, M.; Sharma, K.; Vera, D.R. Molecular Imaging of the Glomerulus via Mesangial Cell Uptake of Radiolabeled Tilmanocept. J. Nucl. Med. 2019, 60, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Sattarzadeh, E.; Amini, M.M.; Kakaei, S.; Khanchi, A. 68Ga-radiolabeled magnetic nanoparticles for PET–MRI imaging. J. Radioanal. Nucl. Chem. 2018, 317, 1333–1339. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254. [Google Scholar] [CrossRef]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Ettlinger, L.; Corbaz, R.; Hütter, R. Zur Systematik der Actinomyceten. Archiv. Mikrobiol. 1958, 31, 326–358. [Google Scholar] [CrossRef]
- Yokoyama, A.; Ohmomo, Y.; Horiuchi, K.; Saji, H.; Tanaka, H.; Yamamoto, K.; Ishil, Y.; Torizuka, K. Deferoxamine, A Promising Bifunctional Chelating Agent for Labeling Proteins with Gallium: Ga-67 DF-HSA: Concise Communication. J. Nucl. Med. 1982, 23, 909–914. [Google Scholar]
- Joaqui-Joaqui, M.A.; Pandey, M.K.; Bansal, A.; Raju, M.V.R.; Armstrong-Pavlik, F.; Dundar, A.; Wong, H.L.; DeGrado, T.R.; Pierre, V.C. Catechol-Based Functionalizable Ligands for Gallium-68 Positron Emission Tomography Imaging. Inorg. Chem. 2020, 59, 12025–12038. [Google Scholar] [CrossRef] [PubMed]
- Govindan, S.V.; Michel, R.B.; Griffiths, G.L.; Goldenberg, D.M.; Mattes, M.J. Deferoxamine as a chelator for 67Ga in the preparation of antibody conjugates. Nucl. Med. Biol. 2005, 32, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Caraco, C.; Aloj, L.; Eckelman, W.C. The gallium–deferoxamine complex: Stability with different deferoxamine concentrations and incubation conditions. Appl. Radiat. Isot. 1998, 49, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Ryser, J.E.; Rose, K.; Jones, R.; Pelegrin, A.; Donath, A.; Egeli, R.; Smith, A.; Offord, R.E. Elimination of Free Radionuclide by a Chelating Agent Improves Tumor-to-Nontumor Ratios Following Radioimmunotargeting with Antibody Labeled with 67Ga. Nucl. Med. Biol. 1998, 25, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Fischer, E.; Rosillo-Lopez, M.; Salzmann, C.G.; Holland, J.P. Multi-functionalised graphene nanoflakes as tumour-targeting theranostic drug-delivery vehicles. Chem. Sci. 2019, 10, 8880–8888. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, S.; White, P.S.; Crumbliss, A.L. Crystal structure of ferrioxamine B: A comparative analysis and implications for molecular recognition. J. Biol. Inorg. Chem. 2001, 6, 810–818. [Google Scholar] [CrossRef]
- Gourni, E.; del Pozzo, L.; Bartholomä, M.; Kiefer, Y.; Meyer, P.T.; Maecke, H.R.; Holland, J.P. Radiochemistry and Preclinical PET Imaging of 68Ga-Desferrioxamine Radiotracers Targeting Prostate-Specific Membrane Antigen. Mol. Imaging 2017, 16, 1536012117737010. [Google Scholar] [CrossRef]
- Ioppolo, J.A.; Caldwell, D.; Beiraghi, O.; Llano, L.; Blacker, M.; Valliant, J.F.; Berti, P.J. 67Ga-labeled deferoxamine derivatives for imaging bacterial infection: Preparation and screening of functionalized siderophore complexes. Nucl. Med. Biol. 2017, 52, 32–41. [Google Scholar] [CrossRef]
- Brandt, M.; Cowell, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. Radiolabelling of the octadentate chelators DFO* and oxoDFO* with zirconium-89 and gallium-68. J. Biol. Inorg. Chem. 2020, 25, 789–796. [Google Scholar] [CrossRef]
- Brown, C.J.M.; Gotsbacher, M.P.; Codd, R. Improved Access to Linear Tetrameric Hydroxamic Acids with Potential as Radiochemical Ligands for Zirconium(IV)-89 PET Imaging. Aust. J. Chem. 2020, 73, 969–978. [Google Scholar] [CrossRef]
- Richardson-Sanchez, T.; Tieu, W.; Gotsbacher, M.P.; Telfer, T.J.; Codd, R. Exploiting the biosynthetic machinery of Streptomyces pilosus to engineer a water-soluble zirconium(iv) chelator. Org. Biomol. Chem. 2017, 15, 5719–5730. [Google Scholar] [CrossRef]
- Noor, A.; van Zuylekom, J.K.; Rudd, S.E.; Roselt, P.D.; Haskali, M.B.; Yan, E.; Wheatcroft, M.; Hicks, R.J.; Cullinane, C.; Donnelly, P.S. Imaging Somatostatin Positive Tumors with Tyr3-Octreotate/Octreotide Conjugated to Desferrioxamine B Squaramide Radiolabeled with either Zirconium-89 or Gallium-68. Bioconjug. Chem. 2021, 32, 1192–1203. [Google Scholar] [CrossRef]
- Noor, A.; van Zuylekom, J.K.; Rudd, S.E.; Waldeck, K.; Roselt, P.D.; Haskali, M.B.; Wheatcroft, M.P.; Yan, E.; Hicks, R.J.; Cullinane, C.; et al. Bivalent Inhibitors of Prostate-Specific Membrane Antigen Conjugated to Desferrioxamine B Squaramide Labeled with Zirconium-89 or Gallium-68 for Diagnostic Imaging of Prostate Cancer. J. Med. Chem. 2020, 63, 9258–9270. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova, S.; Daniskova, A.; Bendova, K.; Novy, Z.; Soural, M.; Petrik, M. [68Ga]Ga-DFO-c(RGDyK): Synthesis and Evaluation of Its Potential for Tumor Imaging in Mice. Int. J. Mol. Sci. 2021, 22, 7391. [Google Scholar] [CrossRef]
- Oroujeni, M.; Xu, T.; Gagnon, K.; Rinne, S.S.; Weis, J.; Garousi, J.; Andersson, K.G.; Löfblom, J.; Orlova, A.; Tolmachev, V. The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule. Pharmaceutics 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.; Valverde, I.E.; Fischer, C.A.; Vomstein, S.; Mindt, T.L. Development of 68Ga- and 89Zr-Labeled Exendin-4 as Potential Radiotracers for the Imaging of Insulinomas by PET. J. Nucl. Med. 2015, 56, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Kaeppeli, S.A.M.; Schibli, R.; Mindt, T.L.; Behe, M. Comparison of desferrioxamine and NODAGA for the gallium-68 labeling of exendin-4. EJNMMI Radiopharm. Chem. 2019, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hisada, H.; Temma, T.; Shimizu, Y.; Kimura, H.; Ono, M.; Nakamoto, Y.; Togashi, K.; Saji, H. Gallium-68-Labeled Anti-HER2 Single-Chain Fv Fragment: Development and In Vivo Monitoring of HER2 Expression. Mol. Imaging Biol. 2015, 17, 102–110. [Google Scholar] [CrossRef]
- Harris, W.R.; Carrano, C.J.; Cooper, S.R.; Sofen, S.R.; Avdeef, A.E.; McArdle, J.V.; Raymond, K.N. Coordination Chemistry of Microbial Iron Transport Compounds. 19. Stability Constants and Electrochemical Behavior of Ferric Enterobactin and Model Complexes. J. Am. Chem. Soc. 1979, 101, 6097–6104. [Google Scholar] [CrossRef]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Schrettl, M.; Helbok, A.; Blatzer, M.; Decristoforo, C. In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl. Med. Biol. 2012, 39, 361–369. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Pfister, J.; Mair, C.; Novy, Z.; Popper, M.; Hajduch, M.; et al. 68Ga-labelled desferrioxamine-B for bacterial infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 372–382. [Google Scholar] [CrossRef]
- Evers, A.; Hancock, R.D.; Martell, A.E.; Motekaitis, R.J. Metal ion recognition in ligands with negatively charged oxygen donor groups. Complexation of iron(III), gallium(III), indium(III), aluminum(III), and other highly charged metal ions. Inorg. Chem. 1989, 28, 2189–2195. [Google Scholar] [CrossRef]
- Guillou, A.; Earley, D.F.; Patra, M.; Holland, J.P. Light-induced synthesis of protein conjugates and its application in photoradiosynthesis of 89Zr-radiolabeled monoclonal antibodies. Nat. Protoc. 2020, 15, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Nostra, B.V.; Viola, N. The Radiopharmaceutical Chemistry of Zirconium-89. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 371–390. [Google Scholar]
- Boros, E.; Ferreira, C.L.; Cawthray, J.F.; Price, E.W.; Patrick, B.O.; Wester, D.W.; Adam, M.J.; Orvig, C. Acyclic Chelate with Ideal Properties for 68Ga PET Imaging Agent Elaboration. J. Am. Chem. Soc. 2010, 132, 15726–15733. [Google Scholar] [CrossRef] [PubMed]
- Platas-Iglesias, C.; Mato-Iglesias, M.; Djanashvili, K.; Muller, R.N.; Elst, L.V.; Peters, J.A.; de Blas, A.; Rodríguez-Blas, T. Lanthanide Chelates Containing Pyridine Units with Potential Application as Contrast Agents in Magnetic Resonance Imaging. Chem. Eur. J. 2004, 10, 3579–3590. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Cawthray, J.F.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. Evaluation of the H2dedpa Scaffold and its cRGDyK Conjugates for Labeling with 64Cu. Inorg. Chem. 2012, 51, 6279–6284. [Google Scholar] [CrossRef]
- Boros, E.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. New Ga derivatives of the H2dedpa scaffold with improved clearance and persistent heart uptake. Nucl. Med. Biol. 2011, 38, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Ferreira, C.L.; Yapp, D.T.T.; Gill, R.K.; Price, E.W.; Adam, M.J.; Orvig, C. RGD conjugates of the H2dedpa scaffold: Synthesis, labeling and imaging with 68Ga. Nucl. Med. Biol. 2012, 39, 785–794. [Google Scholar] [CrossRef]
- Bailey, G.A.; Price, E.W.; Zeglis, B.M.; Ferreira, C.L.; Boros, E.; Lacasse, M.J.; Patrick, B.O.; Lewis, J.S.; Adam, M.J.; Orvig, C. H2azapa: A Versatile Acyclic Multifunctional Chelator for 67Ga, 64Cu, 111In, and 177Lu. Inorg. Chem. 2012, 51, 12575–12589. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Pan, J.; Ferreira, C.L.; Patrick, B.O.; Rebullar, K.; Yapp, D.T.T.; Lin, K.-S.; Adam, M.J.; Orvig, C. Nitroimidazole-Containing H2dedpa and H2CHXdedpa Derivatives as Potential PET Imaging Agents of Hypoxia with 68Ga. Inorg. Chem. 2015, 54, 4953–4965. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Murphy, L.; Cawthray, J.F.; Ross, J.D.; Adam, M.J.; Orvig, C. Novel “bi-modal” H2dedpa derivatives for radio- and fluorescence imaging. J. Inorg. Biochem. 2016, 162, 253–262. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Boros, E.; Patrick, B.O.; Zeisler, S.K.; Kumlin, J.; Adam, M.J.; Schaffer, P.; Orvig, C. Evaluation of H2CHXdedpa, H2dedpa- and H2CHXdedpa-N,N′-propyl-2-NI ligands for 64Cu(II) radiopharmaceuticals. Dalton Trans. 2016, 45, 13082–13090. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Schindler, D.; Schneider, C.; Tan, Y.L.K.; Huh, S.; Ferreira, C.L.; Adam, M.J.; Orvig, C. Synthesis and characterization of lipophilic cationic Ga(III) complexes based on the H2CHXdedpa and H2dedpa ligands and their 67/68Ga radiolabeling studies. RSC Adv. 2016, 6, 103763–103773. [Google Scholar] [CrossRef]
- Saito, K.; Watanabe, H.; Iikuni, S.; Ono, M. Development of novel 67/68Ga-labeled pyridyl benzofuran derivatives as islet amyloid imaging probes. Nucl. Med. Biol. 2022, 106–107, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jaraquemada-Peláez, M.a.d.G.; Cao, Y.; Pan, J.; Lin, K.-S.; Patrick, B.O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. 2018, 58, 2275–2285. [Google Scholar] [CrossRef]
- Wang, X.; Jaraquemada-Peláez, M.d.G.; Cao, Y.; Ingham, A.; Rodríguez-Rodríguez, C.; Pan, J.; Wang, Y.; Saatchi, K.; Häfeli, U.O.; Lin, K.-S.; et al. H2CHXhox: Rigid Cyclohexane-Reinforced Nonmacrocyclic Chelating Ligand for [nat/67/68Ga]Ga3+. Inorg. Chem. 2020, 59, 4895–4908. [Google Scholar] [CrossRef]
- Berry, D.J.; Ma, Y.; Ballinger, J.R.; Tavaré, R.; Koers, A.; Sunassee, K.; Zhou, T.; Nawaz, S.; Mullen, G.E.D.; Hider, R.C.; et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: Tris(hydroxypyridinone) ligands. Chem. Commun. 2011, 47, 7068–7070. [Google Scholar] [CrossRef]
- Imberti, C.; Adumeau, P.; Blower, J.E.; al Salemee, F.; Torres, J.B.; Lewis, J.S.; Zeglis, B.M.; Terry, S.Y.A.; Blower, P.J. Manipulating the In Vivo Behaviour of 68Ga with Tris(Hydroxypyridinone) Chelators: Pretargeting and Blood Clearance. Int. J. Mol. Sci. 2020, 21, 1496. [Google Scholar] [CrossRef]
- Dobbin, P.S.; Hider, R.C.; Hall, A.D.; Taylor, P.D.; Sarpong, P.; Porter, J.B.; Xiao, G.; van der Helm, D. Synthesis, physicochemical properties, and biological evaluation of N-substituted 2-alkyl-3-hydroxy-4(1H)-pyridinones: Orally active iron chelators with clinical potential. J. Med. Chem. 1993, 36, 2448–2458. [Google Scholar] [CrossRef]
- Yue, J.L.; Martell, A.E. Potentiometric and spectrophotometric determination of stabilities of the 1-hydroxy-2-pyridinone complexes of trivalent and divalent metal ions. Inorg. Chim. Acta 1993, 214, 103–111. [Google Scholar] [CrossRef]
- Scarrow, R.C.; Riley, P.E.; Abu-Dari, K.; White, D.L.; Raymond, K.N. Ferric ion sequestering agents. 13. Synthesis, structures, and thermodynamics of complexation of cobalt(III) and iron(III) tris complexes of several chelating hydroxypyridinones. Inorg. Chem. 1985, 24, 954–967. [Google Scholar] [CrossRef]
- Nelson, W.O.; Karpishin, T.B.; Rettig, S.J.; Orvig, C. Aluminum and gallium compounds of 3-hydroxy-4-pyridinones: Synthesis, characterization, and crystallography of biologically active complexes with unusual hydrogen bonding. Inorg. Chem. 1988, 27, 1045–1051. [Google Scholar] [CrossRef]
- Imberti, C.; Terry, S.Y.A.; Cullinane, C.; Clarke, F.; Cornish, G.H.; Ramakrishnan, N.K.; Roselt, P.; Cope, A.P.; Hicks, R.J.; Blower, P.J.; et al. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of αvβ3 Integrin Expression with Gallium-68. Bioconjug. Chem. 2017, 28, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Cullinane, C.; Waldeck, K.; Roselt, P.; Hicks, R.J.; Blower, P.J. Rapid kit-based 68Ga-labelling and PET imaging with THP-Tyr3-octreotate: A preliminary comparison with DOTA-Tyr3-octreotate. EJNMMI Res. 2015, 5, 52. [Google Scholar] [CrossRef]
- Ma, M.T.; Cullinane, C.; Imberti, C.; Torres, J.B.; Terry, S.Y.A.; Roselt, P.; Hicks, R.J.; Blower, P.J. New Tris(hydroxypyridinone) Bifunctional Chelators Containing Isothiocyanate Groups Provide a Versatile Platform for Rapid One-Step Labeling and PET Imaging with 68Ga3+. Bioconjug. Chem. 2016, 27, 309–318. [Google Scholar] [CrossRef]
- Imberti, C.; Chen, Y.-L.; Foley, C.A.; Ma, M.T.; Paterson, B.M.; Wang, Y.; Young, J.D.; Hider, R.C.; Blower, P.J. Tuning the properties of tris(hydroxypyridinone) ligands: Efficient 68Ga chelators for PET imaging. Dalton Trans. 2019, 48, 4299–4313. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Mullen, G.E.D.; Sunassee, K.; Bordoloi, J.; Blower, P.J.; Ballinger, J.R. Simple, mild, one-step labelling of proteins with gallium-68 using a tris(hydroxypyridinone) bifunctional chelator: A 68Ga-THP-scFv targeting the prostate-specific membrane antigen. EJNMMI Res. 2017, 7, 86. [Google Scholar] [CrossRef]
- Blower, J.E.; Cooper, M.S.; Imberti, C.; Ma, M.T.; Marshall, C.; Young, J.D.; Blower, P.J. The Radiopharmaceutical Chemistry of the Radionuclides of Gallium and Indium. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 255–271. [Google Scholar]
- Hofman, M.S.; Eu, P.; Jackson, P.; Hong, E.; Binns, D.; Iravani, A.; Murphy, D.; Mitchell, C.; Siva, S.; Hicks, R.J.; et al. Cold Kit for Prostate-Specific Membrane Antigen (PSMA) PET Imaging: Phase 1 Study of 68Ga-Tris(Hydroxypyridinone)-PSMA PET/CT in Patients with Prostate Cancer. J. Nucl. Med. 2018, 59, 625–631. [Google Scholar] [CrossRef]
- Kulkarni, M.; Hughes, S.; Mallia, A.; Gibson, V.; Young, J.; Aggarwal, A.; Morris, S.; Challacombe, B.; Popert, R.; Brown, C.; et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 674–686. [Google Scholar] [CrossRef]
- Floresta, G.; Keeling, G.P.; Memdouh, S.; Meszaros, L.K.; de Rosales, R.T.M.; Abbate, V. NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines 2021, 9, 367. [Google Scholar] [CrossRef]
- Aime, S.; Bombieri, G.; Cavallotti, C.; Giovenzana, G.B.; Imperio, D.; Marchini, N. An unusual gadolinium ten-coordinated dimeric complex in the series of MRI contrast agents: Na[Gd(H2O)AAZTA]·3H2O. Inorg. Chim. Acta 2008, 361, 1534–1541. [Google Scholar] [CrossRef]
- Aime, S.; Calabi, L.; Cavallotti, C.; Gianolio, E.; Giovenzana, G.B.; Losi, P.; Maiocchi, A.; Palmisano, G.; Sisti, M. [Gd-AAZTA]−: A New Structural Entry for an Improved Generation of MRI Contrast Agents. Inorg. Chem. 2004, 43, 7588–7590. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Uggeri, F.; Giovenzana, G.B.; Bényei, A.; Brücher, E.; Aime, S. Equilibrium and Kinetic Properties of the Lanthanoids(III) and Various Divalent Metal Complexes of the Heptadentate Ligand AAZTA. Chem. Eur. J. 2009, 15, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Elemento, E.M.; Parker, D.; Aime, S.; Gianolio, E.; Lattuada, L. Variation of water exchange dynamics with ligand structure and stereochemistry in lanthanide complexes based on 1,4-diazepine derivatives. Org. Biomol. Chem. 2009, 7, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.A.; Neves, A.; Bortoluzzi, A.J.; Casellato, A.; Anjos, A.d.; Greatti, A.; Xavier, F.R.; Szpoganicz, B. First-Transition-Metal Complexes Containing the Ligands 6-Amino-6-methylperhydro-1,4-diazepine (AAZ) and a New Functionalized Derivative: Can AAZ Act as a Mimetic Ligand for 1,4,7-Triazacyclononane? Inorg. Chem. 2005, 44, 7690–7692. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.P.; Parker, D.; Burchardt, C.; Yufit, D.S.; Zimny, M.; Roesch, F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem. Commun. 2013, 49, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Waldron, B.P. Conformational analysis and synthetic approaches to polydentate perhydro-diazepine ligands for the complexation of gallium(III). Org. Biomol. Chem. 2013, 11, 2827–2838. [Google Scholar] [CrossRef]
- Parker, D.; Waldron, B.P.; Yufit, D.S. Crystallographic and solution NMR structural analyses of four hexacoordinated gallium(III) complexes based on ligands derived from 6-amino-perhydro-1,4-diazepine. Dalton Trans. 2013, 42, 8001–8008. [Google Scholar] [CrossRef]
- Waldron, B. Synthesis and Evaluation of New Ligands for Gallium Radiolabelling. Ph.D. Thesis, University of Durham, Durham, UK, 2013. [Google Scholar]
- Farkas, E.; Vágner, A.; Negri, R.; Lattuada, L.; Tóth, I.; Colombo, V.; Esteban-Gómez, D.; Platas-Iglesias, C.; Notni, J.; Baranyai, Z.; et al. PIDAZTA: Structurally Constrained Chelators for the Efficient Formation of Stable Gallium-68 Complexes at Physiological pH. Chem. Eur. J. 2019, 25, 10698–10709. [Google Scholar] [CrossRef]
- Wu, Z.; Zha, Z.; Choi, S.R.; Plössl, K.; Zhu, L.; Kung, H.F. New 68Ga-PhenA bisphosphonates as potential bone imaging agents. Nucl. Med. Biol. 2016, 43, 360–371. [Google Scholar] [CrossRef]
- Gugliotta, G.; Botta, M.; Giovenzana, G.B.; Tei, L. Fast and easy access to efficient bifunctional chelators for MRI applications. Bioorg. Med. Chem. Lett. 2009, 19, 3442–3444. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Nagel, J.; Sinnes, J.-P.; Roesch, F.; Maina, T. Novel bifunctional DATA chelator for quick access to site-directed PET 68Ga-radiotracers: Preclinical proof-of-principle with [Tyr3]octreotide. Dalton Trans. 2017, 46, 14584–14590. [Google Scholar] [CrossRef] [PubMed]
- Sinnes, J.-P.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, M.; Moon, E.S.; D’Angelo, F.; Geissbühler, L.; Alberts, I.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. Effect of the versatile bifunctional chelator AAZTA5 on the radiometal labelling properties and the in vitro performance of a gastrin releasing peptide receptor antagonist. EJNMMI Radiopharm. Chem. 2020, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Klasen, B.; Moon, E.S.; Rösch, F. AAZTA5-squaramide ester competing with DOTA-, DTPA- and CHX-A″-DTPA-analogues: Promising tool for 177Lu-labeling of monoclonal antibodies under mild conditions. Nucl. Med. Biol. 2021, 96–97, 80–93. [Google Scholar] [CrossRef]
- Greifenstein, L.; Grus, T.; Nagel, J.; Sinnes, J.P.; Rösch, F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA5 for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl. Radiat. Isot. 2020, 156, 108867. [Google Scholar] [CrossRef]
- Sinnes, J.-P.; Bauder-Wüst, U.; Schäfer, M.; Moon, E.S.; Kopka, K.; Rösch, F. 68Ga, 44Sc and 177Lu-labeled AAZTA5-PSMA-617: Synthesis, radiolabeling, stability and cell binding compared to DOTA-PSMA-617 analogues. EJNMMI Radiopharm. Chem. 2020, 5, 28. [Google Scholar] [CrossRef]
- Orteca, G.; Sinnes, J.-P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954. [Google Scholar] [CrossRef]
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.; Parker, D.; Roesch, F. DATATOC: A novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature, EJNMMI Radiopharm. Chem. 2016, 1, 4. [Google Scholar]
- Sinnes, J.-P.; Nagel, J.; Waldron, B.P.; Maina, T.; Nock, B.A.; Bergmann, R.K.; Ullrich, M.; Pietzsch, J.; Bachmann, M.; Baum, R.P.; et al. Instant kit preparation of 68Ga-radiopharmaceuticals via the hybrid chelator DATA: Clinical translation of [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019, 9, 48. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Geraldes, C.F.G.C.; de Lima, J.J.P. Structural and in vivo studies of metal chelates of Ga(III) relevant to biomedical imaging. J. Inorg. Biochem. 2000, 79, 359–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Notni, J.; Šimeček, J.; Hermann, P.; Wester, H.J. TRAP, a Powerful and Versatile Framework for Gallium-68 Radiopharmaceuticals. Chem. Eur. J. 2011, 17, 14718–14722. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Kubíček, V.; Bonnet, C.S.; Hermann, P.; Lukeš, I.; Tóth, É. Dissociation kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.; Sherry, A.; Cacheris, W. Synthesis, Protonation Sequence, and NMR Studies of Polyazamacrocyclic Methylenephosphonates. Inorg. Chem. 1989, 28, 3336–3341. [Google Scholar] [CrossRef]
- Notni, J.; Hermann, P.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Plutnar, J.; Loktionova, N.; Riss, P.J.; Rösch, F.; Lukeš, I. A Triazacyclononane-Based Bifunctional Phosphinate Ligand for the Preparation of Multimeric 68Ga Tracers for Positron Emission Tomography. Chem. Eur. J. 2010, 16, 7174–7185. [Google Scholar] [CrossRef]
- Bianchi, A.; Calabi, L.; Giorgi, C.; Losi, P.; Mariani, P.; Paoli, P.; Rossi, P.; Valtancoli, B.; Virtuani, M. Thermodynamic and structural properties of Gd3+ complexes with functionalized macrocyclic ligands based upon 1,4,7,10-tetraazacyclododecane. J. Chem. Soc. Dalton Trans. 2000, 697–705. [Google Scholar] [CrossRef]
- Notni, J.; Šimeček, J.; Wester, H.-J. Phosphinic Acid Functionalized Polyazacycloalkane Chelators for Radiodiagnostics and Radiotherapeutics: Unique Characteristics and Applications. ChemMedChem 2014, 9, 1107–1115. [Google Scholar] [CrossRef]
- Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. Crystallographic and Computational Characterisation of the Potential PET Tracer 1,4,7-Triazacyclononane-1,4,7-tri(methylenephosphonato)gallium(III). ChemistrySelect 2022, 7, e202103698. [Google Scholar] [CrossRef]
- Brand, C.; Longo, V.A.; Groaning, M.; Weber, W.A.; Reiner, T. Development of a New Folate-Derived Ga-68-Based PET Imaging Agent. Mol. Imaging Biol. 2017, 19, 754–761. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, H.; Li, Y.; Wu, X.; Wang, H.; Cheng, Z. Detecting Vulnerable Atherosclerotic Plaques by 68Ga-Labeled Divalent Cystine Knot Peptide. Mol. Pharm. 2019, 16, 1350–1357. [Google Scholar] [CrossRef]
- Sneddon, D.; Cornelissen, B. Emerging chelators for nuclear imaging. Curr. Opin. Chem. Biol. 2021, 63, 152–162. [Google Scholar] [CrossRef]
- Su, T.; Wang, Y.-B.; Han, D.; Wang, J.; Qi, S.; Gao, L.; Shao, Y.-H.; Qiao, H.-Y.; Chen, J.-W.; Liang, S.-H.; et al. Multimodality Imaging of Angiogenesis in a Rabbit Atherosclerotic Model by GEBP11 Peptide Targeted Nanoparticles. Theranostics 2017, 7, 4791–4804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lang, L.; Wang, Z.; Jacobson, O.; Yung, B.; Zhu, G.; Gu, D.; Ma, Y.; Zhu, X.; Niu, G.; et al. Positron Emission Tomography Imaging of Prostate Cancer with Ga-68-Labeled Gastrin-Releasing Peptide Receptor Agonist BBN7–14 and Antagonist RM26. Bioconjug. Chem. 2018, 29, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, A.; Schottelius, M.; Schwaiger, M.; Wester, H.-J. Preclinical evaluation of [68Ga]NOTA-pentixafor for PET imaging of CXCR4 expression in vivo—A comparison to [68Ga]pentixafor. EJNMMI Res. 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Eisenwiener, K.-P.; Prata, M.I.M.; Buschmann, I.; Zhang, H.-W.; Santos, A.C.; Wenger, S.; Reubi, J.C.; Mäcke, H.R. NODAGATOC, a New Chelator-Coupled Somatostatin Analogue Labeled with [67/68Ga] and [111In] for SPECT, PET, and Targeted Therapeutic Applications of Somatostatin Receptor (hsst2) Expressing Tumors. Bioconjug. Chem. 2002, 13, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Nedrow, J.R.; White, A.G.; Modi, J.; Nguyen, K.; Chang, A.J.; Anderson, C.J. Positron Emission Tomographic Imaging of Copper 64– and Gallium 68–Labeled Chelator Conjugates of the Somatostatin Agonist Tyr3-Octreotate. Mol. Imaging 2014, 13, 7290-2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, X.; Zhang, Z.; Sun, Z.; Loft, M.; Ding, B.; Liu, C.; Xu, L.; Yang, M.; Jiang, Y.; et al. Preclinical Study on GRPR-Targeted 68Ga-Probes for PET Imaging of Prostate Cancer. Bioconjug. Chem. 2016, 27, 1857–1864. [Google Scholar] [CrossRef]
- D’Alessandria, C.; Pohle, K.; Rechenmacher, F.; Neubauer, S.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Kessler, H.; Beer, A.J. In vivo biokinetic and metabolic characterization of the 68Ga-labelled α5β1-selective peptidomimetic FR366. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 953–963. [Google Scholar] [CrossRef]
- Hübner, R.; Cheng, X.; Wängler, B.; Wängler, C. Functional Hybrid Molecules for the Visualization of Cancer: PESIN-Homodimers Combined with Multimodal Molecular Imaging Probes for Positron Emission Tomography and Optical Imaging: Suited for Tracking of GRPR-Positive Malignant Tissue. Chem. Eur. J. 2020, 26, 16349–16356. [Google Scholar] [CrossRef]
- Van der Veen, E.L.; Suurs, F.V.; Cleeren, F.; Bormans, G.; Elsinga, P.H.; Hospers, G.A.P.; Hooge, M.N.L.; de Vries, E.G.E.; de Vries, E.F.J.; Antunes, I.F. Development and Evaluation of Interleukin-2–Derived Radiotracers for PET Imaging of T Cells in Mice. J. Nucl. Med. 2020, 61, 1355–1360. [Google Scholar] [CrossRef]
- André, J.P.; Maecke, H.R.; Zehnder, M.; Macko, L.; Akyel, K.G. 1,4,7-Triazacyclononane-1-succinic acid-4,7-diacetic acid (NODASA): A new bifunctional chelator for radio gallium-labelling of biomolecules. Chem. Commun. 1998, 12, 1301–1302. [Google Scholar] [CrossRef]
- Le Roux, J.; Rubow, S.; Ebenhan, T. A comparison of labelling characteristics of manual and automated synthesis methods for gallium-68 labelled ubiquicidin. Appl. Radiat. Isot. 2021, 168, 109452. [Google Scholar] [CrossRef] [PubMed]
- Bracke, N.; Yao, H.; Wynendaele, E.; Verbeke, F.; Xu, X.; Gevaert, B.; Maes, A.; van de Wiele, C.; Sathekge, M.; de Saeger, S.; et al. In Vitro Functional Quality Characterization of NOTA-Modified Somatropins. Anal. Chem. 2017, 89, 2764–2772. [Google Scholar] [CrossRef]
- Pulido, J.; de Cabrera, M.; Sobczak, A.J.; Amor-Coarasa, A.; McGoron, A.J.; Wnuk, S.F. 4-N-Alkanoyl and 4-N-alkyl gemcitabine analogues with NOTA chelators for 68-gallium labelling. Bioorg. Med. Chem. 2018, 26, 5624–5630. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.-P.; Kozlowski, P.; Jackson, J.; Cunanan, K.M.; Adumeau, P.; Dilling, T.R.; Zeglis, B.M.; Lewis, J.S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60, 8201–8217. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.; Gamre, N.; Lohar, S.P.; Dash, A. A systematic study on the utility of CHX-A″-DTPA-NCS and NOTA-NCS as bifunctional chelators for 177Lu radiopharmaceuticals. Appl. Radiat. Isot. 2017, 127, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Dash, A.; Pillai, M.R.A. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl. Med. Biol. 2013, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; van Leeuwen, F.W.B.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016, 11, 328–339. [Google Scholar] [CrossRef]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis(phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef]
- Passah, A.; Tripathi, M.; Ballal, S.; Yadav, M.P.; Kumar, R.; Roesch, F.; Meckel, M.; Chakraborty, P.S.; Bal, C. Evaluation of bone-seeking novel radiotracer 68Ga-NO2AP-Bisphosphonate for the detection of skeletal metastases in carcinoma breast. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–49. [Google Scholar] [CrossRef]
- Läppchen, T.; Holland, J.P.; Kiefer, Y.; Bartholomä, M.D. Preparation and preclinical evaluation of a 68Ga-labelled c(RGDfK) conjugate comprising the bifunctional chelator NODIA-Me. EJNMMI Radiopharm. Chem. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Läppchen, T.; Kiefer, Y.; Holland, J.P.; Bartholomä, M.D. In vitro and in vivo evaluation of the bifunctional chelator NODIA-Me in combination with a prostate-specific membrane antigen targeting vector. Nucl. Med. Biol. 2018, 60, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, C.; Holland, J.P.; Läppchen, T.; Scherer, H.; Maus, S.; Stemler, T.; Bohnenberger, H.; Ezziddin, S.; Kurz, P.; Bartholomä, M.D. Optimized synthesis and indium complex formation with the bifunctional chelator NODIA-Me. Org. Biomol. Chem. 2018, 16, 7503–7512. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, A.; Läppchen, T.; Weinmann, C.; Bier-Schorr, L.; Keller, M.; Kiefer, Y.; Holland, J.P.; Bartholomä, M.D. Gallium Complexation, Stability, and Bioconjugation of 1,4,7-Triazacyclononane Derived Chelators with Azaheterocyclic Arms. Inorg. Chem. 2017, 56, 9097–9110. [Google Scholar] [CrossRef]
- Šimeček, J.; Schulz, M.; Notni, J.; Plutnar, J.; Kubíček, V.; Havlíčková, J.; Hermann, P. Complexation of Metal Ions with TRAP (1,4,7-Triazacyclononane Phosphinic Acid) Ligands and 1,4,7-Triazacyclononane-1,4,7-triacetic Acid: Phosphinate-Containing Ligands as Unique Chelators for Trivalent Gallium. Inorg. Chem. 2012, 51, 577–590. [Google Scholar] [CrossRef]
- Notni, J.; Steiger, K.; Hoffmann, F.; Reich, D.; Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; Wester, H.-J. Complementary, Selective PET Imaging of Integrin Subtypes α5β1 and αvβ3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J. Nucl. Med. 2016, 57, 460–466. [Google Scholar] [CrossRef]
- Šimeček, J.; Notni, J.; Kapp, T.G.; Kessler, H.; Wester, H.-J. Benefits of NOPO As Chelator in Gallium-68 Peptides, Exemplified by Preclinical Characterization of 68Ga-NOPO–c(RGDfK). Mol. Pharm. 2014, 11, 1687–1695. [Google Scholar] [CrossRef]
- Šimeček, J.; Zemek, O.; Hermann, P.; Notni, J.; Wester, H.-J. Tailored Gallium(III) Chelator NOPO: Synthesis, Characterization, Bioconjugation, and Application in Preclinical Ga-68-PET Imaging. Mol. Pharm. 2014, 11, 3893–3903. [Google Scholar] [CrossRef]
- Poty, S.; Désogère, P.; Šimeček, J.; Bernhard, C.; Goncalves, V.; Goze, C.; Boschetti, F.; Notni, J.; Wester, H.J.; Denat, F. MA-NOTMP: A Triazacyclononane Trimethylphosphinate Based Bifunctional Chelator for Gallium Radiolabelling of Biomolecules. ChemMedChem 2015, 10, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Máté, G.; Šimeček, J.; Pniok, M.; Kertész, I.; Notni, J.; Wester, H.-J.; Galuska, L.; Hermann, P. The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties. Molecules 2015, 20, 13112–13126. [Google Scholar] [CrossRef]
- Reich, D.; Wurzer, A.; Wirtz, M.; Stiegler, V.; Spatz, P.; Pollmann, J.; Wester, H.-J.; Notni, J. Dendritic poly-chelator frameworks for multimeric bioconjugation. Chem. Commun. 2017, 53, 2586–2589. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Pollmann, J.; Schmidt, A.; Reich, D.; Wester, H.-J.; Notni, J. Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results. Mol. Pharm. 2018, 15, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
- Reichart, F.; Maltsev, O.V.; Kapp, T.G.; Räder, A.F.B.; Weinmüller, M.; Marelli, U.K.; Notni, J.; Wurzer, A.; Beck, R.; Wester, H.-J.; et al. Selective Targeting of Integrin αvβ8 by a Highly Active Cyclic Peptide. J. Med. Chem. 2019, 62, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Prata, M.I.M.; André, J.P.; Kovács, Z.; Takács, A.I.; Tircsó, G.; Tóth, I.; Geraldes, C.F.G.C. Gallium(III) chelates of mixed phosphonate-carboxylate triazamacrocyclic ligands relevant to nuclear medicine: Structural, stability and in vivo studies. J. Inorg. Biochem. 2017, 177, 8–16. [Google Scholar] [CrossRef]
- Gai, Y.; Sun, L.; Lan, X.; Zeng, D.; Xiang, G.; Ma, X. Synthesis and Evaluation of New Bifunctional Chelators with Phosphonic Acid Arms for Gallium-68 Based PET Imaging in Melanoma. Bioconjug. Chem. 2018, 29, 3483–3494. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Geraldes, C.F.G.C.; Lima, J.J.P.d. Characterisation of 67Ga3+ complexes of triaza macrocyclic ligands: Biodistribution and clearance studies. Nucl. Med. Biol. 1999, 26, 707–710. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).