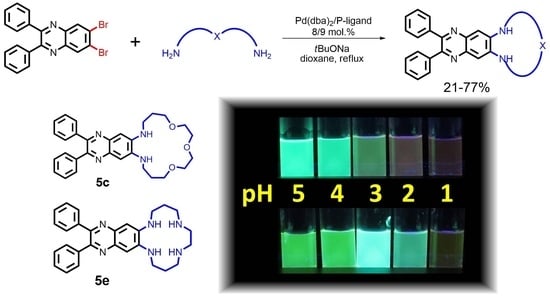
Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution
Abstract
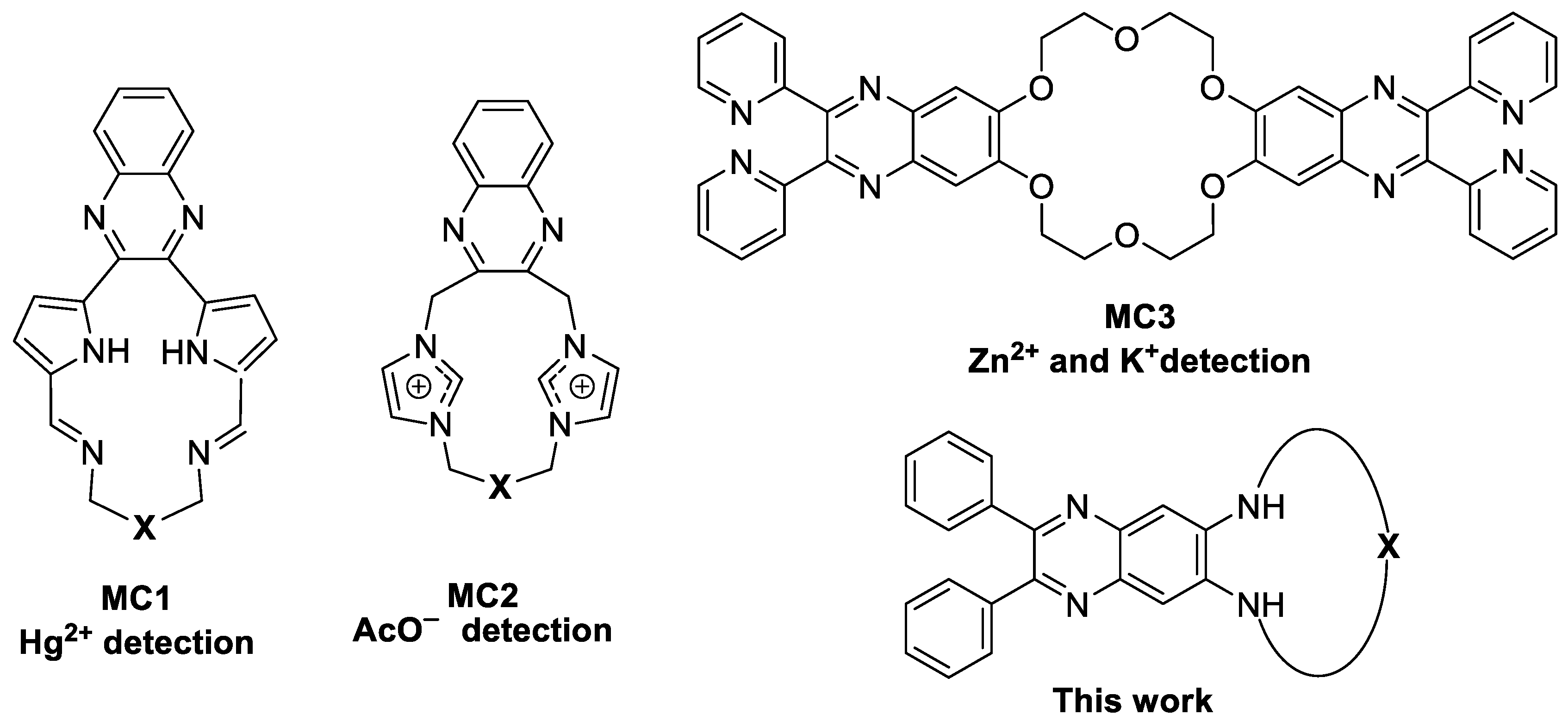
1. Introduction
2. Results and Discussion
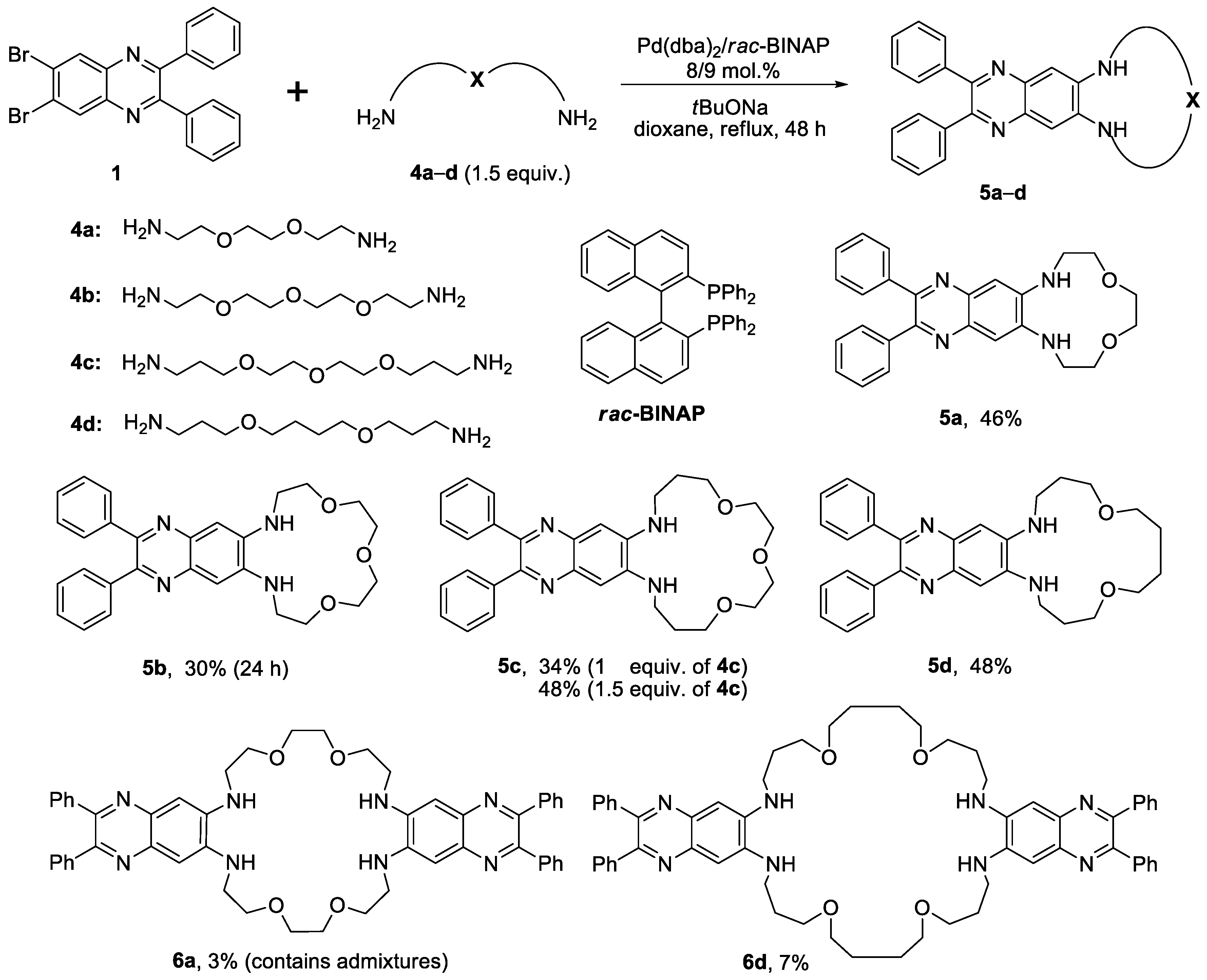
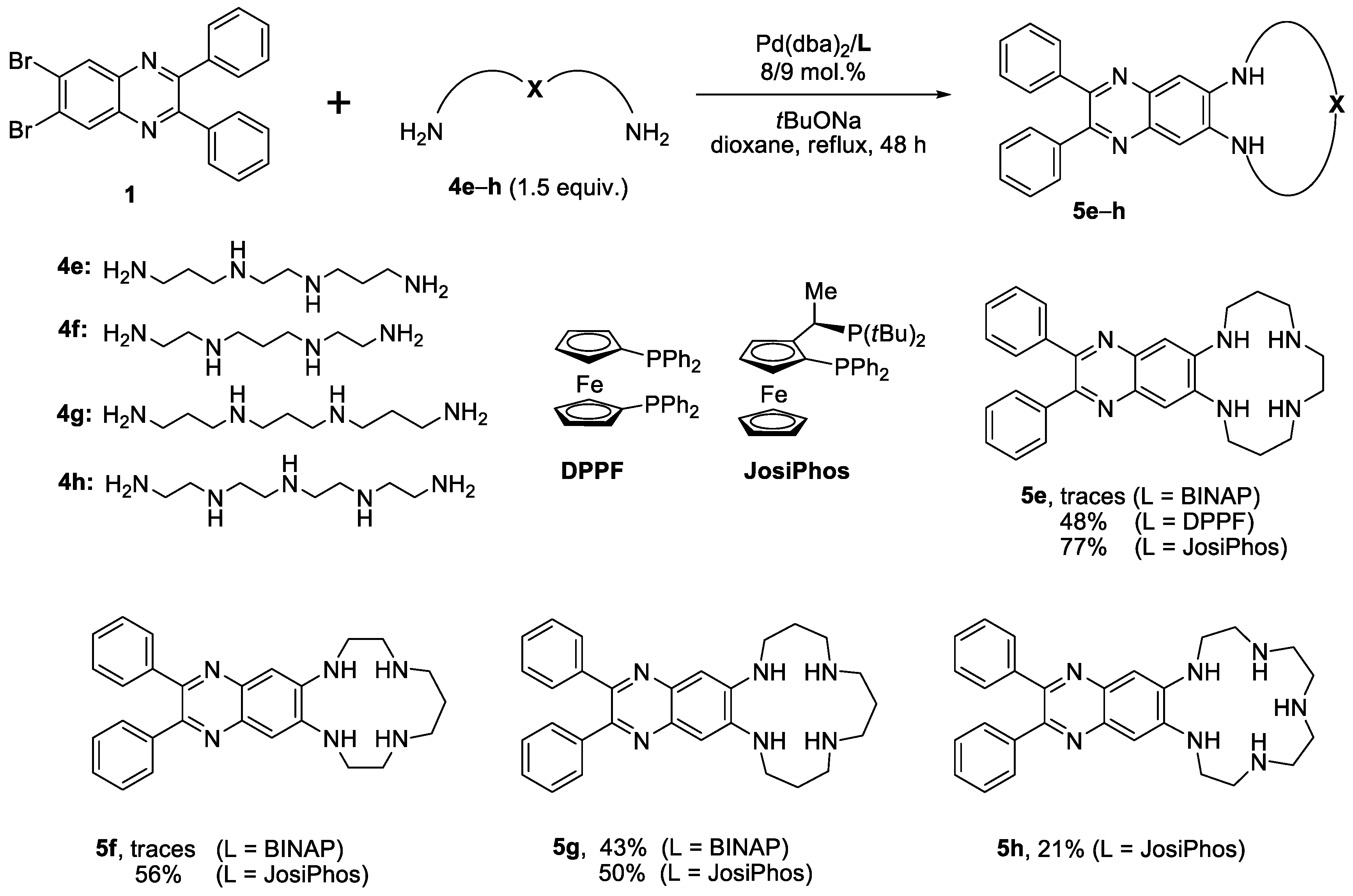
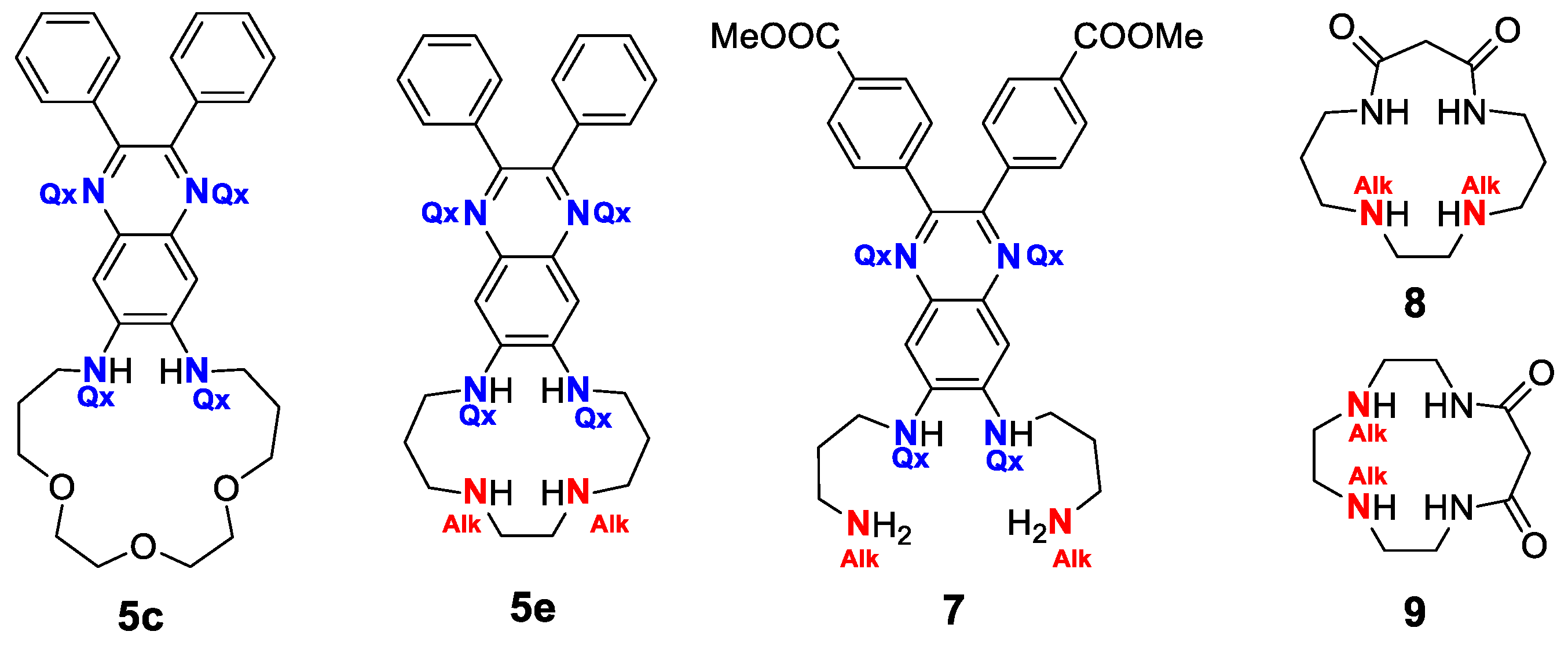
2.1. Synthesis
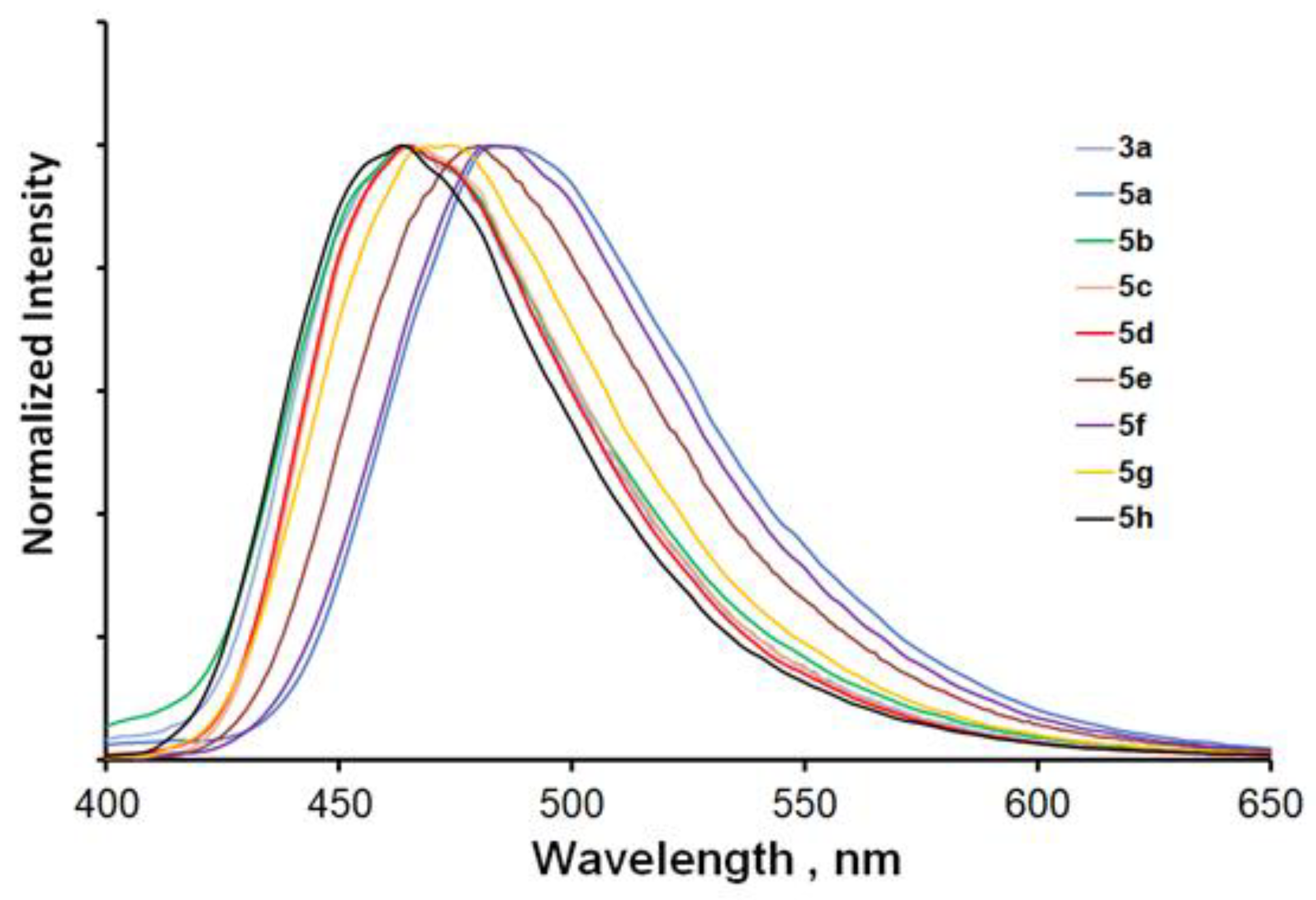
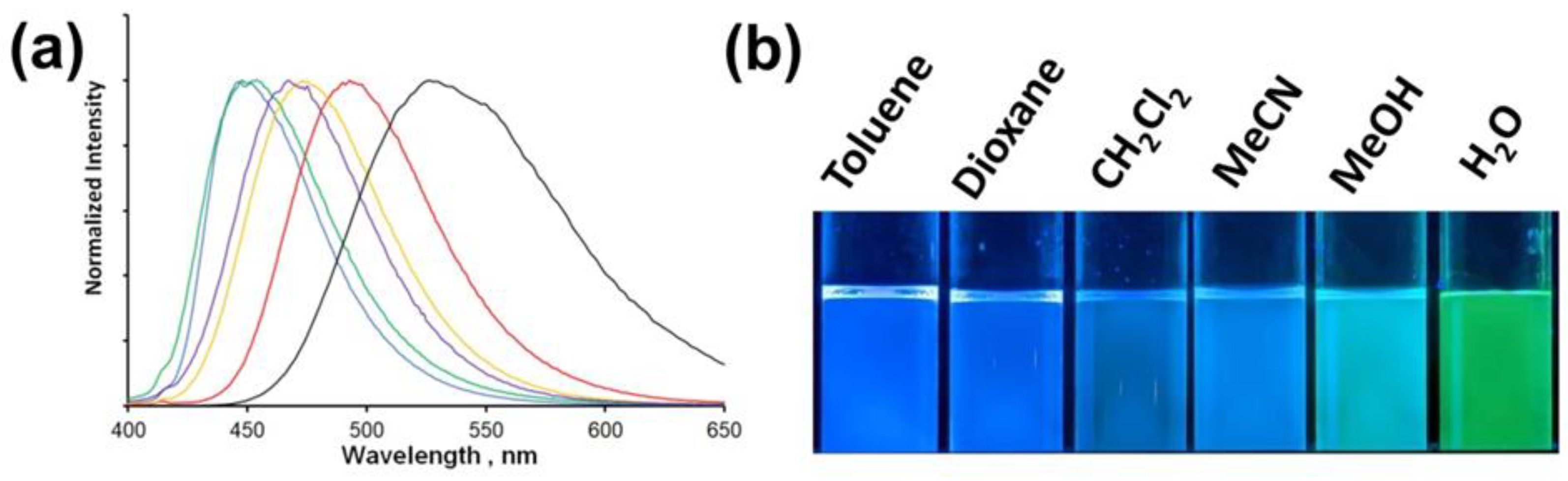
2.2. Optical Properties
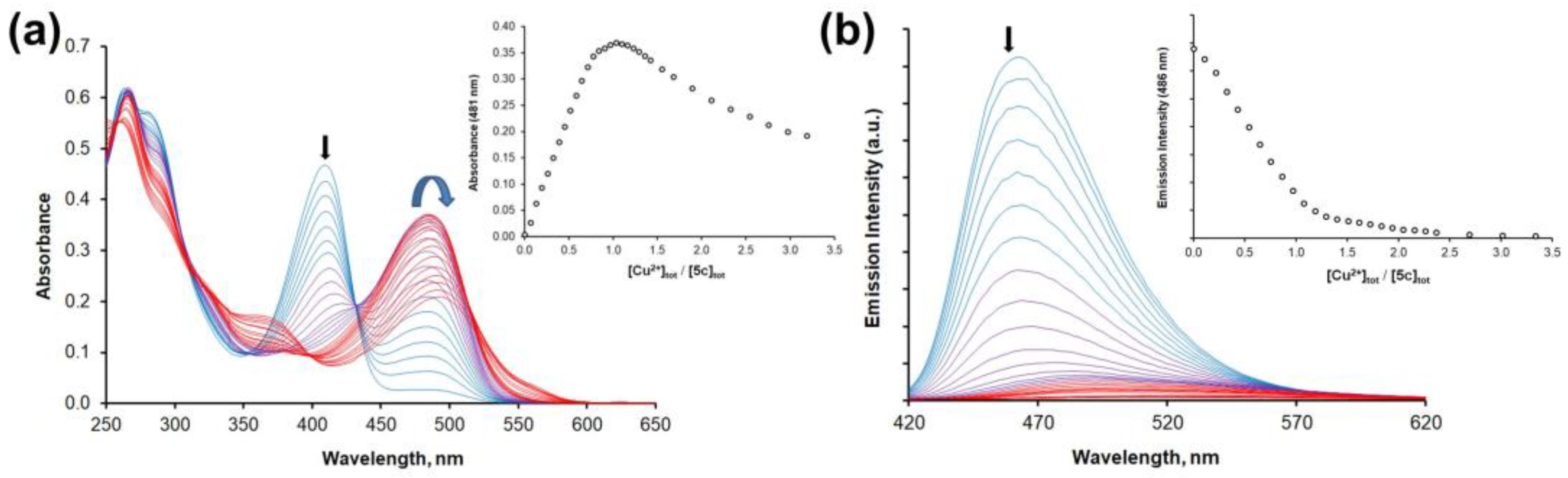
2.3. Detection of Metal Cations in Acetonitrile
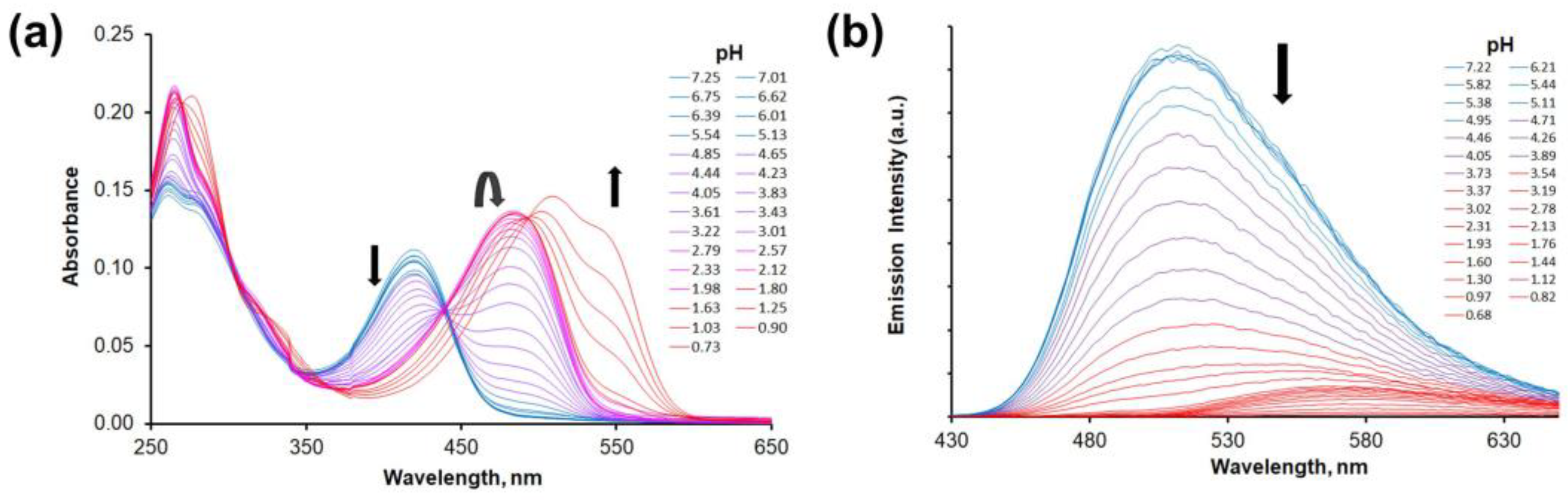
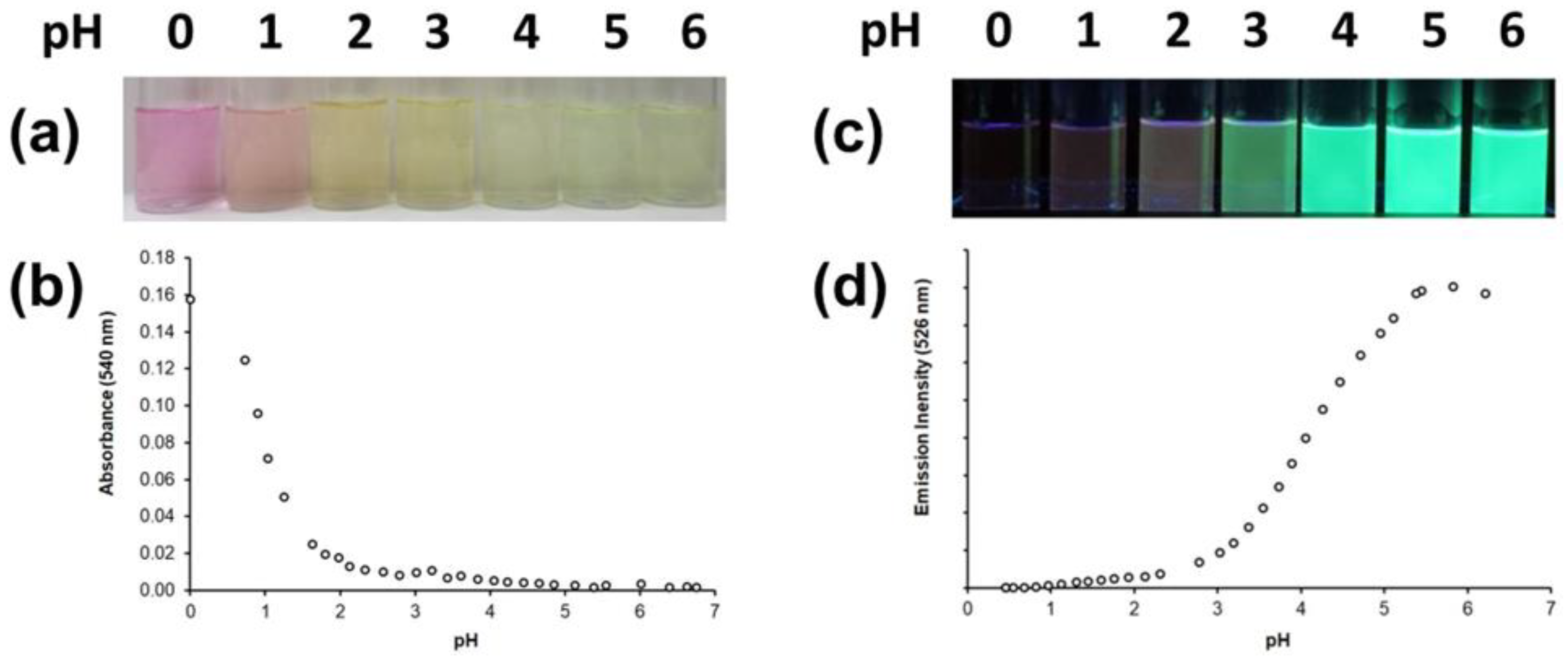
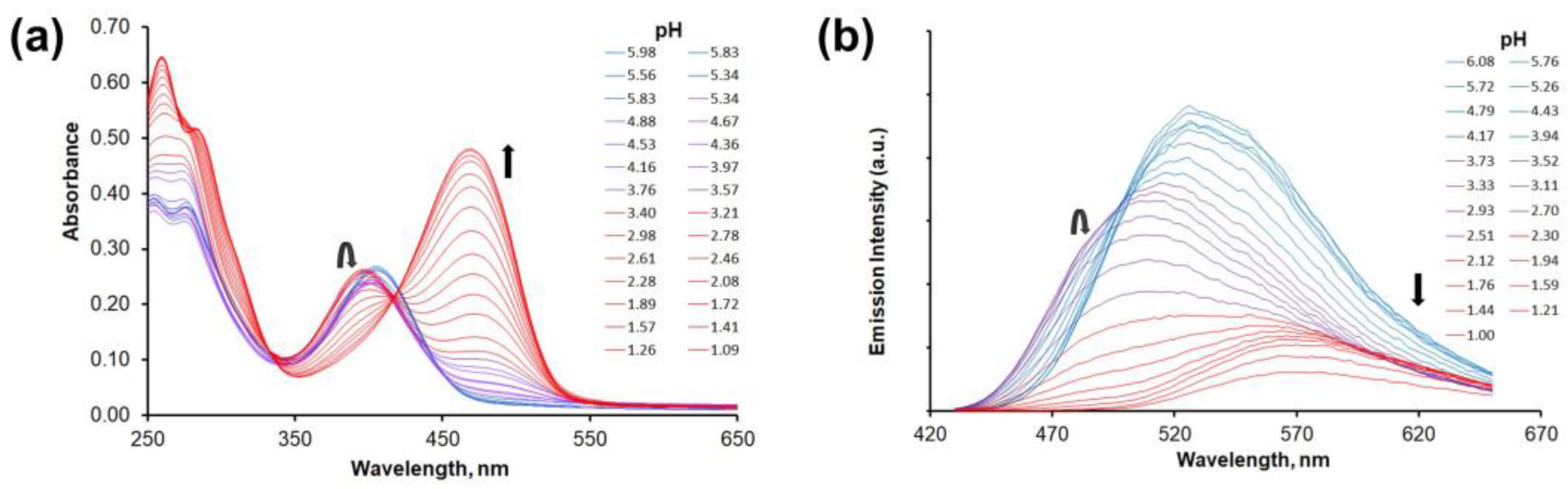
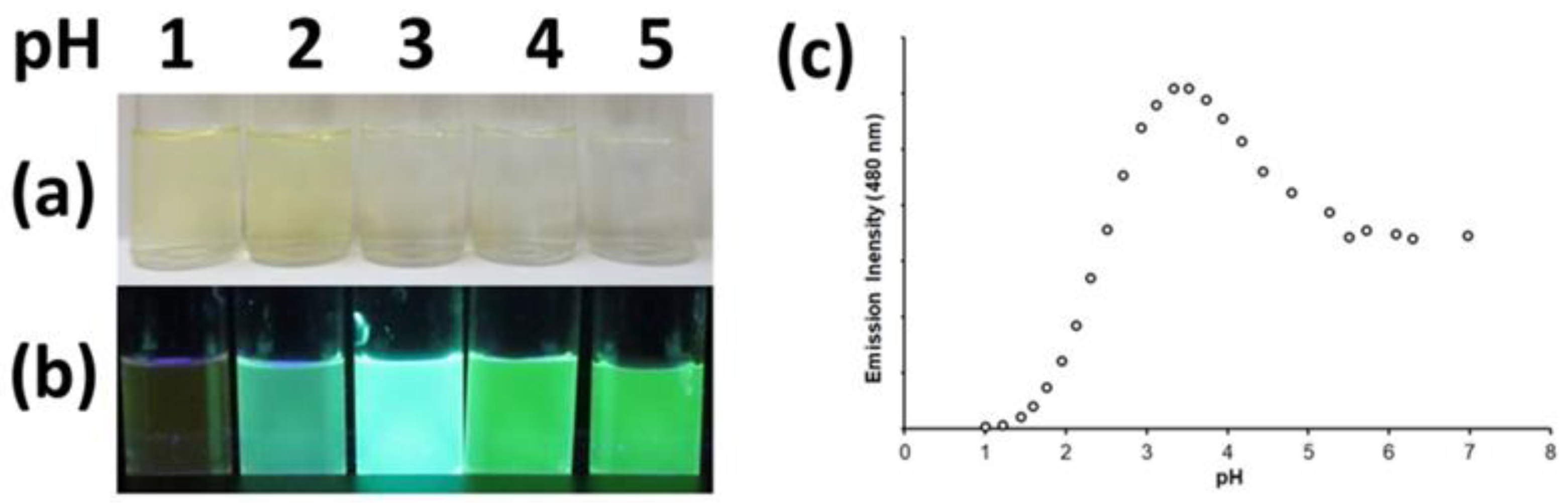
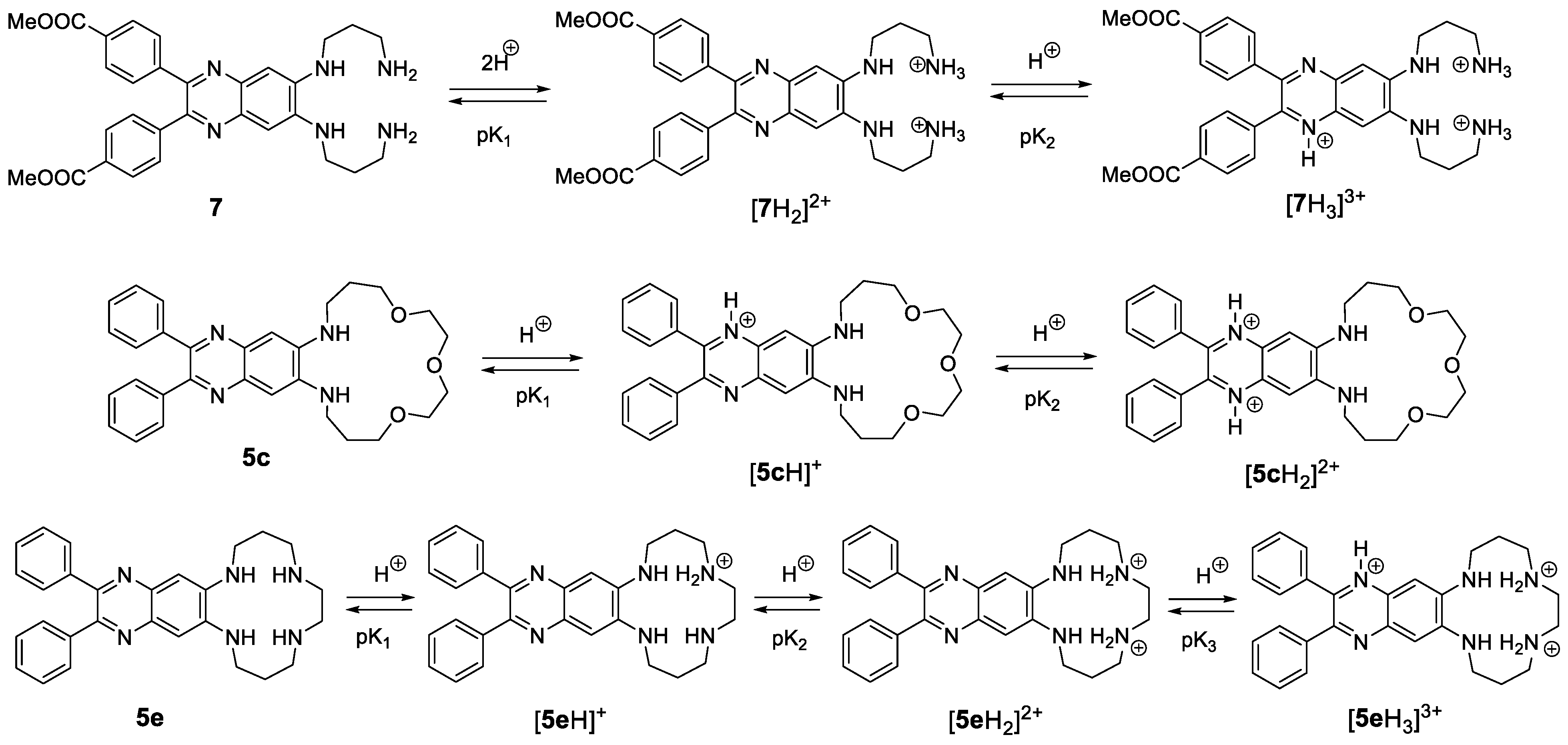
2.4. Protonation Studies
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. Synthesis
3.4. Protonation Studies
3.5. Metal Ions Complexation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, E.; Wang, K.; Guan, Z.-J.; He, H.-H.; Zhang, L.; Zhou, H.-C.; Huang, F.; Fang, Y. Organo-macrocycle-containing hierarchical metal–organic frameworks and cages: Design, structures, and applications. Chem. Soc. Rev. 2022, 51, 8378–8405. [Google Scholar] [CrossRef]
- Sameni, S.; Jeunesse, C.; Matt, D.; Harrowfield, J. Calix[4]arene daisychains. Chem. Soc. Rev. 2009, 38, 2117–2146. [Google Scholar] [CrossRef] [PubMed]
- Brücher, E.; Baranyai, Z.; Tircsó, G. The Future of Biomedical Imaging: Synthesis and Chemical Properties of the DTPA and DOTA Derivative Ligands and Their Complexes. In Biomedical Imaging: The Chemistry of Labels, Probes and Contrast Agents; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 208–260. [Google Scholar]
- Peng, S.; He, Q.; Vargas-Zuniga, G.I.; Qin, L.; Hwang, I.; Kim, S.K.; Heo, N.J.; Lee, C.H.; Dutta, R.; Sessler, J.L. Strapped calix[4]pyrroles: From syntheses to applications. Chem. Soc. Rev. 2020, 49, 865–907. [Google Scholar] [CrossRef] [PubMed]
- Serreli, V.; Lee, C.F.; Kay, E.R.; Leigh, D.A. A molecular information ratchet. Nature 2007, 445, 523–527. [Google Scholar] [CrossRef]
- Liu, S.; Kondratuk, D.V.; Rousseaux, S.A.; Gil-Ramirez, G.; O’Sullivan, M.C.; Cremers, J.; Claridge, T.D.; Anderson, H.L. Caterpillar track complexes in template-directed synthesis and correlated molecular motion. Angew. Chem. Int. Ed. Engl. 2015, 54, 5355–5359. [Google Scholar] [CrossRef]
- Basuray, A.N.; Jacquot de Rouville, H.P.; Hartlieb, K.J.; Kikuchi, T.; Strutt, N.L.; Bruns, C.J.; Ambrogio, M.W.; Avestro, A.J.; Schneebeli, S.T.; Fahrenbach, A.C.; et al. The chameleonic nature of diazaperopyrenium recognition processes. Angew. Chem. Int. Ed. Engl. 2012, 51, 11872–11877. [Google Scholar] [CrossRef]
- Andrews, C.G.; Macdonald, C.L. Crown ether ligation: An approach to low-oxidation-state indium compounds. Angew. Chem. Int. Ed. Engl. 2005, 44, 7453–7456. [Google Scholar] [CrossRef]
- Dailey, K.K.; Rauchfuss, T.B.; Yap, G.P.A.; Rheingold, A.L. Metalloporphyrins as ligands: Synthesis and characterization of[(η6-cymene)-Ru{η5-Ni(OEP)}]2+. Angew. Chem. Int. Ed. Engl. 1996, 35, 1833–1835. [Google Scholar] [CrossRef]
- Horie, M.; Sassa, T.; Hashizume, D.; Suzaki, Y.; Osakada, K.; Wada, T. A crystalline supramolecular switch: Controlling the optical anisotropy through the collective dynamic motion of molecules. Angew. Chem. Int. Ed. Engl. 2007, 46, 4983–4986. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Andreu, D.; Echavarren, A.M.; Prados, P.; De Mendoza, J. A receptor for the enantioselective recognition of phenylalanine and tryptophan under neutral conditions. J. Am. Chem. Soc. 1992, 114, 1511–1512. [Google Scholar] [CrossRef]
- Xue, X.; Lindstrom, A.; Li, Y. Porphyrin-based nanomedicines for cancer treatment. Bioconjugate Chem. 2019, 30, 1585–1603. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Gibson, H.W. Ion pairing and host−guest complexation in low dielectric constant solvents. J. Am. Chem. Soc. 2003, 125, 7001–7004. [Google Scholar] [CrossRef] [PubMed]
- Norvaisa, K.; Flanagan, K.J.; Gibbons, D.; Senge, M.O. Conformational re-engineering of porphyrins as receptors with switchable N-H⋅⋅⋅X-type binding modes. Angew. Chem. Int. Ed. Engl. 2019, 58, 16553–16557. [Google Scholar] [CrossRef]
- Francesconi, O.; Ienco, A.; Moneti, G.; Nativi, C.; Roelens, S. A self-assembled pyrrolic cage receptor specifically recognizes beta-glucopyranosides. Angew. Chem. Int. Ed. Engl. 2006, 45, 6693–6696. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of ion chemosensors based on porphyrin analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Todd, M.H.; Rutledge, P.J. Recent Advances in Macrocyclic Fluorescent Probes for Ion Sensing. Molecules 2017, 22, 200. [Google Scholar] [CrossRef]
- Bottaro, G.; Rizzo, F.; Cavazzini, M.; Armelao, L.; Quici, S. Efficient luminescence from fluorene- and spirobifluorene-based lanthanide complexes upon near-visible Irradiation. Chem. Eur. J. 2014, 20, 4598–4607. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Ferrand, A.-C.; Imbert, D.; Chauvin, A.-S.; Vandevyver, C.D.B.; Bünzli, J.-C.G. Non-cytotoxic, bifunctional EuIII and TbIII luminescent macrocyclic complexes for luminescence resonant energy-transfer experiments. Chem. Eur. J. 2007, 13, 8678–8687. [Google Scholar] [CrossRef]
- da Silva, L.C.; da Costa, E.P.; Freitas, G.R.S.; de Souza, M.A.F.; Araújo, R.M.; Machado, V.G.; Menezes, F.G. Ascorbic acid-based quinoxaline derivative as a chromogenic chemosensor for Cu2+. Inorg. Chem. Commun. 2016, 70, 71–74. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Molina, P.; Tarraga, A. A ferrocene-quinoxaline derivative as a highly selective probe for colorimetric and redox sensing of toxic mercury(II) cations. Sensors 2010, 10, 11311–11321. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Inada, K.; Zhao, L.; Komino, T.; Matsumoto, N.; Ribierre, J.C.; Adachi, C. Organic light emitting diodes with horizontally oriented thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2017, 1216–1223. [Google Scholar] [CrossRef]
- Tibiletti, F.; Simonetti, M.; Nicholas, K.M.; Palmisano, G.; Parravicini, M.; Imbesi, F.; Tollari, S.; Penoni, A. One-pot synthesis of meridianins and meridianin analogues via indolization of nitrosoarenes. Tetrahedron 2010, 66, 1280–1288. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hayashi, N.; Nakamura, M.; Hirai, T. Coumarin–imine–quinoxaline linkage designed based on the strecker reaction as a receptor for fluorometric cyanide anion detection in neutral media. Chem. Lett. 2016, 45, 1294–1296. [Google Scholar] [CrossRef]
- Duke, R.M.; Gunnlaugsson, T. Fluorescent sensing of anions using a bis-quinoxaline amidothiourea based supramolecular cleft; an example of an anion-induced deprotonation event. ChemInform 2010, 51, 5402–5405. [Google Scholar] [CrossRef]
- Shang, X.-F.; Li, J.; Lin, H.; Jiang, P.; Cai, Z.-S.; Lin, H.-K. Anion recognition and sensing of ruthenium(II) and cobalt(II) sulfonamido complexes. Dalton Trans. 2009, 38, 2096–2102. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Munir, I.; al-Rashida, M.; Maria, M.; Ayub, K.; Iqbal, J.; Ludwig, R.; Khan, K.M.; Ali, S.A.; Hameed, A. Novel quinoxaline based chemosensors with selective dual mode of action: Nucleophilic addition and host–guest type complex formation. RSC Adv. 2016, 6, 64009–64018. [Google Scholar] [CrossRef]
- Kruger, P.E.; Mackie, P.R.; Nieuwenhuyzen, M. Optical–structural correlation in a novel quinoxaline-based anion sensor. J. Chem. Soc. Perkin Trans. 2 2001, 2001, 1079–1083. [Google Scholar] [CrossRef]
- Lysandrou, Y.; Newsome, T.; Duty, K.; Mohamed, O.; Markiewicz, J.T. Synthesis, optical and electronic studies of a “clickable” quinoxaline-based pH sensor. J. Photochem. Photobiol. A Chem. 2022, 433, 114183. [Google Scholar] [CrossRef]
- More, Y.W.; Padghan, S.D.; Bhosale, R.S.; Pawar, R.P.; Puyad, A.L.; Bhosale, S.V.; Bhosale, S.V. Proton triggered colorimetric and fluorescence response of a novel quinoxaline compromising a donor-acceptor system. Sensors 2018, 18, 3433. [Google Scholar] [CrossRef]
- Ermakova, E.V.; Cheprakov, A.V.; Bessmertnykh-Lemeune, A. Aminoquinoxaline-based dual colorimetric and fluorescent sensors for pH measurement in aqueous media. Chemosensors 2022, 10, 342. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Z.; Zhong, C.; Xie, G.; Wu, K.; Ma, D.; Yang, C. Tuning the emission from local excited-state to charge-transfer state transition in quinoxaline-based butterfly-shaped molecules: Efficient orange OLEDs based on thermally activated delayed fluorescence emitter. Dyes Pigm. 2017, 141, 325–332. [Google Scholar] [CrossRef]
- Singh, P.S.; Chacko, S.; Kamble, R.M. The design and synthesis of 2,3-diphenylquinoxaline amine derivatives as yellow-blue emissive materials for optoelectrochemical study. New J. Chem. 2019, 43, 6973–6988. [Google Scholar] [CrossRef]
- Upadhyay, A.; Karpagam, S. Synthesis and photo physical properties of carbazole based quinoxaline conjugated polymer for fluorescent detection of Ni2+. Dyes Pigm. 2017, 139, 50–64. [Google Scholar] [CrossRef]
- Cui, W.; Wang, L.; Xiang, G.; Zhou, L.; An, X.; Cao, D. A colorimetric and fluorescence “turn-off” chemosensor for the detection of silver ion based on a conjugated polymer containing 2,3-di(pyridin-2-yl)quinoxaline. Sens. Actuators B Chem. 2015, 207, 281–290. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, M.-S.; Hong, S.-J.; Sessler, J.L.; Lee, C.-H. Selective sensing of snions with calix[4]pyrroles strapped with chromogenic dipyrrolylquinoxalines. J. Org. Chem. 2009, 74, 1065–1069. [Google Scholar] [CrossRef]
- Lücking, U.; Rudkevich, D.M.; Rebek, J. Deep cavitands for anion recognition. ChemInform 2000, 41, 9547–9551. [Google Scholar] [CrossRef]
- Yin, Y.; Sarma, T.; Wang, F.; Yuan, N.; Duan, Z.; Sessler, J.L.; Zhang, Z. Air-stable N, N′-dihydroporphycene: A quinoxaline-fused tetrapyrrolic macrocycle that detects fluoride anion via deprotonation. Org. Lett. 2019, 21, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Maeda, H.; Mizuno, T.; Lynch, V.M.; Furuta, H. Quinoxaline-bridged porphyrinoids. J. Am. Chem. Soc. 2002, 124, 13474–13479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, X.-J.; Wong, W.-Y.; Guo, J.-P.; Wong, W.-K.; Li, Z.-Y. Dipyrrolylquinoxaline-bridged Schiff bases: A new class of fluorescent sensors for mercury(II). Dalton Trans. 2005, 34, 3235–3240. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.J.; Jun, E.J.; Chellappan, K.; Thangadurai, D.; Chandran, R.P.; Hwang, I.-C.; Yoon, J.; Kim, K.S. Quinoxaline−imidazolium receptors for unique sensing of pyrophosphate and acetate by charge transfer. Org. Lett. 2007, 9, 485–488. [Google Scholar] [CrossRef]
- Li, Y.-P.; Yang, H.-R.; Zhao, Q.; Song, W.-C.; Han, J.; Bu, X.-H. Ratiometric and selective fluorescent sensor for Zn2+ as an “Off–On–Off” switch and logic gate. Inorg. Chem. 2012, 51, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Averin, A.D.; Bessmertnykh, A.G.; Denat, F.; Guilard, R. Palladium-catalyzed amination in the synthesis of polyazamacrocycles. Russ. J. Org. Chem. 2010, 46, 947–967. [Google Scholar] [CrossRef]
- Averin, A.D.; Uglov, A.N.; Buryak, A.K.; Beletskaya, I.P. Pd-catalyzed amination of isomeric dibromobiphenyls: Possibilities of one-step synthesis of macrocycles. Mendeleev Commun. 2010, 20, 1–3. [Google Scholar] [CrossRef]
- Ranyuk, E.R.; Averin, A.D.; Beletskaya, I.P. One-step synthesis of chiral azamacrocycles via palladium-catalyzed enantioselective amination of 1,5-dichloroanthraquinone and 1,5-dichloroanthracene. Adv. Synth. Catal. 2010, 352, 2299–2305. [Google Scholar] [CrossRef]
- Yakushev, A.; Chernichenko, N.; Anokhin, M.; Averin, A.; Buryak, A.; Denat, F.; Beletskaya, I. Pd-catalyzed amination in the synthesis of a new Family of macropolycyclic compounds comprising diazacrown ether moieties. Molecules 2014, 19, 940–965. [Google Scholar] [CrossRef]
- Ermakova, E.; Raitman, O.; Shokurov, A.; Kalinina, M.; Selector, S.; Tsivadze, A.; Arslanov, V.; Meyer, M.; Bessmertnykh-Lemeune, A.; Guilard, R. A metal-responsive interdigitated bilayer for selective quantification of mercury(II) traces by surface plasmon resonance. Analyst 2016, 141, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.; Michalak, J.; Meyer, M.; Arslanov, V.; Tsivadze, A.; Guilard, R.; Bessmertnykh-Lemeune, A. Colorimetric Hg2+ sensing in water: From molecules toward low-cost solid devices. Org. Lett. 2013, 15, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Averin, A.D.; Beletskaya, I.P. Synthesis of polymacrocyclic compounds via Pd-catalyzed amination and evaluation of their derivatives as metal detectors. Pure Appl. Chem. 2019, 91, 633–651. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Beletskaya, I.P. Oxaazamacrocycles incorporating the quinoline moiety: Synthesis and the study of their binding properties towards metal cations. New J. Chem. 2016, 40, 5818–5828. [Google Scholar] [CrossRef]
- Abel, A.S.; Mitrofanov, A.Y.; Rousselin, Y.; Denat, F.; Bessmertnykh-Lemeune, A.; Averin, A.D.; Beletskaya, I.P. Ditopic Macrocyclic Receptors with a 4,7-diamino-1,10-phenanthroline fragment for multimodal detection of toxic metal ions. ChemPlusChem 2016, 81, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Averin, A.D.; Grigorova, O.K.; Malysheva, A.S.; Shaferov, A.V.; Beletskaya, I.P. Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors. Pure Appl. Chem. 2020, 92, 1367–1386. [Google Scholar] [CrossRef]
- Shaferov, A.V.; Malysheva, A.S.; Averin, A.D.; Maloshitskaya, O.A.; Beletskaya, I.P. Synthesis and evaluation of the (S)-BINAM derivatives as fluorescent enantioselective detectors. Sensors 2020, 20, 3234. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Su, H.; Si, Y.; Liu, Y.; Peng, Q.; He, J.; Hou, H.; Li, K. An o-phthalimide-based multistimuli-responsive aggregation-induced emission (AIE) system. Mater. Chem. Front. 2019, 3, 50–56. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Averin, A.D. Palladium-catalyzed arylation of linear and cyclic polyamines. Pure Appl. Chem. 2004, 76, 1605–1619. [Google Scholar] [CrossRef]
- Averin, A.D.; Shukhaev, A.V.; Buryak, A.K.; Beletskaya, I.P. Synthesis of polyaza macrocycles by palladium-catalyzed amination of 1,2-dibromobenzene and 2-bromo-1,3-dichlorobenzene. Russ. J. Org. Chem. 2009, 45, 1353. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Bessmertnykh, A.G.; Guilard, R. Palladium-catalyzed synthesis of aryl-substituted polyamine compounds from aryl halides. ChemInform 1997, 38, 2287–2290. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Beletskaya, I.P.; Meyer, M.; Bessmertnykh-Lemeune, A. Ruthenium(II) complexes with (3-polyamino)phenanthrolines: Synthesis and spplication in sensing of Cu(II) ions. Chemosensors 2022, 10, 79. [Google Scholar] [CrossRef]
- Shen, Q.; Hartwig, J.F. [(CyPF-tBu)PdCl2]: An air-stable, one-component, highly efficient catalyst for amination of heteroaryl and aryl halides. Org. Lett. 2008, 10, 4109–4112. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-H.; Chen, X. New fluorescent light-up quinoxalines differentiate between parallel and nonparallel G-quadruplex topologies using different excitation/emission channels. Chem. Commun. 2020, 56, 4168–4171. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Wei, Y.; Lin, J.-S.; Moturu, M.V.R.K.; Chao, W.-S.; Tao, Y.-T.; Chien, C.-H. Doubly ortho-linked quinoxaline/diphenylfluorene hybrids as bipolar, fluorescent chameleons for optoelectronic applications. J. Am. Chem. Soc. 2006, 128, 10992–10993. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Lin, J.T.; Tao, Y.-T.; Chuen, C.-H. Quinoxalines incorporating triarylamines: potential electroluminescent materials with tunable emission characteristics. Chem. Mater. 2002, 14, 2796–2802. [Google Scholar] [CrossRef]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum yield and brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E. Equilibria and kinetics of copper(II) complex formation of a linear and of 13–15-membered macrocyclic dioxo-tetra-amines. J. Chem. Soc. Dalton Trans. 1979, 325–329. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Roznyatovsky, V.A.; Denat, F.; Bessmertnykh-Lemeune, A.; Beletskaya, I.P. 6-Polyamino-substituted quinolines: Synthesis and multiple metal (CuII, HgII and ZnII) monitoring in aqueous media. Org. Biomol. Chem. 2019, 17, 4243–4260. [Google Scholar] [CrossRef]
- Kaganskii, M.M.; Dvoryantseva, G.G.; Elina, A.S. UV spectra and basicity constants of 2-substituted quinoxalines. Chem. Heterocycl. Compounds 1973, 9, 369–373. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Leiner, M.; Hochmuth, P.; Geiger, H. Absorption and fluorescence spectra, pKa values, and fluorescence lifetimes of monohydroxyflavones and monomethoxyflavones. Ber. Bunsenges. Phys. Chem. 1984, 88, 759–767. [Google Scholar] [CrossRef]
- Chambron, J.-C.; Meyer, M. The ins and outs of proton complexation. Chem. Soc. Rev. 2009, 38, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.D.; Motekaitis, R.J.; Mashishi, J.; Cukrowski, I.; Reibenspies, J.H.; Martell, A.E. The unusual protonation constants of cyclam. A potentiometric, crystallographic and molecular mechanics study. J. Chem. Soc. Perkin Trans. 2 1996, 1925–1929. [Google Scholar] [CrossRef]
- Shao, J.; Chang, J.; Chi, C. Linear and star-shaped pyrazine-containing acene dicarboximides with high electron-affinity. Org. Biomol. Chem. 2012, 10, 7045–7052. [Google Scholar] [CrossRef]
- Ukai, T.; Kawazura, H.; Ishii, Y.; Bonnet, J.J.; Ibers, J.A. Chemistry of dibenzylideneacetone-palladium(0) complexes. J. Organomet. Chem. 1974, 65, 253–266. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Beck, M.T.; Nagypál, I. Chemistry of Complex Equilibria; Halsted Press, Ellis Horwood: Chichester, UK, 1990. [Google Scholar]
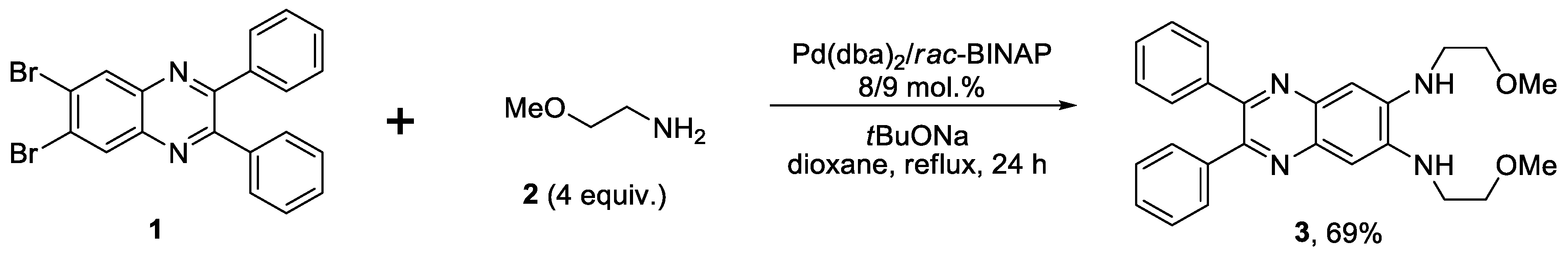
| Compound | λabs, nm (log ε) | λem, nm | Φ, % a | Brightness b, B, Μ−1 cm−1 c |
|---|---|---|---|---|
| 3 | 261 (4.51) 279 (4.50) 404 (4.36) | 465 | 59 | 13,494 |
| 5a | 260 (4.37) 282 (4.34) 408 (4.20) | 485 | 52 | 8241 |
| 5b | 260 (4.48) 279 (4.46) 404 (4.35) | 465 | 48 | 10,746 |
| 5c | 262 (4.38) 282 (4.35) 409 (4.24) | 465 | 44 | 9674 |
| 5d | 263 (4.51) 280 (4.47) 410 (4.41) | 466 | 56 | 14,394 |
| 5e | 257 (4.39) 278 (4.36) 400 (4.19) | 480 | 44 | 6795 |
| 5f | 281 (4.29) 404 (4.11) | 486 | 68 | 8760 |
| 5g | 260 (4.30) 279 (4.29) 404 (4.19) | 464 | 65 | 10,067 |
| 5h | 262 (4.39) 280 (4.37) 406 (4.26) | 465 | 58 | 10,554 |
| Ligand | Complex | log(β) a |
|---|---|---|
| 3 | [Cu(3)]2+ [Cu2(3)]4+ [Cu3(3)]6+ | 7.57(7) 13.3(3) 18.7(2) |
| 5a | [Cu(5a)]2+ [Cu4(5a)3]8+ | 6.53(1) 33.9(3) |
| 5b | [Cu(5b)]2+ [Cu4(5b)3]8+ | 6.73(1) 34.7(1) |
| 5c | [Cu(5c)]2+ [Cu4(5c)2]8+ | 6.47(1) 28.3(3) |
| 5d | [Cu(5d)]2+ [Cu4(5d)2]8+ | 7.01(1) 28.7(1) |
| Compound | Method | pK(Nalk) | pK(Nalk) | pK(NQx) | pK(NQx) |
|---|---|---|---|---|---|
| 5ca | UV-vis b | – | – | 4.10(5) | 0.6(1) |
| FL c | – | – | 3.96(5) | 1.20(5) | |
| 5ea | UV-vis b | {9.5} d | 3.69(5) | 2.36(5) | – e |
| FL c | {9.5} d | 4.01(5) | 2.46(5) | 1.0(1) | |
| 7f | UV-vis b | 10.25 | 10.25 | 2.61 | – |
| FL c | 9.32 | 9.32 | 3.07 | – | |
| 8g | PT h | 9.40 | 6.52 | – | – |
| 9g | PT h | 9.05 | 3.82 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurashov, I.A.; Kharlamova, A.D.; Abel, A.S.; Averin, A.D.; Beletskaya, I.P. Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution. Molecules 2023, 28, 512. https://doi.org/10.3390/molecules28020512
Kurashov IA, Kharlamova AD, Abel AS, Averin AD, Beletskaya IP. Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution. Molecules. 2023; 28(2):512. https://doi.org/10.3390/molecules28020512
Chicago/Turabian StyleKurashov, Igor A., Alisa D. Kharlamova, Anton S. Abel, Alexei D. Averin, and Irina P. Beletskaya. 2023. "Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution" Molecules 28, no. 2: 512. https://doi.org/10.3390/molecules28020512
APA StyleKurashov, I. A., Kharlamova, A. D., Abel, A. S., Averin, A. D., & Beletskaya, I. P. (2023). Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution. Molecules, 28(2), 512. https://doi.org/10.3390/molecules28020512