Synthesis, Structure, and Spectral-Luminescent Properties of Peripherally Fluorinated Mg(II) and Zn(II) Octaphenyltetraazaporphyrins
Abstract
1. Introduction
2. Results and Discussion
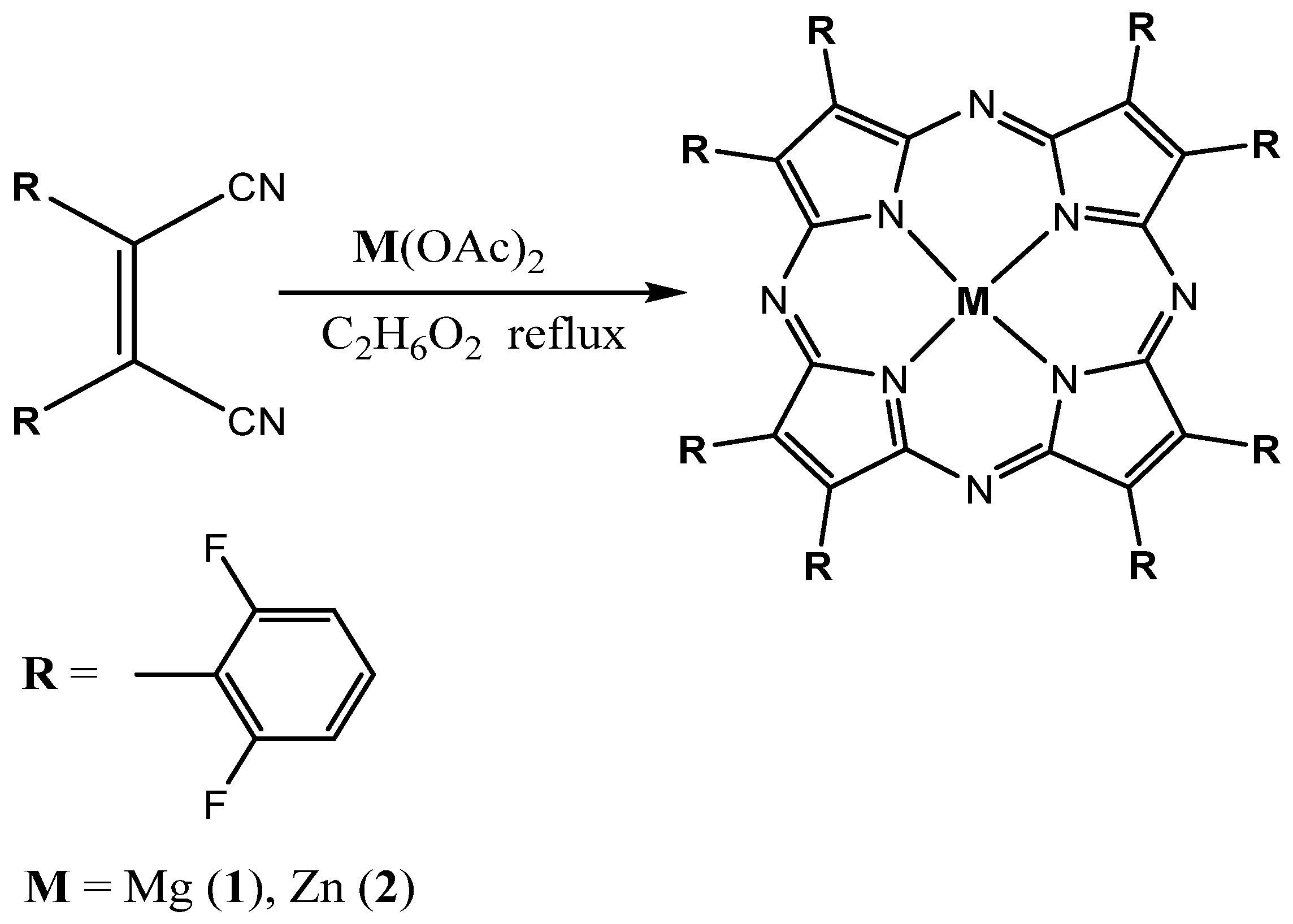
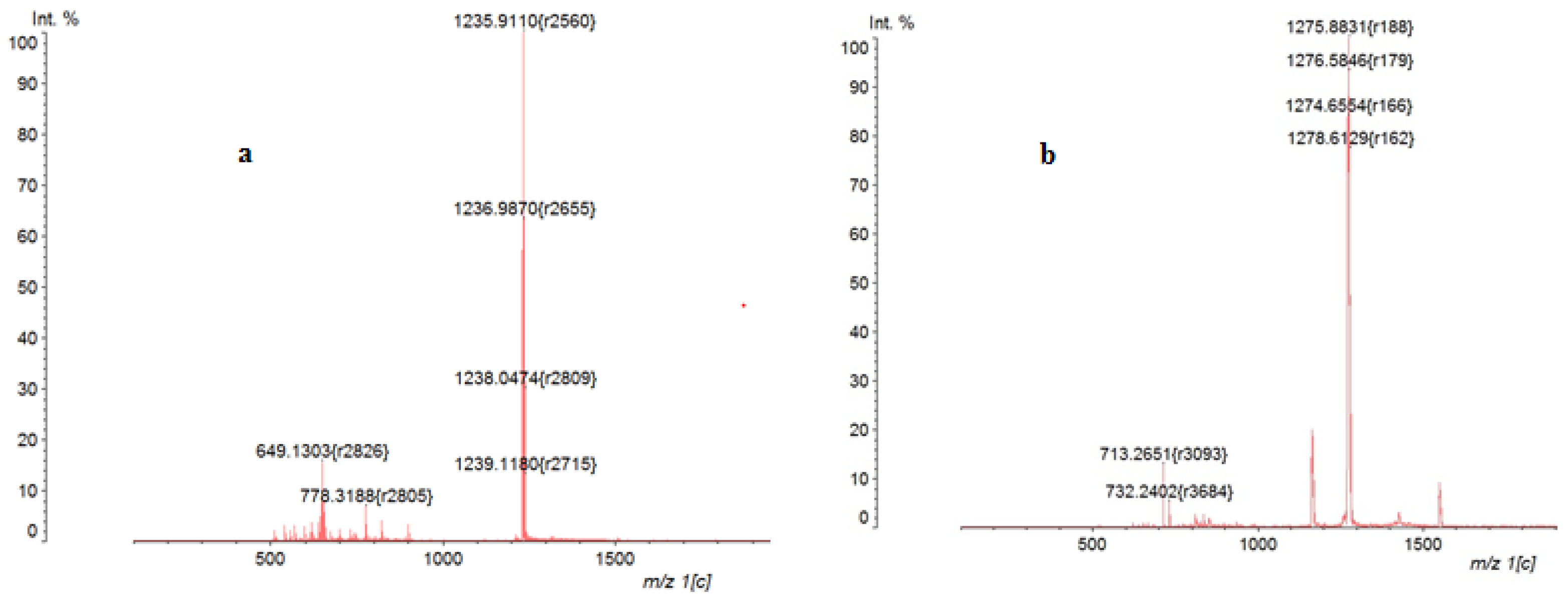
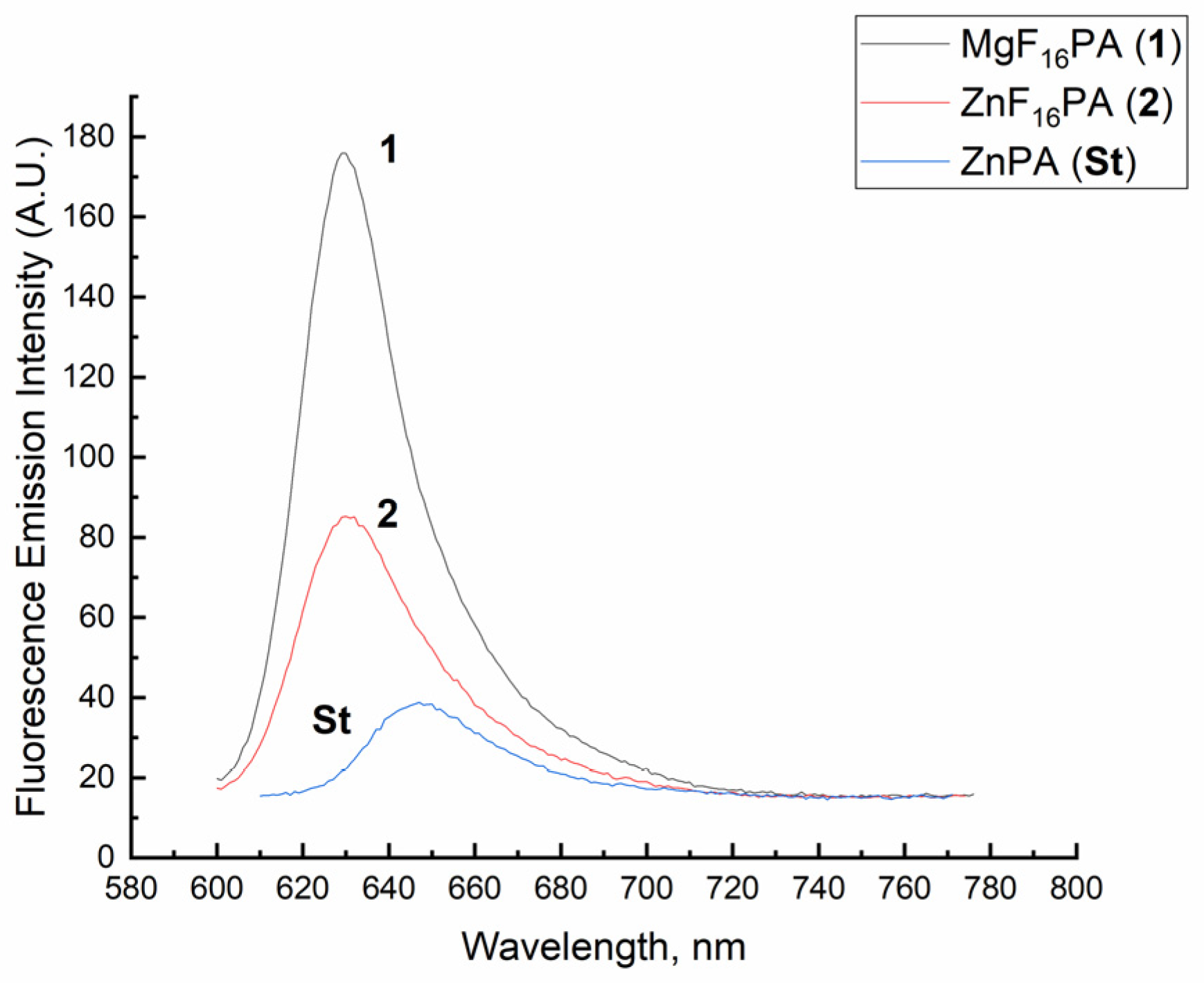
2.1. Synthesis and Spectral-Fluorescence Properties of Mg(II)- and Zn(II)-Octa-(2,6-difluorophenyl)tetraazaporphyrins
2.2. Geometrical Optimization and Energy Characteristics
2.3. Mathematical Simulation for Prediction of Optical Properties
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De la Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 38, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Mshchenko, T.A.; Turubanova, V.D.; Mitroshina, E.V.; Alzeibak, R.; Peskova, N.N.; Lermontova, S.A.; Klapshina, L.G.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Effect of novel porphyrazine photosensitizers on normal and tumor brain cells. J. Biophotonics 2020, 13, e201960077. [Google Scholar] [CrossRef]
- Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B.K.; Kajiwara, T.; Takaishi, S.; Ishikawa, N.; Isshiki, H.; Zhang, Y.F.; et al. Direct observation of lanthanide (III)-phthalocyanine molecules on Au (111) by using scanning tunneling microscopy and scanning tunneling spectroscopy and thin-film field-effect transistor properties of Tb (III)-and Dy (III)-phthalocyanine molecules. J. Am. Chem. 2009, 131, 9967–9976. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Goswami, L.N.; Chen, Y.; Gryshuk, A.; Missert, J.R.; Oseroff, A.; Dougherty, T.J. Nature: A rich source for developing multifunctional agents. Tumor-imaging and photodynamictherapy. Lasers Surg. Med. 2006, 38, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Ghoroghchian, P.P.; Frail, P.R.; Susumu, K.; Blessington, D.; Brannan, A.K.; Bates, F.S.; Chance, B.; Hammer, D.A.; Therien, M.J. Nearinfrared- emissive polymersomes: Self-assembled soft matter for in vivo optical imaging. Proc. Natl. Acad. Sci. USA 2005, 102, 2922–2927. [Google Scholar] [CrossRef]
- Trivedi, E.R.; Blumenfeld, C.M.; Wielgos, T.; Pokropinski, S.; Dande, P.; Hai, T.T.; Barrett Anthony, G.M.; Hoffman, B.M. Multi-gram synthesis of a porphyrazine platform for cellular translocation, conjugation to doxorubicin, and cellular uptake. Tetrahedron Lett. 2012, 53, 5475–5478. [Google Scholar] [CrossRef]
- Chen, L.; Zhanga, Z.; Wang, Y.; Guan, Y.; Deng, K.; Lv, K.; Sun, J.; Li, Z.; Li, M. Photocatalytic properties and electrochemical characteristic of a novel biomimetic oxygenase enzyme photocatalyst iron(II) tetrahydroxymethyl-tetra-(1,4-dithiin)porphyrazine for the degradation of organic pollutants. J. Mol. Catal. 2013, 372, 114–120. [Google Scholar] [CrossRef]
- Trivedi, E.R.; Harney, A.S.; Olive, M.B.; Podgorski, I.; Moin, K.; Sloane, B.F.; Barrett, A.G.M.; Meade, T.J.; Hoffman, B.M. Chiral porphyrazine near-IR optical imaging agent exhibiting preferential tumor accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 1284–1288. [Google Scholar] [CrossRef]
- Goslinski, T.; Tykarska, E.; Kryjewski, M.; Osmalek, T.; Sobiak, S.; Gdaniec, M.; Dutkiewicz, Z.; Mielcarek, J. Potential aluminium (III)-and gallium (III)-selective optical sensors based on porphyrazines. Anal. Sci. 2011, 27, 511–515. [Google Scholar] [CrossRef]
- Klapshina, L.G.; Douglas, W.E.; Grigoryev, I.S.; Korytin, A.I.; Lavrentiev, S.A.; Lopatin, M.A.; Lukyanov, A.Y.; Semenov, V.V.; Gerbierb, P.; Treushnikov, V.M. Novel metal-template assembled highlyfunctionalizedcyanoporphyrazine ytterbium and vanadium complexes for potential photonic and optoelectronic applications. J. Mater. Chem. 2009, 19, 3668–3676. [Google Scholar] [CrossRef]
- Lermontova, S.A.; Grigoryev, I.S.; Shilyagina, N.Y.; Peskova, N.N.; Balalaeva, I.V.; Shirmanova, M.V.; Klapshina, L.G. New porphyrazine macrocycles with high viscosity-sensitive fluorescence parameters. Russ. J. Phys. Chem. 2016, 86, 1330–1338. [Google Scholar] [CrossRef]
- Berezim, B.D. Coordination Compounds of Porphyrins and Phthalocyanine; Wiley: New York, NY, USA; Toronto, ON, Canada, 1981; 286p. [Google Scholar]
- Linstead, R.P.; Weiss, P.T. Phthalocyanines and related compounds. Part XX. Further investigations on tetrabenzporphin and allied substances. J. Chem. Soc. 1950, 11, 2975–2981. [Google Scholar] [CrossRef]
- Chizhova, N.V.; Romanova, A.O. Synthesis of palladium(II) and nickel(II) complexes with tetrabenzoporphine. Russ J. Inorg. Chem. 2007, 52, 1713–1716. [Google Scholar] [CrossRef]
- Sheinin, V.B.; Chizhova, N.V.; Romanova, A.O. Synthesis and spectral characteristics of (tetrabenzoporphyrinato)chloromanganese(III). Russ. J. Gen. Chem. 2010, 80, 351–356. [Google Scholar] [CrossRef]
- Cook, A.H.; Linstead, R.P. Phthalocyanines. PartXI. The preparation of octaphenylporphyrazines from diphenylmaleinnitrile. J. Chem. Soc. 1937, 929–933. [Google Scholar] [CrossRef]
- Stuzhin, P.A.; Goryachev, M.Y.; Ivanova, S.S.; Nazarova, A.; Pimkov, I.; Koifman, O.I. Perfluorinated porphyrazines 1: Synthesis and UV-vis spectral study of perfluorinated octaphenylporphyrazine and its indium(III) complex, [MPA(F5Ph)8](M = 2H, InIII(OH)). J. Porphyr. Phthalocyanines 2013, 17, 905–912. [Google Scholar] [CrossRef]
- Zvezdina, S.V.; Mal’tseva, O.V.; Chizhova, N.V.; Mamardashvili, N.Z. Complexation properties of octa(4-bromophenyl)tetraazaporphyrin and its magnesium(II) complex with salts of d-metals in DMF. Macroheterocycles 2014, 7, 276–280. [Google Scholar] [CrossRef]
- Chizhova, N.V.; Ivanova, Y.B.; Rusanov, A.I.; Khrushkova, Y.V.; Mamardashvili, N.Z. Synthesis and spectral and fluorescent properties of metal complexes of octakis(4-flurophenyl)tetraazaporphyrins. Russ. J. Org. Chem. 2019, 55, 655–661. [Google Scholar] [CrossRef]
- Lebedeva, I.A.; Ivanova, S.S.; Novakova, V.; Zhabanov, Y.A.; Stuzhin, P.A. Perfluorinated porphyrazines. 3. Synthesis, spectral-luminescence and electrochemical properties of perfluorinated octaphenylporphyrazinatozinc(II). J. Fluorine Chem. 2018, 214, 86–93. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; Volume 26, 954p. [Google Scholar]
- Weinhold, F.; Landis, C.R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2001, 2, 91–102. [Google Scholar] [CrossRef]
- Lee, N.; Petrenko, T.; Bergmann, U.; Neese, F.; De Beer, S. Probing valence orbital composition with iron Kβ X-ray emission spectroscopy. J. Am. Chem. Soc. 2010, 132, 9715–9727. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 20, 596p. [Google Scholar]
- Moran, D.; Simmonett, A.C.; Leach, F.E.; Allen, W.D.; Schleyer, P.v.R.; Schaefer, H.F. Popular theoretical methods predict benzene and arenes to be nonplanar. J. Am. Chem. Soc. 2006, 128, 9342–9343. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, A.I.; Dmitrieva, O.A.; Mamardashvili, N.Z.; Tetko, I.V. More is not always better: Local models provide accurate predictions of spectral properties of porphyrins. Int. J. Mol. Sci. 2022, 23, 1201–1212. [Google Scholar] [CrossRef]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online Chemical Modeling Environment (OCHEM):Web Platform for Data Storage, Model Development and Publishing of Chemical Information. Comput. Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Ochem. Available online: https://ochem.eu (accessed on 12 September 2022).
- Tetko, I.V.; Sushko, I.; Pandey, A.K.; Zhu, H.; Tropsha, A.; Papa, E.; Öberg, T.; Todeschini, R.; Fourches, D.; Varnek, A. Critical Assessment of QSAR Models of Environmental Toxicity against Tetrahymena Pyriformis: Focusing on Applicability Domain and Overfitting by Variable Selection. J. Chem. Inf. Model. 2008, 48, 1733–1746. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Ksenofontov, A.A.; Mozgova, V.A.; Gruzdev, M.S.; Chervonova, U.V.; Shelaev, I.V.; Lomova, T.N. Meso-carbazole substituted porphyrin complexes: Synthesis and spectral properties according to experiment, DFT calculations and the prediction by machine learning methods. Dye. Pigment. 2022, 204, 110470–110482. [Google Scholar] [CrossRef]
- Dmitrieva, O.A.; Chizhova, N.V.; Rusanov, A.I.; Koifman, M.O.; Mamardashvili, N.Z. Spectral-fluorescence properties of Zn(II)-octaphenyltetraazaporphyrins. J. Fluoresc. 2020, 30, 657–664. [Google Scholar] [CrossRef] [PubMed]
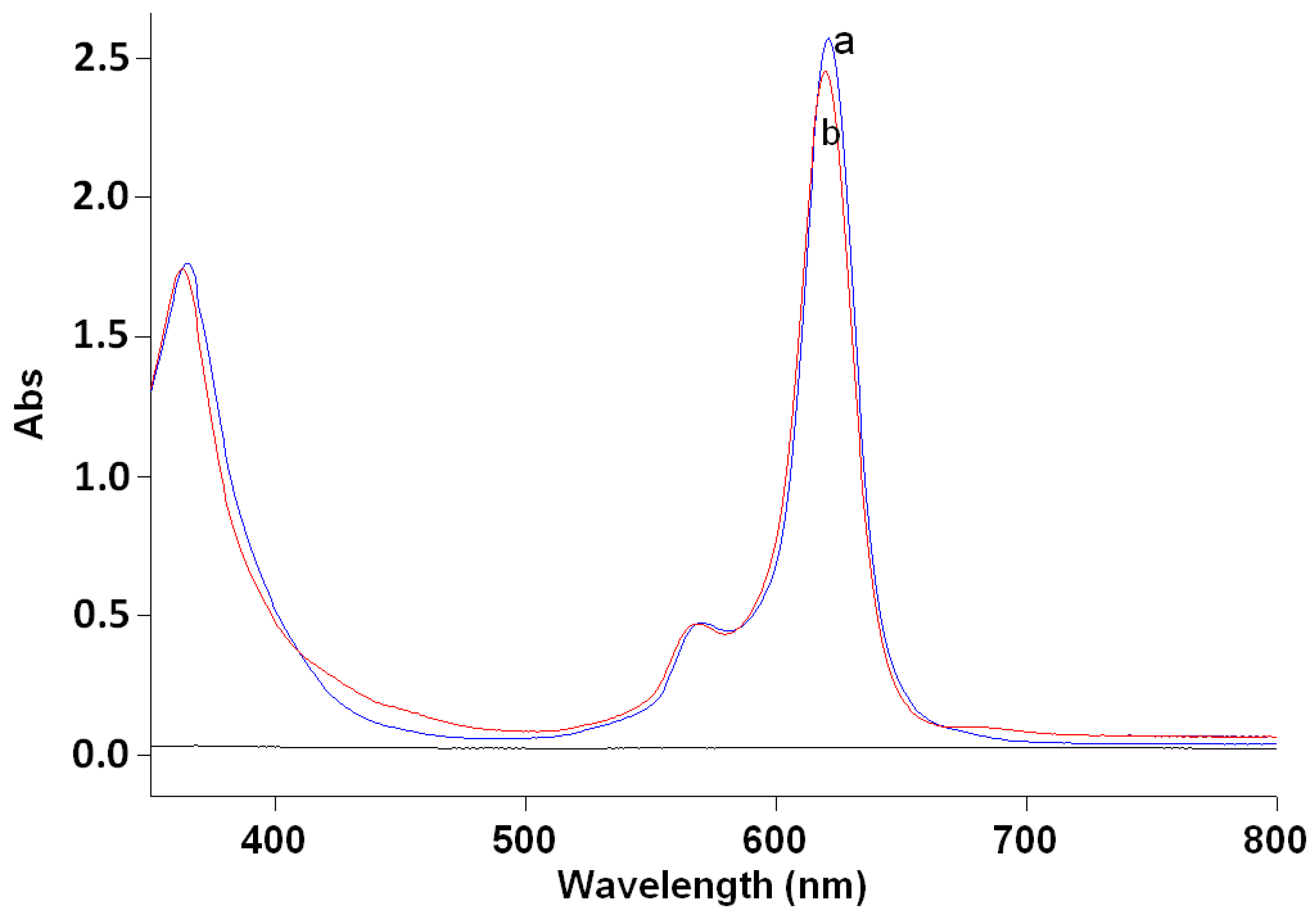
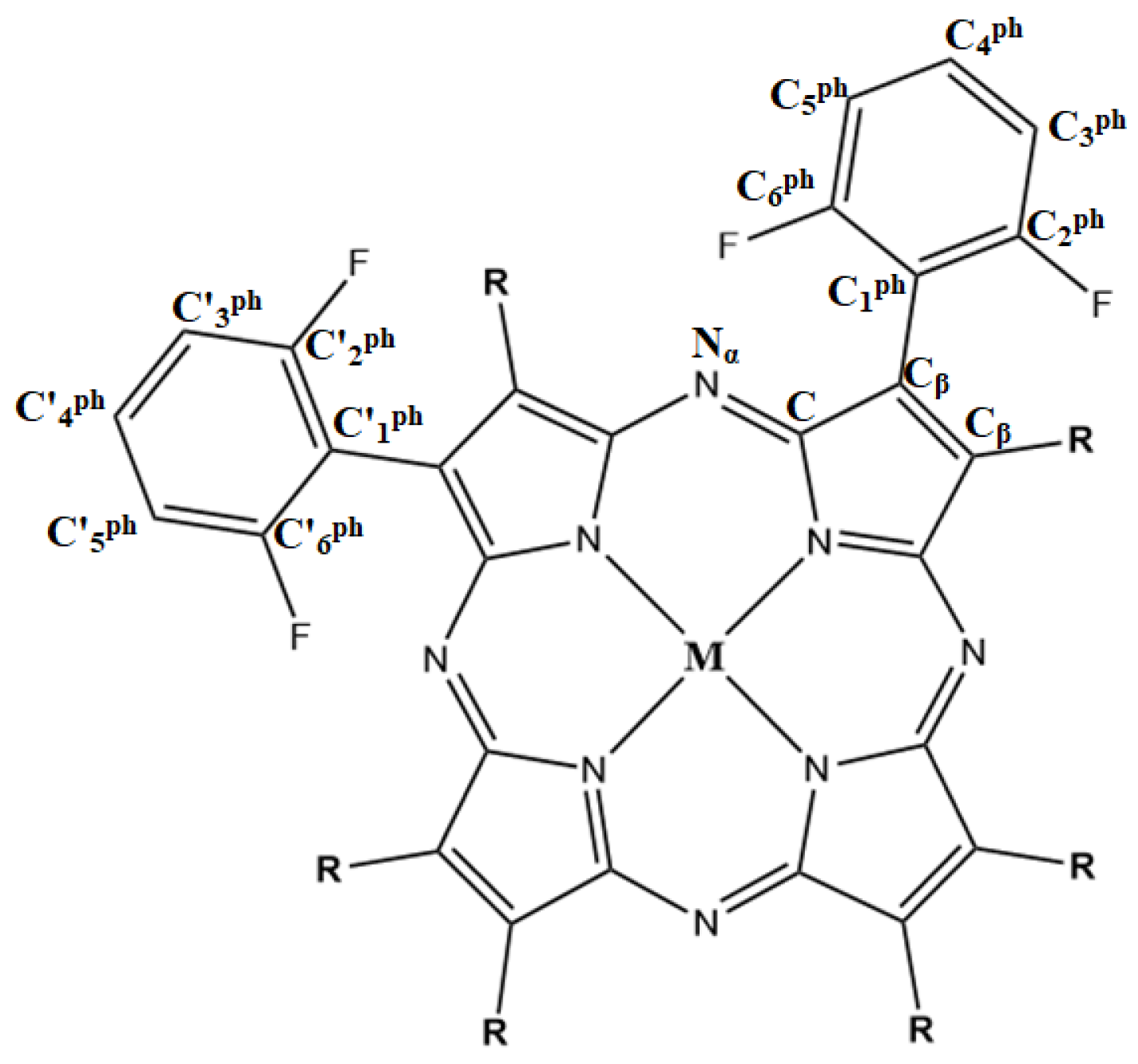
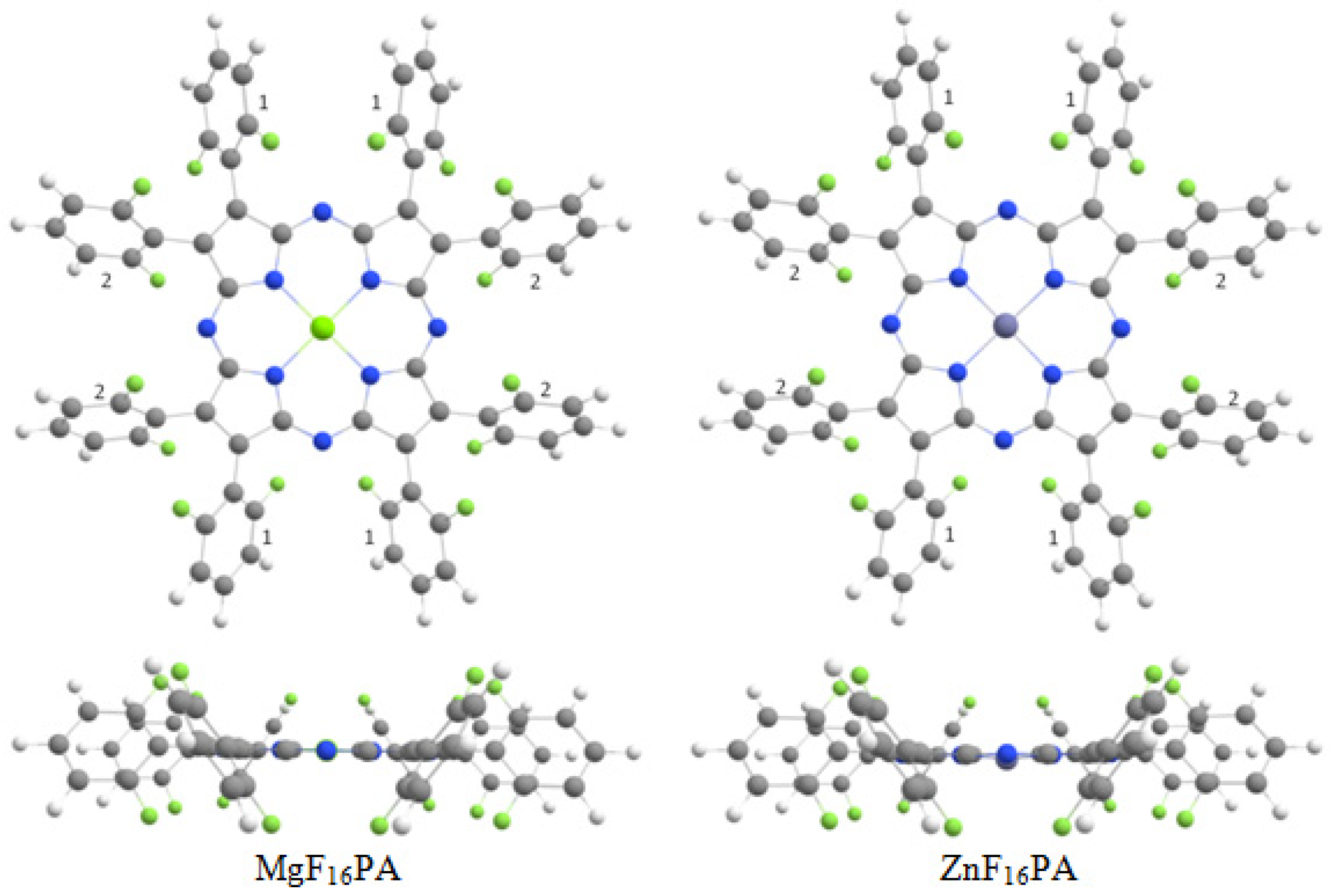
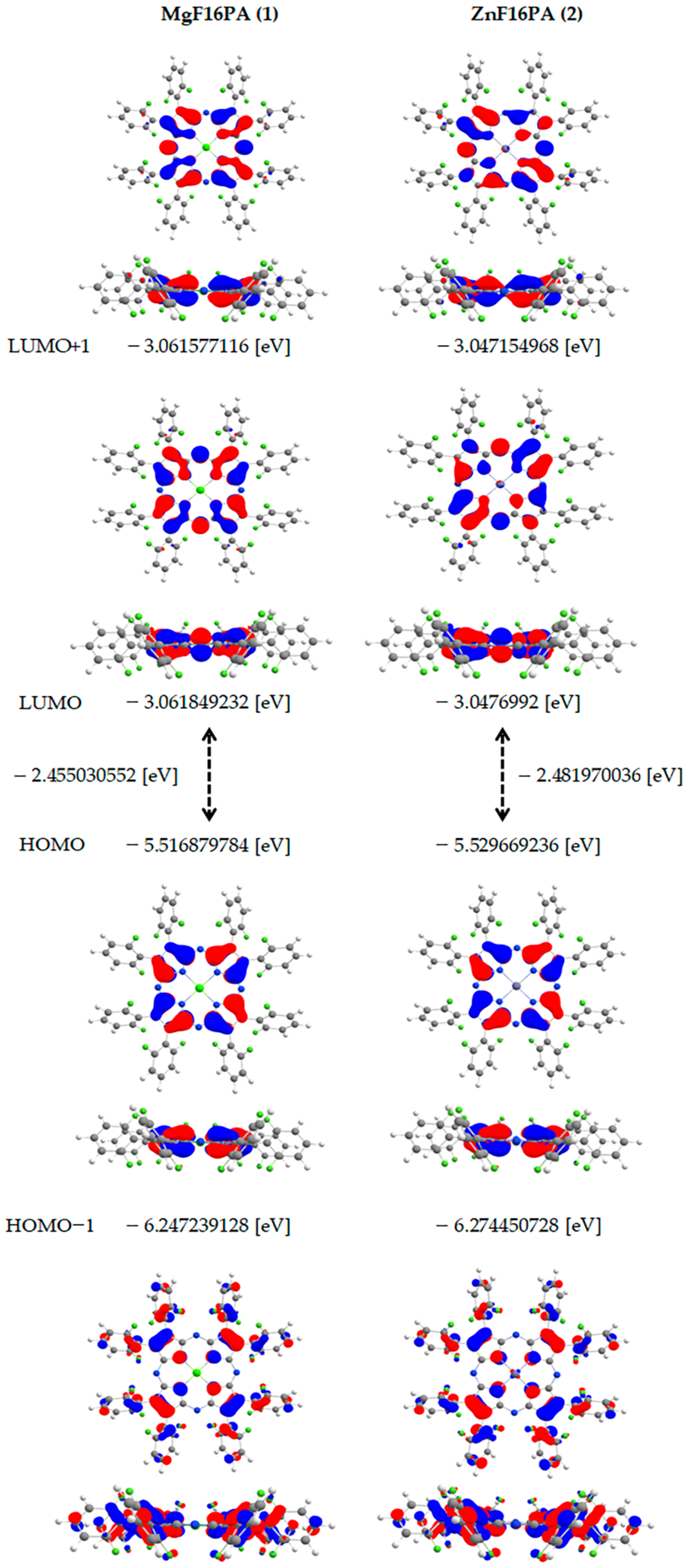
| Complex | Solvent | Band I | Band II | Soret Band |
|---|---|---|---|---|
| Mg(II) | CHCl3 | 621 (5.18) | 570 (4.43) | 365 (5.00) |
| Mg(II) | DMF | 618 (5.18) | 567 (4.42) | 364 (4.96) |
| Zn(II) | CHCl3 | 620 (5.15) | 568 (4.43) | 362 (5.00) |
| Zn(II) | DMF | 621 (5.15) | 569 (4.38) | 362 (4.89) |
| Compound | Qx | Error ± 10% | Reference |
|---|---|---|---|
| MgF8PA * | 0.23 | ±0.023 | [19] |
| ZnF8PA * | 0.17 | ±0.017 | [19] |
| ZnPA | 0.12 | ±0.012 | [20] |
| MgF16PA (1) | 0.29 | ±0.029 | |
| ZnF16PA (2) | 0.18 | ±0.018 | |
| ZnF40PA | 0.19 | ±0.019 | [20] |
| Bond Length, Å | Bond Angle, ° | ||||
|---|---|---|---|---|---|
| Parameters | MgF16PA (1) | ZnF16PA (2) | Parameters | MgF16PA (1) | ZnF16PA (2) |
| Nα-C | 1.335 | 1.332 | C-Nα-C | 124.5 | 123.9 |
| C-Cβ | 1.468 | 1.467 | Nα-C-N | 127.2 | 127.2 |
| Cβ-Cβ | 1.377 | 1.374 | C-N-C | 108.9 | 108.4 |
| Cβ-C1ph Cβ-C′1ph | 1.496 | 1.469 | Nα-C-Cβ | 124.0 | 123.7 |
| C1ph-C2ph C1ph-C6ph C′1ph-C′2ph C′1ph-C′6ph | 1.402 | 1.402 | C-Cβ-Cβ Cβ-Cβ-C | 106.7 106.7 | 106.7 106.6 |
| C2ph-C3ph C6ph-C5ph C′2ph-C′3ph C′6ph-C′5ph | 1.389 | 1.389 | C-Cβ-C1ph C-Cβ-C′1ph Cβ-Cβ-C1ph Cβ-Cβ-C′1ph | 124.7 124.7 128.5 128.6 | 124.8 124.6 128.5 128.7 |
| C3ph-C4ph C5ph-C4ph C′3ph-C′4ph C′5ph-C′4ph | 1.395 | 1.395 | C1ph-C2ph-F C1ph-C6ph-F C1ph-C′2ph-F C1ph-C′6ph-F | 118.3 118.4 118.4 118.4 | 118.3 118.4 118.4 118.4 |
| C3,5ph-H C′3,5ph-H | 1.084 | 1.084 | C1ph-C2ph-C3ph C1ph-C6ph-C5ph C′1ph-C′2ph-C′3ph C′1ph-C′6ph-C′5ph | 123.3 123.0 123.2 123.1 | 123.2 123.0 123.2 123.1 |
| C4ph-H C′4ph-H | 1.085 | 1.085 | C2ph-C3ph-C4ph C6ph-C5ph-C4ph C′2ph-C′3ph-C′4ph C′6ph-C′5ph-C′4ph | 118.6 118.8 118.6 118.8 | 118.6 118.8 118.6 118.8 |
| C2ph-F C′2ph-F | 1.348 | 1.348 | C3ph-C4ph-C5ph C′3ph-C′4ph-C′5ph | 120.5 120.5 | 120.6 120.6 |
| C6ph-F | 1.343 | 1.343 | C2ph-C3ph-H C6ph-C5ph-H C′2ph-C′3ph-H C′6ph-C′5ph-H C3ph-C4ph-H C′3ph-C′4ph-H | 119.2 119.1 119.2 119.1 119.7 119.7 | 119.2 119.1 119.2 119.1 119.7 119.7 |
| C′6ph-F | 1.344 | 1.344 | N-M-N | 90.0 | 89.7 |
| N-M | 1.988 | 1.974 | Dihedral Angle | ||
| Cβ-Cβ-C1ph-C2ph Cβ-Cβ-C1ph-C6ph Cβ-Cβ-C′1ph-C′2ph Cβ-Cβ-C′1ph-C′6ph | 54.2 125.9 53.6 126.8 | 54.7 125.2 53.2 127.1 | |||
| Model | Compound | Soret Band, λ nm Experim. | Soret Band, λ nm Predict. | RMSE | Error, nm |
|---|---|---|---|---|---|
| [27] | ZnF16PA | 364 | 422.7 | 7 ± 3 | 58.7 |
| [32] | 427.2 | 8 ± 4 | 63.2 | ||
| [27] | MgF16PA | 365 | 418.2 | 7 ± 3 | 53.2 |
| [32] | 428.3 | 8 ± 4 | 63.3 | ||
| [27] | ZnF8PA | 382 | 424.6 | 7 ± 3 | 42.6 |
| [32] | 429.9 | 8 ± 4 | 47.9 | ||
| [27] | MgF8PA | 377 | 418.5 | 7 ± 3 | 41.5 |
| [32] | 432.8 | 8 ± 4 | 55.8 | ||
| [27] | ZnPA | 380 * | 427.6 | 7 ± 3 | 47.6 |
| [32] | 439.5 | 8 ± 4 | 59.5 | ||
| [27] | ZnF40PA | 365 | 418.1 | 7 ± 3 | 53.1 |
| [32] | 425.8 | 8 ± 4 | 60.8 |
| Value | Predicted, nm | Experimental, nm | Definition |
|---|---|---|---|
| ML result MgF16PA | 418.2 428.3 | 365 | 53.2 63.3 |
| TD-DFT result MgF16PA | 430 | 365 | 65 |
| ML result ZnF16PA | 422.7 427.2 | 362 | 60.7 65.2 |
| TD-DFT result ZnF16PA | 440 | 362 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanov, A.; Chizhova, N.; Mamardashvili, N. Synthesis, Structure, and Spectral-Luminescent Properties of Peripherally Fluorinated Mg(II) and Zn(II) Octaphenyltetraazaporphyrins. Molecules 2022, 27, 8619. https://doi.org/10.3390/molecules27238619
Rusanov A, Chizhova N, Mamardashvili N. Synthesis, Structure, and Spectral-Luminescent Properties of Peripherally Fluorinated Mg(II) and Zn(II) Octaphenyltetraazaporphyrins. Molecules. 2022; 27(23):8619. https://doi.org/10.3390/molecules27238619
Chicago/Turabian StyleRusanov, Alexey, Natalya Chizhova, and Nugzar Mamardashvili. 2022. "Synthesis, Structure, and Spectral-Luminescent Properties of Peripherally Fluorinated Mg(II) and Zn(II) Octaphenyltetraazaporphyrins" Molecules 27, no. 23: 8619. https://doi.org/10.3390/molecules27238619
APA StyleRusanov, A., Chizhova, N., & Mamardashvili, N. (2022). Synthesis, Structure, and Spectral-Luminescent Properties of Peripherally Fluorinated Mg(II) and Zn(II) Octaphenyltetraazaporphyrins. Molecules, 27(23), 8619. https://doi.org/10.3390/molecules27238619






