Abstract
Traditionally, Cymbopogon citratus is used to treat a variety of ailments, including cough, indigestion, fever, and diabetes. The previous chemical and bioactive research on C. citratus mainly focused on its volatile oil. In this study, 20 non-volatile known compounds were isolated from the dried aerial part of C. citratus, and their structures were elucidated by MS, NMR spectroscopy, and comparison with the published spectroscopic data. Among them, 16 compounds were reported for the first time from this plant. The screening results for antioxidant and α-glucosidase inhibitory activities indicated that compounds caffeic acid (5), 1-O-p-coumaroyl-3-O-caffeoylglycerol (8), 1,3-O-dicaffeoylglycerol (9) and luteolin-7-O-β-D-glucopyranoside (12) had potent antioxidant capacities, with IC50 values from 7.28 to 14.81 μM, 1.70 to 2.15 mol Trolox/mol and 1.31 to 2.42 mol Trolox/mol for DPPH, ABTS, and FRAP, respectively. Meanwhile, compounds 8 and 9 also exhibited significant inhibitory activities against α-glucosidase, with IC50 values of 11.45 ± 1.82 μM and 5.46 ± 0.25 μM, respectively, which were reported for the first time for their α-glucosidase inhibitory activities. The molecular docking result provided a molecular comprehension of the interaction between compounds (8 and 9) and α-glucosidase. The significant antioxidant and α-glucosidase inhibitory activities of compounds 8 and 9 suggested that they could be developed into antidiabetic drugs because of their potential regulatory roles on oxidative stress and digestive enzyme.
1. Introduction
Diabetes mellitus is one of the most serious metabolic diseases in the world, with a high incidence [1]. It is characterized either by insufficient insulin production or insulin resistance caused by genetic and environmental factors, such as obesity, oxidative stress, incorrect dietary components [2,3]. At present, there is a variety of antidiabetic drugs in clinical use, but most of the synthetic antidiabetic drugs have limited therapeutic efficacy and are accompanied by many side effects, such as hypoglycemia, gastrointestinal side effects, and weight gain [4,5]. Meanwhile, natural products have a long history of being used to treat diabetes. Therefore, it might be of great research value to find high-efficiency and low-toxicity hypoglycemic components from traditional antidiabetic plants.
Cymbopogon citratus (C. citratus), which is also known as lemongrass, has a fragrant taste and belongs to the Gramineae family. It is a perennial herbaceous plant that grows mainly in tropical and subtropical regions. It has a very wide range of uses, such as seasoning, medicine, and tea [6]. As a folk medicine, C. citratus can be used to treat inflammation, cough, indigestion, flu, fever, diabetes, and malaria [7,8]. Furthermore, the decoction of C. citratus is commonly used to treat diabetes in Thailand [7]. Previous biological studies of C. citratus have reported that its extracts exhibit diverse therapeutic properties, including hypoglycemic [9], antioxidant [10], antimicrobial [11] and anti-inflammatory activities [12]. The research by Bharti et al. confirmed that C. citratus essential oil could improve the abnormal metabolism of blood glucose, insulin and lipid in Poloxamer 407-induced Wistar rats with type 2 diabetes via increasing the number of β cells and promoting the secretion of GLP-1 [9]. In addition, C. citratus extracts have been proved to possess certain hypoglycemic effects by inhibiting α-glucosidase and α-amylase activities [13].
In recent years, most research on C. citratus has focused on its essential oil. There is about 1–2% essential oil in its leaves [14]. The main components of C. citratus essential oil are limonene, citral, myrcene, geranial, neral and citronellal [15,16], and citral is the crucial ingredient of C. citratus flavor [17]. However, the other chemical constituents of C. citratus and their bioactivities are rarely researched, which might be important for its hypoglycemic function.
α-Glucosidase is an important target in researching hypoglycemic drugs. It can catalyze the hydrolysis of α-1.4-glucosidase, which leads to the hydrolysis of oligosaccharides such as maltose and sucrose in the small intestine [18]. Thus, the inhibition of α-glucosidase activity can slow down glucose production and absorption, and then reduce postprandial blood glucose. The molecular docking study can be used to predict the binding sites and modes of small-molecule compounds to α-glucosidase [19]. Oxidative stress occurs when the production of free radicals exceeds the cell’s inherent antioxidant defense system, which plays an important role in the occurrence and development of diabetes and its complications [20]. Antioxidants play a strong role in resisting oxidative stress [21]. Therefore, natural antioxidants could be effective in preventing diabetes. The 3T3-L1 cell line is generally deemed to be a mature and classical cell line used to study glucose metabolism, adipocyte differentiation, and insulin signal transduction in vitro [22]. Therefore, we studied the hypoglycemic activity of C. citratus via assaying antioxidant capacity, glycosidase inhibitory activity, and glucose uptake rate.
In this study, chemical constituents of the methanol extract of C. citratus were isolated and identified. Furthermore, the antioxidant capacity, α-glucosidase inhibitory activity and the glucose uptake rate of crude extracts and compounds were evaluated. Our work reported the inhibitory activity of 1-O-p-coumaroyl-3-O-caffeoylglycerol (8) and 1,3-O-dicaffeoylglycerol (9) against α-glucosidase for the first time. Meanwhile, the potent antioxidant capacities of compounds 8 and 9 suggested that they are potential natural candidate drugs to inhibit the α-glucosidase activity and oxidative stress in diabetes.
2. Results and Discussion
2.1. Structure Elucidation
Twenty known compounds (Figure 1) were isolated and identified from the dried aerial part of C. citratus, including 4,6-dihydroxy-2-methoxyacetophenone (1) [23], 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (2) [24], 2,4,6-trihydroxy- acetophenone 2-O-β-D-glucoside (3) [25], p-coumaric acid (4) [26], caffeic acid (5) [27], 1-O-feruloylglycerol (6) [28], 1,3-O-di-p-coumaroylglycerol (7) [28], 1-O-p-coumaroyl- 3-O-caffeoylglycerol (8) [29], 1,3-O-dicaffeoylglycerol (9) [29], tricin (10) [30], trcin-7-O-β-D-glucopyranose (11) [31], luteolin-7-O-β-D-glucopyranoside (12) [32], 3,4-diacetoxycinnamic acid (13) [33], glyceroyl monopalmitate (14) [34], allantion (15) [35], adenosine (16) [36], cymbopogonol (17) [37], 7α-hydroxysitosterol (18) [38], stigmasta-5,22-diene-3β,7α-diol (19) [39], ergosterol endoperoxide (20) [38]. Among them, p-coumaric acid (4) was identified by Filipa Tavares et al. from the dried leaves of C. citratus, using HPLC-PDA/ESI-MSn [40]. Caffeic acid (5) was isolated from dried leaves of C. citratus by XiaoLi Bao et al. [41]. Luteolin-7-O-β-D-glucopyranoside (12) was isolated from the dry leaves of C. citratus by Pedro H.O. Borges. [8]. Cymbopogonol (17) was first isolated from the leaf wax of C. citratus by Steven W. Hanson [42]. Except for these four compounds, other compounds were isolated from this species for the first time.
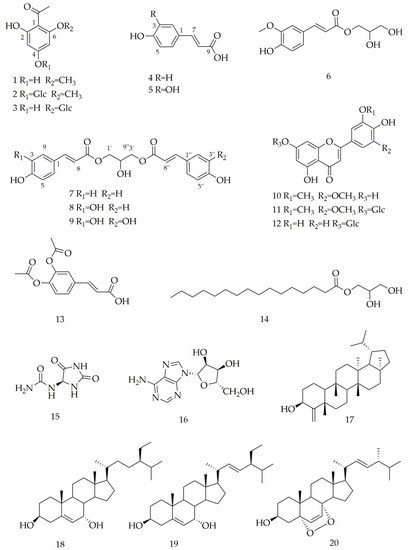
Figure 1.
Chemical structures of isolated compounds from C. citratus.
Compound 1. C9H10O4, ESI-MS: m/z 181 [M-H]–. 1H-NMR (600 MHz, CD3COCD3) δH: 6.03 (1H, d, J = 2.2 Hz, H-3), 5.95 (1H, d, J = 2.2 Hz, H-5), 3.90 (3H, s, OCH3), 2.55 (3H, s, COCH3). 13C-NMR (150 MHz, CD3COCD3) δC: 106.3 (C-1), 168.7 (C-2), 97.0 (C-3), 166.2 (C-4), 92.1 (C-5), 165.1 (C-6), 203.9 (C=O), 33.4 (COCH3), 56.5 (OCH3).
Compound 2. C15H20O9, ESI-MS: m/z 367 [M + Na]+. 1H-NMR (600 MHz, pyridine-d5) δH: 6.73 (1H, d, J = 2.3 Hz, H-3), 6.39 (1H, d, J = 2.3 Hz, H-5), 5.77 (1H, d, J = 7.4 Hz, H-1′), 3.71 (3H, s, OCH3), 2.54 (3H, s, COCH3). 13C-NMR (150 MHz, pyridine-d5) δC: 107.5 (C-1), 167.8 (C-2), 97.9 (C-3), 165.4 (C-4), 92.7 (C-5), 163.9 (C-6), 204.0 (C=O), 33.5 (COCH3), 56.2 (OCH3), 101.5 (C-1′), 74.8 (C-2′), 78.6 (C-3′), 71.3 (C-4′), 79.3 (C-5′), 62.4 (C-6′).
Compound 3. C14H18O9, ESI-MS: m/z 353 [M + Na]+. 1H-NMR (800 MHz, pyridine-d 5) δH: 6.88 (s, 1H, H-3), 6.58 (1H, d, J = 6.4 Hz, H-5), 5.68 (d, J = 6.4 Hz, 1H, H-1′), 2.96 (s, 3H, CH3). 13C-NMR (200 MHz, pyridine-d5) δC: 106.8 (C-1), 163.1 (C-2), 98.7 (C-3), 168.4 (C-4), 96.0 (C-5), 167.5 (C-6), 204.3 (C=O), 34.1 (COCH3), 102.8 (C-1′), 75.2 (C-2′), 79.5 (C-3′), 71.5 (C-4′), 79.6 (C-5′), 62.7 (C-6′).
Compound 4. C9H8O3, ESI-MS: m/z 163 [M-H]–. 1H-NMR (600 MHz, CD3OD) δH: 7.58 (1H, d, J = 15.9 Hz, H-7), 7.44 (2H, d, J = 8.6 Hz, H-2, 6), 6.80 (2H, d, J = 8.6 Hz, H-3, 5), 6.30 (1H, d, J = 15.9 Hz, H-8). 13C-NMR (150 MHz, CD3OD) δC: 127.5 (C-1), 131.2 (C-2), 116.9 (C-3), 161.3 (C-4), 116.9 (C-5), 131.2 (C-6), 146.5 (C-7), 115.9 (C-8), 171.5 (C-9).
Compound 5. C9H8O4, ESI-MS: m/z 181 [M + H]+, 1 H-NMR (500 MHz, CD3OD) δH: 7.52 (1H, d, J = 15.9 Hz, H-7), 7.03 (1H, d, J = 2.1 Hz, H-2), 6.92 (1H, dd, J = 8.2, 2.1 Hz, H-6), 6.77 (1H, d, J = 8.2 Hz, H-5), 6.21 (1H, d, J = 15.9 Hz, H-8). 13C-NMR (125 MHz, CD3OD) δC: 127.8 (C-1), 115.1 (C-2), 146.8 (C-3), 149.4 (C-4), 116.5 (C-5), 122.8 (C-6), 147.0 (C-7), 115.5 (C-8), 171.1 (C-9).
Compound 6. C13H16O6, ESI-MS: m/z 291 [M + Na]+. 1H-NMR (600 MHz, CD3OD) δH: 7.73 (1H, d, J = 15.9 Hz, H-7), 7.27 (1H, d, J = 1.9 Hz, H-2), 7.16 (1H, dd, J = 8.2, 1.9 Hz, H-6), 6.89 (1H, d, J = 8.2 Hz, H-5), 6.28 (1H, d, J = 15.9 Hz, H-8), 4.34 (1H, dd, J = 11.4, 4.3 Hz, H-1′a), 4.25 (1H, d, J = 11.4, 6.3 Hz, H-1′b), 3.96 (3H, s, -OCH3), 3.82 (1H, t, J = 5.4 Hz H-2′), 3.68 (2H, t, J = 5.4 Hz, H-3′). 13C-NMR (150 MHz, CD3OD) δC: 127.8 (C-1), 111.8 (C-2), 150.8 (C-3), 149.5 (C-4), 116.6 (C-5), 124.3 (C-6), 147.2 (C-7), 115.4 (C-8), 169.3 (C-9), 66.7 (C-1′), 71.4 (C-2′), 64.2 (C-3′), 56.6 (OCH3).
Compound 7. C21H20O7. ESI-MS: m/z 407 [M + Na]+. 1H-NMR (600 MHz, CD3COCD3) δH: 7.66 (2H, d, J = 16.1 Hz, H-7, 7″), 7.56 (4H, d, J = 8.6 Hz, H-2, 2″, 6, 6″), 6.90 (4H, d, J = 8.6 Hz, H-3, 3″, 5, 5″), 6.39 (2H, d, J = 16.1 Hz, H-8, 8″), 4.57 (1H, d, J = 5.4 Hz, H-2′), 4.28 (4H, m, H-1′, 3′). 13C-NMR (150 MHz, CD3COCD3) δC: 127.3 (C-1, 1″), 131.4 (C-2, 2″), 117.1 (C-3, 3″), 161.1 (C-4, 4″), 117.1 (C-5, 5″), 131.4 (C-6, 6″), 146.1 (C-7, 7″), 115.6 (C-8, 8″), 167.8 (C-9, 9″), 66.4 (C-1′), 68.6 (C-2′), 66.4 (C-3′).
Compound 8. C21H20O8, ESI-MS: m/z 423 [M + Na]+. 1H-NMR (600 MHz, CD3COCD3) δH: 7.66 (1H, d, J = 16.0 Hz, H-7″), 7.59 (1H, d, J = 16.0 Hz, H-7), 7.56 (2H, d, J = 8.6 Hz, H-2″, 6″), 7.18 (1H, d, J = 1.9 Hz, H-2), 7.06 (1H, dd, J = 8.2, 1.9 Hz, H-6), 6.89 (2H, dd, J = 8.6 Hz, H-3″, 5″), 6.87 (1H, d, J = 8.2 Hz, H-5), 6.39 (1H, d, J = 16.0 Hz, H-8″), 6.32 (1H, d, J = 16.0 Hz, H-8), 4.27 (4H, m, H-1′, 3′), 4.19 (1H, m, H-2′). 13C-NMR (150 MHz, CD3COCD3) δC: 127.4 (C-1), 131.4 (C-2), 116.8 (C-3), 161.0 (C-4), 116.8 (C-5), 131.4 (C-6), 146.7 (C-7), 115.7 (C-8), 167.8 (C-9), 66.4 (C-1′), 68.7 (C-2′), 66.4 (C-3′), 128.0 (C-1″), 115.6 (C-2″), 149.2 (C-3″), 146.5 (C-4″), 116.8 (C-5″), 123.0 (C-6″), 146.1 (C-7″), 115.7 (C-8″), 167.8 (C-9″).
Compound 9. C21H20O9, ESI-MS: m/z 439 [M + Na]+. 1H-NMR (800 MHz, pyridine-d5) δH: 7.97 (2H, d, J=15.8 Hz, H-7, 7″), 7.57 (2H, d, J = 1.7 Hz, H-2, 2″), 7.21 (2H, d, J = 8.1 Hz, H-5, 5″), 7.12 (2H, dd, J = 8.1, 1.7 Hz, H-6, 6″), 6.58 (2H, d, J = 15.8 Hz, H-8, 8″). 13C-NMR (200 MHz, pyridine-d5) δC: 127.2 (C-1, 1″), 116.2 (C-2, 2″), 150.5 (C-3, 3″), 146.5 (C-4, 4″), 117.0 (C-5, 5″), 122.5 (C-6, 6″), 146.5 (C-7, 7″), 115.0 (C-8, 8″), 167.8 (C-9, 9″), 66.6 (C-1′), 68.3 (C-2′), 66.6 (C-3′).
Compound 10. C17H14O7, ESI-MS: m/z 329 [M-H] –. 1H-NMR (500 MHz, pyridine-d5) δH: 7.44 (2H, s, H-2′, 6′), 7.03 (1H, s, H-3), 6.89 (1H, d, J = 2.1 Hz, H-6), 6.76 (1H, d, J = 2.1 Hz, H-8), 3.86 (6H, s, OCH3). 13C-NMR (125 MHz, pyridine-d5) δC: 164.7 (C-2), 104.6 (C-3), 182.8 (C-4), 163.2 (C-5), 100.1 (C-6), 166.0 (C-7), 95.1 (C-8), 158.7 (C-9), 105.0 (C-10), 121.4 (C-1′), 105.3 (C-2′, 6′), 149.4 (C-3′, 5′), 164.7 (C-4′), 56.6 (OCH3).
Compound 11. C23H24O12, ESI-MS: m/z 515 [M + Na]+. 1H-NMR (600 MHz, pyridine-d5) δH: 7.45 (2H, s, H-2′, 6′) 7.20 (1H, d, J = 2.1 Hz, H-8), 7.06 (1H, s, H-3), 6.90 (1H, d, J = 2.1 Hz, H-6), 5.82 (1H, d, J = 6.9 Hz, H-1″), 3.89 (6H, s, OCH3). 13C-NMR (150 MHz, pyridine-d5) δC: 165.4 (C-2), 104.9 (C-3), 183.2 (C-4), 162.9 (C-5), 101.2 (C-6), 164.5 (C-7), 95.9 (C-8), 158.3 (C-9), 107.0 (C-10), 121.2 (C-1′), 105.5 (C-2′, 6′), 150.5 (C-3′, 5′), 142.6 (C-4′), 56.9 (OCH3), 101.1 (C-1″), 75.2 (C-2″), 79.6 (C-3″), 71.4 (C-4″), 79.0 (C-5″), 62.6 (C-6″).
Compound 12. C21H20O11, ESI-MS: m/z 447 [M-H]–. 1H-NMR (600 MHz, pyridine-d5) δH: 7.92 (1H, d, J = 2.0, H-2′), 7.53 (1H, dd, J = 8.3, 2.0 Hz, H-6′), 7.29 (1H, d, J = 8.3 Hz, H-5′), 7.02 (1H, d, J = 1.9 Hz, H-8), 6.96 (1H, s, H-3), 6.87 (1H, d, J = 1.9 Hz, H-6), 5.86 (1H, d, J = 7.7 Hz, H-1″). 13C NMR (150 MHz, C5D5N5) δC: 165.7 (C-2), 104.6 (C-3), 183.3 (C-4), 163.0 (C-5), 101.0 (C-6),164.4 (C-7), 95.7 (C-8),158.3 (C-9), 107.0 (C-10), 123.1 (C-1′), 115.1 (C-2′), 148.3 (C-3′), 152.4 (C-4′), 117.3 (C-5′), 120.1 (C-6′), 102.2 (C-1″), 75.3 (C-2″), 78.9 (C-3″), 71.5 (C-4″), 79.7 (C-5″), 62.8 (C-6″).
Compound 13. C13H12O6. ESI-MS: m/z 287 [M + Na]+. 1H-NMR (500 MHz, CD3OD) δH: 7.63 (1H, d, J = 16.0 Hz, H-7), 7.51 (1H, dd, J = 8.3, 2.0 Hz, H-6), 7.49 (1H, d, J = 2.0 Hz, H-2), 7.25 (1H, d, J = 8.3 Hz, H-5), 6.47 (1H, d, J = 16.0 Hz, H-8), 2.27 (6H, d, J = 4.6 Hz, COCH3). 13C-NMR (125 MHz, CD3OD) δC: 169.0 (C-9), 120.6 (C-8), 144.3 (C-7), 134.7 (C-1), 125.1 (C-2), 144.1 (C-3), 145.1 (C-4), 125.1 (C-5), 127.5 (C-6), 167.0 (COCH3), 169.9 (COCH3), 20.4 (COCH3).
Compound 14. C19H38O4, ESI-MS: m/z 353 [M + Na]+. 1H-NMR (500 MHz, CDCl3) δH: 4.20 (1H, dd, J = 11.7, 4.6 Hz, H-1a), 4.15 (1H, dd, J = 11.7, 6.2 Hz, H-1b), 3.93 (1H, m, H-2), 3.70 (1H, dd, J = 11.4, 3.4 Hz, H-3a), 3.60 (1H, dd, J = 11.4, 5.8 Hz, H-3b), 2.35 (2H, t, J = 7.6 Hz, H-2′), 0.88 (3H, t, J = 7.0 Hz, H-16′). 13C-NMR (125 MHz, CDCl3) δC: 174.6 (C-1), 34.4 (C-2), 32.1 (C-3), 29.9-22.7 (C-4~15), 14.4 (C-16), 65.4(C-1′), 70.5 (C-2′), 63.5 (C-3′).
Compound 15. C4H6N4O3, ESI-MS: m/z 157 [M-H]–. 1H-NMR (600 MHz, DMSO-d6) δH: 10.55 (1H, s, NH-3), 8.07 (1H, s, NH-1), 6.89 (1H, d, J = 8.2 Hz, NH-4), 5.80 (2H, s, NH2-6), 5.24 (1H, d, J = 8.2 Hz, H-4). 13C-NMR (150 MHz, DMSO-d6) δC: 156.8 (C-2), 62.4 (C-4), 173.37 (C-5), 157.4 (C-6).
Compound 16. C10H13N5O4, ESI-MS: m/z 268 [M + H]+. 1H-NMR (600 MHz, pyridine-d5) δH: 8.67 (1H, s, H-8), 8.44 (2H, s, -NH2), 6.75 (1H, d, J = 5.9 Hz, H-1′), 4.33 (1H, d, J = 12.0 Hz, H-5′a), 4.15 (1H, d, J = 12.0 Hz, H-5′b). 13C-NMR (150 MHz, pyridine-d5) δC: 153.8 (C-2), 150.7 (C-4), 122.0 (C-5), 158.2 (C-6), 141.0 (C-8), 91.3 (C-1′), 76.0 (C-2′), 72.9 (C-3′), 88.3 (C-4′), 63.5 (C-5′).
Compound 17. C30H50O, ESI-MS: m/z 449 [M + Na]+. 1H-NMR (500 MHz, CDCl3) δH: 4.84 (1H, d, J = 1.4 Hz, H-23a), 4.78 (1H, d, J = 1.4 Hz, H-23b), 4.31 (1H, t, J = 2.9 Hz, H-3), 1.23 (3H, s, H-24), 0.94 (3H, s, H-25), 0.87 (3H, s, H-26), 0.84 (3H, s, H-27), 0.96 (3H, s, H-28), 0.88 (3H, d, J = 6.6 Hz, H-29), 0.89 (3H, d, J = 6.6 Hz, H-30). 13C-NMR (125 MHz, CDCl3) δC: 15.7 (C-1), 34.9 (C-2), 74.8 (C-3), 161.2 (C-4), 40.1 (C-5), 40.3 (C-6), 15.7 (C-7), 50.0 (C-8), 37.9 (C-9), 59.4 (C-10), 34.9 (C-11), 30.2 (C-12), 39.0 (C-13), 39.7 (C-14), 29.3 (C-15), 32.4 (C-16), 39.73 (C-17), 54.2 (C-18), 48.0 (C-19), 27.7 (C-20), 43.0 (C-21), 30.2 (C-22), 109.3 (C-23), 23.8 (C-24), 18.5 (C-25), 16.5 (C-26), 15.8 (C-27), 33.54 (C-28), 21.0 (C-29), 23.4 (C-30).
Compound 18. C29H50O2, ESI-MS: m/z 453 [M + Na]+. 1H-NMR (500 MHz, CDCl3) δH: 5.60 (1H, dd, J = 5.3, 1.7 Hz, H-6), 3.85 (1H, s, H-7), 3.59 (1H, m, H-3), 1.00 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.84 (3H, d, J = 6.5 Hz, H-27), 0.82 (3H, d, J = 6.8 Hz, H-26), 0.68 (3H, s, H-18). 13C-NMR (125 MHz, CDCl3) δC: 37.0 (C-1), 31.4 (C-2), 71.4 (C-3), 42.0 (C-4), 146.3 (C-5), 123.9 (C-6), 65.4 (C-7), 39.2 (C-8), 42.3 (C-9), 37.4 (C-10), 20.7 (C-11), 39.2 (C-12), 42.2 (C-13), 49.4 (C-14), 24.3 (C-15), 28.3 (C-16), 55.7 (C-17), 11.7 (C-18), 18.3 (C-19), 36.1 (C-20), 18.8 (C-21), 33.9 (C-22), 25.9 (C-23), 45.8 (C-24), 29.1 (C-25), 19.0 (C-26), 19.8 (C-27), 23.1 (C-28), 12.0 (C-29).
Compound 19. C29H48O2. ESI-MS: m/z 451 [M + Na]+. 1H-NMR (800 MHz, CDCl3) δH: 5.60 (1H, d, J = 5.1Hz, H-6), 5.16 (1H, dd, J = 15.2, 8.7 Hz, H-22), 5.02 (1H, dd, J = 15.2, 8.7 Hz, H-23), 3.85 (1H, s, H-7), 3.59 (1H, m, H-3), 1.03 (3H, d, J = 6.6 Hz, H-21), 1.00 (3H, s, H-19), 0.70 (3H, s, H-18). 13C-NMR (200 MHz, CDCl3) δC: 37.0 (C-1), 31.4 (C-2), 71.4 (C-3), 42.0 (C-4), 146.2 (C-5), 123.9 (C-6), 65.3 (C-7), 37.5 (C-8), 42.3 (C-9), 37.5 (C-10), 20.7 (C-11), 39.1 (C-12), 42.3 (C-13), 49.4 (C-14), 24.4 (C-15), 28.9 (C-16), 55.7 (C-17), 11.8 (C-18), 18.2 (C-19), 40.5 (C-20), 21.2 (C-21), 138.2 (C-22), 129.3 (C-23), 51.2 (C-24), 31.9 (C-25), 19.0 (C-26), 21.1 (C-27), 25.4 (C-28), 12.3 (C-29).
Compound 20. C28H44O3, ESI-MS: m/z 451 [M + Na]+. 1H-NMR (500 MHz, CDCl3) δH: 6.50 (1H, d, J = 8.5 Hz, H-7), 6.24 (1H, d, J = 8.5 Hz, H-6), 3.96 (1H, s, H-3), 0.99 (3H, d, J = 6.5, H-21), 0.90 (3H, d, J = 6.5 Hz, H-28), 0.88 (3H, s, H-19), 0.82 (3H, d, J = 8.5 Hz, H-26), 0.81 (3H, d, J = 8.5 Hz, H-27), 0.80 (3H, s, H-18). 13C-NMR (125 MHz, CDCl3) δC: 34.7 (C-1), 30.1(C-2), 66.5 (C-3), 36.9 (C-4), 82.2 (C-5), 135.4 (C-6), 130.7 (C-7), 79.4 (C-8),51.1 (C-9), 36.9 (C-10), 23.4 (C-11), 39.3 (C-12), 44.6 (C-13), 51.7 (C-14), 20.6 (C-15), 28.7 (C-16), 56.2 (C-17), 12.9 (C-18), 18.2 (C-19), 39.7 (C-20), 20.9 (C-21), 135.2 (C-22), 132.3 (C-23), 42.8 (C-24), 33.1 (C-25), 19.6 (C-26), 20.0 (C-27), 17.6 (C-28).
2.2. Antioxidant Activities of Methanolic Extract, Fractions and Isolated Compounds
The methods commonly used to determine the antioxidant capacity of substances include DPPH+, ABTS+, FRAP, and TRAP methods [43], but none of them can comprehensively evaluate the total antioxidant capacity of tested substances. Therefore, the crude methanol extract (CME), different fractions, and isolated compounds were evaluated for their antioxidant activities according to DPPH+, ABTS+, and FRAP assays. As shown in Table 1, the antioxidant activities of CME and different fractions showed consistent results in three different patterns. The CME had antioxidant activity (IC50 = 203.80 ± 21.70 μg/mL for DPPH, 0.61 ± 0.0067 mmol Trolox/g for ABTS, 0.29 ± 0.0051 mmol Trolox/g for FRAP). This result supported the benefits of using C. citratus in food and tea. The n-butanol (n-BuOH) fraction (IC50 = 41.60 ± 3.09 μg/mL for DPPH, 1.20 ± 0.013 mmol Trolox/g for ABTS, 0.82 ± 0.016 mmol Trolox/g for FRAP) showed the most potent antioxidant activity (p < 0.05), and the ethyl acetate (EtOAc) fraction (IC50 = 130.70 ± 8.45 μg/mL for DPPH, 0.96 ± 0.0050 mmol Trolox/g for ABTS, 0.42 ± 0.021 mmol Trolox/g for FRAP) showed moderate antioxidant activity (p < 0.05). In contrast, the petroleum ether (PE) fraction and aqueous fraction (AF) had no antioxidant activities. The potent antioxidant properties of n-BuOH and EtOAc fractions might be due to the antioxidant and free radical scavenging activities of their flavonoid and phenolic compounds [44]. The results suggested that the main antioxidant compounds of C. citratus might present in n-BuOH and EtOAc fractions.

Table 1.
Antioxidant activities of extracts and compounds 1–10, 12 from C. citratus.
Polyphenols and flavonoids are considered to have potential antioxidant activities [45]. Thus, the isolated compounds 1–10 and 12 were screened for their antioxidant activities. As shown in Table 1, compounds 5, 8, 9, and 12 exhibited stronger antioxidant activities (IC50 = 7.28–14.81 μM for DPPH, 1.70–2.15 mol Trolox/mol for ABTS, 1.31–2.42 mol Trolox/mol for FRAP) than ascorbic acid. These results indicated that compounds 5, 8, 9, and 12 had a potent free radical scavenging ability and ferric reducing power, which might be developed into effective natural antioxidants. Compounds 1–4, 6–7, and 10 had no significant activity when IC50 values were greater than 80 μM for DPPH and were not detected to be active for FRAP at 20 μM. Compared with compound 7, compounds 8 and 9 exhibited higher DPPH+ scavenging activities and ferric reducing power, which revealed that the phenolic hydroxyl groups at C-3, 3″ might contribute to the DPPH+ scavenging and ferric reducing abilities. Compound 5 showed more potent antioxidant activities than compound 4, which suggested that the hydroxyl group at C-3 could enhance ferric reducing and DPPH+ scavenging abilities. Interestingly, compounds 2–10 and 12 all showed significant ABTS+ scavenging abilities, which reminds us that we should pay more attention to the antioxidant activities of polyphenols and flavonoids in C. citratus.
2.3. α-Glucosidase Inhibition Activity
α-Glucosidase inhibitors can delay carbohydrate absorption and reduce postprandial hyperglycemia by inhibiting α-glucosidase in upper intestinal epithelial cells and reducing glucose conversion. The results of CME and different fractions are shown in Table 2 and Figure 2a. The PE and EtOAc fractions showed the most potent α-glucosidase inhibition activities (p < 0.05) with IC50 value of 1.77 ± 0.55 μg/mL and 2.47 ± 0.10 μg/mL, respectively. The results are consistent with the previous report [13]. The CME and n-BuOH fractions showed moderate α-glucosidase inhibition activities (p < 0.05) with IC50 value of 7.90 ± 0.55 μg/mL and 7.49 ± 0.34 μg/mL, respectively. The AF exhibited no significant α-glucosidase inhibitory activity with IC50 value > 300 μg/mL. The results indicated that most α-glucosidase inhibitory substances should be small polar compounds present in PE and EtOAc fractions. At the same time, our study further provides scientific evidence for the efficacy of C. citratus as a herbal medicine in the treatment of diabetes, and demonstrates that the possible pathway is through inhibiting α-glucosidase activity.

Table 2.
α-Glucosidase inhibitory activities of extracts and compounds 8–9 from C. citratus.
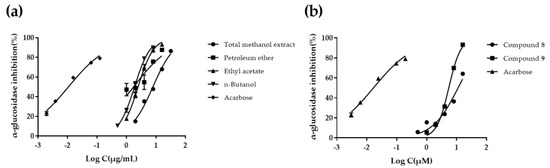
Figure 2.
The α-glucosidase inhibitory effects of C. citratus extracts, and compounds 8, 9. (a) Concentration−response relationship for C. citratus extracts. (b) Concentration−response relationship for compounds 8 and 9. Log C is the logarithm of concentration in μg/mL for C. citratus extracts, and in μM for acarbose and compounds 8, 9 (three independent assays performed in duplicate).
It has been reported in the literature that flavonoid and polyphenols compounds have hypoglycemic activity [46,47]. Therefore, compounds 1–10 and 12 were screened for their α-glucosidase inhibitory activities. The results are shown in Table 3. The inhibitory rates of compounds 8 and 9 on α-glucosidase were 66.96% and 88.85%, respectively, while compounds 1–7, 10 and 12 were less than 50% at 20 μM. Further tests of α-glucosidase inhibitory activities on compounds 8 and 9 were carried out, and the results are shown in Table 2 and Figure 2b. Compounds 8 and 9 showed obvious α-glucosidase inhibitory activities with IC50 values of 11.45 ± 1.82 μM and 5.46 ± 0.25 μM, respectively. This is the first report of the α-glucosidase inhibitory activities of compounds 8 and 9. The α-glucosidase inhibitory activities of compounds 7, 8, and 9 suggested that the phenolic hydroxyl groups at C-3, 3″ might determine whether these types of compounds have α-glucosidase inhibitory activity.

Table 3.
α-Glucosidase inhibitory activities of compounds 1–10 and 12.
2.4. Molecular Docking Study
The molecular docking results showed that 1-O-p-coumaroyl-3-O-caffeoylglycerol (8) and 1,3-O-dicaffeoylglycerol (9) possessed superior binding energy with α-glucosidase (binding energy: −5.19, −5.97 kcal/mol, respectively) (Figure 3.). The surface structures of the ligand−enzyme complex showed that the candidates positioned in the active pocket of α-glucosidase, as illustrated in Figure 3a,c. The results indicated that compound 8 formed two hydrogen-bonding interactions with GLY399, LYS398 residues of the enzyme; the distances were 2.1 and 2.1 Å, respectively. Compound 9 formed a hydrogen-bonding interaction with ASN301 residue of α-glucosidase, and the distance was 2.2 Å. In summary, the results indicated that candidates might bind to the active site of α-glucosidase to inhibit the activity of the enzyme.
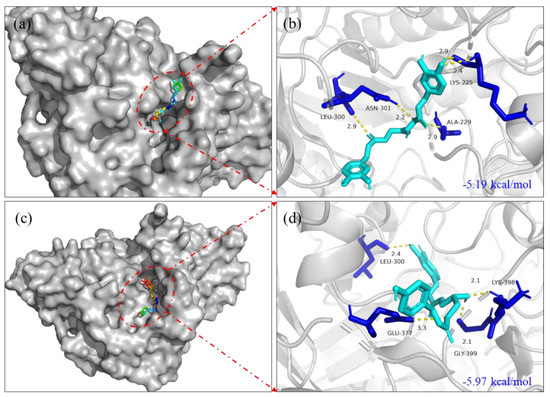
Figure 3.
Molecular docking pictures of 1-O-p-coumaroyl-3-O-caffeoylglycerol (8) and 1,3-O-dicaffeoylglycerol (9) on α-glucosidase of 3WY1. (a) The surface structure of 3WY1−1,3-O-dicaffeoylglycerol. (b) The binding site structure of 3WY1−1,3-O-dicaffeoylglycerol. (c) The surface structure of 3WY1−1-O-p-coumaroyl-3-O-caffeoylglycerol. (d) The binding site structure of 3WY1−1-O-p-coumaroyl-3-O-caffeoylglycerol.
2.5. Glucose Uptake and Cell Viability
To examine the effects of the CME, different fractions and isolated compounds of C. citratus involved with glucose uptake in 3T3-L1 adipocytes were evaluated. As shown in Figure 4a, the insulin group noticeably promoted the glucose uptake rates of 3T3-L1 adipocytes compared to the control group with a statistically significant difference (p < 0.001). It is a pity that compared with the control group, the CME, PE fraction, EtOAc fraction, n-BuOH fraction and AF did not significantly promote glucose uptake in 3T3-L1 adipocytes at 40 and 80 μg/mL (p > 0.05). The results showed that the main hypoglycemic effect of C. citratus may not be achieved by improving glucose uptake in 3T3-L1 adipocytes. As shown in Figure 4c, compound 5 had a weak effect on glucose uptake in 3T3-L1 adipocytes at 20 μM (p < 0.05). Additionally, the results of cell viability (Figure 4b,d) showed that compared with the blank control group, the CME, PE fraction, EtOAc fraction, n-BuOH fraction, AF fraction, and isolated compounds had no cytotoxicity.
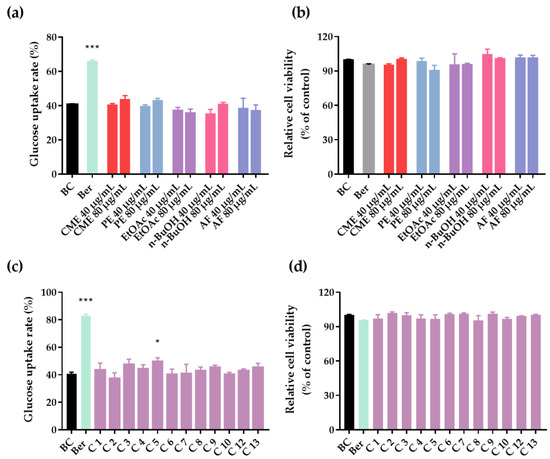
Figure 4.
Glucose uptake and cell viability in 3T3-L1 adipocytes. (a) Glucose uptake rates of C. citratus extract and fractions (CME, PE, EtOAc, n-BuOH, and AF). (b) Relative cell viability of C. citratus extract and fractions. (c) Glucose uptake rates of compounds 1–10 and 12–13. (d) Relative cell viability of compounds 1–10 and 12–13. BC, blank control; Ber, berberine (positive control); C, compound; Compounds at 20 μM and berberine at 10 μg/mL; Data are showed as mean ± SD of three independent experiments; * p < 0.05, *** p < 0.001 versus blank control.
3. Materials and Methods
3.1. Plant Material
The dried aerial part of Cymbopogon citratus (D.C.) Stapf was collected in Jinghong in 2018 and identified by Prof. Yumei Zhang. The voucher specimen (No. 2018006) was deposited in the Innovative Drug Research Group, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.
3.2. Chemicals, Reagents and Cell
NMR was determined by the BrukerAM-400 MHz and Bruker DRX-500 MHZ AVANCE III-600 MHz (Bruker Corporation, Madison, WI, USA) (TMS as internal standard) NMR instrument. EI-MS was determined by the VG AutoSpec 3000 mass spectrometer. Semi-preparative HPLC was run on a Waters e2695 system (Waters Corporation, Milford, MA, USA) with a Waters 2487 detector and an Agilent Zorbax SB-C18 column (250 mm × 9.4 mm, 5 μm) (Agilent Corporation, Santa Clara, CA, USA). Column chromatography was conducted with silica gel (Qingdao Marine Chemical Co. Ltd., Qingdao, China), RP-C18 (Merck KGaA, Darmstadt, Germany), and Sephadex LH-20 (Cytiva Sweden AB, Upsala, Sweden). DPPH (2,2-diphenyl-1-picrylhydrazyl) was purchased from Macklin Biochemical Co. Ltd. (Shanghai, China). FRAP and ABTS+ assays were evaluated by a commercial assay kit (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China). α-Glucosidase (25.4 U/mg), acarbose, 4-nitrophenyl-α-D-glucopyranoside (pNPG), and ascorbic acid were purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). The other chemicals and reagents were purchased from local suppliers. The absorbance was measured by a microplate reader (Molecular Devices, Palo Alto, Santa Clara, CA, USA). The 3T3-L1 mouse preadipocytes were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). High glucose DMEM, low glucose DMEM, Pen-Strep solution (P/S), insulin, certified fetal bovine serum (FBS), special newborn calf serum (NBCS), and phosphate buffered saline (PBS) were purchased from Biological Industries (Shanghai, China). 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone (DEX) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The glucose test kit was purchased from Rongsheng Biotech Co., Ltd. (Shanghai, China). Rosiglitazone (ROSI) was purchased from Meilun Biotech Co., Ltd. (Dalian, Liaoning, China). Dimethyl sulfoxide (DMSO) was obtained from Solarbio (Beijing; China). CellTiter 96® AQueous One Solution Cell Proliferation Assay was also acquired (Promega Corporation, Madison, WI, USA).
3.3. Extraction, Isolation, and Purification
The dried aerial part (18 kg) of C. citratus was powdered and then extracted with industrial methanol three times at room temperature (7 days, 3 days, and 3 days, respectively). The CME (3.02 kg) was obtained by concentration under vacuum, subsequently dissolved by stirring in hot water, and successively partitioned with PE, EtOAc, and n-BuOH, to afford the PE fraction (463.6 g), EtOAc fraction (180.0 g), and n-BuOH fraction (226.1 g).
The PE fraction was subjected to a silica gel column (200–300 mesh, 4 kg) using the gradient elution manner with PE/acetone (10:1, 7:1, 5:1, 2:1, 1:1, 0:1) to gain 10 fractions (Fr.1- Fr.10). Fraction 2 was separated using Sephadex LH-20 column chromatography and recrystallized to gain 17 (20 mg). Fraction 3 was subjected to a silica gel column and further purified by Sephadex LH-20 column chromatography to obtain 20 (7 mg). Fraction 5 was subjected to silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 10 (17 mg), 13 (9 mg), and 14 (37 mg). Fraction 6 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 16 (9 mg) and 19 (7 mg).
The EtOAc fraction was separated on a silica gel column (200–300 mesh, 1.5 kg) using the gradient elution manner with CHCl3/MeOH (30:1, 15:1, 9:1, 4:1, 2:1, 0:1) to obtain 12 fractions (Fr.11- Fr.22). Fraction 15 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography and recrystallizing to obtain 1 (8 mg), 4 (13 mg). Fraction 18 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography and semi-preparative HPLC to obtain 2 (14 mg). Fraction 19 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 6 (5 mg). Fraction 20 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 8 (8 mg). Fraction 22 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography and semi-preparative HPLC to obtain 7 (9 mg). Fraction 22 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography and semi-preparative HPLC to obtain 5 (6 mg), 9 (7 mg), 11 (9 mg).
The n-BuOH fraction was separated on silica gel column (200–300 mesh, 1.5 kg) using the gradient elution manner with CHCl3/MeOH (9:1, 5:1, 3:1, 0:1) to obtain 9 fractions (Fr.23- Fr.31). Fraction 24 was subjected to a silica gel column and further purified by Sephadex LH-20 column chromatography and recrystallizing to obtain 12 (5 mg). Fraction 25 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography and recrystallizing to obtain 3 (6 mg). Fraction 27 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 15 (11 mg). Fraction 30 was subjected to a silica gel column and further purified using Sephadex LH-20 column chromatography to obtain 18 (24 mg).
3.4. Measurement of Antioxidant Activity
The antioxidant activities of the crude extracts and isolated compounds were evaluated by implementing 2,2-diphenyl-1-picrylhydrazyl free radical scavenging (DPPH+), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic free radical scavenging (ABTS+), and the ferric reducing antioxidant power (FRAP) assay.
The DPPH+ assay was measured according to the previous report [48] with slight modifications. Briefly, the crude extracts and chemical compounds were dissolved in anhydrous ethanol at concentrations ranging from 100 to 3200 μg/mL and 20 to 800 μM. The DPPH+ was dissolved with anhydrous methanol to 0.1 mM.
Then 180 μL DPPH solution and 20 μL sample were mixed in each well of a 96-well plate and incubated for 30 min at room temperature in the dark. The absorbance was recorded at 517 nm using a microplate reader. Ascorbic acid was used as a positive control. The DPPH+ scavenging activity was calculated according to the following formula:
A1 = absorbance of the sample group with DPPH, A2 = absorbance of the sample control group without DPPH, A3 = absorbance of the control group with DPPH, and A4 = the absorbance of the blank control.
Inhibition (%) = [1 − (A1 − A2)/(A3 − A4)] × 100
ABTS+ and FRAP assays were measured by commercial assay kits (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China). The experimental method was determined according to the method used in the literature [48]. The crude extracts and chemical compounds were dissolved in anhydrous ethanol at concentrations of 800 μg/mL and 40 μM. The absorbance was measured using a microplate reader at 734 nm in the dark and 593 nm after reaction for 20 min in the dark, respectively. Trolox was used as a standard reference compound to quantitate ABTS+ and FRAP capacity. The results were expressed in µmol Trolox/g or mol Trolox/mol. The standard curve was drawn using Trolox solutions at a range of concentrations.
3.5. α-Glucosidase Inhibition Assay
The α-glucosidase inhibitory activity was measured according to the procedure described in the paper [49], with slight modifications. Briefly, add 50 μL α-glucosidase (0.1 U/mL) and 80 μL phosphate buffered saline (PBS) (pH = 6.9) to each well in 96-well plates, then add 10 μL sample solution (crude extracts and chemical compounds at concentrations ranging from 200 to 6400 μg/mL and 10 to 400 μM) and incubate at 37 °C for 15 min. Immediately, 40 μL PNPG (2.5 mM) was added to each well and reacted at 37 °C for 30 min. In the end, 20 μL Na2CO3 (0.5 mol/L) was added to stop the reaction, and then the absorbance value was measured using a microplate reader at 405 nm. The total volume of the reaction system was 200 μL. The α-glucosidase inhibition activity was calculated according to the following formula:
A1 = the absorbance of the sample group with α-glucosidase, A2 = the absorbance of the sample control group without α-glucosidase, A3 = the absorbance of the control group without samples, and A4 = the absorbance of the blank control without samples and α-glucosidase.
Inhibition (%) = [1 − (A1 − A2)/(A3 − A4)] × 100
Results were expressed as IC50 values calculated according to Prism7.0 software.
3.6. Molecular Docking
Molecular docking was applied to identify the possible binding sites between candidates and α-glucosidase according to a previous study [50]. The crystal structure of halomonas α-glucosidase (PDB ID: 3WY1) was obtained from the Protein Data Bank (PBD), and the three-dimensional structures of the candidates were established through MarvinSketch. The water molecules of α-glucosidase were removed via the PyMOL molecular graphics system (version: 2.2.0) to obtain a stable α-glucosidase structure. AutoDock Tools (ADT, version: 1.5.6) was used to accomplish molecular docking in silico [51]. The cubic grid box dimensions of α-glucosidase were defined as x = 96, y = 98, and z = 118 Å with spacing of 0.686 Å. Finally, the PyMOL molecular graphics system (version: 2.2.0) was used to visualize ligand-enzyme interactions. Based on the minimum energy scoring, the best binding conformations between α-glucosidase and the candidates were selected from all docking results [52].
3.7. 3T3-L1 Preadipocytes Culture and Differentiation
The 3T3-L1 preadipocytes were cultured and differentiated using the method described earlier with slight modifications [53]. The 3T3-L1 preadipocytes were cultured in high glucose DMEM supplemented with 10% NBCS, 1% P/S, and then starved until the cells reached confluence (day 0). Two days later (day 2), the cells were induced for differentiation with high glucose DMEM supplemented with 10% FBS, 1% P/S, IBMX, DEX, Rosi, and insulin. On day 5, the medium was changed to high glucose DMEM containing 10% FBS, 1% P/S, and 100 nM insulin for one day (day 6). Then the cells were completely differentiated into mature adipocytes.
3.8. Glucose Uptake and Cell Viability Assay
The glucose uptake test was carried out according to the procedure described in the paper [53]. The 3T3-L1 adipocytes were inoculated in a 96-well plate at 5 × 104 cells/well for 24 h. Then the berberine (10 μg/mL) and samples (40 μg/mL, 80 μg/mL or 20 μM) were added to individual 3T3-L1 adipocytes and repeated 3 times. After 48 h of culture, the glucose uptake was measured according to the operating instructions of the glucose content determination kit. Following the glucose uptake test, the cell viability was determined using the CellTiter 96® aqueous cell proliferation test [54]. Then 15 μL of CellTiter 96® AQueous One Solution Cell Proliferation Assay reagent was added to each well. Then the absorbance was measured using microplate reader at 490 nm after incubation at 37 °C for 4 h. The cell viability was calculated according to the following formula:
A1 = the absorbance of the blank control, A2 = the absorbance of each fraction group, each compound group, or positive control group.
Cell viability rate (%) = A1/A2 × 100
3.9. Statistical Analysis
Each experiment’s measurements were repeated there times, and all the data were expressed as mean ± standard deviation (SD). The IC50 of DPPH+ scavenging activity and α-glucosidase inhibition activity were calculated according to non-linear regression analysis. The differences between different samples were assessed by using a one-way analysis of variance (ANOVA). If the P value was less than 0.05, the data of samples were considered statistically significant. All analyses were carried out using Graphpad Prism7.0 software (GraphPad Software Inc., San Diego, CA, USA).
4. Conclusions
In this study, 20 compounds, including terpenoids, glycerides, flavones, and their glycosides, aromatic ketones, phenolic acids, alkaloids, and steroids, were isolated from the dried aerial part of C. citratus. Among them, compounds 1–3, 6–11, 13–16, and 18–20 were reported for the first time from this species. The bioactive investigations of the ethanol extract and different fractions of C. citratus exhibited that the EtOAc and n-BuOH fractions had a potent antioxidant effect, and the EtOAc and PE fractions had a potent α-glucosidase inhibitory effect. However, we did not find compounds with α-glucosidase inhibitory activity in the PE fraction, so it would be valuable to study the bioactive compounds in the PE fraction extensively. Compounds 8 and 9 showed a potent antioxidant effect and an α-glucosidase inhibitory effect. The α-glucosidase inhibitory activities of compounds 8 and 9 were reported for the first time. Furthermore, the results revealed that compounds 8 and 9 might be developed as candidate drugs to cure diabetes because of their potential regulatory roles on oxidative stress and digestive enzyme. This study enriched the chemical composition diversity of C. citratus and provided effective evidence for its use in health food and hypoglycemic herbal medicine.
Author Contributions
Conceptualization, H.W., Y.Z.; methodology, H.W., K.Z.; formal analysis, H.W.; investigation, R.Z.; resources, R.Z.; data curation, X.C.; writing—original draft preparation, H.W.; writing—review and editing, Y.Z., K.Z.; visualization, H.W., K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Science and Technology Major Project of Yunnan Province, grant number 202102AA100014, and The Special Project for Environmental Protection in Yunnan Province, grant number E1YN051K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available in the article.
Acknowledgments
We would like to acknowledge Kunming Institute of Botany, Chinese Academy of Sciences for providing NMR and MS facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from authors.
References
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors contributing to Type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AKerblom, H.K.; Knip, M. Putative environmental factors in Type 1 diabetes. Diabetes Metab Rev. 2010, 14, 31–68. [Google Scholar] [CrossRef]
- Page, R.C.L. Chapter 42—Insulin, other hypoglycemic drugs, and glucagon. In Side Effects of Drugs Annual; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 31, pp. 689–702. [Google Scholar]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef] [Green Version]
- Oladeji, O.S.; Adelowo, F.E.; Ayodele, D.T.; Odelade, K.A. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Majewska, E.; Kozlowska, M.; Gruczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation—A review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Borges, P.H.O.; Pedreiro, S.; Baptista, S.J.; Geraldes, C.F.G.C.; Batista, M.T.; Silva, M.M.C.; Figueirinha, A. Inhibition of α-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. J. Ethnopharmacol. 2021, 280, 114470. [Google Scholar] [CrossRef]
- Bharti, S.K.; Kumar, A.; Prakash, O.; Krishnan, S.; Gupta, A.K. Essential oil of Cymbopogon Citratus against diabetes: Validation by In Vivo experiments and computational studies. J. Bioanal. Biomed. 2013, 5, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Briones, M.D.C.; Hernández, L.R.; Guerrero-Beltrán, J.Á. Physicochemical and antioxidant properties of Cymbopogon citratus essential oil. J. Food Res. 2015, 4, 36. [Google Scholar] [CrossRef]
- Bassolé, I.H.; Lamien-Meda, A.; Bayala, B.; Obame, L.C.; Ilboudo, A.J.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 2011, 18, 1070–1074. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef] [PubMed]
- Boaduo, N.K.; Katerere, D.; Eloff, J.N.; Naidoo, V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharm. Biol. 2014, 52, 756–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, L.H.C.; Machado, R.A.F.; Spricigo, C.B.; Pereira, L.K.; Bolzan, A. Extraction of lemongrass essential oil with dense carbon dioxide. J. Supercrit. Fluids 2001, 21, 33–39. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 2002, 50, 1345–1349. [Google Scholar] [CrossRef]
- Bassole, I.H.N.; Nebie, R.; Savadogo, A.; Ouattara, C.T.; Barro, N.; Traore, S.A. Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. Afr. J. Biotechnol. 2005, 4, 1156–1160. [Google Scholar] [CrossRef]
- Saleem, M.; Afza, N.; Anwar, M.A.; Hai, S.M.; Ali, M.S. A comparative study of essential oils of Cymbopogon citratus and some members of the genus Citrus. Nat. Prod. Res. 2003, 17, 369–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Li, J.; Zhang, Z.; He, Y.; Yang, H.; Zhou, P. Inhibition on α-glucosidase activity and non-enzymatic glycation by an anti-oxidative proteoglycan from ganoderma lucidum. Molecules 2022, 27, 1457. [Google Scholar] [CrossRef]
- Tian, T.; Chen, G.Y.; Zhang, H.; Yang, F.Q. Personal glucose meter for α-glucosidase inhibitor screening based on the hydrolysis of maltose. Molecules 2021, 26, 4638. [Google Scholar] [CrossRef]
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ru, Y.; Wang, Z.; He, X.; Kong, K.W.; Zheng, T.; Zhang, X. Phytochemical composition, antioxidant activity, and enzyme inhibitory activities (α-glucosidase, xanthine oxidase, and acetylcholinesterase) of Musella lasiocarpa. Molecules 2021, 26, 4472. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [Green Version]
- Yenesew, A.; Dagne, E.; Müller, M.; Steglich, W. An anthrone, an anthraquinone and two oxanthrones from Kniphofia foliosa. Phytochemistry 1994, 37, 525–528. [Google Scholar] [CrossRef]
- Shen, S.; Ding, X.; Ouyang, M.A.; Wu, Z.J.; Xie, L.H. A new phenolic glycoside and cytotoxic constituents from Celosia argentea. J. Asian Nat. Prod. Res. 2010, 12, 821–827. [Google Scholar] [CrossRef]
- Sun, L.L.; Chen, D.W.; Chen, R.D.; Xie, K.B.; Liu, J.M.; Yang, L.; Dai, J.G. Exploring the aglycon promiscuity of a new glycosyltransferase from Pueraria lobata. Tetrahedron Lett. 2016, 57, 1518–1521. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, H.X.; Cui, Y.L.; Fan, M.X.; Liu, Z.G.; Mei, L.J.; Shao, Y.; Wang, Y.P.; Tao, Y.D. Phenolics from Lagotis brevituba Maxim. Nat. Prod. Res. 2017, 31, 362–366. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, F.R.; Lin, Y.J.; Wang, L.; Chen, J.F.; Wu, Y.C.; Wu, M.J. Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Food Chem. 2007, 105, 48–56. [Google Scholar] [CrossRef]
- Luo, J.G.; Lu, L.; Kong, L.Y. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Delaporte, R.H.; Guzen, K.P.; Laverde, A.; dos Santos, A.R.; Sarragiotto, M.H. Phenylpropanoid glycerols from Tillandsia streptocarpa Baker (Bromeliaceae). Biochem. Syst. Ecol. 2006, 34, 599–602. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Bai, L.; Pan, M.H. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammatory and human cancer cell lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef]
- Lee, S.S.; Baek, N.I.; Baek, Y.S.; Chung, D.K.; Song, M.C.; Bang, M.H. New flavonolignan glycosides from the aerial parts of Zizania latifolia. Molecules 2015, 20, 5616–5624. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.H.; Yin-Tai, N.A.; En-Qi, W.U. Study on chemical constituents of capsella bursa-pastoris. Nat. Prod. Res. Dev. 2014, 26, 50. [Google Scholar]
- Zhou, T.Y.; Ringbeck, B.; Schebb, N.H.; Scherkenbeck, J. Isolation, total synthesis and quantification of caffeoylisocitric acid, a characteristic ingredient of the superfood amaranth. Tetrahedron 2019, 75, 4479–4485. [Google Scholar] [CrossRef]
- Zhang, D.S.; Chen, W.H.; Chen, G.Y.; Han, C.R.; Wang, Y.; Wang, J.; Shang, Q.Q.; Zhao, W.D. Chemical constituents from the stems of Saprosma merrillii Lo. J. Chin. Pharm. Sci. 2013, 48, 964–967. [Google Scholar] [CrossRef]
- Yin, F.; Hu, L.H.; Pan, R.X. Novel dammarane-type glycosides from Gynostemma pentaphyllum. Chem. Pharm. Bull. 2004, 52, 1440–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, J.; Wen, H.X.; Wang, W.D.; Jiao, L.J.; Zhang, L.; Tao, Y.D.; Pei, J.J.; Shao, Y.; Mei, L.J.; Wang, Q.L. Isolation and identification of water-soluble components of Lycium barbarum leaves. Chem. Nat. Compd. 2019, 55, 138–140. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Tsuyuki, T.; Nakamura, N.; Takahashi, T.; Hanson, S.W.; Matsushita, K. Revised structures of cymbopogone and cymbopogonol. Tetrahedron Lett. 1980, 21, 3701–3702. [Google Scholar] [CrossRef]
- Roh, E.M.; Jin, Q.; Jin, H.G.; Shin, J.E.; Choi, E.J.; Moon, Y.H.; Woo, E.R. Structural implication in cytotoxic effects of sterols from Sellaginella tamariscina. Arch. Pharmacal Res. 2010, 33, 1347–1353. [Google Scholar] [CrossRef]
- Zhang, J.M.; Chen, Y.Z.; Li, B.G.; Wang, M.K. Studies on the chemical constituents of aster poliothamnus diels. China J. Chin. Mater. Med. 1997, 22, 103–104. [Google Scholar]
- Tavares, F.; Costa, G.; Francisco, V.; Liberal, J.; Figueirinha, A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus industrial waste as a potential source of bioactive compounds. J. Sci. Food Agric. 2015, 95, 2652–2659. [Google Scholar] [CrossRef]
- Bao, X.-L.; Yuan, H.-H.; Zhao, H.-L.; Fang, X.-X.; Sun, B.; Lan, M.-B. Antioxidant synergisms between cymbopogon citratus polyphenols and a-tocopherol in DPPH radical-scavenging assay. Asian J. Chem. 2015, 27, 3188–3196. [Google Scholar] [CrossRef]
- Hanson, S.W.; Crawford, M.; Koker, M.; Menezes, F.A. Cymbopogonol, a new triterpenoid from Cymbopogon citratus. Phytochemistry 1976, 15, 1074–1075. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2012, 19, 669–675. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Paramasivam, S.; Arulkumar, A. Evaluation of the lemongrass plant (cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 2014, 4, S134–S139. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rahman, S.M.M. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 2011, 44, 672–676. [Google Scholar] [CrossRef]
- Lü, H.; Chen, J.; Li, W.L.; Ren, B.R.; Wu, J.L.; Zhang, H.Q. Hypoglycemic effect of the total flavonoid fraction from Folium Eriobotryae. Phytomedicine 2009, 16, 967–971. [Google Scholar] [CrossRef]
- Huang, D.D.; Jiang, Y.; Chen, W.S.; Yao, F.Y.; Huang, G.H.; Sun, L.N. Evaluation of hypoglycemic effects of polyphenols and extracts from Penthorum chinense. J. Ethnopharmacol. 2015, 163, 256–263. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Zhai, J.; Han, N.; Liu, Z.; Li, S.; Yin, J. Characterization of antioxidant, α-glucosidase and tyrosinase inhibitors from the rhizomes of potentilla anserina L. and their structure–activity relationship. Food Chem. 2021, 336, 127714. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.W.; Wang, R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In Vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complementary Altern. Med. 2019, 19, 207. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kong, K.W.; Wu, D.T.; Liu, H.Y.; Li, H.B.; Zhang, J.R.; Gan, R.Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its alpha-glucosidase inhibitory mechanism. Food Chem. 2021, 374, 131635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, X.-L.; Zhao, X.; Ni, J.-Y.; Wang, H.-L.; Han, M.; Zhang, Y.-M. Antidiabetic potential of catechu via assays for α-glucosidase, α-amylase, and glucose uptake in adipocytes. J. Ethnopharmacol. 2022, 291, 115118. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Addepalli, R.; Netzel, M.E.; Tinggi, U.; Fletcher, M.T.; Sultanbawa, Y.; Osborne, S.A. Antioxidant-rich extracts of Terminalia ferdinandiana interfere with estimation of cell viability. Antioxidants 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).