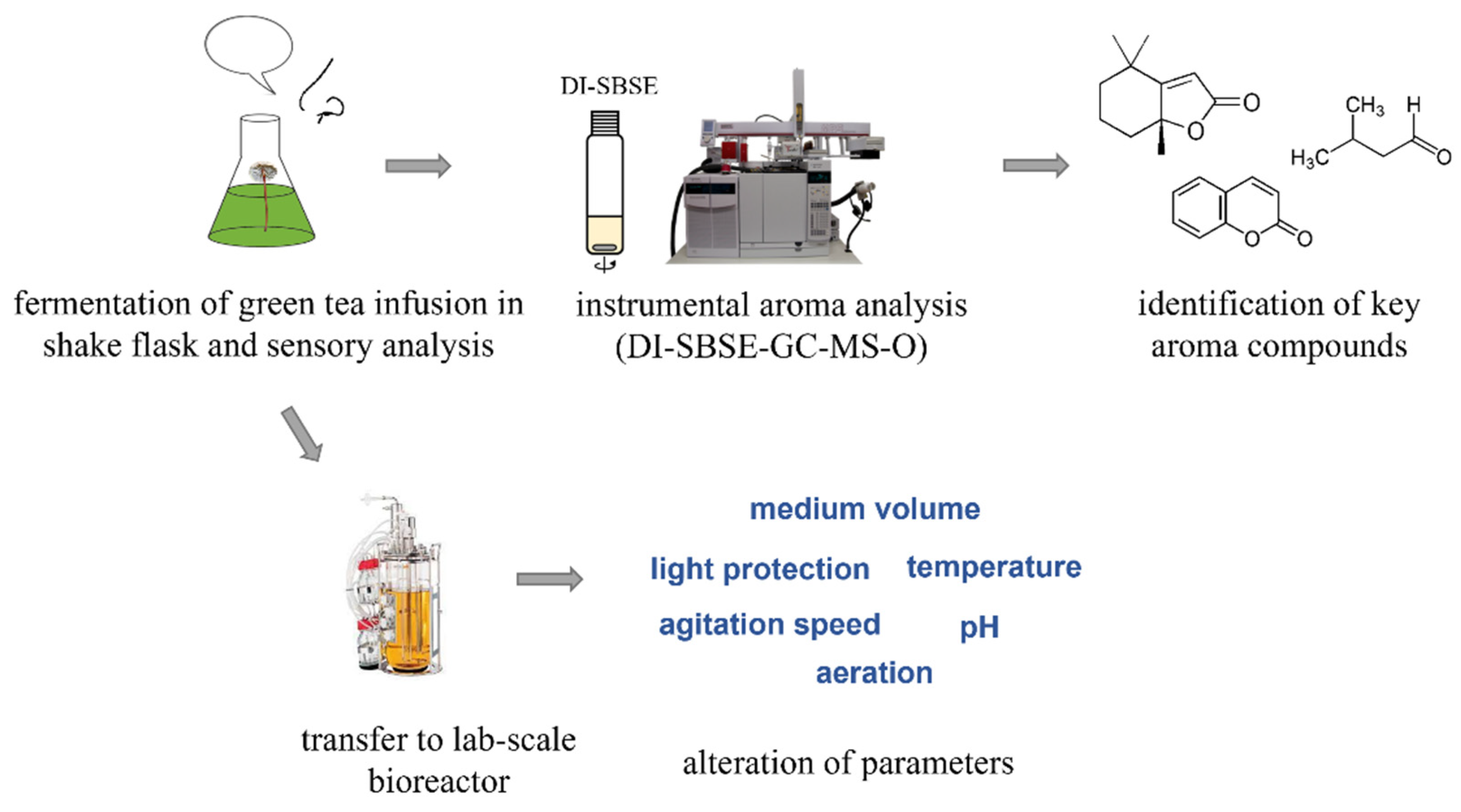
A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative, Quantitative, and Organoleptic Investigation on Chocolate-like Aroma Formed by the Basidiomycete Mycetinis scorodonius
| No. | RI | Compound | Odor Impression | Concentration (µg/L) | Odor Threshold (µg/L) | OAV | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 916 | 2-methylbutanal | chocolate-like, nutty, fermented | 10.17 ± 0.81 | 22.5 § | <1 | RI, odor, MS |
| 2 | 920 | isovaleraldehyde | nutty, chocolate-like, floral | 15.83 ± 0.70 | 0.2 | 79 | RI, odor, MS |
| 3 | 944 | ethanol | ethereal, alcoholic | 3.21 ± 0.01 | 10,000 | <1 | RI, odor, MS |
| 4 | 983 | valeraldehyde | fruity, nutty, chocolate-like | - * | RI, odor | ||
| 5 | 1030 | 2-butanol | sweetish, fruity | 17.22 ± 2.92 | 500 | <1 | RI, odor, MS |
| 6 | 1038 | unknown | fresh, floral | - | - | - | - |
| 7 | 1050 | unknown | fermented, brown, hay-like, malty | - | - | - | - |
| 8 | 1078 | hexanal | fresh, green, grassy | 2.58 ± 0.42 | 4.5 | <1 | RI, odor, MS |
| 9 | 1096 | unknown | chocolate-like, caramel-like | - | - | ||
| 10 | 1115 | isoamyl acetate | sweetish, banana-like, fruity | 23.22 ± 2.25 | 26 | <1 | RI, odor, MS |
| 11 | 1177 | heptanal | fatty, rancid | 15.01 ± 2.98 | 16 | <1 | RI, odor, MS |
| 13 | 1185 | limonene | fruity, citrus-like, herbal | 0.69 ± 0.09 | 10 | <1 | RI, odor, MS |
| 14 | 1199 | eucalyptol | herbal, medicinal, ethereal | 0.33 ± 0.05 | 1.3 | <1 | RI, odor, MS |
| 15 | 1207 | isoamyl alcohol | alcoholic, fruity | 14.72 ± 1.91 | 250 | <1 | RI, odor, MS |
| 16 | 1232 | ethyl hexanoate | wine-like, fruity | - * | - | - | RI, odor |
| 17 | 1239 | unknown | brown, floral, hay-like | - | - | - | - |
| 18 | 1251 | amyl alcohol | fermented, yeast-like, bread-like | - * | - | - | RI, odor |
| 19 | 1254 | pentanol | sweetish, alcoholic | 11.51 ± 0.73 | 4000 | <1 | RI, odor, MS |
| 20 | 1262 | 2-methylpyrazine | brown, musty, earthy | 10.79 ± 2.04 | 60 | <1 | RI, odor, MS |
| 21 | 1279 | octanal | fruity, floral | 0.73 ± 0.14 | 0.7 | 1 | RI, odor, MS |
| 22 | 1293 | hydroxy acetone | pungent, ethereal | 0.23 ± 0.05 | 10 § | <1 | RI, odor, MS |
| 23 | 1298 | unknown | hay-like, green | - | - | - | - |
| 24 | 1308 | (Z)-3-hexenyl acetate | fruity, green, pear-like | - * | - | - | RI, odor |
| 25 | 1317 | 2,5-dimethylpyrazine | peanut-like, nutty, roasted | 25.19 ± 3.83 | 800 | <1 | RI, odor, MS |
| 26 | 1325 | 2,6-dimethylpyrazine | roasted, coffee-like | 2.33 ± 0.11 | 400 | <1 | RI, odor, MS |
| 27 | 1329 | 2-ethylpyrazine | peanut-like, chocolate-like | 1.62 ± 0.21 | 6000 | <1 | RI, odor, MS |
| 28 | 1331 | 6-methyl-5-hepten-2-one | musty, brown | 5.57 ± 0.64 | 50 | <1 | RI, odor, MS |
| 29 | 1350 | unknown | sharp, pungent | - | - | - | - |
| 30 | 1351 | hexanol | fruity, alcoholic, ethereal | 7.71 ± 0.66 | 2500 | <1 | RI, odor, MS |
| 31 | 1374 | unknown | fresh, fruity, citrus-like | - | - | - | - |
| 32 | 1376 | 2-ethylhexyl acetate | herbal, hay-like, sweetish | - * | - | - | RI, odor |
| 33 | 1380 | (Z)-3-hexen-1-ol | green, leafy, citrus-like | 157.72 ± 14.89 | 39 | 4 | RI, odor, MS |
| 34 | 1385 | nonanal | fatty, waxy, brown | 2.70 ± 0.65 | 1 | 3 | RI, odor, MS |
| 35 | 1386 | 2-ethyl-5-methylpyrazine | chocolate-like, coffee-like | -* | - | - | RI, odor |
| 36 | 1398 | 2,3,5-trimethylpyrazine | roasted, chocolate-like, nutty | 5.00 ± 0.66 | 400 | <1 | RI, odor, MS |
| 37 | 1428 | (E)-2-octen-1-al | waxy, fatty, green | - * | - | - | RI, odor |
| 38 | 1445 | (Z)-linalool oxide | woody, terpenic | - * | - | - | RI, odor |
| 39 | 1444 | 2-ethyl-3,6-dimethylpyrazine | coffee-like, chocolate-like | 3.27 ± 0.32 | 0.4 | 8 | RI, odor, MS |
| 40 | 1444 | acetic acid | sourish, vinegar-like | 0.02 ± 0.01 | 2000 | <1 | RI, odor, MS |
| 41 | 1451 | 1-octen-3-ol | earthy, licorice | 1.12 ± 0.03 | 2 | <1 | RI, odor, MS |
| 42 | 1451 | unknown | hay-like | - | - | - | - |
| 43 | 1457 | 2-ethyl-3,5-dimethylpyrazine | cocoa-like, musty, roasted | 1.00 ± 0.19 | 1 | 1 | RI, odor, MS |
| 44 | 1457 | 6-methylhept-5-en-2-ol | sweetish, green | - * | - | - | RI, odor |
| 45 | 1466 | (E)-linalool oxide (furanoid) | woody, terpenic, green | - * | - | - | RI, odor |
| 46 | 1483 | 2-ethyl-1-hexanol | fresh, fruity, sweetish | 15.62 ± 1.47 | 270,000 | <1 | RI, odor, MS |
| 47 | 1488 | decanal | herbal, fresh | 0.93 ± 0.19 | 2 | <1 | RI, odor, MS |
| 48 | 1509 | benzaldehyde | bitter almond-like | 77.13 ± 3.71 | 350 | <1 | RI, odor, MS |
| 49 | 1539 | linalool | floral, sweetish, fruity | 1.45 ± 0.21 | 0.14 | 10 | RI, odor, MS |
| 50 | 1551 | octyl formate | fresh, aromatic | - * | - | - | RI, odor |
| 51 | 1589 | lilac aldehyde D | fresh, floral | - * | - | - | RI, odor |
| 52 | 1593 | terpinen-4-ol | spicy, terpenic | 50.11 ± 4.91 | 150 | <1 | RI, odor, MS |
| 53 | 1597 | unknown | brown, burnt, roasted | - | - | - | - |
| 54 | 1606 | (E)-2-octen-1-ol | green, vegetable-like | - * | - | - | RI, odor |
| 55 | 1615 | γ-butyrolactone | faint, sweetish, rancid | 1.94 ± 0.38 | 5 | <1 | RI, odor, MS |
| 56 | 1628 | phenylacetaldehyde | floral | 2.37 ± 0.13 | 4 | <1 | RI, odor, MS |
| 57 | 1638 | acetophenone | brown, musty, earthy | 115.52 ± 11.13 | 65 | 2 | RI, odor, MS |
| 58 | 1645 | 2,6-dimethyl-5-hepten-1-ol | oily, waxy, musty | - * | - | - | RI, odor |
| 59 | 1688 | α-terpineol | ethereal, terpenic | 31.93 ± 2.85 | 330 | <1 | RI, odor, MS |
| 60 | 1692 | 3-methyl-2(5H)-furanone | chocolate-like, woody, | 67.10 ± 8.15 | 89 | <1 | RI, odor, MS |
| 61 | 1698 | (S)-verbenone | spicy, menthol-like | 22.34 ± 4.01 | 2.59 § | 2 | RI, odor, MS |
| 62 | 1716 | dihydrocarveol | green, mint-like | 2.11 ± 0.62 | 7.5 § | <1 | RI, odor, MS |
| 63 | 1720 | citral | citrus-like | 0.49 ± 0.08 | 32 | <1 | RI, odor, MS |
| 64 | 1725 | (E)-linalool oxide (pyranoid) | terpenic, floral | - * | - | - | RI, odor |
| 65 | 1754 | (E)-linalool 3,7-oxide | hay-like, woody | - * | - | - | RI, odor |
| 66 | 1763 | methyl salicylate | minty, sweetish, floral | 21.01 ± 2.49 | 40 | <1 | RI, odor, MS |
| 67 | 1790 | (Z)-geraniol | sweetish, citrus-like, floral, fruity | 10.27 ± 1.14 | 3.2 | 3 | RI, odor, MS |
| 68 | 1807 | unknown | fermented, malty, chocolate-like | - | - | - | - |
| 69 | 1825 | (Z)-carveol | herbal, spearmint-like, sweetish | 25.87 ± 2.46 | 4 | 6 | RI, odor, MS |
| 70 | 1831 | hexanoic acid | cheesy, fatty | 66.44 ± 2.21 | 3000 | <1 | RI, odor, MS |
| 71 | 1837 | unknown | fruity, citrus-like, caramel-like | - | - | - | - |
| 72 | 1880 | geranyl isovalerate | green, apple-like | - * | - | - | RI, odor |
| 73 | 1898 | phenylethanol | ethereal, fresh, rose-like | 10.73 ± 1.62 | 86 | <1 | RI, odor, MS |
| 74 | 1909 | unknown | earthy, fungal | - | - | - | - |
| 75 | 1934 | (Z)-jasmone | floral, fruity, woody | 2.94 ± 0.39 | 1.9 | 2 | RI, odor, MS |
| 76 | 1956 | unknown | cotton candy-like, sweetish | - | - | - | - |
| 77 | 1977 | β-ionon-5,6-epoxide | fruity, berry-like | - * | - | - | RI, odor |
| 78 | 2014 | unknown | chocolate-like, brown, woody | - | - | - | - |
| 79 | 2025 | unknown | amaretto-like, caramel-like | - | - | - | - |
| 80 | 2049 | octanoic acid | rancid, fatty, vegetable-like, green | 147.62 ± 5.61 | 3000 | <1 | RI, odor, MS |
| 81 | 2085 | unknown | cherry-like, musty, woody | - | - | - | - |
| 82 | 2106 | unknown | ethereal, musty | - | - | - | - |
| 84 | 2124 | unknown | citrus-like, vanilla-like, floral | - | - | - | - |
| 85 | 2156 | nonanoic acid | fatty, coconut-like, waxy | 81.11 ± 3.91 | 3000 | <1 | RI, odor, MS |
| 86 | 2178 | 2-methoxy-4-vinylphenol | roasted, broth-like | 71.11 ± 10.45 | 19 | 4 | RI, odor, MS |
| 87 | 2184 | unknown | brown, hay-like | - | - | - | - |
| 88 | 2222 | methyl anthranilate | fruity | 2.17 ± 0.86 | 7.73 | <1 | RI, odor, MS |
| 89 | 2231 | α-cadinol | brown, woody | - * | - | - | RI, odor |
| 90 | 2263 | decanoic acid | fermented, rancid, sourish, fruity | 71.93 ± 16.06 | 1000 | <1 | RI, odor, MS |
| 91 | 2320 | methyl jasmonate | floral, jasmine-like, citrus-like | - * | - | - | RI, odor |
| 92 | 2332 | dihydroactinidiolide | woody, musk-like, fermented | 638.04 ± 93.05 | 1.85 § | 345 | RI, odor, MS |
| 93 | 2362 | unknown | fresh, vanilla-like, citrus-like | - | - | - | - |
| 94 | 2371 | coumaran | brown, cinnamon-like, spicy | -* | - | - | RI, odor |
| 95 | 2421 | unknown | fruity, sourish | - | - | - | - |
| 96 | 2432 | coumarin | amaretto-like, spicy | 815.95 ± 70.29 | 34 | 24 | RI, odor, MS |
| 97 | 2472 | skatole | floral, pungent, stable-like | 0.74 ± 0.04 | 2 | <1 | RI, odor, MS |
| 98 | 2474 | dodecanoic acid | musty, fatty, sourish | 26.27 ± 2.81 | 10,000 | <1 | RI, odor, MS |
| 99 | 2496 | 5-hydroxymethylfurfural | chamomile-like, waxy | - * | - | - | RI, odor |
| 100 | 2547 | vanillin | vanilla-like, creamy, sweetish | 30.62 ± 2.95 | 35 | <1 | RI, odor, MS |
2.2. Robustness of Bioreactor Process for Natural Chocolate-like Flavor Production
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Green Tea Infusion Preparation
3.3. Fermentation of Green Tea Infusion
3.3.1. Preparation of Pre-Cultures
3.3.2. Fermentation in Shake Flasks
3.3.3. Bioreactor Fermentation
3.4. Direct Immersion Stir Bar Sorptive Extraction (DI-SBSE) for Isolating Volatiles from Fermented Tea Infusion
3.5. Gas Chromatography (GC)
3.6. Compound Identification
3.7. Semi-Quantification, Odor Activity Value (OAV), and Recombination Study
3.8. Data Evaluation and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lomascolo, A.; Stentelaire, C.; Asther, M.; Lesage-Meessen, L. Basidiomycetes as new biotechnological tools to generate natural aromatic flavours for the food industry. Trends Biotechnol. 1999, 17, 282–289. [Google Scholar] [CrossRef]
- Janssens, L.; De Pooter, H.L.; Schamp, N.M.; Vandamme, E.J. Production of flavours by microorganisms. Process Biochem. 1992, 27, 195–215. [Google Scholar] [CrossRef]
- Abraham, B.G.; Berger, R.G. Higher fungi for generating aroma components through novel biotechnologies. J. Agric. Food Chem. 1994, 42, 2344–2348. [Google Scholar] [CrossRef]
- Zhang, Y.; Fraatz, M.A.; Horlamus, F.; Quitmann, H.; Zorn, H. Identification of potent odorants in a novel nonalcoholic beverage produced by fermentation of wort with shiitake (Lentinula edodes). J. Agric. Food Chem. 2014, 62, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Trapp, T.; Zajul, M.; Ahlborn, J.; Stephan, A.; Zorn, H.; Fraatz, M.A. Submerged cultivation of Pleurotus sapidus with molasses: Aroma dilution analyses by means of solid phase microextraction and stir bar sorptive extraction. J. Agric. Food Chem. 2017, 66, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Fraatz, M.A.; Büttner, J.; Salem, A.A.; Rühl, M.; Zorn, H. Wild strawberry-like flavor produced by the fungus Wolfiporia cocos—Identification of character impact compounds by aroma dilution analysis after dynamic headspace extraction. J. Agric. Food Chem. 2021, 69, 14222–14230. [Google Scholar] [CrossRef]
- Rigling, M.; Yadav, M.; Yagishita, M.; Nedele, A.-K.; Sun, J.; Zhang, Y. Biosynthesis of pleasant aroma by enokitake (Flammulina velutipes) with a potential use in a novel tea drink. LWT 2021, 140, 110646. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Riemer, S.J.L.; Stöber, R.; Kaspera, R.; Nimtz, M.; Berger, R.G.; Zorn, H. A novel oxygenase from Pleurotus sapidus transforms valencene to nootkatone. J. Mol. Catal. B Enzym. 2009, 61, 202–207. [Google Scholar] [CrossRef]
- Zelena, K.; Hardebusch, B.; Hülsdau, B.; Berger, R.G.; Zorn, H. Generation of norisoprenoid flavors from carotenoids by fungal peroxidases. J. Agric. Food Chem. 2009, 57, 9951–9955. [Google Scholar] [CrossRef]
- Colpa, D.I.; Fraaije, M.W.; van Bloois, E. DyP-type peroxidases: A promising and versatile class of enzymes. J. Ind. Microbiol. Biotechnol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liers, C.; Aranda, E.; Strittmatter, E.; Piontek, K.; Plattner, D.A.; Zorn, H.; Ullrich, R.; Hofrichter, M. Phenol oxidation by DyP-type peroxidases in comparison to fungal and plant peroxidases. J. Mol. Catal. B Enzym. 2014, 103, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Rigling, M.; Liu, Z.; Hofele, M.; Prozmann, J.; Zhang, C.; Ni, L.; Fan, R.; Zhang, Y. Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem. 2021, 361, 130065. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, P.; Ebeler, S.E. Carotenoid Cleavage Products; ACS Publications: Washington, DC, USA, 2013; ISBN 1947-5918. [Google Scholar]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, P.; Schieberle, P. Evaluation of key odorants in milk chocolate and cocoa mass by aroma extract dilution analyses. J. Agric. Food Chem. 1997, 45, 867–872. [Google Scholar] [CrossRef]
- Beckett, S.T. Industrial Chocolate Manufacture and Use; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1444357557. [Google Scholar]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Odor & Flavor Detection Thresholds in Water. Available online: http://www.leffingwell.com/odorthre.htm (accessed on 14 March 2022).
- Cancho, B.; Fabrellas, C.; Diaz, A.; Ventura, F.; Galceran, M.T. Determination of the odor threshold concentrations of iodinated trihalomethanes in drinking water. J. Agric. Food Chem. 2001, 49, 1881–1884. [Google Scholar] [CrossRef]
- Odor Threshold. Available online: https://www.chemicalbook.com/ChemicalProductProperty_DE_CB6315663.htm (accessed on 14 March 2022).
- Han, Z.-X.; Rana, M.M.; Liu, G.-F.; Gao, M.-J.; Li, D.-X.; Wu, F.-G.; Li, X.-B.; Wan, X.-C.; Wei, S. Data on green tea flavor determinantes as affected by cultivars and manufacturing processes. Data Brief 2017, 10, 492–498. [Google Scholar] [CrossRef]
- Courtin, C.M.; Broekaert, W.F.; Swennen, K.; Aerts, G.; van Craeyveld, V.; Delcour, J.A. Occurrence of arabinoxylo-oligosaccharides and arabinogalactan peptides in beer. J. Am. Soc. Brew. Chem. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Stern, D.J. Studies on popcorn aroma and flavor volatiles. J. Agric. Food Chem. 1997, 45, 837–843. [Google Scholar] [CrossRef]
- Del Mar Caja, M.; Preston, C.; Menzel, M.; Kempf, M.; Schreier, P. Online gas chromatography combustion/pyrolysis−isotope ratio mass spectrometry (HRGC-C/P-IRMS) of (±)-dihydroactinidiolide from tea (Camellia sinensis) and rooibos tea (Aspalathus linearis). J. Agric. Food Chem. 2009, 57, 5899–5902. [Google Scholar] [CrossRef]
- Bosser, A.; Paplorey, E.; Belin, J.-M. A simple way to (.+−.)-dihydroactinidiolide from. beta.-ionone related to the enzymic co-oxidation of. beta.-carotene in aqueous solution. Biotechnol. Prog. 1995, 11, 689–692. [Google Scholar] [CrossRef]
- Schreier, P.; Drawert, F.; Bhiwapurkar, S. Volatile compounds formed by thermal degradation of beta-carotene. Chem. Mikrobiol. Technol. Lebensm. 1979, 6, 90–91. [Google Scholar]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Berger, R.G. Cleavage of β, β-carotene to flavor compounds by fungi. Appl. Microbiol. Biotechnol. 2003, 62, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Nimtz, M.; Berger, R.G. A peroxidase from Lepista irina cleaves β, β-carotene to flavor compounds. Biol. Chem. 2003, 384, 1437–4315. [Google Scholar] [CrossRef]
- Baik, H.-J.; Ko, Y.-S. Studies on the aroma components of roasted and ground coffee. Korean J. Food Sci. Technol. 1996, 28, 15–18. [Google Scholar]
- Co, H.; Sanderson, G.W. Biochemistry of tea fermentation: Conversion of amino acids to black tea aroma constituents. J. Food Sci. 1970, 35, 160–164. [Google Scholar] [CrossRef]
- Self, R.; Rolley, H.L.; Joyce, A.E. Some volatile compounds from cooked potatoes. J. Sci. Food Agric. 1963, 14, 8–14. [Google Scholar] [CrossRef]
- Bailey, S.D.; Mitchell, D.G.; Bazinet, M.L.; Weurman, C. Studies on the volatile components of different varieties of cocoa beans. J. Food Sci. 1962, 27, 165–170. [Google Scholar] [CrossRef]
- Bueno, M.; Marrufo-Curtido, A.; Carrascón, V.; Fernández-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and accumulation of acetaldehyde and strecker aldehydes during red wine oxidation. Front. Chem. 2018, 6, 20. [Google Scholar] [CrossRef]
- Grosshauser, S.; Schieberle, P. Characterization of the key odorants in pan-fried white mushrooms (Agaricus bisporus L.) by means of molecular sensory science: Comparison with the raw mushroom tissue. J. Agric. Food Chem. 2013, 61, 3804–3813. [Google Scholar] [CrossRef]
- Costa, T.M.; Tavares, L.B.B.; de Oliveira, D. Fungi as a source of natural coumarins production. Appl. Microbiol. Biotechnol. 2016, 100, 6571–6584. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and plant phenylalanine ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todaro, C.M.; Vogel, H.C. Fermentation and Biochemical Engineering Handbook; William Andrew: Norwich, NY, USA, 2014; ISBN 1455730467. [Google Scholar]
- Araújo, N.L.; Avelino, K.V.; Halabura, M.I.W.; Marim, R.A.; Kassem, A.S.S.; Linde, G.A.; Colauto, N.B.; do Valle, J.S. Use of green light to improve the production of lignocellulose-decay enzymes by Pleurotus spp. in liquid cultivation. Enzym. Microb. Technol. 2021, 149, 109860. [Google Scholar] [CrossRef] [PubMed]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of food industry side streams by basidiomycetes for production of a vegan protein source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Arjona, D.; Aragón, C.; Aguilera, J.A.; Ramírez, L.; Pisabarro, A.G. Reproducible and controllable light induction of in vitro fruiting of the white-rot basidiomycete Pleurotus ostreatus. Mycol. Res. 2009, 113, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.V.; Gavrilova, V.P.; Yavmetdinov, I.S.; Shleev, S.V.; Stepanova, E.V. Isolation and study of some properties of laccase from the basidiomycetes Cerrena maxima. Biochemistry 2001, 66, 618–622. [Google Scholar]


| Set-Up | Fermentation Time (h) | Odor Impression | Odor Intensity (–) | |
|---|---|---|---|---|
| shake flask | 250 mL | 64 | chocolate-like, malty | 3 |
| bioreactor | 1 L | 68 | chocolate-like, malty | 2.5 |
| 2.5 L | 70 | chocolate-like, woody | 2 | |
| dark | 68 | chocolate-like | 2 | |
| 100 rpm | 68 | chocolate-like, licorice-like | 2.5 | |
| 250 rpm | 68 | chocolate-like, licorice-like | 2 | |
| 18 °C | 68 | chocolate-like | 2.5 | |
| 30 °C | 68 | chocolate-like, malty | 2 | |
| pH 5 | 68 | chocolate-like, sweetish | 1.5 | |
| pH 8 | 68 | chocolate-like, sweetish, salty | 2 | |
| aerated | 68 | chocolate-like, green | 3.5 | |
| Medium Volume (L) | Light Protection | Agitation Speed (rpm) | Temperature (°C) | pH | Aeration | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Standard) | 2.5 | Protected | 100 | 250 | 18 | 30 | 5 | 8 | 100% pO2 | |
| isovaleraldehyde | 7.53 ± 1.13 a | 15.79 ± 1.08 b | 10.89 ± 0.53 b | 3.71 ± 0.11 b | 7.05 ± 1.92 a | 43.32 ± 7.50 b | 22.01 ± 3.68 b | 6.61 ± 0.53 a | 2.11 ± 0.38 b | 68.86 ± 9.89 b |
| octanal | 1.27 ± 0.09 a | 0.39 ± 0.05 b | 1.30 ± 0.15 a | 2.93 ± 0.34 b | 0.74 ± 0.09 b | 3.50 ± 0.69 b | 3.04 ± 0.64 b | 0.63 ± 0.04 b | 0.74 ± 0.12 b | 23.22 ± 5.11 b |
| (Z)-3-hexen-1-ol | 109.62 ± 9.78 a | 111.95 ± 4.87 a | 141.71 ± 34.32 a | 119.98 ± 8.86 a | 115.13 ± 15.54 a | 185.47 ± 20.80 b | 135.14 ± 15.50 b | 128.17 ± 14.86 b | 81.83 ± 7.12 b | 631.31 ± 121.90 b |
| nonanal | 3.13 ± 0.35 a | 1.21 ± 0.17b | 5.91 ± 0.71 b | 9.58 ± 1.01 b | 4.26 ± 0.89 b | 8.38 ± 0.85 b | 5.99 ± 2.25 b | 2.13 ± 0.24 b | 2.84 ± 0.58 a | 103.51 ± 13.71 b |
| 2-ethyl-3,6-dimethylpyrazine | 3.72 ± 0.25 a | 2.79 ± 0.15 b | 6.94 ± 0.47 b | 3.79 ± 0.28 a | 3.57 ± 0.11 a | 9.12 ± 0.69 b | 6.66 ± 0.36 b | 2.93 ± 0.10 a | 6.62 ± 0.63 b | 45.88 ± 6.07 b |
| 2-ethyl-3,5-dimethylpyrazine | 1.06 ± 0.11 a | 0.79 ± 0.04 b | 2.07 ± 0.14 b | 1.04 ± 0.21 a | 3.66 ± 0.14 b | 2.72 ± 0.21 b | 1.96 ± 0.14 b | 0.73 ± 0.07 b | 3.81 ± 0.11 b | 14.29 ± 1.87 b |
| linalool | 1.70 ± 0.16 a | 2.73 ± 0.11 b | 3.71 ± 0.09 b | 2.85 ± 0.02 b | 2.61 ± 0.55 b | 6.38 ± 0.26 b | 5.19 ± 0.33 b | 2.43 ± 0.34 b | 2.18 ± 0.09 b | 64.94 ± 5.96 b |
| acetophenone | 166.45 ± 8.92 a | 74.56 ± 1.36 b | 182.09 ± 44.01 a | 132.69 ± 6.66 b | 131.83 ± 4.23 b | 209.34 ± 22.89 b | 193.69 ± 60.29 a | 162.99 ± 29.97 a | 197.65 ± 22.80 b | 1142.48 ± 162.67 b |
| (S)-verbenone | 21.27 ± 1.95 a | 25.83 ± 1.64 a | 34.59 ± 2.01 b | 17.68 ± 0.66 a | 21.37 ± 3.18 a | 33.99 ± 6.55 b | 15.46 ± 1.44 b | 20.23 ± 1.99 a | 9.88 ± 0.16 b | 157.75 ± 14.78 b |
| (Z)-geraniol | 11.11 ± 0.31 a | 5.63 ± 0.1 b | 24.97 ± 0.53 b | 9.71 ± 0.34 b | 13.52 ± 1.04 b | 12.04 ± 0.46 a | 14.51 ± 0.56 b | 10.07 ± 0.34 a | 0.24 ± 0.03 b | 146.89 ± 10.16 b |
| (Z)-carveol | 40.32 ± 2.11 a | 5.64 ± 0.14 b | 26.46 ± 1.26 b | 26.58 ± 0.94 a | 16.83 ± 1.05 b | 17.75 ± 0.69 b | 30.11 ± 1.94 b | 10.23 ± 0.98 b | 5.09 ± 1.16 b | 418.44 ± 45.01 b |
| (Z)-jasmone | 2.88 ± 0.21 a | 2.73 ± 0.04 a | 7.14 ± 0.12 b | 3.13 ± 0.13 a | 3.37 ± 0.11 a | 8.06 ± 0.19 b | 4.72 ± 0.20 b | 3.57 ± 0.08 a | 3.04 ± 0.32 a | 33.11 ± 2.29 b |
| 2-methoxy-4-vinylphenol | 67.06 ± 10.83 a | 43.32 ± 1.24 b | 104.63 ± 13.09 b | 56.84 ± 3.96 a | 57.57 ± 3.18 a | 120.22 ± 10.07 b | 101.14 ± 7.24 b | 58.17 ± 8.61 a | 53.29 ± 8.81 a | 679.31 ± 80.41 b |
| dihydroactinidiolide | 747.79 ± 41.01 a | 655.08 ± 31.09 b | 803.12 ± 35.57 b | 887.86 ± 43.84 b | 767.72 ± 46.08 a | 717.34 ± 51.46 b | 472.21 ± 17.74 b | 731.02 ± 25.07 a | 816.60 ± 36.45 b | 3384.25 ± 1187.63 b |
| coumarin | 848.01 ± 41.03 a | 789.27 ± 36.82 b | 286.88 ± 52.11 b | 802.07 ± 23.48 a | 790.03 ± 19.85 a | 864.43 ± 45.34 b | 781.81 ± 32.87 b | 897.96 ± 73.13 a | 802.48 ± 39.41 a | 7181.83 ± 1861.63 b |
| Parameter | Standard | Variation | |
|---|---|---|---|
| medium volume | 1 L | 2.5 L | - |
| light protection | not protected from light | in the dark | - |
| agitation rate | 150 rpm | 100 rpm | 250 rpm |
| temperature | 24 °C | 18 °C | 30 °C |
| pH | non-buffered | pH 5 | pH 8 |
| aeration | no active aeration | aeration (100% pO2) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigling, M.; Heger, F.; Graule, M.; Liu, Z.; Zhang, C.; Ni, L.; Zhang, Y. A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules 2022, 27, 2503. https://doi.org/10.3390/molecules27082503
Rigling M, Heger F, Graule M, Liu Z, Zhang C, Ni L, Zhang Y. A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules. 2022; 27(8):2503. https://doi.org/10.3390/molecules27082503
Chicago/Turabian StyleRigling, Marina, Fabienne Heger, Maria Graule, Zhibin Liu, Chen Zhang, Li Ni, and Yanyan Zhang. 2022. "A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius" Molecules 27, no. 8: 2503. https://doi.org/10.3390/molecules27082503
APA StyleRigling, M., Heger, F., Graule, M., Liu, Z., Zhang, C., Ni, L., & Zhang, Y. (2022). A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules, 27(8), 2503. https://doi.org/10.3390/molecules27082503









