Antioxidant and Antiglycation Effects of Cistus × incanus Water Infusion, Its Phenolic Components, and Respective Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Plant Material
2.3. Preparation of Water Infusions
2.4. Reference Standards
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid Content (TFC)
2.7. UHPLC-ESI-qTOF-MS Method
2.8. Antioxidant Activity
2.8.1. DPPH Assay
2.8.2. ABTS Assay
2.8.3. FRAP Assay
2.9. Antiglycation Activity in BSA-MGO Model
2.10. Methylglyoxal Trapping Assay
2.11. Multivariate Modeling
3. Results and Discussion
3.1. Quantification of Phenolics, Flavonoids, and Non-Flavonoid Polyphenols
3.2. UHPLC-ESI-qTOF-MS Profiling of C. incanus Water Infusions
3.3. Antioxidant Potential In Vitro
3.3.1. Antioxidant Effect of C. incanus Water Infusions
3.3.2. Antioxidant Activity of C. incanus Polyphenols and Their Respective Metabolites
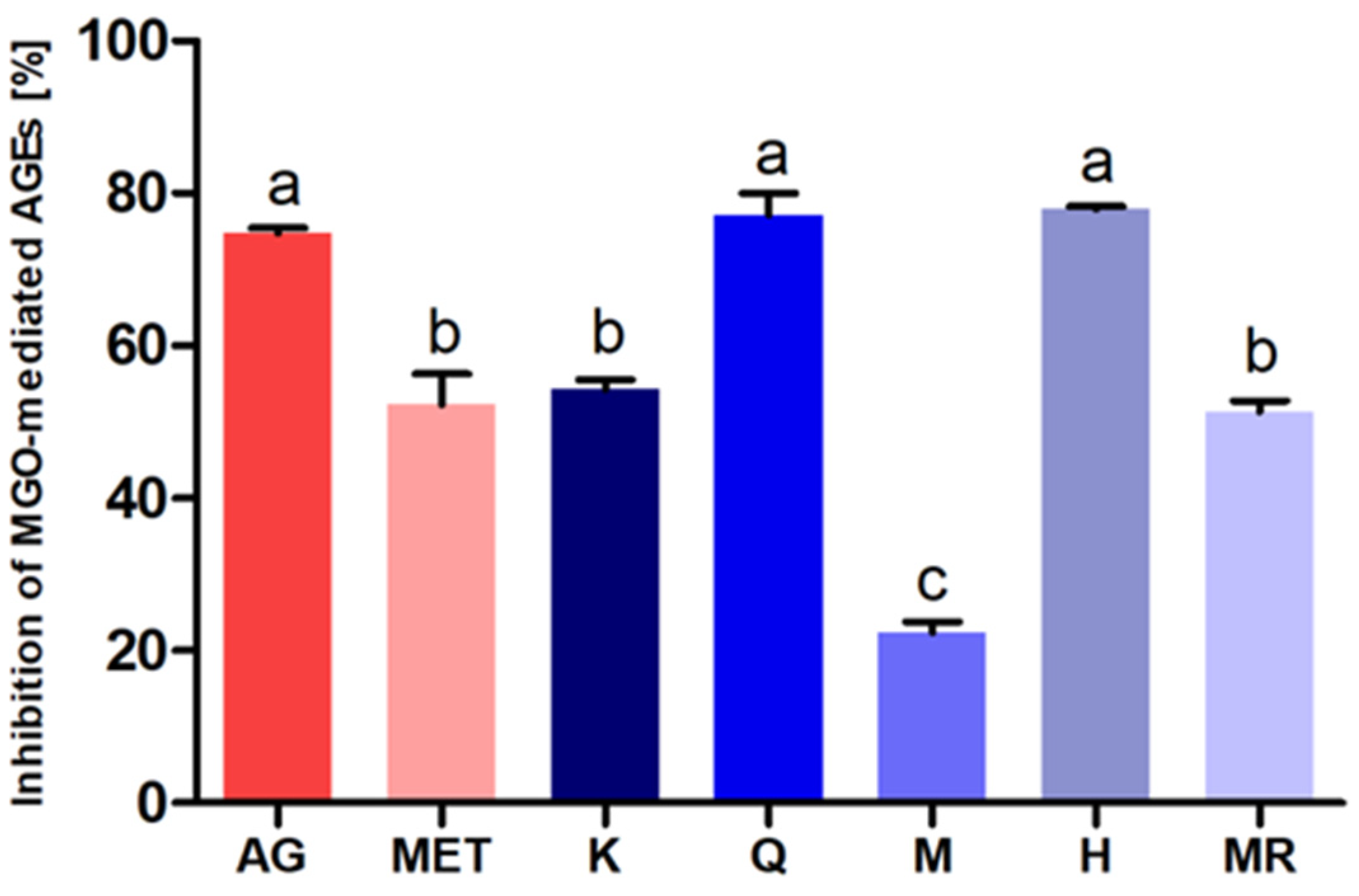
3.4. Antiglycation Potential of C. incanus Flavonols
3.5. Methylglyoxal Trapping Capacity of C. incanus Flavonols
3.6. Correlation between Chemical and Antioxidant Parameters
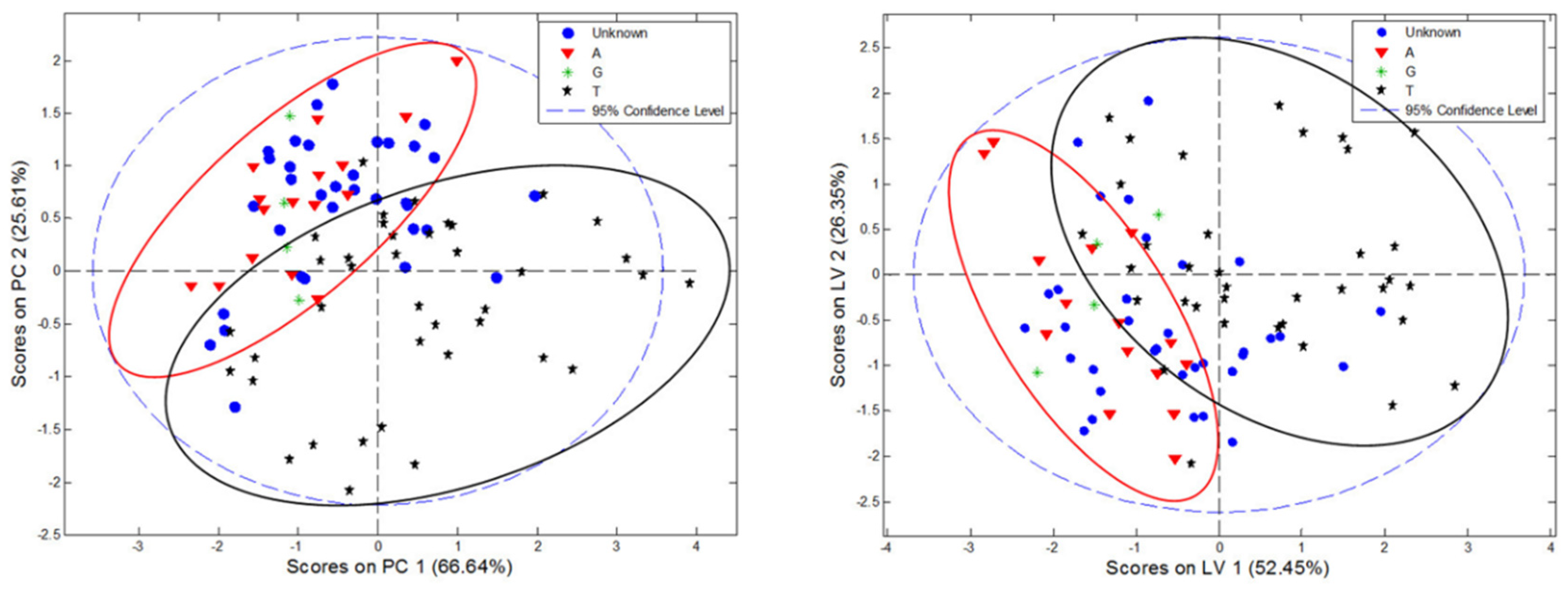
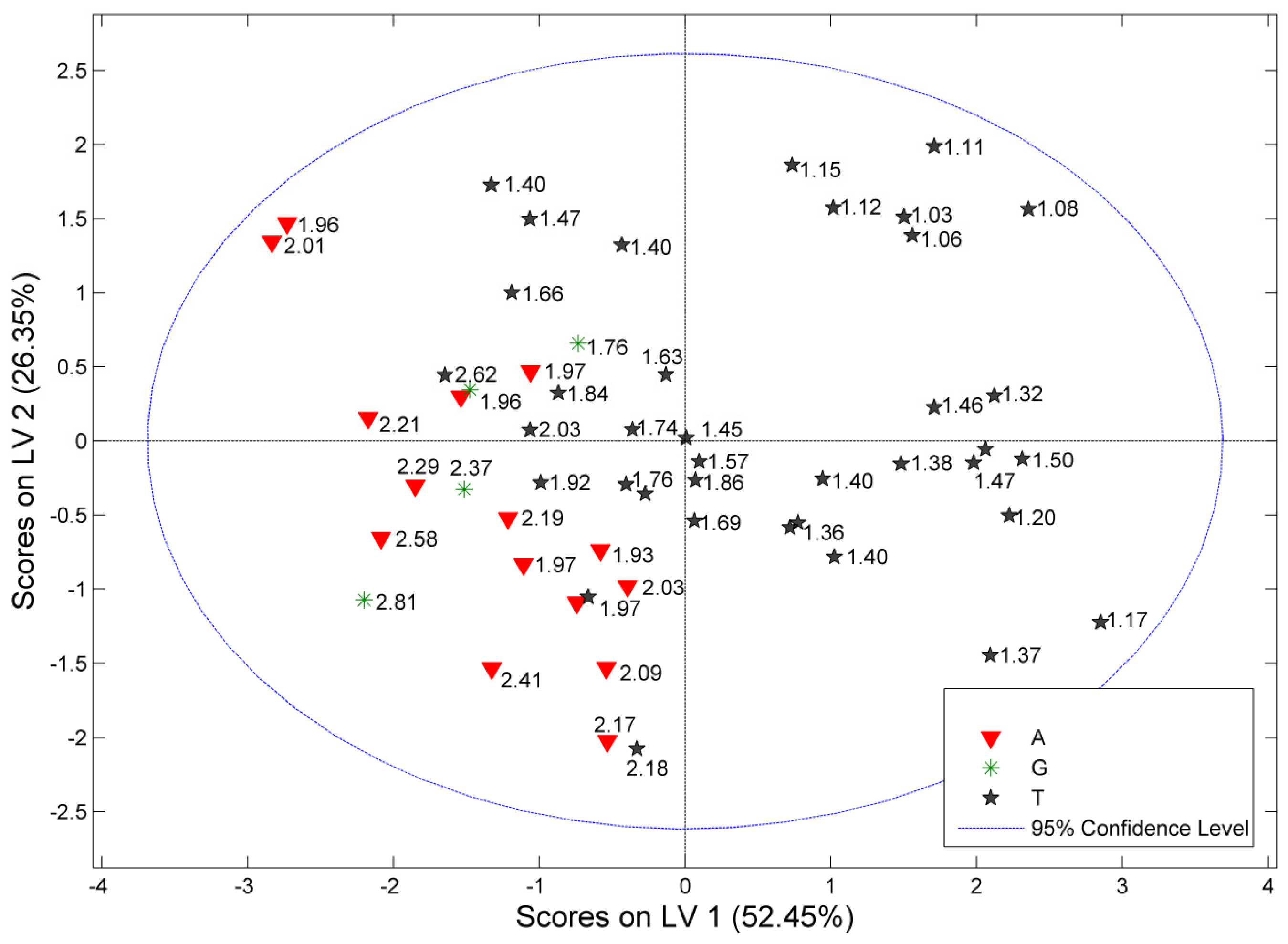
3.7. Multivariate Classification of C. incanus Samples
3.8. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| BSA | bovine albumin serum |
| Ci | Cistus incanus |
| EA | ellagic acid |
| EC50 | half maximal effective concentration |
| GA | gallic acid |
| GAE | gallic acid equivalent |
| IC50 | half maximal inhibitory concentration |
| ME | myricetin equivalent |
| MGO | methylglyoxal |
| QE | quercetin equivalent |
| PCA | principal component analysis |
| RCS | reactive carbonyl species |
| PLS-DA | partial least squares-discriminant analysis |
| ROS | reactive oxygen species |
| SET | single electron transfer |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TPC-TFC | total non-flavonoid polyphenols |
References
- Dimcheva, V.; Karsheva, M. Antioxidant Activity and Polyphenolic Content of the Bulgarian Wild Herb Cistus incanus L. Stored under Different Conditions. J. Chem. Technol. Metall. 2017, 52, 781–790. [Google Scholar]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the Substantiation of Health Claims Related to Various Food(S)/Food Constituent(s) and Protection of Cells from Premature Aging, Antioxidant Activity, Antioxidant Content and Antioxidant Properties, and Protection of DNA, Proteins and Lipids from Oxidative Damage Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1817. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Lin, J.A.; Wu, C.H.; Lu, C.C.; Hsia, S.M.; Yen, G.C. Glycative Stress from Advanced Glycation End Products (AGEs) and Dicarbonyls: An Emerging Biological Factor in Cancer Onset and Progression. Mol. Nutr. Food Res. 2016, 60, 1850–1864. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I. Glycation, Carbonyl Stress and AGEs Inhibitors: A Patent Review. Expert. Opin. Ther. Pat. 2015, 25, 1267–1284. [Google Scholar] [CrossRef]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the Crossroads of the Metabolic Syndrome and Obesity Epidemics. Front. Biosci. (Landmark Ed.) 2019, 24, 186–211. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A Model for Exploring Labdane-Type Diterpenes’ Biosynthesis and a Natural Source of High Value Products with Biological, Aromatic, and Pharmacological Properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Barrajõn-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Falchi, A.; Paolini, J.; Desjobert, J.M.; Melis, A.; Costa, J.; Varesi, L. Phylogeography ofCistus creticus L. on Corsica and Sardinia Inferred by the TRNL-F and RPL32-TRNL Sequences of CpDNA. Mol. Phylogenet. Evol. 2009, 52, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, B.; Vargas, P. Systematics, Character Evolution, and Biogeography ofCistus L. (Cistaceae) Based on ITS, TrnL-TrnF, and MatK Sequences. Mol. Phylogenet. Evol. 2005, 37, 644–660. [Google Scholar] [CrossRef]
- Gülz, P.G.; Herrmann, T.; Hangst, K. Leaf Trichomes in the GenusCistus. Flora 1996, 191, 85–104. [Google Scholar] [CrossRef]
- Čarni, A.; Matevski, V.; Šilc, U. Morphological, Chorological and Ecological Plasticity of Cistus incanus in the Southern Balkans. Plant. Biosyst. 2010, 144, 602–617. [Google Scholar] [CrossRef]
- di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The Polyphenolic Composition of Cistus incanus Herbal Tea and Its Antibacterial and Anti-Adherent Activity against Streptococcus mutans. Planta. Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a Promising Herbal Tea Rich in Bioactive Compounds: LC–MS/MS Determination of Catechins, Flavonols, Phenolic Acids and Alkaloids—A Comparison with Camellia Sinensis, Rooibos and Hoan Ngoc Herbal Tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic Compounds in Cistus incanus Herbal Infusions—Antioxidant Capacity and Thermal Stability during the Brewing Process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Ekiert, H. In Vitro Shoot Cultures of Pink Rock-Rose (Cistus × incanus L.) as a Potential Source of Phenolic Compounds. Acta. Soc. Bot. Pol. 2017, 86, 3563. [Google Scholar] [CrossRef]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Włodarczyk, M.; Starzec, A. Isolation and Structure Elucidation of Cistusin: A New Ellagitannin from Cistus × incanus L. Leaves. Ind. Crops. Prod. 2020, 158, 112971. [Google Scholar] [CrossRef]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The Effect of Cistus incanus Herbal Tea Supplementation on Oxidative Stress Markers and Lipid Profile in Healthy Adults. Cardiol. J. 2021, 28, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Bigagli, E.; Cinci, L.; Gori, A.; Brunetti, C.; Ferrini, F.; Luceri, C. Ethyl Acetate Extract from Cistus × incanus L. Leaves Enriched in Myricetin and Quercetin Derivatives, Inhibits Inflammatory Mediators and Activates Nrf2/HO-1 Pathway in LPS-Stimulated RAW 264.7 Macrophages. Z. Naturforsch. C J. Biosci. 2021, 76, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Konopacka, A.; Waleron, K.; Wesolowski, M. Cistus incanus L. Commercial Products as a Good Source of Polyphenols in Human Diet. Ind. Crop. Prod. 2017, 107, 297–304. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szeremeta, D.; Knaś, M.; Długosz, E.; Ott, P.G.; Kowalska, T.; Sajewicz, M. Antibacterial Potential of the Cistus incanus L. Phenolics as Studied with Use of Thin-Layer Chromatography Combined with Direct Bioautography and In Situ Hydrolysis. J. Chromatogr. A. 2018, 1534, 170–178. [Google Scholar] [CrossRef]
- Kalus, U.; Grigorov, A.; Kadecki, O.; Jansen, J.P.; Kiesewetter, H.; Radtke, H. Cistus incanus (CYSTUS052) for Treating Patients with Infection of the Upper Respiratory Tract. A Prospective, Randomised, Placebo-Controlled Clinical Study. Antivir. Res. 2009, 84, 267–271. [Google Scholar] [CrossRef]
- Rebensburg, S.; Helfer, M.; Schneider, M.; Koppensteiner, H.; Eberle, J.; Schindler, M.; Gürtler, L.; Brack-Werner, R. Potent in Vitro Antiviral Activity of Cistus incanus Extract against HIV and Filoviruses Targets Viral Envelope Proteins. Sci. Rep. 2016, 6, 20394. [Google Scholar] [CrossRef]
- Ait Lahcen, S.; El Hattabi, L.; Benkaddour, R.; Chahboun, N.; Ghanmi, M.; Satrani, B.; Tabyaoui, M.; Zarrouk, A. Chemical Composition, Antioxidant, Antimicrobial and Antifungal Activity of Moroccan Cistus creticus Leaves. Chem. Data Collect. 2020, 26, 100346. [Google Scholar] [CrossRef]
- Vitali, F.; Pennisi, G.; Attaguile, G.; Savoca, F.; Tita, B. Antiproliferative and Cytotoxic Activity of Extracts from Cistus incanus L. and Cistus monspeliensis L. on Human Prostate Cell Lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Jankovics, I.; Borsos, M.; Mirani, S.; Dénes, B. Early Use of Polyphenol-Rich Cistus creticus Extract Containing Nasopharyngeal Spray Is Associated with Significantly Shorter Duration of Symptoms in Mild COVID-19 Patients: A Retrospective Case-Control Study. JCMPHR 2021, 2, 1–5. [Google Scholar] [CrossRef]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The Antiglycoxidative Ability of Selected Phenolic Compounds—An In Vitro Study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed]
- Bialońska, D.; Kasimsetty, S.G.; Khan, S.I.; Daneel, F. Urolithins, Intestinal Microbial Metabolites of Pomegranate ~ellagitannins, Exhibit Potent Antioxidant Activity in a Cell-Based Assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2019. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.-H. Antioxidant Activities of Agrimonia Pilosa Ledeb: In Vitro Comparative Activities of Its Different Fractions. Korean J. Plant. Res. 2014, 27, 642–649. [Google Scholar] [CrossRef][Green Version]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate Phenolics Inhibit Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyl Species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea Polyphenol (-)-Epigallocatechin-3-Gallate: A New Trapping Agent of Reactive Dicarbonyl Species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemometr. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Brereton, R.G.; Lloyd, G.R. Partial Least Squares Discriminant Analysis: Taking the Magic Away. J. Chemom. 2014, 28, 213–225. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Potential of Vasoprotectives to Inhibit Non-Enzymatic Protein Glycation, and Reactive Carbonyl and Oxygen Species Uptake. Int. J. Mol. Sci. 2021, 22, 10026. [Google Scholar] [CrossRef]
- Riehle, P.; Rusche, N.; Saake, B.; Rohn, S. Influence of the Leaf Content and Herbal Particle Size on the Presence and Extractability of Quantitated Phenolic Compounds in Cistus incanus Herbal Teas. J. Agric. Food Chem. 2014, 62, 10978–10988. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Barrajón-Catalán, E.; Micol, V.; Seguera-Carretero, A.; Fernández-Gutiérrez, A. High-Performance Liquid Chromatography with Diode Array Detection Coupled to Electrospray Time-of-Flight and Ion-Trap Tandem Mass Spectrometry to Identify Phenolic Compounds from a Cistus ladanifer Aqueous Extract. Phytochem. Anal. 2010, 21, 307–313. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, 2100053. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various SpanishCistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Saracini, E.; Tattini, M.; Traversi, M.L.; Vincieri, F.F.; Pinelli, P. Simultaneous LC-DAD and LC-MS Determination of Ellagitannins, Flavonoid Glycosides, and Acyl-Glycosyl Flavonoids in Cistus Salvifolius L. Leaves. Chromatographia 2005, 62, 245–249. [Google Scholar] [CrossRef]
- Gürbüz, P.; Koşar, M.; Güvenalp, Z.; Kuruüzüm Uz, A.; Demirezer, L.Ö. Simultaneous Determination of Selected Flavonoids from DifferentCistus Species by Hplc-Pda. Marmara Pharm. J. 2018, 22, 405–410. [Google Scholar] [CrossRef]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Loizzo, M.R.; ben Jemia, M.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and Functional Properties in Prevention of Neurodegenerative Disorders of Five Cistus Species Essential Oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Hopia, A.; Heinonen, M. Antioxidant Activity of Flavonol Aglycones and Their Glycosides in Methyl Linoleate. JAOCS 1999, 76, 139–144. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant Properties of Flavonol Glycosides from Tea. Redox Rep. 1999, 4, 13–16. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Swartz, C.; Maronpot, R.; Davis, J.; Recio, L.; Koyanagi, M.; Hayashi, S. Genotoxicity Evaluation of the Flavonoid, Myricitrin, and Its Aglycone, Myricetin. Food Chem. Toxicol. 2015, 83, 283–292. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence so Far. Evid. Based Complementary. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of Buformin and Metformin on Formation of Advanced Glycation End Products by Methylglyoxal. Clin. Chim. Acta 2005, 358, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Ruggiero-Lopez, D. Metformin Inhibition of Glycation Processes. Diabetes Metab. 2003, 29, 95–103. [Google Scholar] [CrossRef]
- Jung, H.A.; Yoon, N.Y.; Kang, S.S.; Kim, Y.S.; Choi, J.S. Inhibitory Activities of Prenylated Flavonoids from Sophora Flavescens against Aldose Reductase and Generation of Advanced Glycation Endproducts. J. Pharm. Pharmacol. 2010, 60, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Fernando, C.D.; Karunaratne, D.T.; Gunasinghe, S.D.; Cooray, M.C.D.; Kanchana, P.; Udawatte, C.; Perera, P.K. Inhibitory Action on the Production of Advanced Glycation End Products (AGEs) and Suppression of Free Radicals In Vitro by a Sri Lankan Polyherbal Formulation Nawarathne Kalka. BMC Complement. Altern. Med. 2016, 16, 197. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, W. Anti-Glycation and Glycation Reversing Potential of Fenugreek (Trigonella Foenum-Graecum) Seed Extract. Biomed. J. Sci. Tech. Res. 2018, 3, 875. [Google Scholar] [CrossRef]
- Rock, C.A.; Keeney, S.; Zakharchenko, A.; Takano, H.; Spiegel, D.A.; Krieger, A.M.; Ferrari, G.; Levy, R.J. Model Studies of Advanced Glycation End Product Modification of Heterograft Biomaterials: The Effects of In Vitro Glucose, Glyoxal, and Serum Albumin on Collagen Structure and Mechanical Properties. Acta Biomater. 2021, 123, 275–285. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications and other age-related. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory Effect of Naturally Occurring Flavonoids on the Formation of Advanced Glycation Endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Maini, S.; Hodgson, H.L.; Krol, E.S. The UVA and Aqueous Stability of Flavonoids Is Dependent on B-Ring Substitution. J. Agric. Food Chem. 2012, 60, 6966–6976. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal Degradation of Onion Quercetin Glucosides under Roasting Conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics of Glycoxidation of Bovine Serum Albumin by Glucose, Fructose and Ribose and Its Prevention by Food Components. Molecules 2014, 19, 18828–18849. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, X.; Bao, Y.; Zhu, Y.; Zhang, Y.; Li, J.; Peng, Z. Inhibitory Effects of Hyperoside and Quercitrin from Zanthoxylum Bungeanum Maxim. Leaf on 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b]Pyridine Formation by Trapping Phenylacetaldehyde. Eur. Food Res. Technol. 2022, 248, 25–34. [Google Scholar] [CrossRef]
- Yang, B.; Choi, E.; Shim, S.M. Inhibitory activities of kaempferol against methylglyoxal formation, intermediate of advanced glycation end products. Appl. Biol. Chem. 2017, 60, 57–62. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin Inhibits Advanced Glycation End Product Formation via Chelating Metal Ions, Trapping Methylglyoxal, and Trapping Reactive Oxygen Species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, L.; Lv, L.; Sang, S. Trapping Methylglyoxal by Myricetin and Its Metabolites in Mice. J. Agric. Food Chem. 2020, 68, 9408–9414. [Google Scholar] [CrossRef]
| Sample Origin | TPC | TFC | TPC-TFC a [mg/g d.w.] | TPC:TFC | |
|---|---|---|---|---|---|
| GAE [mg/g d.w.] | ME [mg/g d.w.] | ||||
| All samples (n = 102) | Mean | 54.69 ± 8.23 | 31.73 ± 8.55 | 23.14 ± 9.73 | 1.8 ± 0.5 |
| Median | 56.05 | 30.22 | 24.4 | 1.8 | |
| Minimum | 35.39 | 16.7 | 1.33 | 1 | |
| Maximum | 73.09 | 58.68 | 46.18 | 3.1 | |
| Albania (n = 20) | Mean | 55.3 ± 6.97 | 28.92 ± 6.32 | 26.37 ± 6.99 | 2 ± 0.3 |
| Median | 55.03 | 27.07 | 27.97 | 2 | |
| Minimum | 43.24 | 21.44 | 12.7 | 1.3 | |
| Maximum | 73.09 | 41.32 | 39.44 | 2.6 | |
| Greece (n = 4) | Mean | 53.57 ± 4.95 | 24.47 ± 2.19 | 29.1 ± 7.08 | 2.2 ± 0.4 |
| Median | 52.72 | 24.52 | 28.2 | 2.2 | |
| Minimum | 47.74 | 21.7 | 20.6 | 1.8 | |
| Maximum | 61.08 | 27.14 | 39.38 | 2.8 | |
| Turkey (n = 44) | Mean | 52.92 ± 9.13 | 35.96 ± 9.23 | 16.96 ± 8.92 | 1.5 ± 0.4 |
| Median | 53.42 | 33.98 | 17.49 | 1.5 | |
| Minimum | 35.39 | 16.7 | 1.33 | 1 | |
| Maximum | 69.41 | 58.68 | 36.77 | 3.1 |
| No. | Rt [min] | [M − H]− [m/z] | MS/MS [m/z] | Identification | Reference |
|---|---|---|---|---|---|
| 1 | 5.52 | 781.0581 | Punicalin isomers 1 and 2 | [17] | |
| 2 | 5.90 | 781.0583 | [17] | ||
| 3 | 13.57 | 1083.0631/541.0264 a | 781, 601, 301 | Punicalagin isomers 1 and 2 | [23] |
| 4 | 15.90 | 1083.0637/541.0264 a | 781, 601, 451, 301 | [23] | |
| 5 | 16.97 17.36 | 1085.0769/542.026 a | 301 | Terflavin A | [23] |
| 6 | 18.08 18.34 | 1251.0706/625.0285 a | 301 | Cistusin isomers 1 and 2 | [23] |
| 7 | 19.05 | 300.9841 | 257, 229 | Ellagic acid | [50] |
| 8 | 19.92 | 631.098 | 316/317 b | Myricetin-O-galloyl-hexoside (galactoside or glucoside) | [51] |
| 9 | 20.44 | 479.0854 | 316/317 b | Myricetin-3-O-galactoside | [18] |
| 10 | 20.70 | 479.0861 | 316/317 b | Myricetin-3-O-glucoside | [18] |
| 11 | 20.00 | 493.1377 | 313, 179 | Dimethyl-kaempferol-O-hexoside (galactoside or glucoside) | [52] [50] |
| 12 | 21.82 | 449.0751 | 316/317 b | Myricetin-3-O-pentoside (xyloside or arabinoside) | [53] |
| 13 | 22.09 | 463.091 | 316/317 b | Myricitrin (myricetin-3-O-rhamnoside) | [20] |
| 14 | 22.40 | 463.0906 | 300/301 b | Hyperoside (quercetin-3-O-galactoside) | [18] |
| 15 | 22.71 | 463.091 | 300/301 b | Isoquercitrin (quercetin-3-O-glucoside) | [18] |
| 16 | 23.74 | 433.0798 | 300/301 b | Quercetin-3-O-pentoside isomer 1 (xyloside or arabinoside) | [53] |
| 17 | 24.05 | 433.0801 | 300/301 b | Quercetin-3-O-pentoside isomer 2 (xyloside or arabinoside) | [53] |
| 18 | 24.39 | 447.0949 | 300/301 b | Quercitrin(quercetin-3-O-rhamnoside) | [20] |
| 19 | 28.77 | 609.1293 | 301 | Quercetin-O-rhamnoside-O-hexoside (rutin or other isomer) | [20] |
| 20 | 30.27 | 593.1348 | 285 | Tiliroside (6″-O-p-coumaroylastragalin) | [54] |
| Sample Origin | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|
| Inhibition a [%] | GAE [mM/g d.w.] | Inhibition a [%] | GAE [mM/g d.w.] | Fe(II) [mM/g d.w.] | GAE [mM/g d.w.] | ||
| All samples (n = 102) | Mean | 28.12 ± 5.63 | 24.98 ± 5.68 | 27.36 ± 5.92 | 1.39 ± 0.43 | 134.21 ± 42.72 | 29.73 ± 9.76 |
| Median | 28.05 | 24.73 | 28.33 | 1.35 | 128.18 | 28.33 | |
| Minimum | 13.29 | 11.38 | 7.31 | 0.54 | 69.29 | 11.39 | |
| Maximum | 42.65 | 43.37 | 40.31 | 2.79 | 314.28 | 71.89 | |
| Albania (n = 20) | Mean | 25.94 ± 6.16 | 22.54 ± 5.42 | 25.01 ± 6.66 | 1.33 ± 0.47 | 115.39 ± 28.73 | 25.12 ± 6.94 |
| Median | 27.1 | 23.88 | 26.25 | 1.29 | 110.39 | 24.47 | |
| Minimum | 13.29 | 11.38 | 7.31 | 0.54 | 69.29 | 11.39 | |
| Maximum | 35.45 | 30.35 | 36.43 | 2.58 | 168.53 | 37.36 | |
| Greece (n = 4) | Mean | 24.09 ± 1.4 | 20.63 ± 1.19 | 21.83 ± 1.68 | 1.37 ± 0.49 | 107.72 ± 11 | 23.80 ± 3.08 |
| Median | 24.13 | 20.65 | 22.19 | 1.11 | 106.7 | 23.65 | |
| Minimum | 22.09 | 18.91 | 19.18 | 1.05 | 93.26 | 19.61 | |
| Maximum | 26.03 | 22.28 | 23.77 | 2.21 | 124.2 | 28.3 | |
| Turkey (n = 44) | Mean | 30.90 ± 5.31 | 27.02 ± 4.95 | 29.41 ± 6.02 | 1.47 ± 0.45 | 155.22 ± 47.74 | 34.53 ± 10.87 |
| Median | 29.56 | 25.73 | 30.06 | 1.43 | 146.04 | 32.37 | |
| Minimum | 20.35 | 17.42 | 17.56 | 0.84 | 90.92 | 20.15 | |
| Maximum | 42.65 | 38.09 | 40.31 | 2.79 | 314.28 | 71.89 | |
| Sample | DPPH | ABTS | FRAP | |||
|---|---|---|---|---|---|---|
| IC50 [µM] | Inhibition a [%] | IC50 [µM] | Inhibition a [%] | Fe(II) b [µM] | GAE b [µM] | |
| Kaempferol | 6.52 ± 0.31 | 41.17 ± 1.88 | 8.04 ± 0.14 | 33.6 ± 0.24 | 45.99 ± 0.2 | 6.69 ± 0.06 |
| Quercetin | 3.53 ± 0.51 | 63.06 ± 4.15 | 4.30 ± 0.66 | 54.24 ± 8.21 | 116.59 ± 4.26 | 24.44 ± 0.73 |
| Myricetin | 3.06 ± 0.1 | 86.13 ± 2.06 | 3.15 ± 0.18 | 79.8 ± 6.07 | 64.67 ± 0.76 | 12.2 ± 0.22 |
| Hyperoside | 3.91 ± 0.2 | 57.26 ± 2.04 | 7.97 ± 1.1 | 32.28 ± 5.13 | 49.76 ± 1.7 | 4.84 ± 0.29 |
| Myricitrin | 3.31 ± 0.08 | 78.48 ± 1.96 | 3.92 ± 0.21 | 66 ± 2.3 | 62.72 ± 1.62 | 11.63 ± 0.48 |
| Ellagic acid | 2.35 ± 0.07 | 91.27 ± 0.13 | 3.82 ± 0.13 | 59.66 ± 4.18 | 100.99 ± 3.69 | 22.92 ± 1.09 |
| Urolithin A | 16.15 ± 0.63 | 18.11 ± 0.61 | 5.47 ± 0.56 | 49.48 ± 6.11 | 43.13 ± 2.65 | 5.85 ± 0.78 |
| Urolithin B | <0 | <0 | 27.72 ± 3.54 | 7.63 ± 2.07 | 16.41 ± 0.38 | <0 |
| Urolithin C | 4.77 ± 0.03 | 54.54 ± 0.43 | 6.28 ± 0.64 | 42.3 ± 4.15 | 59.73 ± 2.04 | 10.75 ± 0.6 |
| Flavonol or Product | Source | Rt [min] | [M − H]− [m/z] | Adduct or Precursor |
|---|---|---|---|---|
| Kaempferol | S | 32.39 | 285.0411 | |
| 25.67 | 429.0818 | di-MGO-Kaempferol 1 | ||
| 25.77 | 429.0821 | di-MGO-Kaempferol 2 | ||
| 27.98 | 357.0616 | mono-MGO-Kaempferol 1 | ||
| 28.63 | 357.0611 | mono-MGO-Kaempferol 2 | ||
| Quercetin | S | 28.90 | 301.0354 | |
| 23.99 | 445.0788 | di-MGO-Quercetin | ||
| 25.62 | 373.0563 | mono-MGO-Quercetin 1 | ||
| 25.78 | 373.0564 | mono-MGO-Quercetin 2 | ||
| Myricetin | S | - | - | - |
| Hyperoside | S | 22.40 | 463.0886 | |
| 19.00 | 607.1288 | di-MGO -Hyperoside | ||
| 19.65 | 535.1089 | mono-MGO-Hyperoside 1 | ||
| 19.88 | 535.1091 | mono-MGO-Hyperoside 2 | ||
| 20.48 | 535.1094 | mono-MGO-Hyperoside 3 | ||
| 20.70 | 535.1092 | mono-MGO-Hyperoside 4 | ||
| Myricitrin | S | 22.09 | 463.0902 | |
| 19.91 | 535.1097 | mono-MGO-Myricitrin 1 | ||
| 20.24 | 535.1108 | mono-MGO-Myricitrin 2 | ||
| 20.31 | 535.1105 | mono-MGO-Myricitrin 3 | ||
| 20.48 | 535.1101 | mono-MGO-Myricitrin 4 | ||
| 20.59 | 535.1101 | mono-MGO-Myricitrin 5 | ||
| Ci9 | Inf. | 22.09 | 463.0909 | Myricitrin |
| 22.40 | 463.0906 | Hyperoside | ||
| 19.67 | 535.1116 | mono-MGO-Hyperoside 1 | ||
| 19.90 | 535.1134 | mono-MGO-Myricitrin 1 | ||
| 20.24 | 535.1135 | mono-MGO-Myricitrin 2 | ||
| 20.31 | 535.1137 | mono-MGO-Myricitrin 3 | ||
| Ci26 | Inf. | 22.09 | 463.0909 | Myricitrin |
| 22.40 | 463.0906 | Hyperoside | ||
| 19.65 | 535.1115 | mono-MGO-Hyperoside 1 | ||
| 19.88 | 535.1117 | mono-MGO-Hyperoside 2 | ||
| 20.24 | 535.1123 | mono-MGO-Myricitrin 2 |
| Sample Origin | Method | Unit | TPC | TFC | TPC-TFC a |
|---|---|---|---|---|---|
| All (n = 102) | TPC | GAE [mg/g] | - | ||
| TFC | ME [mg/g] | 0.28 | - | ||
| TPC-TFC | [mg/g] | 0.6 | −0.6 | - | |
| DPPH | Inhibition [%] | 0.19 | 0.69 | −0.41 | |
| ABTS | Inhibition [%] | 0.25 | 0.49 | −0.2 | |
| FRAP | Fe(II) [mM/g] | 0.25 | 0.72 | −0.39 | |
| Albania + Greece (n = 24) | TPC | GAE [mg/g] | - | ||
| TFC | ME [mg/g] | 0.39 | - | ||
| TPC-TFC | [mg/g] | 0.61 | −0.49 | - | |
| DPPH | Inhibition [%] | 0.54 | 0.81 | −0.19 | |
| ABTS | Inhibition [%] | 0.48 | 0.45 | 0.08 | |
| FRAP | Fe(II) [mM/g] | 0.29 | 0.88 | −0.49 | |
| Turkey (n = 44) | TPC | GAE [mg/g] | - | ||
| TFC | ME [mg/g] | 0.46 | - | ||
| TPC-TFC | [mg/g] | 0.54 | −0.5 | - | |
| DPPH | Inhibition [%] | 0.24 | 0.51 | −0.26 | |
| ABTS | Inhibition [%] | 0.23 | 0.53 | −0.27 | |
| FRAP | Fe(II) [mM/g] | 0.4 | 0.71 | −0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernacka, K.; Bednarska, K.; Starzec, A.; Mazurek, S.; Fecka, I. Antioxidant and Antiglycation Effects of Cistus × incanus Water Infusion, Its Phenolic Components, and Respective Metabolites. Molecules 2022, 27, 2432. https://doi.org/10.3390/molecules27082432
Bernacka K, Bednarska K, Starzec A, Mazurek S, Fecka I. Antioxidant and Antiglycation Effects of Cistus × incanus Water Infusion, Its Phenolic Components, and Respective Metabolites. Molecules. 2022; 27(8):2432. https://doi.org/10.3390/molecules27082432
Chicago/Turabian StyleBernacka, Karolina, Katarzyna Bednarska, Aneta Starzec, Sylwester Mazurek, and Izabela Fecka. 2022. "Antioxidant and Antiglycation Effects of Cistus × incanus Water Infusion, Its Phenolic Components, and Respective Metabolites" Molecules 27, no. 8: 2432. https://doi.org/10.3390/molecules27082432
APA StyleBernacka, K., Bednarska, K., Starzec, A., Mazurek, S., & Fecka, I. (2022). Antioxidant and Antiglycation Effects of Cistus × incanus Water Infusion, Its Phenolic Components, and Respective Metabolites. Molecules, 27(8), 2432. https://doi.org/10.3390/molecules27082432






