Nigrosporins B, a Potential Anti-Cervical Cancer Agent, Induces Apoptosis and Protective Autophagy in Human Cervical Cancer Ca Ski Cells Mediated by PI3K/AKT/mTOR Signaling Pathway
Abstract
:1. Introduction
2. Material and Method
2.1. Cell Lines and Cultures
2.2. Chemicals and Reagents
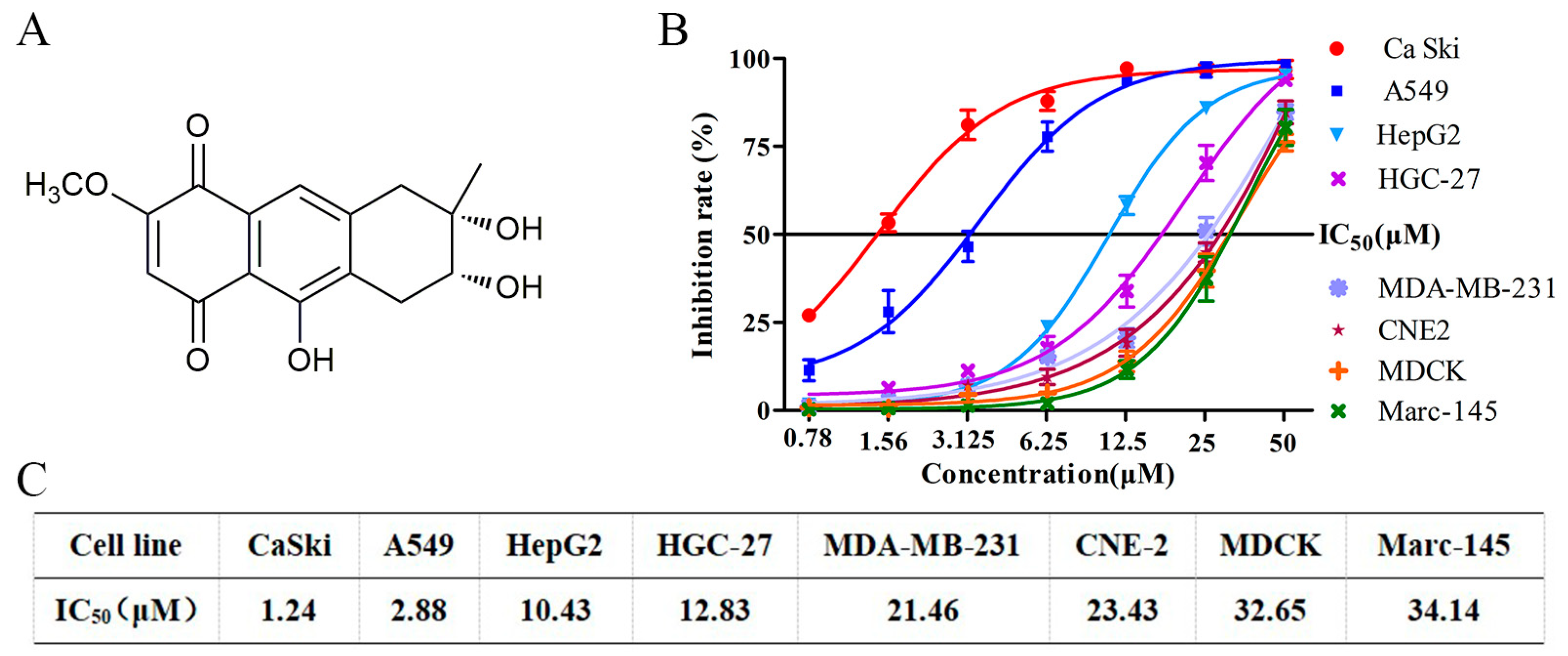
2.3. MTT Assay
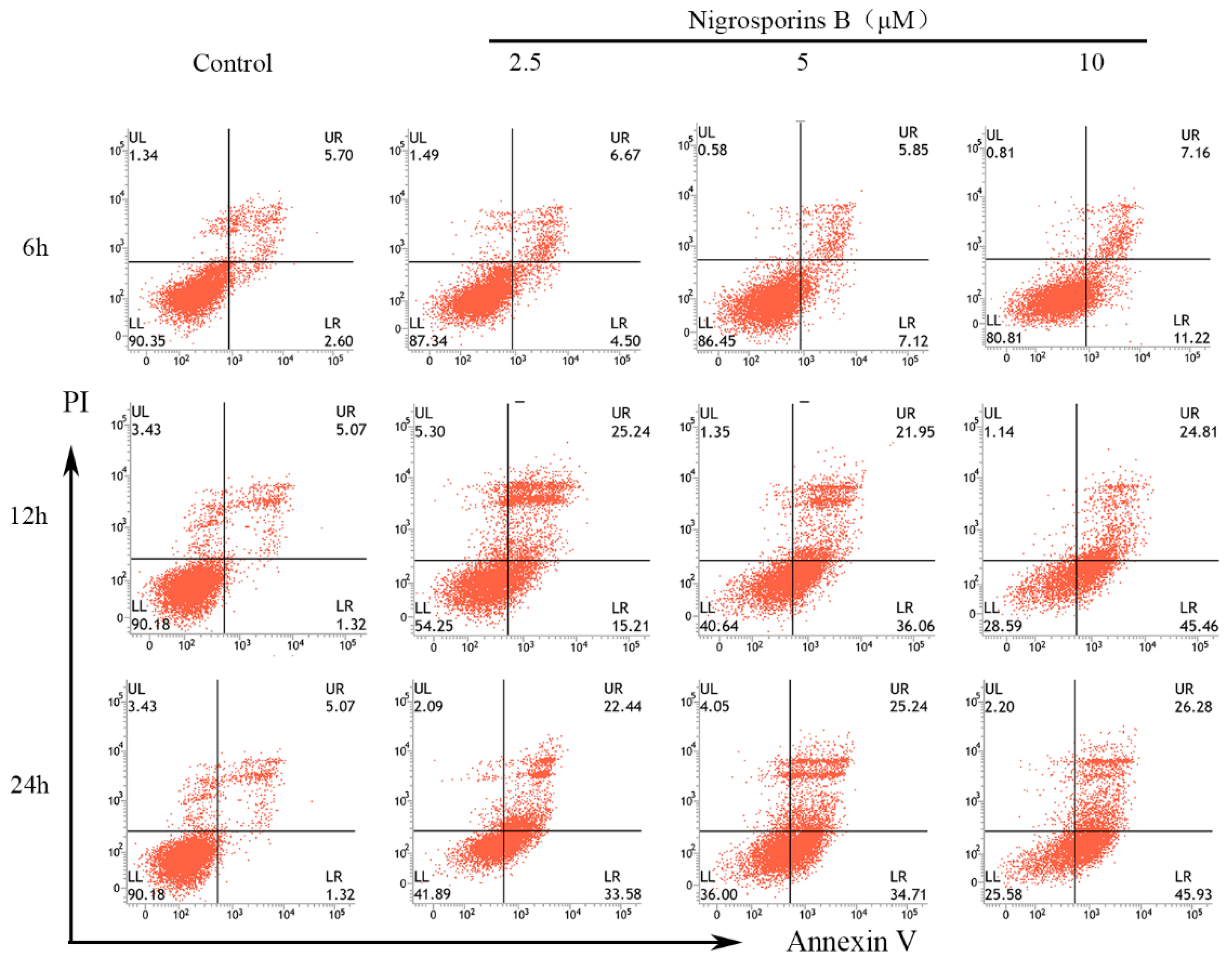
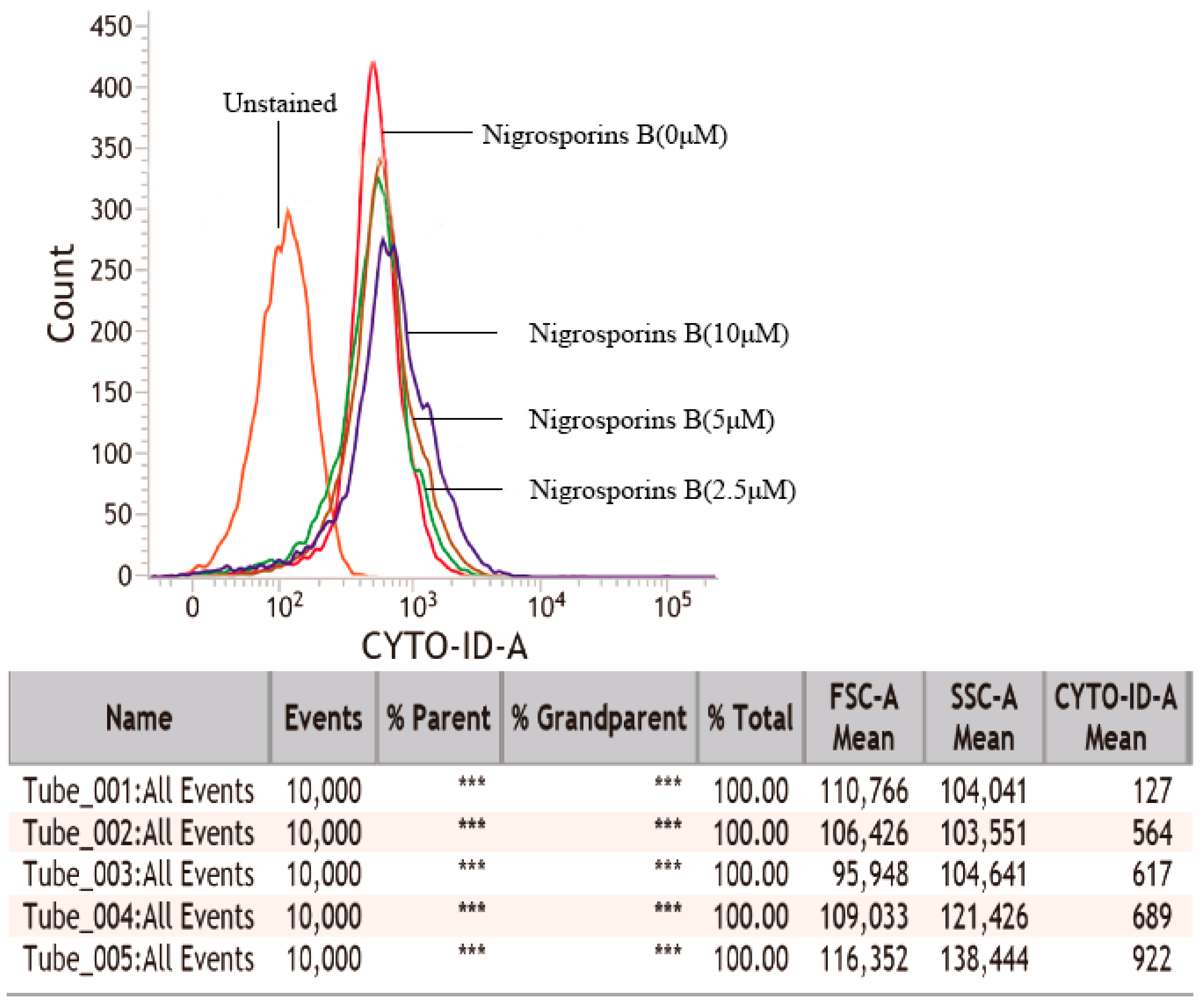
2.4. Flow Cytometry Assay (FCM)
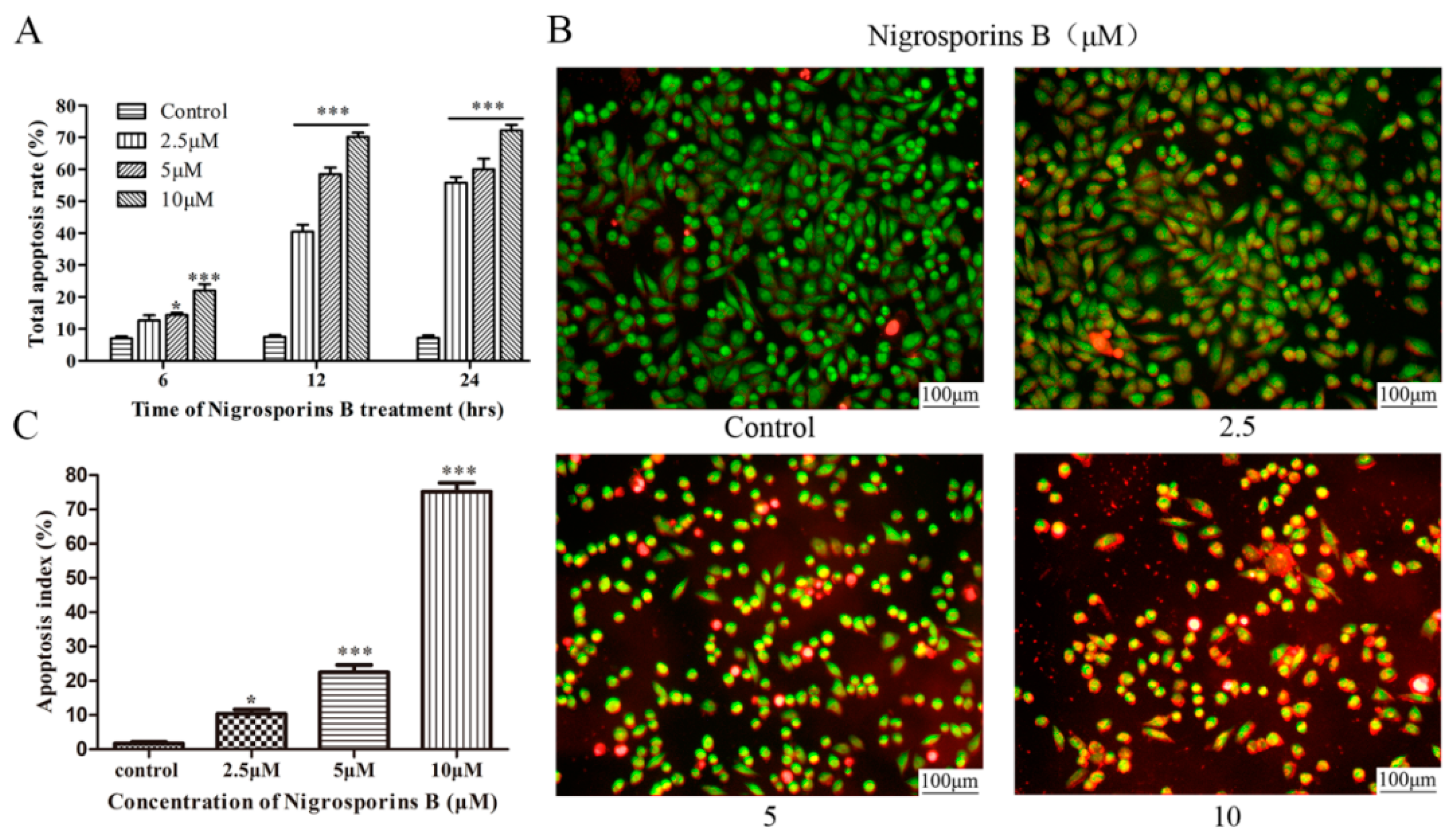
2.5. Acridine Orange/Ethidium Bromide (AO/EB) Dual Fluorescence Staining
2.6. Mitochondrial Membrane Potential (MMP) Assay
2.7. Autophagic Vacuoles Detection
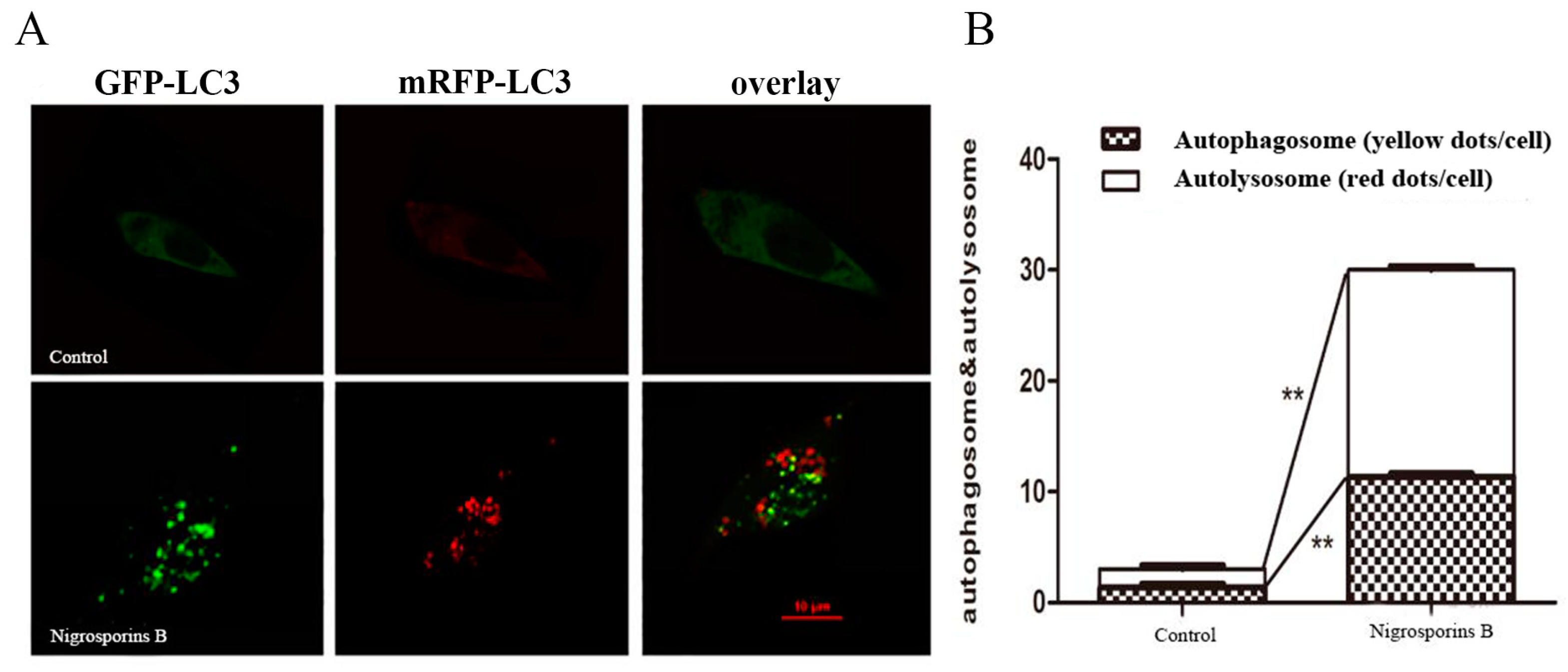
2.8. Autophagic Flux Assessment
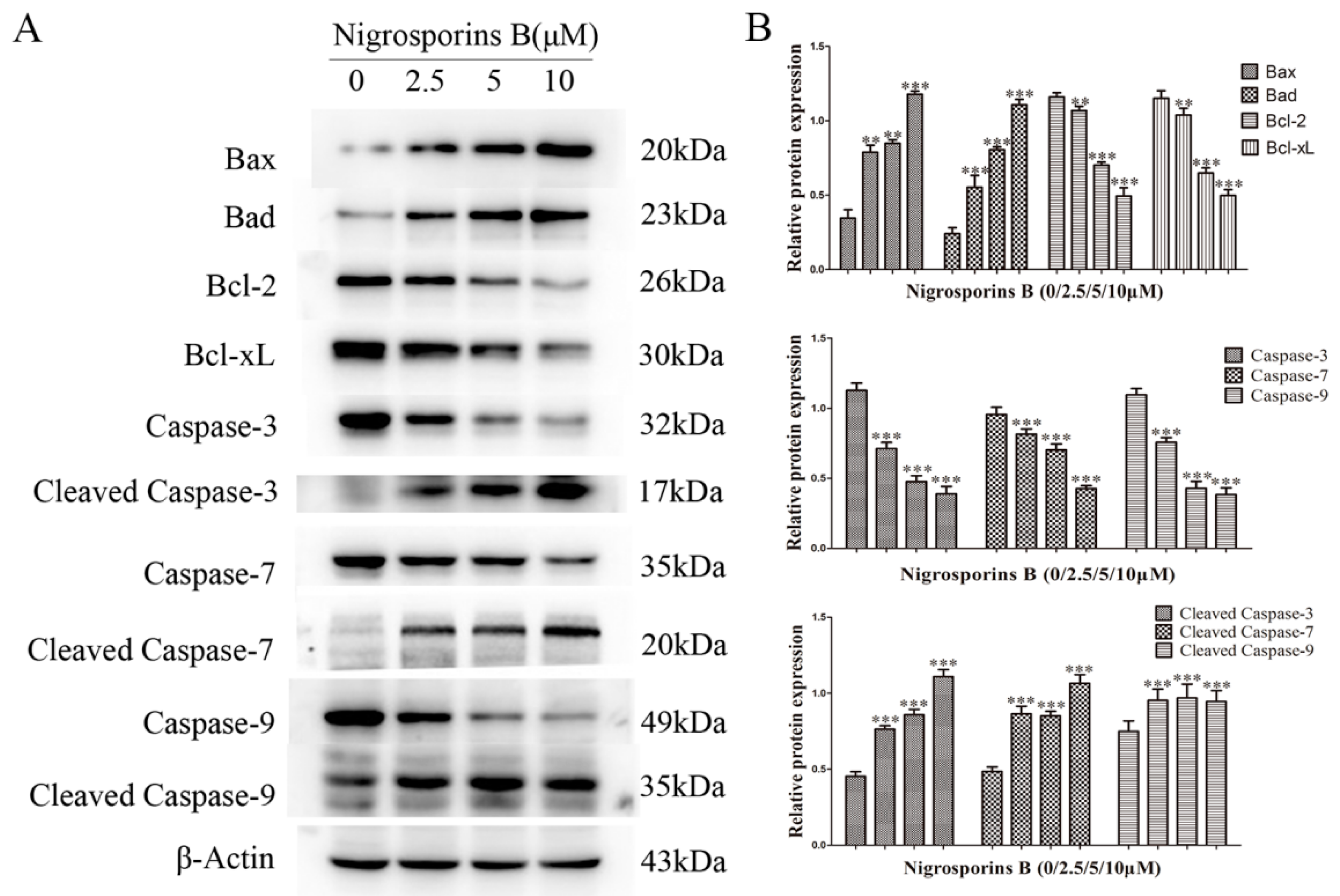
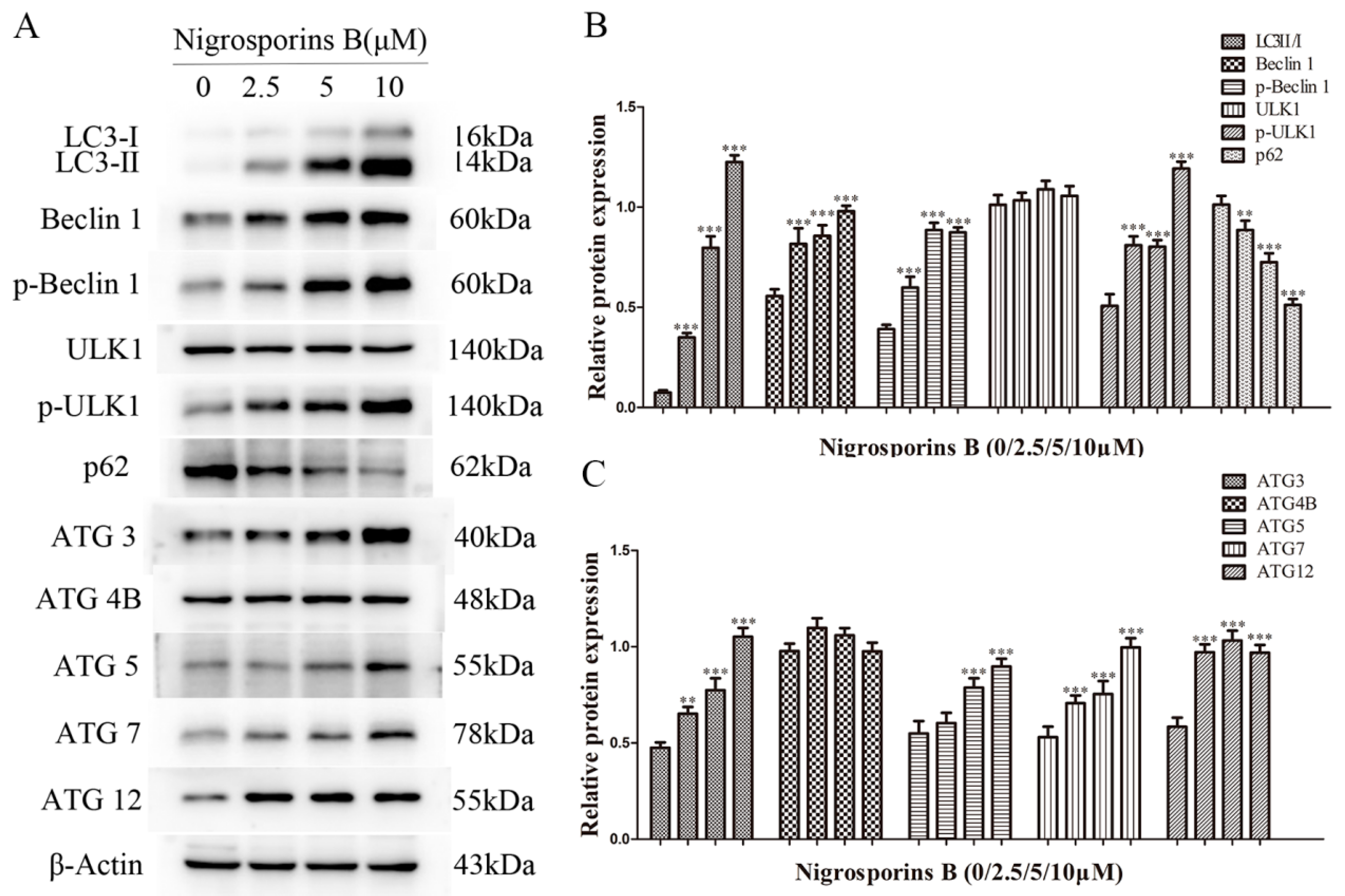
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Nigrosporins B Inhibited the Proliferation of Ca Ski Cells In Vitro
3.2. Nigrosporins B Induced Apoptosis in Ca Ski Cells
3.3. Effect of Nigrosporins B on Mitochondrial Membrane Potential (MMP)
3.4. Effect of Nigrosporins B on Bcl-2 Family and Caspase Family Members
3.5. Nigrosporins B Induced Autophagy in Ca Ski Cells
3.6. Nigrosporins B Altered Expression of Autophagic Related Proteins
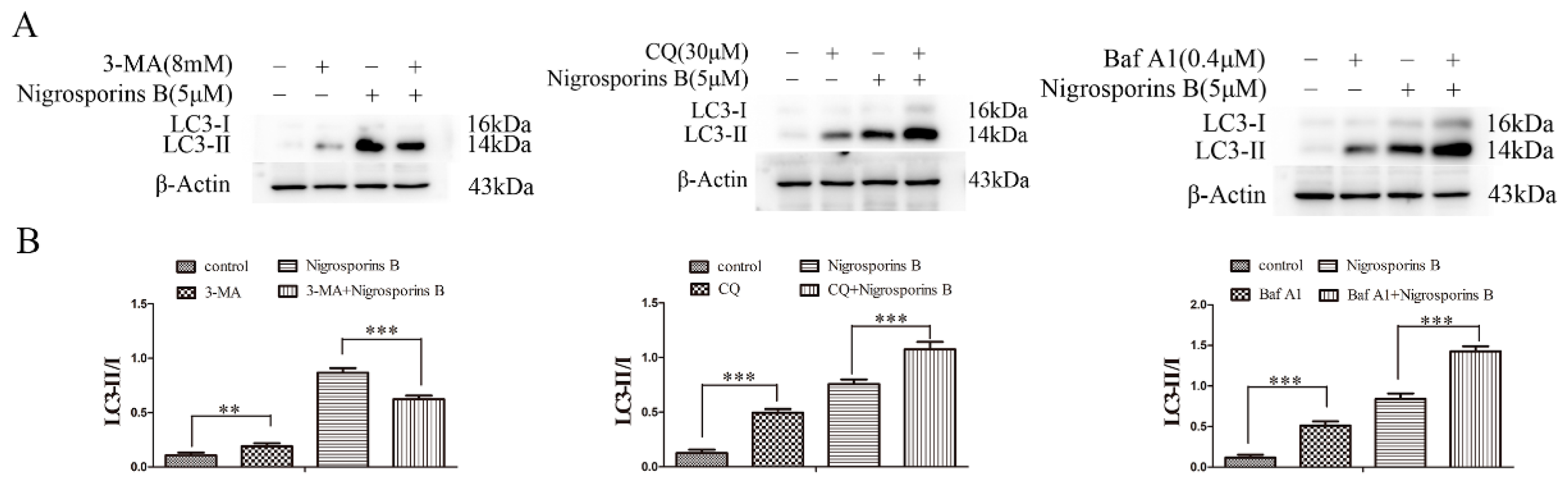
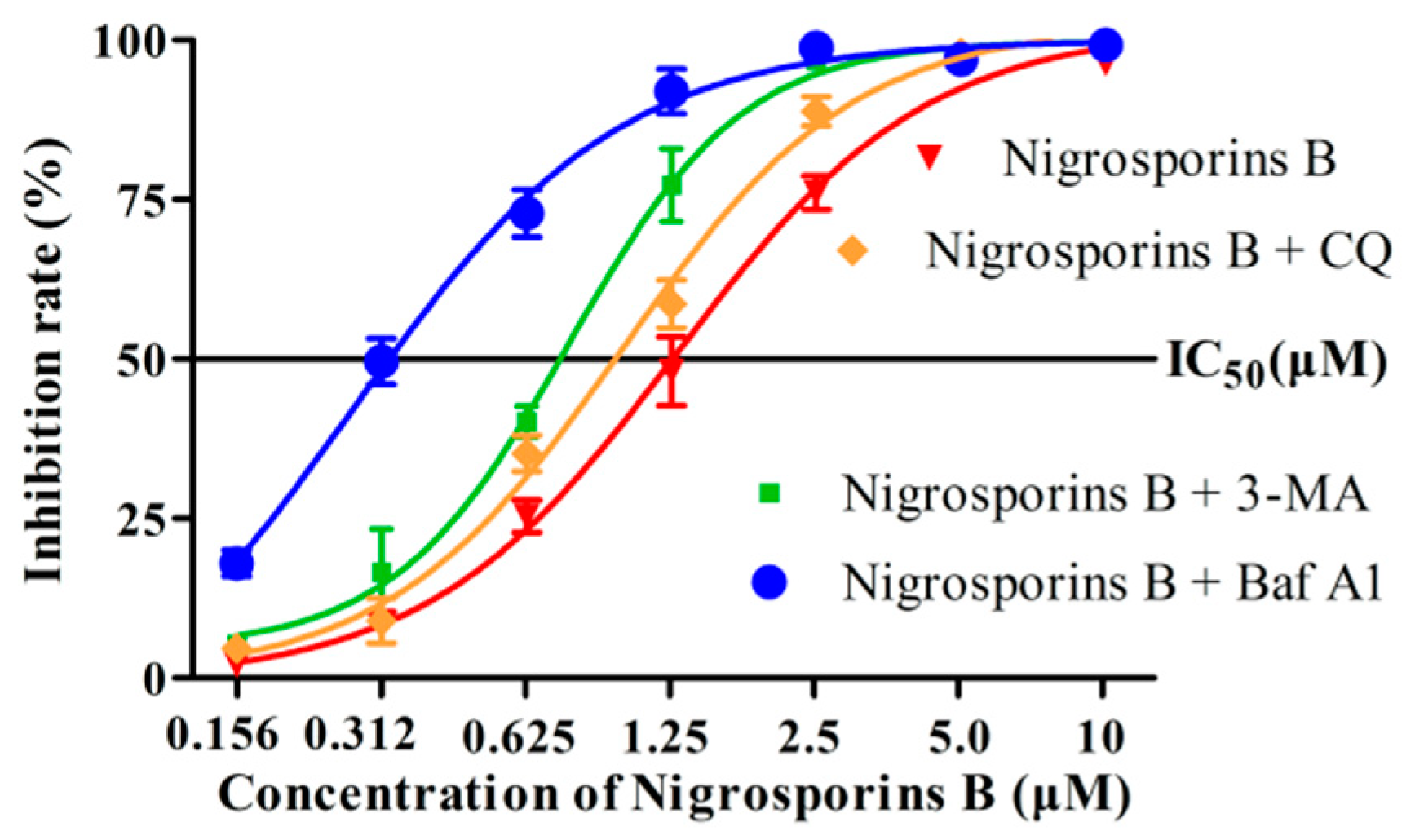
3.7. Inhibition of Autophagy Increased the Cytotoxicity of Nigrosporins B
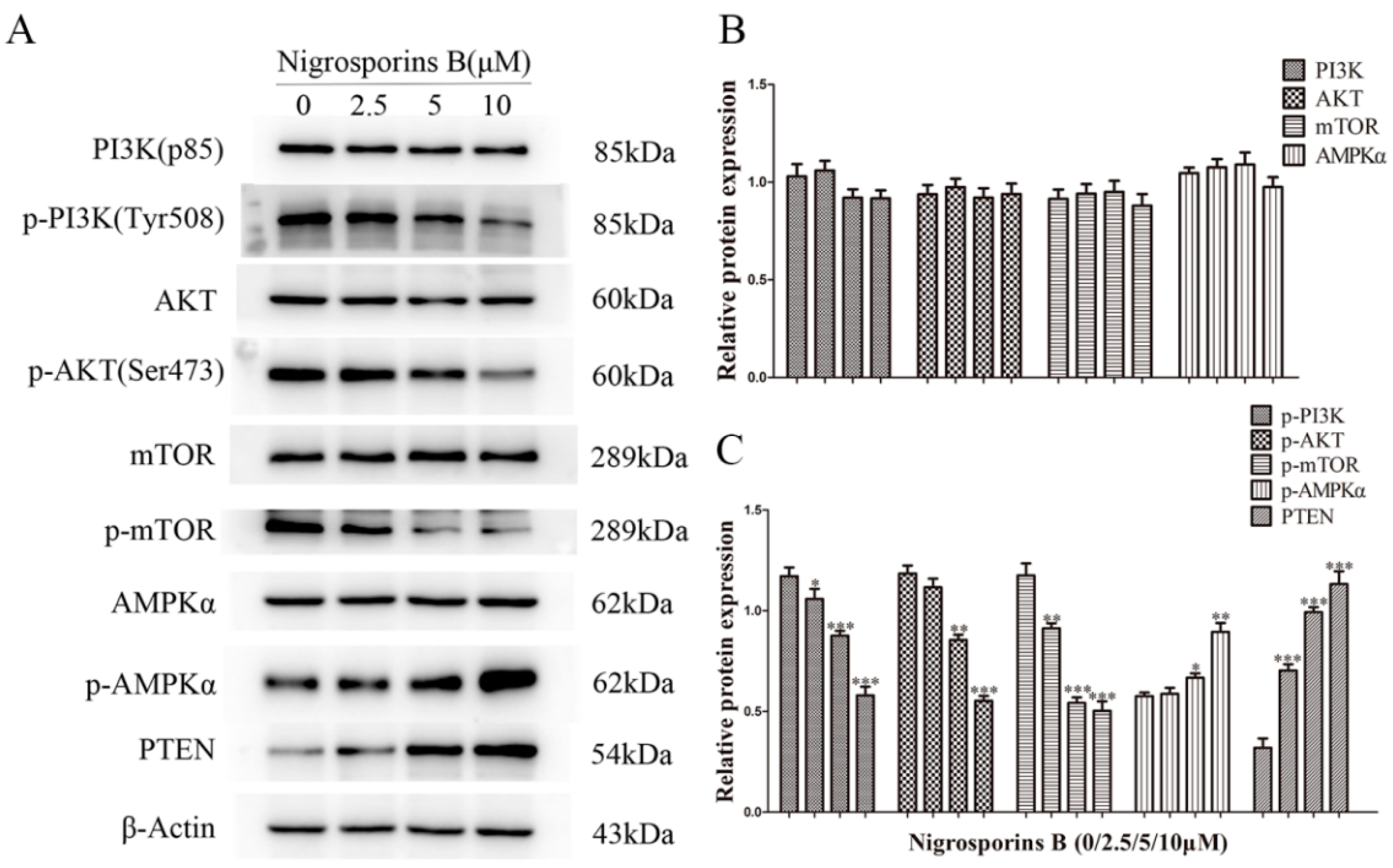
3.8. Nigrosporins B Inhibited the PI3K/AKT/mTOR Signaling Pathway in Ca Ski Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Deep, A.; Sharma, A.K. Current Treatment for Cervical Cancer: An Update. Anti-Cancer Agents Med. Chem. 2020, 20, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.D.; Thorburn, A. Regulation of Apoptosis by Autophagy to Enhance Cancer Therapy. Yale J. Biol. Med. 2019, 92, 707–718. [Google Scholar] [PubMed]
- Ke, B.; Tian, M.; Li, J.; Liu, B.; He, G. Targeting Programmed Cell Death Using Small-Molecule Compounds to Improve Potential Cancer Therapy. Med. Res. Rev. 2016, 36, 983–1035. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [Green Version]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Fairlie, W.D.; Tran, S.; Lee, E.F. Crosstalk between apoptosis and autophagy signaling pathways. Int. Rev. Cell Mol. Biol. 2020, 352, 115–158. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Zhivotovsky, B. Free Radicals in Cross Talk Between Autophagy and Apoptosis. Antioxid. Redox Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, W.; User, S.D.; El-Gazzah, M.; Jaksik, R.; Sajjadi, E.; Rzeszowska-Wolny, J.; Los, M.J. Autophagy, Apoptosis, Mitoptosis and Necrosis: Interdependence Between Those Pathways and Effects on Cancer. Arch. Immunol. Ther. Exp. 2013, 61, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Fujishita, T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr. Top. Microbiol. Immunol. 2017, 407, 153–189. [Google Scholar] [PubMed]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef]
- Matulja, D.; Wittine, K.; Malatesti, N.; Laclef, S.; Turks, M.; Markovic, M.K.; Ambrozic, G.; Markovic, D. Marine Natural Products with High Anticancer Activities. Curr. Med. Chem. 2020, 27, 1243–1307. [Google Scholar] [CrossRef]
- Yang, K.-L.; Wei, M.-Y.; Shao, C.-L.; Fu, X.-M.; Guo, Z.-Y.; Xu, R.-F.; Zheng, C.-J.; She, Z.-G.; Lin, Y.-C.; Wang, C.-Y. Antibacterial Anthraquinone Derivatives from a Sea Anemone-Derived Fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukushima, T.; Tsujino, Y.; Fujimori, T. Nigrosporins A and B, New Phytotoxic and Antibacterial Metabolites Produced by a Fungus Nigrospora oryzae. Biosci. Biotechnol. Biochem. 1997, 61, 1848–1852. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhong, L.; Long, Y.; Li, J.; Wu, J.; Liu, L.; Chen, S.; Lin, Y.; Li, M.; Zhu, X.; et al. Studies on the synthesis of derivatives of marine-derived bostrycin and their structure-activity relationship against tumor cells. Mar. Drugs 2012, 10, 932–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Pridham, K.J.; Sheng, Z. Detecting Autophagy and Autophagy Flux in Chronic Myeloid Leukemia Cells Using a Cyto-ID Fluorescence Spectrophotometric Assay. Methods Mol. Biol. 2016, 1465, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell. Biochem. 2004, 256-257, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S. Caspases and apoptosis. Essays Biochem. 2002, 38, 9–19. [Google Scholar] [CrossRef]
- Shimizu, S.; Yoshida, T.; Tsujioka, M.; Arakawa, S. Autophagic cell death and cancer. Int. J. Mol. Sci. 2014, 15, 3145–3153. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chen, S.; Huang, K.X.; Le, W.D. Why should autophagic flux be assessed? Acta Pharmacol. Sin. 2013, 34, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Yoshida, G.J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: From pathophysiology to treatment. J. Hematol. Oncol. 2017, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-T.; Tan, H.-L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.-N.; Codogno, P.; Shen, H.-M. Dual Role of 3-Methyladenine in Modulation of Autophagy via Different Temporal Patterns of Inhibition on Class I and III Phosphoinositide 3-Kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [Green Version]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Murrow, L.; Debnath, J. ATG12-ATG3 connects basal autophagy and late endosome function. Autophagy 2015, 11, 961–962. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Xie, H.; Hu, T.; Shan, H.; Li, M. Binding Features and Functions of ATG3. Front. Cell Dev. Biol. 2021, 9, 685625. [Google Scholar] [CrossRef]
- Maruyama, T.; Noda, N.N. Autophagy-regulating protease Atg4: Structure, function, regulation and inhibition. J. Antibiot. 2018, 71, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The PTEN-PI3K Axis in Cancer. Biomolecules 2019, 9, 108716. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Kim, J.; Guan, K.-L. AMPK and mTOR in Cellular Energy Homeostasis and Drug Targets. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 381–400. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Elvin, J.A.; Kamath, S.D.; Ali, S.M.; Paintal, A.S.; Restrepo, A.; Berry, E.; Giles, F.J.; Johnson, M.L. FGFR3-TACC3: A novel gene fusion in cervical cancer. Gynecol. Oncol. Rep. 2015, 13, 53–56. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Guo, Z.-Y.; Shao, C.-L.; Zhang, X.-Q.; Cheng, F.; Zou, K.; Chen, J.-F. Nigrosporins B, a Potential Anti-Cervical Cancer Agent, Induces Apoptosis and Protective Autophagy in Human Cervical Cancer Ca Ski Cells Mediated by PI3K/AKT/mTOR Signaling Pathway. Molecules 2022, 27, 2431. https://doi.org/10.3390/molecules27082431
Zhang J, Guo Z-Y, Shao C-L, Zhang X-Q, Cheng F, Zou K, Chen J-F. Nigrosporins B, a Potential Anti-Cervical Cancer Agent, Induces Apoptosis and Protective Autophagy in Human Cervical Cancer Ca Ski Cells Mediated by PI3K/AKT/mTOR Signaling Pathway. Molecules. 2022; 27(8):2431. https://doi.org/10.3390/molecules27082431
Chicago/Turabian StyleZhang, Jing, Zhi-Yong Guo, Chang-Lun Shao, Xue-Qing Zhang, Fan Cheng, Kun Zou, and Jian-Feng Chen. 2022. "Nigrosporins B, a Potential Anti-Cervical Cancer Agent, Induces Apoptosis and Protective Autophagy in Human Cervical Cancer Ca Ski Cells Mediated by PI3K/AKT/mTOR Signaling Pathway" Molecules 27, no. 8: 2431. https://doi.org/10.3390/molecules27082431
APA StyleZhang, J., Guo, Z.-Y., Shao, C.-L., Zhang, X.-Q., Cheng, F., Zou, K., & Chen, J.-F. (2022). Nigrosporins B, a Potential Anti-Cervical Cancer Agent, Induces Apoptosis and Protective Autophagy in Human Cervical Cancer Ca Ski Cells Mediated by PI3K/AKT/mTOR Signaling Pathway. Molecules, 27(8), 2431. https://doi.org/10.3390/molecules27082431






