Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mineral Composition of D. viscosa
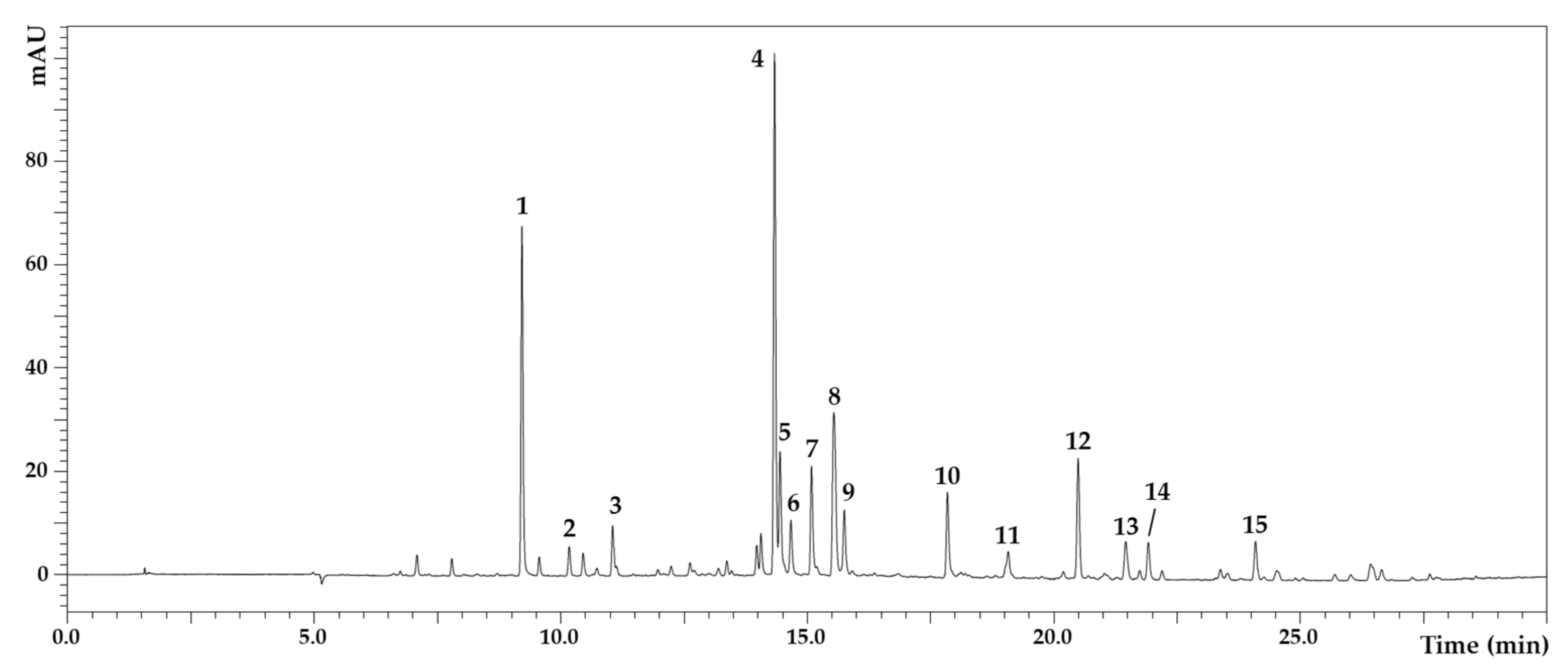
2.2. Polyphenolic Composition of D. viscosa Leaves
2.3. Total Polyphenols and Flavonoids in D. viscosa
2.4. Antioxidant Capacity of D. viscosa
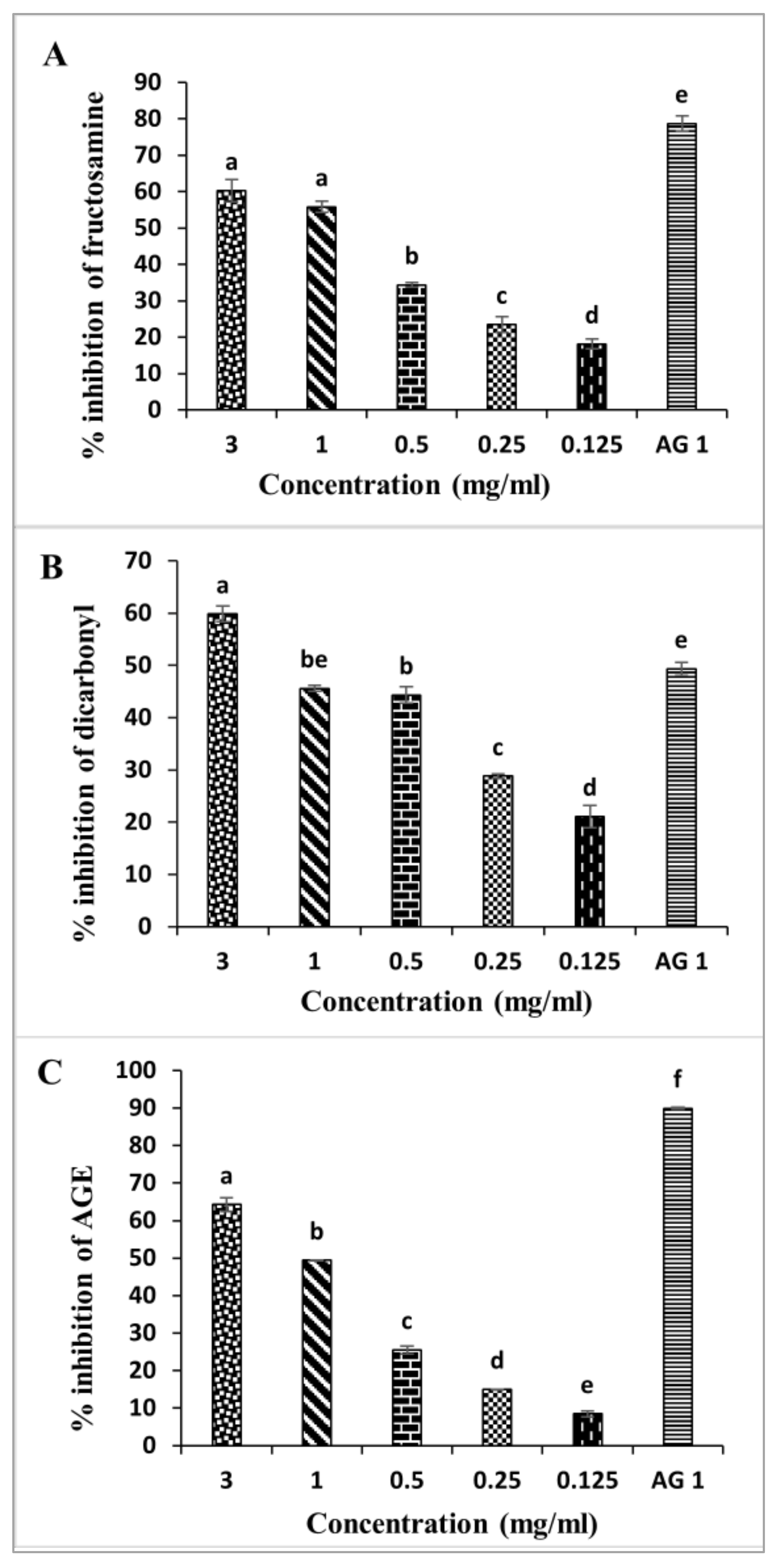
2.5. Inhibition of D. viscosa Leaves on Non-Enzymatic Glycation Process
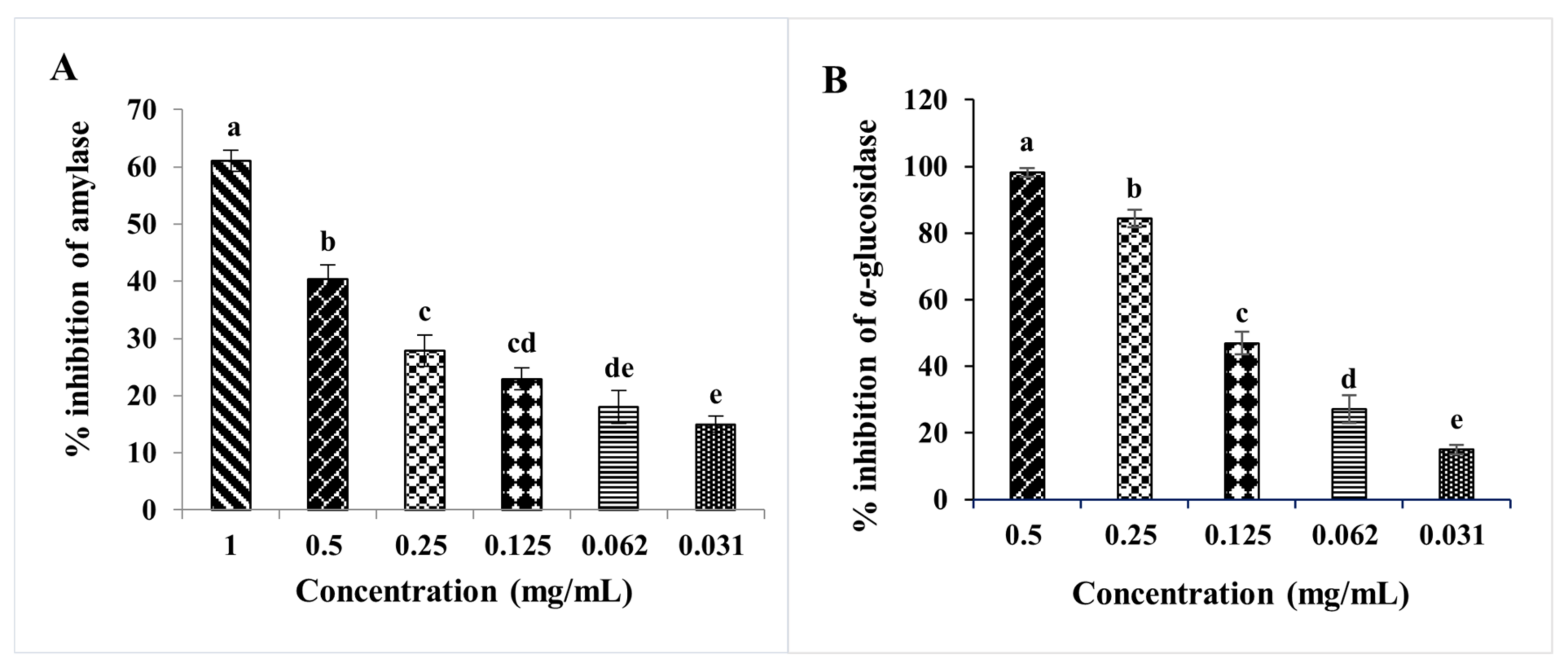
2.6. In Vitro Study of Antidiabetic Activities of D. viscosa Leaf Extracts
2.7. Methanolic Extract Cytotoxicity of D. viscosa on MCF-7 and MDA-MB-468 Cells
2.8. Antibacterial Activity of D. viscosa L. Leaf Extracts
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Mineral Composition Using Inductively Coupled Plasma–Atomic Emission Spectroscopy
3.3. HPLC-DAD-ESI/MS Analysis
3.4. Total Phenolic Content
3.5. Total Flavonoid Content
3.6. DPPH Radical Scavenging Assay
3.7. Radical Scavenging Effect against Acid 2,2′-Azino-bis(3-éthylbenzothiazoline-6-sulphonic) (ABTS+)
3.8. Metal Chelating Activity
3.9. Reducing Power Assay (FRAP)
3.10. Antiglycation Activities
3.10.1. Measurement of Fructosamine Inhibition
3.10.2. Measurement of Dicarbonyl Inhibition
3.11. Antidiabetic Enzymatic Assays
3.11.1. α-Amylase Inhibition Assay
3.11.2. α-Glucosidase Inhibitory Assay
3.12. Cytotoxic Activity
3.12.1. Cell Culture
3.12.2. MTT Assay against Tumor Cells
3.12.3. MTT Assay towards Normal Cells (“PBMCs”)
3.13. Antibacterial Activity
3.13.1. Microorganisms and Growth Conditions
3.13.2. Agar Disc Diffusion Method and Minimum Inhibitory Concentration/Minimum Bactericidal Concentration (MIC/MBC) Determination
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rankou, H.; Culham, A.; Jury, S.L.; Christenhusz, M.J. The Endemic Flora of Morocco. Phytotaxa 2013, 78, 1–69. [Google Scholar]
- El Rhaffari, L.; Zaid, A. Pratique de La Phytothérapie Dans Le Sud-Est Du Maroc (Tafilalet): Un Savoir Empirique Pour Une Pharmacopée Rénovée. Sources Savoir Médicaments Futur 2002, 1, 293–318. [Google Scholar]
- Marmouzi, I.; Bouchmaa, N.; Kharbach, M.; Ezzat, S.M.; Merghany, R.M.; Berkiks, I.; El Jemli, M. Thymelaea genus: Ethnopharmacology, Chemodiversity, and Bioactivities. S. Afr. J. Bot. 2021, 142, 175–192. [Google Scholar]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of Inula viscosa Aiton Leaves. J. Food Saf. 2016, 36, 77–88. [Google Scholar]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar]
- Özkan, E.; Pehlivan Karataş, F.; Birinci Yıldırım, A.; Tas, I.; Eker, I.; Ucar Turker, A. Promising Medicinal Plant Inula viscosa L.: Antiproliferative, Antioxidant, Antibacterial and Phenolic Profiles. Prog. Nutr. 2019, 21, 652–661. [Google Scholar]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules 2021, 26, 3134. [Google Scholar]
- Hepokur, C.; Budak, Y.; Karayel, H.B.; Selvi, B.; Yaylim, İ. Investigation of Cytotoxic Effects of Inula viscosa Extract. Cumhur. Sci. J. 2019, 40, 578–582. [Google Scholar]
- Rhimi, W.; Hlel, R.; Ben Salem, I.; Boulila, A.; Rejeb, A.; Saidi, M. Dittrichia viscosa L. Ethanolic Extract Based Ointment with Antiradical, Antioxidant, and Healing Wound Activities. BioMed Res. Int. 2019, 2019, 4081253. [Google Scholar]
- Konczak, I.; Dalar, A.; Konczak-Islam, K.A. Health Attributes, Antioxidant Properties and Phytochemical Composition of Traditional Medicinal Plants from Eastern Anatolia. In Medicinal Plants; Pereira, D.A.M., Ed.; Nova Publication: New York, NY, USA, 2014; Chapter 7; pp. 183–227. [Google Scholar]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A Nutraceutical Extract from Inula viscosa Leaves: UHPLC-HR-MS/MS Based Polyphenol Profile, and Antioxidant and Cytotoxic Activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by Liquid Chromatography–Mass Spectrometry and Antioxidant Activity of an Ethanolic Extract of Inula viscosa Leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [PubMed]
- Mohti, H.; Taviano, M.F.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Crisafi, G.; Benameur, Q.; Zaid, A.; Miceli, N. Inula viscosa (L.) Aiton Leaves and Flower Buds: Effect of Extraction Solvent/Technique on Their Antioxidant Ability, Antimicrobial Properties and Phenolic Profile. Nat. Prod. Res. 2020, 34, 46–52. [Google Scholar] [PubMed]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; El Hassouni, M. Antioxidant Activities and Total Phenol Content of Inula viscosa Extracts Selected from Three Regions of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 228–233. [Google Scholar]
- Gökbulut, A.; Özhana, O.; Satılmiş, B.; Batçioğlu, K.; Günal, S.; Şarer, E. Antioxidant and Antimicrobial Activities, and Phenolic Compounds of Selected Inula Species from Turkey. Nat. Prod. Commun. 2013, 8, 1934578X1300800417. [Google Scholar]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant Activity of 1, 3-Dicaffeoylquinic Acid Isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Medini, F.; Atik-Bekara, F. In Vitro Evaluation of Antioxidant Activity of the Hydro-Methanolic Extracts of Juniperus oxycedrus Subsp. oxycedrus. Phytothérapie 2013, 11, 244–249. [Google Scholar]
- Adetuyi, F.O.; Karigidi, K.O.; Akintimehin, E.S.; Adeyemo, O.N. Antioxidant Properties of Ageratum conyzoides L. Asteraceae Leaves. Bangladesh J. Sci. Ind. Res. 2018, 53, 265–276. [Google Scholar]
- Gülçin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of Antiradical and Antioxidant Activity of Monodesmosides and Crude Extract from Leontice smirnowii Tuber. Phytomedicine 2006, 13, 343–351. [Google Scholar]
- Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical Characterization, Antioxidant and in Vitro Cytotoxic Activity Evaluation of Juniperus oxycedrus Subsp. oxycedrus Needles and Berries. Molecules 2019, 24, 502. [Google Scholar]
- Adisakwattana, S.; Thilavech, T.; Sompong, W.; Pasukamonset, P. Interaction between Ascorbic Acid and Gallic Acid in a Model of Fructose-Mediated Protein Glycation and Oxidation. Electron. J. Biotechnol. 2017, 27, 32–36. [Google Scholar]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [PubMed] [Green Version]
- Bouchmaa, N.; Ben Mrid, R.; Kabach, I.; Zouaoui, Z.; Karrouchi, K.; Chtibi, H.; Zyad, A.; Cacciola, F.; Nhiri, M. Beta vulgaris subsp. maritima: A Valuable Food with High Added Health Benefits. Appl. Sci. 2022, 12, 1866. [Google Scholar]
- Thornalley, P.J. Dicarbonyl Intermediates in the Maillard Reaction. Ann. N. Y. Acad. Sci. 2005, 1043, 111–117. [Google Scholar] [PubMed]
- Senevirathne, I.G.N.H.; Abeysekera, W.K.S.M.; Abeysekera, W.P.K.M.; Jayanath, N.Y.; Galbada Arachchige, S.P.; Wijewardana, D.C.M.S.I. Antiamylase, Antiglucosidase, and Antiglycation Properties of Millets and Sorghum from Sri Lanka. Evid. Based Complementary Altern. Med. 2021, 2021, 5834915. [Google Scholar]
- Wu, C.-H.; Huang, S.-M.; Lin, J.-A.; Yen, G.-C. Inhibition of Advanced Glycation Endproduct Formation by Foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar]
- Patel, K.; Patel, D.K. Medicinal Importance, Pharmacological Activities, and Analytical Aspects of Hispidulin: A Concise Report. J. Tradit. Complementary Med. 2017, 7, 360–366. [Google Scholar]
- Ceylan, R.; Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Jugreet, S.; Cakır, O.; Ouelbani, R.; Paksoy, M.Y.; Yılmaz, M.A. Enzyme Inhibition and Antioxidant Functionality of Eleven Inula Species Based on Chemical Components and Chemometric Insights. Biochem. Syst. Ecol. 2021, 95, 104225. [Google Scholar]
- Sinéad Lordan, A.; Thomas, J.; Smyth, B. The A-Amylase and a-Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar]
- Boyd, M.R. The NCI in vitro anticancer drug discovery screen. Concept, implementation and operation, 1985–1995. In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Merghoub, N.; Amzazi, S.; Morjani, H. Cytotoxic Effect of Some Moroccan Medicinal Plant Extracts on Human Cervical Cell Lines. J. Med. Plants Res. 2009, 3, 1045–1050. [Google Scholar]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Terryn, C.; Attaleb, M.; Madoulet, C.; Benjouad, A. Inula viscosa Extracts Induces Telomere Shortening and Apoptosis in Cancer Cells and Overcome Drug Resistance. Nutr. Cancer 2016, 68, 131–143. [Google Scholar]
- Mssillou, I.; Agour, A.; Slighoua, M.; Tourabi, M.; Nouioura, G.; Lyoussi, B.; Derwich, E. Phytochemical Characterization, Antioxidant Activity, and in Vitro Investigation of Antimicrobial Potential of Dittrichia viscosa L. Leaf Extracts against Nosocomial Infections. Acta Ecol. Sin. 2021, 15, 289. [Google Scholar]
- Sung, W.S.; Lee, D.G. Antifungal Action of Chlorogenic Acid against Pathogenic Fungi, Mediated by Membrane Disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar]
- Kabach, I.; Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Nhiri, A.Z. Phytochemical Screening, Antioxidant and Cytotoxic Activities of M. Vulgare. Int. J. Pharm. Res. 2019, 11, 338–345. [Google Scholar]
- Miguel, M.; Bouchmaa, N.; Aazza, S.; Gaamoussi, F.; Lyoussi, B. Antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of eleven extracts of Moroccan plants. Fresenius Environ. Bull. 2014, 23, 1–14. [Google Scholar]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese Bayberry (Myrica rubra) Phenolics Mitigated Protein Glycoxidation and Formation of Advanced Glycation End-Products: A Mechanistic Investigation. Food Chem. 2021, 361, 130102. [Google Scholar]
- Huang, Q.; Chai, W.-M.; Ma, Z.-Y.; Ou-Yang, C.; Wei, Q.-M.; Song, S.; Zou, Z.-R.; Peng, Y.-Y. Inhibition of α-Glucosidase Activity and Non-Enzymatic Glycation by Tannic Acid: Inhibitory Activity and Molecular Mechanism. Int. J. Biol. Macromol. 2019, 141, 358–368. [Google Scholar]
- Dong, H.-Q.; Li, M.; Zhu, F.; Liu, F.-L.; Huang, J.-B. Inhibitory Potential of Trilobatin from Lithocarpus Polystachyus Rehd against α-Glucosidase and α-Amylase Linked to Type 2 Diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening Culinary Herbs for Antioxidant and A-glucosidase Inhibitory Activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Nhiri, M.; Ait Mouse, H.; Taoufik, J.; Ansar, M.; Zyad, A. Cytotoxicity of New Pyridazin-3 (2H)-one Derivatives Orchestrating Oxidative Stress in Human Triple-negative Breast Cancer (MDA-MB-468). Arch. Pharm. 2018, 351, 1800128. [Google Scholar]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Bouargalne, Y.; Nhiri, M.; Idir, A.; Taoufik, J.; Ansar, M.; Zyad, A. Reactive Oxygen Species-Mediated Apoptosis and Cytotoxicity of Newly Synthesized Pyridazin-3-Ones In P815 (Murin Mastocytoma) Cell Line. Drug Res. 2019, 69, 528–536. [Google Scholar]
- Bouchmaa, N.; Tilaoui, M.; Boukharsa, Y.; Jaâfari, A.; Mouse, H.A.; Oukerrou, A.; Taoufik, J.; Ansar, M.; Zyad, A. In vitro antitumor activity of newly synthesized pyridazin-3 (2H)-one derivatives via apoptosis induction. Pharm. Chem. J. 2018, 51, 893–901. [Google Scholar]
- Benali, T.; Chtibi, H.; Bouyahya, A.; Khabbach, A.; Hammani, K. Detection of Antioxidant and Antimicrobial Activities in Phenol Components and Essential Oils of Cistus ladaniferus and Mentha suaveolens Extracts. Biomed. Pharmacol. J. 2020, 13, 603–612. [Google Scholar]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and Antioxidant Properties of the Essential Oils and Methanol Extract from Mentha longifolia L. Ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar]

| Mineral Content mg/kg dw | D. viscosa Leaves |
|---|---|
| Macroelements | |
| Ca | 19,511.82 ± 822.77 |
| K | 14,671.00 ± 1582.50 |
| Mg | 5932.00 ± 1043.69 |
| Na | 792.62 ± 79.68 |
| P | 1601.6 ± 357.23 |
| Microelements | |
| Co | 0.224 ± 0.04 |
| Fe | 439.85 ± 61.87 |
| Mn | 78.48 ± 20.73 |
| Zn | 187.21 ± 24.03 |
| Cr | 0.43 ± 0.08 |
| Cu | 12.69 ± 2.93 |
| Se | 4.2 ± 1.09 |
| Heavy metals | |
| Cd | ND |
| N | Tentative Identification | tR (min) | UVmax (nm) | [M-H]− | Extract (mg/L) | Employed Standard for Quantification | References |
|---|---|---|---|---|---|---|---|
| 1 | caffeoylquinic acid | 9.22 | 326 | 353, 191 | 8.69 ± 0.03 | Caffeic acid | [6,10] |
| 2 | caffeic acid | 10.17 | 323 | 179 | 0.17 ± 0.02 | Caffeic acid | [6,10] |
| 3 | di-O-caffeoylquinic acid | 11.06 | 323 | 515, 353 | 0.60 ± 0.01 | Caffeic acid | [6,11] |
| 4 | iso-di-O-caffeoylquinic acid I | 14.33 | 328 | 515, 353 | 13.29 ± 0.06 | Caffeic acid | [6,11] |
| 5 | iso-di-O-caffeoylquinic acid II | 14.45 | 328 | 515, 353 | 3.15 ± 0.08 | Caffeic acid | [6,11] |
| 6 | iso-di-O-caffeoylquinic acid III | 14.66 | 327 | 515, 353 | 0.88 ± 0.01 | Caffeic acid | [6,11] |
| 7 | iso-di-O-caffeoylquinic acid IV | 15.08 | 327 | 515, 353 | 2.35 ± 0.08 | Caffeic acid | [6,11] |
| 8 | unknown | 15.54 | 328 | 695, 405 | - | - | - |
| 9 | Tri-caffeoylglucaric acid | 15.75 | 328 | 695, 371 | 1.41 ± 0.01 | Caffeic acid | [6,11] |
| 10 | unknown | 17.84 | 328 | 428 | - | - | - |
| 11 | padmatin | 19.08 | 288 | 317 | 9.00 ± 0.30 | Naringin | [6,11] |
| 12 | hispidulin | 20.49 | 334 | 299 | 4.40 ± 0.05 | Apigenin | [3,6,11,12,13] |
| 13 | unknown | 21.45 | 290 | 417 | - | - | - |
| 14 | cirsiliol | 21.92 | 358 | 329 | 1.89 ± 0.01 | Apigenin | [6,11] |
| 15 | unknown | 24.09 | 290 | 719, 359 | - | - | - |
| Samples | Extract Yield (%) | Polyphenols (mg GAE/g dw) | Flavonoids (mg QE/g dw) | Reducing Power(mg AAE/g dw) | Antioxidant Properties (IC50 Values; mg/mL) | ||
|---|---|---|---|---|---|---|---|
| DPPH | ABTS | Metal Chelating | |||||
| AE | 17.97 | 144.70 ± 4.33 a | 78.89 ± 2.43 a | 659.44 ± 14.71 a | 0.12 ± 0.004 a | 0.41 ± 0.01 a | 2.39 ± 0.31 a |
| ME | 14.2 | 212.44 ± 13.48 a | 123.05 ± 2.87 b | 944.19 ± 28.44 a | 0.08 ± 0.004 b | 0.22 ± 0.03 b | 0.51 ± 0.03 b |
| IC50 (mg/mL) | ||
|---|---|---|
| α-amylase | α-glucosidase | |
| D. viscosa leaves | 1.381 ± 0.085 a | 0.118 ± 0.02 a |
| Acarbose | 0.046 ± 0.001 b | 0.329 ± 0.041 b |
| Sample Tested | IC50 (µg/mL) Values of Cytotoxity against Tumor Cells | % of Viability in PBMCs | ||||
|---|---|---|---|---|---|---|
| MCF-7 | MDA-MB-468 | PBMCs | Concentration (µg/mL) | |||
| 12.5 | 3.125 | 0.78125 | ||||
| D. viscosa leaves | 2.75 ± 1.2 a | 20.43 ± 2.99 a | >50 a | 88.54 ± 7.16 a | 106.15 ± 4.28 a | 256.98 ± 7.6 a |
| CisP | 0.20 ± 0.0 c | 2.20 ± 0.40 c | 0.27 b | 16.08 ± 3.39 b | 30.08 ± 3.58 b | 37.96 ± 3.44 b |
| Bacterial Strains | Inhibition Zone Diameter (mm) | Concentration of D. viscosa L. (mg/mL) | ||
|---|---|---|---|---|
| D. viscosa L. | Gentamicine | MIC | MBC | |
| Proteus mirabilis | 11.3 ± 0.6 | 18.7 ± 0.6 | 6.79 ± 0.39 × 10−17 | >25 |
| Bacillus subtilis | 10.3 ± 0.6 | 19.7 ± 0.6 | 1.56 ± 2.72 × 10−16 | >25 |
| Staphylococcus aureus | n.e | 15 ± 1 | - | - |
| Pseudomonas aeruginosa | n.e | n.e | - | - |
| Escherichia coli | n.e | 16 ± 1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrid, R.B.; Bouchmaa, N.; Kabach, I.; Zouaoui, Z.; Chtibi, H.; Maadoudi, M.E.; Kounnoun, A.; Cacciola, F.; Majdoub, Y.O.E.; Mondello, L.; et al. Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules 2022, 27, 2108. https://doi.org/10.3390/molecules27072108
Mrid RB, Bouchmaa N, Kabach I, Zouaoui Z, Chtibi H, Maadoudi ME, Kounnoun A, Cacciola F, Majdoub YOE, Mondello L, et al. Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules. 2022; 27(7):2108. https://doi.org/10.3390/molecules27072108
Chicago/Turabian StyleMrid, Reda Ben, Najat Bouchmaa, Imad Kabach, Zakia Zouaoui, Houda Chtibi, Mohammed El Maadoudi, Ayoub Kounnoun, Francesco Cacciola, Yassine Oulad El Majdoub, Luigi Mondello, and et al. 2022. "Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects" Molecules 27, no. 7: 2108. https://doi.org/10.3390/molecules27072108
APA StyleMrid, R. B., Bouchmaa, N., Kabach, I., Zouaoui, Z., Chtibi, H., Maadoudi, M. E., Kounnoun, A., Cacciola, F., Majdoub, Y. O. E., Mondello, L., Zyad, A., & Nhiri, M. (2022). Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules, 27(7), 2108. https://doi.org/10.3390/molecules27072108








