Fungus-Derived 3-Hydroxyterphenyllin and Candidusin A Ameliorate Palmitic Acid-Induced Human Podocyte Injury via Anti-Oxidative and Anti-Apoptotic Mechanisms
Abstract
:1. Introduction
2. Results
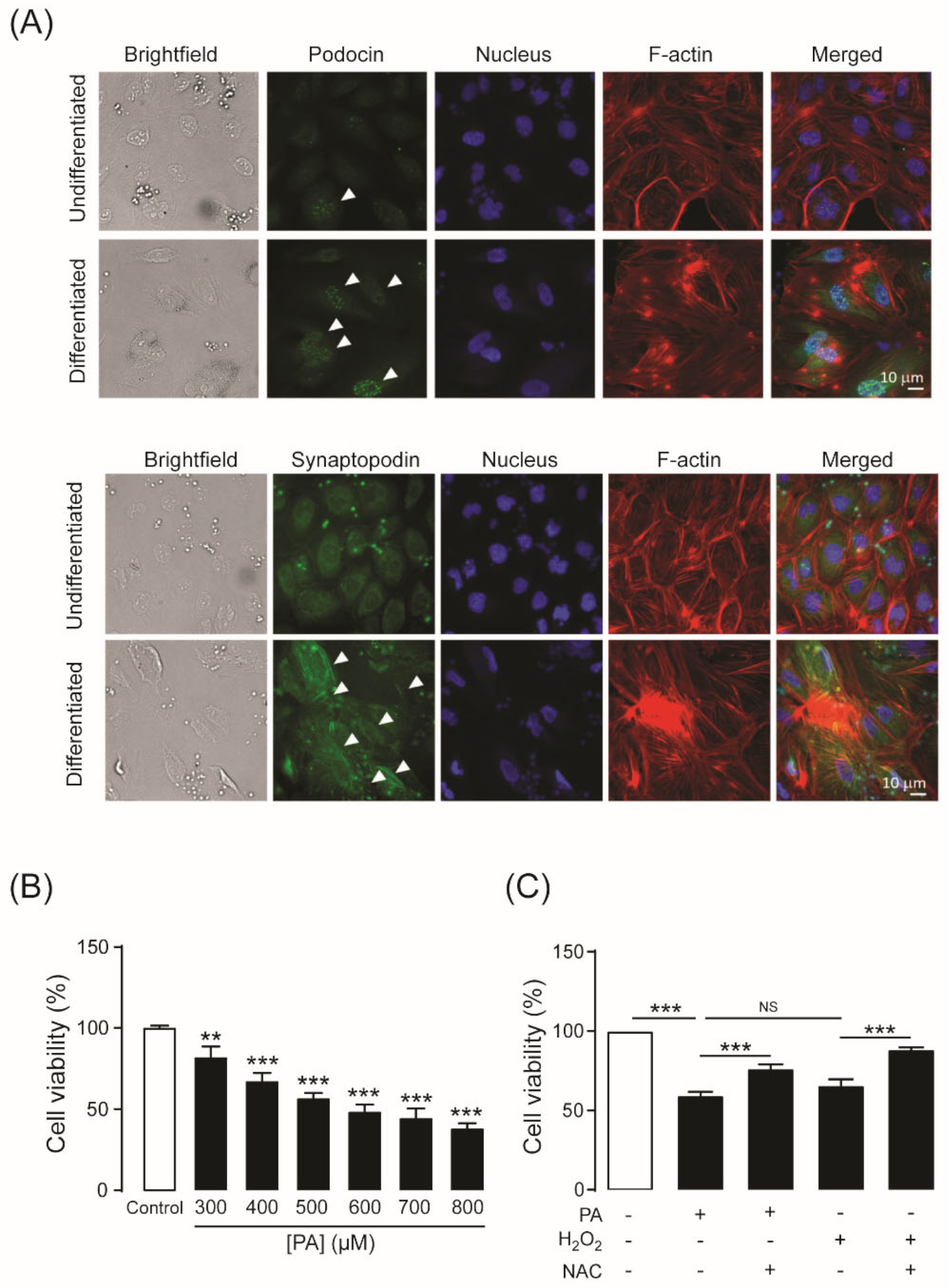
2.1. Establishment of an Experimental Model of PA-Induced Podocyte Death
2.2. Identification of 3-Hydroxyterphenyllin (3-HT) and Candidusin A (CDA) as Inhibitors of PA-Induced Podocyte Injury
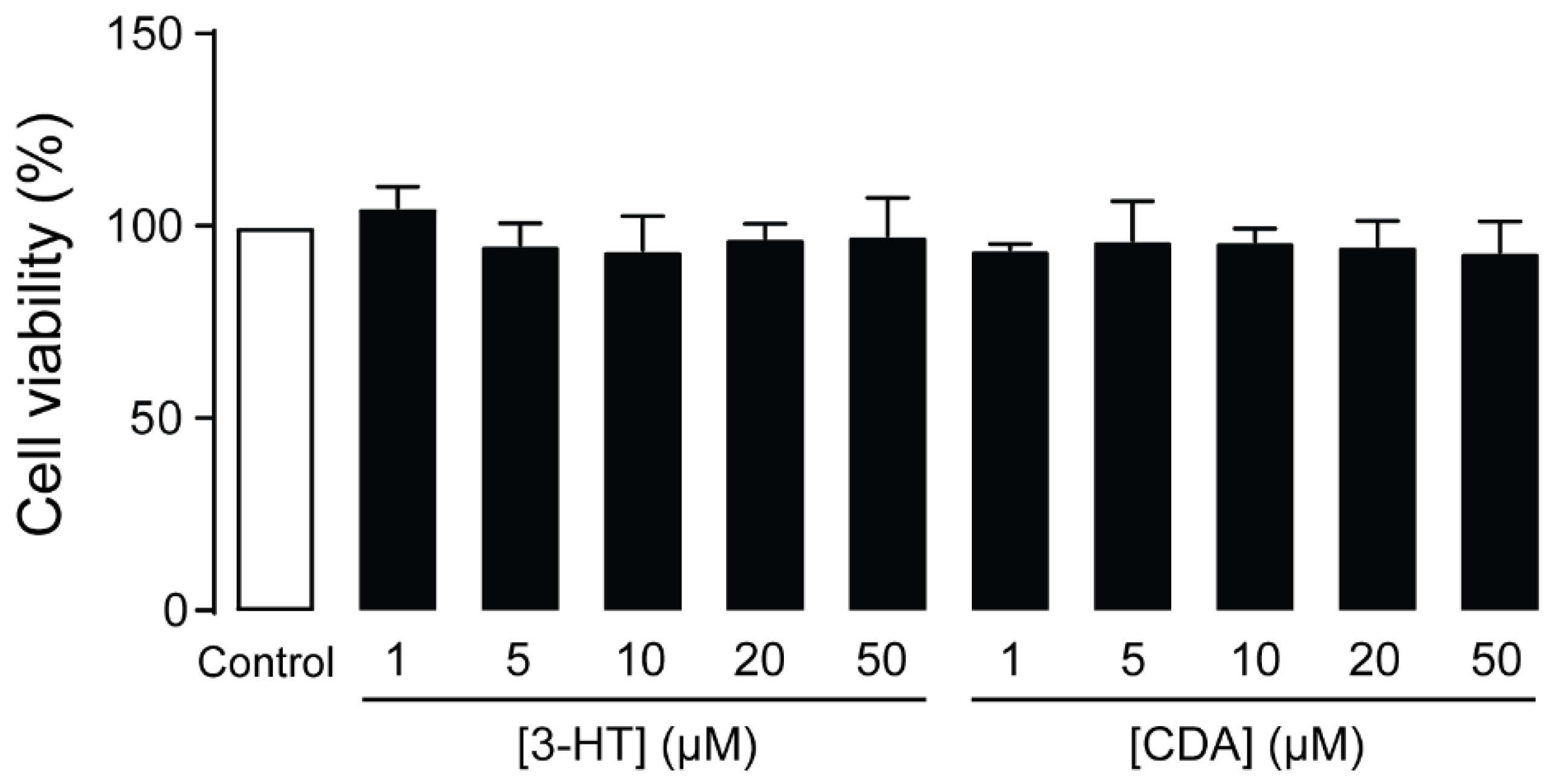
2.3. Cytotoxicity of 3-HT and CDA in Human Podocytes
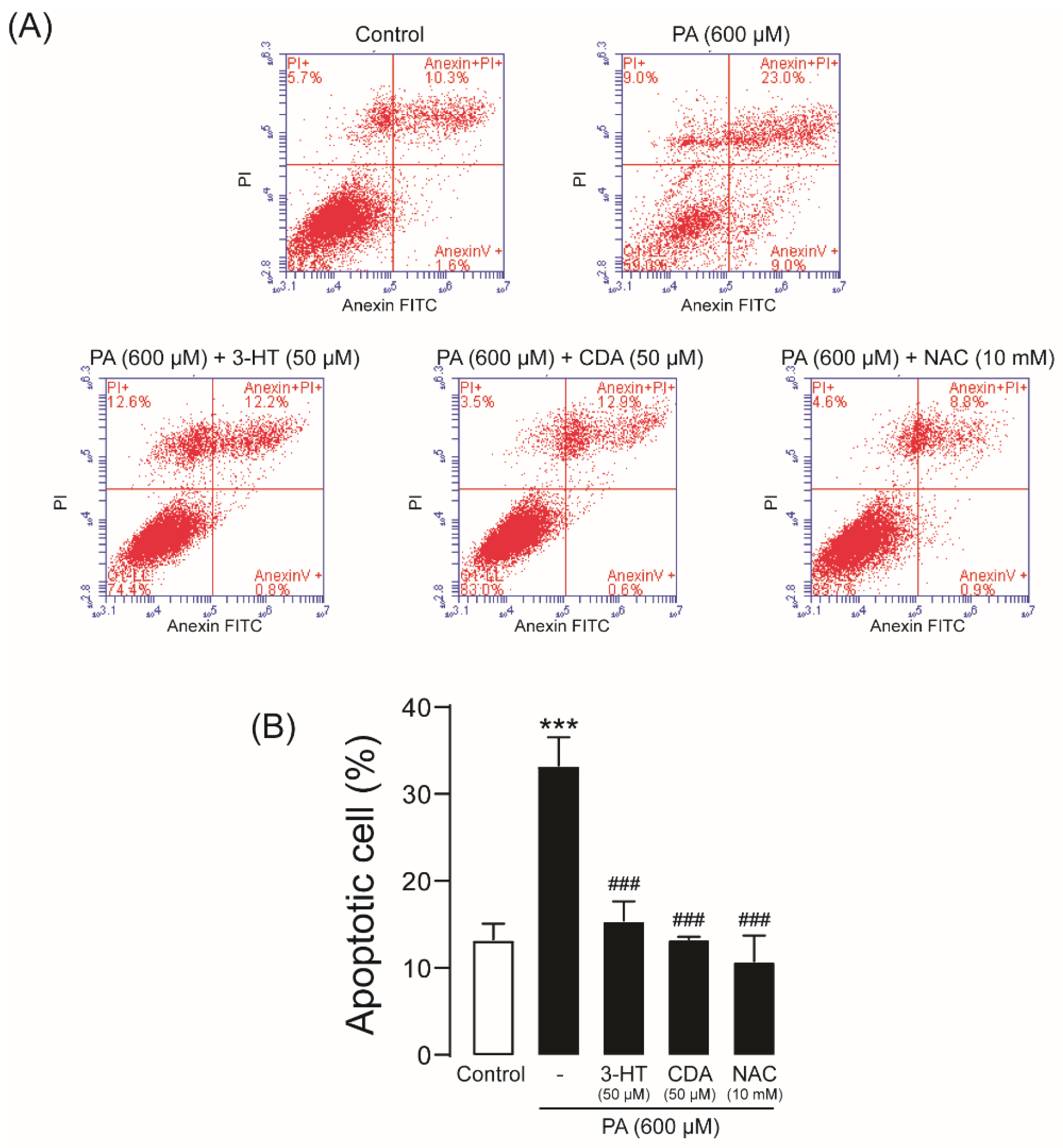
2.4. Effect of 3-HT and CDA on PA-Induced Podocyte Apoptosis
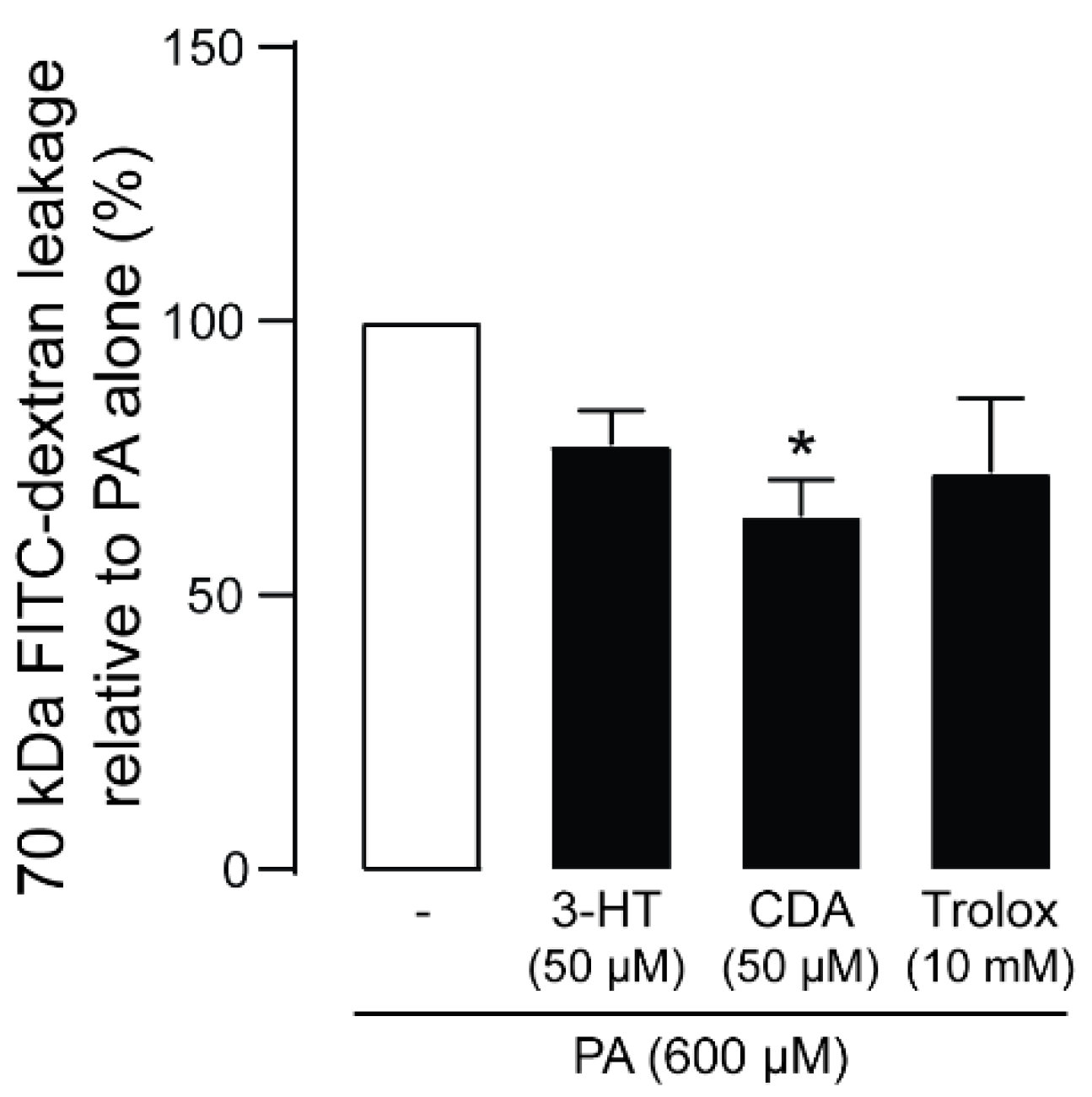
2.5. Effect of 3-HT and CDA on PA-Induced Podocyte Barrier Leakage
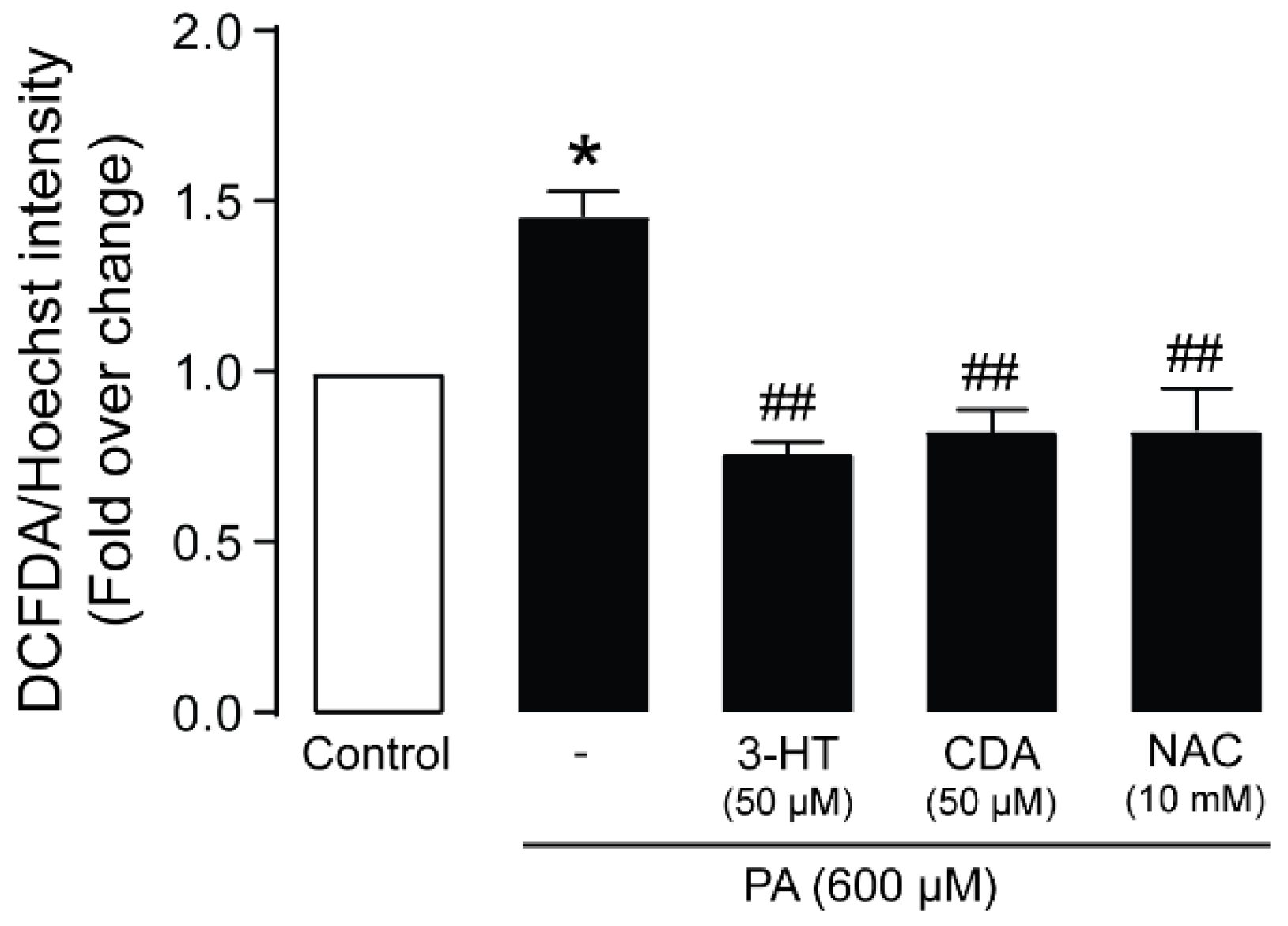
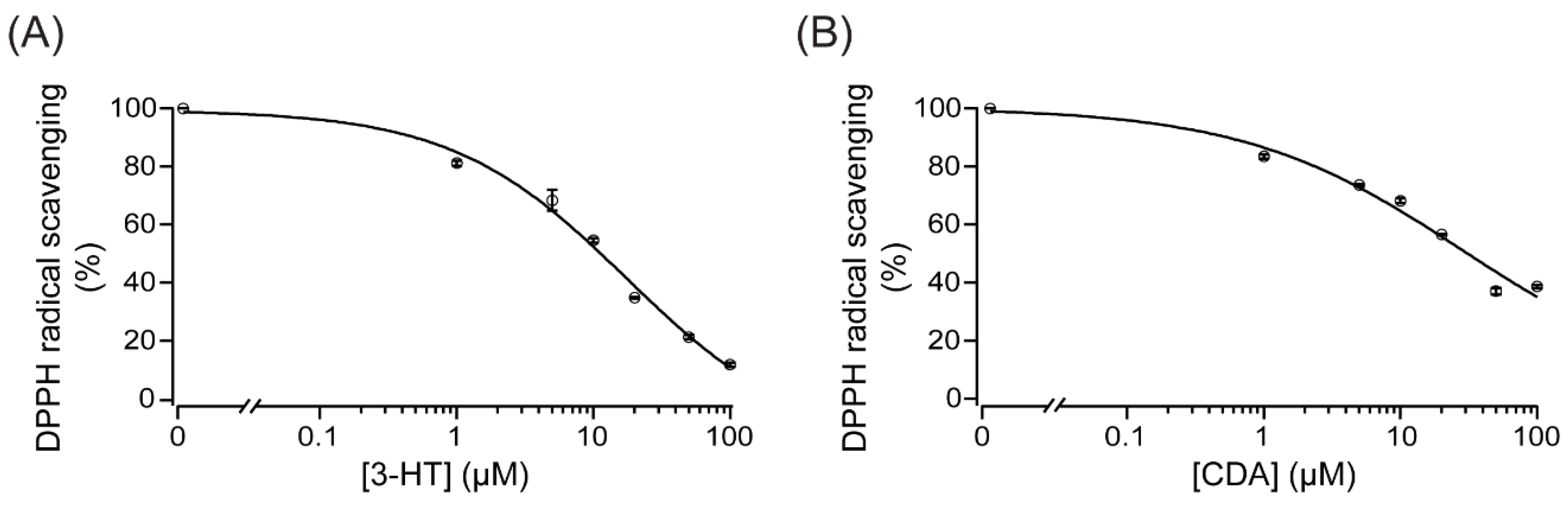
2.6. Anti-Oxidative Mechanisms of 3-HT and CDA
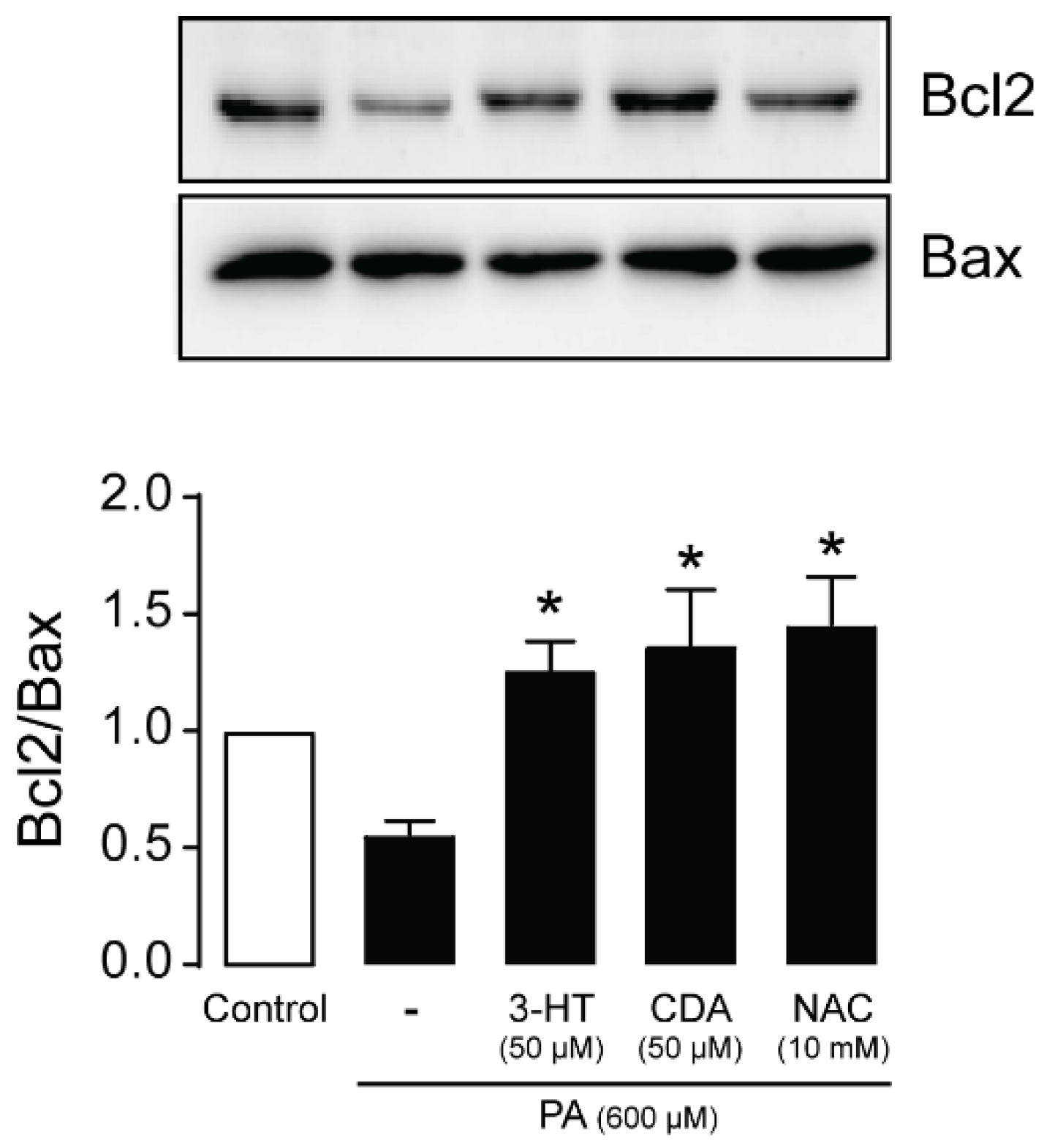
2.7. Effect of 3-HT and CDA on Apoptotic Signaling Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Line, Chemical Reagents, and Antibodies
4.2. Isolation of 3-HT and CDA
4.3. Cell Culture
4.4. Immunofluorescence Staining
4.5. PA Preparation
4.6. Cell Viability Assay
4.7. Flow Cytometric Analysis of Cell Death
4.8. Podocyte Permeability Assay
4.9. Measurement of ROS Generation
4.10. Measurement of Scavenging Activity
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dronavalli, S.; Duka, I.; Bakris, G.L. The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Beladi Mousavi, S.S. Diabetes and end-stage renal disease; a review article on new concepts. J. Renal. Inj. Prev. 2015, 4, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Fineberg, D.; Jandeleit-Dahm, K.A.; Cooper, M.E. Diabetic nephropathy: Diagnosis and treatment. Nat. Rev. Endocrinol. 2013, 9, 713–723. [Google Scholar] [CrossRef]
- Breyer, M.D.; Kretzler, M. Novel avenues for drug discovery in diabetic kidney disease. Expert Opin. Drug Discov. 2018, 13, 65–74. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Dallner, G.; Bentinger, M.; Hussain, S.; Sinha, I.; Yang, J.; Schwank-Xu, C.; Zheng, X.; Swiezewska, E.; Brismar, K.; Valladolid-Acebes, I.; et al. Dehydro-Tocotrienol-beta Counteracts Oxidative-Stress-Induced Diabetes Complications in db/db Mice. Antioxidants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, S.; Hou, Y.; Yi, F. The Updates of Podocyte Lipid Metabolism in Proteinuric Kidney Disease. Kidney Dis. 2021, 7, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Opazo-Rios, L.; Mas, S.; Marin-Royo, G.; Mezzano, S.; Gomez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suk Kang, J.; Son, S.S.; Lee, J.H.; Lee, S.W.; Jeong, A.R.; Lee, E.S.; Cha, S.K.; Chung, C.H.; Lee, E.Y. Protective effects of klotho on palmitate-induced podocyte injury in diabetic nephropathy. PLoS ONE 2021, 16, e0250666. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.A.; Young, K.W.; Andag, U. Targeting the podocyte to treat glomerular kidney disease. Drug Discov. Today 2015, 20, 1228–1234. [Google Scholar] [CrossRef]
- Wharram, B.L.; Goyal, M.; Wiggins, J.E.; Sanden, S.K.; Hussain, S.; Filipiak, W.E.; Saunders, T.L.; Dysko, R.C.; Kohno, K.; Holzman, L.B.; et al. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005, 16, 2941–2952. [Google Scholar] [CrossRef]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef]
- Anil Kumar, P.; Welsh, G.I.; Saleem, M.A.; Menon, R.K. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front. Endocrinol. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Shao, M.; Zhu, Y. Gastrodin inhibits high glucose-induced inflammation, oxidative stress and apoptosis in podocytes by activating the AMPK/Nrf2 signaling pathway. Exp. Ther. Med. 2022, 23, 168. [Google Scholar] [CrossRef]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Geniposide Improves Diabetic Nephropathy by Enhancing ULK1-Mediated Autophagy and Reducing Oxidative Stress through AMPK Activation. Int. J. Mol. Sci. 2021, 22, 1651. [Google Scholar] [CrossRef]
- Xu, S.; Nam, S.M.; Kim, J.H.; Das, R.; Choi, S.K.; Nguyen, T.T.; Quan, X.; Choi, S.J.; Chung, C.H.; Lee, E.Y.; et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015, 6, e1976. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Choi, J.; Lee, H.S. Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J. Cell. Physiol. 2017, 232, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef] [PubMed]
- Hildeman, D.A.; Mitchell, T.; Aronow, B.; Wojciechowski, S.; Kappler, J.; Marrack, P. Control of Bcl-2 expression by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2003, 100, 15035–15040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, H.; Nangaku, M. Podocyte lipotoxicity in diabetic kidney disease. Kidney Int. 2019, 96, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Sosa, M.A.; Fornoni, A. Lipid mediators of insulin signaling in diabetic kidney disease. Am. J. Physiol. Renal. Physiol. 2019, 317, F1241–F1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Lipids mediate podocyte damage. Nat. Rev. Nephrol. 2019, 15, 594. [Google Scholar] [CrossRef]
- Lin, J.S.; Susztak, K. Podocytes: The Weakest Link in Diabetic Kidney Disease? Curr. Diabetes Rep. 2016, 16, 45. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Chen, X.M.; Sun, J.Y.; Jiang, X.S.; Wu, Y.; Yang, S.; Huang, H.Z.; Ruan, X.Z.; Du, X.G. Palmitic Acid-Induced Podocyte Apoptosis via the Reactive Oxygen Species-Dependent Mitochondrial Pathway. Kidney Blood Press. Res. 2018, 43, 206–219. [Google Scholar] [CrossRef]
- Hua, W.; Huang, H.Z.; Tan, L.T.; Wan, J.M.; Gui, H.B.; Zhao, L.; Ruan, X.Z.; Chen, X.M.; Du, X.G. CD36 Mediated Fatty Acid-Induced Podocyte Apoptosis via Oxidative Stress. PLoS ONE 2015, 10, e0127507. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chang, Y.C.; Sheu, F.; Chiang, H.C. Isolation and characterization of antioxidant compounds from Aspergillus candidus broth filtrate. J. Agric. Food Chem. 2001, 49, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Compton, C.; Rankin, G.O.; Cutler, S.J.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. 3-Hydroxyterphenyllin, a natural fungal metabolite, induces apoptosis and S phase arrest in human ovarian carcinoma cells. Int. J. Oncol. 2017, 50, 1392–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, N.; Efthymiou, A.G.; Mather, K.; Chester, N.; Wang, X.; Nath, A.; Rao, M.S.; Steiner, J.P. Compounds with species and cell type specific toxicity identified in a 2000 compound drug screen of neural stem cells and rat mixed cortical neurons. Neurotoxicology 2014, 45, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Chiang, H.C.; Wu, C.H.; Yeh, C.T. The protective effects of Aspergillus candidus metabolites against hydrogen peroxide-induced oxidative damage to Int 407 cells. Food Chem. Toxicol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S128–S138. [Google Scholar] [CrossRef]
- Valentova, K. Cytoprotective Activity of Natural and Synthetic Antioxidants. Antioxidants 2020, 9, 713. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Vidal, C.; Boadle, R.; Thekkedam, C.; Duque, G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol. Open 2013, 2, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [CrossRef]
- Putra, H.N.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Hadsadee, S.; Jungsuttiwong, S.J.T. Caryophyllene sesquiterpenes, chromones and 10-membered macrolides from the marine-derived fungus Pseudopestalotiopsis sp. PSU-AMF45. Tetrahedron 2019, 75, 130530. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhao, B.B.; Lu, C.H.; Huang, J.J.; Shen, Y.M. Two new p-terphenyl derivatives from the marine fungal strain Aspergillus sp. AF119. Nat. Prod. Commun. 2012, 7, 1057–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, Y.; Kawata, A.; Ito, S.; Katayama, T.; Fujisawa, S. Radical-scavenging and Anti-inflammatory Activity of Quercetin and Related Compounds and Their Combinations Against RAW264.7 Cells Stimulated with Porphyromonas gingivalis Fimbriae. Relationships between Anti-inflammatory Activity and Quantum Chemical Parameters. In Vivo 2015, 29, 701–710. [Google Scholar] [PubMed]

| Number | Source | Name of Fungi/Plants |
|---|---|---|
| 1 | Fungi | Aspergillus sp. |
| 2 | Fungi | Pestalotiopsis sp. |
| 3 | Fungi | Trichoderma sp. |
| 4 | Plant | Desmos sp. |
| 5 | Plant | Enicosanthum sp. |
| 6 | Plant | Erythrina sp. |
| 7 | Plant | Friesodielsia sp. |
| 8 | Plant | Garcinia sp. |
| 9 | Plant | Goniothalamus sp. |
| 10 | Plant | Maclura sp. |
| 11 | Plant | Melodorum sp. |
| 12 | Plant | Millettia sp. |
| 13 | Plant | Mitrephora sp. |
| 14 | Plant | Murraya sp. |
| 15 | Plant | Piper sp. |
| 16 | Plant | Pongamia sp. |
| 17 | Plant | Uvaria sp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewin, S.; Changsorn, K.; Sungkaworn, T.; Hiranmartsuwan, P.; Yaosanit, W.; Rukachaisirikul, V.; Muanprasat, C. Fungus-Derived 3-Hydroxyterphenyllin and Candidusin A Ameliorate Palmitic Acid-Induced Human Podocyte Injury via Anti-Oxidative and Anti-Apoptotic Mechanisms. Molecules 2022, 27, 2109. https://doi.org/10.3390/molecules27072109
Kaewin S, Changsorn K, Sungkaworn T, Hiranmartsuwan P, Yaosanit W, Rukachaisirikul V, Muanprasat C. Fungus-Derived 3-Hydroxyterphenyllin and Candidusin A Ameliorate Palmitic Acid-Induced Human Podocyte Injury via Anti-Oxidative and Anti-Apoptotic Mechanisms. Molecules. 2022; 27(7):2109. https://doi.org/10.3390/molecules27072109
Chicago/Turabian StyleKaewin, Suchada, Karn Changsorn, Titiwat Sungkaworn, Peraya Hiranmartsuwan, Wiriya Yaosanit, Vatcharin Rukachaisirikul, and Chatchai Muanprasat. 2022. "Fungus-Derived 3-Hydroxyterphenyllin and Candidusin A Ameliorate Palmitic Acid-Induced Human Podocyte Injury via Anti-Oxidative and Anti-Apoptotic Mechanisms" Molecules 27, no. 7: 2109. https://doi.org/10.3390/molecules27072109
APA StyleKaewin, S., Changsorn, K., Sungkaworn, T., Hiranmartsuwan, P., Yaosanit, W., Rukachaisirikul, V., & Muanprasat, C. (2022). Fungus-Derived 3-Hydroxyterphenyllin and Candidusin A Ameliorate Palmitic Acid-Induced Human Podocyte Injury via Anti-Oxidative and Anti-Apoptotic Mechanisms. Molecules, 27(7), 2109. https://doi.org/10.3390/molecules27072109






