Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides
Abstract
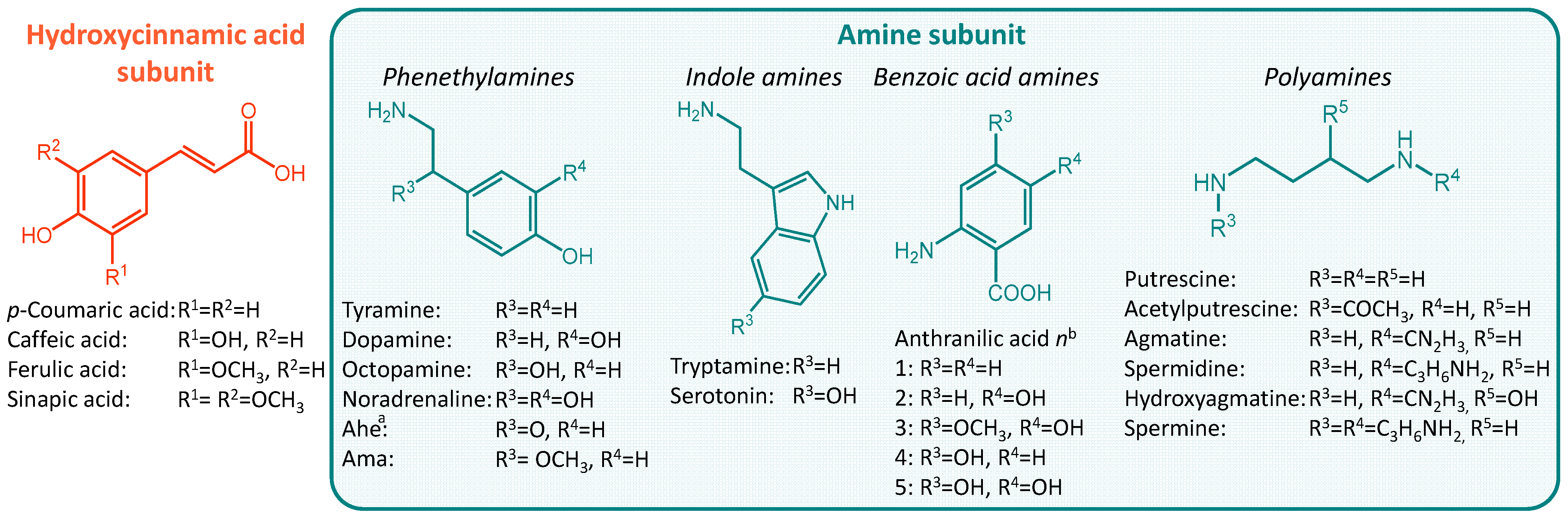
:1. Introduction
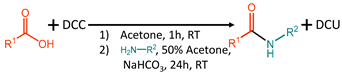
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Information
3.2. General Procedure for the Synthesis of Compounds 1–8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Roumani, M.; Besseau, S.; Gagneul, D.; Robin, C.; Larbat, R. Phenolamides in plants: An update on their function, regulation, and origin of their biosynthetic enzymes. J. Exp. Bot. 2021, 72, 2334–2355. [Google Scholar] [CrossRef] [PubMed]
- Bassard, J.-E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Snooks, H.D.; Sang, S. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef] [PubMed]
- van Zadelhoff, A.; de Bruijn, W.J.; Fang, Z.; Gaquerel, E.; Ishihara, A.; Werck-Reichhart, D.L.; Zhang, P.; Zhou, G.; Franssen, M.C.; Vincken, J.-P. Toward a systematic nomenclature for (neo) lignanamides. J. Nat. Prod. 2021, 84, 956–963. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef] [PubMed]
- Procopio, D.; Siciliano, C.; Trombino, S.; Dumitrescu, D.E.; Suciu, F.; Di Gioia, M.L. Green solvents for the formation of amide linkages. Org. Biomol. 2022, 20, 1137–1149. [Google Scholar] [CrossRef]
- Collins, F.W. Oat phenolics: Avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 1989, 37, 60–66. [Google Scholar] [CrossRef]
- Miyagawa, H.; Ishihara, A.; Kuwahara, Y.; Ueno, T.; Mayama, S. A stress compound in oats induced by victorin, a host-specific toxin from Helminthosporium victoriae. Phytochemistry 1996, 41, 1473–1475. [Google Scholar] [CrossRef]
- Negrel, J.; Smith, T.A. Oxidation of p-coumaroylagmatine in barley seedling extracts in the presence of hydrogen peroxide or thiols. Phytochemistry 1984, 23, 739–741. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kawano, Y.; Uebayasi, M. Induction of adiponectin by natural and synthetic phenolamides in mouse and human preadipocytes and its enhancement by docosahexaenoic acid. Life Sci. 2008, 82, 290–300. [Google Scholar] [CrossRef]
- Todorovic, M.; Perrin, D.M. Recent developments in catalytic amide bond formation. Peptide Sci. 2020, 112, e24210. [Google Scholar] [CrossRef]
- Jaradat, D.S.M. Thirteen decades of peptide synthesis: Key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 2018, 50, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Silva, A.M.; Marques, M.M.B. Sustainable amidation reactions—Recent advances. Eur. J. Org. Chem. 2020, 2020, 2501–2516. [Google Scholar] [CrossRef]
- Fattahi, N.; Ayubi, M.; Ramazani, A. Amidation and esterification of carboxylic acids with amines and phenols by N,N′-diisopropylcarbodiimide: A new approach for amide and ester bond formation in water. Tetrahedron 2018, 74, 4351–4356. [Google Scholar] [CrossRef]
- Stöekigt, J.; Zenk, M. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z. Naturforsch. C 1975, 30, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Muroi, A.; Ishihara, A.; Tanaka, C.; Ishizuka, A.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana. Planta 2009, 230, 517. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rokhum, L. A microwave-assisted highly practical chemoselective esterification and amidation of carboxylic acids. RSC Adv. 2016, 6, 93729–93740. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Rutjes, F.P.; Mecinović, J. Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines. Chem. Commun. 2014, 50, 5763–5766. [Google Scholar] [CrossRef]
- Dalu, F.; Scorciapino, M.A.; Cara, C.; Luridiana, A.; Musinu, A.; Casu, M.; Secci, F.; Cannas, C. A catalyst-free, waste-less ethanol-based solvothermal synthesis of amides. Green Chem. 2018, 20, 375–381. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Nguyen, D.T.; Mecinović, J. Zirconium-catalyzed direct amide bond formation between carboxylic esters and amines. Tetrahedron 2015, 71, 5547–5553. [Google Scholar] [CrossRef]
- Pihlava, J.-M. Identification of hordatines and other phenolamides in barley (Hordeum vulgare) and beer by UPLC-QTOF-MS. J. Cereal Sci. 2014, 60, 645–652. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Sunnerheim, K.; Sundberg, B.; Walsh, K. Stability of oat avenanthramides. Cereal Chem. 2001, 78, 278–281. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, Y.; Jang, H.-J.; Oh, H.-M.; Lim, C.-H.; Lee, S.W.; Rho, M.-C. Study of the UV light conversion of feruloyl amides from Portulaca oleracea and their inhibitory effect on IL-6-induced STAT3 activation. Molecules 2016, 21, 865. [Google Scholar] [CrossRef]
| Compound | Product | Name | Purity (%) a | Conversion (%) b | Yield (mg,%) c |
|---|---|---|---|---|---|
| 1 | 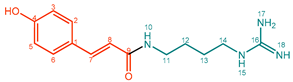 | Coumaroylagmatine (CouAgm) | 99% | 26% | 15.5 mg, 14% |
| 2 | 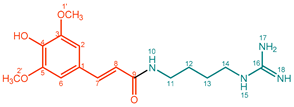 | Sinapoylagmatine (SinAgm) | 99% | 9% | 18.8 mg, 15% |
| 3 | 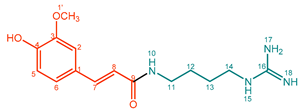 | Feruloylagmatine (FerAgm) | 95% | 31% | 19.4 mg, 19% |
| 4 |  | Feruloyl anthranilate (FerAnt1) d | 91% | 22% | 26.3 mg, 24% |
| 5 | 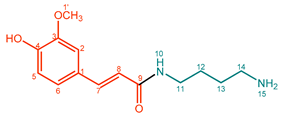 | Feruloylputrescine (FerPut) | 92% | 33% | 21.9 mg, 20% |
| 6 | 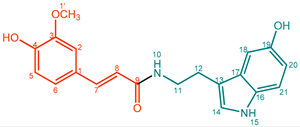 | Feruloylserotonin (FerSrt) | 77% | 44% | 26.7 mg, 21% |
| 7 |  | Feruloyltyramine (FerTrm) | 86% | 53% | 24.5 mg, 21% |
| 8 | 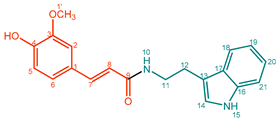 | Feruloyltryptamine (FerTry) | 88% | 20% | 23.1 mg, 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Zadelhoff, A.; Vincken, J.-P.; de Bruijn, W.J.C. Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides. Molecules 2022, 27, 2203. https://doi.org/10.3390/molecules27072203
van Zadelhoff A, Vincken J-P, de Bruijn WJC. Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides. Molecules. 2022; 27(7):2203. https://doi.org/10.3390/molecules27072203
Chicago/Turabian Stylevan Zadelhoff, Annemiek, Jean-Paul Vincken, and Wouter J. C. de Bruijn. 2022. "Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides" Molecules 27, no. 7: 2203. https://doi.org/10.3390/molecules27072203
APA Stylevan Zadelhoff, A., Vincken, J.-P., & de Bruijn, W. J. C. (2022). Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides. Molecules, 27(7), 2203. https://doi.org/10.3390/molecules27072203






