Flavanone Glycosides, Triterpenes, Volatile Compounds and Antimicrobial Activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae)
Abstract
:1. Introduction
2. Results
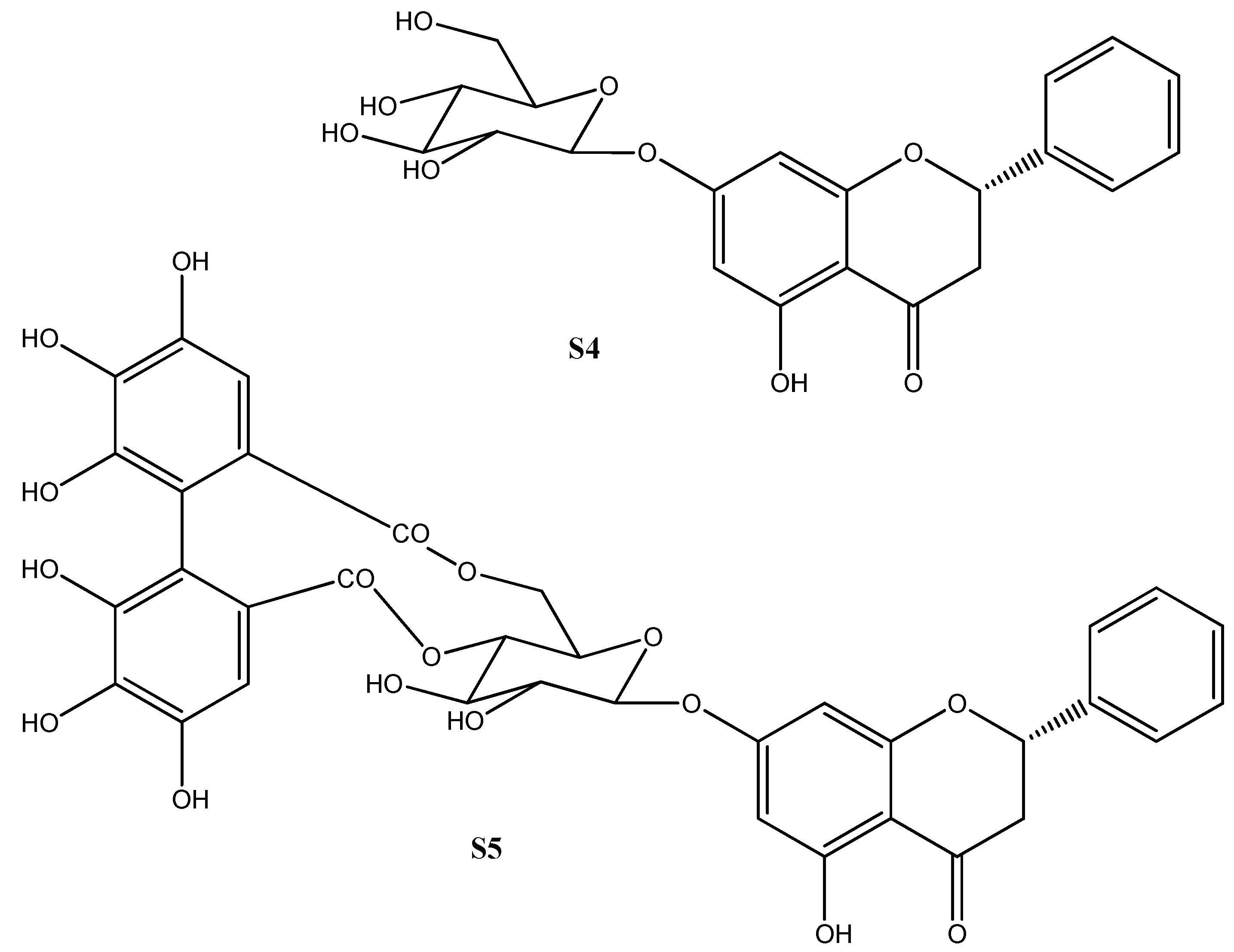
2.1. Non-Volatiles
2.2. Volatile Compounds
2.3. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction, Isolation, and Identification of the Non-Volatile Compounds
3.3. Extraction of the Essential Oils
Analysis of the Essential Oils
3.4. Antimicrobial Analysis
3.4.1. Well Diffusion Test
3.4.2. Minimum Inhibitory Concentration (MIC) and Minimal Microbicidal Concentration (MMC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clausing, G.; Renner, S.S. Molecular Phylogenetics of Melastomataceae and Memecylaceae: Implications for Character Evolution. Am. J. Bot. 2001, 88, 486–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, R.; Almeda, F.; Caddah, M.K.; Martins, A.B.; Meirelles, J.; Michelangeli, F.A.; Weiss, M. Nomenclator Botanicus for the neotropical genus Miconia (Melastomataceae: Miconieae). Phytotaxa 2013, 106, 1–171. [Google Scholar] [CrossRef] [Green Version]
- Baumgratz, J.F.A.; Caddah, M.K.; Chiavegatto, B.; Goldenberg, R.; Guimarães, P.J.F.; Koschnitzke, C.; Kriebel, R.; Lima, L.F.G.; Martins, A.B.; Michelangeli, F.A.; et al. Melastomataceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Disponivel em. Available online: http://Floradobrasil.Jbrj.Gov.Br/Jabot/Floradobrasil/Fb161 (accessed on 29 October 2021).
- Gontijo, D.C.; Gontijo, P.C.; Brandão, G.C.; Diaz, M.A.N.; de Oliveira, A.B.; Fietto, L.G.; Leite, J.P.V. Antioxidant study indicative of antibacterial and antimutagenic activities of an ellagitannin-rich aqueous extract from the leaves of Miconia latecrenata. J. Ethnopharmacol. 2019, 236, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Hoene, F.C. Plantas E Substâncias Vegetais Tóxicas E Medicinais; Graphiears: São Paulo, Brazil, 1939. [Google Scholar]
- Serpeloni, J.M.; Barcelos, G.R.M.; Mori, M.P.; Yanagui, K.; Vilegas, W.; Varanda, E.A.; Cólus, I.M.S. Cytotoxic and mutagenic evaluation of extracts from plant species of the Miconia genus and their influence on doxorubicin-induced mutagenicity: An in vitro analysis. Exp. Toxicol. Pathol. 2011, 63, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Gandhi, S.R.; Passos, F.R.S.; Heimfarth, L.; Pereira, E.W.M.; Monteiro, B.S.; Dos Santos, K.S.; Duarte, M.C.; Abreu, L.S.; Nascimento, Y.M.; et al. Dereplication and quantification of the ethanol extract of Miconia albicans (Melastomaceae) by Hplc-Dad-Esi-/Ms/Ms, and assessment of its anti-hyperalgesic and anti-inflammatory profiles in a mice arthritis-like model: Evidence for involvement of TNF-α, IL-1β and IL-6. J. Ethnopharmacol. 2020, 258, 112938. [Google Scholar] [CrossRef]
- Viegas, F.P.D.; de Castro, A.T.; Castro, A.P.; Siqueira, í; Rosa, W.; Espuri, P.F.; Coelho, L.F.L.; Marques, M.J.; Soares, M.G. In vitro schistosomicidal activity of the crude extract, fractions and Primin, the major active benzoquinone constituent from the leaves of Miconia willdenowii (Melastomaceae). S. Afr. J. Bot. 2017, 111, 365–370. [Google Scholar] [CrossRef]
- Viegas, F.P.D.; Espuri, P.F.; Oliver, J.C.; Silva, N.C.; Dias, A.L.T.; Marques, M.J.; Soares, M.G. Leishmanicidal and antimicrobial activity of primin and primin-containing extracts from Miconia willdenowii. Fitoterapia 2019, 138, 104297. [Google Scholar] [CrossRef]
- Cunha, W.R.; Crevelin, E.J.; Arantes, G.M.; Crotti, M.; Silva, M.L.A.; Furtado, N.A.J.C.; Sérgio, A.; Ferreira, D.S. A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phyther. Res. 2006, 20, 474–478. [Google Scholar] [CrossRef]
- Tarawneh, A.H.; León, F.; Ibrahim, M.A.; Pettaway, S.; Mccurdy, C.R.; Cutler, S.J. Flavanones from Miconia prasina. Phytochem. Lett. 2014, 7, 130–132. [Google Scholar] [CrossRef] [Green Version]
- Lima, T.C.; Matos, S.S.; Carvalho, T.F.; Silveira-Filho, A.J.; Couto, L.P.S.M.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Silva, A.M.O.; Heimfarth, L.; Passos, F.R.S.; et al. Evidence for the Involvement of Il-1β And Tnf-A in anti-inflammatory effect and antioxidative stress profile of the standardized dried extract from Miconia albicans Sw. (Triana) Leaves (Melastomataceae). J. Ethnopharmacol. 2020, 259, 112908. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Maia, J.G.S. Aroma de Flores na Amazônia; Museu Para: Belém, Brazil, 2000. [Google Scholar]
- Gatis-Carrazzoni, A.S.S.G.; Mota, F.V.B.; Leite, T.C.C.; de Oliveira, T.B.; da Silva, S.C.; Bastos, I.V.A.; de Souza Maia, M.B.; Pereira, P.S.; Neto, P.P.M.; de Oliveira Chagas, E.C.; et al. Anti-Inflammatory and Antinociceptive Activities of the Leaf Methanol Extract of Miconia minutiflora (Bonpl.) Dc. and Characterization of Compounds by Uplc-Dad-Qtof-Ms/Ms. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 55–68. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.A.F.; da Silva, M.A.P.; Bezerra, J.W.A.; dos Santos, A.C.B.; Alencar, S.R.; Barbosa, E.A. Atividades biológicas de Miconia spp. Ruiz & Pavon (Melastomataceae Juss.). Gaia Sci. 2017, 11, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Breitmaier, E.; Voelter, W. Carbon-13 Nmr Spectroscopy, 3rd ed.; Vch: Weinhein, Germany, 1987. [Google Scholar]
- Ahmad, V.U.; Rahman, A.U. Handbook of Natural Products Data: Pentacyclic Triterpenoids; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Miranda, M.L.D.; Souza, A.F.; Rodrigues, E.D.; Garcez, F.R.; Garcez, W.S. Constituintes químicos das folhas de riedeliella graciliflora Harms (Leguminosae). Quim. Nova 2012, 35, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and alpha-Amyrin acetate isolated from the stem bark of alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [Green Version]
- Di Pietro, M.E.; Mannu, A.; Mele, A. Nmr Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, X.D.; Xu, L.Z.; Zou, Z.M.; Yang, S.L. A new sterol glycoside from securidaca inappendiculata. J. Asian Nat. Prod. Res. 2005, 7, 649–653. [Google Scholar] [CrossRef]
- Forgo, P.; Kövér, K.E. Gradient enhanced selective experiments in the 1h nmr chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef]
- Nes, W.D.; Norton, R.A.; Benson, M. Carbon-13 Nmr studies on sitosterol biosynthesized from [13c]Mevalonates. Phytochemistry 1992, 31, 805–811. [Google Scholar] [CrossRef]
- Thang, P.T.; Dung, N.A.; Giap, T.H.; Oanh, V.T.K.; Hang, N.T.M.; Huong, T.T.; Thanh, L.N.; Huong, D.T.M.; Van Cuong, P. Preliminary study on the chemical constituents of the leaves of macaranga balansae gagnep. Vietnam J. Chem. 2018, 56, 632–636. [Google Scholar] [CrossRef]
- Hammami, S.; Jannet, H.B.; Bergaoui, A.; Ciavatta, L.; Cimino, G.; Mighri, Z. Isolation and structure elucidation of a flavanone, a flavanone glycoside and vomifoliol from echiochilon fruticosum growing in Tunisia. Molecules 2004, 9, 602–608. [Google Scholar] [CrossRef]
- Hegde, V.R.; Pu, H.; Patel, M.; Das, P.R.; Butkiewicz, N.; Arreaza, G.; Gullo, V.P.; Chan, T.M. Two antiviral compounds from the plant stylogne cauliflora as inhibitors of Hcv Ns3 protease. Bioorg. Med. Chem. Lett. 2003, 13, 2925–2928. [Google Scholar] [CrossRef]
- Taketa, A.T.C.; Breitmaier, E.; Schenkel, E.P. Triterpenes and triterpenoidal glycosides from the fruits of ilex paraguariensis (Maté). J. Braz. Chem. Soc. 2004, 15, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Barroso, P.R.; Otoni, T.J.O.; Mendes, J.P.G.; Machado, E.L.M.; Martins, H.R.; Gregorio, L.E. Analysis of volatiles from aerial parts of miconia ferruginata by Hs-Spme And Gc-Ms. Chem. Nat. Compd. 2017, 53, 167–168. [Google Scholar] [CrossRef]
- Rodrigues, J.; Michelin, D.C.; Rinaldo, D.; Zocolo, G.J.; Dos Santos, L.C.; Vilegas, W.; Salgado, H.R.N. Antimicrobial activity of Miconia Species (Melastomataceae). J. Med. Food 2008, 11, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Celotto, A.C.; Nazario, D.Z.; Spessoto, M.A.; Martins, C.H.G.; Cunha, W.R. Evaluation of the in vitro antimicrobial activity of crude extracts of three Miconia species. Braz. J. Microbiol. 2003, 34, 339–340. [Google Scholar] [CrossRef] [Green Version]
- Cunha, G.O.S.; Terezan, A.P.; Matos, A.P.; Burger, M.C.M.; Vieira, P.C.; Fernandes, J.B.; Da Silva, M.F.G.; Menezes, A.C.S. Antimicrobial activity of isolated compounds and semisynthetic derivatives from Miconia ferruginata. Acta Bras. 2020, 4, 49–52. [Google Scholar] [CrossRef]
- Chen, C.; Wan, C.; Peng, X.; Chen, J. A Flavonone pinocembroside inhibits penicillium italicum growth and blue mold development in ‘newhall’ navel oranges by targeting membrane damage mechanism. Pestic. Biochem. Physiol. 2019, 165, 104505. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Uhplc-Q-Tof/Ms-Based Metabolomics approach reveals the antifungal potential of pinocembroside against citrus green mold phytopathogen. Plants 2020, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of gallic acid against food-related pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Shin, D.W.; Kwon, D.Y. Antibacterial activity of methyl gallate isolated from galla rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef]
- Serna, D.M.O.; Martínez, J.H.I. Phenolics and polyphenolics from melastomataceae species. Molecules 2015, 20, 17818–17847. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Jin, Z.; Dong, J.; Wang, Z.; Jiang, K.; Ye, Y.; Dou, X.; Ding, B. Bioactivity evaluation of pinocembrin derivatives from Penthorum chinense pursh stems. Nat. Prod. Commun. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Silva, S.G.; Cruz, J.N.; Santana De Oliveira, M.; Oliveira, J.; De Moraes, A.A.B.; Da Costa, F.A.M.; Da Costa, K.S.; Diniz Do Nascimento, L.; De Helena Aguiar Andrade, E. First Report on the Annona exsucca dc. essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; Neves Da Cruz, J.; De Jesus Pereira Franco, C.; Silva, S.G.; Da Costa, W.A.; De Oliveira, M.S.; De Aguiar Andrade, E.H. First report on yield and chemical composition of essential oil extracted from Myrcia eximia dc (myrtaceae) from the Brazilian amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, K.D.S.M.; Feitosa, B.D.S.; Cruz, J.N.; Ferreira, O.O.; Franco, C.D.J.P.; Cascaes, M.M.; Oliveira, M.S.D.; Andrade, E.H.D.A. Chemical composition and preliminary toxicity evaluation of the essential oil from Peperomia circinnata link var. circinnata. (Piperaceae) in Artemia salina leach. Molecules 2021, 26, 7359. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Venturieri, G.C.; Guilhon, G.M.S.P.; Souza Filho, A.P.D.S.; Andrade, E.H.D.A. Chemical Composition of volatile compounds in apis mellifera propolis from the Northeast region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Adams, R.P., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. The Nist Mass Spectral Search Program for the Nist/Epa/Nih Mass Spectra Library. In Standard Reference Data Program of the National Institute of Standards and Technology; National Institute of Standards and Technology: Gaithers-Burg, MD, USA, 2011. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 8th ed.; Clinical and Laboratory Standards Institute: Mayne, BC, Canada, 2003. [Google Scholar]
- Cascaes, M.M.; Guilhon, G.M.S.P.; Zoghbi, M.D.G.; Andrade, E.H.A.; Santos, L.S.; Da Silva, J.K.R.; Trovatti Uetanabaro, A.P.; Araújo, I.S. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh Leaves (myrtaceae). Nat. Prod. Res. 2019, 35, 1717–1721. [Google Scholar] [CrossRef]
- Peixoto, R.N.S.; Guilhon, G.M.S.P.; Das Graças, B.; Zoghbi, M.; Araújo, I.S.; Uetanabaro, A.P.T.; Santos, L.S.; Brasil, D.D.S.B. Volatiles, a glutarimide alkaloid and antimicrobial effects of Croton pullei (Euphorbiaceae). Molecules 2013, 18, 3195–3205. [Google Scholar] [CrossRef] [Green Version]
| RIL | RIC | Constituents | Leaf | Branch-1 | Branch-2 |
|---|---|---|---|---|---|
| 801 | 799 | Hexanal | 0.55 | 0.03 | 0.14 |
| 846 | 846 | (2E)-Hexenal | 0.38 | 0.04 | |
| 850 | 851 | (3Z)-Hexenol | 9.73 | 0.03 | 0.1 |
| 854 | 857 | (2E)-Hexenol | 0.77 | ||
| 863 | 864 | Hexanol | 1.39 | 0.1 | |
| 907 | 908 | (2E,4E)-Hexadienal | 0.52 | ||
| 947 | 914 | (2E)-Heptenal | 0.15 | ||
| 952 | 953 | Benzaldehyde | 0.33 | 0.02 | 0.05 |
| 959 | 960 | Heptanol | 0.25 | ||
| 974 | 974 | 1-Octen-3-ol | 8.16 | 0.15 | |
| 981 | 981 | 6-methyl-5-Hepten-2-one | 0.27 | ||
| 984 | 985 | 3-p-Menthene | 0.29 | ||
| 989 | 990 | 6-methyl-5-Hepten-2-ol | 0.28 | 0.05 | |
| 1005 | 1005 | (2E,4E)-Heptadienal | 0.32 | ||
| 1004 | 1008 | (3Z)-Hexenylacetate | 0.54 | ||
| 1024 | 1026 | Limonene | 0.25 | 0.05 | 0.07 |
| 1036 | 1038 | Benzeneacetaldehyde | 0.07 | 0.09 | |
| 1044 | 1044 | (E)-β-Ocimene | 0.35 | ||
| 1063 | 1063 | n-Octanol | 0.23 | ||
| 1086 | 1086 | Terpinolene | 0.14 | ||
| 1089 | 1090 | p-Cymenene | 0.28 | ||
| 1095 | 1096 | Linalool | 1.19 | ||
| 1100 | 1100 | Nonanal | 0.25 | 0.1 | 0.15 |
| 1102 | 1115 | (2E,4E)-Octadienal | 0.07 | ||
| 1142 | 1142 | (3Z)-Hexenylisobutanoate | 0.75 | ||
| 1152 | 1152 | (3Z)-Nonen-1-ol | 0.44 | ||
| 1157 | 1157 | (2E)-Nonen-1-al | 0.15 | 0.11 | 0.12 |
| 1184 | 1183 | (3Z)-Hexenylbutanoate | 9.77 | ||
| 1191 | 1186 | Hexylbutanoate | 0.37 | 0.05 | |
| 1186 | 1188 | α-Terpineol | 0.72 | 0.13 | |
| 1196 | 1196 | Safranal | 0.17 | ||
| 1201 | 1202 | Decanal | 0.13 | ||
| 1210 | 1210 | (2E,4E)-Nonadienal | 0.05 | ||
| 1232 | 1226 | cis-3-Hexenyl isovalerate | 8.65 | ||
| 1249 | 1248 | Geraniol | 0.13 | ||
| 1260 | 1260 | (2E)-Decenal | 0.25 | 0.05 | |
| 1279 | 1275 | cis-3-hexenyl valerate | 0.26 | ||
| 1285 | 1286 | Safrole | 0.18 | 0.09 | |
| 1293 | 1294 | 2-Undecanone | 0.28 | ||
| 1315 | 1315 | (2E,4E)-Decadienal | 0.27 | 0.08 | |
| 1330 | 1330 | Hexyl tiglate | 0.26 | ||
| 1364 | 1365 | Decanoic acid | 0.11 | ||
| 1374 | 1374 | α-Copaene | 0.97 | ||
| 1383 | 1382 | (E)-β-Damascenone | 0.57 | ||
| 1378 | 1385 | (3Z)-Hexenyl hexanoate | 10.91 | ||
| 1417 | 1417 | (E)-Caryophyllene | 2.66 | ||
| 1428 | 1428 | (E)-α-Ionone | 0.78 | ||
| 1432 | 1432 | trans-α-Bergamotene | 0.19 | ||
| 1453 | 1453 | Geranyl acetone | 0.72 | 0.05 | |
| 1452 | 1454 | α-Humulene | 0.98 | ||
| 1487 | 1488 | (E)-β-Ionone | 1.89 | 0.05 | |
| 1495 | 1495 | 2-Tridecanone | 0.34 | ||
| 1505 | 1505 | (E,E)-α-Farnesene | 0.36 | ||
| 1514 | 1515 | β-Curcumene | 1.28 | ||
| 1561 | 1562 | E-Nerolidol | 0.69 | ||
| 1565 | 1566 | Dodecanoic acid | 0.91 | 2.3 | 6.36 |
| 1565 | 1568 | (3Z)-Hexenyl benzoate | 1.93 | ||
| 1594 | 1595 | Ethyl dodecanoate | 0.31 | ||
| 1722 | 1725 | Tetradecanoic acid | 0.59 | 2.6 | 4.48 |
| 1946 | 1940 | Isophytol | 0.34 | 0.29 | |
| 1942 | 1941 | Phytol | 7.34 | 1.41 | |
| 1959 | 1959 | n-Hexadecanoic acid | 1.83 | 32.65 | 42.75 |
| 2029 | 2117 | (9Z,12Z)-Octadecadienoic acid | 48.71 | 35.65 | |
| 2124 | 2130 | Octadecanoic acid | 0.1 | 1.48 | |
| 2500 | 2501 | Pentacosane | 0.2 | 0.44 | |
| 2600 | 2603 | Hexacosane | 0.12 | 0.72 | 0.2 |
| 2700 | 2705 | Heptacosane | 0.12 | 0.38 | 0.09 |
| Total | 85.44 | 88.54 | 94.26 | ||
| Microorganism | MIC (mg·mL−1) | MMC (mg·mL−1) | Control Nist/Chlorf DMSO (mg·mL−1) | |
|---|---|---|---|---|
| Escherichia coli CCMB 261 | 0.625 | 1.25 | R | 5.00 |
| Pseudomonas aeruginosa CCMB 268 | 1.25 | 2.5 | 0.31 | 5.00 |
| Salmonella sp. CCMB 281 | 1.25 | 2.5 | 0.16 | 5.00 |
| Staphylococcus aureus CCMB 262 | 0.625 | 1.25 | 0.31 | 5.00 |
| S. aureus CCMB 263 | 0.625 | 1.25 | 0.31 | 10.00 |
| S. aureus CCMB 285 | 0.625 | 1.25 | R | 10.00 |
| Bacillus cereus CCMB 282 | 0.625 | 1.25 | 0.16 | 5.00 |
| Candida albicans CCMB 286 | 2.5 | 5 | 0.63 | 10.00 |
| C. albicans CCMB 266 | 2.5 | 5 | 0.08 | 10.00 |
| C. parapsilosis CCMB 288 | 5 | 10 | R | 10.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, N.S.; Cascaes, M.M.; da Silva Santos, L.; de Oliveira, M.S.; das Graças Bichara Zoghbi, M.; Araújo, I.S.; Uetanabaro, A.P.T.; de Aguiar Andrade, E.H.; Guilhon, G.M.S.P. Flavanone Glycosides, Triterpenes, Volatile Compounds and Antimicrobial Activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae). Molecules 2022, 27, 2005. https://doi.org/10.3390/molecules27062005
Ferreira NS, Cascaes MM, da Silva Santos L, de Oliveira MS, das Graças Bichara Zoghbi M, Araújo IS, Uetanabaro APT, de Aguiar Andrade EH, Guilhon GMSP. Flavanone Glycosides, Triterpenes, Volatile Compounds and Antimicrobial Activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae). Molecules. 2022; 27(6):2005. https://doi.org/10.3390/molecules27062005
Chicago/Turabian StyleFerreira, Nathália Siso, Márcia Moraes Cascaes, Lourivaldo da Silva Santos, Mozaniel Santana de Oliveira, Maria das Graças Bichara Zoghbi, Isabella Santos Araújo, Ana Paula Trovatti Uetanabaro, Eloisa Helena de Aguiar Andrade, and Giselle Maria Skelding Pinheiro Guilhon. 2022. "Flavanone Glycosides, Triterpenes, Volatile Compounds and Antimicrobial Activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae)" Molecules 27, no. 6: 2005. https://doi.org/10.3390/molecules27062005
APA StyleFerreira, N. S., Cascaes, M. M., da Silva Santos, L., de Oliveira, M. S., das Graças Bichara Zoghbi, M., Araújo, I. S., Uetanabaro, A. P. T., de Aguiar Andrade, E. H., & Guilhon, G. M. S. P. (2022). Flavanone Glycosides, Triterpenes, Volatile Compounds and Antimicrobial Activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae). Molecules, 27(6), 2005. https://doi.org/10.3390/molecules27062005








