Volatile Compound Characterization of Coffee (Coffea arabica) Processed at Different Fermentation Times Using SPME–GC–MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coffee Cherry Moisture
2.2. Dissolved Solids of Coffee Cherries
2.3. Coffee Cherry Color
2.4. Acidity and pH of Coffee Cherries
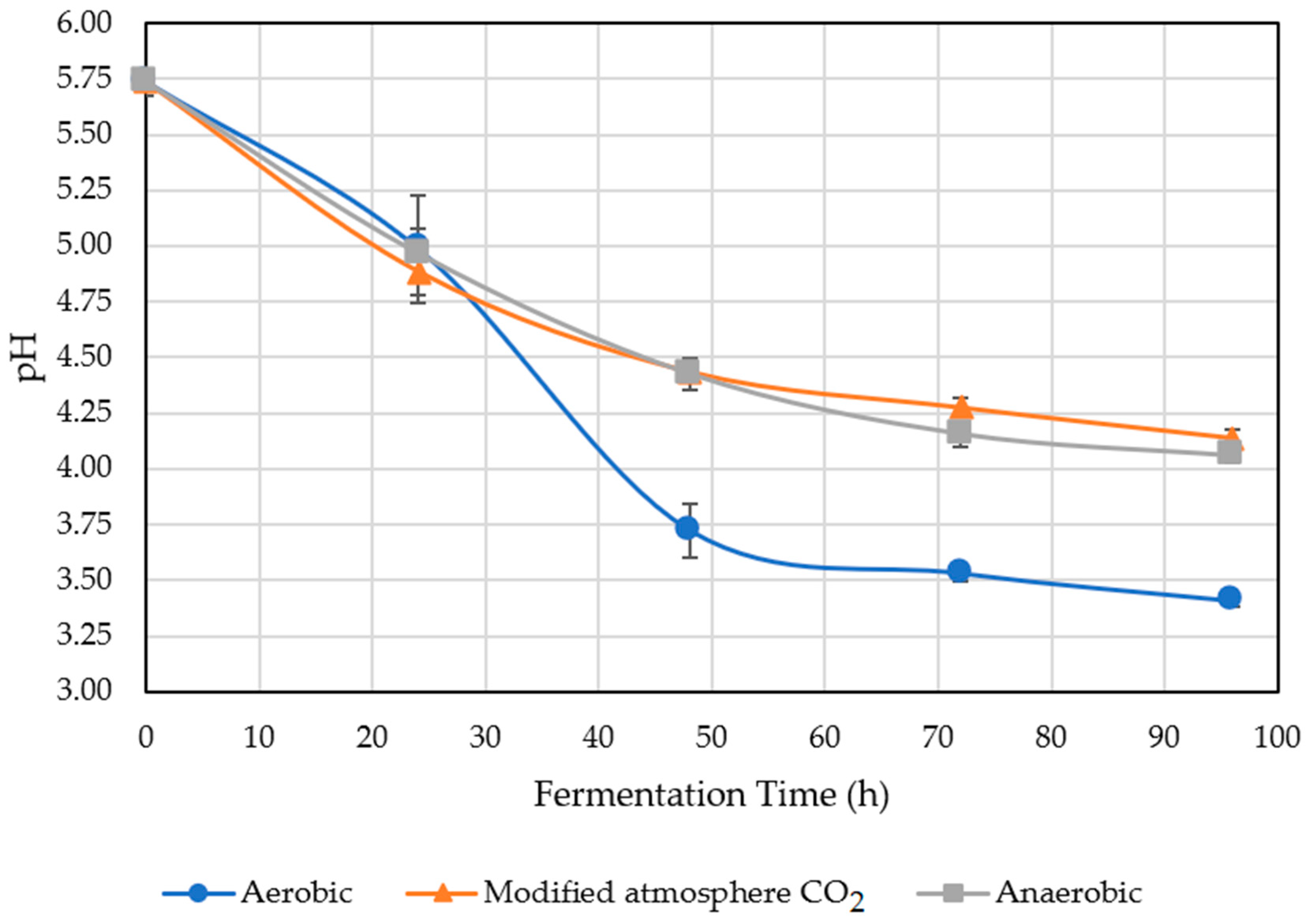
2.5. pH of Treated Coffee
2.6. Acidity of Treated Coffee
2.7. Correlation Analysis: Fermentation Time, pH, and Acidity
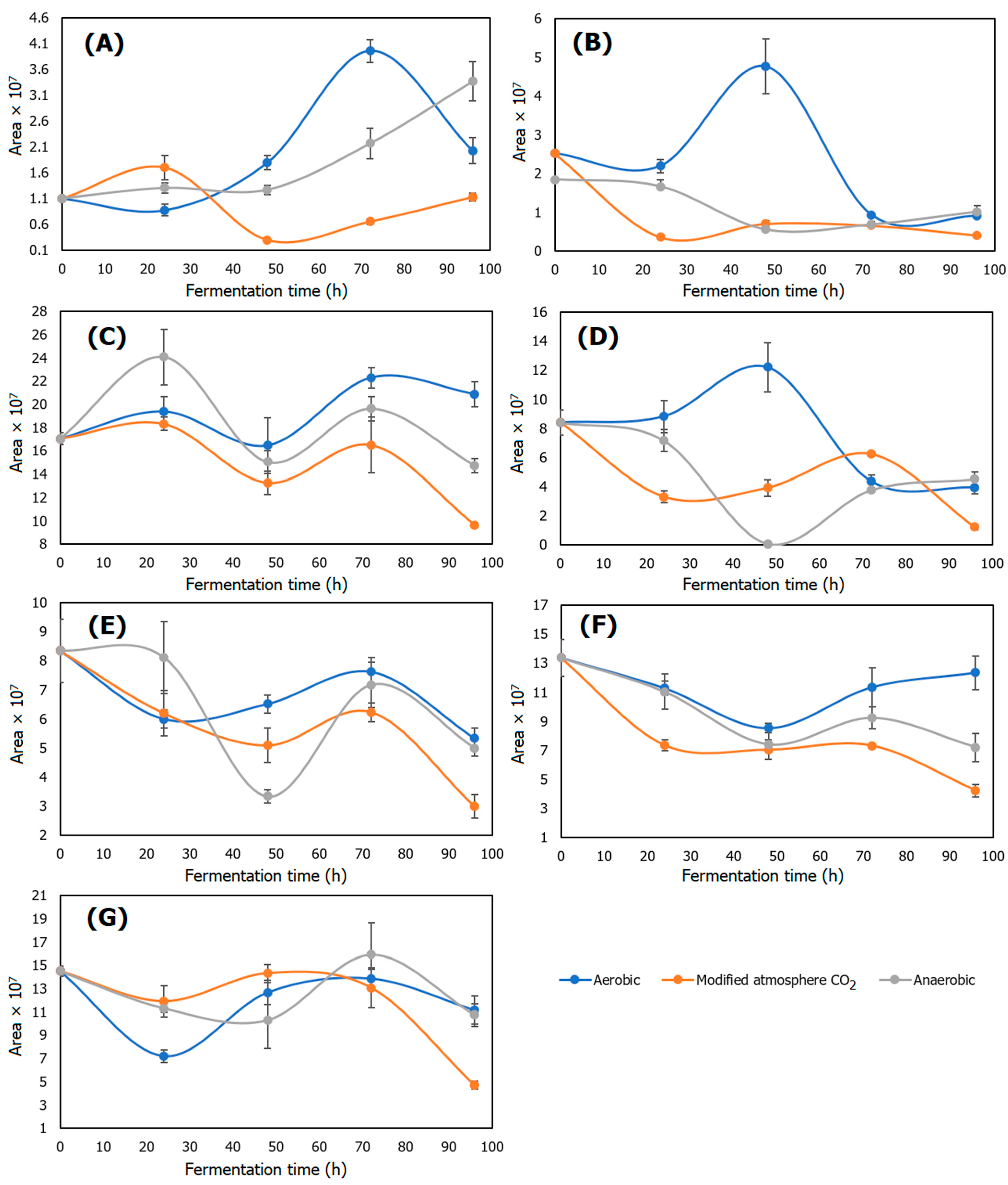
2.8. Volatile Compounds
2.8.1. Acetic Acid
2.8.2. 2-Methylpyrazine
2.8.3. 2-Furancarboxaldehyde
2.8.4. 2-Furanmethanol
2.8.5. 2,6-Dimethylpyrazine
2.8.6. 5-Methylfurfural
2.8.7. 2-Methoxy-4-Vinylphenol
2.9. Correlation Analysis: Volatile Compounds, pH, Acidity, and Fermentation Time
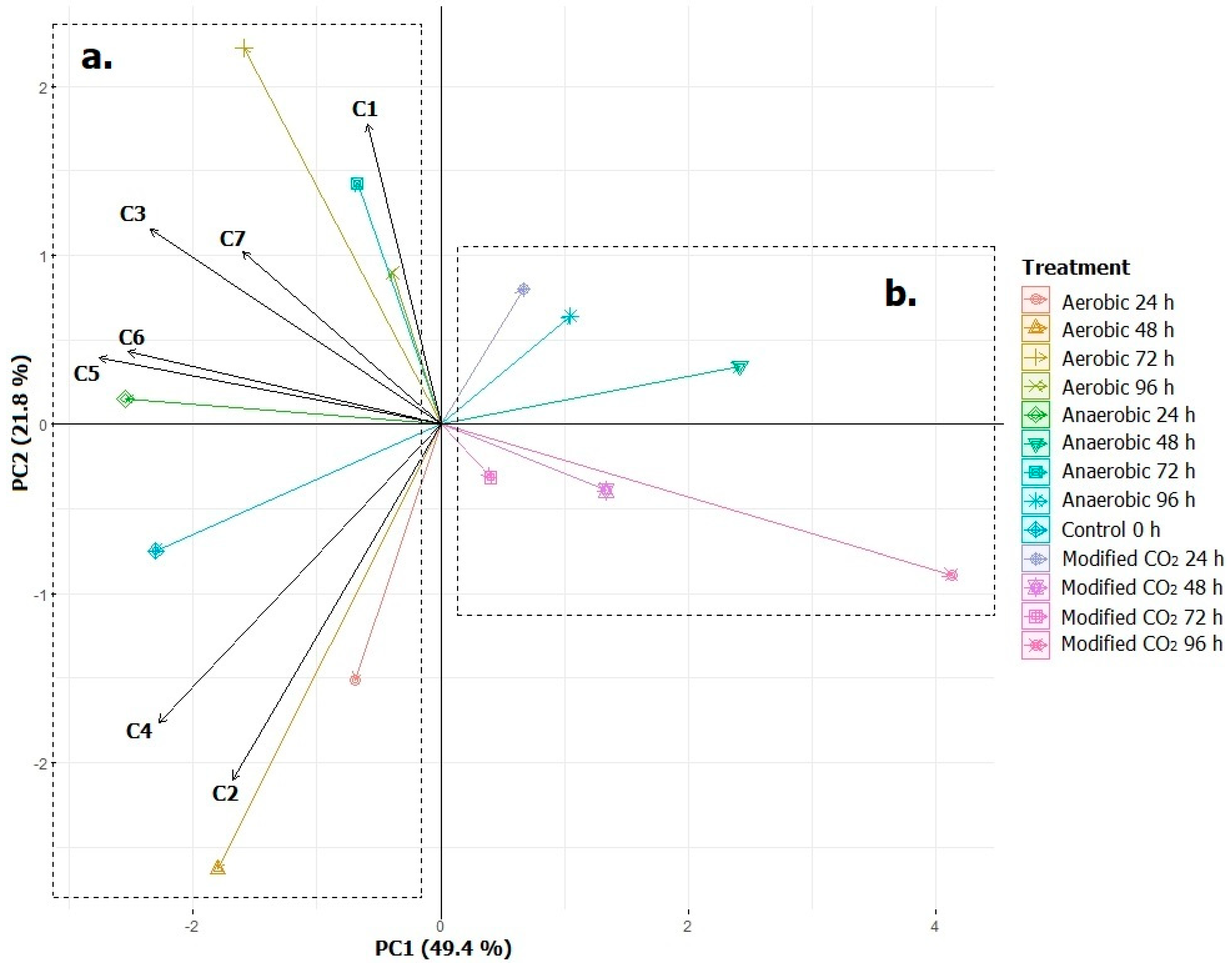
2.10. Principal Components Analysis (PCA)
3. Materials and Methods
3.1. Coffee Processing
3.2. Coffee Cherry Physicochemical Characterization
3.2.1. Moisture
3.2.2. Dissolved Solids
3.2.3. Color
3.2.4. pH and Acidity
3.3. Coffee Fermentation
3.3.1. Aerobic Dry Fermentation
3.3.2. Anaerobic Dry Fermentation
3.3.3. Dry Fermentation in an Atmosphere Modified with CO2
3.4. Coffee Drying, Threshing, and Roasting
3.5. Coffee Pulverization
3.6. Volatile Compounds
3.6.1. Solid Phase Microextraction (SPME)
3.6.2. Gas Chromatography–Mass Spectrometry (GC–MS)
- MS 60.0–61.0, 42.0–46.0: acetic acid;
- MS 94.0–97.0: 2-methylpyrazine and 2-furancarboxaldehyde;
- MS 98.0–99.0: 2-furanmethanol;
- MS 108.0–109.0: 2,6-dimethylpyrazine;
- MS 110.0–112.0: 5-methylfurfural;
- MS 149.0–151.0: 2-methoxy-4-vinylphenol.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abalo, R. Coffee and caffeine consumption for human health. Nutrients 2021, 13, 2918. [Google Scholar] [CrossRef] [PubMed]
- Seninde, D.R.; Chambers, E. Coffee flavor: A review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; Silva, C.F.; da Miguel, M.G.P.C.; de Cordeiro, C.S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef] [Green Version]
- da Mota, M.C.B.; Batista, N.N.; Rabelo, M.H.S.; Ribeiro, D.E.; Borém, F.M.; Schwan, R.F. Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res. Int. 2020, 136, 109482. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Córdoba Castro, N.M.; Guerrero Fajardo, J.E. Caracterización de los procesos tradicionales de fermentación de café en el departamento de Nariño. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 75–83. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Bodner, M.; Morozova, K.; Kruathongsri, P.; Thakeow, P.; Scampicchio, M. Effect of harvesting altitude, fermentation time and roasting degree on the aroma released by coffee powder monitored by proton transfer reaction mass spectrometry. Eur. Food Res. Technol. 2019, 245, 1499–1506. [Google Scholar] [CrossRef]
- Afriliana, A.; Pratiwi, D.; Giyarto, G.; Belgis, M.; Harada, H.; Yushiharu, M.; Taizo, M. Volatile compounds changes in unfermented robusta coffee by re-fermentation using commercial kefir. Nutr. Food Sci. Int. J. 2019, 8, 4–9. [Google Scholar] [CrossRef]
- Yu, J.-M.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of volatile compounds in coffee prepared by various brewing and roasting methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.; Bang, E.; Lee, S.; Rhee, J.K.; Na, Y.C. Comparative evaluation of flavor compounds in fermented green and roasted coffee beans by solid phase microextraction-gas chromatography/mass spectrometry. Flavour Fragr. J. 2019, 34, 365–376. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- de Silva, S.A.; de Queiroz, D.M.; de Pinto, F.A.C.; Santos, N.T. Coffee quality and its relationship with Brix degree and colorimetric information of coffee cherries. Precis. Agric. 2014, 15, 543–554. [Google Scholar] [CrossRef]
- Marín-López, S.M.; Arcila-Pulgarín, J.; Montoya-Restrepo, E.C.; Oliveros-Tascón, C.E. Cambios físicos y químicos durante la maduracíon del fruto de café (Coffea arabica L. var. Colombia). Cenifcafé 2003, 54, 208–225. [Google Scholar]
- Carvajal, J.; Aristizábal, I.; Oliveros, C.; Mejía, J. Colorimetría del fruto de café (Coffea arabica L.) durante su desarrollo y maduración. Rev. Fac. Nal. Agr. Medellín 2011, 64, 6229–6240. [Google Scholar]
- Martins, P.M.M.; Ribeiro, L.S.; da Miguel, M.G.C.P.; Evangelista, S.R.; Schwan, R.F. Production of coffee (Coffea arabica) inoculated with yeasts: Impact on quality. J. Sci. Food Agric. 2019, 99, 5638–5645. [Google Scholar] [CrossRef]
- Silva, P.A.; Rabelo, V.M.; Maria Reis Calixto, J.; De Oliveira Coelho, P.; Rocha De Carvalho Gorski, I. Quality assessment of coffee grown in Campos Gerais, Minas Gerais State, Brazil. Acta Sci. Technol. 2014, 36, 739–744. [Google Scholar] [CrossRef]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- da Silva, B.L.; Pereira, P.V.; Bertoli, L.D.; Silveira, D.L.; Batista, N.N.; Pinheiro, P.F.; de Souza Carneiro, J.; Schwan, R.F.; da Silva, S.A.; Coelho, J.M.; et al. Fermentation of Coffea canephora inoculated with yeasts: Microbiological, chemical, and sensory characteristics. Food Microbiol. 2021, 98, 103786. [Google Scholar] [CrossRef]
- Farah, A. Coffee constituents. Coffee Emerg. Health Eff. Dis. Prev. 2012, 2, 21–58. [Google Scholar] [CrossRef]
- Jackels, S.C.; Jackels, C.F. Characterization of the coffee mucilage fermentation process using chemical indicators: A field study in Nicaragua. J. Food Sci. 2005, 70, C321–C325. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Ijaz, M.U.; Ullah, S.; Pasha, I.; Suleria, H.A.R. Farm to consumer: Factors affecting the organoleptic characteristics of coffee. II: Postharvest processing factors. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1184–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L.L.; Guarçoni, R.C.; Moreli, A.P.; Pinheiro, P.F.; Pinheiro, C.A.; Moreira, T.R.; da Siqueira, E.A.; Ten Caten, C.S. Physicochemical parameters of arabica fermented coffee in different altitudes. Coffee Sci. 2021, 16, e16187. [Google Scholar] [CrossRef]
- Puerta, G.I.; Ríos-Arias, S. Composición química del mucílago de café, según el tiempo de fermentación y refrigeración. Cenicafé 2011, 62, 23–40. [Google Scholar]
- Korhoňová, M.; Hron, K.; Klimčíková, D.; Müller, L.; Bednář, P.; Barták, P. Coffee aroma—Statistical analysis of compositional data. Talanta 2009, 80, 710–715. [Google Scholar] [CrossRef]
- Vasanthy, M.; Ravindran, B.; Chung, W.J.; Chang, S.W. Treatment of coffee cherry pulping wastewater by using lectin protein isolated from Ricinus communis L. seed. J. Water Process Eng. 2021, 39, 101742. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef]
- Thammarat, P.; Kulsing, C.; Wongravee, K.; Leepipatpiboon, N.; Nhujak, T. Identification of volatile compounds and selection of discriminant markers for elephant dung coffee using static headspace gas chromatography—Mass spectrometry and chemometrics. Molecules 2018, 23, 1910. [Google Scholar] [CrossRef] [Green Version]
- Laukaleja, I.; Kruma, Z. Phenolic and volatile compound composition influence to specialty coffee cup quality. Agron. Res. 2019, 17, 1367–1379. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Schwan, R.; Silva, C.; Batista, L. Coffee Fermentation. In Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 677–690. [Google Scholar]
- Bressani, A.P.P.; Batista, N.N.; Ferreira, G.; Martinez, S.J.; Simão, J.B.P.; Dias, D.R.; Schwan, R.F. Characterization of bioactive, chemical, and sensory compounds from fermented coffees with different yeasts species. Food Res. Int. 2021, 150, 110755. [Google Scholar] [CrossRef] [PubMed]
- Puerta, G.I. Factores, procesos y controles en la fermentacion del cafe. Av. Técn. Cenicafé 2012, 1, 1–12. [Google Scholar]
- Wamuyu, K.A.; Richard, K.; Beatrice, M.; Cecilia, K. Effect of different fermentation methods on physicochemical composition and sensory quality of coffee (Coffea arabica). IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 31–36. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the impacts of postharvest processing on the microbiota and metabolite profiles during green coffee bean production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puerta, G.I. Fundamentos del proceso de fermentacion en el beneficio de productos como el cafe. Fed. Nac. Cafe. Colomb. 2010, 1, 1–12. [Google Scholar]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; da Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The crucial role of yeasts in the wet fermentation of coffee beans and quality. Int. J. Food Microbiol. 2020, 333, 108796. [Google Scholar] [CrossRef]
- Avallone, S.; Guyot, B.; Brillouet, J.-M.; Olguin, E.; Guiraud, J.-P. Microbiological and biochemical study of coffee fermentation. Curr. Microbiol. 2001, 42, 252–256. [Google Scholar] [CrossRef]
- Chaichi, M.; Ghasemzadeh-Mohammadi, V.; Hashemi, M.; Mohammadi, A. Furanic compounds and furfural in different coffee products by headspace liquid-phase micro-extraction followed by gas chromatography–mass spectrometry: Survey and effect of brewing procedures. Food Addit. Contam. Part B Surveill. 2015, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. CyTA J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single origin coffee aroma: From optimized flavor protocols and coffee customization to instrumental volatile characterization and chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef] [PubMed]
- Kipkorir, R.; Muhoho, S.; Muliro, P.; Mugendi, B.; Frohme, M.; Broedel, O. Effects of coffee processing technologies on aroma profiles and sensory quality of Ruiru 11 and SL 28 Kenyan coffee varieties. Asian J. Agric. Food Sci. 2015, 3, 178–188. [Google Scholar]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the aroma profile and main key odorants of espresso coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Prakash, I.; Kumar, P.; Om, H.; Basavaraj, K.; Murthy, P.S. Metabolomics and volatile fingerprint of yeast fermented robusta coffee: A value added coffee. LWT 2022, 154, 112717. [Google Scholar] [CrossRef]
- Yu, A.-N.; Zhang, A.-D. The effect of pH on the formation of aroma compounds produced by heating a model system containing L-ascorbic acid with L-threonine/L-serine. Food Chem. 2010, 119, 214–219. [Google Scholar] [CrossRef]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT 2020, 118, 108718. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction–gas chromatographic–time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef]
- Bicchi, C.P.; Panero, O.M.; Pellegrino, G.M.; Vanni, A.C. Characterization of roasted coffee and coffee beverages by solid phase microextraction−gas chromatography and principal component analysis. J. Agric. Food Chem. 1997, 45, 4680–4686. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Vargas, L.F. Evaluation of SDE, SFE and SPME/Gc-Ms for extraction and determination of aroma compounds from Vilcabamba-Ecuadorian roasted coffee. Quím. Nova 2016, 39, 712–719. [Google Scholar] [CrossRef]


| Compound | TR (min) | KI | KIR | Aroma and Taste Description |
|---|---|---|---|---|
| Acetic acid | 3.11 ± 0.04 | 602.90 | 605 | Acid [27], bitter [28], vinegar [6] |
| 2-methylpyrazine | 9.55 ± 0.1 | 878.18 | 876 | Nut [6,20,29], chocolate [28] |
| 2-furancarboxaldehyde | 10.14 ± 0.1 | 892.89 | 851 | Floral [30] |
| 2-furanmethanol | 11.11 ± 0.6 | 915.31 | 891 | Candy [20], burnt [28] |
| 2,6-dimethylpyrazine | 14.20 ± 0.1 | 972.52 | 930 | Cocoa [20], chocolate [29] |
| 5-methylfurfural | 17.01 ± 0.1 | 1028.15 | 982 | Spicy [20], candy [29] |
| 2-methoxy-4-vinylphenol | 34.48 ± 0.04 | 1390.26 | 1330 | Clove [20], spicy [30] |
| Variable | Time | pH | Acidity |
|---|---|---|---|
| Time | 1 | −0.83 *** | 0.90 *** |
| pH | −0.83 *** | 1 | −0.92 *** |
| Acidity | 0.90 *** | −0.92 *** | 1 |
| C1 | 0.41 ** | −0.62 *** | 0.59 *** |
| C2 | −0.33 * | 0.03 ns | −0.25 ns |
| C3 | −0.20 ns | −0.02 ns | −0.04 ns |
| C4 | −0.40 * | 0.16 ns | −0.34 * |
| C5 | −0.49 ** | 0.24 ns | −0.21 ns |
| C6 | −0.37 * | 0.11 ns | −0.14 ns |
| C7 | −0.13 ns | −0.05 ns | 0.19 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galarza, G.; Figueroa, J.G. Volatile Compound Characterization of Coffee (Coffea arabica) Processed at Different Fermentation Times Using SPME–GC–MS. Molecules 2022, 27, 2004. https://doi.org/10.3390/molecules27062004
Galarza G, Figueroa JG. Volatile Compound Characterization of Coffee (Coffea arabica) Processed at Different Fermentation Times Using SPME–GC–MS. Molecules. 2022; 27(6):2004. https://doi.org/10.3390/molecules27062004
Chicago/Turabian StyleGalarza, Gustavo, and Jorge G. Figueroa. 2022. "Volatile Compound Characterization of Coffee (Coffea arabica) Processed at Different Fermentation Times Using SPME–GC–MS" Molecules 27, no. 6: 2004. https://doi.org/10.3390/molecules27062004
APA StyleGalarza, G., & Figueroa, J. G. (2022). Volatile Compound Characterization of Coffee (Coffea arabica) Processed at Different Fermentation Times Using SPME–GC–MS. Molecules, 27(6), 2004. https://doi.org/10.3390/molecules27062004






