Abstract
Some significant compounds present in annatto are geranylgeraniol and tocotrienols. These compounds have beneficial effects against hyperlipidemia and chronic diseases, where oxidative stress and inflammation are present, but the exact mechanism of action of such activities is still a subject of research. This study aimed to evaluate possible mechanisms of action that could be underlying the activities of these molecules. For this, in silico approaches such as ligand topology (PASS and SEA servers) and molecular docking with the software GOLD were used. Additionally, we screened some pharmacokinetic and toxicological parameters using the servers PreADMET, SwissADME, and ProTox-II. The results corroborate the antidyslipidemia and anti-inflammatory activities of geranylgeraniol and tocotrienols. Notably, some new mechanisms of action were predicted to be potentially underlying the activities of these compounds, including inhibition of squalene monooxygenase, lanosterol synthase, and phospholipase A2. These results give new insight into new mechanisms of action involved in these molecules from annatto and Chronic®.
1. Introduction
Lipid disorders, such as dyslipidemia, constitute a significant concern among the overall population and researchers due to their role in hyperlipidemia, hypertension, atherosclerosis, and even insulin resistance. Such aggravation is caused by increased levels of total cholesterol and low-density lipoprotein (LDL) and decreased levels of high-density lipoprotein, which together raise the risk of cardiovascular diseases and metabolic abnormalities [1,2,3,4].
Bixa orellana is the plant species known as “annatto” and “achiote”. This species is studied for some health issues, including inflammation-related conditions and dyslipidemias [5,6,7]. Such health benefits can be at least partly due to the presence of tocotrienols and geranylgeraniol from its composition. Tocotrienols are unsaturated forms of vitamin E known for anti-inflammatory, antioxidant, and lipid-lowering activities, which are higher than those from tocopherols—their saturated counterparts, also parts of the vitamin E group [8,9]. In turn, geranylgeraniol is an intermediate in the biosynthesis of cholesterol, and it is believed to regulate the activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-Coa) reductase negatively.
Both tocotrienols and geranylgeraniol are research subjects due to their biological activities, including cardioprotective and neuroprotective effects, hypolipidemic activity, metabolic disorder prevention, and antitumoral activity [10,11,12]. A fundamental approach in the process of drug discovery is pharmaceutical chemistry. A research can be more efficient through pharmaceutical chemistry by decreasing the necessary time, funds, and number of animals needed.
Some of the parameters often screened in potential new drugs through this approach are biological activity prediction, pharmacokinetic profile, and toxicological potential [13,14]. Hence, by using pharmaceutical chemistry tools, the purpose of this study was to evaluate the pharmaceutical potential of tocotrienols and geranylgeraniol for their main biological activities and possible mechanisms of action. This perspective could hint at safer medications compared with the standard ones.
2. Results and Discussion
2.1. Molecules’ Structure Obtention and Biological Activity Prediction
Tocotrienols and geranylgeraniol are molecules well described and studied in the literature [15,16]. Their structures were obtained from the PubChem database (Figure 1A) and then assessed for possible biological activities and mechanisms of action using the server PASS (prediction of activity spectra for substances) [17,18,19,20].
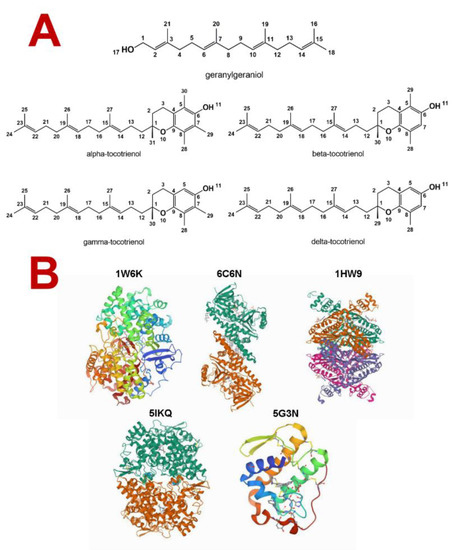
Figure 1.
(A) Molecular structure of geranylgeraniol and tocotrienols. (B) Targets used in the docking simulation with their respective PDB ID. 1W6K: lanosterol synthase complexed with lanosterol; 6C6N: squalene monooxygenase complexed with FAD and CPMPD-4; 1HW9: HMG-CoA reductase complexed with simvastatin; 5IKQ: cyclooxygenase-2 complexed with meclofenamic acid; 5G3N: secreted phospholipase A2 complexed with the inhibitor Azd2716.
Geranylgeraniol had a high probability of activity (Pa) values (>0.7) for the following activities: mucous membrane protection (0.953), lipid metabolism regulation (0.885), TNF expression inhibitor (0.840), antiulcerative (0.770), and antineoplastic (0.743). Still notably, the hypolipidemic activity Pa was 0.686, and antihypercholesterolemic Pa was 0.570, both higher than the probability of inactivity (Pi) (0.015 for both).
Tocotrienols also had significant Pa values for lipid peroxidase inhibition (from 0.941 to 0.989), antioxidant activity (from 0.913 to 0.973), anti-inflammatory activity (from 0.813 to 0.866), antihypercholesterolemic activity (from 0.803 to 0.962), cholesterol synthesis inhibition (from 0.663 to 0.702), among other related activities (Table 1). There are some variations among the isomers, but the class consistently shows high Pa’s tendency to improve the blood lipid profile. It is important to notice that in annatto, the most abundant isomer is δ, according to some authors, which can be up to 90% of the isomer composition [21].

Table 1.
Biological activity prediction of the compounds according to the PASS server.
To corroborate the results predicted by PASS, we further assessed these compounds through SEA (similarity ensemble approach) [22,23]. The outputs of this server are shown in Table 2. Geranylgeraniol had significant values (p-value < 10−10 or max Tanimoto coefficient (MaxTC) > 0.6) for squalene monooxygenase (p-value = 2.6 × 10−27, MaxTC = 0.65) and lanosterol synthase (p-value = 4 × 10−19, MaxTC = 0.40) interaction probability based on similarity with other compounds. Additionally, the server predicted significant interaction probability with phospholipase A2 (p-value = 7.3 × 10−18, MaxTC = 0.3). Tocotrienols had a lower degree of similarity with compounds able to interact with these targets compared with geranylgeraniol; however, the values were still in a considerable range. For squalene monooxygenase interaction, p-values ranged from 2.2 × 10−08 to 8.6 × 10−09, and MaxTC ranged from 0.30 to 0.31; for lanosterol synthase, p-values varied from 1.2 × 10−06 to 2.0 × 10−08, and MaxTC varied from 0.30 to 0.31. Finally, for phospholipase A2, p-values varied from 3 × 10−09 to 6.6 × 10−09, and MaxTC varied from 0.3 to 0.31.

Table 2.
Prediction outputs of the molecules assessed with ligands from the SEA server.
The outputs predicted by PASS and SEA collectively point to these molecules’ tendency to improve the blood lipid profile. However, while in PASS, the most favorable results were achieved by tocotrienols, the highest similarity outputs suggesting that biological action was achieved by geranylgeraniol in SEA. In SEA, the probability of squalene monooxygenase and lanosterol synthase inhibition by tocotrienols was not negligible but was still not high enough. However, it should be kept in mind that these two mechanisms of action are not the only ones that could decrease cholesterol biosynthesis and improve the blood lipid profile. In fact, tocotrienols have been reported to inhibit the mevalonate pathway of HMG-CoA reductase, a pivotal player in cholesterol biosynthesis [24]. While geranylgeraniol was predicted to inhibit lanosterol synthase and monooxygenase in SEA, this was not predicted by PASS. This divergence between the servers could be a negative indicator of these targets, or it could be due to differences in the servers’ training sets, which could give different outcomes.
Reports support a potential role in improving blood lipid profile by geranylgeraniol. For instance, just like tocotrienols, this molecule was shown to decrease HMG-CoA reductase activity [25,26]. Considering the role of this enzyme in cholesterol biosynthesis, this could be a mechanism in which geranylgeraniol exerts its action. Our group reported that the treatment with geranylgeraniol improved blood lipid parameters; however, the molecule was not administrated alone but with tocotrienols [8]. Altogether, the in silico prediction with its known mechanism of action justifies future studies with this molecule alone in treating blood dyslipidemia in vivo.
As mentioned previously, it is believed that this activity may be at least in part due to HMG-CoA reductase inhibition based on previous studies. However, we sought to assess whether more mechanisms were underlying such activity. Hence, molecular docking was performed with the most promising targets.
2.2. Molecular Docking
Molecular docking is a powerful tool in computation chemistry that allows researchers to assess the molecular interactions’ type and intensity between a ligand and a target biomolecule within an active site [27]. A total of five macromolecular targets acquired from PDB were used in GOLD without the cocrystalized ligands (Figure 1B). Three of them are involved in cholesterol metabolism (OSC, SQLE, and HMGR), and two are directly involved in inflammation (PLA2 and COX-2).
Lanosterol synthase (a.k.a. oxidosqualene cyclase (OSC)) is a membrane-bound protein responsible for synthesizing steroids in mammals. Its cyclization reaction forms lanosterol. Due to its role in the synthesis of steroids, this protein is considered a target to hypolipidemic drugs [28]. When complexed with OSC, lanosterol forms hydrogen bonds with the amino acid residues Trp581 and Asp455 [29].
In the docking performed with OSC, geranylgeraniol and tocotrienol had relevant interactions with the receptors’ active-site amino acid residues. The details of such interactions are shown in Table 3, including the interaction type, distances, and docking scores.

Table 3.
Docking interactions of the molecules with OSC.
In Figure 2, it is possible to observe the docking pose in two and three dimensions. It is observed that all molecules could interact with the amino acid residues Asp455 and Trp581 (hydrogen bonds), the same amino acids that can interact with the inhibitor of the enzyme Ro 48-8071, which is considered a structural base for the design of OSC inhibitors. However, the inhibitor performs hydrophobic interactions with Trp581 instead of hydrogen bonds [29].
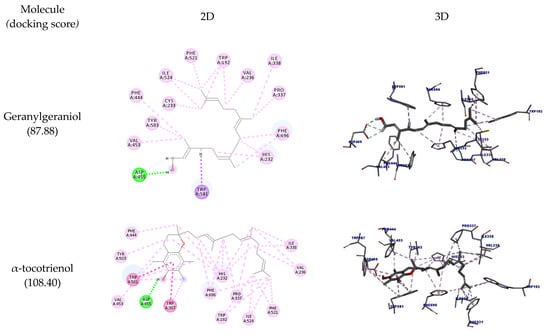
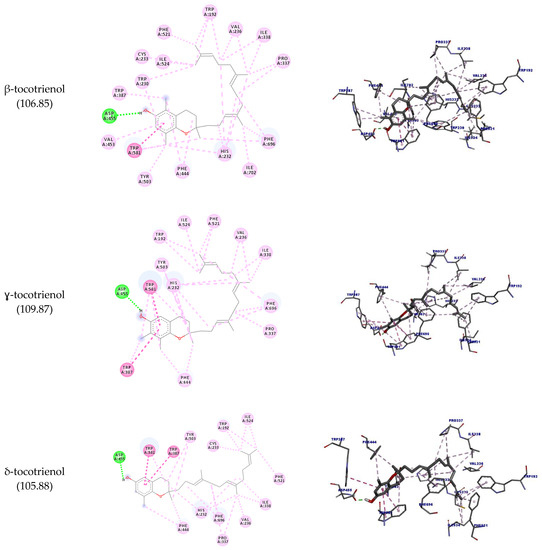
Figure 2.
Two-dimensional and three-dimensional representations of the best docking poses calculated by GOLD with OSC (PDB ID: 1W6K). Pictures produced with Discovery Studio.
Like Ro 48-8071, the molecules could also interact with the residues Trp192 and Phe521, indicating that they can potentially inhibit this enzyme. β-tocotrienol could interact with all the residues mentioned so far plus Trp230, thus performing the same interactions of Ro 48-8071.
Squalene monooxygenase (a.k.a. squalene epoxidase (SQLE)) is the second limiting enzyme in cholesterol biosynthesis accountable to catalyze the conversion of squalene to 2,3(S)-oxidosqualene using flavin adenosine dinucleotide (FAD) as a coenzyme. SQLE inhibition is considered a possible mechanism in treating hypercholesterolemia, fungal infections, and some types of cancer [30]. The docking data with SQLE are shown in Table 4, and the docking poses are depicted in Figure 3.

Table 4.
Docking interactions of the molecules with SQLE.
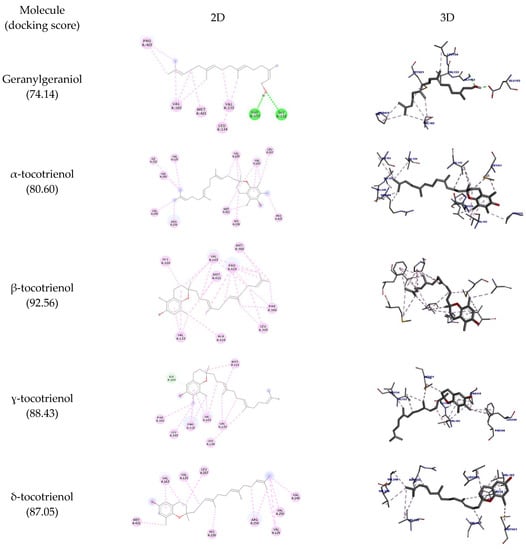
Figure 3.
Two-dimensional and three-dimensional representations of the best docking poses calculated by GOLD with SQLE (PDB ID: 6C6N). Pictures produced with Discovery Studio.
The aromatic groups of the ligand complexed with SQLE (PDB ID: 6C6N) perform nonpolar interactions with the amino acid residues Asp166, Tyr195, Ala322, Leu333, Tyr335, Pro415, Leu416, and Gly418 [30]. Of these residues, only Pro415 could interact with all the molecules tested (hydrophobic interaction) except for δ-tocotrienol. However, other interactions were observed with different amino acid residues. β-tocotrienol was the compound with more interactions with Pro415 (six hydrophobic interactions) and had the highest docking score (92.56).
It is believed that one of the main targets for the hypocholesterolemic activity of tocotrienols is HMG-CoA reductase. This enzyme catalyzes the rate-limiting step in cholesterol biosynthesis [31] and is also targeted by statins, although these molecules inhibit its activity in a different way [8]. As mentioned, there are some reports of HMGR inhibition by geranylgeraniol as well. Here we sought to discover whether the inhibition of these molecules could involve direct binding to HMGR. The docking interactions are detailed in Table 5 and depicted in Figure 4. The results show that the molecules interacted with the amino acid residues Leu562, Leu853, Ala856, and Leu857 through hydrophobic interactions. It is observed that the highest number of interactions and docking score were obtained by γ-tocotrienol (17 interactions; 57.77 docking score), while geranylgeraniol had the lowest (12 and 51.47, respectively).

Table 5.
Docking interactions of the molecules with HMG-CoA reductase.
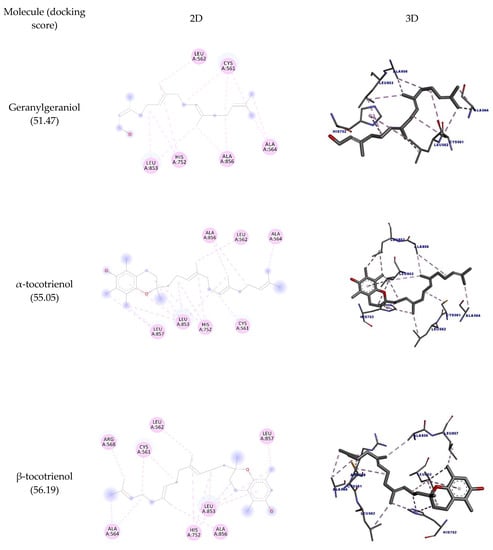
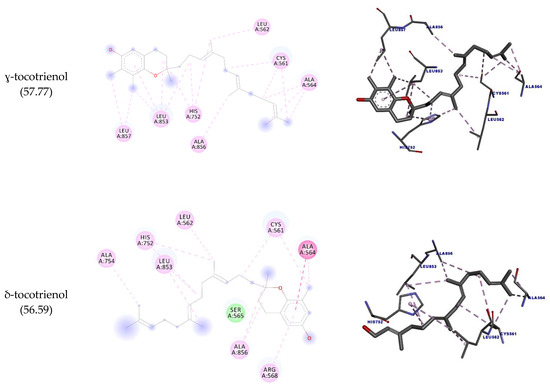
Figure 4.
Two-dimensional and three-dimensional representations of the best docking poses calculated by GOLD with HMG-COA reductase. Pictures produced with Discovery Studio.
Inflammation is tightly associated with lipid and metabolic disturbances [32,33,34]. According to the results predicted by PASS and SEA, geranylgeraniol and tocotrienols may also decrease inflammation. In accordance with our results, it has been reported that geranylgeraniol suppresses the expression of interleukin-1 receptor-associated kinase-1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6), consequently preventing NF-κB excessive activation in LPS-induced inflammatory response in THP-1 cells. In addition, tocotrienols are thought to exert their effects also in part by decreasing the inflammatory cascade [35,36,37,38,39,40].
Since SEA predicted the interaction of all the molecules with phospholipase A2, we performed a docking with this enzyme. We also performed docking with COX-2 because it is a common target for anti-inflammatory compounds (such as the NSAIDs).
COX-2 is an inflammatory enzyme that converts arachidonic acid into prostaglandins, such as prostaglandin H2 [41]. The docking results with COX-2 are shown in Table 6, and the docking poses are depicted in Figure 5. The structure of COX-2 was stored in PDB in a complex with meclofenamic acid, a known inhibitor of this enzyme.

Table 6.
Docking interactions of the molecules with COX-2.
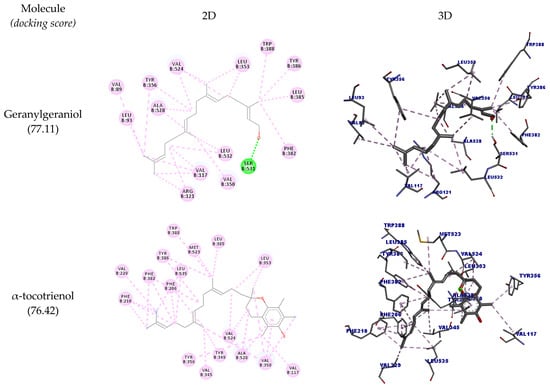
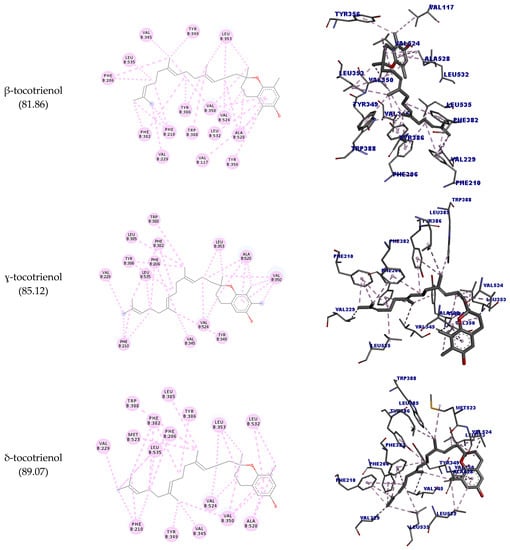
Figure 5.
Two-dimensional and three-dimensional representations of the best docking poses calculated by GOLD with COX-2 (PDB ID: 5IKQ). Pictures produced with Discovery Studio.
The hydrogen bonds between the inhibitor’s carboxylate and the phenolic oxygen of Tyr385 and Ser530 are considered important interactions for the inhibition of this enzyme [42]. It was observed that all the structures could interact with COX-2, but none of them could interact with the amino acid residues Tyr385 and Ser530. The highest docking score was achieved by δ-tocotrienol (89.07), and the other molecules had good scores as well (>70).
Phospholipase A2 is another enzyme involved in the inflammatory response that catalyzes the hydrolysis of two glycerophospholipids and releases two fatty acids and lysophospholipids. The secreted PLA2 is involved in the rate-limiting step of eicosanoid biosynthesis by releasing unesterified arachidonic acid from membrane phospholipids [43].
Table 7 shows all the interactions of this enzyme with geranylgeraniol and tocotrienols, and the best docking poses are depicted in Figure 6. The results show that all molecules interacted with the amino acid residue His47; except for α-tocotrienol, all molecules could interact with Cys28 as well. Most of the molecules assessed could interact with PLA2’s hydrophobic pocket (Leu2, Phe5, His5, Ile9, Ala17, Ala8, Gly22), suggesting this enzyme’s potential inhibition. The highest docking score was achieved by α-tocotrienol (90.64).

Table 7.
Docking interactions of the molecules with PLA2.
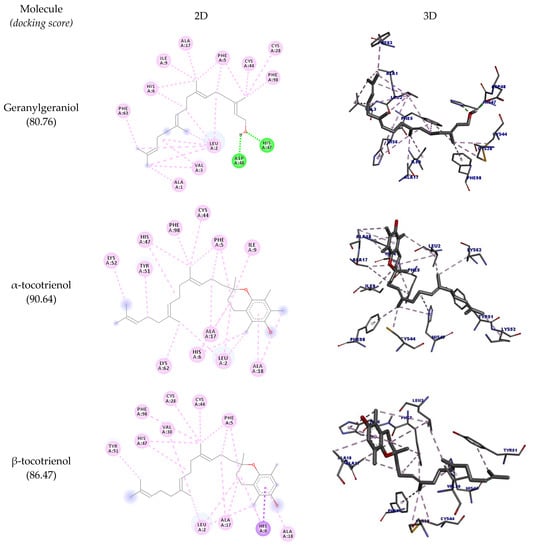
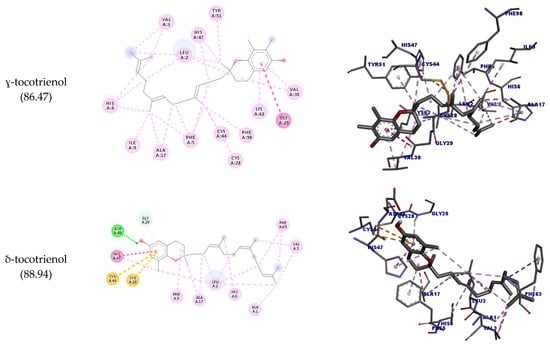
Figure 6.
Two-dimensional and three-dimensional representations of the best docking poses calculated by GOLD with PLA2 (PDB ID: 5G3N). Pictures produced with Discovery Studio.
In the docking studies, it was observed that geranylgeraniol could interact with all the targets assessed. For OSC, SQLE, and PLA2, these interactions were similar to their corresponding crystalized inhibitors, corroborating the predictions by SEA and suggesting a potential hypocholesterolemic and anti-inflammatory activity. Tocotrienols also could interact with the assessed enzymes; notably, β-tocotrienol had an interesting interaction profile with OSC, similar to Ro 48-8071. As regards SQLE, δ-tocotrienol could not interact with the target’s active site amino acid residues, while all others could interact with Pro415, specially β-tocotrienol.
Although all molecules could interact with COX-2, none of these interactions are reported in the literature to inhibit this enzyme activity. For PLA2, an important interaction that inhibits this enzyme is with the amino acid residues His47 and Cys28. All tocotrienols could interact with His47, and all but α-tocotrienol could interact with Cys28 as well (even though this molecule had the highest docking score).
Collectively, the docking supports the biological activity prediction. The results support the hypocholesterolemic and anti-inflammatory potential for geranylgeraniol and tocotrienols, following previous reports in the literature. Although these activities are not new for these molecules, our results suggest some potential new action mechanism that has not been reported, such as lanosterol synthase inhibition, which is different from HMG-CoA reductase inhibition.
2.3. Pharmacokinetic Property Prediction
Despite having a desired biological activity, a compound must effectively reach its therapeutic targets, and for this, the molecule must have a favorable pharmacokinetic profile (absorption, distribution, metabolism, excretion (ADME)). Nowadays, several approaches are available to predict ADME data from compounds [44]. The servers PreADMET and SwissADME were used to indicate such activities based on the compounds’ structures. The data are shown in Table 8.

Table 8.
ADME prediction by PreADMET and SwissADME.
In PreADMET outputs, %HIA represents the human intestinal absorption, which, as the name suggests, refers to the amount of the molecule that is absorbed. HIA is important because most drugs are administered orally and hence need to be absorbed in satisfactory amounts in the gastrointestinal tract [45]. The server PreADMET considers that good drug candidates should have a %HIA of at least 70%. Hence, all the molecules had a great degree of intestinal absorption with %HIA > 97%, and geranylgeraniol had 100%.
SwissADME bases the gastrointestinal absorption and blood–brain barrier permeation on a different model called BOILED-Egg (brain or intestinal estimated permeation method) [46,47]. In this distinct model, geranylgeraniol but not tocotrienols were predicted to be highly permeant to the GI tract due to their high Lop P.
A popular model to assess drug absorption in drug discovery is using Caco-2 or MDCK cells as test systems. PreADMET can predict the molecular permeation in these cells by comparing the molecules from those of its database. According to the server, <4 nm/s represents low permeation, values from 4 to 70 nm/s have intermediate permeation, and values above that represent high permeation. For MDCK, values below 25 represent low permeability, values from 25 to 500 represent intermediate permeation, and values above 500 represent high permeation [48,49].
All molecules assessed had intermediate absorption values in Caco-2 cells, while in MDCK, only geranylgeraniol and δ-tocotrienol had intermediate absorption values, and the others had low values. Overall, geranylgeraniol had superior results to tocotrienols. Among tocotrienols, α-tocotrienol had the highest absorption values (Table 8).
For PreADMET, good drug candidates must have <90% of blood protein binding (BPB) because the molecules should be free to be able to interact with their biological targets [50]. In our prediction, the molecules had an unfavorable BPB profile (higher than 90%). Another distribution parameter assessed was the interaction with P-glycoprotein (P-gp) calculated by SwissADME. This macromolecule is responsible for hampering the intracellular accumulation of potentially toxic compounds and removing them from the CNS through the blood–brain barrier as well [51]. The server predicted that tocotrienols could interact with these targets while geranylgeraniol could not.
Both servers give outputs about blood–brain barrier (BBB) permeation and, hence, have potential to reach the CNS. However, the results are in disagreement. According to PreADMET, compounds with Cbrain/Cblood values higher than 2.0 can cross the BBB, and all the molecules had high values, while in Swiss ADME, which uses the BOILED-Egg model, the molecules were predicted not to cross the BBB. However, these molecules probably cross the BBB according to in vivo data of tocotrienols and other vitamins E in SNC disorders [52,53]. The pharmacokinetics of tocotrienols have been reported in patients with favorable results and safety profiles [54,55].
2.4. Toxicological Property Prediction
The toxicological prediction from geranylgeraniol and tocotrienols were assessed with PreADMET and ProTox-II. This online server is accessible and can help screen possible toxicities from compounds [56]. The prediction outputs are shown in Table 9.

Table 9.
Toxicity prediction in ProTox-II.
All the molecules were predicted to be nonmutagenic in bacteria and nonhepatotoxic, cardiotoxic, immunotoxic, or cytotoxic. The predicted median lethal doses were high, especially for geranylgeraniol. ProTox-II classifies the molecules according to the predicted toxicity from 1 to 6, in which higher values represent less toxic compounds. The highest value was achieved for geranylgeraniol (5), while tocotrienols were classified as 4.
3. Materials and Methods
3.1. Molecules Studied
This study used the major molecules found in the purified annatto oil (PAO) and its granules (Chronic®). The samples were kindly provided by Ages Bioactive Compounds Co. (São Paulo-SP, Brazil). The batch analysis certificate is described as URU200401 (12 March 2020, expiration date: 22 March 2022), composition: bixin (1.7%), tocotrienols (9.59%), and geranylgeraniol (28.32%), as described by Matias Pereira et al. [8].
All structures used were confirmed in the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 1 October 2021) (Figure 1A). The molecules were drawn using ChemDraw [56] and optimized using HyperChem through the semiempirical method RM1 [57].
3.2. Biological Activities Prediction
The prediction of biological activity was based on analysis of the structure–activity relationship of a training set using the PASS server (prediction of activity spectra for substances; http://www.pharmaexpert.ru/passonline, accessed on 1 October 2021), which can predict 4.130 biological activities in the compounds with an average accuracy of 95%. PASS is based on the naïve Bayes classifier approach and multilevel neighborhoods of atoms descriptors. The predicted activities are given as Pa (probability of being active) or Pi (probability to be inactive). Molecules with a Pa superior to 0.7 are considered promising candidates for the given activity; however, molecules with Pa > 0.4 and Pa > Pi could still be good candidates [17,18,19,20].
In addition, the SEA server (similarity ensemble approach; http://sea.bkslab.org/, accessed on 1 November 2021) was used to assess potential targets of the studied molecules. This server predicts small-molecule activity based on the macromolecular targets they interact with, which is inferred according to topology similarity with other molecules’ fingerprints from its database [22,23]. The server gives the p-value as similarity output representing the expected value (E-value) and the max Tanimoto coefficient (MaxTC). In a prediction, the lower the p-value, the more significant it is, evidencing that the prediction is less likely to be by chance; ideally, a prediction should be <10−10 to be highly significant, while a p-value > 1 is considered insignificant. A MaxTC is considered highly significant when the value is >0.6, and insubstantial when <0.3 [22,58].
3.3. Molecular Docking
The docking was performed using the software GOLD (Genetic Optimization for Ligand Docking [59]) using biological targets acquired from Protein Data Bank [60]. A total of five targets were selected: the human lanosterol synthase (an oxidosqualene cyclase (OSC)) complexed with lanosterol, human squalene epoxidase (a.k.a. squalene monooxygenase (SQLE)) complexed with FAD and CPMPD-4, human HMG-CoA reductase (HMGR) complexed with simvastatin, secreted phospholipase A2 (sPLA2) complexed with the inhibitor Azd2716, and human cyclooxygenase-2 (COX-2) complexed with meclofenamic acid (Figure 1B). All the cocrystalized ligands were removed to perform the docking.
Before the dockings, validation was performed for each target by calculating the root mean square deviation (RMSD), which is the root mean square distance of nonhydrogen atoms of the ligand from the crystal structure and their corresponding docked pose. All the crystallized targets had RMSD < 2 Å and considered the upper limit of satisfactory docking [61]. Other parameters assessed were the docking sphere radius and x, y, and z coordinates (Table 10).

Table 10.
Docking validation parameters.
Cocrystallized ligands, ions, and water molecules were removed from the crystallographic structures to perform the docking. Additionally, hydrogens were added to the ligands, and their atomic charge was calculated using HyperChem, as described in [62].
3.4. Pharmacokinetic Prediction
An in silico ADME (absorption, distribution, metabolism, excretion) prediction was performed using the servers PreADMET (https://preadmet.bmdrc.kr/, accessed on 1 November 2021) and SwissADME (http://www.swissadme.ch, accessed on 1 November 2021). These servers can calculate the physicochemical and pharmacokinetic properties of molecules, including human intestinal absorption, Caco-2 cell and MDCK permeability, percentage of plasma protein binding, blood–brain barrier penetration, glycoprotein P interaction, metabolism by P450 cytochromes, among others [46,48,63].
3.5. Toxicological Prediction
The toxicological prediction was performed using ProTox-II. This server can predict different toxicity parameters, such as acute toxicity, organ-specific toxicity, cytotoxicity, carcinogenicity, and immunotoxicity [64].
4. Conclusions
The biological activity results follow what is reported in the literature, mainly for the antioxidant, anti-inflammatory, and antidyslipidemia potential of geranylgeraniol and tocotrienols. The molecular docking corroborated the predicted activities of the servers. Notably, the in silico data presented another mechanism of action that could be involved in the activity of this molecule, which is inhibition of squalene monooxygenase and lanosterol synthase, which will need to be confirmed in vitro.
These in silico data corroborate the use of these molecules against lipid disorders, coronary disease due to cholesterol accumulation, and several chronic diseases in which oxidative stress and inflammatory cascade have a role. Geranylgeraniol and tocotrienols are major molecules from Bixa orellana and Chronic®. The results also point to a good pharmacokinetic profile for these molecules and a good safety profile, according to previously reported experimental data.
Author Contributions
Conceptualization, J.C.T.C. and L.I.d.S.H.-M.; methodology and software, L.I.d.S.H.-M. and M.A.B.; formal analysis, M.A.B. and H.R.d.S.; resources and data curation, J.C.T.C.; writing—original draft preparation, M.A.B., A.V.T.d.L.T.d.S. and A.L.d.N.; writing—review and editing, A.C.M.P.; visualization, A.V.T.d.L.T.d.S., I.R.S.S. and L.F.M.; supervision, J.C.T.C. and L.I.d.S.H.-M.; project administration, J.C.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the contributors to the Pharmaceutical Research Laboratory who provided the Chronic® development and standardization data.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
The samples are available upon request.
References
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Jing, L.; Tian, Y.; Yan, H.; Zhang, B.; Sun, Q.; Dai, D.; Shi, L.; Liu, D.; Yang, Z.; et al. Epidemiology of dyslipidemia and associated cardiovascular risk factors in northeast China: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Pacheco, S.D.G.; Gasparin, A.T.; Jesus, C.H.A.; Sotomaior, B.B.; Ventura, A.C.S.S.B.; Redivo, D.D.B.; Cabrini, D.D.A.; Gaspari Dias, J.D.F.; Miguel, M.D.; Miguel, O.G.; et al. Antinociceptive and Anti-Inflammatory Effects of Bixin, a Carotenoid Extracted from the Seeds of Bixa orellana. Planta Med. 2019, 85, 1216–1224. [Google Scholar] [CrossRef]
- Rivera-Madrid, R.; Aguilar-Espinosa, M.; Cárdenas-Conejo, Y.; Garza-Caligaris, L.E. Carotenoid derivates in achiote (Bixa orellana) seeds: Synthesis and health promoting properties. Front. Plant Sci. 2016, 7, 1406. [Google Scholar] [CrossRef] [Green Version]
- Matias Pereira, A.C.; de Oliveira Carvalho, H.; Gonçalves, D.E.S.; Picanço, K.R.T.; de Lima Teixeira dos Santos, A.V.T.; da Silva, H.R.; Braga, F.S.; Bezerra, R.M.; de Sousa Nunes, A.; Nazima, M.T.S.T.; et al. Co-treatment of purified annatto oil (Bixa orellana l.) and its granules (chronic®) improves the blood lipid profile and bone protective effects of testosterone in the orchiectomy-induced osteoporosis in wistar rats. Molecules 2021, 26, 4720. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Medvedev, O.; Ivanova, A.; Medvedeva, N. Biological properties of tocotrienols. Vopr. Pitan. 2018, 87, 5–16. [Google Scholar] [CrossRef]
- Irwin, J.C.; Fenning, A.S.; Vella, R.K. Geranylgeraniol prevents statin-induced skeletal muscle fatigue without causing adverse effects in cardiac or vascular smooth muscle performance. Transl. Res. 2020, 215, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCully, K.S. Chemical Pathology of Homocysteine VIII. Effects of Tocotrienol, Geranylgeraniol, and Squalene on Thioretinaco Ozonide, Mitochondrial Permeability, and Oxidative Phosphorylation in Arteriosclerosis, Cancer, Neurodegeneration and Aging. Ann. Clin. Lab. Sci. 2020, 50, 567–577. [Google Scholar]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Bolchi, C.; Bavo, F.; Appiani, R.; Roda, G.; Pallavicini, M. 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem. 2020, 200, 112419. [Google Scholar] [CrossRef]
- Song, T.-Q.; Ding, M.-Z.; Zhai, F.; Liu, D.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Engineering Saccharomyces cerevisiae for geranylgeraniol overproduction by combinatorial design. Sci. Rep. 2017, 7, 14991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.L.; Yeganehjoo, H. The potential of isoprenoids in adjuvant cancer therapy to reduce adverse effects of statins. Front. Pharmacol. 2019, 9, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagunin, A.; Zakharov, A.; Filimonov, D.; Poroikov, V. QSAR modelling of rat acute toxicity on the basis of PASS prediction. Mol. Inform. 2011, 30, 241–250. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Dmitriev, A.V.; Pogodin, P.V.; Dubovskaya, V.I.; Ivanov, S.M.; Tarasova, O.A.; et al. Computational platform Way2Drug: From the prediction of biological activity to drug repurposing. Russ. Chem. Bull. 2017, 66, 1832–1841. [Google Scholar] [CrossRef]
- Rudik, A.V.; Dmitriev, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. PASS-based prediction of metabolites detection in biological systems. SAR QSAR Environ. Res. 2019, 30, 751–758. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Liang, L.; Yin, Z.; Lin, J. Improving chemical similarity ensemble approach in target prediction. J. Cheminform. 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.-Y.; Pang, K.-L.; Soelaiman, I.-N. Tocotrienol and Its Role in Chronic Diseases. In Advances in Experimental Medicine and Biology; Gupta, S.C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 928, pp. 97–130. ISBN 9783319413341. [Google Scholar]
- Sever, N.; Song, B.L.; Yabe, D.; Goldstein, J.L.; Brown, M.S.; DeBose-Boydb, R.A. Insig-dependent Ubiquitination and Degradation of Mammalian 3-Hydroxy-3-methylglutaryl-CoA Reductase Stimulated by Sterols and Geranylgeraniol. J. Biol. Chem. 2003, 278, 52479–52490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, M.M.; Elsabrouty, R.; Seemann, J.; Jo, Y.; DeBose-Boyd, R.A. The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase. eLife 2015, 4, e05560. [Google Scholar] [CrossRef]
- Scarpino, A.; Ferenczy, G.G.; Keserű, G.M. Comparative Evaluation of Covalent Docking Tools. J. Chem. Inf. Model. 2018, 58, 1441–1458. [Google Scholar] [CrossRef]
- Morand, O.H.; Aebi, J.D.; Dehmlow, H.; Ji, Y.H.; Gains, N.; Lengsfeld, H.; Himber, J. Ro 48-8071, a new 2,3-oxidosqualene:lanosterol cyclase inhibitor lowering plasma cholesterol in hamsters, squirrel monkeys, and minipigs: Comparison to simvastatin. J. Lipid Res. 1997, 38, 373–390. [Google Scholar] [CrossRef]
- Thoma, R.; Schulz-Gasch, T.; D’Arcy, B.; Benz, J.; Aebi, J.; Dehmlow, H.; Hennig, M.; Stihle, M.; Ruf, A. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 2004, 432, 118–122. [Google Scholar] [CrossRef]
- Padyana, A.K.; Gross, S.; Jin, L.; Cianchetta, G.; Narayanaswamy, R.; Wang, F.; Wang, R.; Fang, C.; Lv, X.; Biller, S.A.; et al. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun. 2019, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, B.; Shukor, M.Y. HMG-CoA Reductase as Target for Drug Development. Methods Mol. Biol. 2020, 2089, 245–250. [Google Scholar] [CrossRef]
- Nepomuceno, R.; de, F. Vallerini, B.; da Silva, R.L.; Corbi, S.C.T.; Bastos, A.D.S.; dos Santos, R.A.; Takahashi, C.S.; Regina, P.; Orrico, S.; Scarel-Caminaga, R.M. Systemic expression of genes related to inflammation and lipid metabolism in patients with dyslipidemia, type 2 diabetes mellitus and chronic periodontitis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2715–2722. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, H.; Liu, X.; Fu, X.; Li, X.; Cao, Q. Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J. Drug Target. 2020, 28, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef]
- Kuhad, A.; Bishnoi, M.; Tiwari, V.; Chopra, K. Suppression of NF-κβ signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009, 92, 251–259. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, W.; Okla, M.; Kang, I.; Moreau, R.; Chung, S. Suppression of NLRP3 inflammasome by γ -tocotrienol ameliorates type 2 diabetes. J. Lipid Res. 2016, 57, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFkB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shridas, P.; Webb, N.R. Diverse Functions of Secretory Phospholipases A2. Adv. Vasc. Med. 2014, 2014, 689815. [Google Scholar] [CrossRef] [Green Version]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Le, J.; Abraham, M.H.; Hersey, A.; Eddershaw, P.J.; Luscombe, C.N.; Boutina, D.; Beck, G.; Sherborne, B.; Cooper, I.; et al. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure—Activity relationship (QSAR) with the Abraham descriptors. J. Pharm. Sci. 2001, 90, 749–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Zoete, V. A Boiled-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.M.V.; de Andrade, F.d.C.P.; Filgueiras, L.A.; de Carvalho Maia, O.A.; Cunha, R.L.; Rodezno, S.V.; Maia Filho, A.L.M.; de Amorim Carvalho, F.A.; Braz, D.C.; Mendes, A.N. preADMET analysis and clinical aspects of dogs treated with the Organotellurium compound RF07: A possible control for canine visceral leishmaniasis? Environ. Toxicol. Pharmacol. 2020, 80, 103470. [Google Scholar] [CrossRef]
- Yamashita, S.; Konishi, K.; Yamazaki, Y.; Taki, Y.; Sakane, T.; Sezaki, H.; Furuyama, Y. New and better protocols for a short-term Caco-2 cell culture system. J. Pharm. Sci. 2002, 91, 669–679. [Google Scholar] [CrossRef]
- Roman, D.L.; Roman, M.; Som, C.; Schmutz, M.; Hernandez, E.; Wick, P.; Casalini, T.; Perale, G.; Ostafe, V.; Isvoran, A. Computational Assessment of the Pharmacological Profiles of Degradation Products of Chitosan. Front. Bioeng. Biotechnol. 2019, 7, 214. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Gumpricht, E.; Rockway, S. Can ω-3 fatty acids and tocotrienol-rich vitamin E reduce symptoms of neurodevelopmental disorders? Nutrition 2014, 30, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A. Pharmacokinetics and Bioavailability of Annatto δ-tocotrienol in Healthy Fed Subjects. J. Clin. Exp. Cardiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Khan, D.A. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Cardoza, C.G.; Vique-Sánchez, J.L. Potential inhibitors of the interaction between ACE2 and SARS-CoV-2 (RBD), to develop a drug. Life Sci. 2020, 256, 117970. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A. Die Geschichte des ChemDraw-Projekts. Angew. Chem. 2014, 126, 11320–11325. [Google Scholar] [CrossRef]
- Nagamani, S.; Kesavan, C.; Muthusamy, K. Atom-based and Pharmacophore-based 3D–QSAR Studies on Vitamin D Receptor (VDR). Comb. Chem. High Throughput Screen. 2018, 21, 329–343. [Google Scholar] [CrossRef]
- Schyman, P.; Liu, R.; Wallqvist, A. General Purpose 2D and 3D Similarity Approach to Identify hERG Blockers. J. Chem. Inf. Model. 2016, 56, 213–222. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, D.; Davis, A.M.; Kleywegt, G.J.; Schmitt, S. An Alternative Method for the Evaluation of Docking Performance: RSR vs RMSD. J. Chem. Inf. Model. 2008, 48, 1411–1422. [Google Scholar] [CrossRef]
- Matias Pereira, A.C.; Sánchez-Ortíz, B.L.; de Melo, E.L.; da Silva Hage-Melim, L.I.; Borges, R.S.; Hu, X.; Carvalho, J.C.T. Perillyl alcohol decreases the frequency and severity of convulsive-like behavior in the adult zebrafish model of acute seizures. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Ruswanto; Siswandono; Richa, M.; Tita, N.; Tresna, L. Molecular docking of 1-benzoyl-3-methylthiourea as anti cancer candidate and its absorption, distribution, and toxicity prediction. J. Pharm. Sci. Res. 2017, 9, 680–684. [Google Scholar]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).