Stability and Formulation of Erlotinib in Skin Creams
Abstract
:1. Introduction
2. Results
2.1. Preliminary Tests
2.1.1. Intrinsic Stability of ERL
2.1.2. Method Validation
2.1.3. Extraction of ERL from the Tablets
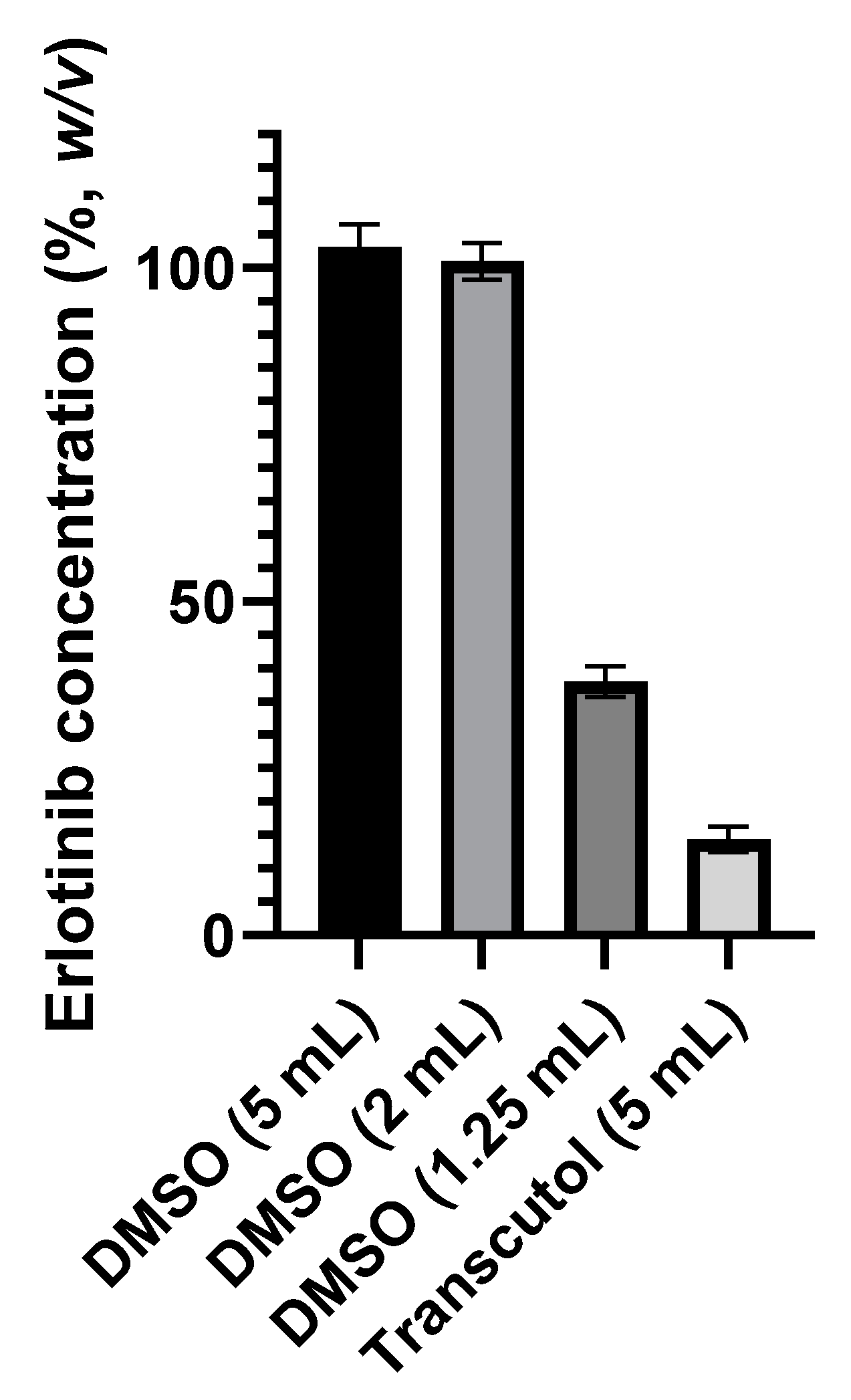
2.1.4. Preliminary Stability Tests
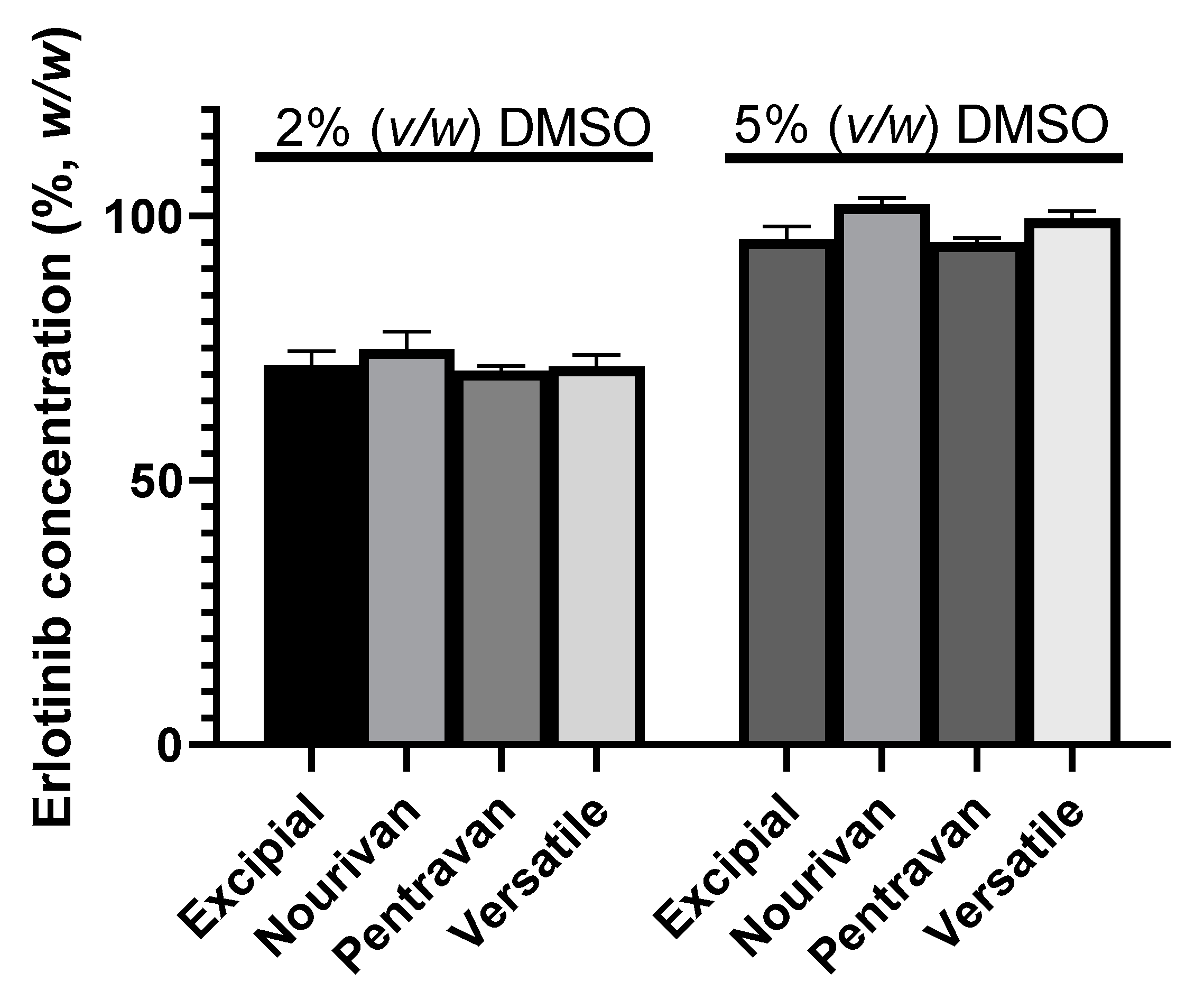
2.2. Stability of ERL under Optimized Conditions of Preparation
3. Discussion
4. Materials and Methods
4.1. Preparation of Test Creams
4.2. Chromatographic Conditions
4.3. Assay Method Validation
4.4. Stability Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsao, M.; Sakurada, A.; Cutz, J.-C.; Zhu, C.; Kamel-Reid, S.; Squire, J.; Lorimer, I.; Zhang, T.; Liu, N.; Daneshmand, M.; et al. Erlotinib in Lung Cancer—Molecular and Clinical Predictors of Outcome. N. Engl. J. Med. 2005, 353, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Greco, C.; Leclerc-Mercier, S.; Chaumon, S.; Doz, F.; Hadj-Rabia, S.; Molina, T.; Boucheix, C.; Bodemer, C. Use of Epidermal Growth Factor Receptor Inhibitor Erlotinib to Treat Palmoplantar Keratoderma in Patients with Olmsted Syndrome Caused by TRPV3 Mutations. JAMA Dermatol. 2020, 156, 191. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.A.; Kies, M.S.; William, W.N.; Johnson, F.M.; Lee, J.J.; Glisson, B.S. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase 2 clinical trial. Cancer 2018, 124, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, T.R.; Griesinger, F. Two Cases of Psoriasis Responding to Erlotinib: Time to Revisiting Inhibition of Epidermal Growth Factor Receptor in Psoriasis Therapy? J. Dermatol. 2012, 225, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kopsky, D.; Bhaskar, A.; Zonneveldt, H.; Keppel Hesselink, J. Topical loperamide for the treatment of localized neuropathic pain: A case report and literature review. J. Pain Res. 2019, 12, 1189–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assmann, T.; Homey, B.; Ruzicka, T. Topical tacrolimus for the treatment of inflammatory skin diseases. Expert Opin. Pharmacother. 2001, 2, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Choi, F.D.; Juhasz, M.L.; Mesinkovska, N.A. Topical ketoconazole: A systematic review of current dermatological applications and future developments. J. Dermatol. Treat. 2019, 30, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Bouchand, C.; Nguyen, D.; Secretan, P.-H.; Vidal, F.; Guery, R.; Auvity, S.; Cohen, J.F.; Lanternier, F.; Lortholary, O.; Cisternino, S.; et al. Voriconazole topical cream formulation: Evidence for stability and antifungal activity. Int. J. Antimicrob. Agents 2020, 56, 106083. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; van Wijk, A.; Smit, E.F.; Postmus, P.E. Side-Effects of Long-Term Administration of Erlotinib in Patients with Non-small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 1477–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, H.; Vemuri, N.M. Chemical and Physicochemical Approaches to Solve Formulation Problems. In The Practice of Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 767–791. [Google Scholar]
- Mahajan, A.A.; Miniyar, P.B.; Patil, A.S.; Waghmare, R.U.; Patil, J.J.; Mohanraj, K.; Tiwari, R.N. Separation, Identification, and Characterization of Degradation Products of Erlotinib Hydrochloride Under ICH-Recommended Stress Conditions by LC, LC-MS/TOF. J. Liq. Chromatogr. Relat. Technol. 2014, 38, 629–639. [Google Scholar] [CrossRef]
- Negreira, N.; Regueiro, J.; de Alda, M.L.; Barceló, D. Degradation of the anticancer drug erlotinib during water chlorination: Non-targeted approach for the identification of transformation products. Water Res. 2015, 85, 103–113. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology; ICH: London, UK, 1994. [Google Scholar]
- Allen, L.V.; Bassani, G.S.; Elder, E.J.; Parr, A.F. Strength and Stability Testing for Compounded Preparations. US Pharmacop. 2014, 1–7, 2021. [Google Scholar]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.R.D.O.; Homem-De-Mello, M.; Silveira, D.; Simeoni, L.A. Advice on Degradation Products in Pharmaceuticals: A Toxicological Evaluation. PDA J. Pharm. Sci. Technol. 2014, 68, 221–238. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. ICH Guidelines Q3C (R8) on Impurities, Guidelines for Residual Solvents; ICH: London, UK, 2021. [Google Scholar]
- Pujeri, S.S.; Khader, A.M.A.; Seetharamappa, J. Validated Stability-Indicating Chromatographic Method for the Assay of Erlotinib Active Pharmaceutical Ingredient. Anal. Lett. 2009, 42, 1855–1867. [Google Scholar] [CrossRef]


| Condition | % Recovery of Erlotinib | Duration of Exposure | Number of Degradation Products Detected |
|---|---|---|---|
| Acidic (0.1 M HCl) | 97.7 | 21 days | 3 |
| Alkaline (0.1 M NaOH) | 98.8 | 21 days | 2 |
| Oxidative stress (3% H2O2) | 91.0 | 8 h | 8 |
| Photolytic (ICH Q1B light conditions) | 60.5 | 24 h | 6 |
| Conditions | Analysis Time | Temperature |
|---|---|---|
| Acidic (0.1 M HCl) | Day 0, 1, 7, 14, 21 | 50 °C |
| Alkaline (0.1 M NaOH) | Day 0, 1, 7, 14, 21 | 50 °C |
| Oxidative stress (3% H2O2) | 0, 8, 24, 48 h | 20 °C |
| Photolytic (ICH Q1B lamp) | 0, 4, 8 h | 20 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.; Secrétan, P.-H.; Cotteret, C.; Jacques-Gustave, E.; Greco, C.; Bodemer, C.; Schlatter, J.; Cisternino, S. Stability and Formulation of Erlotinib in Skin Creams. Molecules 2022, 27, 1070. https://doi.org/10.3390/molecules27031070
Nguyen D, Secrétan P-H, Cotteret C, Jacques-Gustave E, Greco C, Bodemer C, Schlatter J, Cisternino S. Stability and Formulation of Erlotinib in Skin Creams. Molecules. 2022; 27(3):1070. https://doi.org/10.3390/molecules27031070
Chicago/Turabian StyleNguyen, David, Philippe-Henri Secrétan, Camille Cotteret, Emmanuelle Jacques-Gustave, Céline Greco, Christine Bodemer, Joel Schlatter, and Salvatore Cisternino. 2022. "Stability and Formulation of Erlotinib in Skin Creams" Molecules 27, no. 3: 1070. https://doi.org/10.3390/molecules27031070
APA StyleNguyen, D., Secrétan, P.-H., Cotteret, C., Jacques-Gustave, E., Greco, C., Bodemer, C., Schlatter, J., & Cisternino, S. (2022). Stability and Formulation of Erlotinib in Skin Creams. Molecules, 27(3), 1070. https://doi.org/10.3390/molecules27031070