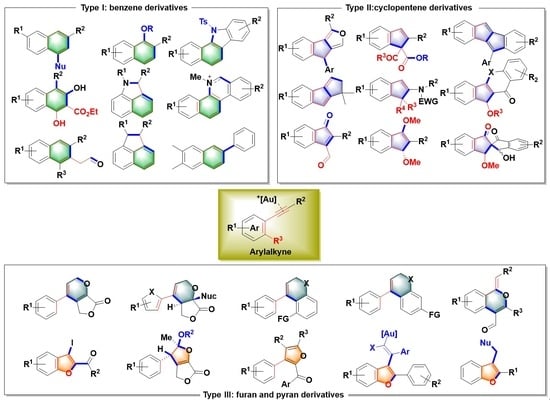
Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation
Abstract
1. Introduction
2. Syntheses of Benzene Derivatives
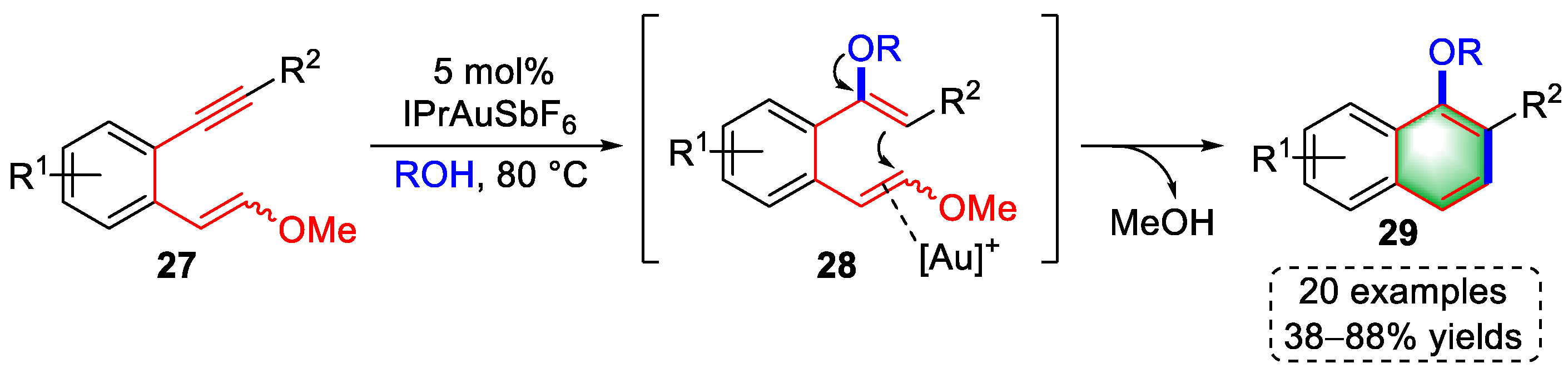
2.1. Arene-Diyne Substrates
2.2. Arene-Enyne Substrates
2.3. Aryne-Enolether Substrates
2.4. Other Arylalkyne Substrates
3. Construction of Cyclopentene Derivatives
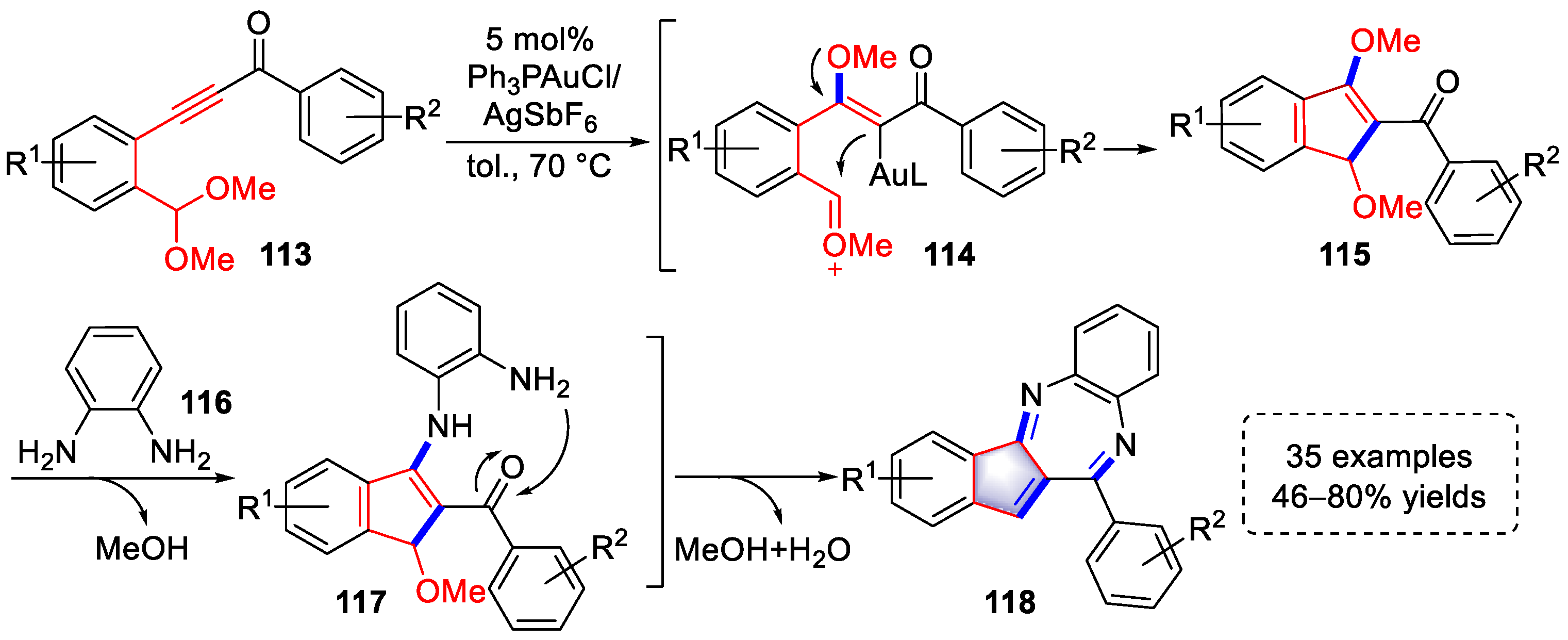
3.1. Arene-Diyne Substrates
3.2. Arene-Enyne Substrates
3.3. Aryne-Enolether Substrates
3.4. Aryne-Acetal Substrates
3.5. Other Arylalkyne Substrates
4. Construction of Furan and Pyran Derivatives
4.1. 1,5-Enyne Substrates
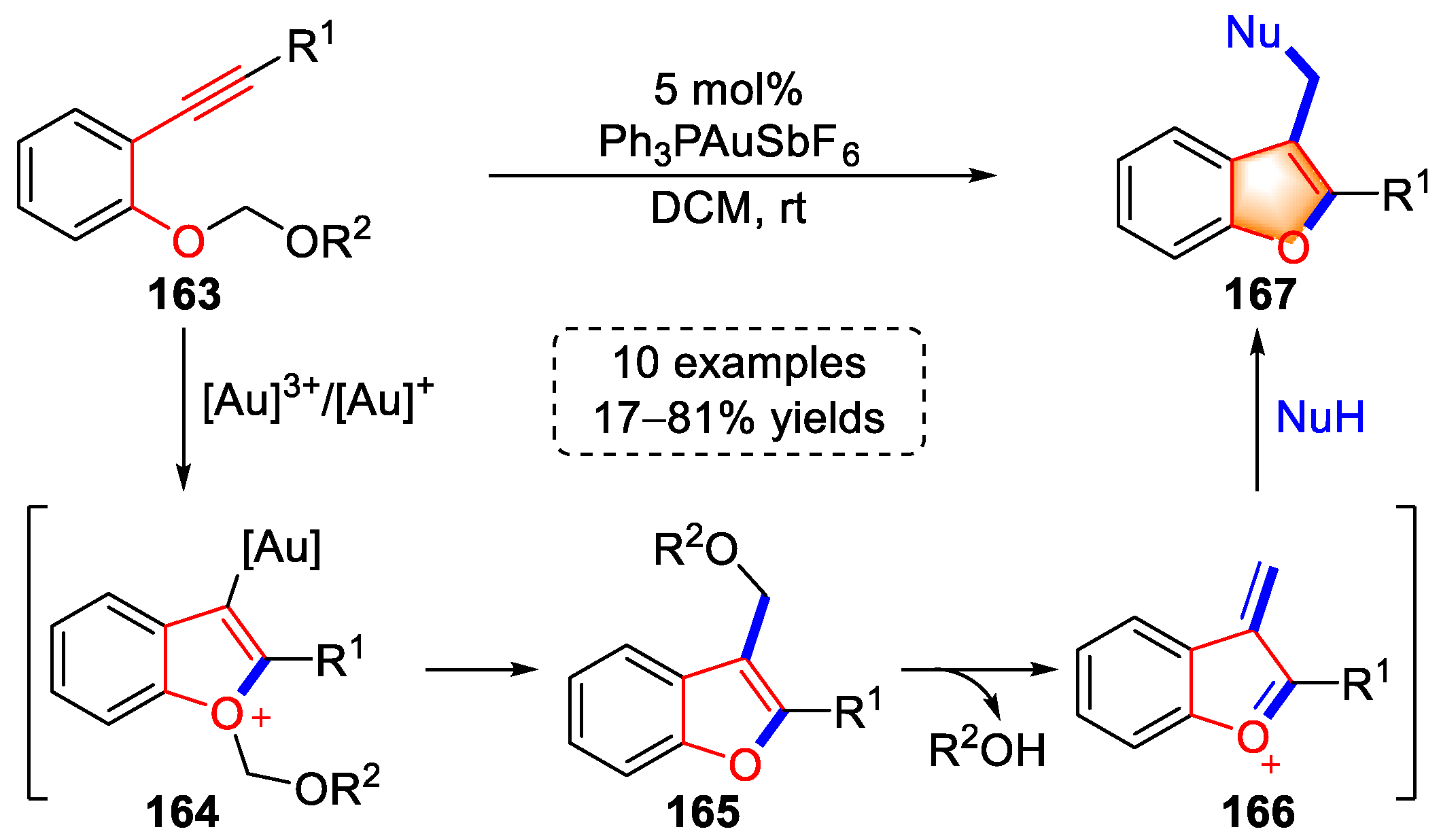
4.2. Alkyne–Phenol Substrates
4.3. Other Arylalkyne Substrates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, G.C.; Sermon, P.A.; Webb, G.; Buchanan, D.A.; Wells, P.B. Hydrogenation over supported gold catalysts. J. Chem. Soc. Chem. Commun. 1973, 13, 444–445. [Google Scholar] [CrossRef]
- Ito, Y.; Sawamura, M.; Hayashi, T. Catalytic asymmetric aldol reaction: Reaction of aldehydes with isocyanoacetate catalyzed by a chiral ferrocenylphosphine-gold(I) complex. J. Am. Chem. Soc. 1986, 108, 6405–6406. [Google Scholar] [CrossRef]
- Gadon, V. Modern gold catalyzed synthesis. Edited by A. Stephen, K. Hashmi and F. Dean Toste. Angew. Chem. Int. Ed. 2012, 51, 11200. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, W.B.; Zhang, J.L. Gold-catalyzed enantioselective annulations. Eur. Chem. J. 2017, 23, 467–512. [Google Scholar] [CrossRef]
- Teles, J.H.; Brode, S.; Chabanas, M. Cationic gold(I) complexes: Highly efficient catalysts for the addition of alcohols to alkynes. Angew. Chem., Int. Ed. 1998, 37, 1415–1418. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Rudolph, M. Gold catalysis in total synthesis. Chem. Soc. Rev. 2008, 37, 1766–1775. [Google Scholar] [CrossRef]
- Wittstock, A.; Bäumer, M. Catalysis by unsupported skeletal gold catalysts. Acc. Chem. Res. 2014, 47, 731–739. [Google Scholar] [CrossRef]
- Friend, C.M.; Hashmi, A.S. Gold catalysis. Acc. Chem. Res. 2014, 47, 729–730. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. Dual Gold Catalysis. Acc. Chem. Res. 2014, 47, 864–876. [Google Scholar] [CrossRef]
- Zhang, L.M. A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation. Acc. Chem. Res. 2014, 47, 877–888. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lackner, A.D.; Toste, F.D. Development of catalysts and ligands for enantioselective gold catalysis. Acc. Chem. Res. 2014, 47, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Obradors, C.; Echavarren, A.M. Gold-catalyzed rearrangements and beyond. Acc. Chem. Res. 2014, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Tang, X.Y.; Shi, M. Gold-catalyzed tandem reactions of methylenecyclopropanes and vinylidenecyclopropanes. Acc. Chem. Res. 2014, 47, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J.; Widenhoefer, R.A. Gold carbenes, gold-stabilized carbocations, and cationic intermediates relevant to gold-catalysed enyne cycloaddition. Chem. Soc. Rev. 2016, 45, 4533–4551. [Google Scholar] [CrossRef]
- Li, W.; Yu, B. Gold-catalyzed glycosylation in the synthesis of complex carbohydrate-containing natural products. Chem. Soc. Rev. 2018, 47, 7954–7984. [Google Scholar] [CrossRef]
- Lu, Z.C.; Hammond, G.B.; Xu, B. Improving homogeneous cationic gold catalysis through a mechanism-based approach. Acc. Chem. Res. 2019, 52, 1275–1288. [Google Scholar] [CrossRef]
- Greiner, L.C.; Matsuoka, J.; Inuki, S.; Ohno, H. Azido-alkynes in gold(I)-catalyzed indole syntheses. Chem. Rev. 2021, 21, 3897–3910. [Google Scholar] [CrossRef]
- Li, D.Y.; Zang, W.Q.; Bird, M.J.; Hyland, C.J.T.; Shi, M. Gold-catalyzed conversion of highly strained compounds. Chem. Rev. 2021, 121, 8685–8755. [Google Scholar] [CrossRef]
- Mato, M.; Franchino, A.; Garci, A.M.C.; Echavarren, A.M. Gold-catalyzed synthesis of small rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of medium-sized rings by gold catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef]
- Ghosh, T.; Chatterjee, J.; Bhakta, S. Gold-catalyzed hydroarylation reactions: A comprehensive overview. Org. Biomol. Chem. 2022, 20, 7151–7187. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Braun, I.; Rudolph, M.; Rominger, F. The role of gold acetylides as a selectivity trigger and the importance of gem-diaurated species in the gold-catalyzed hydroarylating-aromatization of arene-diynes. Organometallics 2012, 31, 644–661. [Google Scholar] [CrossRef]
- Naoe, S.; Suzuki, Y.; Hirano, K.; Inaba, Y.; Oishi, S.; Fujii, N.; Ohno, H. Gold(I)-catalyzed regioselective inter-/intramolecular addition cascade of di- and triynes for direct construction of substituted naphthalenes. J. Org. Chem. 2012, 77, 4907–4916. [Google Scholar] [CrossRef]
- Yu, L.Z.; Wei, Y.; Shi, M. Synthesis of polysubstituted polycyclic aromatic hydrocarbons by gold-catalyzed cyclization–oxidation of alkylidenecyclopropane-containing 1,5-enynes. ACS Catal. 2017, 7, 4242–4247. [Google Scholar] [CrossRef]
- Ikeuchi, T.; Inuki, S.; Oishi, S.; Ohno, H. Gold(I)-catalyzed cascade cyclization reactions of allenynes for the synthesis of fused cyclopropanes and acenaphthenes. Angew. Chem. Int. Ed. 2019, 58, 7792–7796. [Google Scholar] [CrossRef]
- Komatsu, H.; Ikeuchi, T.; Tsuno, H.; Arichi, N.; Yasui, K.; Oishi, S.; Inuki, S.; Fukazawa, A.; Ohno, H. Construction of tricyclic nitrogen heterocycles by a gold(I)-catalyzed cascade cyclization of allenynes and application to polycyclic π-electron systems. Angew. Chem. Int. Ed. 2021, 60, 27019–27025. [Google Scholar] [CrossRef]
- Fu, J.Y.; Li, B.B.; Wang, X.X.; Wang, H.; Deng, M.H.; Yang, H.L.; Lin, B.; Cheng, M.S.; Yang, L.; Liu, Y.X. Collective total syntheses of benzo[c]phenanthridine alkaloids via a sequential transition metal-catalyzed pot-economic approach. Org. Lett. 2022, 24, 8310–8315. [Google Scholar] [CrossRef]
- Liu, Y.X.; Guo, J.; Liu, Y.; Wang, X.Y.; Wang, Y.S.; Jia, X.Y.; Wei, G.F.; Chen, L.Z.; Xiao, J.Y.; Cheng, M.S. Au(I)-catalyzed triple bond alkoxylation/dienolether aromaticity-driven cascade cyclization to naphthalenes. Chem. Commun. 2014, 50, 6243–6245. [Google Scholar] [CrossRef]
- Peng, X.S.; Zhu, L.F.; Hou, Y.Q.; Pang, Y.D.; Li, Y.M.; Fu, J.Y.; Yang, L.; Lin, B.; Liu, Y.X.; Cheng, M.S. Access to benzo[a]carbazoles and indeno[1,2-c]quinolines by a gold(I)-catalyzed tunable domino cyclization of difunctional 1,2-diphenylethynes. Org. Lett. 2017, 19, 3402–3405. [Google Scholar] [CrossRef]
- Xiong, Z.L.; Zhang, X.H.; Li, Y.M.; Peng, X.S.; Fu, J.Y.; Guo, J.; Xie, F.K.; Jiang, C.G.; Lin, B.; Liu, Y.X.; et al. Syntheses of 12H-benzo[a]xanthen-12-ones and benzo[a]acridin-12(7H)-ones through Au(I)-catalyzed Michael addition/6-endo-trig cyclization/aromatization cascade annulation. Org. Biomol. Chem. 2018, 16, 7361–7374. [Google Scholar] [CrossRef]
- Fu, J.Y.; Li, B.B.; Wang, X.G.; Liang, Q.D.; Peng, X.S.; Yang, L.; Wan, T.; Wang, X.X.; Lin, B.; Cheng, M.S. Au(I)-catalyzed 6-endo-dig cyclizations of aromatic 1,5-enynes to 2-(naphthalen-2-yl)anilines leading to divergent syntheses of benzo[α]carbazole, benzo[c,h]cinnoline and dibenzo[i]phenanthridine derivatives. Chin. J. Chem. 2022, 40, 46–52. [Google Scholar] [CrossRef]
- Shu, C.; Chen, C.B.; Chen, W.X.; Ye, L.W. Flexible and practical synthesis of anthracenes through gold-catalyzed cyclization of o-alkynyldiarylmethanes. Org. Lett. 2013, 15, 5542–5545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, S.F.; Niu, Y.J.; Liu, C.J.; Zhu, L.F.; Li, Y.M.; Cui, S.S.; Xiong, Z.L.; Cheng, M.S.; Lin, B.; Liu, Y.X. Gold(I)-initiated cycloisomerization/Diels-Alder/retro-Diels-Alder cascade strategy to biaryls. J. Org. Chem. 2017, 82, 9066–9074. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Q.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-catalyzed regiodivergent annulations of diazo-alkynes controlled by Et3N(HF)3. ACS Catal. 2021, 11, 15203–15211. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.F.; Xie, X.; Liu, J.; Liu, Y.H. Gold-catalyzed furan/yne cyclizations for the regiodefined assembly of multisubstituted protected 1-naphthols. J. Org. Chem. 2012, 77, 1915–1921. [Google Scholar] [CrossRef]
- Song, X.R.; Xia, X.F.; Song, Q.B.; Yang, F.; Li, Y.X.; Liu, X.Y.; Liang, Y.M. Gold-catalyzed cascade reaction of hydroxy enynes for the synthesis of oxanorbornenes and naphthalene derivatives. Org. Lett. 2012, 14, 3344–3347. [Google Scholar] [CrossRef]
- Taguchi, M.; Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Synthesis of fused carbazoles by gold-catalyzed tricyclization of conjugated diynes via rearrangement of an N-propargyl group. Org. Lett. 2015, 17, 6250–6253. [Google Scholar] [CrossRef]
- Kale, B.S.; Liu, R.S. Gold-catalyzed aromatizations of 3-ene-5-siloxy-1,6-diynes with nitrosoarenes to enable 1,4-N,O-functionalizations: One-pot construction of 4-hydroxy-3-aminobenzaldehyde cores. Org. Lett. 2019, 21, 8434–8438. [Google Scholar] [CrossRef]
- Milian, A.; Garcia-Garcia, P.; Perez-Redondo, A.; Sanz, R.; Vaquero, J.J.; Fernandez-Rodriguez, M.A. Selective synthesis of phenanthrenes and dihydrophenanthrenes via gold-catalyzed cycloisomerization of biphenyl embedded trienynes. Org. Lett. 2020, 22, 8464–8469. [Google Scholar] [CrossRef]
- Koshikawa, T.; Nagashima, Y.; Tanaka, K. Gold-catalyzed [3 + 2] annulation, carbenoid transfer, and C–H insertion cascade: Elucidation of annulation mechanisms via benzopyrylium intermediates. ACS Catal. 2021, 11, 1932–1937. [Google Scholar] [CrossRef]
- Wang, H.F.; Guo, L.N.; Fan, Z.B.; Tang, T.H.; Zi, W.W. Gold-catalyzed formal hexadehydro-Diels-Alder/carboalkoxylation reaction cascades. Org. Lett. 2021, 23, 2676–2681. [Google Scholar] [CrossRef]
- Bharath Kumar, P.; Raju, C.E.; Chandubhai, P.H.; Sridhar, B.; Karunakar, G.V. Gold(I)-catalyzed regioselective cyclization to access cyclopropane-fused tetrahydrobenzochromenes. Org. Lett. 2022, 24, 6761–6766. [Google Scholar] [CrossRef]
- Fu, J.Y.; Li, B.B.; Zhou, Z.F.; Cheng, M.S.; Yang, L.; Liu, Y.X. Formal total synthesis of macarpine via a Au(I)-catalyzed 6-endo-dig cycloisomerization strategy. Beilstein J. Org. Chem. 2022, 18, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Samala, S.; Mandadapu, A.K.; Saifuddin, M.; Kundu, B. Gold-catalyzed sequential alkyne activation: One-pot synthesis of NH-carbazoles via cascade hydroarylation of alkyne/6-endo-dig carbocyclization reactions. J. Org. Chem. 2013, 78, 6769–6774. [Google Scholar] [CrossRef]
- Li, N.; Lian, X.L.; Li, Y.H.; Wang, T.Y.; Han, Z.Y.; Zhang, L.; Gong, L.Z. Gold-catalyzed direct assembly of aryl-annulated carbazoles from 2-alkynyl arylazides and alkynes. Org. Lett. 2016, 18, 4178–4181. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, D.T.; Niu, Z.J.; Li, M.; Shi, W.Y.; Wang, C.T.; Wei, W.X.; Liang, Y.M. Gold-catalyzed tandem annulations of pyridylhomopropargylic alcohols with propargyl alcohols. Org. Lett. 2021, 23, 832–836. [Google Scholar] [CrossRef]
- Pandit, Y.B.; Liu, R.S. Dynamic kinetic resolution in gold-catalyzed (4 + 2)-annulations between alkynyl benzaldehydes and allenamides to yield enantioenriched all-carbon diarylalkylmethane derivatives. Org. Lett. 2022, 24, 548–553. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Braun, I.; Nosel, P.; Schadlich, J.; Wieteck, M.; Rudolph, M.; Rominger, F. Simple gold-catalyzed synthesis of benzofulvenes-gem-diaurated species as “instant dual-activation” precatalysts. Angew. Chem. Int. Ed. 2012, 51, 4456–4460. [Google Scholar] [CrossRef]
- Wurm, T.; Bucher, J.; Duckworth, S.B.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Catalyzed generation of vinyl cations from 1,5-diynes. Angew. Chem. Int. Ed. 2017, 56, 3364–3368. [Google Scholar] [CrossRef]
- Hu, C.; Farshadfar, K.; Dietl, M.C.; Cervantes-Reyes, A.; Wang, T.; Adak, T.; Rudolph, M.; Rominger, F.; Li, J.; Ariafard, A.; et al. Gold-catalyzed [5,5]-rearrangement. ACS Catal. 2021, 11, 6510–6518. [Google Scholar] [CrossRef]
- Sanjuan, A.M.; Virumbrales, C.; Garcia-Garcia, P.; Fernandez-Rodriguez, M.A.; Sanz, R. Formal [4 + 1] cycloadditions of β,β-diaryl-substituted ortho-(alkynyl)styrenes through gold(I)-catalyzed cycloisomerization reactions. Org. Lett. 2016, 18, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Virumbrales, C.; El-Remaily, M.; Suarez-Pantiga, S.; Fernandez-Rodriguez, M.A.; Rodriguez, F.; Sanz, R. Gold(I) catalysis applied to the stereoselective synthesis of indeno[2,1-b]thiochromene derivatives and seleno analogues. Org. Lett. 2022, 24, 8077–8082. [Google Scholar] [CrossRef]
- Guo, J.; Peng, X.S.; Wang, X.Y.; Xie, F.K.; Zhang, X.H.; Liang, G.D.; Sun, Z.H.; Liu, Y.X.; Cheng, M.S.; Liu, Y. A gold-catalyzed cycloisomerization/aaerobic oxidation cascade strategy for 2-aryl indenones from 1,5-enynes. Org. Biomol. Chem. 2018, 16, 9147–9151. [Google Scholar] [CrossRef]
- Zi, W.W.; Toste, F.D. Gold(I)-catalyzed enantioselective carboalkoxylation of alkynes. J. Am. Chem. Soc. 2013, 135, 12600–12603. [Google Scholar] [CrossRef]
- Jiang, C.G.; Xiong, Z.L.; Jin, S.F.; Gao, P.; Tang, Y.Z.; Wang, Y.S.; Du, C.; Wang, X.Y.; Liu, Y.; Lin, B.; et al. A Au(I)-catalyzed hydrogen bond-directed tandem strategy to synthesize indeno-chromen-4-one and indeno-quinolin-4-one derivatives. Chem. Commun. 2016, 52, 11516–11519. [Google Scholar] [CrossRef]
- Yamada, T.; Park, K.; Tachikawa, T.; Fujii, A.; Rudolph, M.; Hashmi, A.S.K.; Sajiki, H. Gold-catalyzed cyclization of 2-alkynylaldehyde cyclic acetals via hydride shift for the synthesis of indenone derivatives. Org. Lett. 2020, 22, 1883–1888. [Google Scholar] [CrossRef]
- Xie, F.K.; Zhang, B.; Chen, Y.Y.; Jia, H.W.; Sun, L.; Zhuang, K.T.; Yin, L.L.; Cheng, M.S.; Lin, B.; Liu, Y.X. A gold(I)-catalyzed tandem cyclization to benzo[b]indeno[1,2-e][1,4]diazepines from o-phenylenediamines and ynones. Adv. Synth. Catal. 2020, 362, 3886–3897. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, S.; Ding, Z.X.; Hou, A.B.; Fu, J.Y.; Su, H.W.; Cheng, M.S.; Lin, B.; Yang, L.; Liu, Y.X. Gold(I)-catalyzed tandem intramolecular methoxylation/double aldol condensation strategy yielding 2,2′-spirobi[indene] derivatives. Org. Lett. 2022, 24, 6777–6782. [Google Scholar] [CrossRef]
- Bao, M.; Chen, J.Z.; Pei, C.; Zhang, S.J.; Lei, J.P.; Hu, W.H.; Xu, X.F. Gold-catalyzed ketene dual functionalization and mechanistic insights: Divergent synthesis of indenes and benzo[d]oxepines. Sci. Chin. Chem. 2021, 64, 778–787. [Google Scholar] [CrossRef]
- Thilmany, P.; Guarnieri-Ibáñez, A.; Jacob, C.; Lacour, J.; Evano, G. Straightforward synthesis of indenes by gold-catalyzed intramolecular hydroalkylation of ynamides. ACS Org. Inorg. Au 2021, 2, 53–58. [Google Scholar] [CrossRef]
- Greiner, L.C.; Arichi, N.; Inuki, S.; Ohno, H. Gold(I)-catalyzed benzylic C(sp3)-H functionalizations: Divergent synthesis of indole[a]- and [b]-fused polycycles. Angew. Chem. Int. Ed. 2022, e202213653. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Feng, L.S.; Wang, Y.L.; Zhang, F.; Bai, L.Y.; Deng, J.L. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266–276. [Google Scholar] [CrossRef]
- Jin, S.F.; Jiang, C.G.; Peng, X.S.; Shan, C.H.; Cui, S.S.; Niu, Y.Y.; Liu, Y.; Lan, Y.; Liu, Y.X.; Cheng, M.S. Gold(I)-catalyzed angle strain controlled strategy to furopyran derivatives from propargyl vinyl ethers: Insight into the regioselectivity of cycloisomerization. Org. Lett. 2016, 18, 680–683. [Google Scholar] [CrossRef]
- Liu, Y.X.; Jin, S.F.; Wang, Y.S.; Cui, S.S.; Peng, X.S.; Niu, Y.Y.; Du, C.; Cheng, M.S. A gold(I)-catalyzed substituent-controlled cycloisomerization of propargyl vinyl ethers to multi-substituted furofuran and furopyran derivatives. Chem. Commun. 2016, 52, 6233–6236. [Google Scholar] [CrossRef]
- Ding, D.; Mou, T.; Feng, M.G.; Jiang, X.F. Utility of ligand effect in homogenous gold catalysis: Enabling regiodivergent π-bond-activated cyclization. J. Am. Chem. Soc. 2016, 138, 5218–5221. [Google Scholar] [CrossRef]
- Obata, T.; Suzuki, S.; Nakagawa, A.; Kajihara, R.; Noguchi, K.; Saito, A. Gold-catalyzed domino synthesis of functionalized benzofurans and tetracyclic isochromans via formal carboalkoxylation. Org. Lett. 2016, 18, 4136–4139. [Google Scholar] [CrossRef]
- Fernandez-Canelas, P.; Rubio, E.; Gonzalez, J.M. Oxyacylation of iodoalkynes: Gold(I)-catalyzed expeditious access to benzofurans. Org. Lett. 2019, 21, 6566–6569. [Google Scholar] [CrossRef]
- Wu, J.W.; Wei, C.B.; Zhao, F.; Du, W.Q.; Geng, Z.S.; Xia, Z.H. Gold(I)-catalyzed tandem cyclization/hydroarylation of o-alkynylphenols with haloalkynes. J. Org. Chem. 2022, 87, 14374–14383. [Google Scholar] [CrossRef]
- Bao, M.; Qian, Y.; Su, H.; Wu, B.; Qiu, L.H.; Hu, W.H.; Xu, X.F. Gold(I)-catalyzed and H2O-mediated carbene cascade reaction of propargyl diazoacetates: Furan synthesis and mechanistic insights. Org. Lett. 2018, 20, 5332–5335. [Google Scholar] [CrossRef]
- Zhao, J.D.; Xu, W.; Xie, X.; Sun, N.; Li, X.D.; Liu, Y.H. Gold-catalyzed oxidative cyclizations of {o-(alkynyl)phenyl propargyl} silyl ether derivatives involving 1,2-enynyl migration: Synthesis of functionalized 1H-isochromenes and 2H-pyrans. Org. Lett. 2018, 20, 5461–5465. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Zhu, Y.; Li, Y.; Yu, B. Mechanistic insights into the gold(I)-catalyzed activation of glycosyl ortho-alkynylbenzoates for glycosidation. J. Am. Chem. Soc. 2013, 135, 18396–18405. [Google Scholar] [CrossRef]
- Aparece, M.D.; Vadola, P.A. Gold-catalyzed dearomative spirocyclization of aryl alkynoate esters. Org. Lett. 2014, 16, 6008–6011. [Google Scholar] [CrossRef]
- Mallampudi, N.A.; Reddy, G.S.; Maity, S.; Mohapatra, D.K. Gold(I)-catalyzed cyclization for the synthesis of 8-hydroxy-3- substituted isocoumarins: Total synthesis of exserolide F. Org. Lett. 2017, 19, 2074–2077. [Google Scholar] [CrossRef]
- Hamada, N.; Yamaguchi, A.; Inuki, S.; Oishi, S.; Ohno, H. Gold(I)-catalyzed oxidative cascade cyclization of 1,4-diyn-3-ones for the construction of tropone-fused furan scaffolds. Org. Lett. 2018, 20, 4401–4405. [Google Scholar] [CrossRef]
- Wagner, P.; Ghosh, N.; Gandon, V.; Blond, G. Solvent effect in gold(I)-catalyzed domino reaction: Access to furopyrans. Org. Lett. 2020, 22, 7333–7337. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Ding, D.; Sun, Y.X.; Dong, J.L. Gold(III)-catalyzed three-component coupling reaction (TCC) selective toward furans. Org. Lett. 2013, 15, 2884–2887. [Google Scholar] [CrossRef]
- Hosseyni, S.; Su, Y.J.; Shi, X.D. Gold catalyzed synthesis of substituted furan by intermolecular cascade reaction of propargyl alcohol and alkyne. Org. Lett. 2015, 17, 6010–6013. [Google Scholar] [CrossRef]
- Campeau, D.; León Rayo, D.F.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-catalyzed reactions of specially activated alkynes, allenes, and alkenes. Chem. Rev. 2021, 121, 8756–8867. [Google Scholar] [CrossRef]
- Shandilya, S.; Gogoi, M.P.; Dutta, S.; Sahoo, A.K. Gold-catalyzed transformation of ynamides. Chem. Rec. 2021, 21, 4123–4149. [Google Scholar] [CrossRef]
- Bag, D.; Sawant, S.D. Heteroarene-tethered functionalized alkyne metamorphosis. Chem. Eur. J. 2021, 27, 1165–1218. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Tlahuext-Aca, A.; Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 2016, 49, 2261–2272. [Google Scholar] [CrossRef]



































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Su, H.; Sun, Y.; Zhang, S.; Cheng, M.; Liu, Y. Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation. Molecules 2022, 27, 8956. https://doi.org/10.3390/molecules27248956
Yang L, Su H, Sun Y, Zhang S, Cheng M, Liu Y. Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation. Molecules. 2022; 27(24):8956. https://doi.org/10.3390/molecules27248956
Chicago/Turabian StyleYang, Lu, Hongwei Su, Yue Sun, Sen Zhang, Maosheng Cheng, and Yongxiang Liu. 2022. "Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation" Molecules 27, no. 24: 8956. https://doi.org/10.3390/molecules27248956
APA StyleYang, L., Su, H., Sun, Y., Zhang, S., Cheng, M., & Liu, Y. (2022). Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation. Molecules, 27(24), 8956. https://doi.org/10.3390/molecules27248956