On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 2: EGA-FTIR
Abstract
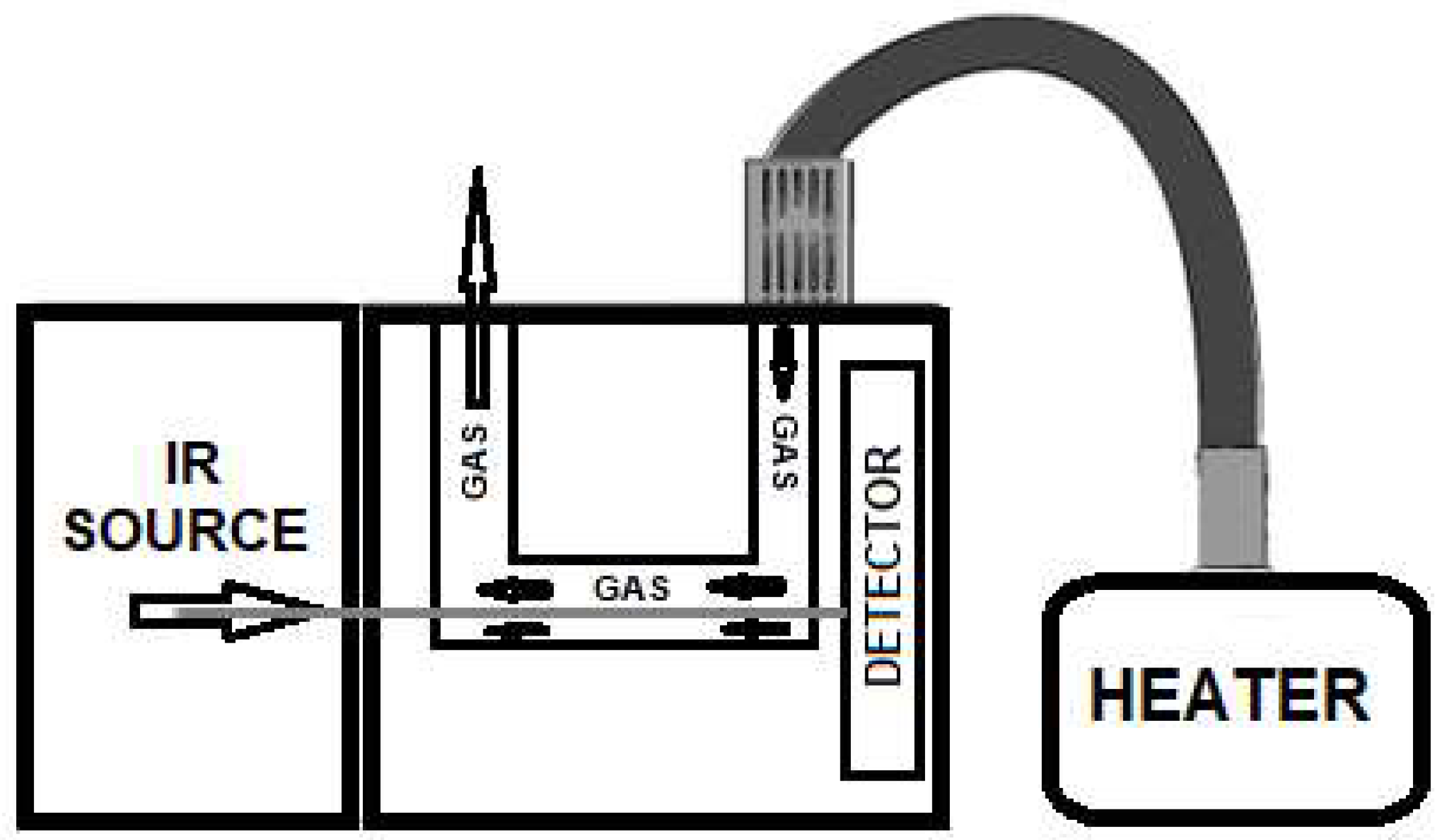
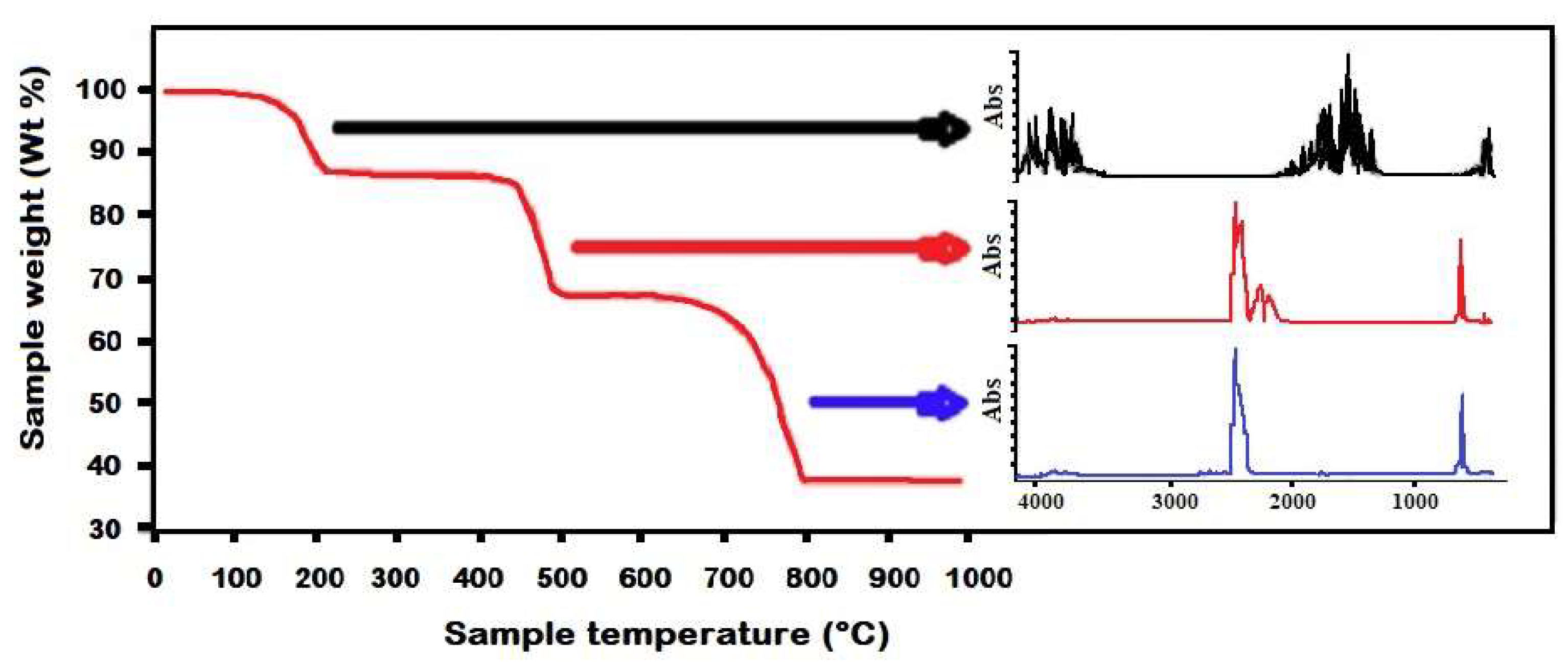
1. Introduction
2. Applications to Polymers
3. Applications to Complexes and Compounds
4. Applications to Metal–Organic Frameworks
5. Applications to Catalyst
6. Applications to Flame Retardants
7. Applications to Epoxy Resins
8. Applications to Biomass
9. Applications to Oil and Bitumen
10. Applications to Cellulose, Lignin and Lignite
11. Applications to Materials and Composites
12. Applications to Nanoparticles and Nanomaterials
13. Applications to Zeolites
14. Applications to Clays
15. Applications to Coal
16. Applications to Membranes
17. Applications to Coatings
18. Applications to Environment and Health
19. Applications to Sewage Sludge
20. Applications to Sulphur
21. Applications to Tobacco
22. Applications to Explosives and Propellants
23. Applications to Waste Materials
24. Applications to Food
25. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGA | Evolved gas Analysis |
| TG | ThermoGravimetry |
| TGA | ThermoGravimetric Analysis |
| MS | Mass Spectrometry |
| QMS | Quadrupole Mass Spectrometry |
| FTIR | Fourier-Transform InfraRed spectroscopy |
| UV | UltraViolet spectroscopy |
| XRD | X-Ray Diffraction |
| PMMA | Polymethylmethacrilate |
| DART | Direct analysis in real time |
| DFT | Density Functional Theory |
| DSC | Differential Scanning Calorimetry |
| DTA | Differential Thermal Analysis |
| ICP-OES | Inductively Coupled Plasma—Optical Emission Spectroscopy |
| MOF | metal Organic Framework |
| DRIFTs | Diffuse Reflectance Infrared Fourier-Transform Spectroscopy |
| SEM | Scanning Electron Microscopy |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| FID | Flame Ionization Detector |
| HS-GC-MS | Head Space GasChromatography/Mass Spectrometry |
| Py-GC/MS | Pyrolysis-GasChromatography/Mass Spectrometry |
| PCA | Principal Component Analysis |
| OLTI-EGA | On-Line Thermally Induced Evolved Gas Analysis |
| NMR | Nuclear Magnetic Resonance |
References
- Materazzi, S. Thermogravimetry—Infrared Spectroscopy (TG-FTIR) Coupled Analysis. Appl. Spectrosc. Rev. 1997, 32, 385–404. [Google Scholar] [CrossRef]
- Materazzi, S. Mass Spectrometry Coupled to Thermogravimetry (TG-MS) for Evolved Gas Characterization: A Review. Appl. Spectrosc. Rev. 1998, 33, 189–218. [Google Scholar] [CrossRef]
- Materazzi, S.; Curini, R. On-Line Evolved Gas Analysis by Infrared Spectroscopy Coupled to Thermoanalytical Instruments. Appl. Spectrosc. Rev. 2001, 36, 1. [Google Scholar] [CrossRef]
- Materazzi, S.; Curini, R. The Coupling of Mass Spectrometry with Thermoanalytical Instruments: Applications of Evolved Gas Analysis. Appl. Spectrosc. Rev. 2001, 36, 169. [Google Scholar] [CrossRef]
- Materazzi, S.; Gentili, A.; Curini, R. Applications of Evolved Gas Analysis: Part 1: EGA by Infrared Spectroscopy. Talanta 2006, 68, 489–496. [Google Scholar] [CrossRef]
- Materazzi, S.; Gentili, A.; Curini, R. Applications of Evolved Gas Analysis. Part 2: EGA by Mass Spectrometry. Talanta 2006, 69, 781–794. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S. Evolved Gas Analysis by Infrared Spectroscopy. Appl. Spectrosc. Rev. 2010, 45, 241–273. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S. Evolved Gas Analysis by Mass Spectrometry. Appl. Spectrosc. Rev. 2011, 46, 261–340. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S. Recent Applications of Evolved Gas Analysis by Infrared Spectroscopy (IR-EGA). Appl. Spectrosc. Rev. 2013, 48, 654–689. [Google Scholar] [CrossRef]
- Risoluti, R.; Fabiano, M.A.; Gullifa, G.; Vecchio Ciprioti, S.; Materazzi, S. FTIR-Evolved Gas Analysis in Recent Thermoanalytical Investigations. Appl. Spectrosc. Rev. 2017, 52, 39–72. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S. Mass Spectrometry for Evolved Gas Analysis: An Update. Appl. Spectrosc. Rev. 2019, 54, 87–116. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Barone, L.; Papa, E.; Materazzi, S. On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 1: EGA-MS. Molecules 2022, 27, 3518. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Chetwynd, A.J.; Kelly, C.A.; Stark, C.; Valsami-Jones, E.; Krause, S.; Lynch, I. An Untargeted Thermogravimetric Analysis-Fourier Transform Infrared-Gas Chromatography-Mass Spectrometry Approach for Plastic Polymer Identification. Environ. Sci. Technol. 2021, 55, 8721–8729. [Google Scholar] [CrossRef] [PubMed]
- Łyszczek, R.; Rusinek, I.; Sienkiewicz-Gromiuk, J.; Iwan, M.; Pavlyuk, O. 3-D Lanthanide Coordination Polymers with the Flexible 1,3-Phenylenediacetate Linker: Spectroscopic, Structural and Thermal Investigations. Polyhedron 2019, 159, 93–101. [Google Scholar] [CrossRef]
- Budrugeac, P.; Cucos, A.; Dascălu, R.; Atkinson, I.; Osiceanu, P. Application of Model-Free and Multivariate Nonlinear Regression Methods for Evaluation of the Kinetic Scheme and Kinetic Parameters of Thermal Decomposition of Low Density Polyethylene. Thermochim. Acta 2022, 708, 179138. [Google Scholar] [CrossRef]
- Ostasz, A.; Kirillov, A.M. Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers. Polymers 2020, 12, 1329. [Google Scholar] [CrossRef]
- Tang, W.; Zeng, T.; Hu, J.; Li, J.; Yang, R. Investigation on the Thermal Decomposition of the Elastomer Containing Fluoroolefin Segment by DSC-TG-MS-FTIR. Polym. Adv. Technol. 2021, 32, 4880–4890. [Google Scholar] [CrossRef]
- Godiya, C.B.; Gabrielli, S.; Materazzi, S.; Pianesi, M.S.; Stefanini, N.; Marcantoni, E. Depolymerization of Waste Poly(Methyl Methacrylate) Scraps and Purification of Depolymerized Products. J. Environ. Manag. 2019, 231, 1012–1020. [Google Scholar] [CrossRef]
- Pannase, A.M.; Singh, R.K.; Ruj, B.; Gupta, P. Decomposition of Polyamide via Slow Pyrolysis: Effect of Heating Rate and Operating Temperature on Product Yield and Composition. J. Anal. Appl. Pyrolysis 2020, 151, 104886. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation Markers and Plasticizer Loss of Cellulose Acetate Films during Ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Yu, Y.; Chen, T.; Song, N.; Chen, Z.; Lin, Z.; Jiang, J. Effects of Melamine Polyphosphate on Explosion Characteristics and Thermal Pyrolysis Behavior of Polyamide 66 Dust. J. Loss Prev. Process Ind. 2022, 78, 104820. [Google Scholar] [CrossRef]
- Yin, N.; Song, Y.; Zhou, J.; Tian, Y.; Yang, A. Effects of Direct Coal Liquefaction Residue Additions on Low-Rank Pulverized Coal during Co-Pyrolysis. Mater. Sci. Forum 2020, 999, 167–177. [Google Scholar] [CrossRef]
- Goedecke, C.; Dittmann, D.; Eisentraut, P.; Wiesner, Y.; Schartel, B.; Klack, P.; Braun, U. Evaluation of Thermoanalytical Methods Equipped with Evolved Gas Analysis for the Detection of Microplastic in Environmental Samples. J. Anal. Appl. Pyrolysis 2020, 152, 104961. [Google Scholar] [CrossRef]
- Caliskan, E.; Shishatskiy, S.; Neumann, S.; Abetz, V.; Filiz, V. Investigation of the Side Chain Effect on Gas and Water Vapor Transport Properties of Anthracene-Maleimide Based Polymers of Intrinsic Microporosity. Polymers 2022, 14, 119. [Google Scholar] [CrossRef]
- Scala, A.; Piperno, A.; Micale, N.; Mineo, P.G.; Abbadessa, A.; Risoluti, R.; Castelli, G.; Bruno, F.; Vitale, F.; Cascio, A.; et al. “Click” on PLGA-PEG and Hyaluronic Acid: Gaining Access to Anti-Leishmanial Pentamidine Bioconjugates. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 106, 2778–2785. [Google Scholar] [CrossRef]
- Gacki, M.; Kafarska, K.; Wolf, W.M. A Supramolecular Polymeric Chain in the Cobalt(II) Complex with Diclofenac: Synthesis, Crystal Structure, Spectroscopic, Thermal and Antioxidant Activity. J. Coord. Chem. 2019, 72, 3481–3494. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Abaas, M. Comparison of Oxidation Behavior of Linear and Branched Alkanes. Fuel Process. Technol. 2019, 188, 203–211. [Google Scholar] [CrossRef]
- Zapała, L.; Kosińska, M.; Woźnicka, E.; Byczyński, Ł.; Ciszkowicz, E.; Lecka-Szlachta, K.; Zapała, W.; Chutkowski, M. Comparison of Spectral and Thermal Properties and Antibacterial Activity of New Binary and Ternary Complexes of Sm(III), Eu(III) and Gd(III) Ions with N-Phenylanthranilic Acid and 1,10-Phenanthroline. Thermochim. Acta 2019, 671, 134–148. [Google Scholar] [CrossRef]
- Świderski, G.; Łyszczek, R.; Wojtulewski, S.; Kalinowska, M.; Świsłocka, R.; Lewandowski, W. Comparison of Structural, Spectroscopic, Theoretical and Thermal Properties of Metal Complexes (Zn(II), Mn(II), Cu(II), Ni(II) and Co(II)) of Pyridazine-3-Carboxylic Acid and Pyridazine-4-Carboxylic Acids. Inorg. Chim. Acta 2020, 512, 119865. [Google Scholar] [CrossRef]
- Ferenc, W.; Osypiuk, D.; Sarzyński, J.; Głuchowska, H. Complexes of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) with Ligand Formed by Condensation Reaction of Isatin with Glutamic Acid. Eclética Química 2020, 45, 12–27. [Google Scholar] [CrossRef]
- Zapała, L.; Kosińska-Pezda, M.; Byczyński, Ł.; Zapała, W.; Maciołek, U.; Woźnicka, E.; Ciszkowicz, E.; Lecka-Szlachta, K. Green Synthesis of Niflumic Acid Complexes with Some Transition Metal Ions (Mn(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II)). Spectroscopic, Thermoanalytical and Antibacterial Studies. Thermochim. Acta 2021, 696, 178814. [Google Scholar] [CrossRef]
- Worzakowska, M.; Sztanke, M.; Sztanke, K. Decomposition Course of Anticancer Active Imidazolidine-Based Hybrids with Diethyl Butanedioate Studied by TG/FTIR/QMS-Coupled Method. J. Anal. Appl. Pyrolysis 2019, 143, 104686. [Google Scholar] [CrossRef]
- Głuchowska, H.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Effect of N,N′-Dimethylformamide Solvent on Structure and Thermal Properties of Lanthanide(III) Complexes with Flexible Biphenyl-4,4′-Dioxydiacetic Acid. J. Therm. Anal. Calorim. 2022, 147, 1187–1200. [Google Scholar] [CrossRef]
- Rasi, S.; Ricart, S.; Obradors, X.; Puig, T.; Roura-Grabulosa, P.; Farjas, J. Effect of Triethanolamine on the Pyrolysis of Metal-Propionate-Based Solutions. J. Anal. Appl. Pyrolysis 2019, 143, 104685. [Google Scholar] [CrossRef]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Heterometallic Di- and Trinuclear CuIILnIII (LnIII = La, Ce, Pr, Nd) Complexes with an Alcohol-Functionalized Compartmental Schiff Base Ligand: Syntheses, Crystal Structures, Thermal and Magnetic Studies. Polyhedron 2020, 188, 114703. [Google Scholar] [CrossRef]
- Materazzi, S.; Foti, C.; Crea, F.; Risoluti, R.; Finamore, J. Biomimetic Complexes of Divalent Cobalt and Zinc with N-Heterocyclic Dicarboxylic Ligands. Thermochim. Acta 2014, 580, 7–12. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S.; Wo, L.W.; De Angelis Curtis, S. TG-MS and TG-FTIR Studies of Imidazole-Substituted Coordination Compounds: Co(II) and Ni(II)-Complexes of Bis(1-Methylimidazol-2-Yl)Ketone. Thermochim. Acta 2012, 543, 183–187. [Google Scholar] [CrossRef]
- Risoluti, R.; Piazzese, D.; Napoli, A.; Materazzi, S. Study of [2-(2′-Pyridyl)Imidazole] Complexes to Confirm Two Main Characteristic Thermoanalytical Behaviors of Transition Metal Complexes Based on Imidazole Derivatives. J. Anal. Appl. Pyrolysis 2016, 117, 82–87. [Google Scholar] [CrossRef]
- Materazzi, S. The Decomposition Mechanism of Noradrenaline Complexes with Transition-Metal Ions: A Coupled TG-FT-IR Study. Thermochim. Acta 1998, 319, 131–138. [Google Scholar] [CrossRef]
- Materazzi, S.; Aquili, S.; Bianchetti, C.; D’Ascenzo, G.; Kadish, K.M.; Bear, J.L. The Decomposition Mechanism of New Solid-State 4(5)-Aminoimidazole-5(4)-Carboxamide Coordination Compounds. Thermochim. Acta 2004, 409, 145–150. [Google Scholar] [CrossRef]
- Materazzi, S.; Finamore, J.; Risoluti, R.; Napoli, A. Biomimetic Complexes of Co(II), Cu(II) and Ni(II) with 2-Aminomethylbenzimidazole. EGA-MS Characterization of the Thermally Induced Decomposition. Microchem. J. 2014, 115, 27–31. [Google Scholar] [CrossRef]
- Materazzi, S.; Risoluti, R.; Napoli, A. EGA-MS Study to Characterize the Thermally Induced Decomposition of Co(II), Ni(II), Cu(II) and Zn(II) Complexes with 1,1-Diaminobutane-Schiff Base. Thermochim. Acta 2015, 606, 90–94. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fabiano, M.A.; Wo, L.W.; Materazzi, S. Biomimetic Complexes of Cd(II), Mn(II), and Zn(II) with 2-Aminomethylbenzimidazole. EGA/MS Characterization of the Thermally Induced Decomposition. Russ. J. Gen. Chem. 2017, 87, 300–304. [Google Scholar] [CrossRef]
- Materazzi, S.; Gullifa, G.; Fabiano, M.A.; Frati, P.; Santurro, A.; Scopetti, M.; Fineschi, V.; Risoluti, R. New Frontiers in Thermal Analysis: A TG/Chemometrics Approach for Postmortem Interval Estimation in Vitreous Humor. J. Therm. Anal. Calorim. 2017, 130, 549–557. [Google Scholar] [CrossRef]
- Materazzi, S.; Napoli, A.; Risoluti, R.; Finamore, J.; D’Arienzo, S. Characterization of Thermally Induced Mechanisms by Mass Spectrometry-Evolved Gas Analysis (EGA-MS): A Study of Divalent Cobalt and Zinc Biomimetic Complexes with N-Heterocyclic Dicarboxylic Ligands. Int. J. Mass Spectrom. 2014, 365–366, 372–376. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fabiano, M.A.; Materazzi, S. Biomimetic Complexes of Co(II), Mn(II), and Ni(II) with 2-Propyl-4,5-Imidazoledicarboxylic Acid. EGA-MS Characterization of the Thermally Induced Decomposition. Russ. J. Gen. Chem. 2015, 85, 2374–2377. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fabiano, M.A.; Iona, R.; Zuccatosta, F.; Wo, L.W.; Materazzi, S. Divalent Transition Metal Complexes of 2-(Pyridin-2-Yl)Imidazole: Evolved Gas Analysis Predicting Model to Provide Characteristic Coordination. Russ. J. Gen. Chem. 2017, 87, 2915–2921. [Google Scholar] [CrossRef]
- Risoluti, R.; Fabiano, M.A.; Gullifa, G.; Wo, L.W.; Materazzi, S. Biomimetic Complexes of Cd(II), Mn(II), and Zn(II) with 1,1-Diaminobutane–Schiff Base. EGA/MS Study of the Thermally Induced Decomposition. Russ. J. Gen. Chem. 2017, 87, 564–568. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Carcassi, E.; Buiarelli, F.; Wo, L.W.; Materazzi, S. Modeling Solid State Stability for Speciation: A Ten-Year Long Study. Molecules 2019, 24, 3013. [Google Scholar] [CrossRef]
- Wang, W.-T.; Liu, S.-H.; Cheng, Y.-F.; Wang, Y.; Yu, C.-F. Evaluation of Thermal Decomposition Characteristics and Potential Hazards of 1-n-Butyl-3-Methylimidazolium Dicyanamide by STA, ARC, and TG-FTIR. J. Therm. Anal. Calorim. 2022, 147, 11127–11137. [Google Scholar] [CrossRef]
- González-Rivera, J.; Husanu, E.; Mero, A.; Ferrari, C.; Duce, C.; Tinè, M.R.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Insights into Microwave Heating Response and Thermal Decomposition Behavior of Deep Eutectic Solvents. J. Mol. Liq. 2020, 300, 112357. [Google Scholar] [CrossRef]
- Medeiros, R.S.; Ferreira, A.P.G.; Cavalheiro, E.T.G. Revisiting the Thermal Behavior of Dipyrone. J. Therm. Anal. Calorim. 2022, 147, 6287–6299. [Google Scholar] [CrossRef]
- Yao, H.; Jiang, J.; Li, B.; Ni, L.; Ni, Y.; Yao, X. Investigation of Pyrolysis Kinetics, Mechanism and Thermal Stability of Tert-Butyl Peroxy-2-Ethyl Hexanoate. Process Saf. Environ. Prot. 2022, 160, 734–748. [Google Scholar] [CrossRef]
- Vlasyuk, D.; Łyszczek, R. Effect of Different Synthesis Approaches on Structural and Thermal Properties of Lanthanide(III) Metal–Organic Frameworks Based on the 1H-Pyrazole-3,5-Dicarboxylate Linker. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3534–3548. [Google Scholar] [CrossRef]
- Velikova, N.; Spassova, I. Bifunctional Mesoporous Hybrid Sol-Gel Prepared Silicas for CO2 Adsorption. J. Sol-Gel Sci. Technol. 2021, 100, 326–340. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Li, W.; Huang, X.; Luan, S.; Hou, X.; Zhang, M.; Wang, Q. CO2 Mediated Fabrication of Hierarchically Porous Metal-Organic Frameworks. Microporous Mesoporous Mater. 2019, 277, 154–162. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, Z.; Zhong, D.; Qin, Z.; Zhou, N.; Li, W. Effect of Two-Dimensional Zeolitic Imidazolate Frameworks-L on Flame Retardant Property of Thermoplastic Polyurethane Elastomers. Polym. Adv. Technol. 2021, 32, 2072–2081. [Google Scholar] [CrossRef]
- Dascălu, I.-A.; Mikhalyova, E.A.; Shova, S.; Bratanovici, B.-I.; Ardeleanu, R.; Marangoci, N.; Lozan, V.; Roman, G. Synthesis, Crystal Structure and Luminescent Properties of Isoreticular Lanthanide–Organic Frameworks Based on a Tetramethyl-Substituted Terphenyldicarboxylic Acid. Polyhedron 2021, 194, 114929. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Yi, J.; Qin, Z.; Wang, C.; Sun, Z.; Xu, Y.; Xu, S.; Zhao, F. Two Novel Heterobimetallic Metal-Organic Frameworks for the Enhanced Catalytic Thermolysis and Laser Ignition of CL-20. Mater. Today Chem. 2022, 23, 100676. [Google Scholar] [CrossRef]
- Yousef, S.; Kiminaitė, I.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Catalytic Pyrolysis Kinetic Behaviour of Glass Fibre-Reinforced Epoxy Resin Composites over ZSM-5 Zeolite Catalyst. Fuel 2022, 315, 123235. [Google Scholar] [CrossRef]
- Tong, W.; Xie, Y.; Hu, W.; Peng, Y.; Liu, W.; Li, Y.; Zhang, Y.; Wang, Y. A Bifunctional CoP/N-Doped Porous Carbon Composite Derived from a Single Source Precursor for Bisphenol A Removal. RSC Adv. 2020, 10, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Eimontas, J.; Striūgas, N.; Abdelnaby, M.A.; Yousef, S. Catalytic Pyrolysis Kinetic Behavior and TG-FTIR-GC–MS Analysis of Metallized Food Packaging Plastics with Different Concentrations of ZSM-5 Zeolite Catalyst. Polymers 2021, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nishu; Yellezuome, D.; Chai, M.; Li, C.; Liu, R. Catalytic Pyrolysis of Biomass over Fe-Modified Hierarchical ZSM-5: Insights into Mono-Aromatics Selectivity and Pyrolysis Behavior Using Py-GC/MS and TG-FTIR. J. Energy Inst. 2021, 99, 218–228. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, H.; Ma, Q.; Yang, L.; Ma, L.; Wu, Y.; Zhang, P. Catalytic Upgrading of Coal Volatiles with Fe2O3 and Hematite by TG-FTIR and Py-GC/MS. Fuel 2022, 313, 122667. [Google Scholar] [CrossRef]
- Müller, A.; Völs, P.; Störr, B.; Mertens, F. Comparative Study of the CO2 Methanation Activity of Hydrotalcite-Based Nickel Catalysts Generated by Using Different Reduction Protocols. Catal. Lett. 2022, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Li, Y.; Ma, X.; Yan, Q. Effects of High-Energy Multicore Ferrocene-Based Catalysts on the Thermal Decomposition of Ammonium Perchlorate. Appl. Organomet. Chem. 2022, 36, e6605. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Zhuo, J.; Yao, Q. Investigation of a Ni-Modified MCM-41 Catalyst for the Reduction of Oxygenates and Carbon Deposits during the Co-Pyrolysis of Cellulose and Polypropylene. ACS Omega 2020, 5, 20299–20310. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. A Bio-Derived Char Forming Flame Retardant Additive for Nylon 6 Based on Crosslinked Tannic Acid. Thermochim. Acta 2020, 693, 178750. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Bourbigot, S. A Case Study of Polyether Ether Ketone (I): Investigating the Thermal and Fire Behavior of a High-Performance Material. Polymers 2020, 12, 1789. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Ma, C.; Zhou, F.; Hu, Y.; Schartel, B. A Liquid Phosphorous Flame Retardant Combined with Expandable Graphite or Melamine in Flexible Polyurethane Foam. Polym. Adv. Technol. 2022, 33, 326–339. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Zhang, H.; Cui, J.; Guo, J.; Yang, B. Butyltriphenylphosphine-Based Chelate Borates Influenced on Flame Retardancy of Polystyrene Composite Containing Self-Expanded Intumescent Flame Retardants. J. Appl. Polym. Sci. 2021, 138, 50650. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rajasudha, V.; Mahendran, A.R.; Vijayakumar, C.T. Castor Oil and HER Based Polyurethanes: Synthesis, Characterization and Structure-Thermal Stability Relation. Polym. Korea 2021, 45, 511–519. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, S.; Liu, M.; Wen, P.; Liu, X.; Tang, G.; Xu, X. Bio-Based Trivalent Phytate: A Novel Strategy for Enhancing Fire Performance of Rigid Polyurethane Foam Composites. J. Renew. Mater. 2022, 10, 1201–1220. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, L.-L.; Zhao, B. A Novel Aminothiazole-Based Cyclotriphosphazene Derivate towards Epoxy Resins for High Flame Retardancy and Smoke Suppression. Polym. Degrad. Stab. 2021, 190, 109651. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, C.; Liu, S.; Wang, P.; Zhang, G. A Novel Flame Retardant Based on Polyhydric Alcohols and P–N Synergy for Treatment of Cotton Fabrics. Cellulose 2021, 28, 1781–1793. [Google Scholar] [CrossRef]
- Li, S.; Zhong, L.; Huang, S.; Wang, D.; Zhang, F.; Zhang, G. A Novel Flame Retardant with Reactive Ammonium Phosphate Groups and Polymerizing Ability for Preparing Durable Flame Retardant and Stiff Cotton Fabric. Polym. Degrad. Stab. 2019, 164, 145–156. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Wang, S.; Li, Y.; Jin, X.; He, W.; Liu, J.; Zhu, P.; Dong, C. A Novel Flame-Retardant System toward Polyester Fabrics: Flame Retardant, Anti-Dripping and Smoke Suppression. J. Polym. Res. 2022, 29, 180. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, G.; Wang, P.; Dai, F. A Novel ε-Polylysine-Derived Durable Phosphorus-nitrogen-based Flame Retardant for Cotton Fabrics. Cellulose 2021, 28, 3807–3822. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Xu, Y.-J.; Jiang, Z.; Dong, C.; Liu, Y.; Zhu, P. Ecofriendly Flame-Retardant Cotton Fabrics: Preparation, Flame Retardancy, Thermal Degradation Properties, and Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 19246–19256. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Zhang, C.; Qi, Z.; Li, L.; Zhu, P. Flame Retardancy and Thermal Behavior of Wool Fabric Treated with a Phosphorus-Containing Polycarboxylic Acid. Polymers 2021, 13, 4111. [Google Scholar] [CrossRef]
- Luo, H.; Rao, W.; Zhao, P.; Wang, L.; Liu, Y.; Yu, C. An Efficient Organic/Inorganic Phosphorus–Nitrogen–Silicon Flame Retardant towards Low-Flammability Epoxy Resin. Polym. Degrad. Stab. 2020, 178, 109195. [Google Scholar] [CrossRef]
- Dai, K.; Deng, Z.; Liu, G.; Wu, Y.; Xu, W.; Hu, Y. Effects of a Reactive Phosphorus-Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin. Polymers 2020, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, T.; Ding, Z.; Li, C.; Lu, S. Characterization of Thermal Decomposition and Combustion for Commercial Flame-Retardant Rubber Floor Cloth in TG–FTIR and FPA. J. Therm. Anal. Calorim. 2019, 135, 3453–3461. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Dong, C.; Liu, J.; Kong, D.; Sun, H.; Lu, Z. Comparison of Differences in the Flame Retardancy of Cotton Fabrics Caused by the Introduction of Cyclic Polysiloxane into P/N Organic Coatings. New J. Chem. 2021, 45, 17131–17142. [Google Scholar] [CrossRef]
- Liu, J.; Dong, C.; Zhang, Z.; Sun, H.; Kong, D.; Lu, Z. Durable Flame Retardant Cotton Fabrics Modified with a Novel Silicon–Phosphorus–Nitrogen Synergistic Flame Retardant. Cellulose 2020, 27, 9027–9043. [Google Scholar] [CrossRef]
- Yang, Y.; Haurie, L.; Wen, J.; Zhang, S.; Ollivier, A.; Wang, D.-Y. Effect of Oxidized Wood Flour as Functional Filler on the Mechanical, Thermal and Flame-Retardant Properties of Polylactide Biocomposites. Ind. Crop. Prod. 2019, 130, 301–309. [Google Scholar] [CrossRef]
- Shao, N.; Qu, Y.; Hou, L.; Hu, Y.; Tian, Z.; Gao, Y.; Zhu, X. Effect of Starch-Based Flame Retardant on the Thermal Degradation and Combustion Properties of Reconstituted Tobacco Sheet. Cellulose 2021, 28, 741–755. [Google Scholar] [CrossRef]
- Shao, N.; Xue, F.; Hou, L.; Li, D.; Gao, Y.; Zhu, X. Effects of Ternary Potassium Containing Intumescent Flame Retardant Coating on the Combustion and Thermal Degradation Properties of Reconstituted Tobacco Sheet. Thermochim. Acta 2019, 678, 178310. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S.; Tau, F.; Russo, A.; Romolo, F.S. Towards Innovation in Paper Dating: A MicroNIR Analytical Platform and Chemometrics. Analyst 2018, 143, 4394–4399. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, L.; Li, D.; Li, B.; Geng, Z.; Zhu, X. Effects of β-Cyclodextrin Quaternary Flame-Retardant Coating on Combustion and Thermal Degradation Behavior of Reconstituted Tobacco Sheet. Tob. Sci. Technol. 2021, 54, 52–62. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, L.; Zhang, P.; Han, Z.; Chen, D.; Liu, X.; Zhou, Z. Effect of Aluminum Diethylphosphinate on Flame Retardant and Thermal Properties of Rigid Polyurethane Foam Composites. J. Therm. Anal. Calorim. 2020, 140, 625–636. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Liang, M.; Yuan, T.; Rong, H. Effect of Aluminum Hydroxide (ATH) on Flame Retardancy and Smoke Suppression Properties of SBS-Modified Asphalt. Road Mater. Pavement Des. 2021, 1–18. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Effect of Aluminum Leaching Pretreatment on Catalytic Pyrolysis of Metallised Food Packaging Plastics and Its Linear and Nonlinear Kinetic Behaviour. Sci. Total Environ. 2022, 844, 157150. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Deng, D.; Zhou, L.; Zhang, P.; Tang, G. Flame Retardancy and Thermal Degradation of Rigid Polyurethane Foams Composites Based on Aluminum Hypophosphite. Mater. Res. Express 2019, 6, 105365. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Yu, F.; Liu, M.; Yang, S.; Tang, G.; Xu, X.; Wang, B.; Liu, X. Flame Retardant Rigid Polyurethane Foam Composites Based on Iron Tailings and Aluminum Phosphate: A Novel Strategy for Utilizing Industrial Solid Wastes. Polym. Adv. Technol. 2021, 32, 4826–4839. [Google Scholar] [CrossRef]
- Hu, Q.; Zou, L.; Liu, Z.; Chen, J.; Liu, J.; Liu, X. Flame-Retardant Polyurethane Elastomer Based on Aluminum Salt of Monomethylphosphinate. J. Therm. Anal. Calorim. 2021, 143, 2953–2961. [Google Scholar] [CrossRef]
- Dagdag, O.; El Gouri, M.; Safi, Z.S.; Wazzan, N.; Safi, S.K.; Jodeh, S.; Hamed, O.; Haldhar, R.; Verma, C.; Ebenso, E.E. Flame Retardancy of an Intumescent Epoxy Resin Containing Cyclotriphosphazene: Experimental, Computational and Statistical Studies. Iran. Polym. J. Engl. Ed. 2021, 30, 1169–1179. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, Y.; Jiang, M.; Liu, P. Flame Retardant Polyacrylonitrile Fiber Modified with Hydrazine Hydrate and Copper Ions. J. Text. Inst. 2022, 1–10. [Google Scholar] [CrossRef]
- Cheng, G.; Xuan, Z.; Tang, Z.; Tian, S.; Sha, F.; Ding, G.; Wan, X. Flame-Retardant Behavior and Mechanism of the SBR/MMT Composites Modified by Melamine Matrix Modifier. J. Appl. Polym. Sci. 2021, 138, 50632. [Google Scholar] [CrossRef]
- Zhang, D.; Pei, M.; Wei, K.; Tan, F.; Gao, C.; Bao, D.; Qin, S. Flame-Retardant Properties and Mechanism of Polylactic Acid-Conjugated Flame-Retardant Composites. Front. Chem. 2022, 10, 894112. [Google Scholar] [CrossRef]
- Mustata, F.; Rosu, D.; Varganici, C.-D.; Rosu, L.; Rosca, I.; Tudorachi, N. Assessing the Thermal and Fungal Behavior of Eco-Friendly Epoxy Thermosets Derived from Vegetable Oils for Wood Protective Coatings. Prog. Org. Coat. 2021, 163, 106612. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and Mechanical Properties of DGEBA-Based Epoxy Resin Cured with a Cycloaliphatic Hardener: Combined Action of Silica, Melamine and DOPO-Derivative. Mater. Des. 2020, 193, 108862. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched Phosphorus Flame Retardants: Multifunctional Additives for Epoxy Resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. Improvement of the Flame Retardant and Thermomechanical Properties of Epoxy Resins by a Vanillin-Derived Cyclotriphosphazene-Cored Triazole Compound. Polym. Degrad. Stab. 2022, 204, 110088. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Cui, Y.; Ye, J.; Chen, D.; Yuan, Y.; Liu, X.; Liu, M.; Peng, C.; Wu, Z. Liquid Oxygen Compatibility of Epoxy Matrix and Carbon Fiber Reinforced Epoxy Composite. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106771. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, C.; Dai, S.; Wang, J.; Pan, Z.; Zhou, H. Function of Chitosan in a DOPO-Based Flame Retardant Modified Epoxy Resin. J. Appl. Polym. Sci. 2022, 139, 51593. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, P.-W.; Xiong, K.-K.; Liu, H.-H.; Zhao, P.-H.; Liu, Y.-Q. Impacts of Multi-Element Flame Retardants on Flame Retardancy, Thermal Stability, and Pyrolysis Behavior of Epoxy Resin. Polym. Degrad. Stab. 2019, 167, 217–227. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, M.-Q.; Yu, J.; Nie, S.-Q.; Zhang, D.-Q.; Qin, S.-H. Synergistic Flame-Retardant Effect of Epoxy Resin Combined with Phenethyl-Bridged DOPO Derivative and Graphene Nanosheets. Chin. J. Polym. Sci. Engl. Ed. 2019, 37, 79–88. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.; Shen, R.; Wang, Z.; Wang, Q. Thermal Behavior and Smoke Characteristics of Glass/Epoxy Laminate and Its Foam Core Sandwich Composite. J. Therm. Anal. Calorim. 2020, 141, 1173–1182. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Matrix Matters: Hyperbranched Flame Retardants in Aliphatic and Aromatic Epoxy Resins. Polym. Degrad. Stab. 2019, 170, 108986. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Sulfur’s Role in the Flame Retardancy of Thio-Ether–Linked Hyperbranched Polyphosphoesters in Epoxy Resins. Eur. Polym. J. 2020, 122, 109390. [Google Scholar] [CrossRef]
- Mustata, F.; Tudorachi, N. Synthesis and Thermal Characterization of Some Hardeners for Epoxy Resins Based on Castor Oil and Cyclic Anhydrides. Ind. Crop. Prod. 2021, 159, 113087. [Google Scholar] [CrossRef]
- Tian, B.; Xu, L.; Jing, M.; Liu, N.; Tian, Y. A Comprehensive Evaluation on Pyrolysis Behavior, Kinetics, and Primary Volatile Formation Pathways of Rice Husk for Application to Catalytic Valorization. Fuel Process. Technol. 2021, 214, 106715. [Google Scholar] [CrossRef]
- Ding, B.; Liu, H.; Li, F. Analysis of Pyrolysis and Volatile Products Characteristics of Jatropha Curcas Shell. Guocheng Gongcheng Xuebao Chin. J. Process Eng. 2022, 22, 366–375. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An Investigation into the Pyrolysis and Oxidation of Bio-Oil from Sugarcane Bagasse: Kinetics and Evolved Gases Using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 Gasification with CaO Looping for Syngas Production in a Fixed-Bed Reactor. Renew. Energy 2021, 167, 652–661. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Evrendilek, F.; Xie, W.; Kuo, J.; Buyukada, M. Bioenergy and Emission Characterizations of Catalytic Combustion and Pyrolysis of Litchi Peels via TG-FTIR-MS and Py-GC/MS. Renew. Energy 2020, 148, 1074–1093. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A State-of-the-Art Review on Thermochemical Conversion of Biomass for Biofuel Production: A TG-FTIR Approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass Pyrolysis Technologies for Value-Added Products: A State-of-the-Art Review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Yasmin, T.; Asghar, A.; Ahmad, M.S.; Mehmood, M.A.; Nawaz, M. Biorefinery Potential of Typha Domingensis Biomass to Produce Bioenergy and Biochemicals Assessed through Pyrolysis, Thermogravimetry, and TG-FTIR-GCMS-Based Study. Biomass Convers. Biorefin. 2021, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Li, S.; Wang, X.; Lin, R. Comparative Study on the Two-Step Pyrolysis of Different Lignocellulosic Biomass: Effects of Components. J. Anal. Appl. Pyrolysis 2020, 152, 104966. [Google Scholar] [CrossRef]
- Tahir, M.H.; Irfan, R.M.; Hussain, M.B.; Alhumade, H.; Al-Turki, Y.; Cheng, X.; Karim, A.; Ibrahim, M.; Rathore, H.A. Catalytic Fast Pyrolysis of Soybean Straw Biomass for Glycolaldehyde-Rich Bio-Oil Production and Subsequent Extraction. ACS Omega 2021, 6, 33694–33700. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Siciliano, C.; Mazzotti, F.; Donna, L.D.; Risoluti, R.; Napoli, A. Protein Extraction, Enrichment and MALDI MS and MS/MS Analysis from Bitter Orange Leaves (Citrus aurantium). Molecules 2020, 25, 1485. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Domenico, M.D.; Bolzan, A.; Machado, R.A.F.; Marangoni, C. Evaluating the Bioenergy Potential of Cupuassu Shell through Pyrolysis Kinetics, Thermodynamic Parameters of Activation, and Evolved Gas Analysis with TG/FTIR Technique. Thermochim. Acta 2022, 711, 179187. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y.; Luo, B. Effect of Potassium on the Pyrolysis of Biomass Components: Pyrolysis Behaviors, Product Distribution and Kinetic Characteristics. Waste Manag. 2021, 121, 255–264. [Google Scholar] [CrossRef]
- Bezerra, R.D.C.D.F.; Rodrigues, F.E.A.; Arruda, T.B.M.G.; Moreira, F.B.D.F.; Chaves, P.O.B.; Assunção, J.C.D.C.; Ricardo, N.M.P.S. Babassu-Oil-Based Biolubricant: Chemical Characterization and Physicochemical Behavior as Additive to Naphthenic Lubricant NH-10. Ind. Crop. Prod. 2020, 154, 112624. [Google Scholar] [CrossRef]
- Nardella, F.; Duce, C.; Ribechini, E. Analytical Pyrolysis and Thermal Analysis to Chemically Characterise Bitumen from Italian Geological Deposits and Neolithic Stone Tools. J. Anal. Appl. Pyrolysis 2021, 158, 105262. [Google Scholar] [CrossRef]
- Abduhani, H.; Tursun, Y.; Abulizi, A.; Talifu, D.; Huang, X. Characteristics and Kinetics of the Gas Releasing during Oil Shale Pyrolysis in a Micro Fluidized Bed Reactor. J. Anal. Appl. Pyrolysis 2021, 157, 105187. [Google Scholar] [CrossRef]
- Sasar, M.; Johnston, C.T.; Santagata, M. Characterization and Dynamics of Residual Organics in Oil Sands Fluid Fine Tailings. Energy Fuels 2022, 36, 6881–6892. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Y.; Li, S.; Wang, Q.; Hong, W.; Jiang, H.; Li, H.; Jiang, M. Pyrolysis Characteristics of Oil Shale Semi-Coke and Its Extracted Bitumen. J. Anal. Appl. Pyrolysis 2021, 156, 105120. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Y.; Li, S.; Wang, Q.; Hong, W.; Jiang, H.; Li, H.; Jiang, M. Pyrolytic Characteristics of Fushun Oil Shale and Its By-Products. J. Therm. Anal. Calorim. 2022, 147, 5255–5267. [Google Scholar] [CrossRef]
- Shi, H.; Ouyang, Q.; Wang, J.-Y.; Zhu, P.-L.; Hao, J.-W.; Huang, X.-B. Accelerating the Formation of the Conjugated Ladder Structure of Poly(Acrylonitrile-Co-Vinyl Acetate) by Cross-Linked Poplar Lignin Doped with Boron Phosphate. Mater. Res. Express 2020, 7, 055309. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chang, L.; Zi, C.; Liang, G.; Zhang, D.; Xie, W. Analyses on Thermal Stability of Lignites and Its Derived Humic Acids. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wang, C.; Zhu, X. Catalytic Effects of Ammonium Dihydrogen Phosphate on the Pyrolysis of Lignocellulosic Biomass: Selective Production of Furfural and Levoglucosenone. Fuel Process. Technol. 2020, 209, 106525. [Google Scholar] [CrossRef]
- Sidi-Yacoub, B.; Oudghiri, F.; Belkadi, M.; Rodríguez-Barroso, R. Characterization of Lignocellulosic Components in Exhausted Sugar Beet Pulp Waste by TG/FTIR Analysis. J. Therm. Anal. Calorim. 2019, 138, 1801–1809. [Google Scholar] [CrossRef]
- Liang, J.; Chen, J.; Wu, S.; Liu, C.; Lei, M. Comprehensive Insights into Xylan Structure Evolution via Multi-Perspective Analysis during Slow Pyrolysis Process. Fuel Process. Technol. 2019, 186, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Ding, M.; Wang, M.; Xu, X. Construction of a Phytic Acid–Silica System in Wood for Highly Efficient Flame Retardancy and Smoke Suppression. Materials 2021, 14, 4164. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Lin, X. Construction of Strong Non-Covalent Interactions for Preparation of Flame-Retarded Acrylic Pressure-Sensitive Adhesives with Improved Shear and Peel Strengths. J. Appl. Polym. Sci. 2022, 139, 52122. [Google Scholar] [CrossRef]
- Zhao, S.; Bi, X.; Sun, R.; Niu, M.; Pan, X. Density Functional Theory and Experimental Study of Cellulose Initial Degradation Stage under Inert and Oxidative Atmosphere. J. Mol. Struct. 2020, 1204, 127543. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Bi, Y.; Tian, L.; Wang, X. Determination of Pyrolysis Characteristics and Thermo-Kinetics to Assess the Bioenergy Potential of Phragmites Communis. Energy Convers. Manag. 2020, 207, 112510. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Li, Y.; Xin, Y.; Li, S. Enhancing and Upgrading Bio-Oil during Catalytic Pyrolysis of Cellulose: The Synergistic Effect of Potassium Cation and Different Anions Impregnation. Fuel Process. Technol. 2019, 193, 338–347. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into Biomass Pyrolysis Mechanism Based on Cellulose, Hemicellulose, and Lignin: Evolution of Volatiles and Kinetics, Elucidation of Reaction Pathways, and Characterization of Gas, Biochar and Bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Li, P.; Wu, M.; Zhang, X.; Zhang, X.; Zhu, G. Investigations on Cunninghamia Lanceolate Cedar Wood Pyrolysis by Thermogravimetric-Fourier Transform Infrared Analysis and a Modified Discrete Distributed Activation Energy Model Kinetic Method. Energy Fuels 2019, 33, 12499–12507. [Google Scholar] [CrossRef]
- Li, T.; Zhang, G.; Xie, Z.; Chen, Z.; Xu, S.; Zhang, Y.; Xiong, C.; Xu, S. Analysis and Safety Evaluation of Flue Gas Produced by an Organic Fireproof Plugging Materials Pyrolysis. Huagong Jinzhan/Chem. Ind. Eng. Prog. 2021, 40, 4108–4115. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, T.; Zhang, Y.; Wang, Q.; Li, G. Characterization of the Incense Ingredients of Cultivated Grafting Kynam by TG-FTIR and HS-GC-MS. Fitoterapia 2020, 142, 104493. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Zhang, H.; Olanya, O.M. Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels. Molecules 2022, 27, 316. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Li, Y.; Huang, L.; Qiu, J.; Sun, X.; Cang, Z.; Lv, J. Applying Feasibility Investigation of Resin Modifying Agent in Asphalt Pavement Materials. Adv. Civ. Eng. 2021, 2021, 9362400. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Chen, S.; Zhao, F.; Qiu, L.; Meng, Z.; Ding, L.; Wang, B.; Pan, Q. Comparative Thermal Research on Energetic Molecular Perovskite Structures. Molecules 2022, 27, 805. [Google Scholar] [CrossRef]
- Huo, H.; Ma, Y.; Cheng, Y.; Cao, J. 3D Carbon Aerogel from Waste Corrugated Cardboard as a Photothermal Reservoir for Solar Steam Generation. Environ. Sci. Pollut. Res. 2022, 29, 23936–23948. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Ma, Y. Bio-Inspired Hierarchical Porous Activated Carbon Aerogel from Waste Corrugated Cardboard for Adsorption of Oxytetracycline from Water. Biomass Convers. Biorefin. 2022, 1–18. [Google Scholar] [CrossRef]
- Ma, Y. Carbon Aerogel from Waste Corrugated Cardboard: Facile Preparation, Characterization, and Application to Solar Steam Generation and Adsorption. Korean J. Chem. Eng. 2022, 39, 1775–1787. [Google Scholar] [CrossRef]
- Yang, D.; Mo, W.; Zhang, S.; Li, B.; Hu, D.; Chen, S. A Graphene Oxide Functionalized Energetic Coordination Polymer Possesses Good Thermostability, Heat Release and Combustion Catalytic Performance for Ammonium Perchlorate. Dalton Trans. 2020, 49, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, Z.; Wei, J.; Li, Y.; Xiang, D.; Wu, Y.; Que, Y. A Phosphorous-Containing Bio-Based Furfurylamine Type Benzoxazine and Its Application in Bisphenol-A Type Benzoxazine Resins: Preparation, Thermal Properties and Flammability. Polymers 2022, 14, 1597. [Google Scholar] [CrossRef]
- Yuan, Q.; Yao, B.; Huang, Z.-R.; Huang, Q. Developing a Liquid and Curable Two-Component Precursor System for Fabrication of SiC(N)-Based Composites. Ceram. Int. 2019, 45, 24007–24013. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part II: Thermal Degradation, Gas Phase, and Elemental Analyses. Polymers 2020, 12, 2238. [Google Scholar] [CrossRef]
- Fan, L.; Yang, L.; Lin, Y.; Fan, G.; Li, F. Enhanced Thermal Stabilization Effect of Hybrid Nanocomposite of Ni–Al Layered Double Hydroxide/Carbon Nanotubes on Polyvinyl Chloride Resin. Polym. Degrad. Stab. 2020, 176, 109153. [Google Scholar] [CrossRef]
- Xiao, D.; Zheng, M.-T.; Gohs, U.; Wagenknecht, U.; Voit, B.; Wang, D.-Y. Highly Efficient Flame Retardant and Smoke Suppression Mechanism of Polypropylene Nanocomposites Based on Clay and Allylamine Polyphosphate. J. Appl. Polym. Sci. 2022, 139, 52311. [Google Scholar] [CrossRef]
- Geng, L.; Cai, Y.; Lu, L.; Zhang, Y.; Li, Y.; Chen, B.; Peng, X.-F. Highly Strong and Conductive Carbon Fibers Originated from Bioinspired Lignin/Nanocellulose Precursors Obtained by Flow-Assisted Alignment and in Situ Interfacial Complexation. ACS Sustain. Chem. Eng. 2021, 9, 2591–2599. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, B.; Liu, M.; Yang, Y.; Liu, X.; Chen, D.; Wang, B.; Tang, G.; Liu, X. Fire Performance of Piperazine Phytate Modified Rigid Polyurethane Foam Composites. Polym. Adv. Technol. 2021, 32, 4531–4546. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Xiao, W.; Li, J.; Han, Z.; Wang, B. Mechanism of Two Typical Binders BR and F2604 on Thermal Decomposition of HMX. ACS Omega 2021, 6, 2025–2033. [Google Scholar] [CrossRef]
- Liu, M.; Feng, Z.; Zhao, R.; Wang, B.; Deng, D.; Zhou, Z.; Yang, Y.; Liu, X.; Liu, X.; Tang, G. Enhancement of Fire Performance for Rigid Polyurethane Foam Composites by Incorporation of Aluminum Hypophosphite and Expanded Graphite. Polym. Bull. 2022, 79, 10991–11012. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Tang, G.; Long, H.; Wang, B.; Zhang, H.; Ji, Y.; Yang, Y. Fire Retarded Polyurethane Foam Composites Based on Steel Slag/Ammonium Polyphosphate System: A Novel Strategy for Utilization of Metallurgical Solid Waste. Polym. Adv. Technol. 2022, 33, 452–463. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J. Facile Preparation of Magnetic Porous Carbon Monolith from Waste Corrugated Cardboard Box for Solar Steam Generation and Adsorption. Biomass Convers. Biorefin. 2022, 12, 2185–2202. [Google Scholar] [CrossRef]
- Kunc, F.; Kodra, O.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. A Multi-Method Approach for Quantification of Surface Coatings on Commercial Zinc Oxide Nanomaterials. Nanomaterials 2020, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Pielichowska, K.; Nowicka, K. Analysis of Nanomaterials and Nanocomposites by Thermoanalytical Methods. Thermochim. Acta 2019, 675, 140–163. [Google Scholar] [CrossRef]
- Celluzzi, A.; Paolini, A.; D’Oria, V.; Risoluti, R.; Materazzi, S.; Pezzullo, M.; Casciardi, S.; Sennato, S.; Bordi, F.; Masotti, A. Biophysical and Biological Contributions of Polyamine-Coated Carbon Nanotubes and Bidimensional Buckypapers in the Delivery of Mirnas to Human Cells. Int. J. Nanomed. 2017, 13, 1–18. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, C.-Y.; Lu, S.; Cheng, Y.; Zhang, H.-P. Experimental Insight into Catalytic Mechanism of Transition Metal Oxide Nanoparticles on Combustion of 5-Amino-1H-Tetrazole Energetic Propellant by Multi Kinetics Methods and TG-FTIR-MS Analysis. Fuel 2019, 245, 78–88. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Zhang, D.; Liu, C.-C.; Lu, S.; Zhang, H.-P. Experimental Investigation on Combustion Behaviors and Reaction Mechanisms for 5-Aminotetrazole Solid Propellant with Nanosized Metal Oxide Additives under Elevated Pressure Conditions. Appl. Therm. Eng. 2019, 162, 114207. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Carcassi, E.; Masotti, A.; Materazzi, S. TGA/Chemometrics Addressing Innovative Preparation Strategies for Functionalized Carbon Nanotubes. J. Pharm. Anal. 2020, 10, 351–355. [Google Scholar] [CrossRef]
- Attia, N.F.; Nour, M.; Hassan, M.; Mohamed, G.; Oh, H.; Mahmoud, M. Effect of Type of Organic Modifier on the Clay Layered-Based Nanocomposites Flammability and Toxic Gases Emission. J. Thermoplast. Compos. Mater. 2020, 35, 1488–1509. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Lai, X.; Li, H.; Wu, T. Improvement of Platinum Nanoparticles-Immobilized α-Zirconium Phosphate Sheets on Tracking and Erosion Resistance of Silicone Rubber. Compos. Part B Eng. 2019, 176, 107203. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.; Zinnatullin, A.L.; Rodionov, N.O.; Vagizov, F.G.; Osin, Y.N.; Yakimova, L.S. Improving Heavy Oil Oxidation Performance by Oil-Dispersed CoFe2O4 Nanoparticles in In-Situ Combustion Process for Enhanced Oil Recovery. Fuel 2021, 285, 119216. [Google Scholar] [CrossRef]
- Yang, T.-H.; Liu, J.-X.; Li, B.-S.; Zhai, Y.-M.; Wang, J.; Tong, B.-L. Effect of Ca Modified HZSM-5 Zeolites on Catalytic Pyrolysis of Oil Shale. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2021, 49, 137–144. [Google Scholar] [CrossRef]
- Liang, X.; Peng, X.; Xia, C.; Yuan, H.; Zou, K.; Huang, K.; Lin, M.; Zhu, B.; Luo, Y.; Shu, X. Improving Ti Incorporation into the BEA Framework by Employing Ethoxylated Chlorotitanate as Ti Precursor: Postsynthesis, Characterization, and Incorporation Mechanism. Ind. Eng. Chem. Res. 2021, 60, 1219–1230. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Zou, L.; Cai, S.; Liu, X.; Liu, J.; Xie, X. Synergistic Effect of Zeolite on the Nitrogen-Containing Phosphinate Salt-Based Acrylonitrile–Butadiene–Styrene Flame-Retardant Composite. J. Polym. Res. 2022, 29, 6. [Google Scholar] [CrossRef]
- Pires, M.; Murariu, M.; Cardoso, A.M.; Bonnaud, L.; Dubois, P. Thermal Degradation of Poly(Lactic Acid)–Zeolite Composites Produced by Melt-Blending. Polym. Bull. 2020, 77, 2111–2137. [Google Scholar] [CrossRef]
- Silvero, E.Z.; Albiol, J.G.; Díaz-Canales, E.M.; Vaquer, M.J.V.; Gómez-Tena, M.P. Application of Evolved Gas Analysis Technique for Speciation of Minor Minerals in Clays. Minerals 2020, 10, 824. [Google Scholar] [CrossRef]
- Mo, C.; Zhao, J.; Zhang, D. Real-Time Measurement of Mechanical Behavior of Granite During Heating–Cooling Cycle: A Mineralogical Perspective. Rock Mech. Rock Eng. 2022, 55, 4403–4422. [Google Scholar] [CrossRef]
- Pulidori, E.; Lluveras-Tenorio, A.; Carosi, R.; Bernazzani, L.; Duce, C.; Pagnotta, S.; Lezzerini, M.; Barone, G.; Mazzoleni, P.; Tiné, M.R. Building Geopolymers for CuHe Part I: Thermal Properties of Raw Materials as Precursors for Geopolymers. J. Therm. Anal. Calorim. 2022, 147, 5323–5335. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Lv, J.; Feng, S.; Ci, J. Early Properties and Microstructure Evolution of Alkali-Activated Brick Powder Geopolymers at Varied Curing Humidity. J. Build. Eng. 2022, 54, 104674. [Google Scholar] [CrossRef]
- Ariskina, K.A.; Yuan, C.; Abaas, M.; Emelianov, D.A.; Rodionov, N.; Varfolomeev, M.A. Catalytic Effect of Clay Rocks as Natural Catalysts on the Combustion of Heavy Oil. Appl. Clay Sci. 2020, 193, 105662. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Jiang, X.; Shen, J. An Improved Parallel Reaction Model Applied to Coal Pyrolysis. Fuel Process. Technol. 2021, 211, 106608. [Google Scholar] [CrossRef]
- Xi, Z.; Wang, X.; Li, M.; Wang, X. Characteristic Analysis of Pulverized Coal Combustion. Combust. Sci. Technol. 2021, 193, 1605–1622. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Wang, W.; Luo, C.; Mei, D. Chemical Looping Combustion of Lignite with the CaSO4–CoO Mixed Oxygen Carrier. J. Energy Inst. 2020, 93, 1229–1241. [Google Scholar] [CrossRef]
- Bi, H.; Wang, C.; Lin, Q.; Jiang, X.; Jiang, C.; Bao, L. Combustion Behavior, Kinetics, Gas Emission Characteristics and Artificial Neural Network Modeling of Coal Gangue and Biomass via TG-FTIR. Energy 2020, 213, 118790. [Google Scholar] [CrossRef]
- Li, B.; Liu, G.; Sun, W.; Ye, L.; Bi, M.; Gao, W. Experimental and Theoretical Study on Kinetic Behaviour of Coal Gangue and Raw Coal Using Model Reconstruction. J. Therm. Anal. Calorim. 2021, 144, 463–477. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wang, Z.-Q.; Cheng, X.-X.; Ma, C.-Y. Investigation into the Effect of Fe2O3 on Combustion Characteristics and Kinetic of Coal/Char Co-Combustion Based on TG-FTIR. K. Cheng Je Wu Li Hsueh Pao/J. Eng. Thermophys. 2019, 40, 938–944. [Google Scholar]
- Ni, Z.; Bi, H.; Jiang, C.; Wang, C.; Tian, J.; Zhou, W.; Sun, H.; Lin, Q. Investigation of the Co-Pyrolysis of Coal Slime and Coffee Industry Residue Based on Machine Learning Methods and TG-FTIR: Synergistic Effect, Kinetics and Thermodynamic. Fuel 2021, 305, 121527. [Google Scholar] [CrossRef]
- Yao, Q.; Li, Y.; Tang, X.; Gao, J.; Wang, R.; Zhang, Y.; Sun, M.; Ma, X. Separation of Petroleum Ether Extracted Residue of Low Temperature Coal Tar by Chromatography Column and Structural Feature of Fractions by TG-FTIR and PY-GC/MS. Fuel 2019, 245, 122–130. [Google Scholar] [CrossRef]
- Wang, Q.; Kawano, Y.; Yu, L.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Development of High-Performance Sub-Nanoporous SiC-Based Membranes Derived from Polytitanocarbosilane. J. Membr. Sci. 2020, 598, 117688. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Zakarauskas, K.; Praspaliauskas, M.; Abdelnaby, M.A. Pyrolysis Kinetic Behavior and TG-FTIR-GC–MS Analysis of Metallised Food Packaging Plastics. Fuel 2020, 282, 118737. [Google Scholar] [CrossRef]
- Passauer, L. A Case Study on the Thermal Degradation of an Acrylate-Type Polyurethane Wood Coating Using Thermogravimetry Coupled with Evolved Gas Analysis. Prog. Org. Coat. 2021, 157, 106331. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Gu, T.; Yang, R.; Fu, Z.; Yan, B.; Chen, G. Effects of Torrefaction on the Formation and Distribution of Dioxins during Wood and PVC Pyrolysis: An Experimental and Mechanistic Study. J. Anal. Appl. Pyrolysis 2021, 157, 105240. [Google Scholar] [CrossRef]
- Liu, W.-J.; Shao, Z.-G.; Xu, Y. Emission Characteristics of Nitrogen and Sulfur Containing Pollutants during the Pyrolysis of Oily Sludge with and without Catalysis. J. Hazard. Mater. 2021, 401, 123820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, R. Energy Conversion and Utilization System of Municipal Solid Waste. Energy Eng. J. Assoc. Energy Eng. 2020, 117, 89–98. [Google Scholar] [CrossRef]
- Risoluti, R.; Canepari, S.; Frati, P.; Fineschi, V.; Materazzi, S. “2n Analytical Platform” to Update Procedures in Thanatochemistry: Estimation of Post Mortem Interval in Vitreous Humor. Anal. Chem. 2019, 91, 7025–7031. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fineschi, V.; Frati, P.; Materazzi, S. Application of Innovative TGA/Chemometric Approach for Forensic Purposes: The Estimation of the Time since Death in Contaminated Specimens. Diagnostics 2021, 11, 121. [Google Scholar] [CrossRef]
- Caprari, P.; Massimi, S.; Diana, L.; Sorrentino, F.; Maffei, L.; Materazzi, S.; Risoluti, R. Hemorheological Alterations and Oxidative Damage in Sickle Cell Anemia. Front. Mol. Biosci. 2019, 6, 142. [Google Scholar] [CrossRef]
- Risoluti, R.; Caprari, P.; Gullifa, G.; Massimi, S.; Sorrentino, F.; Buiarelli, F.; Materazzi, S. New Methods for Thalassemia Screening: TGA/Chemometrics Test Is Not Influenced by the Aging of Blood Samples. Microchem. J. 2019, 146, 374–380. [Google Scholar] [CrossRef]
- Risoluti, R.; Caprari, P.; Gullifa, G.; Diana, L.; Luciani, M.; Amato, A.; Materazzi, S. TGA/Chemometric Test Is Able to Detect the Presence of a Rare Hemoglobin Variant Hb Bibba. Front. Mol. Biosci. 2019, 6, 101. [Google Scholar] [CrossRef]
- Risoluti, R.; Caprari, P.; Gullifa, G.; Massimi, S.; Maffei, L.; Sorrentino, F.; Carcassi, E.; Materazzi, S. An Innovative Multilevel Test for Hemoglobinopathies: TGA/Chemometrics Simultaneously Identifies and Classifies Sickle Cell Disease from Thalassemia. Front. Mol. Biosci. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Materazzi, S.; Sorrentino, F.; Maffei, L.; Caprari, P. Thermogravimetric Analysis Coupled with Chemometrics as a Powerful Predictive Tool for SS-Thalassemia Screening. Talanta 2016, 159, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Caprari, P.; Gullifa, G.; Sorrentino, F.; Maffei, L.; Massimi, S.; Carcassi, E.; Materazzi, S. Differential Diagnosis of Hereditary Hemolytic Anemias in a Single Multiscreening Test by TGA/Chemometrics. Chem. Commun. 2020, 56, 7557–7560. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Caprari, P.; Gullifa, G.; Massimi, S.; Sorrentino, F.; Maffei, L.; Materazzi, S. Innovative Screening Test for the Early Detection of Sickle Cell Anemia. Talanta 2020, 219, 121243. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fabiano, M.A.; Sorrentino, F.; Caprari, P.; Materazzi, S. Advances in Thermoanalytical Techniques: May Aspirin Interfere with ß-Thalassemia Diagnosis? J. Therm. Anal. Calorim. 2018, 134, 1299–1306. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S.; Sorrentino, F.; Bozzi, C.; Caprari, P. Update on Thalassemia Diagnosis: New Insights and Methods. Talanta 2018, 183, 216–222. [Google Scholar] [CrossRef]
- Materazzi, S.; Caprari, P.; Gullifa, G.; Massimi, S.; Carcassi, E.; Risoluti, R. Development of a Novel Test for the Identification of Hereditary Erythrocyte Membrane Defects by TGA/Chemometrics. Analyst 2020, 145, 4452–4456. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Buiarelli, F.; Materazzi, S. Real Time Detection of Amphetamine in Oral Fluids by MicroNIR/Chemometrics. Talanta 2020, 208, 120456. [Google Scholar] [CrossRef]
- Risoluti, R.; Pichini, S.; Pacifici, R.; Materazzi, S. Miniaturized Analytical Platform for Cocaine Detection in Oral Fluids by MicroNIR/Chemometrics. Talanta 2019, 202, 546–553. [Google Scholar] [CrossRef]
- Borucka, M.; Celiński, M.; Sałasińska, K.; Gajek, A. Identification of Volatile and Semi-Volatile Organic Compounds Emitted during Thermal Degradation and Combustion of Triadimenol. J. Therm. Anal. Calorim. 2020, 139, 1493–1506. [Google Scholar] [CrossRef]
- Catauro, M.; Naviglio, D.; Risoluti, R.; Vecchio Ciprioti, S. Sol–Gel Synthesis and Thermal Behavior of Bioactive Ferrous Citrate–Silica Hybrid Materials. J. Therm. Anal. Calorim. 2018, 133, 1085–1092. [Google Scholar] [CrossRef]
- Ludovici, M.; Kozul, N.; Materazzi, S.; Risoluti, R.; Picardo, M.; Camera, E. Influence of the Sebaceous Gland Density on the Stratum Corneum Lipidome. Sci. Rep. 2018, 8, 11500. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhong, D.; Liu, W. Catalytic Co-Pyrolysis of Oil Sludge and Biomass over ZSM-5 for Production of Aromatic Platform Chemicals. Chemosphere 2022, 291, 132912. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-Pyrolysis of Sewage Sludge and Rice Husk by TG–FTIR–MS: Pyrolysis Behavior, Kinetics, and Condensable/Non-Condensable Gases Characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Liu, W.; Dai, L.; Qiu, R. Co-Pyrolysis of Sewage Sludge and Hydrochar with Coals: Pyrolytic Behaviors and Kinetics Analysis Using TG-FTIR and a Discrete Distributed Activation Energy Model. Energy Convers. Manag. 2020, 203, 112226. [Google Scholar] [CrossRef]
- Liu, Z.; Asghar, A.; Hou, C.; Ali, I.; Naqvi, S.R.; Wang, N.; Zhu, H.; Mehmood, M.A.; Liu, C.-G. Co-Pyrolysis of the Chinese Liquor Industry Waste and Bamboo Waste, Elucidation of the Pyrolysis Reaction Chemistry, and TG-FTIR-MS Based Study of the Evolved Gases. Fuel 2022, 326, 124976. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, F.; Li, J.; Lyu, Y.; Song, X.; Xu, C.; Ma, W.; Chen, G. Comparing Pyrolysis Characteristics of Daqing Multi-Source Oil Sludge. Huagong Jinzhan/Chem. Ind. Eng. Prog. 2021, 40, 421–433. [Google Scholar] [CrossRef]
- Gu, J.; Fan, H.; Wang, Y.; Zhang, Y.; Yuan, H.; Chen, Y. Co-Pyrolysis of Xylan and High-Density Polyethylene: Product Distribution and Synergistic Effects. Fuel 2020, 267, 116896. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, J.; He, Y.; Evrendilek, F.; Buyukada, M.; Xie, W.; Sun, S. Co-Pyrolytic Mechanisms, Kinetics, Emissions and Products of Biomass and Sewage Sludge in N2, CO2 and Mixed Atmospheres. Chem. Eng. J. 2020, 397, 125372. [Google Scholar] [CrossRef]
- Xiao, X.; Tan, J.-K.; Yuan, J.-K.; Fang, P.; Huang, J.-H.; Tang, Z.-J.; Wu, H.-W.; Hu, S.-L. Dual Role of O2 Concentration on the Reducing Gases Produced and NO Reduction during Sewage Sludge Combustion in Pilot Scale Cement Precalciner. Waste Manag. 2022, 137, 100–109. [Google Scholar] [CrossRef]
- Du, B.; Yu, Z.; Tian, Y.; Ma, X. Effects of Baking Soda on Co-Hydrothermal Carbonization of Sewage Sludge and Chlorella Vulgaris: Improved the Environmental Friendliness of Hydrochar Incineration Process. J. Environ. Chem. Eng. 2021, 9, 106404. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, J.; Li, T.; Jiang, X.; Ma, S.; Cen, W.; Jiang, W. A Regenerable N-Rich Hierarchical Porous Carbon Synthesized from Waste Biomass for H2S Removal at Room Temperature. Sci. Total Environ. 2021, 768, 144452. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Cai, B.; Fu, L.; Liang, M.; Shi, X.; Wang, B.; Deng, N.; Li, B. Analysis of Pyrolysis Characteristics and Kinetics of Cigar Tobacco and Flue-Cured Tobacco by TG-FTIR. Beiträge Table Int./Contrib. Tob. Res. Int. 2021, 30, 29–43. [Google Scholar] [CrossRef]
- Gu, W.; Yu, Z.; Fang, S.; Dai, M.; Chen, L.; Ma, X. Effects of Hydrothermal Carbonization on Catalytic Fast Pyrolysis of Tobacco Stems. Biomass Convers. Biorefin. 2020, 10, 1221–1236. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.; Wang, P.; Jin, Y.; Bao, S.; Wang, X.; Zhang, Y.; Zhang, X.; Guan, M.; Li, Y.; et al. Effects of Glycerin/Propylene Glycol Ratios on Pyrolysis and Smoke Release of Tobacco Granules. Tob. Sci. Technol. 2022, 55, 50–58. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Wang, C.; Wang, P.; Wang, X.; Zhang, Y.; Zhou, S.; Zhang, X.; Jin, Y.; Guan, M.; et al. Effects of Tobacco Types on Pyrolysis and Smoke Release of Tobacco Granules. Tob. Sci. Technol. 2022, 55, 42–50. [Google Scholar] [CrossRef]
- Risoluti, R.; Gregori, A.; Schiavone, S.; Materazzi, S. “Click and Screen” Technology for the Detection of Explosives on Human Hands by a Portable MicroNIR-Chemometrics Platform. Anal. Chem. 2018, 90, 4288–4292. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Hu, Y.; Bai, Y.; Guo, Z.; Fan, D.; Ma, H. A Series of Guanidine Salts of 3,6-Bis-Nitroguanyl-1,2,4,5-Tetrazine: Green Nitrogen-Rich Gasgenerating Agent. RSC Adv. 2020, 10, 36287–36294. [Google Scholar] [CrossRef]
- Wang, K.; Xue, B.; Chen, J.-G.; He, Z.-H.; Ji, Y.; Wang, B.; Lu, J.; Liu, Z.-W.; Liu, Z.-T. A Combined Experimental and Theoretical Study of the Thermal Decomposition Mechanism and Kinetics of Ammonium Dinitramide (ADN). New J. Chem. 2020, 44, 6833–6844. [Google Scholar] [CrossRef]
- Chen, J.; Li, B.; Cao, C.-Y.; Zhou, S.-Y.; Xie, L.-F.; Zhang, D. Catalytic Co-Pyrolysis of 5-Amino-1H-Tetrazole Assembled with Copper and Boron Powder: Pyrolysis Kinetics and Reaction Mechanism. Fuel 2022, 314, 122783. [Google Scholar] [CrossRef]
- Lu, Y.; He, J.; Tong, W.; Hou, J.; Han, J.; Yang, L. Insights into Thermal Behavior and Gas Evolution Characteristics of ZnCP and CdCP by TG-FTIR-GC/MS Analysis. J. Anal. Appl. Pyrolysis 2022, 163, 105495. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, N.; Zhang, T.; Gong, H.; Ma, H.; An, T.; Zhao, F.; Hu, R. Compatibility and Thermal Decomposition Mechanism of Nitrocellulose/Cr2O3 Nanoparticles Studied Using DSC and TG-FTIR. RSC Adv. 2019, 9, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Santamaria, S.; Carvel, R. Critical Factors Determining the Onset of Backdraft Using Solid Fuels. Fire Technol. 2020, 56, 937–957. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, S.; Wen, X.; Sang, C.; Luo, Y. Effect of Mixed Isocyanate Curing Agents on the Performance of In Situ-Prepared HTPE Binder Applied in Propellant. Propellants Explos. Pyrotech. 2021, 46, 428–439. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, H.; Yao, E.; Yu, Z.; An, T.; Zhao, F.; Yu, X. Influence of Tailored CuO and Al/CuO Nanothermites on the Thermocatalytic Degradation of Nitrocellulose and Combustion Performance of AP/HTPB Composite Propellant. Cellulose 2021, 28, 8671–8691. [Google Scholar] [CrossRef]
- Ferreira, E.N.; Arruda, T.B.M.G.; Rodrigues, F.E.A.; Arruda, D.T.D.; da Silva Júnior, J.H.; Porto, D.L.; Ricardo, N.M.P.S. Investigation of the Thermal Degradation of the Biolubricant through TG-FTIR and Characterization of the Biodiesel—Pequi (Caryocar brasiliensis) as Energetic Raw Material. Fuel 2019, 245, 398–405. [Google Scholar] [CrossRef]
- EL-Sayed, S.A. Review of Thermal Decomposition, Kinetics Parameters and Evolved Gases during Pyrolysis of Energetic Materials Using Different Techniques. J. Anal. Appl. Pyrolysis 2022, 161, 105364. [Google Scholar] [CrossRef]
- Long, F.; Cao, X.; Jiang, X.; Zhai, Q.; Zhao, J.; Yu, S.; Jiang, J.; Xu, J. An Effective Strategy for Waste Oil Deoxygenation and Upgrading for Hydrocarbon Biofuels Production: A Computational and Experimental Investigation. J. Energy Inst. 2022, 100, 109–119. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Liu, H.; Ding, B. Characteristics of Waste Cooking Oil Pyrolysis by TG-FTIR-MS and TG-GC/MS. Zhongnan Daxue Xuebao Ziran Kexue Ban/J. Cent. South Univ. Sci. Technol. 2021, 52, 1297–1306. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Yu, Z.; Deng, T.; Chen, X.; Chen, L.; Dai, M. A Study on Catalytic Co-Pyrolysis of Kitchen Waste with Tire Waste over ZSM-5 Using TG-FTIR and Py-GC/MS. Bioresour. Technol. 2019, 289, 121585. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. A New Strategy for Butanol Extraction from COVID-19 Mask Using Catalytic Pyrolysis Process over ZSM-5 Zeolite Catalyst and Its Kinetic Behavior. Thermochim. Acta 2022, 711, 179198. [Google Scholar] [CrossRef]
- Xu, G.; Cai, X.; Wang, S.; Fang, B.; Wang, H.; Zhu, Y. Characteristics, Kinetics, Infrared Analysis and Process Optimization of Co-Pyrolysis of Waste Tires and Oily Sludge. J. Environ. Manag. 2022, 316, 115278. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, S.; Wang, J.; Xiang, L.; Qin, B.; Zuo, Z.; Guo, D. Copyrolysis of Tire Powder and Engine Oil: Reaction Behavior and Kinetics. Asia-Pac. J. Chem. Eng. 2020, 15, e2486. [Google Scholar] [CrossRef]
- Wang, F.; Gao, N.; Magdziarz, A.; Quan, C. Co-Pyrolysis of Biomass and Waste Tires under High-Pressure Two-Stage Fixed Bed Reactor. Bioresour. Technol. 2022, 344, 126306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yan, B.; Tao, J.; Li, J.; Kumari, L.; Tafa Oba, B.; Akintayo Aborisade, M.; Ali Jamro, I.; Chen, G. Co-Pyrolysis of de-Oiled Microalgal Biomass Residue and Waste Tires: Deeper Insights from Thermal Kinetics, Behaviors, Drivers, Bio-Oils, Bio-Chars, and in-Situ Evolved Gases Analyses. Chem. Eng. J. 2022, 446, 137160. [Google Scholar] [CrossRef]
- Wang, F.; Gao, N.; Quan, C.; López, G. Investigation of Hot Char Catalytic Role in the Pyrolysis of Waste Tires in a Two-Step Process. J. Anal. Appl. Pyrolysis 2020, 146, 104770. [Google Scholar] [CrossRef]
- Sun, L.; Wen, Y.; Liu, Q.; Li, D.; Lyu, L.; Pei, J.; Zhang, J.; Li, R. A Laboratory Investigation into the Effect of Waste Non-Tire Rubber Particles on the Performance Properties of Terminal Blend Rubberized Asphalt Binders. Constr. Build. Mater. 2021, 313, 125409. [Google Scholar] [CrossRef]
- Xia, W.; Xu, T.; Wang, H.; Pan, Y. Combustion Kinetics of Asphalt Binder Components and the Release Processes of Gaseous Products. Combust. Flame 2019, 206, 322–333. [Google Scholar] [CrossRef]
- Zhu, K.; Qin, X.; Wang, Y.; Lin, C.; Wang, Q.; Wu, K. Effect of the Oxygen Concentration on the Combustion of Asphalt Binder. J. Anal. Appl. Pyrolysis 2021, 160, 105370. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. “Lab-on-Click” Detection of Illicit Drugs in Oral Fluids by MicroNIR-Chemometrics. Anal. Chem. 2019, 91, 6435–6439. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. MicroNIR/Chemometrics: A New Analytical Platform for Fast and Accurate Detection of Δ9-Tetrahydrocannabinol (THC) in Oral Fluids. Drug Alcohol Depend. 2019, 205, 107578. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Materazzi, S.; Gregori, A.; Ripani, L. Early Detection of Emerging Street Drugs by near Infrared Spectroscopy and Chemometrics. Talanta 2016, 153, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR Investigation on the Co-Pyrolysis of the Waste HDPE, PP, PS and PET under High Heating Conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Gautam, R.; Vinu, R.; Vaithyanathan, P. Analytical Fast Pyrolysis of Nitrogen-Rich Mosquito Species via Pyrolysis-FTIR and Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2020, 146, 104766. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Chen, W.-H.; Ong, H.C.; Sheen, H.-K.; Chang, J.-S.; Hsieh, T.-H.; Ling, T.C. Effects of Dry and Wet Torrefaction Pretreatment on Microalgae Pyrolysis Analyzed by TG-FTIR and Double-Shot Py-GC/MS. Energy 2020, 210, 118579. [Google Scholar] [CrossRef]
- Yan, B.; Li, Z.; Jiao, L.; Li, J.; Chen, G.; Yang, G. Chemical Looping Gasification of Chlorella: Parametric Optimization, Reaction Mechanisms, and Nitrogen-Containing Pollutants Emission. Fuel 2021, 289, 119987. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Zhang, X.; Hu, X.; Liu, T.; Guo, Q. Chemical Looping Staged Conversion of Microalgae with Calcium Ferrite as Oxygen Carrier: Pyrolysis and Gasification Characteristics. J. Anal. Appl. Pyrolysis 2021, 156, 105129. [Google Scholar] [CrossRef]
- Farooq, W.; Ali, I.; Raza Naqvi, S.; Sajid, M.; Abbas Khan, H.; Adamu, S. Evolved Gas Analysis and Kinetics of Catalytic and Non-Catalytic Pyrolysis of Microalgae Chlorella sp. Biomass With Ni/θ-Al2O3 Catalyst via Thermogravimetric Analysis. Front. Energy Res. 2021, 9, 775037. [Google Scholar] [CrossRef]
- Niu, X.; Xu, Y.; Shen, L. Effect of Torrefaction on the Evolution of Carbon and Nitrogen during Chemical Looping Gasification of Rapeseed Cake. Chem. Eng. J. 2022, 450, 138134. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Evolution of Carbon and Nitrogen during Chemical Looping Gasification of Rapeseed Cake with Ca-Fe Oxygen Carrier. Chem. Eng. J. 2022, 431, 134232. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.; Li, H.; Huang, X.; Liu, X.; Guo, L.; Huang, L. Characterization of Moxa Floss Combustion by TG/DSC, TG-FTIR and IR. Bioresour. Technol. 2019, 288, 121516. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Jiang, X.; Lv, G.; Yan, J.; Lin, X.; Song, H.; Cao, J. Chemical Synthesis Residual Pyrolysis and Combustion: Kinetics and Evolved Gases Investigated by TG-FTIR. J. Therm. Sci. 2020, 29, 108–114. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Z.; Zhang, X.; Ma, X. Co-Combustion Behavior of Municipal Solid Waste and Food Waste Anaerobic Digestates: Combustion Performance, Kinetics, Optimization, and Gaseous Products. J. Environ. Chem. Eng. 2021, 9, 106028. [Google Scholar] [CrossRef]
- Torres, M.; Portugau, P.; Castiglioni, J.; Cuña, A.; Yermán, L. Co-Combustion Behaviours of a Low Calorific Uruguayan Oil Shale with Biomass Wastes. Fuel 2020, 266, 117118. [Google Scholar] [CrossRef]
- Ma, Z.; Cheng, L.; Wang, Q.; Li, L.; Luo, G.; Zhang, W. Co-Combustion Characteristics and CO2 Emissions of Low-Calorific Multi-Fuels by TG-FTIR Analysis. Energy 2022, 252, 123919. [Google Scholar] [CrossRef]
- Ni, Z.; Bi, H.; Jiang, C.; Tian, J.; Sun, H.; Zhou, W.; Lin, Q. Research on the Co-Pyrolysis of Coal Gangue and Coffee Industry Residue Based on Machine Language: Interaction, Kinetics, and Thermodynamics. Sci. Total Environ. 2022, 804, 150217. [Google Scholar] [CrossRef]
- Ni, Z.; Bi, H.; Jiang, C.; Sun, H.; Zhou, W.; Qiu, Z.; Lin, Q. Research on the Co-Pyrolysis of Coal Slime and Cellulose Based on TG-FTIR-MS, Artificial Neural Network, and Principal Component Analysis. Fuel 2022, 320, 123960. [Google Scholar] [CrossRef]
- Wu, F.-H.; Lu, Y.-H.; Chen, G.-B.; Lin, H.-T.; Lin, T.-H. Co-Combustion Characteristics of Black Liquor and Waste Oil Sludge. Int. J. Energy Res. 2022, 46, 6065–6080. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Hu, S.; Yuan, H.; Chen, Y. Co-Pyrolysis and Co-Gasification of Biomass and Polyethylene: Thermal Behaviors, Volatile Products and Characteristics of Their Residues. J. Energy Inst. 2019, 92, 1926–1935. [Google Scholar] [CrossRef]
- Hou, Y.; Feng, Z.; He, Y.; Gao, Q.; Ni, L.; Su, M.; Ren, H.; Liu, Z.; Hu, W. Co-Pyrolysis Characteristics and Synergistic Interaction of Bamboo Residues and Disposable Face Mask. Renew. Energy 2022, 194, 415–425. [Google Scholar] [CrossRef]
- Song, Y.; Yin, N.; Yao, D.; Ma, Q.; Zhou, J.; Lan, X. Co-Pyrolysis Characteristics and Synergistic Mechanism of Low-Rank Coal and Direct Liquefaction Residue. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2675–2689. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, W.; Bi, H.; Ni, Z.; Sun, H.; Lin, Q. Co-Pyrolysis of Coal Slime and Cattle Manure by TG–FTIR–MS and Artificial Neural Network Modeling: Pyrolysis Behavior, Kinetics, Gas Emission Characteristics. Energy 2022, 247, 123203. [Google Scholar] [CrossRef]
- Yin, H.; Huang, X.; Song, X.; Miao, H.; Mu, L. Co–Pyrolysis of de–Alkalized Lignin and Coconut Shell via TG/DTG–FTIR and Machine Learning Methods: Pyrolysis Characteristics, Gas Products, and Thermo–Kinetics. Fuel 2022, 329, 125517. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Liu, J.; Evrendilek, F.; Buyukada, M. CO2-Assisted Co-Pyrolysis of Textile Dyeing Sludge and Hyperaccumulator Biomass: Dynamic and Comparative Analyses of Evolved Gases, Bio-Oils, Biochars, and Reaction Mechanisms. J. Hazard. Mater. 2020, 400, 123190. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Evrendilek, F.; Kuo, J.; Buyukada, M. Combustion Behaviors of Pteris vittata Using Thermogravimetric, Kinetic, Emission and Optimization Analyses. J. Clean. Prod. 2019, 237, 117772. [Google Scholar] [CrossRef]
- Zhu, X.; He, M.; Xu, Z.; Luo, Z.; Gao, B.; Ruan, R.; Wang, C.-H.; Wong, K.-H.; Tsang, D.C.W. Combined Acid Pretreatment and Co-Hydrothermal Carbonization to Enhance Energy Recovery from Food Waste Digestate. Energy Convers. Manag. 2022, 266, 115855. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Huang, Q.; Yan, J. Hierarchical Porous Structure Formation Mechanism in Food Waste Component Derived N-Doped Biochar: Application in VOCs Removal. Chemosphere 2022, 291, 132702. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, W.-H. Co-Torrefaction Followed by Co-Combustion of Intermediate Waste Epoxy Resins and Woody Biomass in the Form of Mini-Pellet. Int. J. Energy Res. 2020, 44, 9317–9332. [Google Scholar] [CrossRef]
- Tian, L.; Lin, K.; Zhao, Y.; Zhao, C.; Huang, Q.; Zhou, T. Combustion Performance of Fine Screenings from Municipal Solid Waste: Thermo-Kinetic Investigation and Deep Learning Modeling via TG-FTIR. Energy 2022, 243, 122783. [Google Scholar] [CrossRef]
- Tahir, M.H.; Cheng, X.; Irfan, R.M.; Ashraf, R.; Zhang, Y. Comparative Chemical Analysis of Pyrolyzed Bio Oil Using Online TGA-FTIR and GC-MS. J. Anal. Appl. Pyrolysis 2020, 150, 104890. [Google Scholar] [CrossRef]
- Kajda-Szcześniak, M.; Czop, M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy. Energies 2022, 15, 1516. [Google Scholar] [CrossRef]
- Qin, X.; Sun, X.; Hou, R.; Yin, Y. Decision Support for Solution of Straw Waste into Asphalt Material Based on TG-FTIR. Green Mater. 2020, 9, 84–96. [Google Scholar] [CrossRef]
- Tang, X.; Chen, Z.; Liu, J.; Chen, Z.; Xie, W.; Evrendilek, F.; Buyukada, M. Dynamic Pyrolysis Behaviors, Products, and Mechanisms of Waste Rubber and Polyurethane Bicycle Tires. J. Hazard. Mater. 2021, 402, 123516. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Ding, Z.; Fu, J.; Evrendilek, F.; Xie, W.; He, Y. Dynamic Pyrolytic Reaction Mechanisms, Pathways, and Products of Medical Masks and Infusion Tubes. Sci. Total Environ. 2022, 842, 156710. [Google Scholar] [CrossRef]
- Li, W.-H.; Ma, Z.-Y.; Yan, J.-H.; Huang, Q.-X.; Jiang, X.-G. Evolution and Distribution Characteristics of Fluorine during the Incineration of Fluorine-Containing Waste in a Hazardous Waste Incinerator. J. Zhejiang Univ. Sci. A 2019, 20, 564–576. [Google Scholar] [CrossRef]
- Qin, Z.-M.; Wang, P.-C.; Yang, R.; Chen, H.-B. Fast Pyrolysis of Silicones at Low Temperatures Catalyzed by Anatase Titanium Dioxide. Polym. Degrad. Stab. 2020, 182, 109387. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A New Look towards the Thermal Decomposition of Chitins and Chitosans with Different Degrees of Deacetylation by Coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Liang, R.; Liang, Q.; Li, Z.; Zhou, Q.; Li, L.; Sun, W. Assessment of the Thermal Hazards and Oxidization Mechanism of Coloured Corn Starch Dust by TG–FTIR. J. Loss Prev. Process Ind. 2021, 69, 104380. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Materazzi, S. Assessing the Quality of Milk Using a Multicomponent Analytical Platform. Front. Chem. 2020, 8, 614718. [Google Scholar] [CrossRef]
- Buiarelli, F.; Bernardini, F.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Simonetti, G.; Risoluti, R. Extraction, Purification, and Determination by HPLC of Quercetin in Some Italian Wines. Food Anal. Methods 2018, 11, 3558–3562. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. Development of a “Single-Click” Analytical Platform for the Detection of Cannabinoids in Hemp Seed Oil. RSC Adv. 2020, 10, 43394–43399. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. Monitoring of Cannabinoids in Hemp Flours by MicroNIR/Chemometrics. Talanta 2020, 211, 120672. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. The Detection of Cannabinoids in Veterinary Feeds by MicroNIR/Chemometrics: A New Analytical Platform. Analyst 2020, 145, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gao, Y.; Ma, Y. Facile Preparation of Activated Carbon Foam via Pyrolysis of Waste Bread under CO2 Atmosphere. Biomass Convers. Biorefin. 2019, 9, 521–529. [Google Scholar] [CrossRef]
- de Carvalho, G.C.; de Moura, M.F.V.; de Castro, H.G.C.; da Silva Júnior, J.H.; da Silva, H.E.B.; dos Santos, K.M.; Rocha, Z.M.S. Influence of the Atmosphere on the Decomposition of Vegetable Oils: Study of the Profiles of FTIR Spectra and Evolution of Gaseous Products. J. Therm. Anal. Calorim. 2020, 140, 2247–2258. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gullifa, G.; Barone, L.; Papa, E.; Materazzi, S.; Risoluti, R. On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 2: EGA-FTIR. Molecules 2022, 27, 8926. https://doi.org/10.3390/molecules27248926
Gullifa G, Barone L, Papa E, Materazzi S, Risoluti R. On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 2: EGA-FTIR. Molecules. 2022; 27(24):8926. https://doi.org/10.3390/molecules27248926
Chicago/Turabian StyleGullifa, Giuseppina, Laura Barone, Elena Papa, Stefano Materazzi, and Roberta Risoluti. 2022. "On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 2: EGA-FTIR" Molecules 27, no. 24: 8926. https://doi.org/10.3390/molecules27248926
APA StyleGullifa, G., Barone, L., Papa, E., Materazzi, S., & Risoluti, R. (2022). On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 2: EGA-FTIR. Molecules, 27(24), 8926. https://doi.org/10.3390/molecules27248926







