W-SBA-15 as an Effective Catalyst for the Epoxidation of 1,5,9-Cyclododecatriene
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
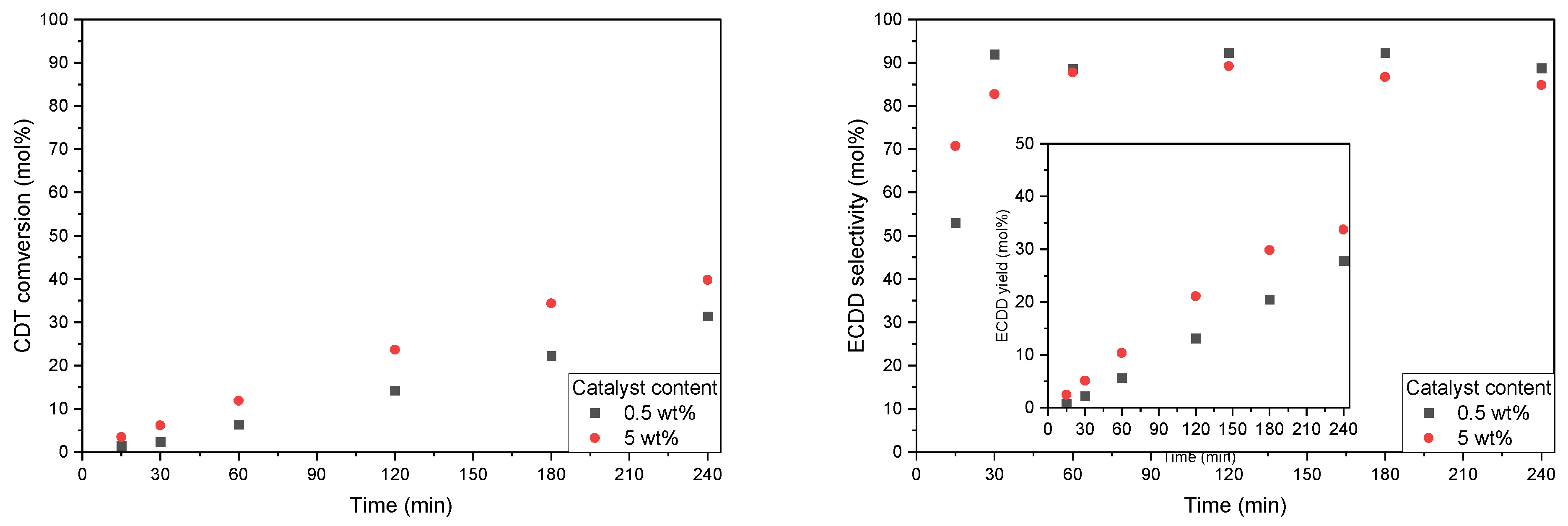
2.2. Catalytic Tests
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lewandowski, G.; Kujbida, M.; Wróblewska, A. Epoxidation of 1,5,9-Cyclododecatriene with Hydrogen Peroxide under Phase-Transfer Catalysis Conditions: Influence of Selected Parameters on the Course of Epoxidation. React. Kinet. Mech. Catal. 2021, 132, 983–1001. [Google Scholar] [CrossRef]
- Oenbrink, G.; Schiffer, T. Cyclododecanol, Cyclododecanone, and Laurolactam. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527306732. [Google Scholar]
- Wołosiak, A.; Lewandowski, G.; Milchert, E. Catalytic Activity of H3PW12O40/Aliquat 336 in Epoxidation of (Z,E,E)-1,5,9-Cyclododecatriene to €-1,2-Epoxy-(Z,E)-5,9-Cyclododecadiene. Ind. Eng. Chem. Res. 2011, 50, 7101–7108. [Google Scholar] [CrossRef]
- Fankhauser, P. Unsaturated Macrocyclic Epoxide as Perfuming Ingredient. U.S. Patent App 2017/0,058,236, 2 March 2017. [Google Scholar]
- Fankhauser, P. Cyclododecadienone Derivatives as Perfuming Ingredients. U.S. Patent App 2016/0,060,569, 3 March 2016. [Google Scholar]
- Wróblewska, A.; Kujbida, M.; Lewandowski, G.; Kamińska, A.; Koren, Z.C.; Michalkiewicz, B. Epoxidation of 1,5,9-Cyclododecatriene with Hydrogen Peroxide over Ti-MCM-41 Catalyst. Catalysts 2021, 11, 1402. [Google Scholar] [CrossRef]
- Duan, Y.Z.; Cao, Y.J.; Shang, X.L.; Jia, D.M.; Li, C.H. Synthesis of G-C3N4/W-SBA-15 Composites for Photocatalytic Degradation of Tetracycline Hydrochloride. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2140–2149. [Google Scholar] [CrossRef]
- Chang, F.; Wang, J.; Luo, J.; Sun, J.; Hu, X. Synthesis, Characterization, and Visible-Light-Driven Photocatalytic Performance of W-SBA15. J. Colloid Interface Sci. 2016, 468, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.A.; Zhang, Y. Ni/W/SBA-15 Catalyst for Carbon Dioxide Reforming of Methane. In Proceedings of the 4th International Conference on Renewable Energy, Resources & Sustainable Technologies, Shenzhen, China, 30–31 December 2016; Volume 112, pp. 40–46. [Google Scholar]
- Bhowmik, S.; Enjamuri, N.; Marimuthu, B.; Darbha, S. C-O Hydrogenolysis of C3-C4 Polyols Selectively to Terminal Diols over Pt/W/SBA-15 Catalysts. Catalysts 2022, 12, 1070. [Google Scholar] [CrossRef]
- Zuo, G.Z.; Xu, Y.B.; Zheng, J.; Jiang, F.; Liu, X.H. Investigation on Converting 1-Butene and Ethylene into Propene via Metathesis Reaction over W-Based Catalysts. RSC Adv. 2018, 8, 8372–8384. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.M.; Wang, J.K.; Wang, B.; Guo, Z.M.; Lv, Z.G. Highly Effective Green Oxidation of Aldehydes Catalysed by Recyclable Tungsten Complex Immobilized in Organosilanes-Modified SBA-15. Microporous Mesoporous Mater. 2019, 277, 84–94. [Google Scholar] [CrossRef]
- Jin, M.; Guo, Z.; Ge, X.; Lv, Z. Tungsten-Based Organic Mesoporous SBA-15 Materials: Characterization and Catalytic Properties in the Oxidation of Cyclopentene to Glutaric Acid with H2O2. React. Kinet. Mech. Catal. 2018, 125, 1139–1157. [Google Scholar] [CrossRef]
- Chiker, F.; Nogier, J.P.; Launay, F.; Bonardet, J.L. New Ti-SBA Mesoporous Solids Functionnalized under Gas Phase Conditions: Characterisation and Application to Selective Oxidation of Alkenes. Appl. Catal. A Gen. 2003, 243, 309–321. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, C.; Li, M.; Gao, B.; Wang, X.; Zheng, X. Synthesis and Characterization of Mesoporous, Tungsten-Containing Molecular Sieve Composites. J. Non. Cryst. Solids 2009, 355, 2209–2215. [Google Scholar] [CrossRef]
- Dimitrov, L.; Palcheva, R.; Spojakina, A.; Jiratova, K. Synthesis and Characterization of W-SBA-15 and W-HMS as Supports for HDS. J. Porous Mater. 2011, 18, 425–434. [Google Scholar] [CrossRef]
- Maheswari, R.; Pachamuthu, M.P.; Ramanathan, A.; Subramaniam, B. Synthesis, Characterization, and Epoxidation Activity of Tungsten-Incorporated SBA-16 (W-SBA-16). Ind. Eng. Chem. Res. 2014, 53, 18833–18839. [Google Scholar] [CrossRef]
- Bérubé, F.; Kleitz, F.; Kaliaguine, S. A Comprehensive Study of Titanium-Substituted SBA-15 Mesoporous Materials Prepared by Direct Synthesis. J. Phys. Chem. C 2008, 112, 14403–14411. [Google Scholar] [CrossRef]
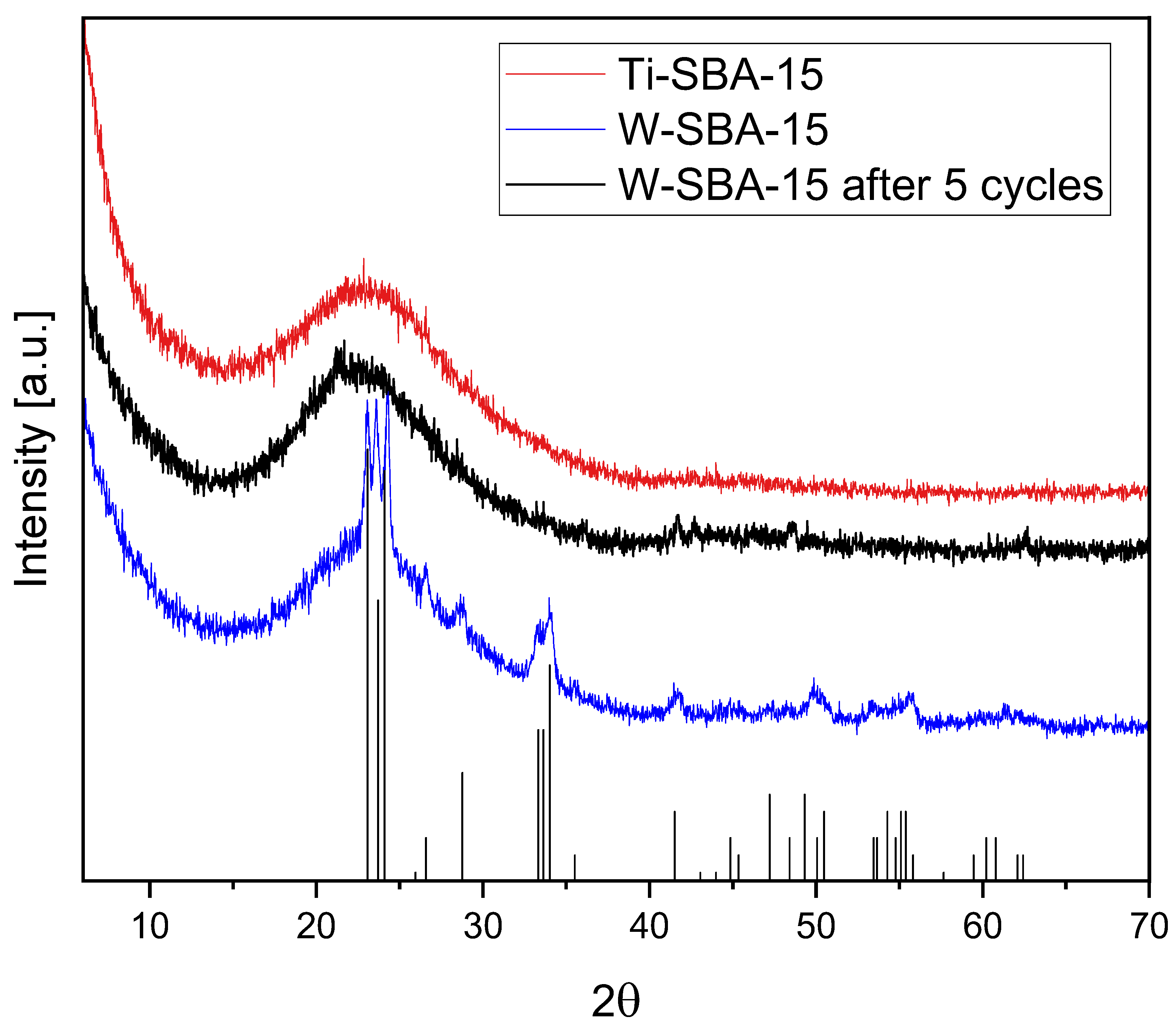
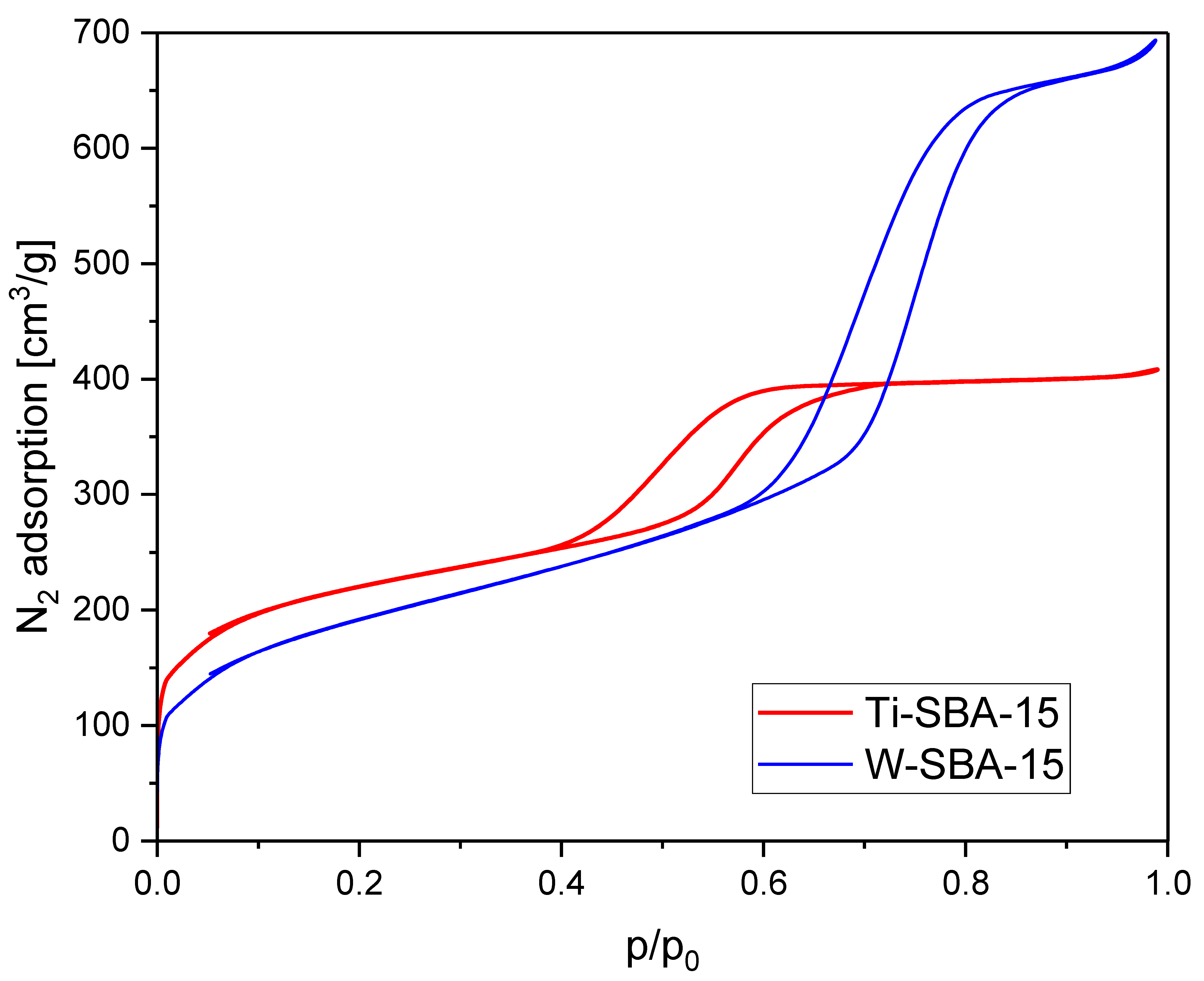
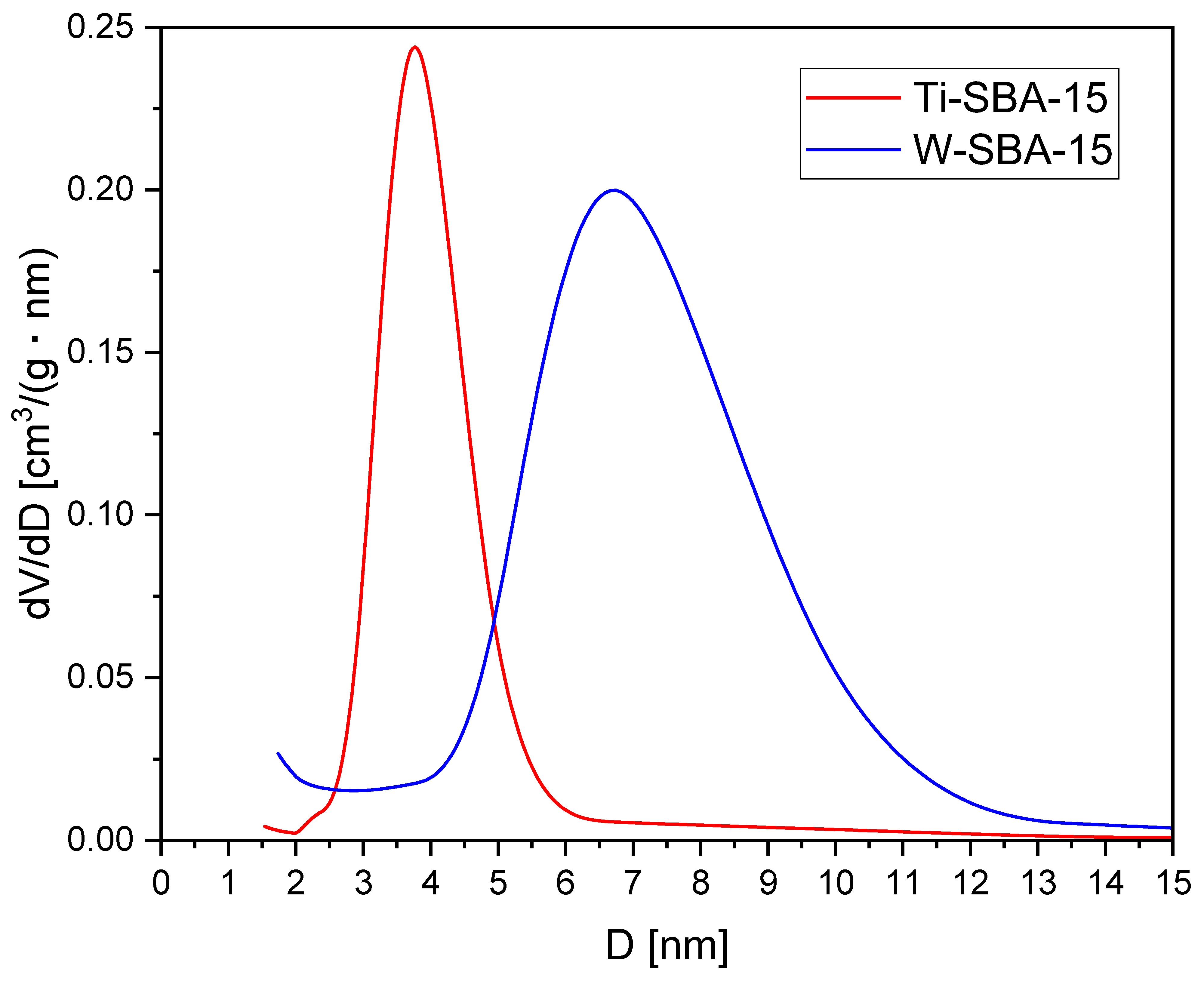
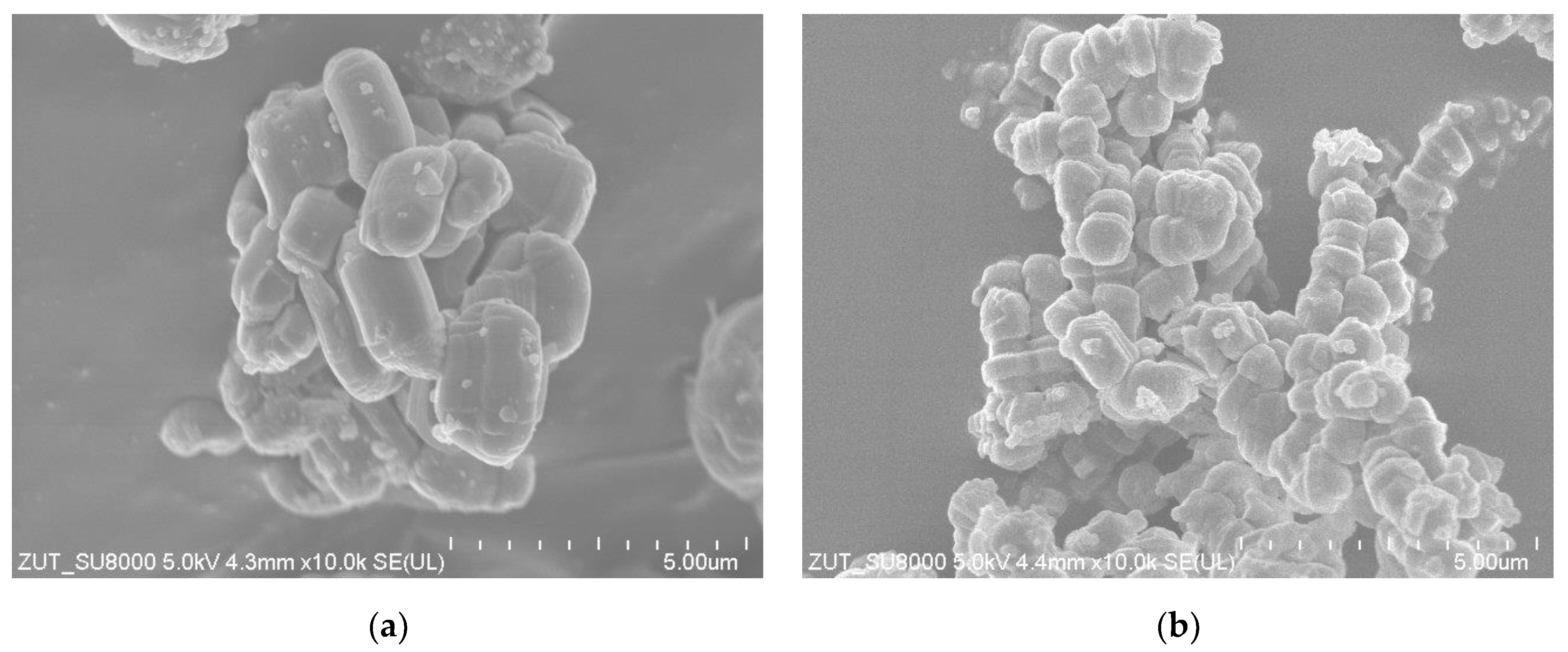

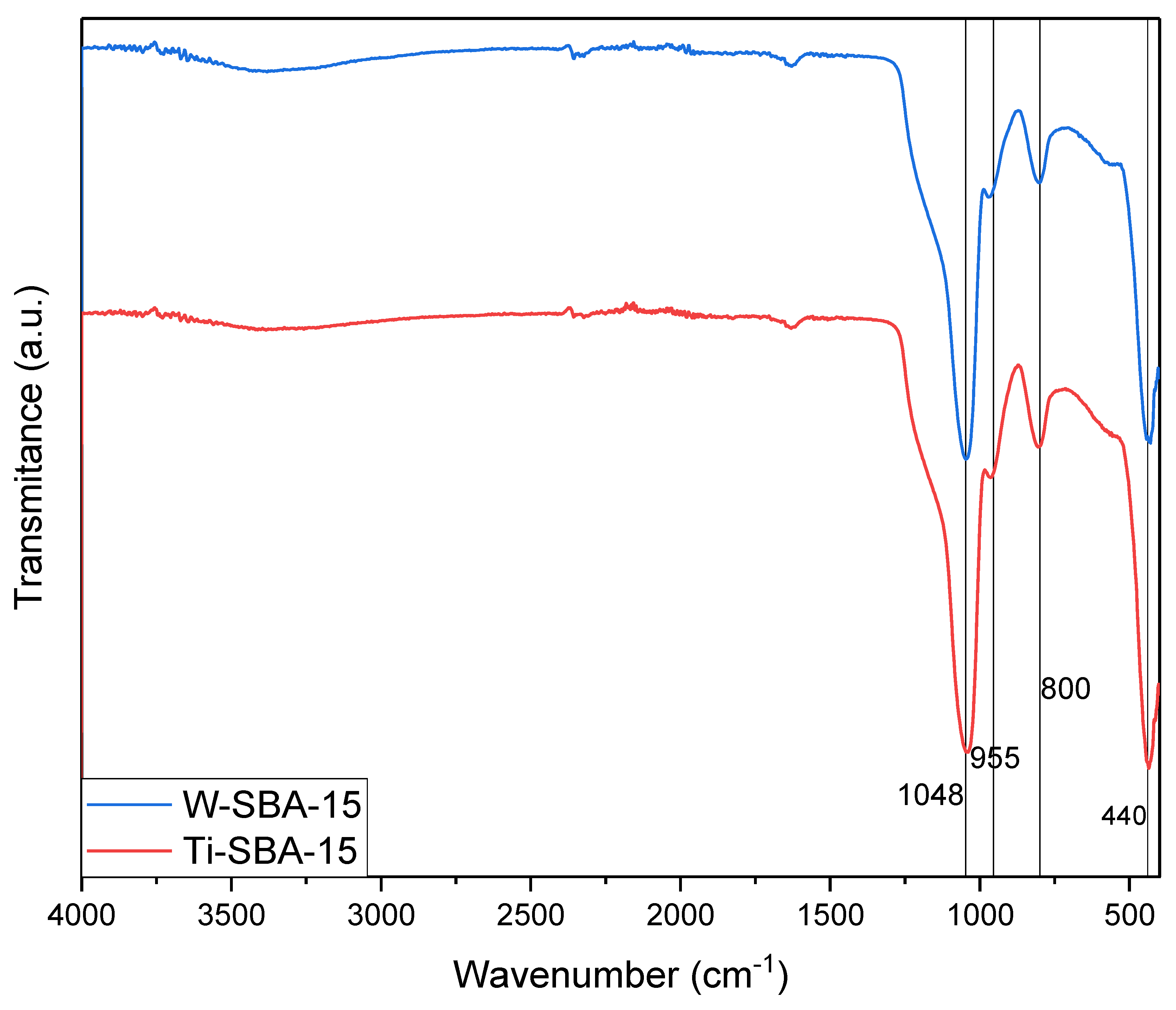
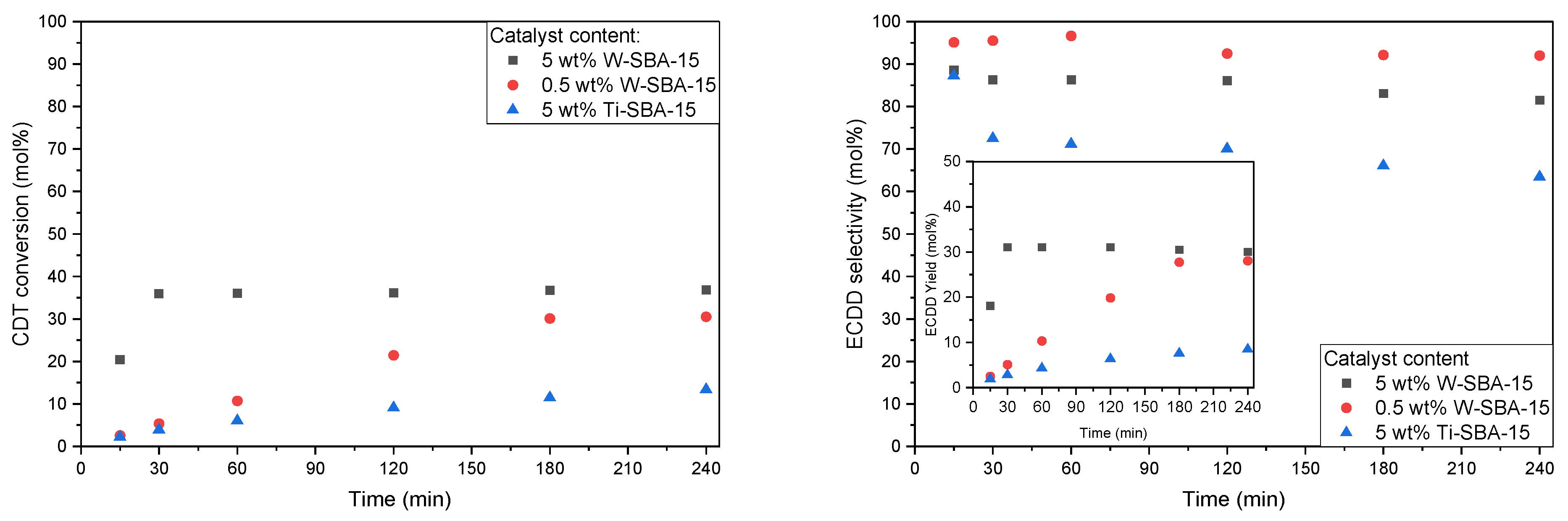
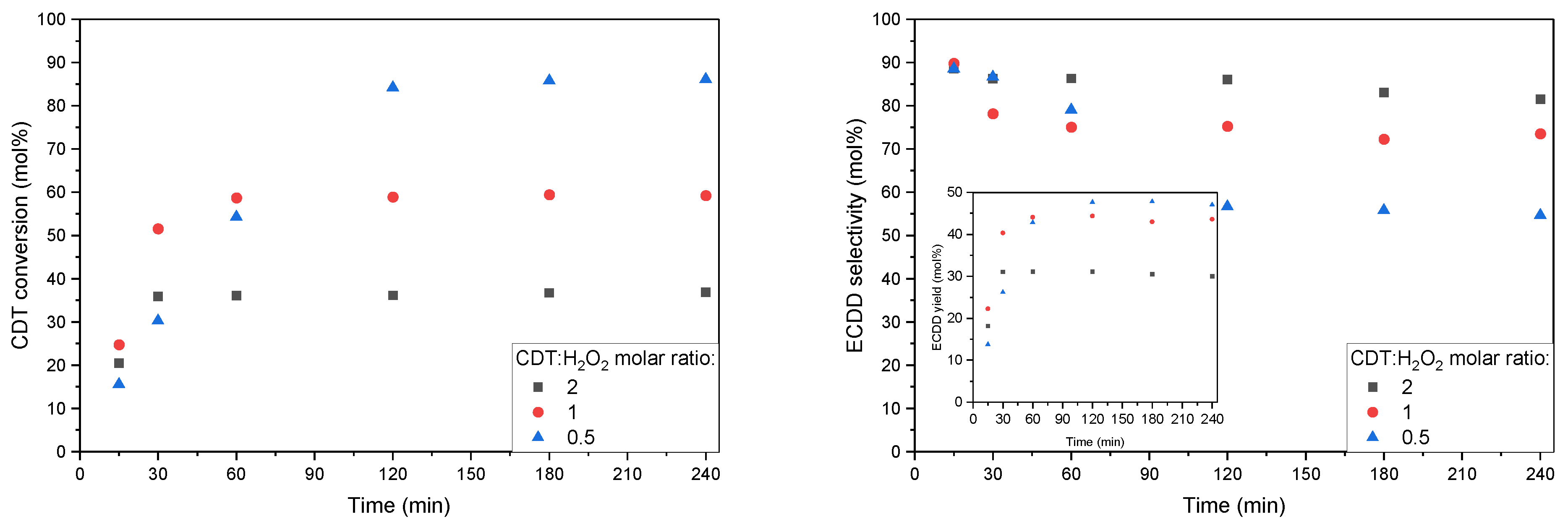
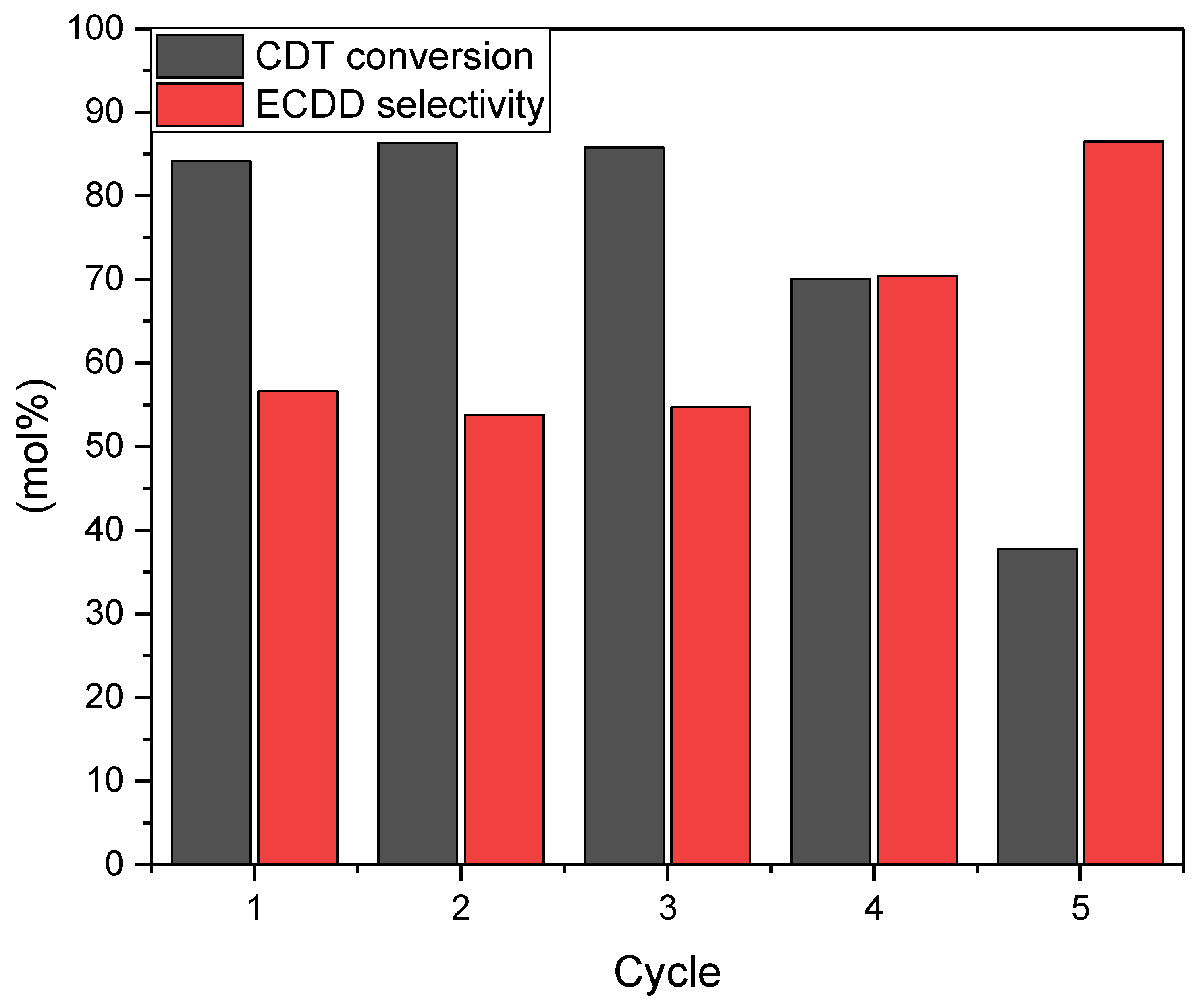

| Catalyst | SBET [m2/g] | Vtot [cm3/g] | Vmic [cm3/g]: |
|---|---|---|---|
| Ti-SBA-15 | 773 | 0.634 | 0.165 |
| W-SBA-15 | 664 | 1.08 | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujbida, M.; Wróblewska, A.; Lewandowski, G.; Miądlicki, P.; Michalkiewicz, B. W-SBA-15 as an Effective Catalyst for the Epoxidation of 1,5,9-Cyclododecatriene. Molecules 2022, 27, 8769. https://doi.org/10.3390/molecules27248769
Kujbida M, Wróblewska A, Lewandowski G, Miądlicki P, Michalkiewicz B. W-SBA-15 as an Effective Catalyst for the Epoxidation of 1,5,9-Cyclododecatriene. Molecules. 2022; 27(24):8769. https://doi.org/10.3390/molecules27248769
Chicago/Turabian StyleKujbida, Marcin, Agnieszka Wróblewska, Grzegorz Lewandowski, Piotr Miądlicki, and Beata Michalkiewicz. 2022. "W-SBA-15 as an Effective Catalyst for the Epoxidation of 1,5,9-Cyclododecatriene" Molecules 27, no. 24: 8769. https://doi.org/10.3390/molecules27248769
APA StyleKujbida, M., Wróblewska, A., Lewandowski, G., Miądlicki, P., & Michalkiewicz, B. (2022). W-SBA-15 as an Effective Catalyst for the Epoxidation of 1,5,9-Cyclododecatriene. Molecules, 27(24), 8769. https://doi.org/10.3390/molecules27248769








