Impact of TiO2 Reduction and Cu Doping on Bacteria Inactivation under Artificial Solar Light Irradiation
Abstract
1. Introduction
2. Results
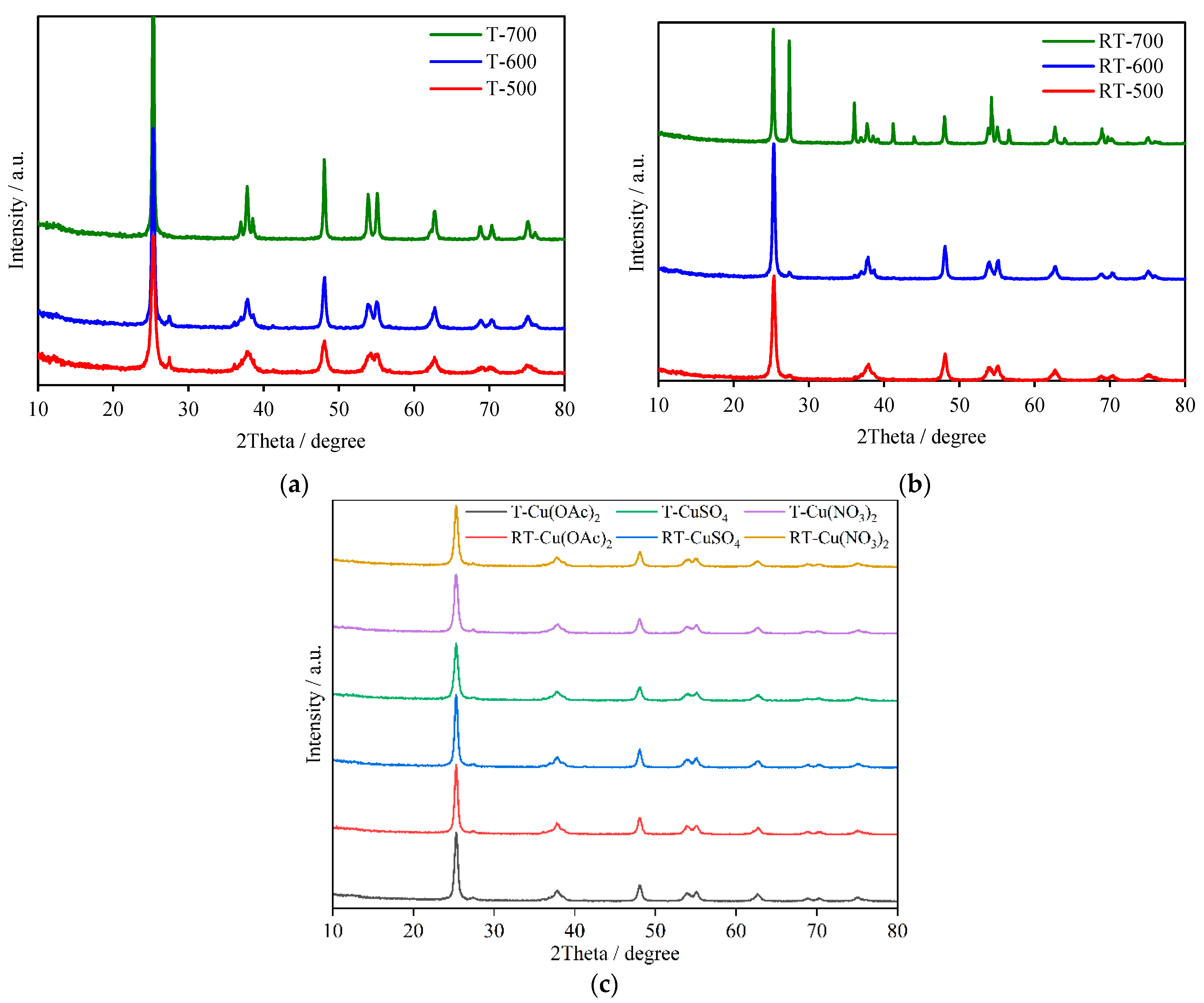
2.1. X-ray Diffraction and TEM Analyses
2.2. X-ray Fluorescence Spectroscopy
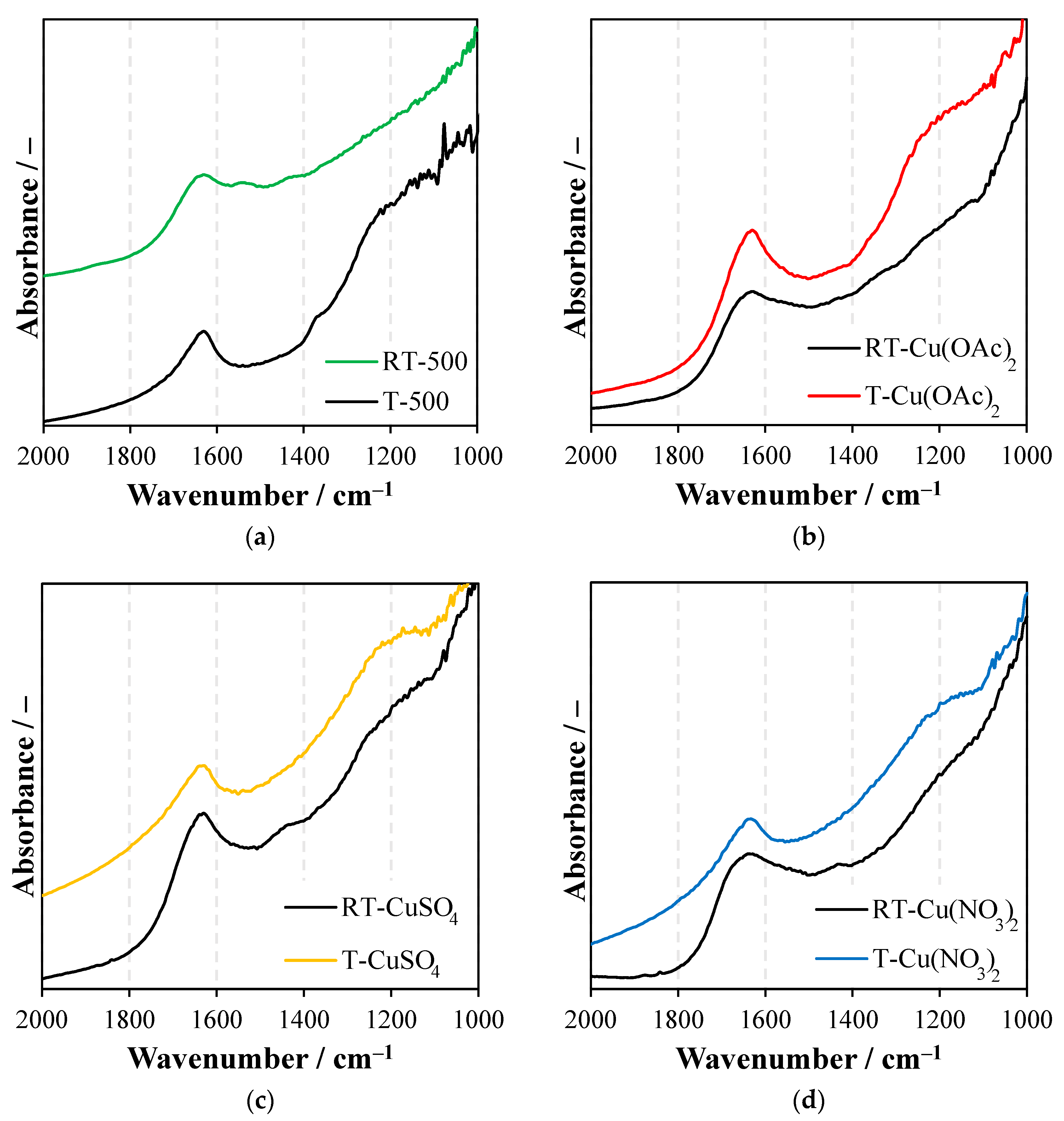
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
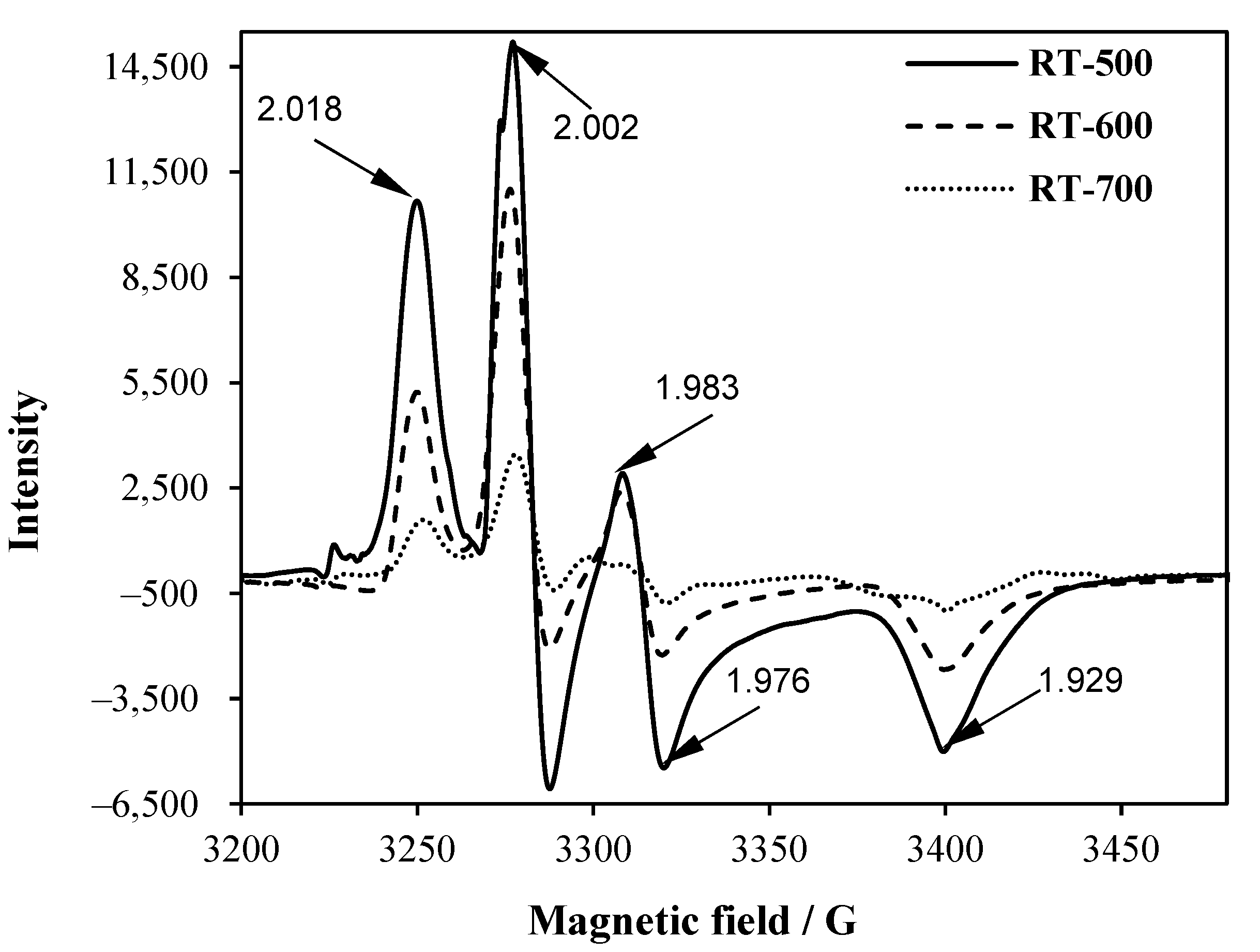
2.4. EPR Spectroscopy
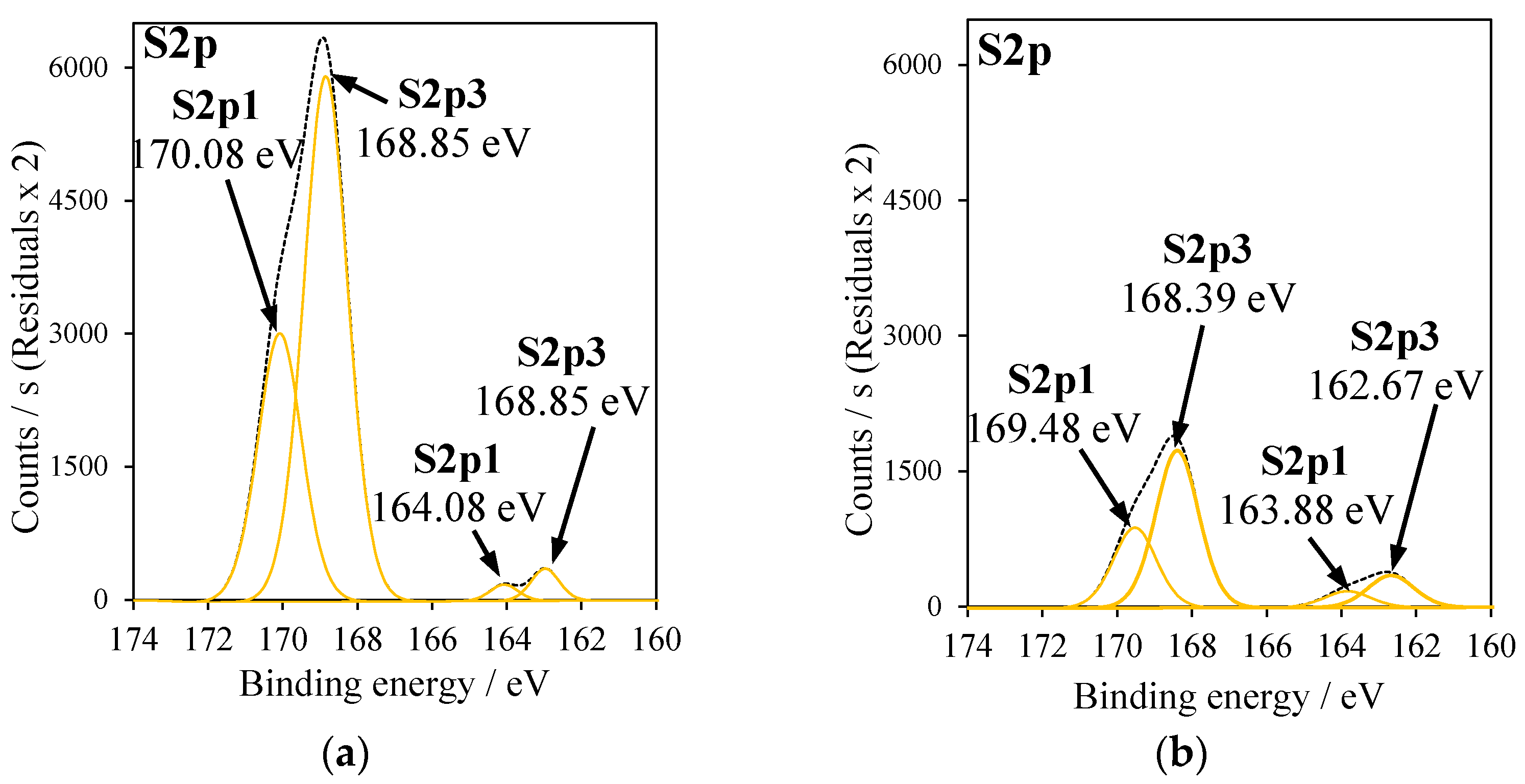
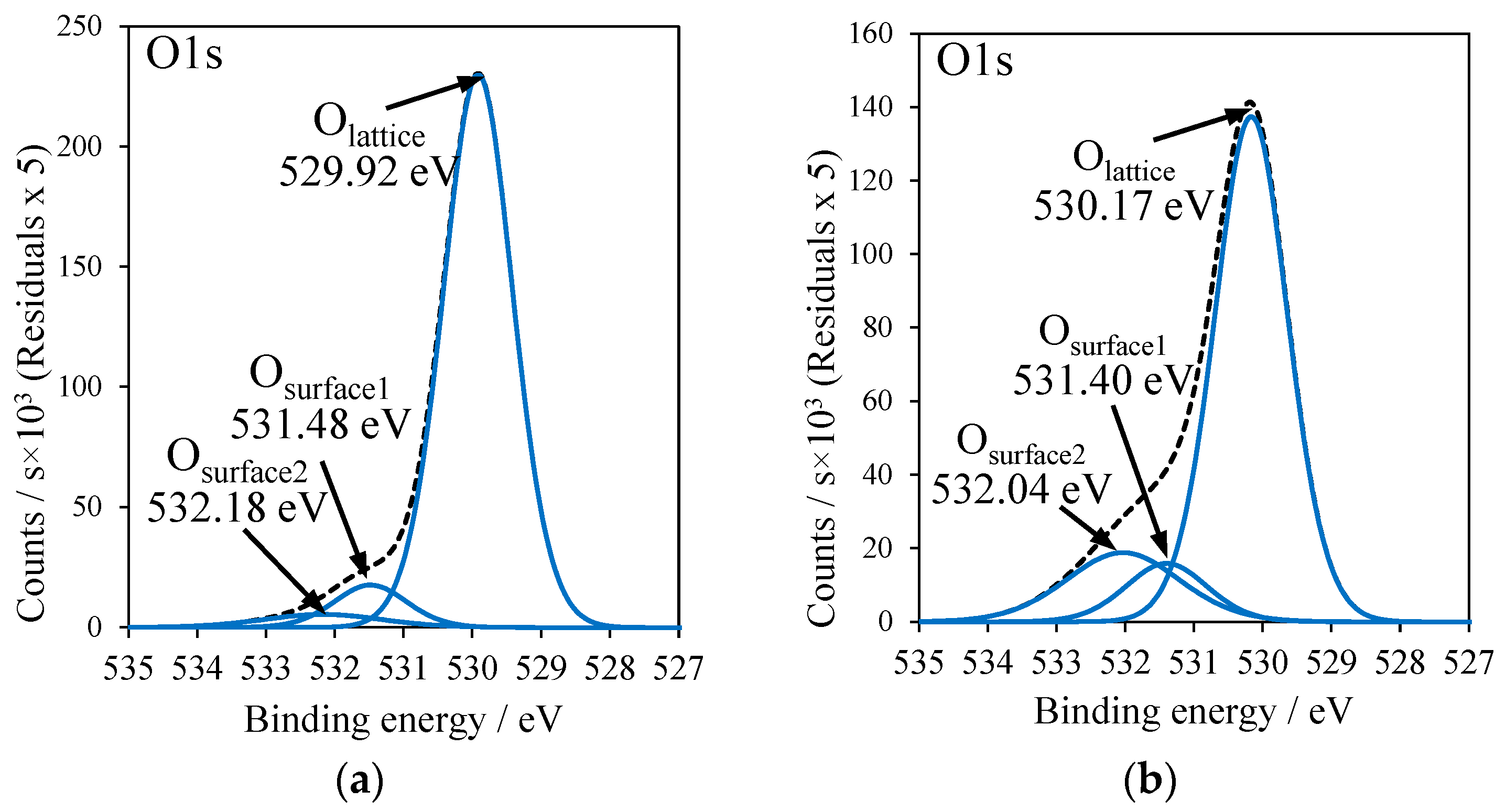
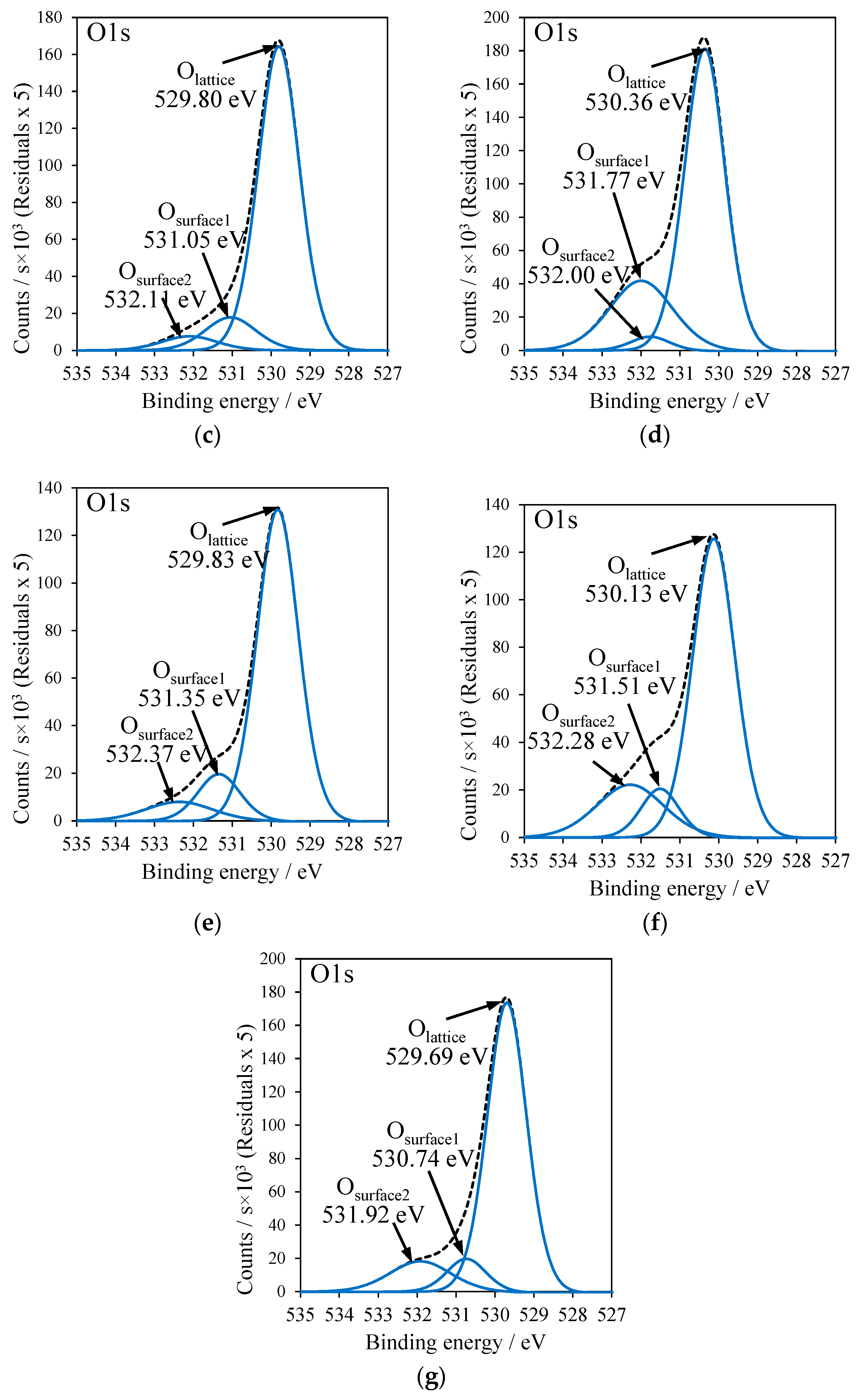
2.5. X-ray Photoelectron Spectroscopy
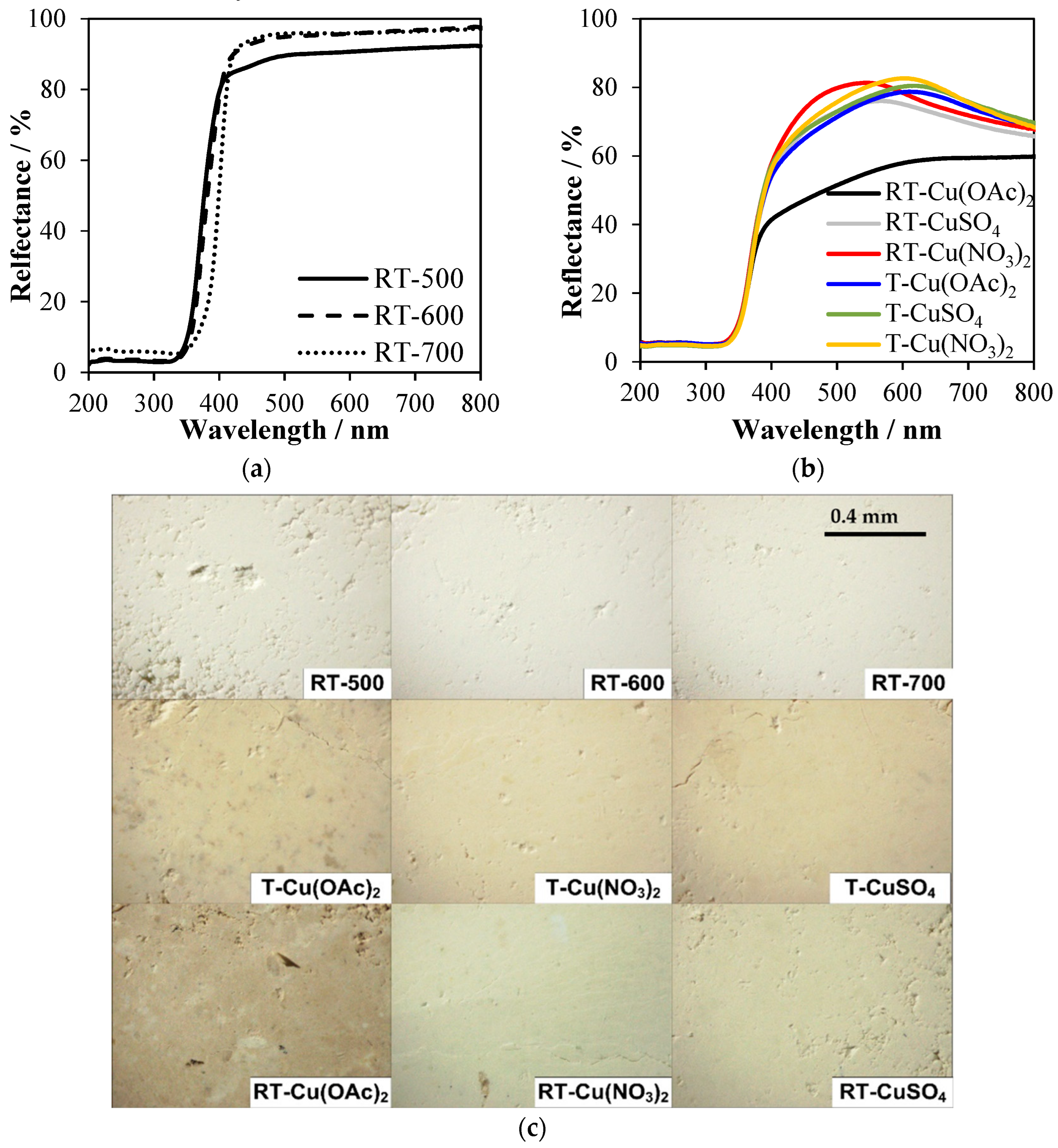
2.6. UV-Vis Spectroscopy
2.7. Zeta Potential and pH
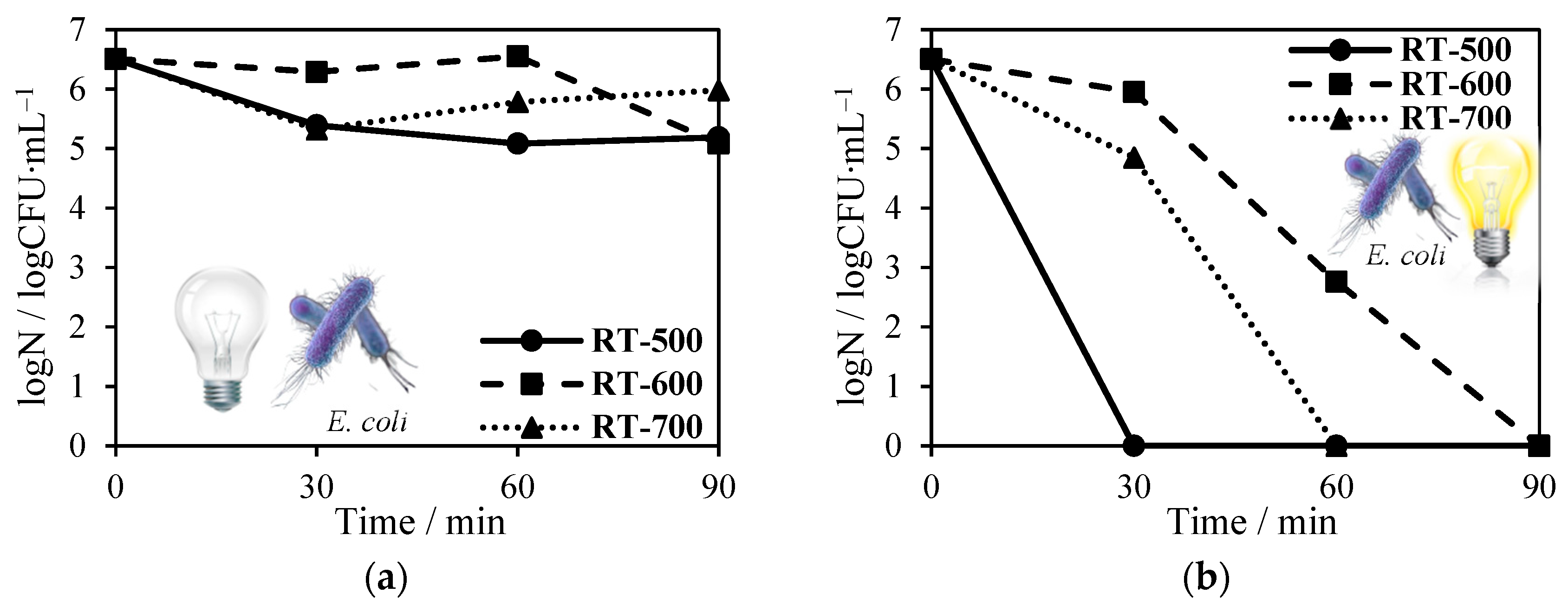
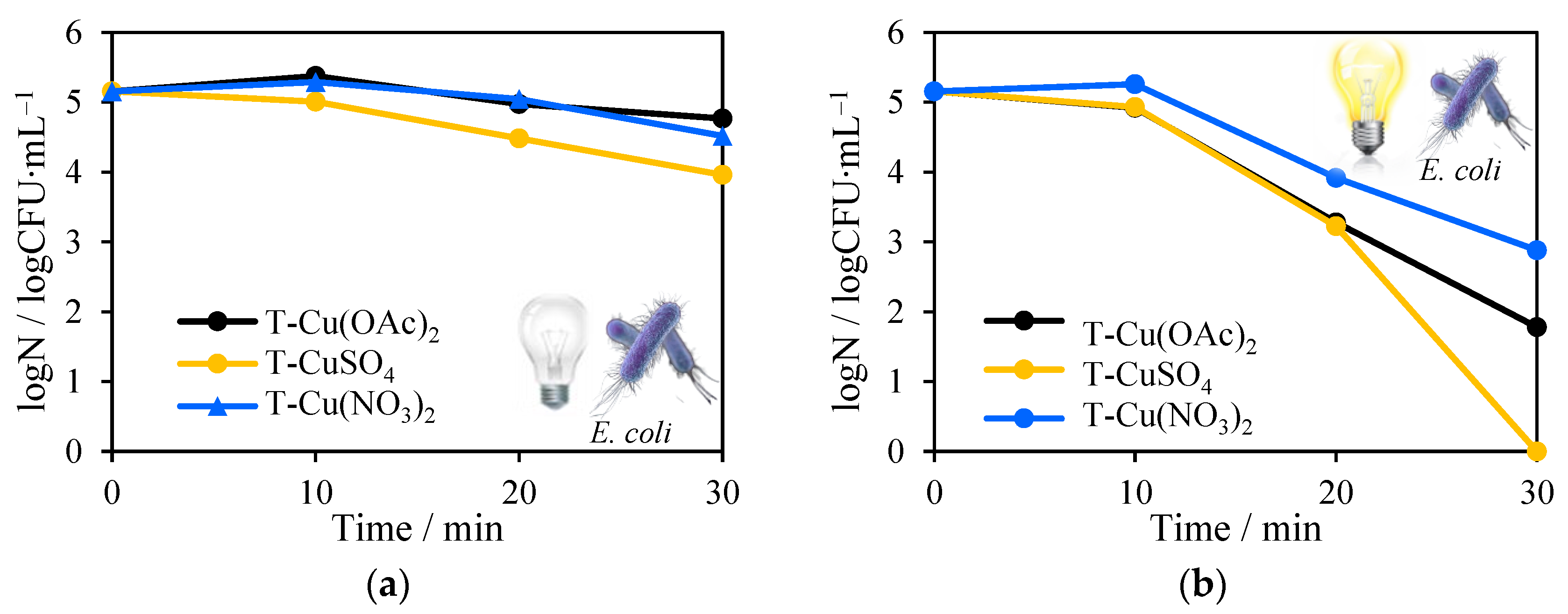
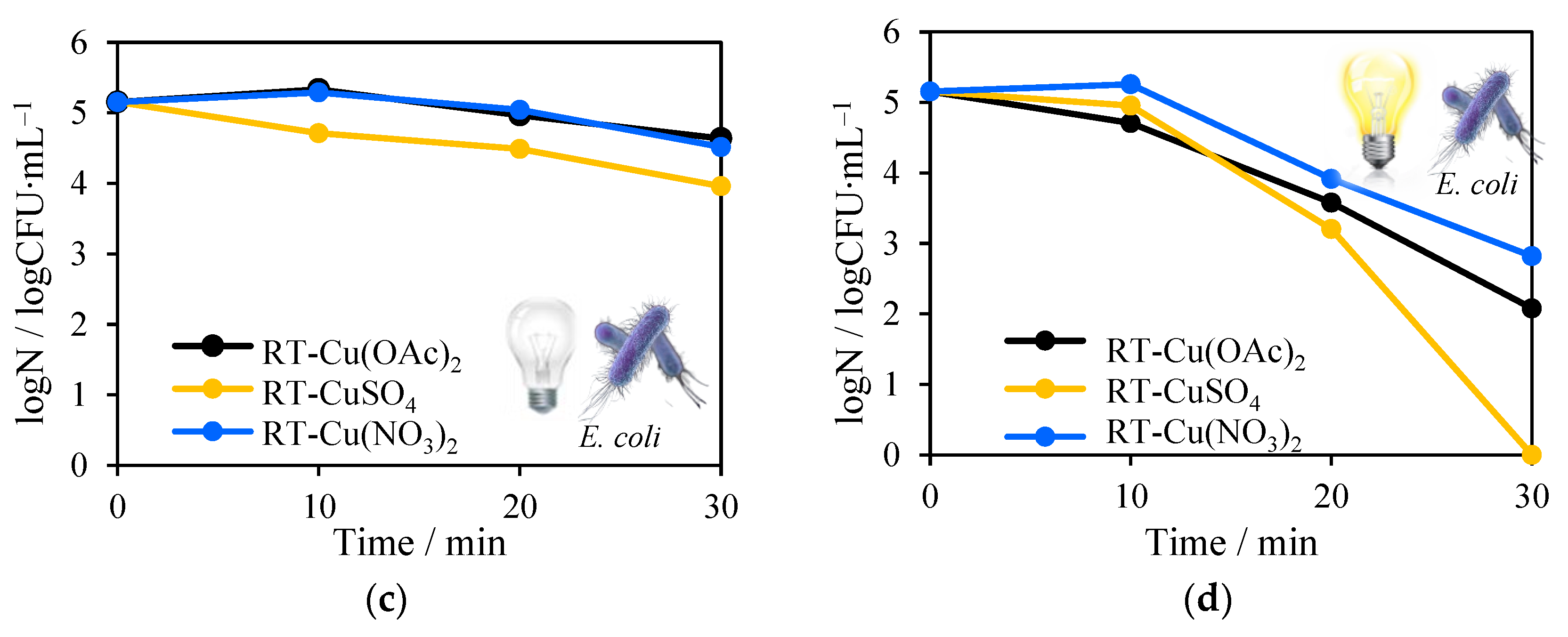
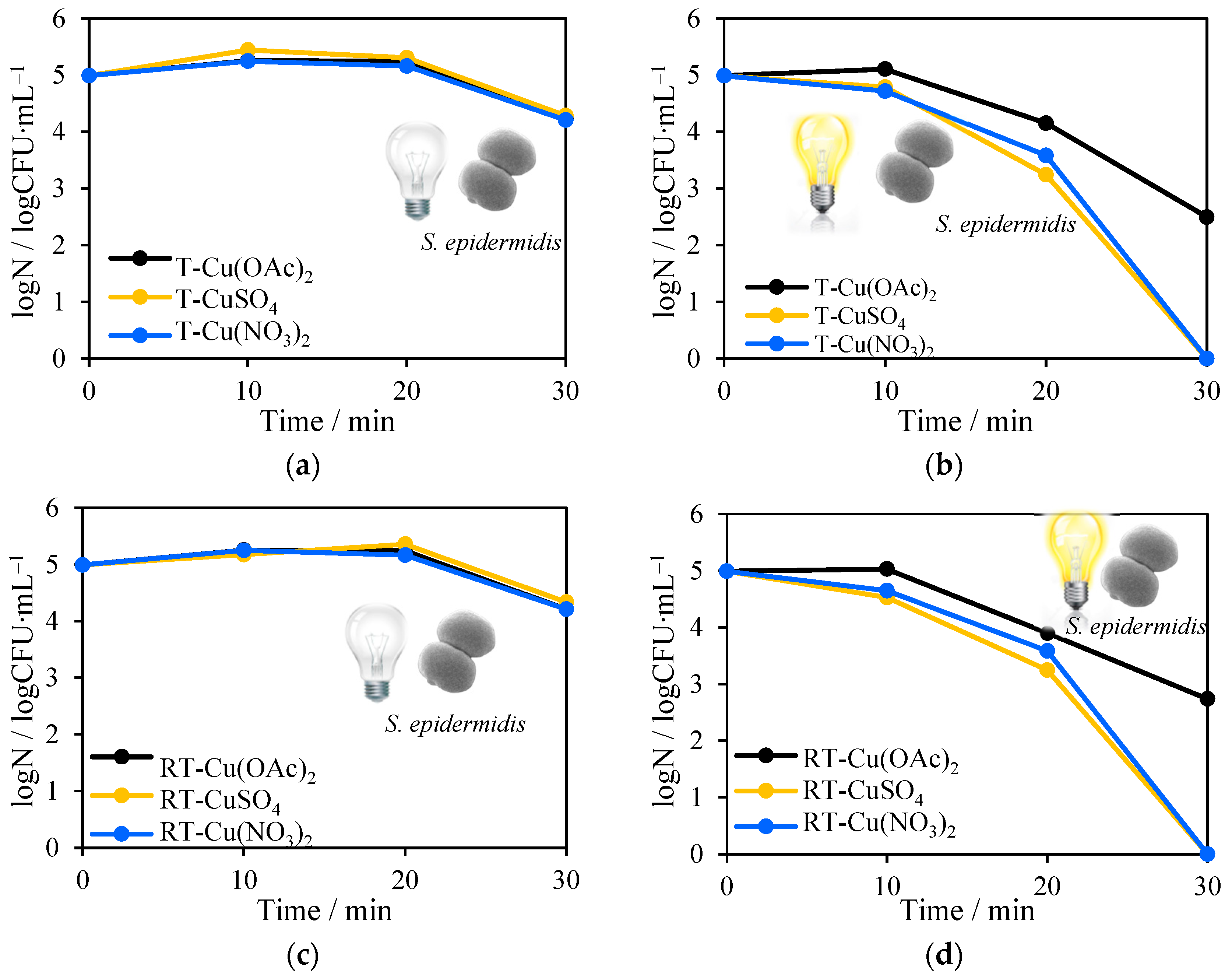
2.8. Antimicrobial Tests towards Escherichia Coli and Staphylococcus Epidermidis Inactivation in the Presence of Solar Light
2.9. Reactive Radical Formation
3. Discussion
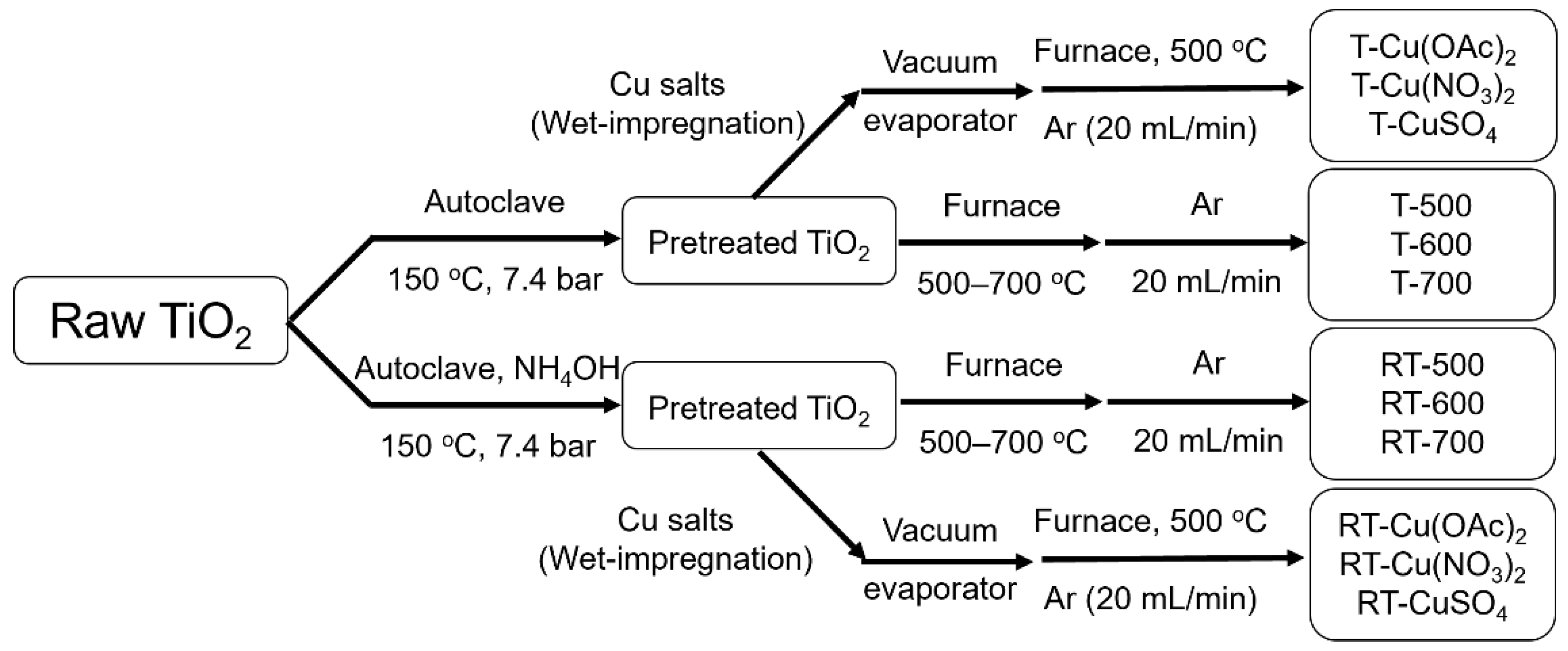
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leung, Y.H.; Xu, X.; Ma, A.P.Y.; Liu, F.; Ng, A.M.C.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišić, A.B.; Lee, P.K.H.; et al. Toxicity of ZnO and TiO2 to Escherichia Coli Cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Chen, S.; Ge, Z.; Yang, H.; Tang, J. Fabrication of Cu/TiO2 Nanocomposite: Toward an Enhanced Antibacterial Performance in the Absence of Light. Mater. Lett. 2012, 83, 154–157. [Google Scholar] [CrossRef]
- Rahmawati, F.; Kusumaningsih, T.; Hapsari, A.; Hastuti, A. Ag and Cu Loaded on TiO2/Graphite as a Catalyst for Escherichia Coli-Contaminated Water Disinfection. Chem. Pap. 2010, 64, 557–565. [Google Scholar] [CrossRef]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of Solar Simulated N-Doped TiO2 Photocatalysis on the Inactivation and Antibiotic Resistance of an E. Coli Strain in Biologically Treated Urban Wastewater. Appl. Catal. B Environ. 2014, 144, 369–378. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient Visible-Light Induced Photocatalysis on Nanoporous Nitrogen-Doped Titanium Dioxide Catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H.; Chen, C.; Li, X.; Niu, M.C.; Gao, X.Y.; Yang, Y.T. Effect of Calcination Temperature on the Structure and Visible-Light Photocatalytic Activities of (N, S and C) Co-Doped TiO2 Nano-Materials. Appl. Surf. Sci. 2015, 332, 172–180. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts 2021, 11, 978. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Skrzypska, A.; Felczak, P.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Nishiguchi, H.; Toyoda, M. Role of the Hydroxyl Groups Coordinated ToTiO2 Surface on the Photocatalytic Decomposition of Ethylene at Different Ambient Conditions. Catalysts 2022, 12, 386. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Fuks, H.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Impact of TiO2 Surface Defects on the Mechanism of Acetaldehyde Decomposition under Irradiation of a Fluorescent Lamp. Catalysts 2021, 11, 1281. [Google Scholar] [CrossRef]
- Rychtowski, P.; Orlikowski, J.; Żołnierkiewicz, G.; Tryba, B. Mechanism of Hydroxyl Radicals Formation on the Reduced Rutile. Mater. Res. Bull. 2022, 147, 111643. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Wang, X.; Yao, J.; Zhai, M.; Liu, B.; Liang, C.; Shi, H. Highly Efficient Ti3+ Self-Doped TiO2 Co-Modified with Carbon Dots and Palladium Nanocomposites for Disinfection of Bacterial and Fungi. J. Hazard. Mater. 2021, 413, 125318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, M.; Liu, X.; Wang, X.; Liu, S.; Han, B.; Liu, B.; Shi, H. Copper Modified Ti3+ Self-Doped TiO2 Photocatalyst for Highly Efficient Photodisinfection of Five Agricultural Pathogenic Fungus. Chem. Eng. J. 2020, 387, 124171. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact Killing and Antimicrobial Properties of Copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Lopez, T.; Cuevas, J.L.; Ilharco, L.; Ramírez, P.; Rodríguez-Reinoso, F.; Rodríguez-Castellón, E. XPS Characterization and E. Coli DNA Degradation Using Functionalized Cu/TiO2 Nanobiocatalysts. Mol. Catal. 2018, 449, 62–71. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Pulgarin, C.; Lavanchy, J.-C.; Kiwi, J. Growth of TiO2/Cu Films by HiPIMS for Accelerated Bacterial Loss of Viability. Surf. Coat. Technol. 2013, 232, 804–813. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the Mechanism of Photocatalytic Reactions on CuxO@TiO2 Core–Shell Photocatalysts. J. Mater. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Sun, H.; Wang, X.; Lian, J. Preparation and Photocatalytic Performance of Cu-Doped TiO2 Nanoparticles. Trans. Nonferrous Met. Soc. China 2015, 25, 504–509. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, X.; Liu, K.; Wang, N. Preparation of Novel Cu/TiO2 Mischcrystal Composites and Antibacterial Activities for Escherichia Coli under Visible Light. Ceram. Int. 2017, 43, 9658–9663. [Google Scholar] [CrossRef]
- Khraisheh, M.; Wu, L.; Al-Muhtaseb, A.H.; Al-Ghouti, M.A. Photocatalytic Disinfection of Escherichia Coli Using TiO2 P25 and Cu-Doped TiO2. J. Ind. Eng. Chem. 2015, 28, 369–376. [Google Scholar] [CrossRef]
- Carvalho, H.W.P.; Batista, A.P.L.; Hammer, P.; Ramalho, T.C. Photocatalytic Degradation of Methylene Blue by TiO2–Cu Thin Films: Theoretical and Experimental Study. J. Hazard. Mater. 2010, 184, 273–280. [Google Scholar] [CrossRef]
- Zong, M.; Bai, L.; Liu, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B. Antibacterial Ability and Angiogenic Activity of Cu-Ti-O Nanotube Arrays. Mater. Sci. Eng. C 2017, 71, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tryba, B.; Rychtowski, P.; Markowska-Szczupak, A.; Przepiórski, J. Photocatalytic Decomposition of Acetaldehyde on Different TiO2-Based Materials: A Review. Catalysts 2020, 10, 1464. [Google Scholar] [CrossRef]
- Topalian, Z.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Spectroscopic Study of the Photofixation of SO2 on Anatase TiO2 Thin Films and Their Oleophobic Properties. ACS Appl. Mater. Interfaces 2012, 4, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.-S.; Ke, S.C. EPR Investigation of TiO 2 Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Howe, R.F.; Gratzel, M. EPR Observation of Trapped Electrons in Colloidal Titanium Dioxide. J. Phys. Chem. 1985, 89, 4495–4499. [Google Scholar] [CrossRef]
- Amano, F. Hydrogen Reduced Rutile Titanium Dioxide Photocatalyst. In Titanium Dioxide; Janus, M., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3413-8. [Google Scholar]
- Siriwardane, R.V.; Poston, J.A. Interaction of H2S with Zinc Titanate in the Presence of H2 and CO. Appl. Surf. Sci. 1990, 45, 131–139. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Peng, S.; Lu, G.; Li, S. Modification of TiO2 with Sulfate and Phosphate for Enhanced Eosin Y-Sensitized Hydrogen Evolution under Visible Light Illumination. Photochem. Photobiol. Sci. 2013, 12, 1903–1910. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Song, D.; An, C.; Wang, J. Catalysis of a Nanometre Solid Super Acid of SO42−/TiO2 on the Thermal Decomposition of Ammonium Nitrate. Nanomater. Nanotechnol. 2016, 6, 23. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Min, B.-K.; Cho, M.H. Microbial Fuel Cell Assisted Band Gap Narrowed TiO2 for Visible Light-Induced Photocatalytic Activities and Power Generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.-J.; Song, Y.; Sun, Z. Reduced TiO2 Rutile Nanorods with Well-Defined Facets and Their Visible-Light Photocatalytic Activity. Chem. Commun. 2014, 50, 2755–2757. [Google Scholar] [CrossRef]
- Pitchaimuthu, S.; Honda, K.; Suzuki, S.; Naito, A.; Suzuki, N.; Katsumata, K.; Nakata, K.; Ishida, N.; Kitamura, N.; Idemoto, Y.; et al. Solution Plasma Process-Derived Defect-Induced Heterophase Anatase/Brookite TiO 2 Nanocrystals for Enhanced Gaseous Photocatalytic Performance. ACS Omega 2018, 3, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Luciu, I.; Bartali, R.; Laidani, N. Influence of Hydrogen Addition to an Ar Plasma on the Structural Properties of TiO2−x Thin Films Deposited by RF Sputtering. J. Phys. D Appl. Phys. 2012, 45, 345302. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, A.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an Electronic Structure of N-TiO2 on Its Photocatalytic Activity towards Decomposition of Acetaldehyde under UV and Fluorescent Lamps Irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Sordo, C.; Cruz, C. Kinetics of the Photocatalytic Disinfection of Escherichia Coli Suspensions. Appl. Catal. B Environ. 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.M.; Ali, S.H.; Hassan, A.A.; Kassem, T.S.E.; Van Patten, P.G. How Does Photocatalytic Activity Depend on Adsorption, Composition, and Other Key Factors in Mixed Metal Oxide Nanocomposites? Colloid Interface Sci. Commun. 2021, 40, 100341. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial Properties of TiO2 Modified with Reduced Graphene Oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef]
- Wyness, A.J.; Paterson, D.M.; Defew, E.C.; Stutter, M.I.; Avery, L.M. The Role of Zeta Potential in the Adhesion of E. Coli to Suspended Intertidal Sediments. Water Res. 2018, 142, 159–166. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic Degradation of Pathogenic Bacteria with AgI/TiO2 under Visible Light Irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef]





| Sample | Mean Crystallite Size/nm | |
|---|---|---|
| Anatase | Rutile | |
| Raw TiO2 | 12 | 19 |
| T-500 | 17 | 70 |
| T-600 | 26 | 51 |
| T-700 | 48 | – |
| RT-500 | 21 | 24 |
| RT-600 | 30 | 37 |
| RT-700 | 56 | 177 |
| T-Cu(OAc)2 | 20 | 14 |
| RT-Cu(OAc)2 | 22 | 23 |
| T-CuSO4 | 16 | 16 |
| RT-CuSO4 | 23 | 16 |
| T-Cu(NO3)2 | 18 | 20 |
| RT-Cu(NO3)2 | 19 | 26 |
| Sample | XRF Mass Content/% | ||
|---|---|---|---|
| Ti | Cu | S | |
| Raw TiO2 | 84.26 | – | 1.54 |
| T-Cu(OAc)2 | 77.63 | 1.65 | 0.72 |
| RT-Cu(OAc)2 | 80.70 | 1.62 | 0.11 |
| T-CuSO4 | 77.38 | 1.60 | 1.52 |
| RT-CuSO4 | 81.42 | 1.59 | 0.40 |
| T-Cu(NO3)2 | 77.16 | 1.67 | 1.24 |
| RT-Cu(NO3)2 | 80.66 | 1.50 | 0.14 |
| Sample | Cu2O/CuO | Olattice/Osurface | Osurface1/Osurface2 | Ti2p BE/eV |
|---|---|---|---|---|
| RT-500 | – | 89.3/10.7 | 66.6/33.4 | 458.55 |
| T-Cu(OAc)2 | 89.3/10.7 | 74.5/25.5 | 37.7/62.3 | 458.96 |
| RT-Cu(OAc)2 | 77.9/22.1 | 83.6/16.4 | 68.2/31.8 | 458.55 |
| T-CuSO4 | 86.2/13.8 | 71.2/28.8 | 10.8/89.2 | 459.14 |
| RT-CuSO4 | 86.7/13.3 | 79.7/20.3 | 60.8/39.2 | 458.57 |
| T-Cu(NO3)2 | 71.9/28.1 | 70.1/29.9 | 34.4/65.6 | 458.88 |
| RT-Cu(NO3)2 | 84.0/16.0 | 78.4/21.6 | 40.8/59.2 | 458.46 |
| Sample | Solution | pH | Zeta Potential/mV |
|---|---|---|---|
| RT-500 | NaCl | - | −12.43 |
| RT-600 | - | −9.15 | |
| RT-700 | - | −10.78 | |
| T-Cu(OAc)2 | 5.47 | −7.23 | |
| RT-Cu(OAc)2 | 6.54 | −7.37 | |
| T-CuSO4 | 4.43 | −6.91 | |
| RT-CuSO4 | 6.18 | −8.40 | |
| T-Cu(NO3)2 | 4.48 | −8.28 | |
| RT-Cu(NO3)2 | 6.47 | −11.50 | |
| RT-500 | H3PO4 | - | −24.07 |
| RT-600 | - | −25.90 | |
| RT-700 | - | −25.49 | |
| T-Cu(OAc)2 | 7.25 | −24.60 | |
| RT-Cu(OAc)2 | 7.27 | −24.90 | |
| T-CuSO4 | 7.24 | −25.00 | |
| RT-CuSO4 | 7.24 | −25.10 | |
| T-Cu(NO3)2 | 7.23 | −24.40 | |
| RT-Cu(NO3)2 | 7.25 | −26.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychtowski, P.; Paszkiewicz, O.; Román-Martínez, M.C.; Lillo-Ródenas, M.Á.; Markowska-Szczupak, A.; Tryba, B. Impact of TiO2 Reduction and Cu Doping on Bacteria Inactivation under Artificial Solar Light Irradiation. Molecules 2022, 27, 9032. https://doi.org/10.3390/molecules27249032
Rychtowski P, Paszkiewicz O, Román-Martínez MC, Lillo-Ródenas MÁ, Markowska-Szczupak A, Tryba B. Impact of TiO2 Reduction and Cu Doping on Bacteria Inactivation under Artificial Solar Light Irradiation. Molecules. 2022; 27(24):9032. https://doi.org/10.3390/molecules27249032
Chicago/Turabian StyleRychtowski, Piotr, Oliwia Paszkiewicz, Maria Carmen Román-Martínez, Maria Ángeles Lillo-Ródenas, Agata Markowska-Szczupak, and Beata Tryba. 2022. "Impact of TiO2 Reduction and Cu Doping on Bacteria Inactivation under Artificial Solar Light Irradiation" Molecules 27, no. 24: 9032. https://doi.org/10.3390/molecules27249032
APA StyleRychtowski, P., Paszkiewicz, O., Román-Martínez, M. C., Lillo-Ródenas, M. Á., Markowska-Szczupak, A., & Tryba, B. (2022). Impact of TiO2 Reduction and Cu Doping on Bacteria Inactivation under Artificial Solar Light Irradiation. Molecules, 27(24), 9032. https://doi.org/10.3390/molecules27249032










