Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Collection
2.2. Preparation of Extract
2.3. Total Polyphenols
2.4. Flavonoids Content
2.5. HPLC-DAD Analysis
2.6. Antioxidant Capacity
2.6.1. DPPH Test
2.6.2. FRAP Test
2.6.3. TAC Test
2.7. Antimicrobial Properties of Extracts of S. elaeagnifolium Leaves and Fruits
2.7.1. Antimicrobial Activity Examination
2.7.2. Minimum Inhibitory Concentration (MIC) Determination
2.8. In Silico Studies
2.9. Statistical Analysis
3. Results and Discussion
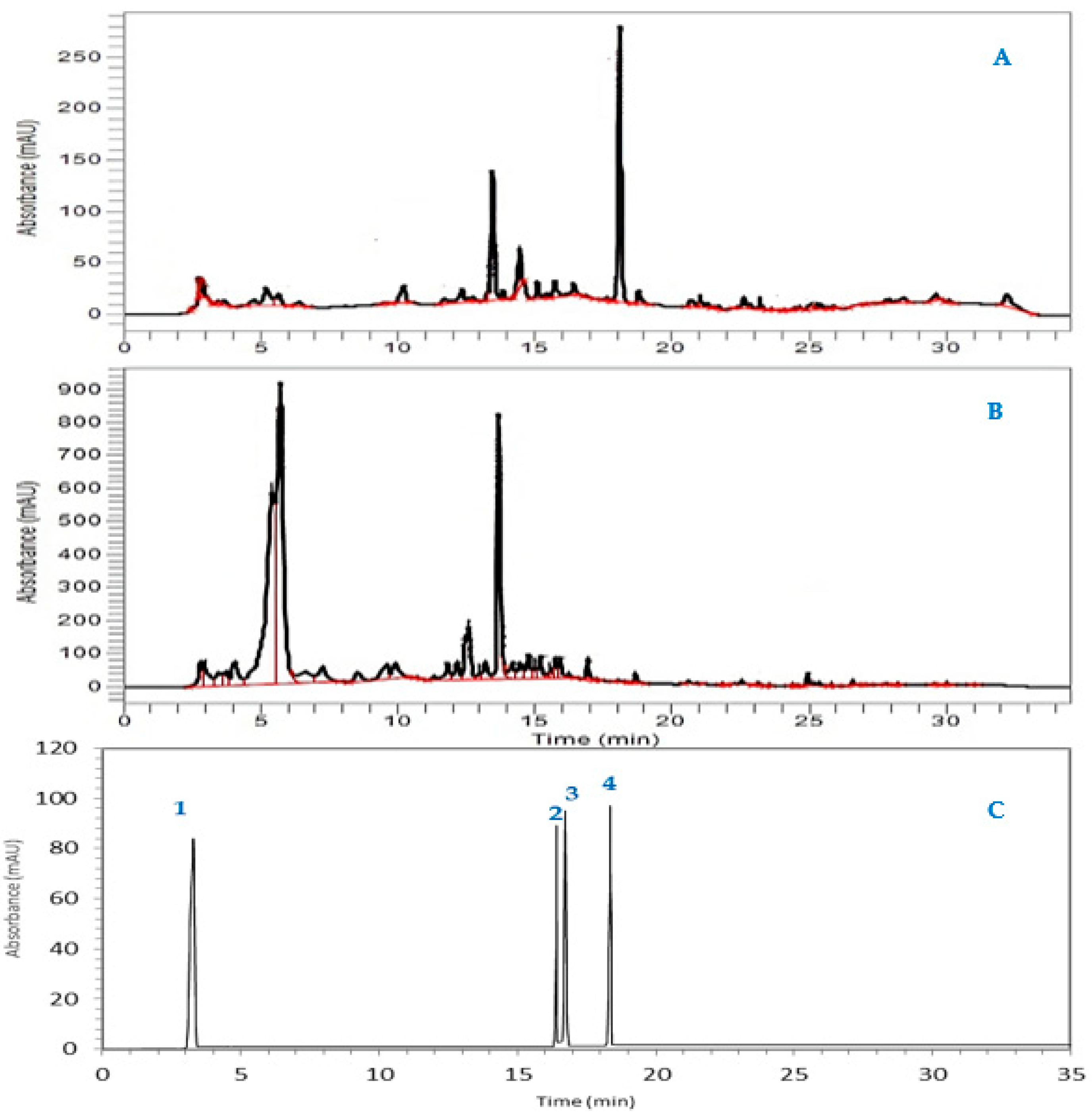
3.1. Analysis of the Chemical Composition of SF and SFr
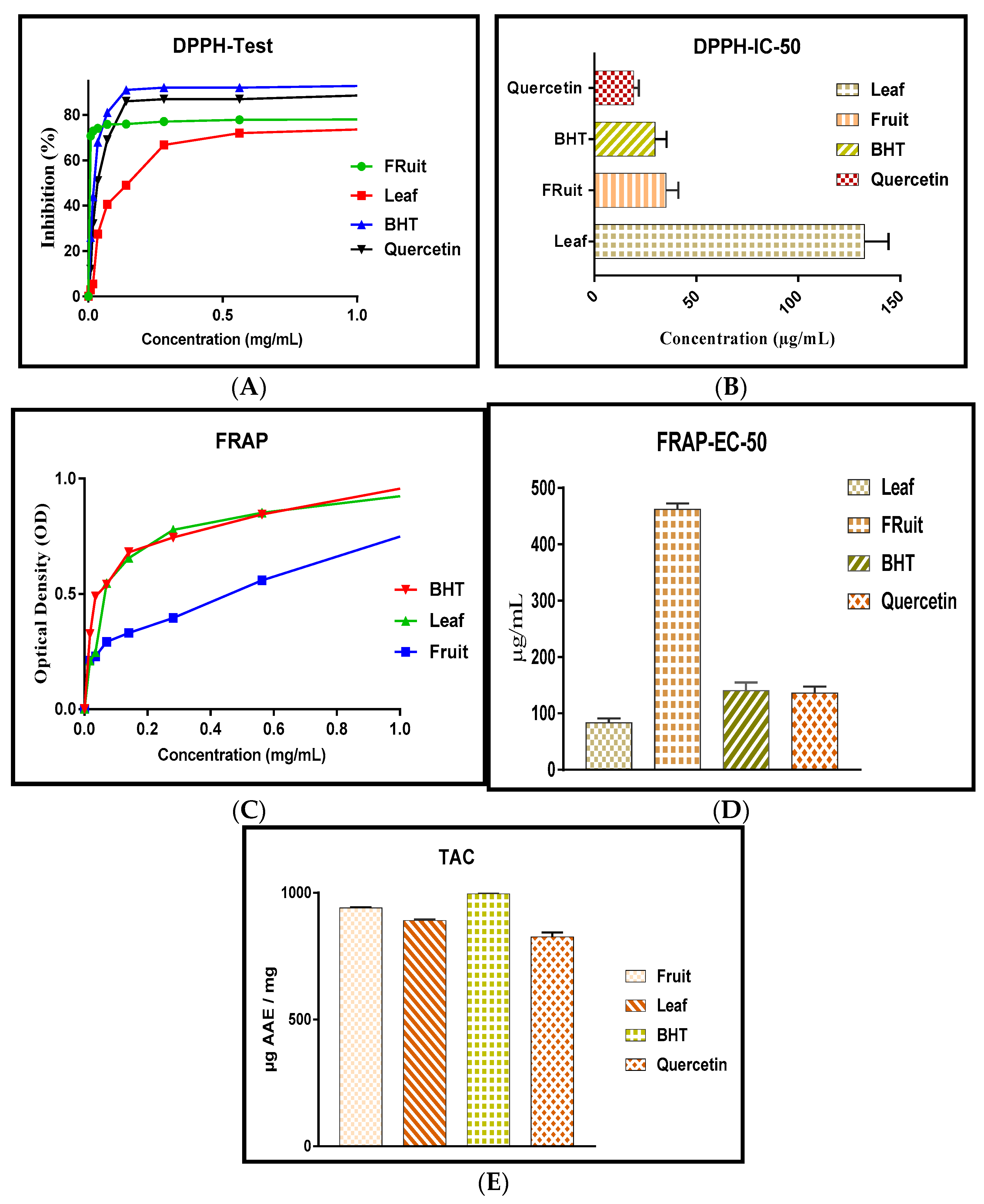
3.2. Antioxidant Activity
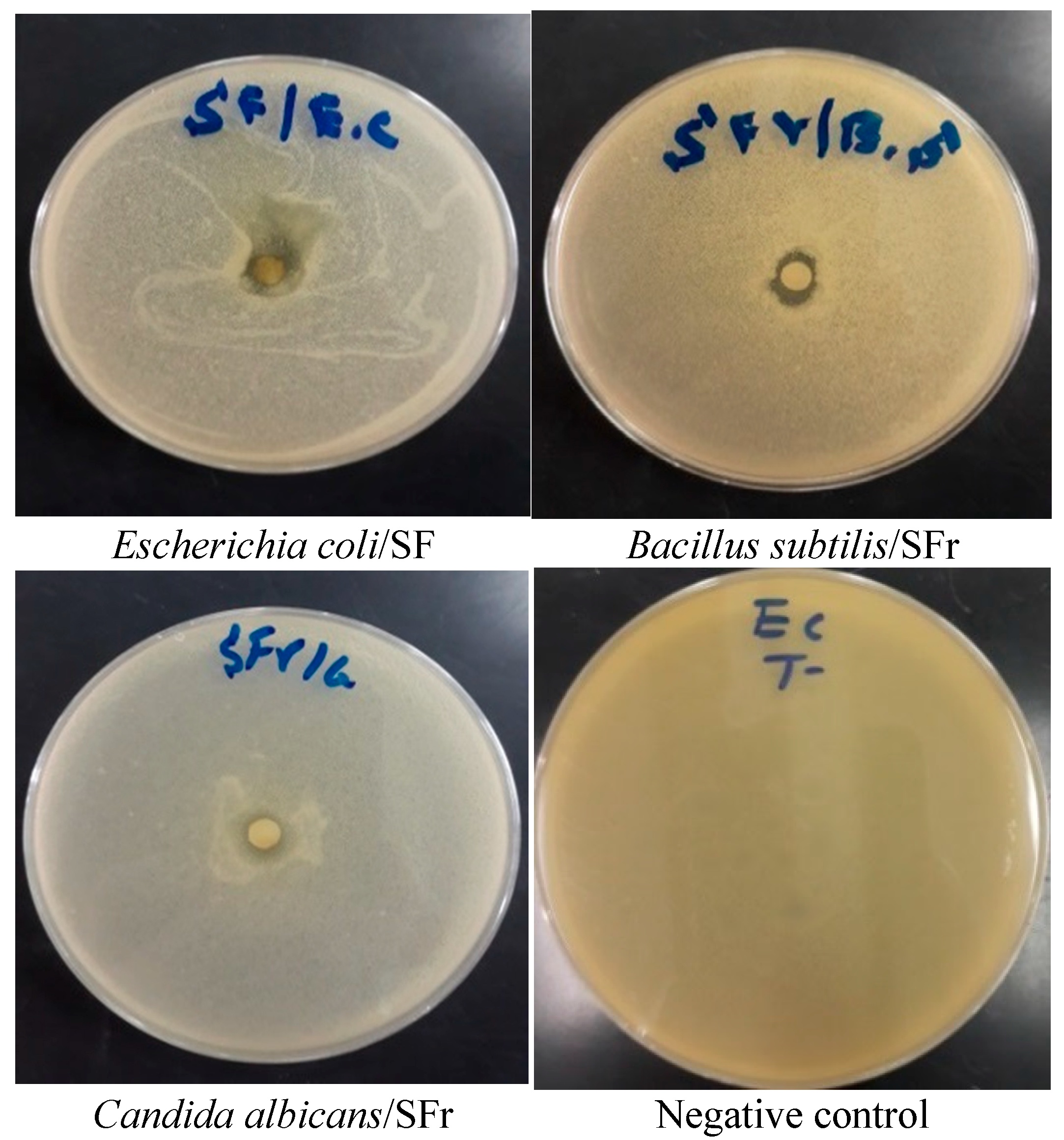
3.3. Antimicrobial Activity
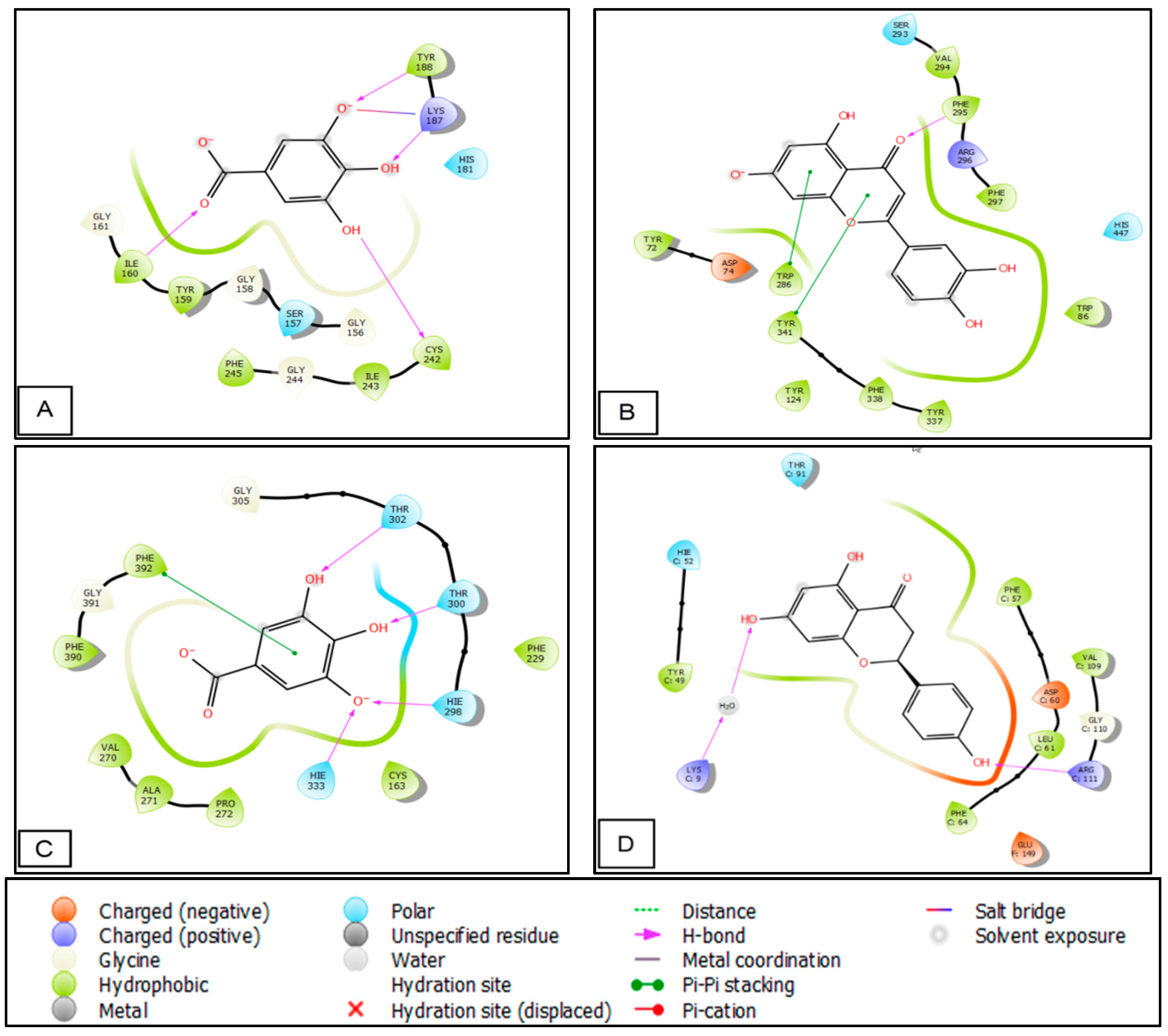
3.4. In Silico Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Egamberdieva, D.; Mamedov, N.; Ovidi, E.; Tiezzi, A.; Craker, L. Phytochemical and Pharmacological Properties of Medicinal Plants from Uzbekistan: A Review. J. Med. Act. Plants 2017, 5, 59–75. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Isabelle, M.; Cherki, M.; Khalil, A. Marrubium vulgare Extract Inhibits Human-LDL Oxidation and Enhances HDL-Mediated Cholesterol Efflux in THP-1 Macrophage. Life Sci. 2007, 80, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Enwa, F. A Review on the Phytochemical Profile and the Antibacterial Susceptibility Pattern of Some Clinical Isolates to the Ethanolic Leaves Extract of Moringa Oleifera LAM (Moringaceae). Int. J. Adv. Res. 2013, 1, 226–238. [Google Scholar]
- Kara, M.; Assouguem, A.; Abdou R., Z.; Bahhou, J. Phytochemical Content and Antioxidant Activity of Vinegar Prepared from Four Apple Varieties by Different Methods. Trop. J. Nat. Prod. Res. 2021, 5, 1578–1585. [Google Scholar] [CrossRef]
- Ullah, M.; Mehmood, S.; Khan, R.A.; Ali, M.; Fozia, F.; Waqas, M.; Ullah, R.; Alotaibi, A.; Sultan, M.A. Sultan Assessment of Antidiabetic Potential and Phytochemical Profiling of Viscum Album, a Traditional Antidiabetic Plant. J. Food Qual. 2022, 2022, 5691379. [Google Scholar] [CrossRef]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis Vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.; Pinto, R.; Queiroz, M.; Rodrigues, M.; Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of Bioactive Compounds from White Grape (Vitis Vinifera L.) Stems as Potential Antimicrobial Agents for Human Health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S.; Ercisli, S.; Assouguem, A.; Alotaibi, A.; Ullah, R. Bioactive Phytochemicals and Quenching Activity of Radicals in Selected Drought-Resistant Amaranthus Tricolor Vegetable Amaranth. Antioxidants 2022, 11, 578. [Google Scholar] [CrossRef]
- Ullah, R.; Alqahtani, A.S. GC-MS Analysis, Heavy Metals, Biological, and Toxicological Evaluation of Reseda muricata and Marrubium vulgare Methanol Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 2284328. [Google Scholar] [CrossRef]
- Manoko, M.L.K.; van Weerden, G.M.; van Berg, R.G.; Mariani, C. A New Tetraploid Species of Solanum L. Sect. Solanum (Solanaceae) from Tanzania. PhytoKeys 2012, 16, 65–74. [Google Scholar] [CrossRef] [Green Version]
- El-Shaboury, G.A.; Al-Wadi, H.M.; Badr, A. Biodiversity of Some Solanum Species from Southwestern Saudi Arabia’s Highlands. Bot. Lett. 2021, 168, 246–255. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.; Kamaly, O.; Assouguem, A.; Badiaa, L.; Benjelloun, A. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef] [PubMed]
- Al-hamaideh, K.; Dmour, I.; El-Elimat, T.; Afifi, F. UPLC-MS Profile and Anti-Proliferative Activity of the Berries of an Aggressive Wild-Growing Weed: Solanum elaeagnifolium Cav. (Solanaceae). Trop. J. Nat. Prod. Res. 2020, 4, 1131–1138. [Google Scholar] [CrossRef]
- Badawy, A.; Zayed, R.; Ahmed, S.; Hassanean, H. Phytochemical and Pharmacological Studies of Solanum elaeagnifolium Growing in Egypt. J. Nat. Prod. 2013, 6, 156–167. [Google Scholar]
- Hawas, U.W.; Soliman, G.M.; El-Kassem, L.T.A.; Farrag, A.R.H.; Mahmoud, K.; León, F. A New Flavonoid C-Glycoside from Solanum elaeagnifolium with Hepatoprotective and Curative Activities against Paracetamol-Induced Liver Injury in Mice. Z. Für Nat. C 2013, 68, 19–28. [Google Scholar]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.-Z.; Alshawwa, S.; Kamaly, O.; El Moussaoui, A.; Badiaa, L.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 14. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of Antioxidant Capacity and Total Phenolic Content of Different Fractions of Selected Microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic Compounds and Antioxidant Activities of Buckwheat (Fagopyrum esculentum Moench) Hulls and Flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Gandhiappan, J.; Rengasamy, R. Comparative Study on Antioxidant Activity of Different Species of Solanaceae Family. Adv. Appl. Sci. Res. 2012, 3, 1538–1544. [Google Scholar]
- Abdelfattah, E.M.; Aimad, A.; Bourhia, M.; Chebbac, K.; Salamatullah, A.M.; Soufan, W.; Nafidi, H.-A.; Aboul-Soud, M.A.M.; Ouahmane, L.; Bari, A. Insecticidal and Antifungal Activities of Chemically-Characterized Essential Oils from the Leaves of Withania frutescens L. Life 2022, 12, 88. [Google Scholar] [CrossRef]
- Koncić, M.Z.; Kremer, D.; Karlović, K.; Kosalec, I. Evaluation of Antioxidant Activities and Phenolic Content of Berberis Vulgaris L. and Berberis Croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Akka, A.A.; Choubbane, A.; Akhazzane, M.; Aboul-soud, M.A.M.; et al. Essential Oils from Leaves of Juniperus thurifera L., Exhibiting Antioxidant, Antifungal and Antibacterial Activities against Antibiotic-Resistant Microbes. Horticulturae 2022, 8, 321. [Google Scholar] [CrossRef]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Ouaritini, Z.B.; Salamatullah, A.M.; Alzahrani, A.; Aboul-soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Beniaich, G.; Hafsa, O.; Maliki, I.; Bin Jardan, Y.A.; El Moussaoui, A.; Chebaibi, M.; Agour, A.; Zouirech, O.; Nafidi, H.-A.; Khallouki, F. GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea Radiata Essential Oils. Horticulturae 2022, 8, 886. [Google Scholar] [CrossRef]
- Rezouki, S.; Allali, A.; Bouchra, L.; Eloutassi, N.; Fadli, M. The Impact of the Harvesting Period and Drying Conditions on the Essential Oil Yield of Rosmarinus Officinalis, Thymus Satureioides and Origanum Compactum from the Taza-Taounate Region. Asian J. Agric. Biol. 2021, 2021, 202004251. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Zahra, J.F.; Almehdi, A.; Elmsellem, H.; Benbrahim, K.F.; Bari, A. Antibacterial, Antifungal and Antioxidant Activity of Total Polyphenols of Withania frutescens L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Effect of Organic Solvents and Water Extraction on the Phytochemical Profile and Antioxidant Activity of Clitoria ternatea Flowers. ACS Food Sci. Technol. 2021, 1, 1567–1577. [Google Scholar] [CrossRef]
- Feki, H.; Koubaa, I.; Damak, M. Secondary Metabolites and Antioxidant Activity of Seed Extracts from Solanum elaeagnifolium Cav. Mediterr. J. Chem. 2014, 2, 639–647. [Google Scholar] [CrossRef]
- Houda, M.; Derbré, S.; Jedy, A.; Tlili, N.; Legault, J.; Richomme, P.; Limam, F.; Saidani-Tounsi, M. Combined Anti-AGEs and Antioxidant Activities of Different Solvent Extracts of Solanum elaeagnifolium Cav (Solanacea) Fruits during Ripening and Related to Their Phytochemical Compositions. EXCLI J. 2014, 13, 1029–1042. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and Growing Region Determine the Antioxidant Polyphenolic Concentration and Composition of Apples Grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef]
- Kara, M.; Assouguem, A.; Benmessaoud, S.; El Fadili, M.; Alshawwa, S.; Kamaly, O.; Saghrouchni, H.; Zerhouni, R.; Bahhou, J. Contribution to the Evaluation of Physicochemical Properties, Total Phenolic Content, Antioxidant Potential, and Antimicrobial Activity of Vinegar Commercialized in Morocco. Molecules 2022, 27, 770. [Google Scholar] [CrossRef]
- Moussaoui, A.E.; Bourhia, M.; Jawhari, F.Z.; Khalis, H.; Chedadi, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Alotaibi, A.; et al. Responses of Withania frutescens (L.) Pauquy (Solanaceae) Growing in the Mediterranean Area to Changes in the Environmental Conditions: An Approach of Adaptation. Front. Ecol. Evol. 2021, 9, 666005. [Google Scholar] [CrossRef]
- Alonso, Á.; Castro-Mejías, R.; Rodrı́guez, M.C.; Guillén, D.; Barroso, C. Study of the Antioxidant Power of Brandies and Vinegars Derived from Sherry Wines and Correlation with Their Content in Polyphenols. Food Res. Int. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Gamar, A.; Berrekhis, F.; Zair, T.; Hilali, F.E. Correlation of Total Polyphenolic Content with Antioxidant Activity of Hydromethanolic Extract and Their Fractions of the Salvia Officinalis Leaves from Different Regions of Morocco. J. Chem. 2021, 2021, e8585313. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Rajalakshmi, P.; Pugalenthi, M. Phytochemical Screening and In Vitro Antioxidant Activity of Lantana camara L. and Solanum elaeagnifolium C. Int. J. Bot. Stud. 2016, 1, 26–29. [Google Scholar]
- Balavivekananthan, S.; Xavier, T.; Kumar, S.; Sabitha, R. Antibacterial Activity of Solanum elaeagnifolium Cav. Stem and Leaf Extract against Human Pathogenic Bacteria. Res. J. Pharm. Technol. 2021, 14, 1339–1345. [Google Scholar] [CrossRef]
- Nitiema, L.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A. In Vitro Antimicrobial Activity of Some Phenolic Compounds (Coumarin and Quercetin) Against Gastroenteritis Bacterial Strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Kumar, P. A Review on the Pharmaceutical Activity of Solanum Surattense. GSC Adv. Res. Rev. 2021, 7, 038–044. [Google Scholar] [CrossRef]
- Gębarowska, E.; Łyczko, J.; Rdzanek, M.; Wiatrak, B.; Pląskowska, E.; Gołębiowska, H.; Kuźniarski, A.; Gębarowski, T. Evaluation of Antimicrobial and Chemopreventive Properties and Phytochemical Analysis of Solanum nigrum L. Aerial Parts and Root Extracts. Appl. Sci. 2022, 12, 6845. [Google Scholar] [CrossRef]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal Effects of Phytocompounds on Candida Species Alone and in Combination with Fluconazole. Int. J. Antimicrob. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef]


| Fruit Extract | Leaf Extract | |
|---|---|---|
| Polyphenols | 0.153 ± 0.001 mg EAG/g Ext | 0.11 ± 0.01 mg EAG/g Ext |
| Flavonoids | 0.015 ± 0.001 mg QE/g Ext | 0.004 ± 0.0002 mg QE/g Ext |
| Compounds | Time (min) | Area % | |
|---|---|---|---|
| SF | SFr | ||
| Luteolin | 3.440 | 0.51 | 1.12 |
| Quercetin | 16.421 | 1.22 | 1.03 |
| Gallic acid | 16.883 | 0.40 | 0.22 |
| Naringenin | 18.781 | 1.93 | 0.97 |
| S. aureus | E. coli | B. subtilis | P. mirabilis | C. albicans | |
|---|---|---|---|---|---|
| SF | 0.00 ± 0.00 a | 12.33 ± 0.57 a | 0.00 ± 0.00 a | 11.33 ± 0.57 a | 0.00 ± 0.00 a |
| SFr | 8.66 ± 0.57 b | 0.00 ± 0.00 b | 10.67 ± 0.57 b | 9.67 ± 0.57 b | 9.00 ± 0.50 b |
| Ampicillin | 8.00 ± 0.00 b | 10 ± 0.00 c | Rs | Rs | - |
| Fluconazole | - | - | - | - | Rs |
| S. aureus | E. coli | B. subtilis | P. mirabilis | C. albicans | |
| SF | - | 2.5 ± 0.00 a | - | 1.25 ± 0.00 a | - |
| SFr | 2.5 ± 0.00 a | - | 2.5 ± 0.00 a | 1.25 ± 0.00 a | 0.31 ± 0.00 a |
| Ampicillin | 5.00 ± 0.00 b | 5.00 ± 0.00 b | - | - | - |
| Fluconazole | - | - | - | - | - |
| 2CDU | 4EY7 | 1FJ4 | 3Q8U | |||||
|---|---|---|---|---|---|---|---|---|
| Glide score (kcal/mol) | Glide energy (Kcal/mol) | Glide score (kcal/mol) | Glide energy (Kcal/mol) | Glide score (kcal/mol) | Glide energy (Kcal/mol) | Glide score (kcal/mol) | Glide energy (Kcal/mol) | |
| Gallic acid | −6.561 | −30.439 | −6.271 | −25.092 | −6.756 | −28.225 | −5.368 | −32.578 |
| Luteolin | −6.328 | −42.269 | −8.416 | −40.362 | −6.503 | −37.449 | −6.145 | −31.288 |
| Quercetin | −6.23 | −46.008 | −8.173 | −44.48 | −5.912 | −36.099 | −5.95 | −36.585 |
| Naringenin | −5.712 | −36.157 | −8.244 | −39.955 | −6.703 | −34.484 | −6.477 | −19.174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslamti, M.; Metouekel, A.; Chelouati, T.; El Moussaoui, A.; Barnossi, A.E.; Chebaibi, M.; Nafidi, H.-A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; et al. Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules 2022, 27, 8688. https://doi.org/10.3390/molecules27248688
Bouslamti M, Metouekel A, Chelouati T, El Moussaoui A, Barnossi AE, Chebaibi M, Nafidi H-A, Salamatullah AM, Alzahrani A, Aboul-Soud MAM, et al. Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules. 2022; 27(24):8688. https://doi.org/10.3390/molecules27248688
Chicago/Turabian StyleBouslamti, Mohammed, Amira Metouekel, Tarik Chelouati, Abdelfattah El Moussaoui, Azeddin El Barnossi, Mohamed Chebaibi, Hiba-Allah Nafidi, Ahmad Mohammad Salamatullah, Abdulhakeem Alzahrani, Mourad A. M. Aboul-Soud, and et al. 2022. "Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches" Molecules 27, no. 24: 8688. https://doi.org/10.3390/molecules27248688
APA StyleBouslamti, M., Metouekel, A., Chelouati, T., El Moussaoui, A., Barnossi, A. E., Chebaibi, M., Nafidi, H.-A., Salamatullah, A. M., Alzahrani, A., Aboul-Soud, M. A. M., Bourhia, M., Lyoussi, B., & Benjelloun, A. S. (2022). Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules, 27(24), 8688. https://doi.org/10.3390/molecules27248688











