Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review
Abstract
1. Introduction
2. Methodology
3. Regional (Folkloric) Name
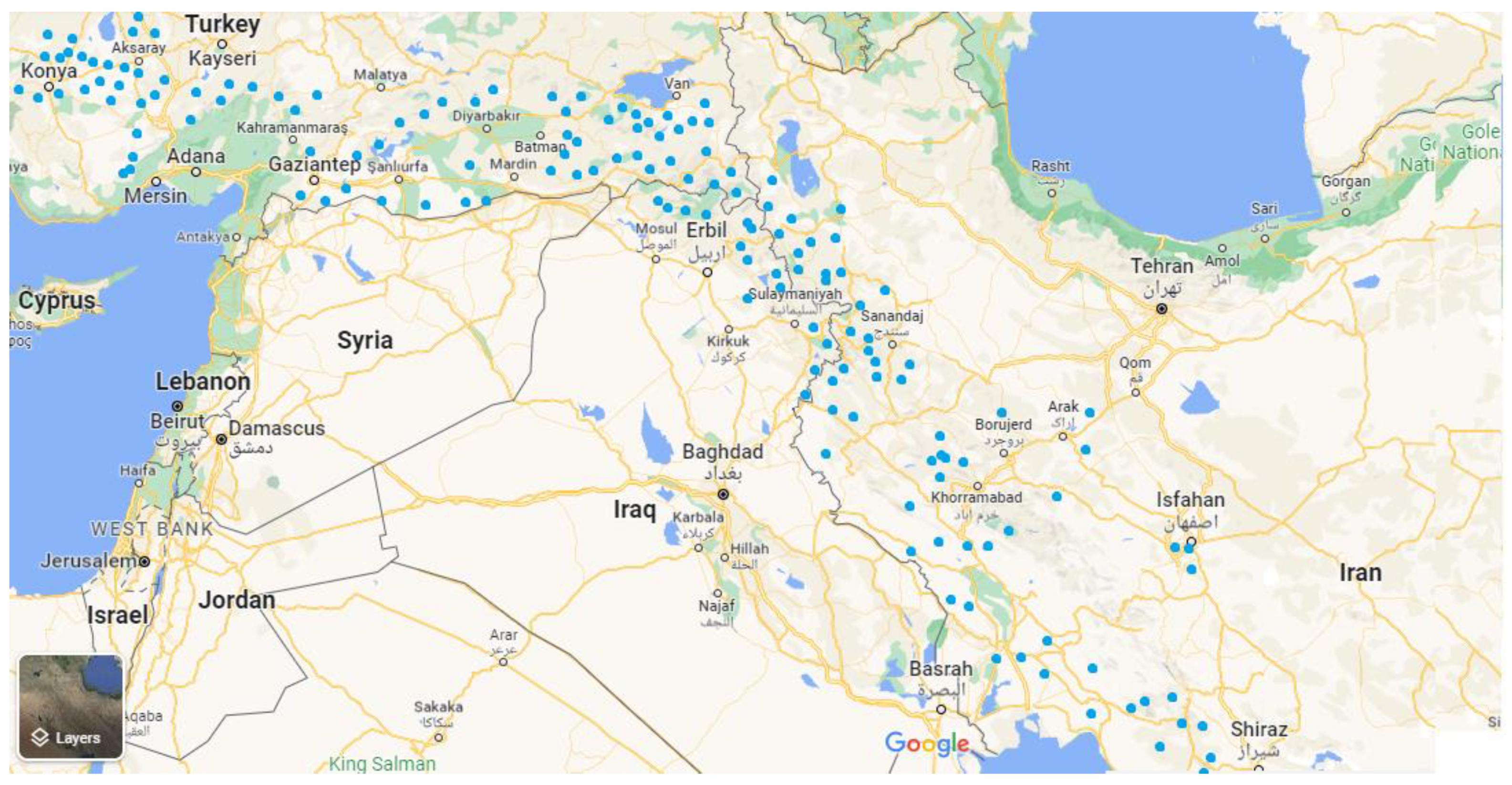
4. Regional Distribution
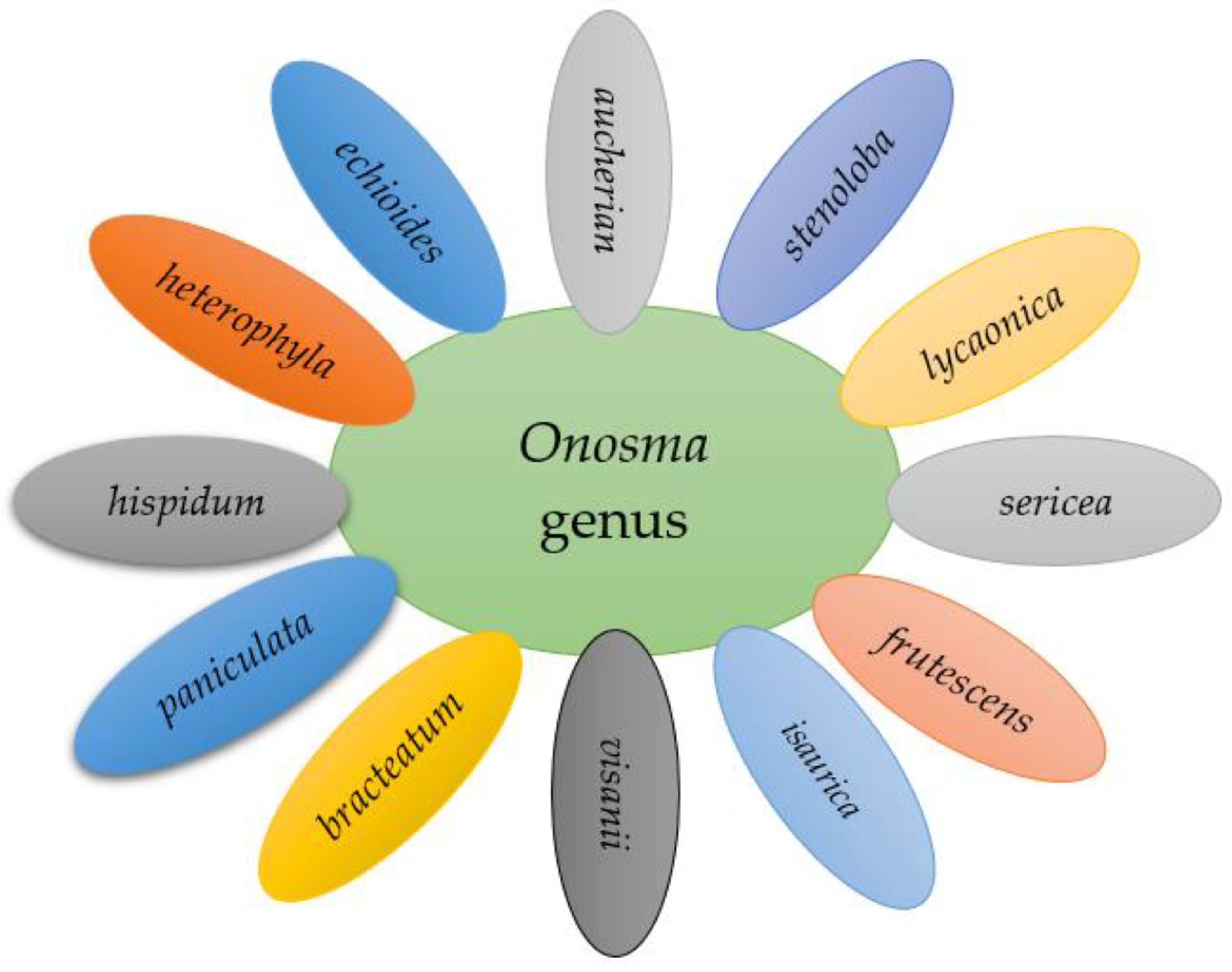
5. Onosma Taxonomy
6. Traditional Use
| Species | Traditional Name | Country of Habitat | Medicinal Parts | Medicinal Use |
|---|---|---|---|---|
| O. alborosea | Safeen mountain, Shaqlawa district, Iraqi Kurdistan | Aerial parts | Sedative, heart diseases, kidney disorders [33] | |
| O. orientalis | Safeen mountain, Shaqlawa district, Iraqi Kurdistan | Aerial parts | Sedative [33] | |
| O. armeniacum | Turkey, Anatolia | Leaves | healing wound, peptic ulcers, burns, dyspnea, hoarseness, hemorrhoids, and abdominal pains [34] | |
| O. argentatum, | Turkey | roots | Wound healing [35] | |
| O. chlorotricum | Iran, Lorestan | roots | Wound healing [36] | |
| O. hispidum | Iran (Korrassan) | roots | headache, wounds, insect stings and bits, inflammatory diseases, while its flowers are used for cardiovascular problems [37] and as a dye and a substitute for alkanet [38] | |
| O. bracteatum Wall | Gaozaban, Sedge | India, Nepal, Kashmir, and in the northwestern Himalayas | Roots, flowers | asthma, respiratory problems, tonic, alterative, demulcent, diuretic, spasmolytic, rheumatoid arthritis, diuretic, and antileprotic [40,41]. |
| O. sericeum | Turkey, Adıyaman | roots | As curatives for cutaneous wounds and burns [13] | |
| O. microcarpum | Turkey, Il’yca district, Erzurum province | Roots and leaves | Wound healing [36] | |
| O. echioides | Turkey | Leaves and flowers | Laxatives for children and as a cordial, stimulant for orthopedic and cardiac problems [46] | |
| O. paniculata | China | roots | Anticancer [44] | |
| O. aucheriana | Turkey | Roots, leaves, flowers | itchiness, leucoderma, bronchitis, abdominal pain, strangury, fever, wounds, burns, and urinary calculi. Stimulants and cardio-tonics. Laxative, purgative, and as wound remedy [45] |
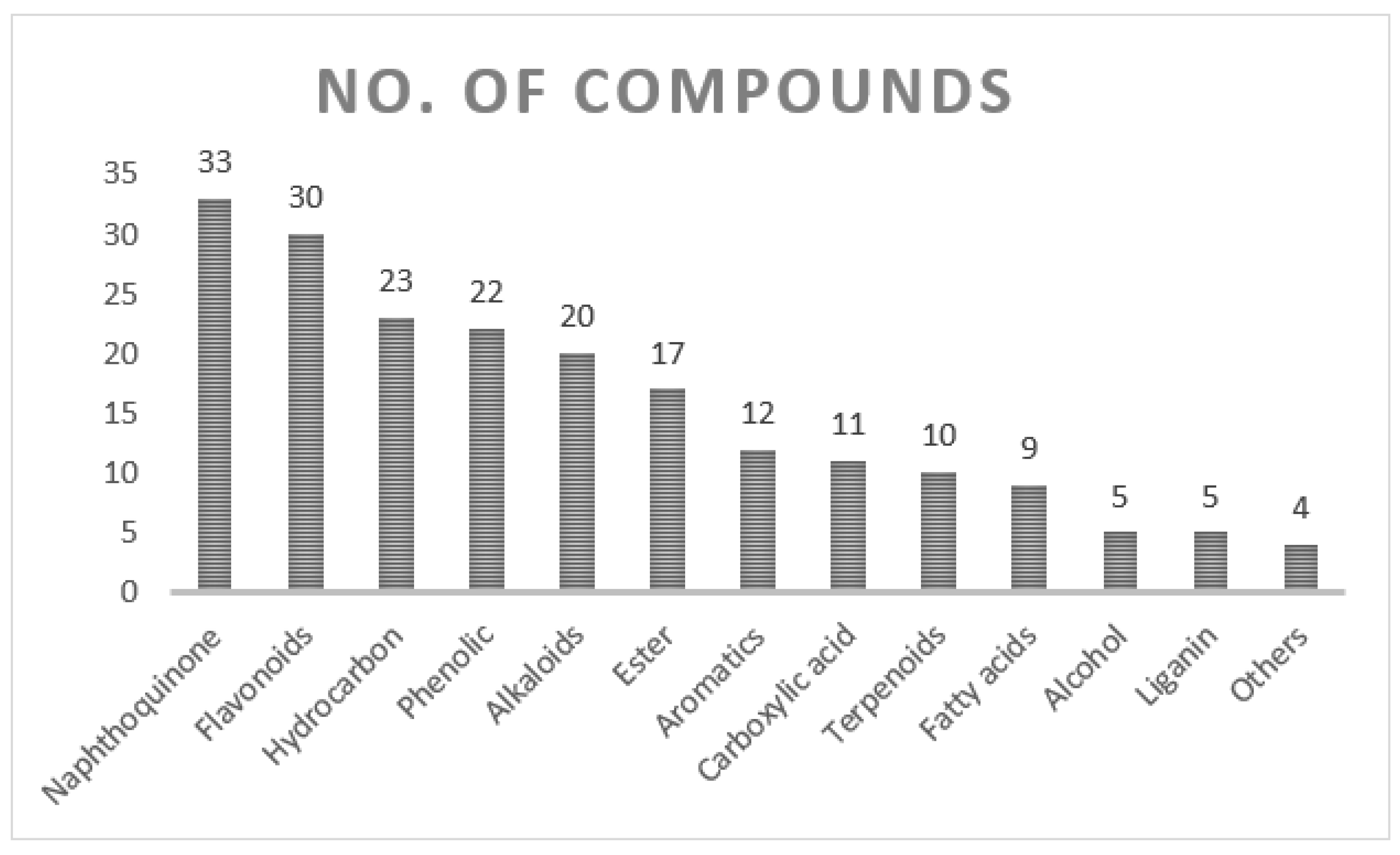
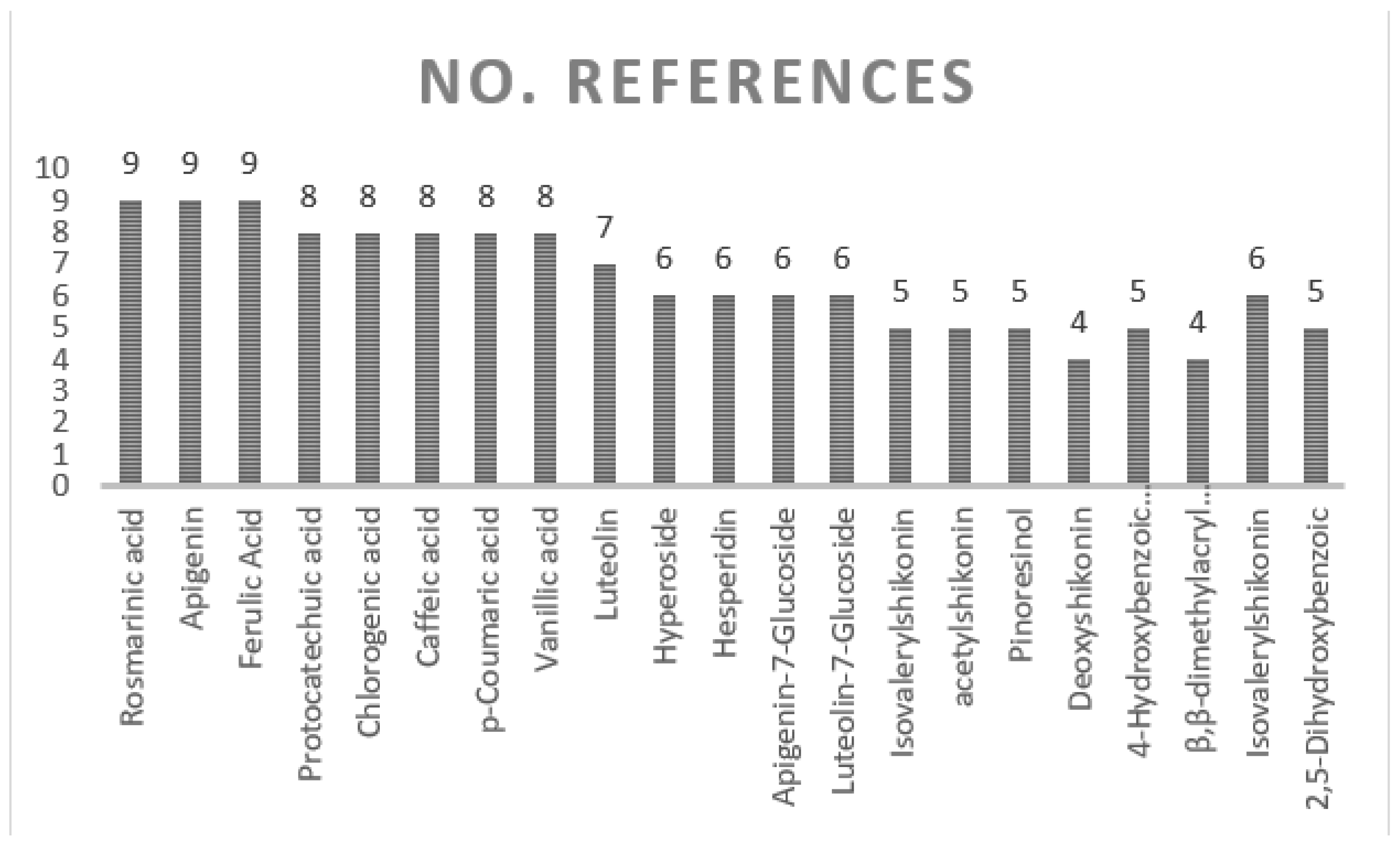
7. Chemical Profile of Onosma Species
8. Toxicity Study of the Onosma Species
8.1. Toxicity In Vivo Experiment
8.2. Genotoxicity and Mutagenicity
9. Pharmacological Activity of the Onosma Species
9.1. Antibacterial Activity
9.2. Antifungal Activity
9.3. Antioxidant Activity
9.4. Cytotoxicity Activity
9.5. Enzyme Inhibitory Activity
9.5.1. Antidiabetic Activity
9.5.2. Alzheimer’s Disease
9.5.3. Anti-Tyrosinase Activity
9.5.4. Anti-Lipoxygenases Activity
10. Other Biological Activity
10.1. Parasiticidal Activity
10.2. Anti-Inflammatory and Analgesic Activity
10.3. Gastric-Ulcerogenic Activity
10.4. Treatment and Prevention of COVID-19
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binzet, R.; Kandemir, I.; Orcan, N. Palynological classification of Onosma L. (Boraginaceae) species from east Mediterranean region in Turkey. Acta Bot. Croat. 2010, 69, 259–274. [Google Scholar]
- Güzel, Ö.; Duman, S.; Yılmaz, S.; Pirhan, A.F.; Bedir, E. Screening of Onosma Species for Cytotoxic Activity. Proceedings 2017, 1, 1048. [Google Scholar]
- Rechinger, K.H. (Ed.) Reidl Onosma L. In Flora Iranica, Akademische Druck-u; Verlagsanstalt: Graz, Austria, 1967. [Google Scholar]
- Onosma, R.H. Flora of Turkey and the East Aegean Islands; Devis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1978; Volume 6, pp. 326–376. [Google Scholar]
- Ahmad, S.A. Onosma hawramanensis (Boraginaceae), a New Species from Kurdistan, Iraq. Harvard Pap. Bot. 2014, 19, 201–202. [Google Scholar] [CrossRef]
- Mehrabian, A.R.; Mozaffarian, V. Seven new species of Onosma L. (Boraginaceae) with emphasis on their habitats in Iran. Taiwania 2018, 63, 366–388. [Google Scholar] [CrossRef]
- Cecchi, L.; Coppi, A.; Selvi, F. Onosma juliae (boraginaceae), a new species from Southern Turkey, with remarks on the systematics of Onosma in the irano-turanian region. Phytotaxa 2016, 288, 201–213. [Google Scholar] [CrossRef]
- Attar, F.; Amini Rad, M.; Mirtadzadini, M. Onosma Alburzensis (Boraginaceae), a New Species From Central Alburz Mountains, North Iran. Iran. J. Bot. 2021, 27, 78–83. [Google Scholar] [CrossRef]
- Shilov, S.V.; Ustenova, G.O.; Kiyekbayeva, L.N.; Korotetskiy, I.S.; Kudashkina, N.V.; Zubenko, N.V.; Parenova, R.A.; Jumagaziyeva, A.B.; Iskakbayeva, Z.A.; Kenesheva, S.T. Component Composition and Biological Activity of Various Extracts of Onosma gmelinii (Boraginaceae). Int. J. Biomater. 2022, 2022, 4427804. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tlili, N. Onosma inexspectata and Onosma armenum as Novel Sources of Phytochemicals with Determination by High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS/MS) with Evaluation of the Antioxidant and Enzyme Inhibitory Capacities. Anal Lett 2022, 55, 1068–1079. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Marjani, M.; Poursharifi, N.; Marjani, A. Effects of Onosma dichroanthum Boiss. root extract on AGS human gastric cancer cell-line. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Kundaković, T.; Stanojković, T.; Juranić, Z.; Kovačević, N. Cytotoxicity in vitro of naphthazarin derivatives from Onosma arenaria. Phyther. Res. 2006, 20, 602–604. [Google Scholar] [CrossRef]
- Doğan Çalhan, S.; Gündoğan, M. Evaluation of changes in the biological activity of Onosma sericeum Willd (Boraginaceae) based on collection time and extraction solvent, and determination of its mineral and trace element composition. J. Turkish Chem. Soc. Sect. A Chem. 2019, 6, 355–364. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crops Prod. 2020, 154, 112632. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Ceylan, R.; Zengin, G.; Matić, S.; Jurić, T.; Diuzheva, A.; Jeko, J.; Cziáky, Z.; Aktumsek, A. Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition. Ind. Crops Prod. 2020, 144, 112053. [Google Scholar] [CrossRef]
- Jabbar, A.A. Onosma mutabilis: Phytochemical composition, antioxidant, cytotoxicity, and acute oral toxicity. Food Sci. Nutr. 2021, 9, 5755–5764. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Ali, N.; Bashir, S.; Choudhary, M.I.; Azam, S.; Khan, I. Parasiticidal, antifungal and antibacterial activities of Onosma griffithii Vatke. Afr. J. Biotechnol. 2009, 8, 5084–5087. [Google Scholar]
- Özgen, U.; Houghton, P.J.; Ogundipe, Y.; Coşkun, M. Antioxidant and antimicrobial activities of Onosma argentatum and Rubia peregrina. Fitoterapia 2003, 74, 682–685. [Google Scholar] [CrossRef]
- Kilinç, N. Molecular Mechanisms of Possible Action of Naphthoquinones from Onosma in the Treatment and Prevention of COVID-19. Cauc. J. Sci. 2021, 8, 173–185. [Google Scholar] [CrossRef]
- Mehrabian, A.; Sheidai, M.; Noormohammadi, Z.; Mehrabian, A. Palynological diversity in the genus Onosma L. (Boraginaceae) of Iran Shahid Beheshti University, GC, Faculty of Biological Sciences, Tehran, Iran. Ann. Biol. Res. 2012, 3, 3885–3893. [Google Scholar]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.D.; Popovic, S.L.; Zaric, M.M.; Djurdjevic, P.M.; Markovic, S.D.; Baskic, D.D. Naphthoquinone rich Onosma visianii Clem (Boraginaceae) root extracts induce apoptosis and cell cycle arrest in HCT-116 and MDA-MB-231 cancer cell lines. Nat. Prod. Res. 2018, 32, 2712–2716. [Google Scholar] [CrossRef]
- Sagratini, G.; Cristalli, G.; Giardinà, D.; Gioventù, G.; Maggi, F.; Ricciutelli, M.; Vittori, S. Alkannin/shikonin mixture from roots of Onosma echioides (L.) L.: Extraction method study and quantification. J. Sep. Sci. 2008, 31, 945–952. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K. Onosma L.: A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140–151. [Google Scholar] [CrossRef]
- Rajapara, A.M.; Mamta, B. Shah the Genus Onosma L.: A Comprehensive Review. EPRA Int. J. Res. Dev. 2021, 7838, 219–227. [Google Scholar] [CrossRef]
- Joshi, M.C. Hand Book of Indian Medicinal Plants; Scientific Reports: Jodhpor, India, 2019. [Google Scholar]
- Khan, M.N.; Tariq, M.; Akhtar, J.; Khan, M.A. Study of a Controversial Unani Drug Gaozaban—A review. World J. Pharm. Res. 2018, 7, 213–223. [Google Scholar] [CrossRef]
- Khajoei Nasab, F.; Mehrabian, A.; Mostafavi, H. Mapping the current and future distributions of Onosma species endemic to Iran. J. Arid Land 2020, 12, 1031–1045. [Google Scholar] [CrossRef]
- Moradi Zeinab, H.; MEHRABIAN, A.; Naghizadeh, S.; MOSTAFAVI, H.; Khajoi Nasab, F. Distribution patterns, diversity and conservation priorities of Onosma L. (Boraginaceae Juss.) in some sections of the northwestern geomorphologic unit of Iran. Environ. Sci. 2019, 17, 73–94. [Google Scholar]
- Youssef, S. Endemic Plant Species of Iraq: From Floristic Diversity to Critical Analysis Review. J. Duhok Univ. 2020, 23, 90–105. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma ambigens: Phytochemical composition, antioxidant and enzyme inhibitory activity. Ind. Crops Prod. 2020, 154, 112651. [Google Scholar] [CrossRef]
- Luebert, F.; Cecchi, L.; Frohlich, M.W.; Gottschling, M.; Guilliams, C.M.; Hasenstab-Lehman, K.E.; Hilger, H.H.; Miller, J.S.; Mittelbach, M.; Nazaire, M.; et al. Familial classification of the Boraginales. Taxon 2016, 65, 502–522. [Google Scholar] [CrossRef]
- Mehrabian, A.R.; Sheidai, M.; Mozaffarian, V. Micromorphology of leaf trichomes in Onosma (Boraginaceae) and their systematic relevance in Iran. Phytol. Balc. 2014, 20, 33–48. [Google Scholar]
- Abdullah, F.O.; Hussain, F.H.S.; Sardar, A.S.; Vita-Finzi, P.; Vidari, G. Phytochemistry and Ethnopharmacology of Medicinal Plants Used on Safeen Mountain in the Kurdistan Region of Iraq. Nat. Prod. Commun. 2016, 11, 1923–1927. [Google Scholar] [CrossRef]
- Cadirci, E.; Suleyman, H.; Aksoy, H.; Halici, Z.; Ozgen, U.; Koc, A.; Ozturk, N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem. Biol. Interact. 2007, 170, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Reidl, H. Additional notes on Cwoiswa-Species (Boraginaceae) from Turkey. Linzer Biol. Beitr. 1987, 19, 461–465. [Google Scholar]
- Özgen, U.; Coşkun, M.; Kazaz, C.; Seçen, H. Naphthoquinones from the roots of Onosma argentatum Hub.-Mor. (Boraginaceae). Turkish J. Chem. 2004, 28, 451–454. [Google Scholar]
- Ghahremaninejad, F.; Joharchi, M.; Vitek, E. New plant records for Khorassan province, Iran. Ann. Naturhist. Mus. Wien B 2005, 106, 255–293. [Google Scholar]
- Ahmad, I.; Anis, I.; Malik, A.; Nawaz, S.A.; Choudhary, M.I. Cholinesterase Inhibitory Constituents from Onosma hispida. Chem. Pharm. Bull. 2003, 51, 412–414. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, H.; Wang, Z.-N.; Luo, H.-Z.; Jia, A.-Q. Phytochemical constituents of Onosma bracteatum Wall. Phytochem. Lett. 2021, 45, 1–5. [Google Scholar] [CrossRef]
- Badruddeen; Fareed, S.; Siddiqui, H.H.; Haque, S.E.; Khalid, M.; Akhtar, J. Psychoimmunomodulatory effects of Onosma bracteatum wall. (Gaozaban) on stress model in sprague dawley rats. J. Clin. Diagn. Res. 2012, 6, 1356–1360. [Google Scholar]
- Ved, D.; Sureshchandra, S.T.; Barve, V.; Srinivas, V.; Sangeetha, S.; Ravikumar, K. Envis Newsletter. Envis Newsl. Med. Plants Envis Cent Med. Plants 2016, 1, 49–116. [Google Scholar]
- Binzet, R.; Akçin, Ö.E. The anatomical properties of two Onosma L. (Boraginaceae) species from Turkey. J. Med. Plants Res. 2012, 6, 3288–3294. [Google Scholar]
- Kandemir, A.; Hedge, I.C. An Anomalous New Ferulago (Apiaceae) from Eastern Turkey. Willdenowia 2007, 37, 273–276. [Google Scholar] [CrossRef]
- Rinner, B.; Kretschmer, N.; Knausz, H.; Mayer, A.; Boechzelt, H.; Hao, X.-J.; Heubl, G.; Efferth, T.; Schaider, H.; Bauer, R. A petrol ether extract of the roots of Onosma paniculatum induces cell death in a caspase dependent manner. J. Ethnopharmacol. 2010, 129, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mašković, P.Z.; Diamanto, L.D.; Vujic, J.M.; Cvetanović, A.D.; Radojković, M.M.; Gadžurić, S.B.; Zengin, G. Onosma aucheriana: A source of biologically active molecules for novel food ingredients and pharmaceuticals. J. Funct. Foods 2015, 19, 479–486. [Google Scholar] [CrossRef]
- Kandemir, A.; Türkmen, Z. The flora of Üzümlü-Sakaltutan (Erzincan-Gümüv’hane). Turk. J. Bot. 2008, 32, 265–304. [Google Scholar]
- Saravanakumar, K.; Sarikurkcu, C.; Sarikurkcu, R.T.; Wang, M.-H. A comparative study on the phenolic composition, antioxidant and enzyme inhibition activities of two endemic Onosma species. Ind. Crops Prod. 2019, 142, 111878. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sarikurkcu, C.; Sahinler, S.S.; Sarikurkcu, R.B.; Wang, M.-H. Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species. Plants 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Sahinler, S.S.; Tepe, B. Onosma aucheriana, O. frutescens, and O. sericea: Phytochemical profiling and biological activity. Ind. Crops Prod. 2020, 154, 112633. [Google Scholar] [CrossRef]
- Kirkan, B.; Sarikurkcu, C.; Zengin, G. Bioactive constituents, antioxidant effects and enzyme inhibitory properties of two Onosma species (Onosma trapezuntea and O. rigidum). S. Afr. J. Bot. 2021, 145, 142–148. [Google Scholar] [CrossRef]
- Sihoglu Tepe, A. Determination of the Chemical Composition, Antioxidant, and Enzyme Inhibitory Activity of Onosma mollis DC. J. Chem. 2021, 2021, 5405365. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kirkan, B.; Ozer, M.S.; Ceylan, O.; Atilgan, N.; Cengiz, M.; Tepe, B. Chemical characterization and biological activity of Onosma gigantea extracts. Ind. Crops Prod. 2018, 115, 323–329. [Google Scholar] [CrossRef]
- Ahmad, I.; Nawaz, S.A.; Afza, N.; Malik, A.; Fatima, I.; Khan, S.B.; Ahmad, M.; Choudhary, M.I. Isolation of onosmins A and B, lipoxygenase inhibitors from Onosma hispida. Chem. Pharm. Bull. 2005, 53, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Yildiz, I.; Aydin, A.; Genc, N. Antiproliferative and cytotoxic effects of bioactive compounds isolated from Onosma bourgaei. Med. Oncol. 2022, 39, 116. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.M.; Abdel-Ghani, A.E.; Wink, M. Pyrrolizidine alkaloids from Onosma arenaria (Boraginaceae). Biochem. Syst. Ecol. 2003, 31, 477–485. [Google Scholar] [CrossRef]
- Rder, E.; Wiedenfeld, H.; Krger, R.; Teppner, H. Pyrrolizidinalkaloide dreier Onosma-Sippen (Boraginaceae-Lithospermeae). Phyton (B Aires) 1993, 33, 41–49. [Google Scholar]
- Mellidis, A.S.; Papageorgiou, V.P. Naphthazarins from Onosma heterophylla. J. Nat. Prod. 1987, 50, 618–619. [Google Scholar] [CrossRef]
- Sut, S.; Pavela, R.; Kolarčik, V.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Dall’Acqua, S.; Benelli, G. Identification of Onosma visianii Roots Extract and Purified Shikonin Derivatives as Potential Acaricidal Agents against Tetranychus urticae. Molecules 2017, 22, 1002. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Popovic, S.L.; Zaric, M.M.; Baskic, D.D.; Krstic, G.B.; Tesevic, V.V.; Kacaniova, M.M. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI J. 2017, 16, 73–88. [Google Scholar]
- Hu, Y.; Jiang, Z.; Leung, K.S.-Y.; Zhao, Z. Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography. Anal. Chim. Acta 2006, 577, 26–31. [Google Scholar] [CrossRef]
- Mellidis, A.S.; Papageorgiou, V.P. Lipids from roots of Onosma heterophylla. Phytochemistry 1987, 26, 842–843. [Google Scholar] [CrossRef]
- Kretschmer, N.; Rinner, B.; Deutsch, A.J.A.; Lohberger, B.; Knausz, H.; Kunert, O.; Blunder, M.; Boechzelt, H.; Schaider, H.; Bauer, R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma Cells. J. Nat. Prod. 2012, 75, 865–869. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, H.D.G.L.; Peng, Y.; Li, S.S. Comparative study on enantiomeric excess of main akannin/shikonin derivatives isolated from the roots of three endemic Boraginaceae plants in China. Biomed. Chromatogr. 2011, 25, 1067–1075. [Google Scholar] [CrossRef]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric natural products: Occurrence and biogenesis. Angew. Chem. Int. Ed. Engl. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Er-bu, A.; Liang, X.; Luan, S.; He, C.; Yin, L.; Yin, Z.; Zou, Y.; Li, L.; Song, X. Determination of the main naphthoquinones in Onosma hookeri Clarke. var. longiforum Duthie and its optimization of the ultrasound-assisted extraction using response surface methodology. J. Food Sci. 2021, 86, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Mehrotra, S. Naphthaquinones from some Boraginaceous Taxa-A Chemical Review.pdf. Nat. Prod. Sci. 1996, 2, 75–85. [Google Scholar]
- Damianakos, H.; Sotiroudis, G.; Chinou, I. Pyrrolizidine alkaloids from Onosma erecta. J. Nat. Prod. 2013, 76, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Yuldasheva, N.K.; Ul’chenko, N.T.; Glushenkova, A.I. Lipids from fruits of Solenanthus turkestanicus and Onosma irrigans. Chem. Nat. Compd. 2013, 49, 599–602. [Google Scholar] [CrossRef]
- Yıldız, G.; Köse, Y.B.; Kürkçüoğlu, M. Essential oil composition of Onosma isaurica boiss. & heldr. and Onosma bulbotrichum dc. from tokat, Turkey. Nat. Volatiles Essent. Oils 2020, 7, 30–33. [Google Scholar]
- Kostić, A.Ž.; Mačukanović-Jocić, M.P.; Milinčić, D.D.; Petrović, J.D.; Gašić, U.M.; Gligorijević, N.N.; Jarić, S.V.; Soković, M.D.; Tešić, Ž.L.; Pešić, M.B. Hieracium waldsteinii (Asteraceae) and Onosma stellulata (Boraginaceae) as a Source of Antioxidant and Antimicrobial Agents. Chem. Biodivers. 2022, 19, e202200069. [Google Scholar] [CrossRef]
- Ozgen, U.; Miloglu, F.D.; Bulut, G. Quantitative determination of shikonin derivatives with UV-Vis spectrophotometric methods in the roots of Onosma nigricaule. Rev. Anal. Chem. 2011, 30, 59–63. [Google Scholar] [CrossRef]
- Tosun, A.; Akkol, E.K.; Bahadir, O.; Yeşilada, E. Evaluation of anti-inflammatory and antinociceptive activities of some Onosma L. species growing in Turkey. J. Ethnopharmacol. 2008, 120, 378–381. [Google Scholar] [CrossRef]
- Shoaib, A.; Siddiqui, H.H.; Badruddeen; Dixit, R.K. Evaluation of Noxious Consequence of Bark Extract of Onosma echioides Linn Root: Hematology, Biochemistry, and Histopathological Findings. J. Diet. Suppl. 2020, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Redvzić, A.; Redvzić, S.; Sejdic, N. Genotoxic Effects of Aquatic Extract Of Endemic Plant Onosma Stellulata Waldst. & Kit. (Boraginaceae). Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 346–347. [Google Scholar]
- Maskovic, P.Z.; Mira, A.C.; Pavlovic, M.; Vujosevic, M.R.; Blagojevic, J.V.; Djuric, M.; Moracanin, S.V.; Djukic, D.A. A study on the ethanolic extract of Onosma aucheriana biological and toxicological evaluation. Rev. Chim. 2016, 67, 2511–2518. [Google Scholar]
- Kumar, A.; Kaur, V.; Pandit, K.; Tuli, H.S.; Sak, K.; Jain, S.K.; Kaur, S. Antioxidant Phytoconstituents from Onosma bracteata Wall. (Boraginaceae) Ameliorate the CCl4 Induced Hepatic Damage: In Vivo Study in Male Wistar Rats. Front. Pharmacol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Binzet, R.; Binzet, G.; Gumus, I.; Turunc, E.; Solmaz, U.; Keskin, E.; Dogen, A.; Arslan, H. Chemical Composition and Antimicrobial Activity of Essential Oil and Various Extracts of Onosma sieheana Hayek Roots. J. Essent. Oil Bear. Plants 2019, 22, 94–104. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.A.; Ayaz, S.; Ahamd, I. Antibacterial and antifungal studies of the crude extract and solvent fractions of Onosma khyberianum. Pharmacologica 2013, 4, 525–528. [Google Scholar]
- Dousti, B.; Nabipor, F. Antibacterial and antifungal activity of Onosma essential oils compared to four common antibioticsMON ANTIBIOTICS. In Proceedings of the 21st International Congress of Microbiology of Iran, Tehran, Iran, 18 August 2020. MEDISM21_019. [Google Scholar]
- Ning, W.; Zhao, Q.; Xia, Z. Effects of Fungal Elicitor on Shikonin Derivatives Formation in Onosma paniculatum Cell Cultures. Chin. Sci. Abstr. Ser. B 1995, 14, 33. [Google Scholar]
- Menghani, E.; Sudhanshu, R.N.; Mittal, S. Free radical scavenging capacity and antioxidant activity of Onosma bracteatum. Int. J. Pharm. Res. Dev. 2011, 4, 16–20. [Google Scholar]
- Zengin, G.; Mahomoodally, M.F.; Picot-Allain, C.M.N.; Cakmak, Y.S.; Uysal, S.; Aktumsek, A. In vitro tyrosinase inhibitory and antioxidant potential of Consolida orientalis, Onosma isauricum and Spartium junceum from Turkey. S. Afr. J. Bot. 2019, 120, 119–123. [Google Scholar] [CrossRef]
- Albaqami, J.; Myles, E.L.; Tiriveedhi, V.; Boadi, W.; Driggins, S.N. The Effect of Onosma bracteatum in cancer cells. MOJ Bioequivalence Bioavailab. 2018, 5, 321–325. [Google Scholar] [CrossRef]
- Asghar, M.; Islam, M.; Saeed, H.; Imtiaz, F.; Saleem, B.; Saleem, Z.; Qamar, S.; Iqtedar, M. Investigations on Onosma Hispidum wall root extracts for in-vitro antidiabetic, proliferative and cytotoxic effects. J. Anim. Plant Sci. 2018, 28, 1339–1347. [Google Scholar]
- Nadri, M.; Dehpour, A.A.; Yaghubi, S.; Fathi, H.; Ataee, R. Effects of the Anti-diabetic and Anti-neuropathy Effects of Onosma Dichroanthum in an Experimental Model of Diabetes by Streptozocin in Mice. Iran J. Endocrinol. Metab. 2017, 19, 161–169. [Google Scholar]
- Chen, W.-C.; Tseng, T.-S.; Hsiao, N.-W.; Lin, Y.-L.; Wen, Z.-H.; Tsai, C.-C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.-C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Imran, H.; Rahman, A.; Sohail, T.; Taqvi, S.I.H.; Yaqeen, Z. Onosma bracteatum wall: A Potent analgesic agent. Bangladesh J. Med. Sci. 2018, 17, 36–41. [Google Scholar] [CrossRef]

| Kingdom | Plantea |
|---|---|
| Phylum | Tracheophyta |
| Class | Angiosperms |
| Order | Boraginales |
| Family | Boraginaceae |
| Subfamily | Boraginoideae |
| Genus | Onosma L. |
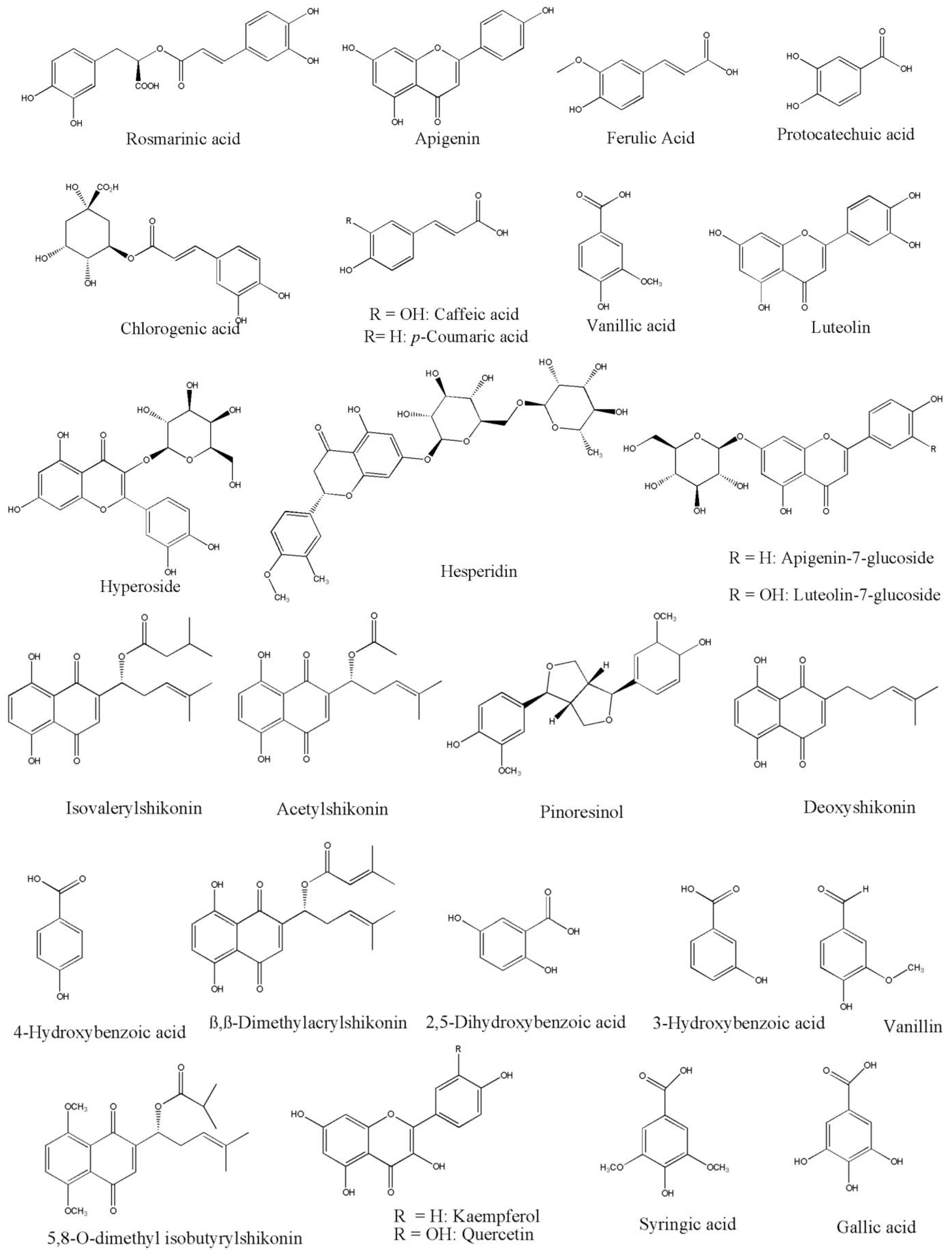
| No. | Chemical Names | Organic Class | Plant Species | Distribution in Plant | Reference | |
|---|---|---|---|---|---|---|
| 1 | Hyperoside | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. trapezuntea, O. rigidum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,47,48,49,50,51] | |
| 2 | Hesperidin | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 3 | Vanillic acid | Aromatics | O. isaurica, O. bracteosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. bracteatum, O. inexspectata, O. armenum, O. hispidum, O. mollis | Aerial parts | [10,14,30,39,47,49,51,52] | |
| 4 | Pinoresinol | Phenolics | O. isaurica, O. bracteosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. sericea, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,49,51] | |
| 5 | Apigenin-7-glucoside | Flaconoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 6 | Apigenin | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. hispida, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51,53,54] | |
| 7 | Ferulic acid | Phenolics | O. isaurica, O. bracteosa, O. sericea, O. lycaonica, O. papillosa. O. aucheriana, O. gigantea, O. pulchra O. frutescens, O. inexspectata, O. armenum, O. hispidum, O. mollis | Aerial parts | [10,14,15,47,48,49,51,52,53,55] | |
| 8 | Luteolin-7-glucoside | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 9 | Luteolin | Flavonoid | O. isaurica, O. bracteosa, O. stenoloba, O. lycaonica, O. papillosa. O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. inexspectata, O. armenum, O. sericea, O. mollis | Aerial parts | [10,14,15,47,48,49,51,53] | |
| 10 | Rosmarinic acid | Aromatic | O. isaurica, O. inexspectata, O. armenum, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. bracteatum, O. trapezuntea, O. rigidum, O. inexspectata, O. armenum, O. mutabilis, O. mollis | Aerial parts | [10,14,16,30,39,45,47,48,50,51,53] | |
| 11 | 3-Hydroxybenzoic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. pulchra, O. aucheriana, O. sericea, O. inexspectata, O. armenum | Aerial parts | [10,14,47,49,51] | |
| 12 | Protocatechuic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. ambigens, O. bracteatum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [14,30,39,45,47,49,51,53] | |
| 13 | Chlorogenic acid | Quinic acids | O. isaurica, O. bracteosa, O. ambigens, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. trapezuntea, O. rigidum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [14,30,45,47,49,50,51,53] | |
| 14 | Gentisic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. lycaonica, O. papillosa, O. mollis | Aerial parts | [14,47,48,49] | |
| 15 | Caffeic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa. O. aucheriana, O. gigantea, O. pulchra, O. bracteatum, O. inexspectata, O. armenum | Aerial parts | [10,14,39,45,47,48,51,53] | |
| 16 | p-Coumaric acid | Aromatics | O. isaurica, O. bracteosa, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. lycaonica, O. papillosa, O. ambigens, O. inexspectata, O. armenum | Aerial parts | [10,14,30,45,46,47,48,49,51,53] | |
| 17 | Salvianic acid A | Phenolics | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 18 | Verbascoside | Phenolics | O. sericea, O. aucheriana, | Aerial parts | [15,49] | |
| 19 | Rosmarinic acid-O-hexoside | Aromatics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 20 | Apigenin-O-hexoside | Flavonoid | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 21 | Methyl caffeate | Phenolics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 22 | Apigenin-O-rhamnosylhexoside | Phenolics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 23 | Diosmin | Flavonoid | O. sericea | Aerial parts | [15] | |
| 24 | O-Methylrosmarinic acid isomer | Phenolics | O. sericea | Aerial parts | [15] | |
| 25 | Tricin | Flavonoid | O. sericea | Aerial parts | [15] | |
| 26 | Cirsiliol | Flavonoid | O. sericea | Aerial parts | [15] | |
| 27 | Diosmetin | Flavonoid | O. sericea | Aerial parts | [15,55] | |
| 28 | Stearic acid | Fatty acid | O. sericea | Aerial parts | [15] | |
| 29 | Intermedine | Ester | O. stenoloba, O. alborosea, O. arenaria | Aerial parts, roots | [15,56] | |
| 30 | Lycopsamine | Alkaloid | O. stenoloba | Aerial parts | [15] | |
| 31 | Caffeoylshikimic acid isomer | Phenolics | O. stenoloba | Aerial parts | [15] | |
| 32 | Heliosupine | Pyrrolizidine Alkaloids | O. stenoloba | Aerial parts | [15] | |
| 33 | Vicenin-2 | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 34 | Echimidine | Pyrrolizidine Alkaloids | O. stenoloba | Aerial parts | [15] | |
| 35 | Isoferulic acid | Aromatics | O. stenoloba | Aerial parts | [15] | |
| 36 | Rosmarinic acid-di-Ohexoside | Aromatics | O. stenoloba | Aerial parts | [15] | |
| 37 | Quercetin-O-hexoside | Flavonoid | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 38 | Kaempferol-O-hexoside | Ester | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 39 | Isorhamnetin-O-rhamnosylhexoside | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 40 | Trihydroxyisoflavone | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 41 | Ursolic acid | O. stenoloba | Aerial parts | [15] | ||
| 42 | 4-Hydroxybenzoic acid | Triterpenoids | O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. gigantea, O. aucheriana, O. bracteatum | Aerial parts | [14,30,39,48,49] | |
| 43 | Eriodictyol | Carboxylic acid | O. lycaonica, O. papillosa. | Aerial parts | [48] | |
| 44 | Vanillin | Phenolics | O. lycaonica, O. papillosa. O. pulchra, O. frutescens, O.aucherian, O. sericea | Aerial parts | [14,48,49,57] | |
| 45 | (+)-Catechin | Flavonoid | O. lycaonica, O. papillosa O. frutescens | Aerial parts | [48,49,57] | |
| 46 | Homoprotocatechuic acid | Phenolics | O. lycaonica, O. papillosa | Aerial parts | [48,57] | |
| 47 | Acetylshikonin | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 48 | Shikonin derivatives | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 49 | Acetyl shikonin | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 50 | Shikonin derivatives | Naphthoquinones | O. visianii | Roots | [59] | |
| 51 | Shikonin derivatives | Naphthoquinones | O. visianii | Roots | [59] | |
| 52 | Isobutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59] | |
| 53 | Isovalerylshikonin | Naphthoquinones | O. visianii, O. paniculata, O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum, O. echioides, O. heterophylla | Roots | [21,22,59,60,61,62] | |
| 54 | α-methylbutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59] | |
| 55 | 5,8-O-dimethyl deoxyshikonin | Naphthoquinones | O. visianii | Roots | [59,63] | |
| 56 | 5,8-O-dimethyl isobutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59,61] | |
| 57 | deoxyshikonin | Naphthoquinones | O. visianii, O. paniculata, paniculatum | Roots | [21,59,60,62,63] | |
| 58 | Acetylshikonin | Naphthoquinones | O. visianii, O. confertum, O. echioides, O. setosum, O. paniculata, paniculatum | Roots | [21,59,60,61,64] | |
| 59 | β-Hydroxyisovalerylshikonin | Naphthoquinones | O. paniculata, O. heterophylla | Roots | [63,65] | |
| 60 | β,β-dimethylacrylshikonin | Naphthoquinones | O. paniculata, O. confertum, O. exsertum, O. waltonii, O. paniculatum, hookeri, Onosma hookeri, Onosma zerizaminum | Roots | [62,63,64,65] | |
| 61 | Methylbutyrylshikonin | Naphthoquinones | O. paniculata | Roots | [63] | |
| 62 | Isovalerylshikonin | Naphthoquinones | O. paniculata | Roots | [63] | |
| 63 | Gallic acid | Fatty acid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,45,49] | |
| 64 | Quercetin | Flavonoid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,45,49] | |
| 65 | Syringic acid | Fatty acid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,49] | |
| 66 | Shikonin derivatives | Hydrocarbon | O. mutabilis | Aerial parts | [16] | |
| 67 | Shikonin derivatives | naphthoquinones | O. mutabilis | Aerial parts | [16] | |
| 68 | 3-O-Methyl-d-glucose | Hydrocarbon | O. mutabilis | Aerial parts | [16] | |
| 69 | 24,25-Dihydroxycholecalciferol | Vitamin D | O. mutabilis | Aerial parts | [16] | |
| 70 | β-Sitosterol | Phytosterol | O. mutabilis, O. heterophylla | Aerial parts, roots | [16,65] | |
| 71 | Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite | Phenolics | O. mutabilis | Aerial parts | [16] | |
| 72 | p-Hydroxybenzoic acid | Carboxylic acid | O. gigantea, O. aucheriana, O. bracteatum | Aerial parts | [39,45,53] | |
| 73 | trans-Cinnamic acid | Cinnamic acid | O. gigantea | Aerial parts | [53] | |
| 74 | Kaempferol | Flavonoid | O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49,53] | |
| 75 | 3,4-Dihydroxyphenylacetic acid | Catechol | O. pulchra | Aerial parts | [14] | |
| 76 | Taxifolin | Flavonoid | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 77 | Sinapic acid | Aromatics | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 78 | Eriodictyol | Flavonoid | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 79 | Shikonin derivatives | Naphthoquinones | O. echioides | Aerial parts | [64] | |
| 80 | Pulmonarioside C | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 81 | 9′-Methoxyl salvianolic acid | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 82 | 4-O-(E)-p-coumaroyl-l-threonic acid | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 83 | Coumarin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 84 | Umbelliferone | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 85 | Scopoletin | Aromatics | F | Aerial parts | [39] | |
| 86 | 6,7-Dimethoxycoumarin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 87 | Esculetin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 88 | Caffeic acid methyl ester | Ester | O. bracteatum | Aerial parts | [39] | |
| 89 | 1-O-Caffeoyl glycerol | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 90 | Latifolicinin C | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 91 | Oresbiusin A | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 92 | Ethyl 3-(3, 4-dihydroxyphenyl)lactate | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 93 | 4, 5-Dihydroxy-3-methoxybenzoic acid | Carboxylic acid | O. bracteatum | Aerial parts | [39] | |
| 94 | 5-Hydroxymethyl-furoic acid | Furoic acid | O. bracteatum | Aerial parts | [39] | |
| 95 | 3,4-Dihydroxybenzyl alcohol | Alcohol | O. bracteatum | Aerial parts | [39] | |
| 96 | Rosmarinic acid methyl ester | Ester | O. bracteatum | Aerial parts | [39] | |
| 97 | Salviaflaside methyl ester | Ester | O. bracteatum | Aerial parts | [39] | |
| 98 | 9′-(2,3-Dihydroxypropyl)-rosmarinic acid | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 99 | p-Coumarinic acid ester of trigonotin | Ester | O. bracteatum | Aerial parts | [39] | |
| 100 | Echiumin A | Liganin | O. bracteatum | Aerial parts | [39] | |
| 101 | Ternifoliuslignan A | Liganin | O. bracteatum | Aerial parts | [39] | |
| 102 | Ternifoliuslignan D | Liganin | O. bracteatum | Aerial parts | [39] | |
| 103 | Eritrichin | Liganin | O. bracteatum | Aerial parts | [39] | |
| 104 | Shikonin derivatives | Naphthoquinon | O. bracteatum | Aerial parts | [39] | |
| 105 | Kaempferol 3-O-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside] | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 106 | Kaempferol 3-O-[α-l-rhamno pyranosyl-(1→6)-β-d-glucopyranoside] | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 107 | Impecylone A | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 108 | Tigloylshikonin | Naphthoquinon | O. hookeri | Roots | [66] | |
| 109 | Acetyl shikonin | Naphthoquinon | O. hispidum | Roots | [67] | |
| 110 | Alkannan | Naphthoquinon | O. hispidum, O. echioides | Roots | [67] | |
| 111 | Deoxyshikonin | Naphthoquinon | O. hispidum, O. echioides, O. confertum | Roots | [62,67] | |
| 112 | 7-O-acetylechinatine N-oxide | Alkaloid | O. erects | Roots | [68] | |
| 113 | Viridinatine N-oxide stereoisomer | Alkaloid | O. erects | Roots | [68] | |
| 114 | 7-Epi-echimiplatine Noxide | Alkaloid | O. erects | Roots | [68] | |
| 115 | Onosmerectine N-oxide | Alkaloid | O. erects | Roots | [68] | |
| 116 | Acid 2,3-dimethyl-2,3,4-trihydroxypentanoic acid | Alkaloid | O. erects | Roots | [68] | |
| 117 | Acyloin 4-methyl-2-hydroxypentanon | Alkaloid | O. erects | Roots | [68] | |
| 118 | 2-Methyl-n-butyrylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, hookeri, O. confertum | Roots | [62] | |
| 119 | β-Acetoxyisovalerylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum | Roots | [62] | |
| 120 | Isobutylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum | Roots | [62] | |
| 121 | Alkannin | Naphthoquinon | O. echioides, O. paniculata | Roots | [65] | |
| 122 | Shikonin | Naphthoquinon | O. caucasicum, O. conferitum, O. hookeri, O. livanovii, O. polyphyllum, O. tauricum, O. sericium, O. setosum, O. visianii, O. zerizaminium | Roots | [65] | |
| 123 | β,β-dimethylacrylalkannin | Naphthoquinon | O. heterophylla, O. hookeri, O. paniculata | Roots | [65] | |
| 124 | Heliotridine | Alkaloid | O. heterophyllum | Roots | [58] | |
| 125 | Necine derivative (1-methyl-8(-pyrrolizine) | Alkaloid | O. heterophyllum | Roots | [58] | |
| 126 | Acetylintermedine | Alkaloid | O. alborosea, O. arenaria | Roots | [56,57] | |
| 127 | O7-Acetyllycopsamine | Alkaloid | O. alborosea, O. arenaria | Roots | [56,57] | |
| 128 | 5,6-Dihydro-7,9-dimethoxy 7H- pyrrolizine | Alkaloid | O. arenaria | Roots | [56] | |
| 129 | 7-Acetylretronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 130 | 9-(Butyryl-2-ene) supinidine | O. arenaria | Roots | [56] | ||
| 131 | 7-Acetyl-9-(2-methylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 132 | 7-Acetyl-9-(2,3-dimethylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 133 | 7-Acetyl-9-(2-hydroxy-3-methylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 134 | 3′-Acetylsupinine | Alkaloid | O. arenaria | Roots | [56] | |
| 135 | 7-Acetyl-9-(2,3-dihydroxybutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 136 | Uplandicine | Pyrrolizines | O. arenaria | Roots | [56] | |
| 137 | Palmitic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 138 | Oleic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 139 | Linolenic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 140 | γ-Linolenic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 141 | Stearidonic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 142 | monoenoic acids 20:1, 22:1, and 24:1 | Fatty acid | O. irrigans | Fruits | [69] | |
| 143 | Hexahydrofarnesyl acetone | Fatty acid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 144 | Phytol | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 145 | Farnesyl acetone | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 146 | Hexadecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 147 | Hexyl hexanoate | Ester | O. isaurica | Aerial parts | [70] | |
| 148 | (E)-2-Decenal | Medium-chain aldehyde Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 149 | 1-Hexadecene | Unsaturated aliphatic Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 150 | Safranal | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 151 | Heptadecane | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 152 | Dodecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 153 | E)-2-Undecenal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 154 | Tridecanal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 155 | (E)-Geranyl acetone | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 156 | 1-Isobutyl-4-isopropyl-2,2-dimethyl succinate | Dicarboxylic acid | O. bulbotrichum | Aerial parts | [70] | |
| 157 | Neophytadiene isomer I | Terpenoid | O. isaurica | Aerial parts | [70] | |
| 158 | Tetradecanal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 159 | (E)-β-Ionone | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 160 | Neophytadiene | Sesquiterpenoid | O. isaurica | Aerial parts | [70] | |
| 161 | Pentadecanal | Sydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 162 | (E)-Nerolidol | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 163 | Hexadecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 164 | 3,4-Dimethyl-5-pentylidene-2(5H)-furanone | Phenolics | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 165 | 3,4-Dimethyl-5-pentyl-5H-furan-2-one | Phenolics | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 166 | Carvacrol | Monoterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 167 | Tricosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 168 | (2E, 6E)-Farnesol | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 169 | Tetracosane | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 170 | Pentacosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 171 | Geranyl linalool | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 172 | Heptacosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 173 | Nonacosane | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 174 | 1-Docosene | Unsaturated aliphatic Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 175 | isorhamnetin-3-O-rutinoside | Flavonoid | O. stellulata | Aerial parts | [71] | |
| 176 | sinapic acid | Aromatic | O. stellulata | Aerial parts | [71] | |
| 177 | Deoxyshikonin [2-(4-methyl-pent-3-enyl)-5,8-dihydroxynaphthalene-1,4-dione] | Naphthoquinon | O. nigricaule | roots | [72] | |
| 178 | β, β - Dimethylacrylshikonin (5,8-Dihydroxy-2-[1-(β, β -dimethy lacryloyloxy)-4-methyl-3-pentenyl]-1,4-naphthalenedion] | Naphthoquinon | O. nigricaule | Roots | [72] | |
| 179 | Acetyl shikonin [(+)-Acetic acid 1-(5,8-dihydroxy-1,4- dioxo-1,4-dihydro-naphthalen-2-yl)-4-methyl-pent-3-enyl ester] | Naphthoquinon | O. nigricaule | Roots | [72] | |
| 180 | 2-[(4-methylbenzyl)amino]benzoic acid | Carboxylic acid | O. hispida | Whole plant | [54] | |
| 181 | Methyl 2-[(4-methylbenzyl)amino]benzoate | Flavonoid | O. hispida | Whole plant | [54] | |
| 182 | 6,4′-Dimethoxy-3,5,7-trihydroxyflavone | Flavonoid | O. hispida | Whole plant | [54] | |
| 183 | apigenin 7-O-β-d-glucoside | Flavonoid | O. hispida | Whole plant | [54] | |
| 184 | Paraffins | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 185 | n-Dodecane | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 186 | n-Decatrian | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 187 | Methyl dodecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 188 | Methyl tetradecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 189 | Methyl 4-methyl tetradodecan-9,12 dien-oate | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 190 | Methyl 4-methyl tetradodec-9-ene-oate | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 191 | Methyl 4-methyl hexadec-9-ene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 192 | Methyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 193 | Ethyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 194 | Isopropyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 195 | Methyl octadeca-9,12,15-triene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 196 | Methyl octadeca-9,12-diene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 197 | Methyl octadec-9-ene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 198 | Methyl octadecanoate | Methyl ester | O. heterophylla | Roots | [65] | |
| 199 | diosmetin-7-O-β-glucoside | Aromatics | O. bourgaei | Aerial parts | [55] | |
| 200 | allantoin | Imidazoles | O. bourgaei | Aerial parts | [55] | |
| 201 | globoidnan A | Liganin | O. bourgaei | Aerial parts | [55] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbar, A.A.; Abdullah, F.O.; Hassan, A.O.; Galali, Y.; Hassan, R.R.; Rashid, E.Q.; Salih, M.I.; Aziz, K.F. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules 2022, 27, 8687. https://doi.org/10.3390/molecules27248687
Jabbar AA, Abdullah FO, Hassan AO, Galali Y, Hassan RR, Rashid EQ, Salih MI, Aziz KF. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules. 2022; 27(24):8687. https://doi.org/10.3390/molecules27248687
Chicago/Turabian StyleJabbar, Ahmed Aj., Fuad O. Abdullah, Abdullah Othman Hassan, Yaseen Galali, Rawaz Rizgar Hassan, Essa Q. Rashid, Musher Ismael Salih, and Kareem Fattah Aziz. 2022. "Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review" Molecules 27, no. 24: 8687. https://doi.org/10.3390/molecules27248687
APA StyleJabbar, A. A., Abdullah, F. O., Hassan, A. O., Galali, Y., Hassan, R. R., Rashid, E. Q., Salih, M. I., & Aziz, K. F. (2022). Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules, 27(24), 8687. https://doi.org/10.3390/molecules27248687