Abstract
Phenylpropanoids and flavonoids are specialized metabolites frequently reported as involved in plant defense to biotic or abiotic stresses. Their biosynthetic accumulation may be constitutive and/or induced in response to external stimuli. They may participate in plant signaling driving plant defense responses, act as a physical or chemical barrier to prevent invasion, or as a direct toxic weapon against microbial or insect targets. Their protective action is described as the combinatory effect of their localization during the host’s interaction with aggressors, their sustained availability, and the predominance of specific compounds or synergy with others. Their biosynthesis and regulation are partly deciphered; however, a lot of gaps in knowledge remain to be filled. Their mode of action on microorganisms and insects probably arises from an interference with important cellular machineries and structures, yet this is not fully understood for all type of pests and pathogens. We present here an overview of advances in the state of the art for both phenylpropanoids and flavonoids with the objective of paving the way for plant breeders looking for natural sources of resistance to improve plant varieties. Examples are provided for all types of microorganisms and insects that are targeted in crop protection. For this purpose, fields of phytopathology, phytochemistry, and human health were explored.
1. Introduction
Plant secondary metabolites are involved in various biological functions and play a role in plant interactions with their environment, particularly under biotic and abiotic stresses. While some of these metabolites play a fundamental role in the attraction of pollinators and in chemical ecology, others are involved in coping with stressful stimuli, as reviewed in [1,2,3,4,5,6]. They were not considered essential for plant growth and development when they were first discovered and were qualified as “secondary”. Nevertheless, nuances in the definition of secondary metabolites have emerged over the past two decades. Because of their pivotal role in the plasticity and response of plants to various environmental stimuli, some authors rather refer to them as “specialized” metabolites, while “central” is used for primary metabolites [7,8,9]. Moreover, the advent of high-throughput sequencing has allowed the publication of the genomes of several species and highlighted that the genes involved in the biosynthesis pathways of these metabolites occupy a significant place in a large array of genomes [10]. As we fully adhere to the concept of high importance of these metabolites, the term “specialized metabolites” will be used throughout this paper.
Metabolomics and functional genomics technologies have accelerated the large-scale exploration of plant specialized metabolites and the key enzymes involved in their biosynthesis [11,12]. The objective of the present review was to compile data about phenylpropanoids and flavonoids because of their wide range of biological activities and particularly their significant involvement in numerous mechanisms of plant adaptation to the environment.
The phenylpropanoid pathway (PPP) results in the accumulation of many families of compounds, such as phenylpropanoids, flavonoids, lignins, monolignols, phenolic acids, stilbenes and coumarins [13]. The flavonoid family includes subfamilies of molecules classified according to their structure, e.g., flavones, isoflavones, anthocyanidins, flavonols, flavanols, flavanones, aurones or chalcones. Each subfamily comprises a large diversity of molecules as a result of various conjugation processes through C- or O-methylation, sulfation, or glycosylation [14,15,16].
In stress-free conditions, flavonoids play a role in the development of plant reproductive organs and seeds, such as pollen tube germination and growth or seed maturation, dormancy and longevity [17,18]. They are also involved in plant attractiveness to pollinators through the color or scent they confer to flowers [19]. Finally, they also play a role in plant–microorganism communication for the establishment of symbiosis, such as in legume–rhizobium interactions during nodulation [18,19].
In adverse abiotic conditions, they can mediate defense responses. For instance, under water stress, plants have to deal with concomitant oxidative stress caused by reactive oxygen species (ROS) to prevent cellular damage. For this purpose, high antioxidant activity could be necessary to limit lipid peroxidation of cell membranes [20,21]. This may be obtained through the upregulation of genes involved in the phenolic flavonoid biosynthesis described in Figure 1. For example, in Chrysanthemum morifolium L. cultivars exposed to water stress, genes encoding enzymes phenylalanine ammonia-lyase (PAL), chalcone isomerase (CHI) and flavanone 3-hydroxylase (F3H) were upregulated, leading to an increased production of antioxidant flavonoids [22]. Biosynthesis of caffeic acid derivatives and flavonoid glycosides was also strongly enhanced during salt and UV stresses [23]. The mutation of several genes coding for a Myeloblastosis (MYB) transcription factor, a chalcone synthase (CHS) and a few chalcone isomerases was reported to alter the freezing tolerance of Arabidopsis thaliana (L.) Heynh. [24]. Under excess of solar radiation, flavonoids strongly accumulate in leaves and glandular trichomes of Phillyrea latifolia L. [25] leading authors to suggest their protective role in the integrated mechanisms of acclimation of P. latifolia to excessive light.
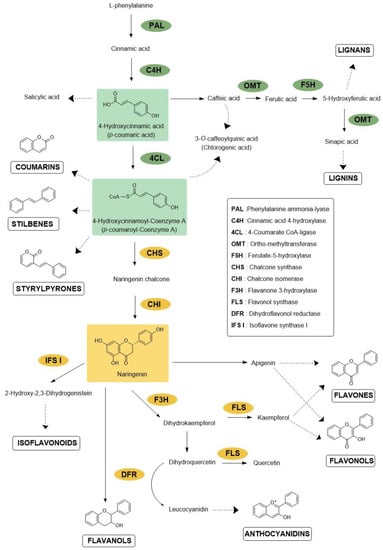
Figure 1.
Main steps of the phenylpropanoid and flavonoid pathways. Enzymes mentioned in this paper are shown in green and yellow for central phenylpropanoid pathways and flavonoid biosynthesis, respectively. Complete arrows refer to one step in the biosynthetic pathway, whereas dashed arrows represent undetailed pathways leading to one molecule or molecule subfamilies.
Finally, in a context of biotic stresses, plant defense may be mediated by the action of flavonoids and phenylpropanoid compounds acting indirectly as signaling molecules, or directly through the toxic effect of phytoanticipins (constitutively accumulated active compounds in plant tissues) and phytoalexins (newly synthesized active compounds following pathogen detection) [26,27,28]. Their accumulation or the importance of the expression of genes involved in their biosynthesis has indeed been demonstrated in regards of resistance to biotic stresses [29,30,31,32]. This has been described in the literature from two points of view. On the one hand, research focusing on basal defense addressed the different types of constitutive defense mechanisms, from physical to chemical barriers. Cell wall reinforcement involving phenylpropanoid derivatives is one of them [30,32,33,34,35]. As an example, the abundance of phenylpropanoids in maize (Zea mays L.) grain pericarps was thought to limit disease symptoms in genotypes resistant to Fusarium graminearum and Fusarium verticillioides, as well as against maize weevil (Sitophilus zeamais (Motsch.) [35,36,37]. On the other hand, research focusing on induced resistance investigated the potential toxicity of these metabolites that may suppress or limit the pathogenicity of invaders. Although the study of this direct effect played by bioactive compounds against pathogens has been promoted by a rising interest in deciphering the molecular dialogue between the host and the pathogen, these mechanisms are still poorly described in plant science. In contrast, the biological activity of plant-derived compounds—especially flavonoids—on human pathogenic microorganisms has been notably investigated in the field of drug development [38,39]. Moreover, potential intracellular targets of some flavones have been discovered when searching for natural anti-inflammatory compounds [40].
In order to help breeding for plants resistant to pests and diseases or plants receptive to biopesticides, this review covers the state of the art on the molecular and mechanistic diversity of phenylpropanoid or flavonoid derivatives potentially involved in plant resistance to biotic stresses. This is presented according to the nature of the targeted pathogen, including the highlights of the findings in human health research.
2. Protection against Microbes
2.1. Bacterial Targets
A wide range of studies have explored the involvement of the PPP in mediating a response to phytopathogenic bacteria. For example in the tobacco (Nicotiana tabacum L.)–Pseudomonas syringae pathosystem, an infection-induced increase was shown for the flavonoids and other phenylpropanoid derivative content, especially regarding coumaric acid content [32]. Similarly, flavonoid glycosides and hydroxycinnamic acid production significantly increased in orange leaves (Citrus sinensis L.) infected by Candidatus liberibacter asiaticus [29]. In potato (Solanum tuberosum L.) tubers, rutin (quercetin-3-O-rutinoside) (Table 1) and nicotiflorin (kaempferol-3-O-rutinoside) were shown to be related with resistance to Pectobacterium atrosepticum, a necrotrophic bacterial pathogen [41]. Finally, resistance to Erwinia carotovora subsp. carotovora of transgenic potato tubers accumulating a high content of pelargonidin-3-O-rutinoside-5-O-glucopyranoside and peonidin-3-O-rutinoside-5-O-glucopyranoside both acylated with p-coumaric acid, was double that of untransformed plants with lower amounts of these anthocyanins (Table 1) [42].
In a context of plant protection, in order to develop biological alternatives to synthetic phytoprotectants, the biological actions of phenylpropanoids have been largely investigated through in vitro tests. However, according to [43], an efficient protection may probably not arise from any significant toxicity towards the microorganism, but rather from defense elicitation of the host. Therefore, to reach an efficient pest management, we suggest exploring both the direct or indirect modes of action of these compounds. As a consequence, there is a need to delve into the question of their biosynthesis and targets.
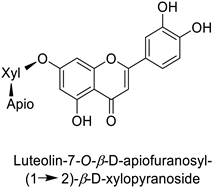
By studying the postinoculation transcriptomic shift, some genes encoding enzymes at a crucial step in the PPP were found to rapidly respond, whereas others were induced much later in the onset of the response to infection [31,32]. In soybean (Glycine max (L.) Merr.) infected with Pseudomonas syringae, CHS, F3H and isoflavone synthase I (IFS I) genes were among the earliest to be activated [31]. In tobacco infected with the same bacterium, PAL, cinnamic acid 4-hydroxylase (C4H), 4-coumarate:CoA-ligase (4CL), some ortho-methyltransferases (OMT) and ferulate-5-hydroxylase (F5H) genes were the most expressed [32]. At this stage, although the discovery of these potential genes of interest may pave the way for the development of new plant varieties resistant to bacteria, little is known about the biological targets of the enzymes related to these genes. Plant-oriented literature only reports that some PPP compounds can target vital functionalities of cellular processes and jeopardize bacterial survival without details about the mechanisms implemented. For example, fragarin isolated from strawberry (Fragaria ananassa (Weston) Duchesne ex Rozier) leaves caused cell death by disrupting cell membrane integrity in Clavibacter michiganensis [44,45]. Interesting data from research in medical microbiology may nonetheless provide leads, as a number of authors have proposed an elaborated description of the mechanism of action for phenylpropanoids to find natural antibiotics for human health [46,47]. Thus, the antimicrobial action of flavonoid glycosides isolated from the aerial parts of Graptophyllum grandulosum Turrill—chrysoeriol-7-O-β-d-xylopyranoside, luteolin-7-O-β-d-apiofuranosyl-(1→2)-β-d-xylopyranoside, chrysoeriol-7-O-β-d-apiofuranosyl-(1→2)-β-d-xylopyranoside, chrysoeriol-7-O-α-l-rhamnopyranosyl-(1→6)-β-d-(4″-hydrogenosulfate) glucopyranoside and isorhamnetin-3-O-rutinoside (Table 1)—was reported to cause cell lysis in S. aureus due to alteration of membrane permeability, with a minimum inhibitory concentration (MIC) ranging from 4 to 8 µg.mL−1 [48]. This membrane fluidity alteration may arise from the upregulation of genes responsible for a rearrangement of the membrane fatty acid proportions, as observed in S. aureus and E. coli exposed to a relatively low concentration of naringenin (Table 1) (with a MIC of 1.84 mM and 3.64 mM respectively) [49]. The physicochemical properties of this class of compounds are involved in their ability to cross the bacterial wall and lipidic membranes to reach their intracellular targets [50]. A low capacity of cell penetration could explain why the cytotoxicity of some molecules may go unnoticed in the framework of in vitro tests on a whole-cell scale, in spite of an apparent effect observed on isolated cellular components [51].
The cell walls of Gram-positive and Gram-negative bacteria are structurally divergent. The wall of Gram-positive bacteria comprises a cytoplasmic membrane underneath a periplasmic space mainly composed of peptidoglycans, whereas Gram-negative bacteria have an additional membrane on the outer side, coated by lipopolysaccharides, rendering bacteria of this type rather less permeable to relatively more hydrophobic compounds. Therefore, this could explain the discrepancy in the antibacterial activity of the same active compound. This was observed with naringenin [49] whose growth inhibition was more perceptible on a Gram-positive (S. aureus) than a Gram-negative bacterium (E. coli). Chlorogenic acid (Table 1) can cause dismantling of the outer membrane in Shigella dysenteriae (Gram-negative). It was hypothesized that its acidic carboxyl group may chelate stabilizing cations in the lipopolysaccharide layer, which further disrupts the outer membrane structure [52]. Although it is clear that a certain level of lipophilicity of the compound is required to interact with and pass through the cell membrane [53,54], manipulating other properties—e.g., by adding sugar moieties—can improve the interaction with enzyme active sites, compared to aglycones [15,40]. Therefore, studying the structure–activity relationships of the specialized metabolites in regard to the physical barrier of the bacteria is undoubtedly essential in searching of efficient antibacterial compounds.
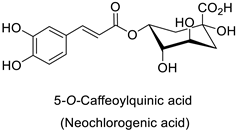
Compounds with better penetrability exhibit stronger effects on key functionalities in the intracellular milieu. First of all, the electron transport respiratory chain, and thus cell survival, can be dramatically affected. This has been demonstrated in a case study on Micrococcus luteus: for licochalcones A and C (Table 1), two retrochalcones isolated from Chinese licorice (Glycyrrhiza inflata L.) roots compromised the enzymatic activity of the NADH-cytochrome C reductase [51]. In addition, the antibacterial activity of the compound of interest may consist in preventing cell proliferation, through a direct interference with cell division processes. In E. coli, the polymerization of an important cytoskeletal protein FtsZ—required for cytokinesis—was hindered by the action of chlorogenic acid, leading to inhibition of cell division (Table 1) [55]. A molecular modeling study suggested that chlorogenic acid made hydrogen bonds and hydrophobic interactions to various residues of this protein, hence altering its conformation and disabling the GTPase activity preceding polymerization [55].
These few discoveries illustrate the action of molecules from the PPP family at different subcellular levels, targeting essential functionalities and fundamental structural components of the bacterial cell. The investigation could be transposed to plant-pathogenic bacteria to support previous hypotheses on plant disease resistance through the biological activity of phenylpropanoid derivatives.
2.2. Fungal Targets
Since the early 2010s, the accessibility of metabolomic tools has hugely favored the identification of specialized PPP metabolites correlated with plant resistance to fungi and oomycetes [56,57,58,59,60]. O-glycosylated flavonoids are the most frequently reported in such host–pathogen interactions. The mechanistic studies published so far followed an overall trend emphasizing a quantitative aspect and/or a spatiotemporal dimension of phenolic derivative accumulation in plant tissues associated with resistance to fungi.
Quantitative aspects can be illustrated by a case study on carrot (Daucus carota L.) leaves. Genotypes more resistant to Alternaria dauci contained significantly higher levels of feruloylquinic acid, as well as 4′-O- and 7-O-glycosides of apigenin, luteolin (Table 1) and chrysoeriol, compared to susceptible genotypes [57]. Such differences in PPP metabolites between resistant and susceptible genotypes may be found in numerous other pathosystems providing support for plant breeders. Nevertheless, with the same compounds, an opposite correlation can be found between metabolite contents and disease resistance depending on the pathogen. This was specifically observed in potato tubers enriched in rutin and nicotiflorin that turned out to be resistant to Pectobacterium atrosepticum, as mentioned above, but at the same time susceptible to Phytophthora infestans, a biotrophic fungal pathogen [41]. A systemic approach is therefore required to make sure that breeding for an increased content of a specific specialized metabolite involved in resistance to one pathogen will not decrease resistance to another one.
Spatial aspects can be exemplified by maize—Fusarium graminearum and maize—Fusarium verticillioides pathosystems, where a high content in ferulic acid in the grain pericarp was linked to a lesser disease extent in resistant genotypes [36,37]. Such a specific localization of the defense metabolite seems to be a very strategic way to prevent pathogen invasion. For example, in cotton (Gossypium hirsutum L. and Gossypium barbadense L.), catechin and gallocatechin (Table 1) were predominant near the Verticillium dahliae infection site in the vessels, creating a toxic environment that confined the pathogen to the vessel lumens [61]. This local accumulation contributed to prevent the systemic spread of the vascular disease through the formation of tyloses. This mechanism was also observed in grapevine (Vitis vinifera L.) defense against Phaeomoniella chlamydospora and Phaeoacremonium species [62]. Similarly, the resistance of barley (Hordeum vulgare L.) to mildew (Blumeria graminis) was attributed to the accumulation of light-absorbing compounds—suggested to be phenylpropanoids—in the papilla of the coleoptiles [63].
Temporality can be illustrated by the resistance of the date palm tree (Phoenix dactylifera L.) to fungal diseases caused by Fusarium oxysporum, likely driven by a quantitative differential in 5-O-caffeoyl-shikimic acid content, particularly at physiological stage 3 (ripening of dates) [64]. The phenotypic contrast between resistant and susceptible cultivars to a fungal disease is often attributed to an early or constitutive availability of the compound of interest in the tissues leading to an efficient defense response, e.g., preformed chlorogenic acid in tobacco plants resistant to Cercospora nicotianae [65]. Similarly, studies on the barley (Gibberella zeae) pathosystem led to the identification of 194 metabolites constitutively present in a resistant genotype and significantly more accumulated than in a susceptible one [56]. Among them were kaempferol-3-O-rhamnopyranoside, naringenin-7-O-glucopyranoside, kaempferol-3-O-rhamnopyranoside-7-O-glucopyranoside, kaempferol-3-O-glucopyranoside-7-O-rhamnopyranoside, and kaempferol-3-O-sophoroside-7-O-rhamnopyranoside. Such a constitutive accumulation of flavonoids in the context of disease resistance in various plant–fungus pathosystems is now becoming a general trend described in many studies [56,66,67,68]. In addition to an early or constitutive synthesis of the defense compounds, their maintenance at a high concentration over time, i.e., as long as the disease pressure is high, is equally important [67]. Therefore, plant resistance to fungal diseases is not only more complex than a simple quantitative differential between resistant and susceptible genotypes at the time of infection, but its durability also depends on the stability of metabolite contents in the tissues over time. Thus, according to the development cycles of the disease and those of the plant, an efficient metabolic ratio must be maintained.
A multidisciplinary approach combining reverse genetic tools with biochemical characterization of the resulting proteins has led to a better understanding of the biosynthesis of phenylpropanoid derivatives mediating fungal disease resistance. Firstly, due to the upstream position of the PAL gene in the PPP, modifications would deprive the plant from the biosynthesis of a lot of compounds driven by downstream genes in the pathway. A tobacco PAL mutant (PAL-suppressed YE-6-16 transformant) exhibited a rapid expansion of lesions after infection by Cercospora nicotianae, whereas PAL gene overexpression resulted in reduced disease symptoms [65,69]. In Arabidopsis thaliana, inactivation of a gene encoding a CHS led to a decreased anthocyanin content and lower resistance to Verticillium dahliae [70]. In contrast, overexpression of CHS-, CHI- and dihydroflavonol reductase (DFR-) encoding genes in flax (Linum usitatissimum L.) was correlated with increased resistance to Fusarium species through an increased flavonoid content [71]. Overexpression and mutation of an R2R3 MYB transcription factor clearly impacted resistance to Dothiorella gregaria in poplar (Populus tomentosa Carr.) through enhanced and decreased proanthocyanidin content, respectively [72]. As supplementary evidence, chemical inhibition of a CHS enzyme and downregulation of the corresponding CHS gene in cucumber (Cucumis sativus L.) resulted in nearly complete suppression of induced resistance towards Podosphaera xanthii [73].
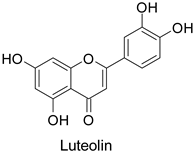
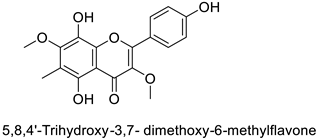
The antifungal activity exerted by phenylpropanoids, and flavonoids has been investigated, corroborating the metabolomic and functional genomic-driven hypothesis on their link to disease resistance. Flavonoids extracted from the needles of Picea neoveitchii Mast., used at 1 mg·mL−1, exhibited very interesting antifungal activities: kaempferol-7-O-(2″-E-p-coumaroyl)-α-l-arabinofuranoside exhibited strong activity against Fusarium oxysporum with a relative inhibitory percentage of 108.1%, while 5,7,4′-trihydroxy-3,8,-dimethoxy-6-C-methylflavone, 5,8,4′-trihydroxy-3,7-dimethoxy-6-C-methylflavone, 7-methoxy-6-C-methylkaempferol and kaempferol-7-O-(2″-E-p-coumaroyl)-α-l-arabinofuranoside were active against Rhizoctonia solani, with 49.5%, 53.3%, 95.3% and 49.5% relative inhibitory percentages, respectively, (Table 1) [58]. These compounds were as active as carbendazim, a synthetic chemical fungicide used against these two pathogens. Other flavonoids, such as eriodictyol, homoeriodictyol, dihydroquercetin, and luteolin (Table 1) isolated from Ficus sarmentosa, var. henryi (King) Corner, were effective against pathogenic fungi, e.g., Fusarium graminearum and Septoria zeicola. Among these flavonoids, luteolin showed the strongest inhibitory activity, with half-maximal inhibitory concentration (IC50) values of 56.38 and 81.48 mg·L−1 to each fungus, respectively [74]. Finally, in an in vivo assay, cherry tomatoes sprayed with laurel (Laurus nobilis L.) oil containing about 44% of eugenol and 30% of cinnamaldehyde (Table 1) were less infected by Alternaria alternata after 5 days of storage at 25 °C than control without oil. More precisely, the proportion of decayed tomatoes treated with 1 mg·mL−1 of laurel oil was reduced by 86.4% [75].
Despite all these investigations on biosynthesis and targets, little is known about the mode of action involved in the described fungicidal activities. Some studies have assessed the effect of phenylpropanoid derivatives on the integrity of plant fungal pathogens. Among them is the study of essential oil from laurel leaves mentioned above: fungicidal activity was evidenced with invaginations and folds in the cell wall of the fungus and drastically reduced sporulation.
Medical research suggested another mechanism: a high concentration of a bioactive caffeic acid derivative like chlorogenic acid in the extracellular environment was efficient to disrupt the lipid membrane of Candida albicans, Trichosporon beigelii and Malassezia furfur, leading to ion leakage and break of the intracellular equilibrium (Table 1) [76]. Apigenin (Table 1) isolated from the leaves of Aster yomena (Kitam.) Honda had the same effect on C. albicans: it caused intracellular calcium and potassium leakage and led to osmotic imbalance [77]. The capacity of some compounds to cross cell boundaries suggests that they might reach and interfere with nuclear components. Despite the lack of clear evidence of a nucleic acid–flavonoid interaction, apoptosis-associated DNA fragmentation and chromatin condensation was observed in Candida glabrata following treatment with 4 µg·mL−1 of glabridin (Table 1), an isoflavan mainly found in Glycyrrhiza glabra L. roots [78]. These mechanisms may also occur in plant fungal pathogens and should be explored by plant prebreeders.
2.3. Viral Targets
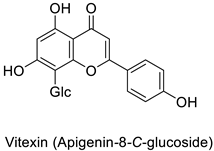
Plant exposure to viral agents activated salicylic acid (SA) biosynthesis and induced the biosynthesis of other defense metabolites from the PPP to initiate systemic acquired resistance (SAR). This was shown for the sugarcane mosaic virus that causes dwarf mosaic disease on maize [79]. As it is hosted by a broad range of economically important plant species, such as tobacco and tomato (Solanum lycopersicum L.), specific attention has been paid to the tobacco mosaic virus (TMV) in the exploration of the antiviral activity of candidate specialized metabolites [80]. A more precise study pointed out that while 5-O-caffeoylquinic acid and quercetin abounded at the TMV infection site in tobacco leaves, kaempferol was predominant in a more remote part of the plant that exhibited SAR (Table 1) [11].
Most of the publications pointed out the role of quercetin and kaempferol (Table 1) in triggering the defense response of the host plant, rather than a direct action on viral particles, e.g., in the Datura stramonium L.-TMV and Chenopodium amaranticolor H.J. Coste and A.Reyn.—TMV pathosystems [81]. Other studies mention a correlation between the metabolite content and plant resistance to viruses, without further determining whether they are potentially harmful or not to viral particles.
In medical research, one of the earliest works carried on murine leukemia viruses (MLVs) and human immunodeficiency viruses (HIVs) revealed that 1 µg·mL−1 and 2 µg·mL−1 of 5,6,7-trihydroxyflavone (baicalein) inhibited the activity of their respective reverse transcriptases by 90% (Table 1) [82]. A similar trial on HIV showed the same trend, with approximately 90% of reverse transcriptase inhibition at 200 µg·mL−1 of hinokiflavone and robustaflavone (Table 1) isolated from Rhus succedanea L. [83]. This surely brings evidence that phenylpropanoid derivatives could limit viral reproduction within the host, although the way they inhibit the biological function of this strategic enzyme remains unclear. These observations need to be transposed to the framework of plant pathology studies to determine the putative direct effects of these compounds on plant viruses.
3. Protection against Insects
Among macroscopic pests of cultivated plants, insects are a concern, not only because of their direct damaging effect mainly linked to the herbivorous activity of their larvae but also because of indirect damage through their ability to transmit microbial pathogens to the host plant.
3.1. Herbivore Targets
Efforts have been made to explore the natural defense mechanisms of plants against herbivores. The defensive compounds are either produced constitutively or in response to plant damage, and affect feeding, growth, and survival of herbivores. In addition, plants also release volatile organic compounds that attract the natural enemies of the herbivores, as reviewed in [84]. Phenylpropanoids and flavonoids are mentioned among these defensive compounds preventing plants from insect invasion. For example, chlorogenic acid and feruloylquinic acid (Table 1) discriminate resistant and susceptible genotypes of chrysanthemum (Dendranthema grandiflora (Ramat.) Kitam.) to thrips (Frankliniella occidentalis) with higher amounts of both molecules in thrip-resistant genotypes [85]. Similarly high contents of quercetin, chlorogenic acid and rutin (Table 1) in wild-cultivated crosses of groundnut plants (Arachis hypogaea L. x Arachis kempff-mercadoi Krapov. and W.C. Greg.) were linked to their resistance to Spodoptera litura (Fab.) [86]. In carrot leaves, the flavone luteolin and the phenylpropanoid sinapic acid significantly differentiated thrip-resistant cultivars from susceptible ones (Table 1) [87]. In some cases of plant resistance to insects, the ratio of the specific metabolites was preponderant over their respective contents, as in carrot, where resistance to the fly Psila rosae F. is positively correlated with high luteolin-7-O-glucopyranoside/kaempferol-3-O-glucopyranoside and methyluteolin-7-O-glucopyranoside/kaempferol-3-O-glucopyranoside ratios [88].
Modes of action were investigated either in artificial bioassays or in planta. Chlorogenic acid was shown to significantly injure larval growth rate and larval survival capacity of F. occidentalis thrips when fed artificial diets including 5% chlorogenic acid (Table 1) [85]. Quercetin mostly contained in leaf extracts of castor beans (Ricinus communis L.) caused the death of adults and had remarkable ovicidal and oviposition-deterrent activity against Callosobruchus chinensis L., a common species of beetle found in the bean weevil subfamily and known to be a pest to many stored legumes (Table 1) [89]. Some compounds do not have direct insecticidal activity, but their physical and chemical properties can improve the solubility of other compounds and thus their penetration and efficacy. This sort of synergy was illustrated with non-PPP metabolites in an in vitro analysis that revealed an up to 19-fold increase in penetration of camphor in a binary mixture with 1,8-cineole through the larval integument of the cabbage looper (Trichoplusia ni) in comparison to camphor alone, the most toxic ratio being 60:40 1,8-cineole:camphor (LD50 = 186.9 μg/insect) [90,91]. Such a synergy could probably be searched within PPP metabolites. As mentioned before for fungal targets, one metabolite may have opposite actions against different targets, e.g., chlorogenic acid is on the one hand auxiliary to control thrips but on the other hand is promoting oviposition on carrot leaves by the black swallowtail (Papilio polyxenes Fabr.) [92]. An integrative approach is therefore needed to ensure that the protection strategy against one target does not increase severity of the disorders caused by another agent.
3.2. Vector Targets
Not only can insects cause direct damage to plants but they can also be vectors transmitting economically threatening diseases, such as Pierce’s disease, caused by the bacterium Xyllella fastidiosa [93], the grapevine yellow disease caused by phytoplasmas [94] or other major crop viruses. Therefore, limiting an epidemic by preventing the contact of the host with disease-carrying vectors is a major preoccupation in crop management. In this regard, Su and collaborators [95] addressed this dimension by linking metabolic changes in tomato leaves to vector behavior. They showed that whiteflies (Bemisia tabaci) actively recognized plants previously attacked by conspecifics due to decreased terpenoid and flavonoid contents. By treating tomato plants infested by B. tabaci with naringenin they increased their content of rutin, kaempferol-rhamnopyranoside, quercetin-trisaccharide, 3-O-methylmyricetin and anthocyanin up to the same level as those measured in noninfested plants and showed that the preference of B. tabaci for oviposition on previously infested plants was reversed (Table 1). As a consequence of reduced B. tabaci population, both the pest and the vectored virus—e.g., the tomato yellow leaf curl virus (TYLCV)—damage can be decreased. Moreover, whiteflies fed less on the phloem of flavonoid-rich tomato leaves, and the spread of TYLCV was reduced [96]. The authors pointed out that their findings rather suggest an impediment of the host–vector interaction than an antiviral activity of flavonoids, as disease expression was only delayed. Similarly, flavonoid accumulation was observed after infection of grapevine by the Flavescence dorée phytoplasma. This flavonoid accumulation was thought to repel the insect vectors afterwards [97].
The genes of the PPP involved in plant resistance to insects, considered direct herbivores or disease vectors, are poorly documented. Susceptibility of carrot roots to larval damage caused by the fly Psila rosae correlated with semiquantitatively estimated accumulation of PAL1 and PAL3 mRNAs in leaves [88]. The two genes responsible for their biosynthesis were expressed at a higher level in resistant lines than in susceptible ones. The PPP genes whose overexpression was correlated with the metabolic changes described above in the tomato–B. tabaci experiment [95] were the genes coding for CHS, CHI, flavonol synthase (FLS) and DFR.
In the field of drug discovery, phenylpropanoids and flavonoids are increasingly explored to develop eco-friendly insecticides targeting the vectors of major human diseases, such as mosquitoes [98,99,100,101,102,103]. At a time when resistance to conventional insecticides is alarming, recent works aimed at improving insecticide efficiency by overcoming the resistance mechanisms of insects. As a matter of fact, the activity of the CYP6AA3 and CYP6P7 cytochrome P450 monooxygenases of mosquito—known to detoxify insecticides such as pyrethroids—was inhibited by four flavones (apigenin, 5-hydroxy-7,8-dimethoxyflavone, 5-hydroxy-7,8,2′,3′-tetramethoxyflavone, and 5,4′-dihydroxy-7,8,2′,3′-tetramethoxyflavone) from Andrographis paniculata Nees (Table 1) [104]. Other studies focused more on the insect’s vital component and have investigated the neurotoxicity of plant phenylpropanoids to the target insects. On this basis, the mortality of Aedes aegypti was attributed to the inhibition of acetylcholinesterase activity by the phenylpropanoids asaricin, isoasarone and trans-asarone from Piper sarmentosum Roxb. leaf extracts (Table 1) [105]. This strategy may deserve to be explored for crop protection against insects.
4. Prospects
The health benefits conferred by some plant-derived foodstuffs and beverages have greatly encouraged the exploration of the bioactive compounds involved and enhanced the investigation of their production in plant tissues [106,107,108]. This has paved the way for the understanding of the mechanisms of action of some phenylpropanoid derivatives on human bacterial and fungal agents, but also on viruses [76,82,83,109]. On this basis, knowledge from medical research could be a pioneer in understanding how plant-derived bioactive compounds negatively influence plant pathogen development (Figure 2).
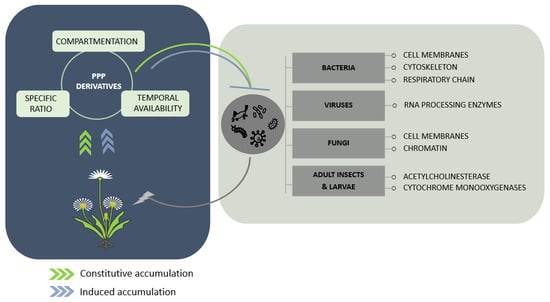
Figure 2.
Potential intracellular targets of flavonoids and phenylpropanoids in microorganisms and insects aggressing plants. Biosynthetic accumulation can be constitutive and/or induced in response to external stimuli. The protective action of phenylpropanoids and flavonoids is described as the combinatory effect of their localization during the host’s interaction with aggressors, their sustained availability, and the promotion of specific compounds over others among the same subfamily or their putative synergy. Their action on microorganisms and insects probably arises from interference with important cellular machineries and structures, but this is not fully understood for all types of pests and pathogens.
An overview of the breakthroughs cited in this review clearly shows that some molecules exhibit a universal action regardless of the nature of the pathogen (summarized in Table 1). Chlorogenic acid, quercetin and other flavonoids are most frequently mentioned [55,56,76,89,95]. This universality has been exploited in the development of multidisease-resistant crops mediated by phenylpropanoids and flavonoids, e.g., myricetin (a flavonol) from tomato, to a vast array of herbivore insects [110]. Further described in [43], defense priming by rutin application on different host plants helped inducing SA-mediated defense responses against various bacterial pathogens. Similarly, quercetin induced the expression of defense-related genes in apple fruits [111]. Supporting these findings, the recent characterization of a gene in the PPP of maize corroborated the place held by these families of compounds: phenylpropanoids and flavonoids have become a promising and sustainable source of multiresistance [112]. However, despite this apparent potential in the expression of multiresistance, their action appeared to be more complex, as their accumulation in a given host can be perceived differently by various pathogens [41,92]. A preliminary systemic investigation based on metabolite contents should be undertaken prior to their selection for disease resistance. Efforts are still to be made in plant health research to understand the mechanisms of action of this family of compounds to better use them. Finally, it will be necessary to evaluate not only the content of metabolites of interest but also their location in the plant organs. Indeed, at the tissue level, phenylpropanoids can be found in grain pericarps and in various leaf tissues, such as glandular trichomes, cuticle, epidermis and mesophyll [25,36,113,114]. Immunolocalization of PAL and CHS in Primula kewensis W. Wats. suggested that flavonoid biosynthesis occurred in the head of glandular cells [115]. The concentration in aglycones and their glycosides may vary, considering different levels of the leaf tissue, suggesting the specificity of their function [116]. At the cellular level, phenylpropanoid derivatives are stored in vacuoles, but can also be detected in the cell wall [117]. This accumulation inside or at the peripheral sites of the cell/tissue/organs may signify the formation of physical or chemical barriers preventing pathogen or pest invasion (direct effect) or an involvement in plant signaling to mediate defense responses or plant-to-plant communication. Therefore, depending on the mechanisms of action, the metabolite content required to be efficient for plant protection may be different.

Table 1.
Overview of main active PPP compounds and their putative mechanisms of action. Molecules are sorted in two families, phenylpropanoids and flavonoids, and are alphabetically presented within the families. The table does not include all the compounds cited in the text, but only those that have been documented either through genetic studies on segregating progenies, functional validation of candidate genes explaining their biosynthesis, direct toxicity tests on the targets, or histological observations of the effects of the compound on the target. Compounds for which the literature only relates a correlation between resistance level and metabolite content are not described. Concentration range is given for the compounds with biological activity reported in the corresponding references. MIC, minimum inhibitory concentration; EC50, half-maximal effective concentration; IC50, half-maximal inhibitory concentration; MC50, half larval mortality concentration.
Table 1.
Overview of main active PPP compounds and their putative mechanisms of action. Molecules are sorted in two families, phenylpropanoids and flavonoids, and are alphabetically presented within the families. The table does not include all the compounds cited in the text, but only those that have been documented either through genetic studies on segregating progenies, functional validation of candidate genes explaining their biosynthesis, direct toxicity tests on the targets, or histological observations of the effects of the compound on the target. Compounds for which the literature only relates a correlation between resistance level and metabolite content are not described. Concentration range is given for the compounds with biological activity reported in the corresponding references. MIC, minimum inhibitory concentration; EC50, half-maximal effective concentration; IC50, half-maximal inhibitory concentration; MC50, half larval mortality concentration.
| Compound | Host | Targets | Concentration | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Phenylpropanoids | |||||
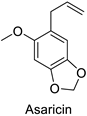 | Piper sarmentosum | Aedes aegypti | IC50 = 0.73 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at the infection site | [11] |
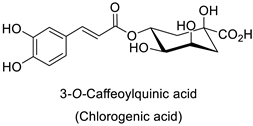 | Human | Escherichia coli | IC50 = 69.55 ± 3.6 µM | Inhibition of FtsZ polymerase (a cell division protein) | [55] |
| Shigella dysenteriae | MIC = 20 µg·mL−1 | Bacterial outer membrane disintegration | [52] | ||
| Candida albicans, Trichosporon beigelii and Malassezia furfur | MIC = 40–80 µg·mL−1 | Deterioration of membrane electrical potential | [76] | ||
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.67 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
| Chrysanthemum (Dendranthema grandiflora) | Frankliniella occidentalis | 5% in culture medium | Reduction of larval growth rate and adults survival rate | [85] | |
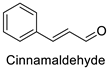 | Tomato (Solanum lycopersicum) | Alternaria alternata | MIC = 800 µg·mL−1 (Essential oil containing 30% of cinnamaldehyde and 44% of eugenol) | Cell wall invaginations and distortions | [75] |
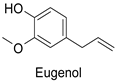 | Tomato (Solanum lycopersicum) | Alteranaria alternata | MIC = 800 µg·mL−1 (Essential oil containing 30% of cinnamaldehyde and 44% of eugenol) | Cell wall invaginations and distortions | [75] |
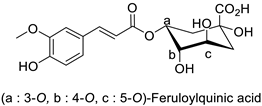 | Chrysanthemum (Dendranthema grandiflora) | Frankliniella occidentalis | 5% in culture medium | Reduction of larval growth rate and adults survival rate | [85] |
| Carrot (Daucus carota) | Alternaria dauci | - | - | [57] | |
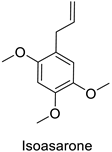 | Piper sarmentosum | Aedes aegypti | IC50 = 0.92 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
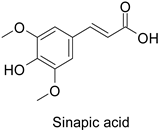 | Carrot (Daucus carota) | Psila rosae | 0.35–2.11 mg·g−1 | Insecticidal activity | [87] |
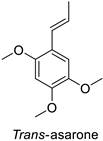 | Piper sarmentosum | Aedes aegypti | IC50 = 15.75 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
| Flavonoids | |||||
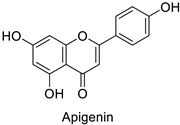 | Human | Candida albicans | 5µg·mL−1 | Cell shrinkage due to membrane disruption | [77] |
| Anopheles minimus | IC50 = 2.38 µM | Inhibition of cytochrome P450 monooxygenases | [104] | ||
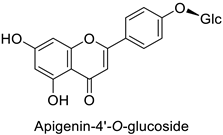 | Carrot (Daucus carota) | Alternaria dauci | - | - | [57] |
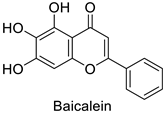 | Human | Murine leukemia viruses (MLVs) | 90% of inhibition at 1 µg·mL−1 | Inhibition of reverse transcriptase activity | [82] |
| Human immunodeficiency viruses (HIVs) | 90% of inhibition at 2 µg·mL−1 | ||||
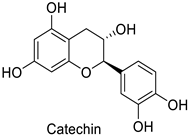 | Cotton (Gossypium hirsutum and Gossypium barbadense) | Verticillium dahliae | - | Confinement of the pathogen at the infection site | [61] |
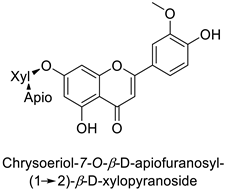 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
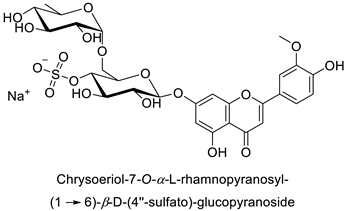 | |||||
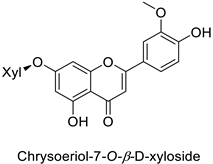 | |||||
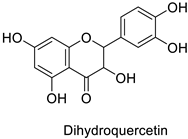 | - | Fusarium graminearum | IC50 = 124.27 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 160.32 mg·L−1 | |||
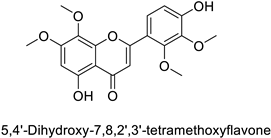 | Human | Anopheles minimus | IC50 = 5.91–16.6 µM | Inhibition of cytochrome P450 monooxygenases | [104] |
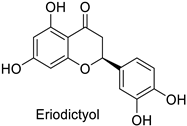 | - | Fusarium graminearum | IC50 = 162.71 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 247.32 mg·L−1 | |||
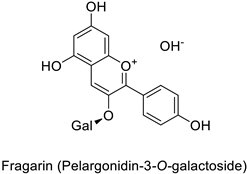 | Strawberry (Fragaria ananassa) | Clavibacter michiganensis | EC50 = 0.07 µM | Cell membrane permeabilization | [44,45] |
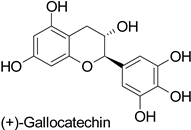 | Cotton (Gossypium hirsutum and Gossypium barbadense) | Verticillium dahliae | - | Confinement of the pathogen at the infection site | [61] |
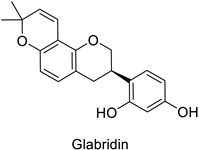 | Human | Candida glabrata | MIC = 4–16 µg·mL−1 | DNA fragmentation and chromatin condensation | [78] |
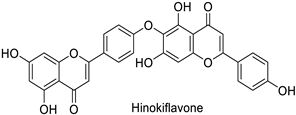 | Human | Human immunodeficiency virus | IC50 = 62 µM | Inhibition of reverse transcriptase activity | [83] |
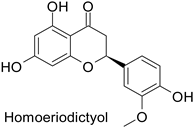 | - | Fusarium graminearum | IC50 = 274.78 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 240.31 mg·L−1 | |||
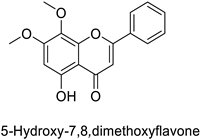 | Human | Anopheles minimus | IC50 = 7.24–8.90 µM | Inhibition of cytochrome P450 monooxygenases | [104] |
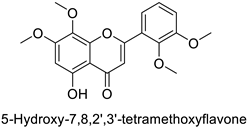 | IC50 = 6.45–8.35 µM | ||||
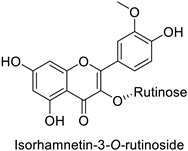 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
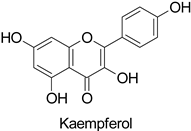 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at SAR site | [11] |
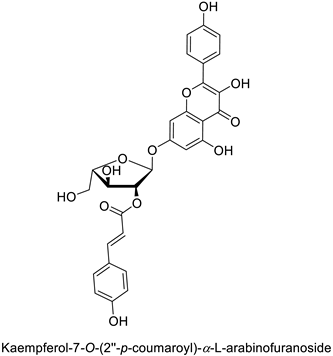 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 49.5% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
| Fusarium oxysporum | 108.1% of growth inhibition at 1 mg·mL−1 | ||||
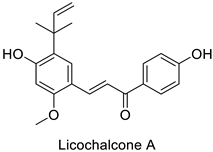 | Human | Micrococcus luteus | MIC = 1.56 µg mL−1 | Inhibition of NADH-cytochrome C reductase | [51] |
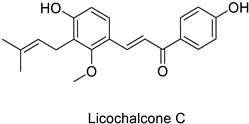 | MIC = 6.25 µg mL−1 | ||||
 | Carrot (Daucus carota) | Psila rosae | 0.35–2.11 mg·g−1 | Insecticidal activity | [87] |
| - | Fusarium graminearum | IC50 = 56.38 mg·L−1 | Mycelial growth inhibition | [74] | |
| - | Septoria zeicola | IC50 = 81.48 mg·L−1 | |||
 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
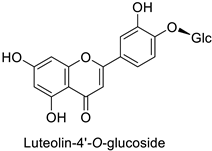 | Carrot (Daucus carota) | Alternaria dauci | - | - | [57] |
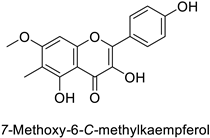 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 95.3% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
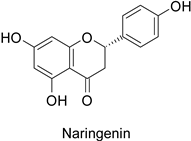 | Human | Staphylococcus aureus | MIC = 1.84 mM | Membrane fatty acid rearrangement leading to membrane integrity and fluidity alteration. | [49] |
| Escherichia coli | MIC = 3.64 mM | ||||
| Tomato (Solanum lycopersicum) | Bemisia tabaci | 10 µM | Oviposition deterrent | [95] | |
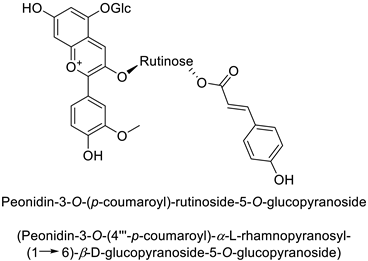 | Potato (Solanum tuberosum) | Erwinia carotovora | - | - | [42] |
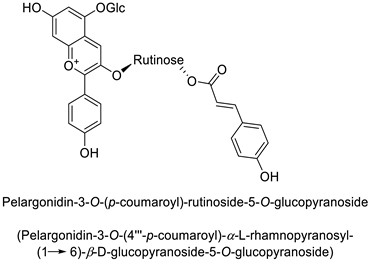 | |||||
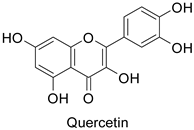 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at the infection site | [11] |
| Datura stramonium and Chenopodium amaranticolor | 250 µM | Putative host defense mediation through salicylic acid biosynthesis | [81] | ||
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.73 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
| Stored legumes | Callosobruchus chinensis L. | 3 mg·mL−1 (leaf extract mainly containing quercetin) | Insecticidal activity | [89] | |
| 6 mg·mL−1 (leaf extract mainly containing quercetin) | Oviposition deterrent Ovicidal activity | ||||
| Datura stramonium and Chenopodium amaranticolor | Tobacco mosaic virus (TMV) | 250 µM | Putative host defense mediation through kaempferol and salicylic acid biosynthesis | [81] | |
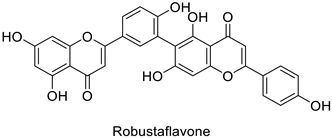 | Human | Human immunodeficiency virus | IC50 = 65 µM | Inhibition of reverse transcriptase activity | [83] |
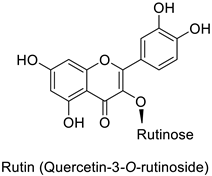 | Potato (Solanum tuberosum) | Pectobacterium atrosepticum | 88 µg·mL−1 | - | [41] |
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.60 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
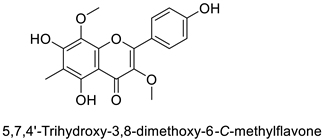 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 49.5% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
 | 53.3% of growth inhibition at 1 mg·mL−1 | ||||
 | Datura stramonium and Chenopodium amaranticolor | Tobacco mosaic virus (TMV) | 250 µM | Putative host defense mediation through kaempferol and salicylic acid biosynthesis | [81] |
* According to linear multiple regression equation.
Author Contributions
Conceptualization, C.K. and M.-L.R.; validation, M.B.; formal analysis, all authors; resources, all authors; writing—original draft preparation, C.K. and M.-L.R.; writing—review and editing, J.-J.H., V.L.C., L.H., E.G. and M.B.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministère de l’Enseignement Supérieur et de la Recherche and the RFI Objectif Végétal of the Pays de la Loire, grant name Flarescad, and the APC was funded by RFI Objectif Végétal of the Pays de la Loire.
Acknowledgments
Annie Buchwalter for English review and Andrea Ghidini for his priceless advices.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vining, L.C. Functions of Secondary Metabolites. Annu. Rev. Microbiol. 1990, 44, 395–427. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Bednarek, P. Chemical Warfare or Modulators of Defence Responses—The Function of Secondary Metabolites in Plant Immunity. Curr. Opin. Plant Biol. 2012, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary Metabolites in Plant Innate Immunity: Conserved Function of Divergent Chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant Secondary Metabolites in Nectar: Impacts on Pollinators and Ecological Functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A.; Ziegler, J.; Vogt, T. Specialized Plant Metabolites: Diversity and Biosynthesis. In Ecological Biochemistry: Environmental and Interspecies Interactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 14–37. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar]
- Kim, J.; Buel, C.R. A Revolution in Plant Metabolism: Genome-Enabled Pathway Discovery. Plant Physiol. 2015, 169, 1532–1539. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Hollander, J.G.; Lefeber, A.W.M.; Erkelens, C.; Nuzillard, J.M.; Verpoorte, R. NMR Metabolomics to Revisit the Tobacco Mosaic Virus Infection in Nicotiana Tabacum Leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef]
- Tugizimana, F.; Djami-Tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic Analysis of Defense-Related Reprogramming in Sorghum Bicolor in Response to Colletotrichum Sublineolum Infection Reveals a Functional Metabolic Web of Phenylpropanoid and Flavonoid Pathways. Front. Plant Sci. 2019, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Hannoufa, A.; Varin, L.; Ibrahim, R.K. Spatial Distribution of Flavonoid Conjugates in Relation to Glucosyltransferase and Sulfotransferase Activities in Flaveria Bidentis. Plant Physiol. 1991, 97, 259–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zagrean-Tuza, C.; Mot, A.C.; Chmiel, T.; Bende, A.; Turcu, I. Sugar Matters: Sugar Moieties as Reactivity-Tuning Factors in Quercetin: O-Glycosides. Food Funct. 2020, 11, 5293–5307. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Pharmacognosie: Phytochimie et Plantes Médicinales; Tec & doc Cachan: Paris, France, 1999; ISBN 2-7430-0315-4. [Google Scholar]
- Samanta, A.; Das, G.; Das, S. Roles of Flavonoids in Plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Haraguchi, H.; Yoshida, N.; Ishikawa, H.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Protection of Mitochondrial Functions against Oxidative Stresses by Isoflavans from Glycyrrhiza Glabra. J. Pharm. Pharmacol. 2010, 52, 219–223. [Google Scholar] [CrossRef]
- Rackova, L.; Firakova, S.; Kostalova, D.; Stefek, M.; Sturdik, E.; Majekova, M. Oxidation of Liposomal Membrane Suppressed by Flavonoids: Quantitative Structure-Activity Relationship. Bioorganic Med. Chem. 2005, 13, 6477–6484. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The Biosynthesis of Flavonoids Is Enhanced Similarly by UV Radiation and Root Zone Salinity in L. vulgare Leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids Are Determinants of Freezing Tolerance and Cold Acclimation in Arabidopsis Thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids Accumulate in Leaves and Glandular Trichomes of Phillyrea Latifolia Exposed to Excess Solar Radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-Wide Analysis of Phenylpropanoid Defence Pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Oros, G.; Kállai, Z. Phytoanticipins: The Constitutive Defense Compounds as Potential Botanical Fungicides. In Bioactive Molecules in Plant Defense: Signaling in Growth and Stress; Springer International Publishing: Cham, Switzerland, 2019; pp. 179–229. ISBN 9783030271657. [Google Scholar]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Hijaz, F.M.; Manthey, J.A.; Folimonova, S.Y.; Davis, C.L.; Jones, S.E.; Reyes-De-Corcuera, J.I. An HPLC-MS Characterization of the Changes in Sweet Orange Leaf Metabolite Profile Following Infection by the Bacterial Pathogen Candidatus Liberibacter Asiaticus. PLoS ONE 2013, 8, e79485. [Google Scholar] [CrossRef]
- Tan, B.A.; Daim, L.D.J.; Ithnin, N.; Ooi, T.E.K.; Md-Noh, N.; Mohamed, M.; Mohd-Yusof, H.; Appleton, D.R.; Kulaveerasingam, H. Expression of Phenylpropanoid and Flavonoid Pathway Genes in Oil Palm Roots during Infection by Ganoderma Boninense. Plant Gene 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Zabala, G.; Zou, J.; Tuteja, J.; Gonzalez, D.O.; Clough, S.J.; Vodkin, L.O. Transcriptome Changes in the Phenylpropanoid Pathway of Glycine Max in Response to Pseudomonas Syringae Infection. BMC Plant Biol. 2006, 6, 26. [Google Scholar] [CrossRef]
- Szatmári, Á.; Zvara, Á.; Móricz, Á.M.; Besenyei, E.; Szabó, E.; Ott, P.G.; Puskás, L.G.; Bozsó, Z. Pattern Triggered Immunity (PTI) in Tobacco: Isolation of Activated Genes Suggests Role of the Phenylpropanoid Pathway in Inhibition of Bacterial Pathogens. PLoS ONE 2014, 9, e102869. [Google Scholar] [CrossRef]
- Matern, U.; Grimmig, B.; Kneusel, R.E. Plant Cell Wall Reinforcement in the Disease-Resistance Response: Molecular Composition and Regulation. Can. J. Bot. 1995, 73, 511–517. [Google Scholar] [CrossRef]
- Eynck, C.; Séguin-Swartz, G.; Clarke, W.E.; Parkin, I.A.P. Monolignol Biosynthesis Is Associated with Resistance to Sclerotinia Sclerotiorum in Camelina Sativa. Mol. Plant Pathol. 2012, 13, 887–899. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Bergvinson, D.J.; Burt, A.J.; Ramputh, A.I.; Díaz-Pontones, D.M.; Arnason, J.T. The Role of Pericarp Cell Wall Components in Maize Weevil Resistance. Crop Sci. 2004, 44, 1546–1552. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalán, C.A.N. Phenylpropanoids from Maize Pericarp: Resistance Factors to Kernel Infection and Fumonisin Accumulation by Fusarium Verticillioides. Eur. J. Plant Pathol. 2013, 135, 105–113. [Google Scholar] [CrossRef]
- Bily, A.C.; Reid, L.M.; Taylor, J.H.; Johnston, D.; Malouin, C.; Burt, A.J.; Bakan, B.; Regnault-Roger, C.; Pauls, K.P.; Arnason, J.T.; et al. Dehydrodimers of Ferulic Acid in Maize Grain Pericarp and Aleurone: Resistance Factors to Fusarium Graminearum. Phytopathology 2003, 93, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef]
- Crascì, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating in Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef]
- Kröner, A.; Marnet, N.; Andrivon, D.; Val, F. Nicotiflorin, Rutin and Chlorogenic Acid: Phenylpropanoids Involved Differently in Quantitative Resistance of Potato Tubers to Biotrophic and Necrotrophic Pathogens. Plant Physiol. Biochem. 2012, 57, 23–31. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Jafra, S.; Oszmiański, J.; Szopa, J. Ectopic Expression of Anthocyanin 5-O-Glucosyltransferase in Potato Tuber Causes Increased Resistance to Bacteria. J. Agric. Food Chem. 2005, 53, 272–281. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Li, Y.; Wang, Y.; Li, M.; Wang, Y.; Ding, X.; Chu, Z. Rutin-Mediated Priming of Plant Resistance to Three Bacterial Pathogens Initiating the Early SA Signal Pathway. PLoS ONE 2016, 11, e0146910. [Google Scholar] [CrossRef]
- Filippone, M.P.; Diaz-Ricci, J.C.; Castagnaro, A.P.; Farías, R.N. Effect of Fragarin on the Cytoplasmic Membrane of the Phytopathogen Clavibacter Michiganensis. Mol. Plant-Microbe Interact. 2001, 14, 925–928. [Google Scholar] [CrossRef]
- Filippone, M.P.; Diaz Ricci, J.; Mamaní De Marchese, A.; Farías, R.N.; Castagnaro, A. Isolation and Purification of a 316 Da Preformed Compound from Strawberry (Fragaria Ananassa) Leaves Active against Plant Pathogens. FEBS Lett. 1999, 459, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of Flavonoids on Antimicrobial Activity of Microorganisms Present in Dental Plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.D.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Flavonoid Glycosides from Graptophyllum Grandulosum and Their Mechanism of Antibacterial Action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of Membrane Properties and Fatty Acids Biosynthesis-Related Genes in Escherichia Coli and Staphylococcus Aureus: Implications for the Antibacterial Mechanism of Naringenin. Biochim. Biophys. Acta Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza Inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Soudaminikkutty, R.; Narasumani, M.L.; Doble, M. Phenylpropanoids Inhibit Protofilament Formation of Escherichia Coli Cell Division Protein FtsZ. J. Med. Microbiol. 2011, 60, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass Spectrometry-Based Metabolomics Application to Identify Quantitative Resistance-Related Metabolites in Barley against Fusarium Head Blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Koutouan, C.; Clerc, V.L.; Baltenweck, R.; Claudel, P.; Halter, D.; Hugueney, P.; Hamama, L.; Suel, A.; Huet, S.; Merlet, M.H.B.; et al. Link between Carrot Leaf Secondary Metabolites and Resistance to Alternaria Dauci. Sci. Rep. 2018, 8, 13746. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, W.; Du, X.; Zhang, H.; Lin, L.; Xu, H. Chemical Constituents of Picea Neoveitchii. Phytochemistry 2011, 72, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Muth, D.; Narożna, D.; Mądrzak, C.; Stobiecki, M.; Kachlicki, P. Changes of Phenolic Secondary Metabolite Profiles in the Reaction of Narrow Leaf Lupin (Lupinus angustifolius) Plants to Infections with Colletotrichum Lupini Fungus or Treatment with Its Toxin. Metabolomics 2013, 9, 575–589. [Google Scholar] [CrossRef]
- Yogendra, K.N.; Kushalappa, A.C.; Sarmiento, F.; Rodriguez, E.; Mosquera, T. Metabolomics Deciphers Quantitative Resistance Mechanisms in Diploid Potato Clones against Late Blight. Funct. Plant Biol. 2015, 42, 284–298. [Google Scholar] [CrossRef]
- Mace, M.E.; Bell, A.A.; Stipanovic, R.D. Histochemistry and Identification of Flavanols in Verticillium Wilt-Resistant and -Susceptible Cottons. Physiol. Plant Pathol. 1978, 13, 143–149. [Google Scholar] [CrossRef]
- Del Rio, J.A.; Gonzalez, A.; Fuster, M.D.; Botia, J.M.; Gomez, P.; Frias, V.; Ortuño, A. Tylose Formation and Changes in Phenolic Compounds of Grape. Phytopathol. Mediterr. 2001, 40, 394–399. [Google Scholar]
- Aist, J.R.; Gold, R.E.; Bayles, C.J.; Morrison, G.H.; Chandra, S.; Israel, H.W. Evidence That Molecular Components of Papillae May Be Involved in Ml-o Resistance to Barley Powdery Mildew. Physiol. Mol. Plant Pathol. 1988, 33, 17–32. [Google Scholar] [CrossRef]
- Ziouti, A.; EL Modafar, C.; Fleuriet, A.; EL Boustani, S.; Macheix, J.J. Phenolic Compounds in Date Palm Cultivars Sensitive and Resistant to Fusarium Oxysporum. Biol. Plant. 1996, 38, 451–457. [Google Scholar] [CrossRef]
- Maher, E.A.; Bate, N.J.; Ni, W.; Elkind, Y.; Dixon, R.A.; Lamb, C.J. Increased Disease Susceptibility of Transgenic Tobacco Plants with Suppressed Levels of Preformed Phenylpropanoid Products. Proc. Natl. Acad. Sci. USA 1994, 91, 7802–7806. [Google Scholar] [CrossRef] [PubMed]
- Ardila, H.D.; Martínez, S.T.; Higuera, B.L. Levels of Constitutive Flavonoid Biosynthetic Enzymes in Carnation (Dianthus Caryophyllus L.) Cultivars with Differential Response to Fusarium Oxysporum f. Sp. Dianthi. Acta Physiol. Plant. 2013, 35, 1233–1245. [Google Scholar] [CrossRef]
- Melake-Berhan, A.; Butler, L.G.; Ejeta, G.; Menkir, A. Grain Mold Resistance and Polyphenol Accumulation in Sorghum. J. Agric. Food Chem. 1996, 44, 2428–2434. [Google Scholar] [CrossRef]
- Wang, Y.C.; Qian, W.J.; Li, N.N.; Hao, X.Y.; Wang, L.; Xiao, B.; Wang, X.C.; Yang, Y.J. Metabolic Changes of Caffeine in Tea Plant (Camellia Sinensis (L.) O. Kuntze) as Defense Response to Colletotrichum Fructicola. J. Agric. Food Chem. 2016, 64, 6685–6693. [Google Scholar] [CrossRef]
- Shadle, G.L.; Wesley, S.V.; Korth, K.L.; Chen, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid Compounds and Disease Resistance in Transgenic Tobacco with Altered Expression of L-Phenylalanine Ammonia-Lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef]
- Lei, K.J.; Zhang, L.; Du, X.Y.; An, Y.; Chang, G.H.; An, G.Y. A Chalcone Synthase Controls the Verticillium Disease Resistance Response in Both Arabidopsis Thaliana and Cotton. Eur. J. Plant Pathol. 2018, 152, 769–781. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering Flax with Increased Flavonoid Content and Thus Fusarium Resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef]
- Fofana, B.; Benhamou, N.; Mcnally, D.J.; Labbé, C.; Séguin, A.; Bélanger, R.R. Suppression of Induced Resistance in Cucumber Through Disruption of the Flavonoid Pathway. Phytopathology 2004, 95, 114–123. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Tian, Y.; Shen, L.; Xu, H.H. Antifungal Flavonoids from Ficus Sarmentosa Var. Henryi (King) Corner. Agric. Sci. China 2010, 9, 690–694. [Google Scholar] [CrossRef]
- Xu, S.; Yan, F.; Ni, Z.; Chen, Q.; Zhang, H.; Zheng, X. In Vitro and in Vivo Control of Alternaria Alternata in Cherry Tomato by Essential Oil from Laurus Nobilis of Chinese Origin. J. Sci. Food Agric. 2014, 94, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. Antifungal Action of Chlorogenic Acid against Pathogenic Fungi, Mediated by Membrane Disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.R.; Lee, D.G. Apigenin Induces Cell Shrinkage in Candida Albicans by Membrane Perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef]
- Moazeni, M.; Hedayati, M.T.; Nabili, M.; Mousavi, S.J.; Abdollahi Gohar, A.; Gholami, S. Glabridin Triggers Over-Expression of MCA1 and NUC1 Genes in Candida Glabrata: Is It an Apoptosis Inducer? J. Mycol. Med. 2017, 27, 369–375. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z.; et al. Maize Phenylalanine Ammonia-Lyases Contribute to Resistance to Sugarcane Mosaic Virus Infection, Most Likely through Positive Regulation of Salicylic Acid Accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef]
- Scholthof, K.-B. Tobacco Mosaic Virus. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Krcatović, E.; Rusak, G.; Bezić, N.; Krajacić, M. Inhibition of Tobacco Mosaic Virus Infection by Quercetin and Vitexin. Acta Virol. 2008, 52, 119–124. [Google Scholar]
- Ono, K.; Nakane, H.; Fukushima, M.; Chermann, J.C.; Barré-Sinoussi, F. Inhibition of reverse transcriptase activity by a flavonoid compound, 5, 6, 7-trihydroxyflavone. Biochem. Biophys. Res. Commun. 1989, 160, 982–987. [Google Scholar] [CrossRef]
- Lin, Y.; Anderson, H.; Flavin, M.T.; Pai, Y.S.; Mata-greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F. In Vitro Anti-HIV Activity of Biflavonoids Isolated from Rhus Succedanea and Garcinia Multiflora. J. Nat. Prod. 1997, 3864, 884–888. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of Chlorogenic Acid as a Resistance Factor for Thrips in Chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, N.; Kranthi, K.R.; Jadhav, D.R.; Kranthi, S.; Chandra, S. Influence of Foliar Chemical Compounds on the Development of Spodoptera Litura (Fab.) in Interspecific Derivatives of Groundnut. J. Appl. Entomol. 2004, 128, 321–328. [Google Scholar] [CrossRef]
- Leiss, K.A.; Cristofori, G.; van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G.L. An Eco-Metabolomic Study of Host Plant Resistance to Western Flower Thrips in Cultivated, Biofortified and Wild Carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef]
- Simlat, M.; Stobiecki, M.; Szklarczyk, M. Accumulation of Selected Phenolics and Expression of PAL Genes in Carrots Differing in Their Susceptibility to Carrot Fly (Psila Rosae F.). Euphytica 2013, 190, 253–266. [Google Scholar] [CrossRef][Green Version]
- Upasani, S.M.; Kotkar, H.M.; Mendki, P.S.; Maheshwari, V.L. Partial Characterization and Insecticidal Properties of Ricinus Communis L Foliage Flavonoids. Pest Manag. Sci. 2003, 59, 1349–1354. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Penetration-Enhancement Underlies Synergy of Plant Essential Oil Terpenoids as Insecticides in the Cabbage Looper, Trichoplusia Ni. Sci. Rep. 2017, 7, 42432. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Enhanced Cuticular Penetration as the Mechanism for Synergy of Insecticidal Constituents of Rosemary Essential Oil in Trichoplusia Ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- Feeny, P.; Sachdev, K.; Rosenberry, L.; Carter, M. Luteolin 7-O-(6″-O-Malonyl)-β-d-Glucopyranoside and Trans-Chlorogenic Acid: Oviposition Stimulants for the Black Swallowtail Butterfly. Phytochemistry 1988, 27, 3439–3448. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Nunney, L. How Do Plant Diseases Caused by Xylella Fastidiosa Emerge? Plant Dis. 2015, 99, 1457–1467. [Google Scholar] [CrossRef]
- Stoepler, T.M.; Wolf, T.K. North American Grapevine Yellows Disease: Current Knowledge and Management Recommendations for Wine Growers; Virginia Cooperative Extension: Blacksburg, VA, USA, 2013; Publication AREC-48P. [Google Scholar]
- Su, Q.; Chen, G.; Mescher, M.C.; Peng, Z.; Xie, W.; Wang, S.; Wu, Q.; Liu, J.; Li, C.; Wang, W.; et al. Whitefly Aggregation on Tomato Is Mediated by Feeding-Induced Changes in Plant Metabolites That Influence the Behaviour and Performance of Conspecifics. Funct. Ecol. 2018, 32, 1180–1193. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato Plant Flavonoids Increase Whitefly Resistance and Reduce Spread of Tomato Yellow Leaf Curl Virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Ferrandino, A.; Caciagli, P.; Kedrina, O.; Schubert, A.; Palmano, S. Metabolic and Transcript Analysis of the Flavonoid Pathway in Diseased and Recovered Nebbiolo and Barbera Grapevines (Vitis Vinifera L.) Following Infection by Flavescence Dorée Phytoplasma. Plant Cell Environ. 2014, 37, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Kumar, P.; Poonia, S. Larvicidal Activity and GC-MS Analysis of Flavonoids of Vitex Negundo and Andrographis Paniculata against Two Vector Mosquitoes Anopheles Stephensi and Aedes Aegypti. J. Vector Borne Dis. 2013, 50, 171–178. [Google Scholar] [PubMed]
- El-Akhal, F.; Guemmouh, R.; Zerrouq, F.; Ez Zoubi, Y.; El Ouali Lalami, A. Struggle against Vector-Borne Diseases: Phytochemical Screening and Larvicidal Activity of Hydro-Ethanolic Extract of Ocimum Basilicum in North East of Morocco against the Larvae of Malaria Vector Mosquito Anopheles Labranchiae (Diptera: Culicidae). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 284–288. [Google Scholar]
- Muema, J.M.; Njeru, S.N.; Colombier, C.; Marubu, R.M. Methanolic Extract of Agerantum Conyzoides Exhibited Toxicity and Growth Disruption Activities against Anopheles Gambiae Sensu Stricto and Anopheles Arabiensis Larvae. BMC Complement. Altern. Med. 2016, 16, 475. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Souza, M.S.R.; Teles, Y.C.F.; Oliveira, L.H.G.; Lima, J.B.; Conceição, A.S.; Nunes, F.C.; Silva, T.M.S.; De Fátima Vanderlei De Souza, M. New Sulphated Flavonoids and Larvicidal Activity of Helicteres Velutina K. Schum (Sterculiaceae). Molecules 2018, 23, 2784. [Google Scholar] [CrossRef]
- Ferreira, M.D.L.; Fernandes, D.A.; Nunes, F.C.; Teles, Y.C.F.; Rolim, Y.M.; da Silva, C.M.; de Albuquerque, J.B.L.; Agra, M.D.F.; de Souza, M.D.F.V. Phytochemical Study of Waltheria Viscosissima and Evaluation of Its Larvicidal Activity against Aedes Aegypti. Rev. Bras. Farmacogn. 2019, 29, 582–590. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Melo, D.R.; Franco, C.T.; Maturano, R.; Fabri, R.L.; Daemon, E. Evaluation of Eugenol and (E)-Cinnamaldehyde Insecticidal Activity against Larvae and Pupae of Musca Domestica (Diptera: Muscidae). J. Med. Entomol. 2020, 57, 181–186. [Google Scholar] [CrossRef]
- Kotewong, R.; Duangkaew, P.; Srisook, E.; Sarapusit, S.; Rongnoparut, P. Structure–Function Relationships of Inhibition of Mosquito Cytochrome P450 Enzymes by Flavonoids of Andrographis Paniculata. Parasitol. Res. 2014, 113, 3381–3392. [Google Scholar] [CrossRef]
- Hematpoor, A.; Liew, S.Y.; Chong, W.L.; Azirun, M.S.; Lee, V.S.; Awang, K. Inhibition and Larvicidal Activity of Phenylpropanoids from Piper Sarmentosum on Acetylcholinesterase against Mosquito Vectors and Their Binding Mode of Interaction. PLoS ONE 2016, 11, e155265. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea Pachycephala Rech.F. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 897. [Google Scholar]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The Influence of Light Quality on the Production of Bioactive Metabolites—Verbascoside, Isoverbascoside and Phenolic Acids and the Content of Photosynthetic Pigments in Biomass of Verbena officinalis L. Cultured in Vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and Biofilm Inhibitory Activities of Gallic, Caffeic, and Chlorogenic Acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Vosman, B.; van’t Westende, W.P.C.; Henken, B.; van Eekelen, H.D.L.M.; de Vos, R.C.H.; Voorrips, R.E. Broad Spectrum Insect Resistance and Metabolites in Close Relatives of the Cultivated Tomato. Euphytica 2018, 214, 46. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; De Girolamo, A.; Ippolito, A.; González-Candelas, L. Characterization of Genes Associated with Induced Resistance against Penicillium Expansum in Apple Fruit Treated with Quercetin. Postharvest Biol. Technol. 2010, 56, 1–11. [Google Scholar] [CrossRef]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; El Kasmi, F.; Yang, L.; Teixeira, P.; et al. A Gene Encoding Maize Caffeoyl-CoA O-Methyltransferase Confers Quantitative Resistance to Multiple Pathogens. Nat. Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- Muravnik, L.E.; Kostina, O.V.; Shavarda, A.L. Glandular Trichomes of Tussilago Farfara (Senecioneae, Asteraceae). Planta 2016, 244, 737–752. [Google Scholar] [CrossRef]
- Alvarez, D.V.; Hernández, M.S.; Hernández, V.A.G.; Engleman, E.M.; Damián Nava, A. Flavonoids in Psidium Guajava L. Leaves. Hortic. Int. J. 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Schöpker, H.; Kneisel, M.; Beerhues, L.; Robenek, H.; Wiermann, R. Phenylalanine Ammonia-Lyase and Chalcone Synthase in Glands OfPrimula Kewensis (W. Wats): Immunofluorescence and Immunogold Localization. Planta 1995, 196, 712–719. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the Role of Flavonoids in the Integrated Mechanisms of Response of Ligustrum Vulgare and Phillyrea Latifolia to High Solar Radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.P. Tissue Localization of Phenolic Compounds in Plants by Confocal Laser Scanning Microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
