Abstract
The pharmacological actions of benzylisoquinoline alkaloids are quite substantial, and have recently attracted much attention. One of the principle benzylisoquinoline alkaloids has been found in the unripe seed capsules of Papaver somniferum L. Although it lacks analgesic effects and is unrelated to the compounds in the morphine class, it is a peripheral vasodilator and has a direct effect on vessels. It is reported to inhibit the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) phosphodiesterase in smooth muscles, and it has been observed to increase intracellular levels of cAMP and cGMP. It induces coronary, cerebral, and pulmonary artery dilatation and helps to lower cerebral vascular resistance and enhance cerebral blood flow. Current pharmacological research has revealed that papaverine demonstrates a variety of biological activities, including activity against erectile dysfunction, postoperative vasospasms, and pulmonary vasoconstriction, as well as antiviral, cardioprotective, anti-inflammatory, anticancer, neuroprotective, and gestational actions. It was recently demonstrated that papaverine has the potential to control SARS-CoV-2 by preventing its cytopathic effect. These experiments were carried out both in vitro and in vivo and require an extensive understanding of the mechanisms of action. With its multiple mechanisms, papaverine can be considered as a natural compound that is used to develop therapeutic drugs. To validate its applications, additional research is required into its precise therapeutic mechanisms as well as its acute and chronic toxicities. Therefore, the goal of this review is to discuss the major studies and reported clinical studies looking into the pharmacological effects of papaverine and the mechanisms of action underneath these effects. Additionally, it is recommended to conduct further research via significant pharmacodynamic and pharmacokinetic studies.
Keywords:
Papaver somniferum; opium; benzylisoquinoline; papaverine; alkaloid; antiviral; anticancer; SARS-CoV-2 1. Introduction
Since ancient times, medicinal plants have played a key role in traditional medicine systems. Phytochemicals are being mined more frequently to find novel leads in the drug discovery process or to find better alternatives to existing ones [1,2,3,4,5]. Around 75% of the global population, mostly from developing countries, depend primarily on traditional herbal medicines due to their affordability and environmentally beneficial qualities [6].
According to estimates, 20% of plant species produce 12,000 alkaloids combined, many of which have been used in both traditional and modern-day medicine for centuries. Among these, about 2500 of the substances are members of a structurally diverse class of metabolites known as benzylisoquinoline alkaloids (BIAs), which also includes the opiate drugs morphine and codeine. Additionally, the benzylisoquinoline alkaloid family is a prominent class of plant-derived chemicals that has shown a wide range of pharmacological activity, including antibacterial, antitussive, antispasmodic, and anticancer properties [7,8].
A prominent benzylisoquinoline alkaloid is papaverine, which can be obtained from Papaverine somniferum L. (opium poppy). The opium alkaloids include papaverine, morphine, codeine, thebaine, noscapine, and narceine, as well as a small percentage of some other compounds. Various pieces of traditional research evidence have demonstrated opium alkaloids in Chinese and Indian herbal medicine to be effective at treating a variety of ailments, including chronic cough, rectum prolapse, diarrhea, dysentery, and gastrointestinal issues. In addition, papaverine has also been incorporated in therapeutic settings to treat erectile dysfunction, smooth muscle spasms, and spasms associated with gastrointestinal problems. Scientists have also found papaverine as a nonselective phosphodiesterase (PDE) inhibitor in mammals, boosting the amount of cAMP and cGMP available for cell signaling [9]. Hence, this secondary metabolite demands further exploration and requires pharmacological research investigations. Therefore, the current study focuses on the molecular mechanisms of papaverine’s pharmacological potential as they have been investigated in diverse experimental models.
2. Natural Source of Papaverine
Due to their phytochemical composition, members of the genus Papaver (family: Papaveraceae) are recognized for their therapeutic benefits. The most significant Papaver species that contributes phytochemicals for drug development is Papaver somniferum L. (opium poppy), which is highly produced in countries such as Afghanistan, Myanmar, Mexico, Laos, Turkey, Czechia, and Spain. Other commonly cultivated Papaver species include P. bracteatum Lindl. (Persian poppy), P. rhoeas L. (common poppy or corn poppy), P. dubium L., P. pseudo-orientale Medw., and P. orientale L., which are grown at high altitudes in north and northwest Iran, Russia, the Caucasia region, Europe, and America [10]. P. somniferum L. produces papaverine naturally in its unripe seed capsules. A total of 40 alkaloids have been found in the plant; however, morphine (10–15%), noscapine (4–5%), codeine (1–3%), papaverine (1–3%), and thebaine (1–3%) are the five primary alkaloids. The prevalence of papaverine in Indian species ranges from 0.5% to 3% [11].
3. Chemistry of Papaverine
Benzylisoquinoline alkaloids hold a prominent place in alkaloid chemistry as they serve as in vivo precursors to many other naturally occurring isoquinolines. They are either 1,2,3,4-tetrahydro, as in coclaurine and N-nororientaline, or fully aromatic, as in papaverine, palaudine, and escholamine. Ring A in the benzylisoquinoline alkaloids may possess two or three oxygenated substituents, while ring C has only one or two substituents [12].
Papaverine, also known by its IUPAC nomenclature 1-[(3,4-dimethoxy phenyl) methyl]-6,7-dimethoxyisoquinoline, is one of the principal benzylisoquinoline alkaloids found in P. somniferum [13,14]. Naturally, it is produced as a byproduct of morphine, codeine, and narcotine synthesis. Its m/z ratio was determined to be 340.15417 [15]. It is a neutral solid that only slightly dissolves in water [16]. There are four methoxy groups in papaverine. Even if the molecule lacks a TV-methyl group, it still functions as a tertiary base. It is considered a pyridine derivative because it may be reduced to a secondary amine by adding four hydrogen atoms, with the heterocyclic ring fused to a benzene ring [12] (Figure 1).
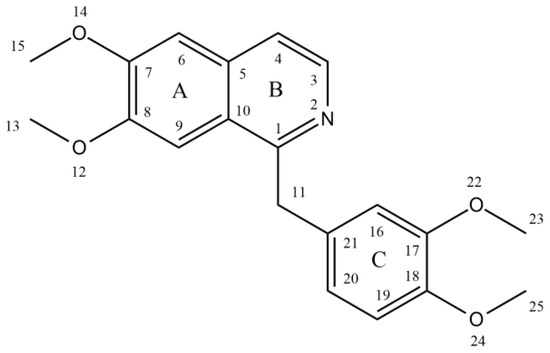
Figure 1.
Structure of papaverine.
Guido Goldschmiedt first illustrated the structure of the papaverine between the years of 1885 and 1898. By forming methiodide and demonstrating the presence of four methoxy groups per mole, he established the existence of a tertiary nitrogen atom. Under different conditions, the base was oxidized with potassium permanganate to produce various related compounds (Figure 2).
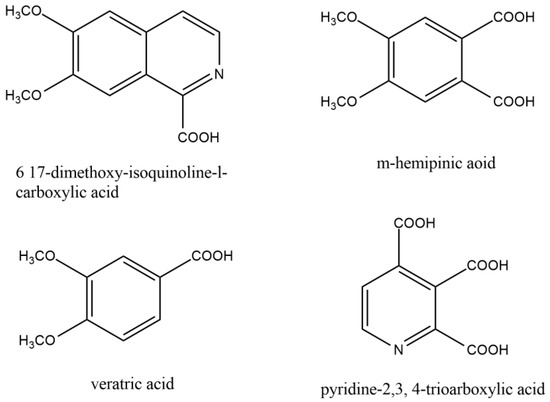
Figure 2.
Similar compounds of papaverine helped to elucidate the structure of papaverine.
The current structure was recognized as papaverine based on the evidence mentioned above and other relevant data. Following the successful synthesis of papaverine in 1909, Pictet and Gams validated the molecular structure [14].
4. Biosynthesis of Papaverine
Two units of tyrosine contribute as the precursors for the biosynthesis of papaverine, and the intermediate products include (S)- norcoclaurine, laudanine, norlaudanine, reticuline, norreticuline, tetrahydropapaverine, and dihydropapaverine. Recent investigations revealed that the primary pathway of papaverine biosynthesis in the opium poppy has been identified by systematic silencing of benzylisoquinoline alkaloid biosynthetic genes.
There are two suggested metabolic pathways for (S)-norcoclaurine. One involves only N-demethylated intermediates (the NH pathway), whereas the other involves (S)-reticuline and involves a number of N-methylated intermediates (the NCH3 pathway) [13,17,18]. The NH route advances via (S)- norreticuline [13,19,20] (Figure 3), whereas the NCH3 route involves (S)-reticuline [13,15,20,21] (Figure 4).
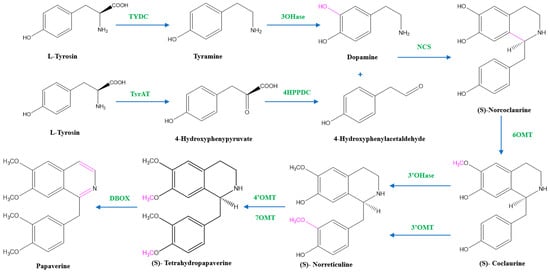
Figure 3.
The NH pathway of papaverine synthesis. TYDC = tyrosine decarboxylase, TyrAT = L-tyrosine aminotransferase, 4HPPDC = 4-hydroxyphenylpyruvate decarboxylase, 3OHase = tyramine 3-hydroxylase, NCS = norcoclaurine synthase, 6OMT = norcoclaurine-6-O-methyltransferase, 3’OHase = 3’ hydroxylase, 3’OMT = 3′-O-methyltransferase, 4’OMT = 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase, 7OMT = norreticuline 7-O-methyltransferase, DBOX = dihydrobenzophenanthridine oxidase.
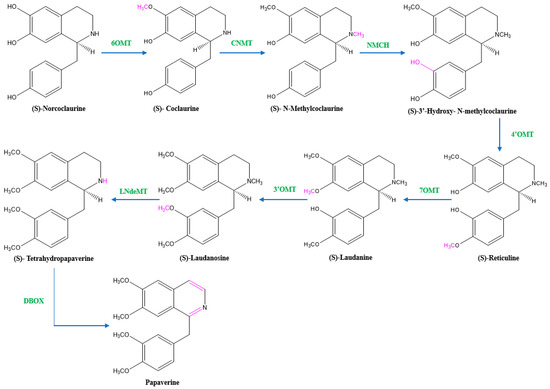
Figure 4.
The NCH3 pathway of papaverine synthesis. 6OMT = norcoclaurine-6-O-methyltransferase, CNMT = coclaurine-N-methyltransferase, NMCH = (S)-N-methylcoclaurine 3′-hydroxylase, 4’OMT = 4′-O-methyltransferase, 7OMT = reticuline 7-O-methyltransferase, 3’OMT = 3′-O-methyltransferase, LNdeMT = laudanosine N-demethylase, DBOX = dihydrobenzophenanthridine oxidase.
The first step in papaverine biosynthesis is the condensation of two L-tyrosine derivatives, 4-hydroxyphenylacetaldehyde (4HPAA) and dopamine, which is accomplished through decarboxylation, meta-hydroxylation, and transamination to produce the precursor to all other benzylisoquinoline alkaloids, (S)-norcoclaurine. Tyrosine decarboxylase (TYDC) and tyramine 3-hydroxylase (3OHase) transform L-tyrosine into tyramine and dopamine, respectively. L-tyrosine can be transaminated by L-tyrosine aminotransferase (TyrAT) in the production of 4HPAA, and then decarboxylated by an enzyme identified as 4-hydroxyphenylpyruvate decarboxylase (4HPPDC). Norcoclaurine synthase (NCS) is the enzyme that catalyzes the condensation of (S)-norcoclaurine from 4HPAA and dopamine.
Norcoclaurine-6-O-methyltransferase (6OMT) first transforms (S)-norcoclaurine into (S)-coclaurine. In the NH pathway, (S)-coclaurine first undergoes 3′ hydroxylation by 3′ hydroxylase (3′OHase) and then is converted to (S)-norreticuline by 3′-O-methyltransferase (3′OMT). On the other hand, in the NCH3 pathway, (S)-coclaurine is taken up by coclaurine N-methyltransferase (CNMT) to yield (S)-N-methylcoclaurine. (S)-N-methylcoclaurine is hydroxylated to 3′-hydroxy-N-methylcoclaurine by (S)-N-methylcoclaurine 3′-hydroxylase (NMCH), which is then transformed into (S)-reticuline by 3′-hydroxy-N-Methylcoclaurine 4′-O-methyltransferase (4′OMT). It is interesting to note that only NMCH has been reported to exhibit strict stereoisomer and substrate specificity, accepting only (S)-N-methylcoclaurine and rejecting either the corresponding (R)-N-methylcoclaurine or N-desmethyl compounds. As a distinction, the O- and N-methyltransferases often accept a wide range of (R)- and (S)-tetrahydroisoquinolines [19]. The enzyme reticuline 7-O-methyltransferase (7OMT) can further methylate reticuline to produce laudanine, which can then be fully O-methylated to laudanosine by 3′-O-methyltransferase.
The final steps in papaverine biosynthesis comprise the oxidation of the fully O-methylated and N-desmethyl molecule tetrahydropapaverine by dihydrobenzophenanthridine oxidase (DBOX).
Advanced quantum chemical density functional theory (DFT) calculations, as well as diffuse reflectance (Ds), experimental electronic absorption (EAs), matrix-associated laser desorption ionization (MALDI) coupled with Orbitrap imaging mass spectrometry (MS), fluorescence spectroscopy (Fs), and circular dichroic (CD) have been used for theoretical and experimental elucidation of the papaverine biosynthetic pathway via (S)-reticuline (the NCH3 pathway) [19,20,22].
5. Mechanism of Action of Papaverine
Papaverine is recognized as the most effective smooth muscle relaxant, as it acts directly on smooth muscle by exerting a strong vasodilating effect. It has been observed to boost intracellular levels of cAMP and cGMP by blocking the cAMP and cGMP phosphodiesterase in smooth muscles (Figure 5) [23,24,25,26,27]. Inhibiting the release of calcium from the intracellular space and obstructing calcium ion channels in the cell membrane are two other ways that papaverine may work [28].
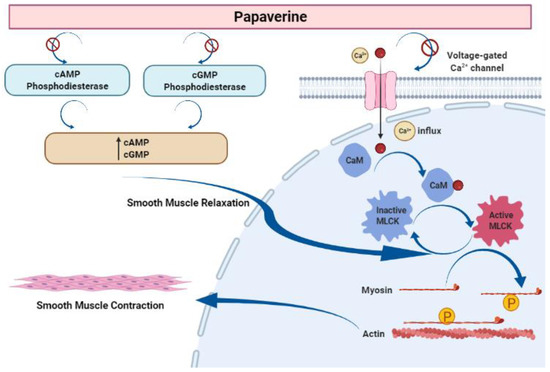
Figure 5.
Mechanism of action of papaverine in smooth muscle relaxation. Smooth muscle contraction requires five steps: After the increase in intracellular Ca2+ concentration from the extracellular fluid, these ions bind to a protein called calmodulin (CaM). This complex activates a protein called myosin light-chain kinase (MLCK) (papaverine inhibits this step), which subsequently phosphorylates light chains of myosin heads, increasing the myosin ATPase activity. Finally, active myosin cross-bridges slide along actin and create muscle tension to contract the cell.
6. Pharmacological Properties of Papaverine
Papaverine has been demonstrated to be a particular PDE10A phosphodiesterase inhibitor, which is mostly found in the striatum of the brain. The chronic injection of it into mice resulted in motor and cognitive deficits as well as elevated anxiety. Other studies have suggested that it may also have an antipsychotic effect. However, not all research has supported this theory [29,30]. Nevertheless, papaverine has been approved for the treatment of GIT, bile duct, and ureter spasmolytic disorders [31].
6.1. Activity against Erectile Dysfunction (ED)
PDE5 inhibitors are used as the first-line therapy for ED [32]. As a popular vasodilator, their usage has been observed to improve penile impotence [33]. Its ability to treat ED and impotence has been known for a long time. By far, the largest number of studies have been published demonstrating the effectiveness of papaverine in treating erectile dysfunction (ED) and impotence [34]. Three simultaneous and synergistic processes work together to maintain normal erectile function: (1) relaxation of the cavernosal smooth muscle, (2) an increase in penile arterial inflow caused by neurological activity, and (3) a restriction of venous outflow from the penis. These processes occur due to the following: (1) the activation of cGMP-dependent protein kinase G (PKG); (2) the activation of cGMP-dependent ion channels that reduce intracellular Ca2+ by Ca2+ sequestration and/or extrusion; (3) the opening of K+ channels, causing the hyperpolarization of corpus cavernosum smooth muscle cells; and (4) the activation of myosin light-chain phosphatases. The objective of ED pharmacotherapy is to develop novel pharmacological targets that inhibit the contractile systems (α-adrenoceptor antagonists) and activate (e.g., prostaglandin E1 (PGE1), NO-donors, and forskolin) or augment (e.g., PDE inhibitors and gene therapy) the vasodilatory systems to produce greater trabecular smooth muscle relaxation of the corpora cavernosa [35,36,37] (Figure 6).
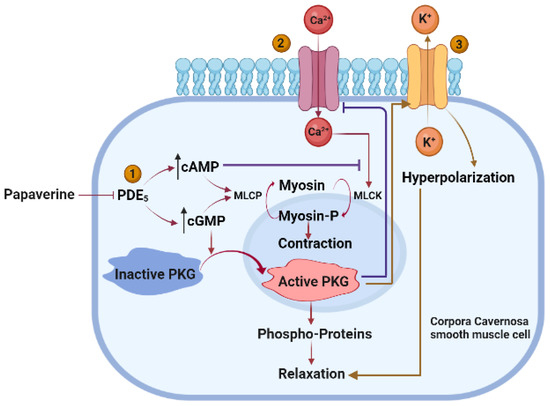
Figure 6.
Mechanism of action of papaverine in relaxation of cavernosal smooth muscle. Papaverine blocks cAMP and cGMP phosphodiesterase to raise the concentration of cAMP and cGMP, which further releases MLCP that dephosphorylates myosin, resulting in smooth muscle relaxation and increased cGMP that activates PKG and leads to smooth muscle relaxation (1). Activated PKG lowers Ca2+ influx. Ca2+ activates MLCK, which contracts smooth muscle via myosin phosphorylation (2). Papaverine induces efflux of K+ with subsequent hyperpolarization and relaxation of corpora cavernosa smooth muscle cells.
Intracavernous papaverine was found to play a vital role in the management of male erectile failure in a study on 48 patients with psychogenic impotence. Intracavernous papaverine induces erection by several mechanisms. It relaxes the smooth muscles of sinusoids and increases the arterial flow to the corpora. The use of papaverine has also been linked to increased venous outflow resistance [34,38]. Research conducted on 17 men with organic impotence revealed papaverine gel to cause a noticeably larger cavernous artery diameter [39]. Although its use as a monotherapy to treat impotence was initially questioned due to priapism being a significant side effect (which occurs in 15–18% of patients), physicians soon discovered that this side effect was dose-dependent and only occurs in patients with neurogenic impotence [40,41]. To lessen toxicity and priapism, papaverine was combined with phentolamine and PGE1 [35]. By raising the amount of intracellular cyclic adenosine monophosphate, relaxing the smooth muscle of the cavernous body and helicine arteries, and inhibiting the enzyme phosphodiesterase, papaverine and phentolamine were able to significantly increase erections in various experiments [42,43]. It has been observed that papaverine’s effectiveness is equal to that of oral sildenafil in a different trial that involved 31 male patients who had ED injuries and were in the early stages of paraplegia [44]. Recently, it was discovered that a new topical formulation using lyotropic liquid crystal (LLC) systems and papaverine-HCl was a suitable and efficient substitute for the injectable formulation in the treatment of ED [34]. According to a study conducted in vitro, the substance increases the motility of post-thaw sperm [34].
6.2. Activity against Pulmonary Vasoconstriction
The protective effects of papaverine on the lungs have been demonstrated through various mechanisms. In a model of pulmonary embolism caused by autologous blood clots in rabbit lungs that were isolated and perfused, papaverine was discovered to lessen pulmonary vasoconstriction and edema. In the pulmonary vascular bed, papaverine can diminish the vasoconstrictor response to ET-1, TxA2, and serotonin without altering their release. It is generally known that PDE inhibitors also have antiplatelet effects due to their ability to raise cAMP levels. Papaverine, in particular, has been demonstrated to reduce platelet aggregation brought on by ADP as well. Such an outcome might have contributed to the better results observed in the papaverine-treated group in [45]. It was discovered that it inhibits voltage-gated Ca2+ channels in a concentration-dependent manner, resulting in the relaxation of tracheal smooth muscle [46]. Papaverine with nifedipine effectively decreased the pulmonary artery vasoconstriction brought on by ECS (Euro-Collins solution). The elevation in cAMP caused by papaverine may improve lung preservation. Additionally, it has been noted to reduce Ca2+ influx via cell membranes [34].
6.3. Postoperative Vasospasm
To significantly minimize postoperative vasospasms and maintain regular vascular morphology throughout antispasmodic therapy, papaverine-loaded electrospun fibrous membranes were developed [47]. In a rabbit model, it was discovered that the intra-arterial route is more effective for lowering autologous blood-induced cerebral vasospasms [48]. For a very long time, papaverine has been used to prevent vasospasms induced by subarachnoid hemorrhage [49]. In numerous investigations, including in patients who had aneurysmal subarachnoid hemorrhages, the effectiveness of papaverine in avoiding vasospasms was validated. Papaverine can be given either on its own or in conjunction with transluminal balloon angioplasty. In these circumstances, papaverine has been found to improve cerebral oxygenation, increase the angiographic vessel diameter, decrease the extended cerebral circulation time, and boost cerebral blood flow in an effort to prevent cerebral infarction. Here, a significant barrier is the short-lived nature of papaverine [50,51,52,53]. A sustained-release formulation that can be implanted intracranially might minimize this, and it would also lower the likelihood of hypotension during surgery [54]. Nevertheless, a different trial involving 31 patients with a subarachnoid hemorrhage-related vasospasm found no additional benefits from papaverine when compared to the medical treatment of vasospasms alone. The authors came to the conclusion that changing the time or indications for therapeutic intervention could be advantageous [49]. Another retrospective study on nine consecutive patients with acute large-artery occlusion treated with a stent retriever and intra-arterial papaverine demonstrated an increase in the caliber and flow of the infused arteries, suggesting a safe and effective method of treating cerebral vasospasms following mechanical thrombectomy in acute ischemic stroke [55]. Another investigation of 27 patients with a subarachnoid hemorrhage-related symptomatic vasospasm discovered that intra-arterial papaverine consistently reduces cerebral circulation time [56]. Following intra-arterial infusion of papaverine, individuals with symptomatic vasospasms showed an improvement in cerebral oxygenation as well as a reduction in cerebral lactic acidosis [57].
6.4. Antiviral Properties
Papaverine is also recognized for its antiviral activities against different human viruses and the murine retrovirus, MSV-Harvey. It has been hypothesized that, at least for the measles virus, interference with cellular DNA synthesis directly, competitive and reversible binding to the DNA molecule, or an increase in endogenous cAMP will impede viral RNA synthesis and the phosphorylation of viral proteins [58]. In a study, papaverine suppressed the viral growth of measles in neuroblastoma cells by inhibiting the synthesis of viral RNAs in a dose-dependent and reversible manner [59]. It was reported to show effective antiviral activity by inhibiting the replication of the CMV virus. The mechanism of action underlying the relaxing effect of these drugs on smooth muscle may prevent at least the initial cell rounding, and it is possible that a critical physiologic event(s) (e.g., the rise in intracellular free Ca2+) may be important to both early cellular responses and CMV replication [60]. Papaverine inhibited the replication of HIV in H9 cell lines by blocking the RT activity and p24 expression. It also showed inhibiting activity in the peripheral blood mononuclear cell (PBMC) culture by influencing the viral markers RT and p24 [58,61]. It inhibited the replication of the measles virus in neural cells [62]. Papaverine showed a dose-dependent inhibition of multiple strains of influenza virus when A/WSN/33 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40 were used in a study [63]. In a very recent study, papaverine revealed its ability to inhibit the SARS-CoV-2 cytopathicity in the human epithelial colorectal adenocarcinoma cell line, Caco-2 [64] (Table 1).

Table 1.
Antiviral properties of papaverine.
6.5. Cardiovascular Activity
Papaverine exhibited potent cardioprotective effects by diverse mechanisms. It directly stimulated the sinus rate and atrial contractility by demonstrating positive chronotropic and inotropic effects on an isolated atrial preparation from a dog, which again points to the inhibition of PDE and accumulated cAMP. Additionally, papaverine may partially trigger the release of catecholamines from adrenergic nerve fibers and may interfere with the process of adenosine uptake. It is hypothesized that papaverine may directly stimulate atrial contractility and SA nodal pacemaker activity [65]. Similar effects were found in another study where papaverine displayed positive inotropic effects on atrial preparation, whereas in ventricular preparation, it did not affect the force of contraction significantly [66]. Papaverine inhibits both hKv1.5 and native hKv1.5 channels in a concentration, voltage, state, and time-dependent manner. This interaction shows that papaverine may change cardiac excitability in vivo [67].
6.6. Anti-Inflammatory Activity
Through the cAMP/PKA and MEK/Erk pathways, papaverine reduced the expression of proinflammatory factors and inhibited the activation of primary retinal microglia caused by LPS, and the MEK/Erk pathway may be partially regulated by cAMP/PKA, which can provide theoretical and experimental support for its protection of the central nervous system [68]. Yoshikawa et al. first noticed that papaverine could prevent the release of TNF-α and IL-1β in LPS-induced BV2 cells [69]. Similar activities were reported in another study where papaverine prevented the production of nitric oxide and proinflammatory cytokines in LPS-stimulated microglia [31]. Furthermore, it appeared to have anti-inflammatory effects in mouse models by inhibiting high mobility group box 1-mediated inflammatory responses [70]. Similar effects were demonstrated in LPS-stimulated macrophages and microglia where papaverine suppressed TNF-α [68,71], IL1β, and the NF-κB signaling pathway [72], thus proving its potential to treat neurodegenerative diseases.
6.7. Anticancer Activity
It was found that papaverine effectively induced a morphological change and inhibited proliferation and the invasive potential of human prostate cell lines PC-3, DU145, and LNCaP primarily through its PDE-inhibiting capability, which resulted in raised cAMP levels [73]. Similar effects were reported on the LNCaP cell line due to a synergistic effect induced by a combination of papaverine and prostaglandin E2 (PGE2) [74] and on PC-3 by inducing apoptosis and cell cycle arrest along with the downregulation of NFkB and the PI3K/Akt signaling pathway [75]. The phytochemical has been reported to exhibit cytotoxic effects on cancerous HT29, T47D, and HT1080 cell lines without affecting the noncancerous mouse NIH3T3 cell line as compared to doxorubicin, a widely used anticancer drug. The mechanism behind it was selective DNA damage and the induction of apoptosis on cancerous cell lines [76]. It expressed a cytotoxic effect against cancer stem cells, especially human breast cancer cell line MCF-7, by arresting the cell cycle in the G1 phase and inducing apoptosis [77]. Antiproliferative activity of the compound was reported on hepatocarcinoma cell line HepG-2 as it affected the telomerase activity [78]. It has been proven to be an effective radiosensitizing agent that reduces the rate of oxygen consumption through the inhibition of mitochondrial complex I. Thus, the compound was found to improve the response to radiation therapy and is a potential candidate for tumor hypoxia treatment [9]. Papaverine is found to prevent cell migration and delay zebrafish development by suppressing the kit-signaling pathway [79]. The compound significantly inhibited the proliferation of human glioblastoma cell lines U87MG and T98G and the tumor volume in the U87MG xenograft mouse model [80,81]. The papaverine–Au(III) complex was reported to have better cytotoxic activities against human breast cancer MCF-7 cells and hepatocellular carcinoma HepG-2 cells than papaverine itself, and the inhibiting ability was higher than that of cisplatin against MCF-7 [16]. Caroverine, which is one derivative of papaverine, prevented the expression of VEGF, which is a well-known tumor-promoting factor [82]. In another study, a papaverine oxidation product that is a 6a,12a-diazadibenzo-[a,g]fluorenylium derivative inhibited the MCF-7 cell line by blocking the G0/G1 phase of the cell cycle and telomerase activity [83]. An investigation involving S. cerevisiae and docking and molecular dynamic simulation studies showed evidence that papaverine induces ROS-mediated apoptosis and inhibits Bcr-Abl downstream signaling [84] (Table 2).

Table 2.
Anticancer properties of papaverine.
6.8. Neuroprotective Effect
Papaverine may also exert neuroprotective effects to treat neuropsychiatric diseases such as schizophrenia and depression [85,86]. In a clinical trial involving three female patients with tardive dyskinesia, it was revealed that daily 300 to 600 mg doses of papaverine improved the dyskinesia condition without showing any side effects. The authors concluded that the effects were due to the inhibition of the dopamine pathway, which might be the reason for dyskinetic movements [87]. The study was again conducted with a larger number of patients via the oral administration of sustained-release 150 mg papaverine capsules. Two out of nine patients showed clinical improvements [88]. Oro-facial dyskinesia was improved in another clinical trial conducted on 150 patients [89]. Papaverine was also found to potentiate nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells in a concentration-dependent manner [90]. By significantly raising the levels of BDNF, synapsin-IIa, DCX, pCREB, IL-10, and GSH in various brain regions while significantly lowering the levels of TNF-α, IL-6, and TBARS, the drug was found to restore the basic behavioral phenotype in autism spectrum disorder [9]. Another study revealed that the substance may be useful in reducing the ischemic infarct volume, suggesting that it may be used to treat cerebral ischemia in clinical practice [91]. By modifying the NF-B and CREB signaling pathways, it prevents the activation of the NLRP3 inflammasome, which reduces microglial activation and neuronal cell death. As a result, it could be a promising treatment for Parkinson’s disease, which is exacerbated by systemic inflammation [92]. In the subacute MPTP/P animal model of Parkinson’s disease, the data revealed that papaverine reduces neuroinflammation and MMP-3 production, which prevents dopaminergic neuronal cell death and α-synuclein aggregation. In light of this, it might be a viable medication for the management of Parkinson’s disease [93]. Papaverine enhanced cognitive function in a mouse model with Huntington’s disease by inhibiting PDE10, resulting in cAMP-responsive element-binding protein (CREB) phosphorylation and GluA1 [94]. It also provided efficient protection to the spinal cord during descending thoracic and thoracoabdominal aortic aneurysm repair surgery by perfusing the spinal cord [1]. In another study, papaverine revealed its direct effect on synaptic vesicles, which was exhibited via the increase in norepinephrine and dopamine-β-hydroxylase from isolated perfused cat spleen [95]. Furthermore, papaverine temporarily increased sublingual microcirculatory blood flow in septic shock patients who needed vasoconstrictors to maintain blood pressure during fluid resuscitation without affecting systemic hemodynamics [96].
6.9. Gestational Activity
In the 1990s, research on papaverine and its derivatives revealed that they could shorten the time needed for the first stage of labor. A specific inhibitor of PDE 4, drotaverine hydrochloride, is a homolog of papaverine. Because of its ability to relax smooth muscles, it was found to be beneficial in accelerating cervical dilation [97]. Madhu et al. found that women who were treated with drotaverine through the latent phase of labor had a significantly shorter time between the administration of the medication and the delivery of the fetus compared to women who were treated with a placebo. The study involved 146 women who gave birth vaginally (182 min compared to 245 min with a placebo) [98]. Another retrospective comparative investigation of 498 pregnant women indicated that short-term prenatal exposure to papaverine adjusted for indication was not linked to preterm births, cesarean birth, reduced birth weight, small gestational age, or perinatal death [99]. Additionally, it was claimed that the medication worked well to lower pre-eclamptic patients’ blood pressure [100].
6.10. Other Activities
Papaverine hydrochloride is equally as effective as sodium diclofenac for the short-term relief of acute renal colic pain, and it may be advantageous in patients with contraindications according to a prospective, single-blind clinical study that involved 86 patients with acute renal colic who were given 120 mg intravenous papaverine hydrochloride [101]. Another study found that the injection of alprostadil and papaverine into the spermatic cord protected against ischemia/reperfusion injury following right-side testes torsion and reduced histological alterations following testicular ischemia-reperfusion injury [102].
7. Limitations of the Study
Executing an ideal drug discovery and development process is one of the primary challenges for the pharmaceutical research community. ADME is a critical step in the drug design process that investigates the fate of a drug molecule after ingestion. Notably, drug metabolism studies are critical processes for optimizing the lead compounds with optimal PK/PD features, identifying new chemical entities based on the discovery of active metabolites, minimizing potential safety liabilities due to the development of reactive or toxic metabolites, comparing preclinical metabolism in animals with humans to guarantee that animals used in experiments have the potential to adequately cover human metabolites and support human dose predictions, and so on [103]. However, ADME and the PK/PD parameters of papaverine were not evident in this review. When it comes to drug attrition during the clinical stage of development, compound failure rates because of the toxicity prior to human testing are relatively high, and they may account for up to 30% of the loss. In order to establish an anticipated safe dose range and to gather knowledge on drug distribution, organ-specific toxicity, and metabolism, toxicology studies in at least two nonhuman species are typically utilized [104]. The toxicological parameters of papaverine were not defined in this review. Proper translation and determination of the maximum recommended starting dose in humans is a critical task in new drug development and research [105]. No specific dose of papaverine was studied in this review. Moreover, study data are not available for use in lactating mothers and pediatric and geriatric patients. The three-dimensionality of molecules is intimately related to the clinical success of drug candidates [106], which was not elaborated on in this review. The efficacy of traditional medicines is frequently the consequence of a synergistic interaction between numerous components, targets, and pathways [107]. This review did not include the positive or negative synergistic effects of possible analogs of papaverine found in opium. Possible side effects of papaverine include priapism, penile fibrosis, and arrythmia [108]. No studies have been conducted on whether these side effects can be utilized as a secondary usage via repurposing; e.g., metformin is the first-line therapy of type II diabetes, and it can be repurposed as an antiobesity drug for both diabetic and nondiabetic patients [109,110].
8. Discussion and Future Recommendations
Papaverine has been proven to be a high-value opioid alkaloid in the field of therapeutics either in solitude or in combination with other metabolites/molecules [9]. It was approved by the Food and Drug Administration (FDA) of the United States as a vasodilator to be predominantly used in the treatment of cerebral vasospasms and coronary circulation [108]. Several preclinical and clinical studies also demonstrated its potential efficacy against pulmonary vasoconstriction, erectile dysfunction, postoperative vasospasms, some particular viral infections, inflammation, cardiac excitability, carcinoma, neurological disturbances, gestational difficulties, pre-eclampsia, acute renal colic pain, and ischemia-reperfusion injury, as well as other muscle spasm-oriented complications [87,88,91,101]. Some of the notable mechanisms underlying the different pharmacological actions include vasodilation, the activation of cGMP and cAMP-dependent biomolecules, the inhibition of vasoconstrictor responses to biomolecules, interference with certain viral nucleic acids, the inhibition of cytokine release (such as TNF-α, IL-1β, and NF-κB), the apoptosis of diseased cells, the potentiation of neurite outgrowth, the alteration of different biomolecular signaling pathways, etc., which are discussed throughout this review. As a consequence, the multiple bioactive capabilities of papaverine suggest that it may also be an effective natural phytoconstituent in disease management. Moreover, synthetic drugs consist of several drawbacks, such as a lack of bioavailability, cost-effective issues, drug resistance issues, unexpected adverse effects, etc. [111]. To combat these drawbacks, there is a need to search for lead compounds among the natural substances [112]. Plants are a present from the Earth that have been providing us vital phytochemicals for thousands of years [113,114]. Bioactive phytochemicals from natural sources play pivotal roles in drug discovery and development. Almost 80% of all currently available drugs are either directly derived from plant or are a modified version [115,116]. Alkaloids are a very important class of bioactive phytochemicals that play a significant role in drug discovery [117]. Thus, papaverine is a potential natural drug candidate that may be utilized in the near future. Researchers should carry out several studies on the papaverine alkaloid, including by studying the determination and revision of its PK/PD parameters, the therapeutic index, safety and toxicological profiles, dosage, drug–drug interactions, drug-food interactions, and other important parameters. Considering all these factors, papaverine should be subjected to extensive research to establish it as a novel drug and/or lead compound. This review will provide future researchers with important insights for further studies on this conspicuous alkaloid.
9. Conclusions
The majority of the alkaloids isolated from the opium poppy seed, such as morphine and codeine, have analgesic properties; nevertheless, papaverine varies from the opium group of alkaloids both chemically and therapeutically. While the majority of the primary alkaloid chemicals derived from the opium poppy are narcotic and have an analgesic effect, the majority of papaverine’s pharmacological usage is as a non-narcotic, non-analgesic smooth muscle relaxant and vasodilator. Papaverine is a recognized inhibitor of phosphodiesterases. Papaverine is FDA-approved and is already in clinical use as a vasodilator. It is gaining more and more attention for use in other biological activities. Within the scope of this work, we described multiple bioactive properties of papaverine in addition to the molecular mechanisms behind such activities. Both in vitro and in vivo as well as clinical studies showed that papaverine possessed considerable pharmacological properties besides its vasodilator effects. As a result, it is a vital potential candidate both for the discovery of novel drugs and the development of the existing drug. As a matter of fact, its antiviral and anticancer actions both exhibit unique mechanisms of action that show considerable potential for treating their respective illnesses, which demonstrates that papaverine is a prominent candidate for use in the research and development of new antiviral and anticancer medications. In addition, toxicological research must be carried out to establish the substance’s safety for use in other pharmaceutical applications.
Author Contributions
Conceptualization, S.A. (Sania Ashrafi), S.A. (Safaet Alam), A.S., A.R. and B.K.; investigation, S.A. (Sania Ashrafi), S.A. (Safaet Alam), A.S., A.R., N.U.E., F.T.R., M.M. and M.N.P.; writing—original draft preparation, S.A. (Sania Ashrafi), S.A. (Safaet Alam), A.S., A.R., N.U.E., F.T.R. and T.S.; writing—review and editing, S.A. (Sania Ashrafi), S.A. (Safaet Alam), N.U.E., T.S. and B.K.; visualization, S.A. (Sania Ashrafi) and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0038), and the innovation network support Program through the INNOPOLIS funded by Ministry of Science and ICT (2022-IT-RD-0205-01-101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Sania Ashrafi is grateful to Rumman Adib for his continuous support and motivation throughout the work.
Conflicts of Interest
There are no known financial or research-based conflicts of interest among the authors of this research work and article.
References
- Arora, L.; Hosn, M.A. Spinal Cord Perfusion Protection for Thoraco-Abdominal Aortic Aneurysm Surgery. Curr. Opin. Anaesthesiol. 2019, 32, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Grdina, D.J.; Murley, J.S.; Kataoka, Y. Radioprotectants: Current Status and New Directions. Oncology 2002, 63, 2–10. [Google Scholar] [CrossRef]
- Weiss, J.F.; Landauer, M.R. Protection against Ionizing Radiation by Antioxidant Nutrients and Phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Alam, S.; Emon, N.; Shahriar, S.; Richi, F.; Haque, M.; Islam, M.; Sakib, S.; Ganguly, A. Pharmacological and Computer-Aided Studies Provide New Insights into Millettia Peguensis Ali (Fabaceae). Saudi Pharm. J. 2020, 28, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, S.; Alam, S.; Emon, N.; Ahsan, M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis. Molecules 2022, 27, 5972. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Emdadul, M.; Mukul, H.; Millat, M.S.; Saif, M.; Rashed, U. Phytochemical Nature and Pharmacological Evaluation of Chloroform Extract of Pandanus Fascicularis L. (Fruits): An in Vivo Study Open Access. Artic. J. Bioanal. Biomed. 2017, 9, 4. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Fossati, E.; Bourgeois, L.; Burton, E.; Gold, N.D.; Martin, V.J.J. Reconstituting Plant Secondary Metabolism in Saccharomyces Cerevisiae for Production of High-Value Benzylisoquinoline Alkaloids. Methods Enzymol. 2016, 575, 195–224. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Facchini, P.J. Alkaloid Biosynthesis: Metabolism and Trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.T.; Koong, A.C.; Giaccia, A.J.; Yu, B.; et al. Papaverine and Its Derivatives Radiosensitize Solid Tumors by Inhibiting Mitochondrial Metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.J.; Pentea, M.; Sarac, I.; Küşümler, A.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxid. Med. Cell. Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef]
- Peter, K. V Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0857095684. [Google Scholar]
- Shamma, M. The Isoquinoline Alkaloids Chemistry and Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Desgagné-Penix, I.; Facchini, P.J. Systematic Silencing of Benzylisoquinoline Alkaloid Biosynthetic Genes Reveals the Major Route to Papaverine in Opium Poppy. Plant J. 2012, 72, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mi, J.Q.; He, J.T.; Guo, Z.Q.; Zhao, M.P.; Chang, W.B. Development of an Indirect Competitive ELISA for the Determination of Papaverine. Talanta 2005, 66, 1005–1011. [Google Scholar] [CrossRef]
- Han, X.; Lamshöft, M.; Grobe, N.; Ren, X.; Fist, A.; Kutchan, T.; Spiteller, M.; Zenk, M. The Biosynthesis of Papaverine Proceeds via (S)-Reticuline. Phytochemistry 2010, 71, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Srivastava, A.; Agrawal, L.; Raj, R.; Jaidi, M.; Raj, S.K.; Gupta, S.; Dixit, R.; Singh, P.C.; Tripathi, T.; Sidhu, O.P.; et al. Ageratum Enation Virus Infection Induces Programmed Cell Death and Alters Metabolite Biosynthesis in Papaver Somniferum. Front. Plant Sci. 2017, 8, 1172. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Pathak, S.; Kumar, R.S.; Dhar, Y.V.; Pandey, A.; Shukla, S.; Trivedi, P.K. 3′O-Methyltransferase, Ps3′OMT, from Opium Poppy: Involvement in Papaverine Biosynthesis. Plant Cell Rep. 2019, 38, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, G.A.W.; Facchini, P.J. Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. On the Biosynthetic Pathway of Papaverine via (S)-Reticuline—Theoretical vs. Experimental Study. Nat. Prod. Commun. 2012, 7, 581–586. [Google Scholar] [CrossRef]
- Pathak, S.; Lakhwani, D.; Gupta, P.; Mishra, B.K.; Shukla, S.; Asif, M.H.; Trivedi, P.K. Comparative Transcriptome Analysis Using High Papaverine Mutant of Papaver Somniferum Reveals Pathway and Uncharacterized Steps of Papaverine Biosynthesis. PLoS ONE 2013, 8, e65622. [Google Scholar] [CrossRef]
- Pienkny, S.; Brandt, W.; Schmidt, J.; Kramell, R.; Ziegler, J. Functional Characterization of a Novel Benzylisoquinoline O-methyltransferase Suggests Its Involvement in Papaverine Biosynthesis in Opium Poppy (Papaver somniferum L.). Plant J. 2009, 60, 56–67. [Google Scholar] [CrossRef]
- Abusnina, A.; Lugnier, C. Therapeutic Potentials of Natural Compounds Acting on Cyclic Nucleotide Phosphodiesterase Families. Cell. Signal. 2017, 39, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.; Das, M.; Goswami, S.; Singh, M.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Dong, H.H.; Guang, Y.B.; Tae, K.Y.; Byung, S.S.; Yong, G.K.; Chul, J.K. The Effect of Papaverine on Ion Channels in Rat Basilar Smooth Muscle Cells. Neurol. Res. 2007, 29, 544–550. [Google Scholar] [CrossRef]
- Gomes, D.; Joubert, A.; Visagie, M. In Vitro Effects of Papaverine on Cell Proliferation, Reactive Oxygen Species, and Cell Cycle Progression in Cancer Cells. Molecules 2021, 26, 6388. [Google Scholar] [CrossRef]
- Shimizu, K.; Yoshihara, E.; Takahashi, M.; Gotoh, K.; Orita, S.; Urakawa, N.; Nakajyo, S. Mechanism of Relaxant Response to Papaverine on the Smooth Muscle of Non-Pregnant Rat Uterus. J. Smooth Muscle Res. 2000, 36, 83–91. [Google Scholar] [CrossRef]
- Liu, J.K.; Couldwell, W.T. Intra-Arterial Papaverine Infusions for the Treatment of Cerebral Vasospasm Induced by Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2005, 2, 124–132. [Google Scholar] [CrossRef]
- Hebb, A.L.O.; Robertson, H.A.; Denovan-Wright, E.M. Phosphodiesterase 10A Inhibition Is Associated with Locomotor and Cognitive Deficits and Increased Anxiety in Mice. Eur. Neuropsychopharmacol. 2008, 18, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Breier, M.; Ko, D.; Thangaraj, N.; Marzan, D.E.; Swerdlow, N.R. Evaluating the Antipsychotic Profile of the Preferential PDE10A Inhibitor, Papaverine. Psychopharmacology 2009, 203, 723–735. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Park, J.-S.; Leem, Y.-H.; Park, J.-E.; Kim, D.-Y.; Choi, Y.-H.; Park, E.-M.; Kang, J.L.; Kim, H.-S. The Phosphodiesterase 10 Inhibitor Papaverine Exerts Anti-Inflammatory and Neuroprotective Effects via the PKA Signaling Pathway in Neuroinflammation and Parkinson’s Disease Mouse Models. J. Neuroinflamm. 2019, 16, 246. [Google Scholar] [CrossRef]
- Bakr, A.M.; El-Sakka, A.A.; El-Sakka, A.I. Considerations for Prescribing Pharmacotherapy for the Treatment of Erectile Dysfunction. Expert Opin. Pharmacother. 2020, 22, 821–834. [Google Scholar] [CrossRef]
- Fusi, F.; Manetti, F.; Durante, M.; Sgaragli, G.; Saponara, S. The Vasodilator Papaverine Stimulates L-Type Ca2+ Current in Rat Tail Artery Myocytes via a PKA-Dependent Mechanism. Vascul. Pharmacol. 2016, 76, 53–61. [Google Scholar] [CrossRef]
- Berkó, S.; Zsikó, S.; Deák, G.; Gácsi, A.; Kovács, A.; Budai-Szűcs, M.; Pajor, L.; Bajory, Z.; Csányi, E. Papaverine Hydrochloride Containing Nanostructured Lyotropic Liquid Crystal Formulation as a Potential Drug Delivery System for the Treatment of Erectile Dysfunction. Drug Des. Dev. Ther. 2018, 12, 2923–2931. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Champion, H.C.; Hellstrom, W.J.G.; Kadowitz, P.J. Pharmacotherapy for Erectile Dysfunction. Trends Pharmacol. Sci. 2000, 21, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, C.G. Narrative Review Current Diagnosis and Management of Erectile Dysfunction. MJA 2019, 210, 469–476. [Google Scholar] [CrossRef]
- Padma-Nathan, H.; Christ, G.; Adaikan, G.; Becher, E.; Brock, G.; Carrier, S.; Carson, C.; Corbin, J.; Francis, S.; Debusk, R.; et al. Pharmacotherapy for Erectile Dysfunction. J. Sex. Med. 2010, 7, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Usta, M.; Erdoğru, T.; Tefekil, A.; Köksal, T.; Yücel, B.; Kadioğlu, A. Honeymoon Impotence: Psychogenic or Organic in Origin? Urology 2001, 57, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; El-Kamel, A.; Khalil, S. Systemic Enhancement of Papaverine Transdermal Gel for Erectile Dysfunction. Drug Dev. Ind. Pharm. 2012, 38, 912–922. [Google Scholar] [CrossRef]
- Kilic, M.; Serefoglu, E.C.; Ozdemir, A.T.; Balbay, M.D. The Actual Incidence of Papaverine-Induced Priapism in Patients with Erectile Dysfunction Following Penile Colour Doppler Ultrasonography. Andrologia 2010, 42, 1–4. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Hatzichristou, D.G. A Comparative Review of the Options for Treatment of Erectile Dysfunction: Which Treatment for Which Patient? Drugs 2005, 65, 1621–1650. [Google Scholar] [CrossRef]
- Bella, A.J.; Brock, G.B. Intracavernous Pharmacotherapy for Erectile Dysfunction. Endocrine 2004, 23, 149–155. [Google Scholar] [CrossRef]
- Claro, J.; Aboim, J.; Maríngolo, M.; Andrade, E.; Aguiar, W.; Nogueira, M.; Nardozza Junior, A.; Srougi, M. Intracavernous Injection in the Treatment of Erectile Dysfunction after Radical Prostatectomy: An Observational Study. Sao Paulo Med. J. 2001, 119, 135–137. [Google Scholar] [CrossRef]
- Yildiz, N.; Gokkaya, N.K.O.; Koseoglu, F.; Gokkaya, S.; Comert, D. Efficacies of Papaverine and Sildenafil in the Treatment of Erectile Dysfunction in Early-Stage Paraplegic Men. Int. J. Rehabil. Res. 2011, 34, 44–52. [Google Scholar] [CrossRef]
- Trejo, H.E.; Urich, D.; Pezzulo, A.A.; Caraballo, J.C.; Gutiérrez, J.; Castro, I.J.; Centeno, G.R.; Sánchez De León, R. Beneficial Effects of Hydrocortisone and Papaverine on a Model of Pulmonary Embolism Induced by Autologous Blood Clots in Isolated and Perfused Rabbit Lungs. Respirology 2007, 12, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Nakajima, T.; Hisada, T.; Sugimoto, T.; Kurachi, Y. On the Mechanism of Papaverine Inhibition of the Voltage-Dependent Ca++ Current in Isolated Smooth Muscle Cells from the Guinea Pig Trachea. J. Pharmacol. Exp. Ther. 1992, 263, 194–200. [Google Scholar] [PubMed]
- Zhu, W.; Liu, S.; Zhao, J.; Liu, S.; Jiang, S.; Li, B.; Yang, H.; Fan, C.; Cui, W. Highly Flexible and Rapidly Degradable Papaverine-Loaded Electrospun Fibrous Membranes for Preventing Vasospasm and Repairing Vascular Tissue. Acta Biomater. 2014, 10, 3018–3028. [Google Scholar] [CrossRef]
- Liu, H.-M.; Tu, Y.-K. The Efficacy of Papaverine Administration by Different Routes for the Treatment of Experimental Acute Cerebral Vasospasm. J. Clin. Neurosci. 2002, 9, 561–565. [Google Scholar] [CrossRef]
- Biondi, A.; Ricciardi, G.K.; Puybasset, L.; Abdennour, L.; Longo, M.; Chiras, J.; Van Effenterre, R. Intra-Arterial Nimodipine for the Treatment of Symptomatic Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage: Preliminary Results. Am. J. Neuroradiol. 2004, 25, 1067–1076. [Google Scholar] [PubMed]
- Firlik, K.; Kaufmann, A.; Firlik, A.; Jungreis, C.; Yonas, H. Intra-Arterial Papaverine for the Treatment of Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage. Surg. Neurol. 1999, 51, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sayama, C.M.; Liu, J.K.; Couldwell, W.T. Update on Endovascular Therapies for Cerebral Vasospasm Induced by Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Focus 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Kerz, T.; Boor, S.; Beyer, C.; Welschehold, S.; Schuessler, A.; Oertel, J. Effect of Intraarterial Papaverine or Nimodipine on Vessel Diameter in Patients with Cerebral Vasospasm after Subarachnoid Hemorrhage. Br. J. Neurosurg. 2012, 26, 517–524. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, H.-J.; Ko, Y.; Kim, Y.-S.; Kim, D.-W.; Kim, J.-M. Effectiveness of Papaverine Cisternal Irrigation for Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage and Measurement of Biomarkers. Neurol. Sci. 2014, 35, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Vajkoczy, P.; Horn, P.; Bauhuf, C.; Munch, E.; Hubner Ing, U.; Thome, C.; Poeckler-Schoeninger, C.; Roth, H.; Schmiedek, P. Effect of Intra-Arterial Papaverine on Regional Cerebral Blood Flow in Hemodynamically Relevant Cerebral Vasospasm. Stroke 2001, 32, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Baltsavias, G.; Yella, S.; Al Shameri, R.A.; Luft, A.; Valavanis, A. Intra-Arterial Administration of Papaverine during Mechanical Thrombectomy for Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 41–47. [Google Scholar] [CrossRef]
- Dabus, G.; Nogueira, R. Current Options for the Management of Aneurysmal Subarachnoid Hemorrhage-Induced Cerebral Vasospasm: A Comprehensive Review of the Literature. Interv. Neurol. 2013, 2, 30–51. [Google Scholar] [CrossRef]
- Badjatia, N.; Topcuoglu, M.A.; Pryor, J.C.; Rabinov, J.D.; Ogilvy, C.S.; Carter, B.S.; Rordorf, G.A. Preliminary Experience with Intra-Arterial Nicardipine as a Treatment for Cerebral Vasospasm. Am. J. Neuroradiol. 2004, 25, 819–826. [Google Scholar]
- Yu, D.; Morris-Natschke, S.; Lee, K. New Developments in Natural Products-based Anti-AIDS Research. Med. Res. Rev. 2007, 27, 108–132. [Google Scholar] [CrossRef]
- Rima, B.; Duprex, W. Molecular Mechanisms of Measles Virus Persistence. Virus Res. 2005, 111, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B. Antiviral Compounds from Plants; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351078177. [Google Scholar]
- Dereli, F.; Ilhan, M.; Belwal, T. Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Griffin, D.E.; Lin, W.H.; Pan, C.H. Measles Virus, Immune Control, and Persistence. FEMS Microbiol. Rev. 2012, 36, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Leser, G.P.; Lamb, R.A. Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses. J. Virol. 2020, 94, e01888-19. [Google Scholar] [CrossRef]
- Ellinger, B.; Bojkova, D.; Zaliani, A.; Cinatl, J.; Claussen, C.; Westhaus, S.; Keminer, O.; Reinshagen, J.; Kuzikov, M.; Wolf, M.; et al. A SARS-CoV-2 Cytopathicity Dataset Generated by High-Content Screening of a Large Drug Repurposing Collection. Sci. Data 2021, 8, 70. [Google Scholar] [CrossRef]
- Pluta, R.; Hansen-Schwartz, J.; Dreier, J.; Vajkoczy, P.; Macdonald, R.; Nishizawa, S.; Kasuya, H.; Wellman, G.; Keller, E.; Zauner, A.; et al. Cerebral Vasospasm Following Subarachnoid Hemorrhage: Time for a New World of Thought. Neurol. Res. 2009, 31, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Polur, H.; Joshi, T.; Workman, C.T.; Lavekar, G.; Kouskoumvekaki, I. Back to the Roots: Prediction of Biologically Active Natural Products from Ayurveda Traditional Medicine. Mol. Inform. 2011, 30, 181–187. [Google Scholar] [CrossRef]
- Choe, H.; Lee, Y.K.; Lee, Y.T.; Choe, H.; Ko, S.H.; Joo, C.U.; Kim, M.H.; Kim, G.S.; Eun, J.S.; Kim, J.H.; et al. Papaverine Blocks HKv1.5 Channel Current and Human Atrial Ultrarapid Delayed Rectifier K+ Currents. J. Pharmacol. Exp. Ther. 2003, 304, 706–712. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y. Cascade Signals of Papaverine Inhibiting LPS-Induced Retinal Microglial Activation. J. Mol. Neurosci. 2019, 68, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Suzumura, A.; Tamaru, T.; Takayanagi, T.; Sawada, M. Effects of Phosphodiesterase Inhibitors on Cytokine Production by Microglia. Mult. Scler. J. 1999, 5, 126–133. [Google Scholar] [CrossRef]
- Tamada, K.; Nakajima, S.; Ogawa, N.; Inada, M.; Shibasaki, H.; Sato, A.; Takasawa, R.; Yoshimori, A.; Suzuki, Y.; Watanabe, N.; et al. Papaverine Identified as an Inhibitor of High Mobility Group Box 1/Receptor for Advanced Glycation End-Products Interaction Suppresses High Mobility Group Box 1-Mediated Inflammatory Responses. Biochem. Biophys. Res. Commun. 2019, 511, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kawano, Y. Inhibitory Effects of Herbal Alkaloids on the Tumor Necrosis Factor-.ALPHA. and Nitric Oxide Production in Lipopolysaccharide-Stimulated RAW264 Macrophages. Chem. Pharm. Bull. 2011, 59, 388–391. [Google Scholar] [CrossRef]
- Dang, Y.; Mu, Y.; Wang, K.; Xu, K.; Yang, J.; Zhu, Y.; Luo, B. Papaverine Inhibits Lipopolysaccharide-Induced Microglial Activation by Suppressing NF-ΚB Signaling Pathway. Drug Des. Dev. Ther. 2016, 10, 851. [Google Scholar] [CrossRef]
- Gomes, D.; Joubert, A.; Visagie, M. In Vitro Effects of Papaverine on Cell Migration and Vascular Endothelial Growth Factor in Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 4654. [Google Scholar] [CrossRef]
- Shimizu, T.; Ohta, Y.; Ozawa, H.; Matsushima, H.; Takeda, K. Papaverine Combined with Prostaglandin E2 Synergistically Induces Neuron-like Morphological Changes and Decrease of Malignancy in Human Prostatic Cancer LNCaP Cells. Anticancer Res. 2000, 20, 761–767. [Google Scholar]
- Huang, H.; Li, L.-J.; Zhang, H.-B.; Wei, A.-Y. Papaverine Selectively Inhibits Human Prostate Cancer Cell (PC-3) Growth by Inducing Mitochondrial Mediated Apoptosis, Cell Cycle Arrest and Downregulation of NF-ΚB/PI3K/Akt Signalling Pathway. J. BUON 2017, 22, 112–118. [Google Scholar]
- Afzali, M.; Ghaeli, P.; Khanavi, M.; Parsa, M.; Montazeri, H.; Ghahremani, M.H.; Ostad, S.N. Non-Addictive Opium Alkaloids Selectively Induce Apoptosis in Cancer Cells Compared to Normal Cells. DARU J. Pharm. Sci. 2015, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Sajadian, S.; Vatankhah, M.; Majdzadeh, M.; Kouhsari, S.M.; Ghahremani, M.H.; Ostad, S.N. Cell Cycle Arrest and Apoptogenic Properties of Opium Alkaloids Noscapine and Papaverine on Breast Cancer Stem Cells. Toxicol. Mech. Methods 2015, 25, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells—Inhibition of Telomerase and Induction of Senescence. Molecules 2014, 19, 11846–11859. [Google Scholar] [CrossRef]
- Hultman, K.A.; Scott, A.W.; Johnson, S.L. Small Molecule Modifier Screen for Kit-Dependent Functions in Zebrafish Embryonic Melanocytes. Zebrafish 2008, 5, 279–287. [Google Scholar] [CrossRef]
- Inada, M.; Sato, A.; Shindo, M.; Yamamoto, Y.; Akasaki, Y.; Ichimura, K.; Tanuma, S.-I. Anticancer Non-Narcotic Opium Alkaloid Papaverine Suppresses Human Glioblastoma Cell Growth. Anticancer Res. 2019, 39, 6743–6750. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Shindo, M.; Kobayashi, K.; Sato, A.; Yamamoto, Y.; Akasaki, Y.; Ichimura, K.; Tanuma, S. Anticancer Effects of a Non-Narcotic Opium Alkaloid Medicine, Papaverine, in Human Glioblastoma Cells. PLoS ONE 2019, 14, e0216358. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H.; Rohr-Udilova, N.; Gille, L.; Bieberschulte, W.; Jurek, D.; Marian, B.; Schulte-Hermann, R. Ubiquinol and the Papaverine Derivative Caroverine Prevent the Expression of Tumour- Promoting Factors in Adenoma and Carcinoma Colon Cancer Cells Induced by Dietary Fat. BioFactors 2005, 25, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rubis, B.; Kaczmarek, M.; Szymanowska, N.; Galezowska, E.; Czyrski, A.; Juskowiak, B.; Hermann, T.; Rybczynska, M. The Biological Activity of G-Quadruplex DNA Binding Papaverine-Derived Ligand in Breast Cancer Cells. Investig. New Drugs 2009, 27, 289–296. [Google Scholar] [CrossRef]
- Parcha, P.K.; Sarvagalla, S.; Ashok, C.; Sudharshan, S.J.; Dyavaiah, M.; Coumar, M.S.; Rajasekaran, B. Repositioning Antispasmodic Drug Papaverine for the Treatment of Chronic Myeloid Leukemia. Pharmacol. Rep. 2021, 73, 615–628. [Google Scholar] [CrossRef]
- So, H.; Chau, C.; Chiu, W.; Ho, K.; Lo, C.; Yim, S.; Sham, P. Analysis of Genome-Wide Association Data Highlights Candidates for Drug Repositioning in Psychiatry. Nat. Neurosci. 2017, 20, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Strick, C.A. Treating Neuropsychiatric Disorders with PDE10A Inhibitors. Drug Discov. Today Ther. Strateg. 2006, 3, 527–532. [Google Scholar] [CrossRef]
- Gardos, G.; Granacher, R.P.; Cole, J.O.; Sniffin, C. The Effects of Papaverine in Tardive Dyskinesia. Prog. Neuropsychopharmacol. 1979, 3, 543–550. [Google Scholar] [CrossRef]
- El-Sayeh, H.G.; Lyra da Silva, J.P.; Rathbone, J.; Soares-Weiser, K. Non-Neuroleptic Catecholaminergic Drugs for Neuroleptic-Induced Tardive Dyskinesia. Cochrane Database Syst. Rev. 2006, 1. [Google Scholar] [CrossRef]
- Soares-Weiser, K.; Rathbone, J.; Ogawa, Y.; Shinohara, K.; Bergman, H. Miscellaneous Treatments for Antipsychotic-Induced Tardive Dyskinesia. Cochrane Database Syst. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Itoh, K.; Ishima, T.; Kehler, J.; Hashimoto, K. Potentiation of NGF-Induced Neurite Outgrowth in PC12 Cells by Papaverine: Role Played by PLC-γ, IP3 Receptors. Brain Res. 2011, 1377, 32–40. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Q.; Gu, H.; Zhang, Y.; Wei, P.; Qi, Y.; Liu, J.; Wang, Z. Pluripotent Anti-Inflammatory Immunomodulatory Effects of Papaverine against Cerebral Ischemic-Reperfusion Injury. J. Pharmacol. Sci. 2020, 144, 69–75. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Papaverine Exerts Neuroprotective Effect by Inhibiting NLRP3 Inflammasome Activation in an MPTP-Induced Microglial Priming Mouse Model Challenged with LPS. Biomol. Ther. 2021, 29, 295. [Google Scholar] [CrossRef] [PubMed]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kang, J.L.; Kim, H.S. Papaverine Inhibits α-Synuclein Aggregation by Modulating Neuroinflammation and Matrix Metalloproteinase-3 Expression in the Subacute MPTP/P Mouse Model of Parkinson’s Disease. Biomed. Pharmacother. 2020, 130, 110576. [Google Scholar] [CrossRef]
- Giralt, A.; Saavedra, A.; Carretón, O.; Arumí, H.; Tyebji, S.; Alberch, J.; Pérez-Navarro, E. PDE10 Inhibition Increases GluA1 and CREB Phosphorylation and Improves Spatial and Recognition Memories in a Huntington’s Disease Mouse Model. Hippocampus 2013, 23, 684–695. [Google Scholar] [CrossRef]
- Otto, C. Heartbeat: Improving Diagnosis and Management of Aortic Valve Disease. Heart 2018, 104, 1809. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.; Yao, G.; Wang, Z. Papaverine Improves Sublingual Blood Flow in Patients with Septic Shock. J. Surg. Res. 2015, 195, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Saravala, H. Instrumental and Analytical Study of Active Pharmaceutical Ingredients and Bio Active Molecules. Ph.D. Thesis, Saurashtra University, Rajkot, India, 2011. [Google Scholar]
- Madhu, C.; Mahavarkar, S.; Bhave, S. A Randomised Controlled Study Comparing Drotaverine Hydrochloride and Valethamate Bromide in the Augmentation of Labour. Arch. Gynecol. Obstet. 2010, 282, 11–15. [Google Scholar] [CrossRef]
- Gueta, I.; Braun, A.; Gilan, A.; Berlin, M.; Kohn, E.; Barchel, D.; Markovits, N.; Berkovitch, M.; Loebstein, R. Pregnancy Outcomes Following Gestational Exposure to Papaverine: An Observational Comparative Study. Br. J. Clin. Pharmacol. 2021, 87, 3910–3915. [Google Scholar] [CrossRef] [PubMed]
- Kublickiene, K.; Lindblom, B.; Krüger, K.; Nisell, H. Preeclampsia: Evidence for Impaired Shear Stress–Mediated Nitric Oxide Release in Uterine Circulation. Am. J. Obstet. Gynecol. 2000, 183, 160–166. [Google Scholar] [CrossRef]
- Snir, N.; Moskovitz, B.; Nativ, O.; Margel, D.; Sandovski, U.; Sulkes, J.; Livne, P.M.; Lifshitz, D.A. Papaverine Hydrochloride for the Treatment of Renal Colic: An Old Drug Revisited. A Prospective, Randomized Study. J. Urol. 2008, 179, 1411–1414. [Google Scholar] [CrossRef]
- Karagoz, M.A.; Doluoglu, O.G.; Ünverdi, H.; Resorlu, B.; Sunay, M.M.; Demirbas, A.; Karakan, T.; Aydin, A. The Protective Effect of Papaverine and Alprostadil in Rat Testes after Ischemia and Reperfusion Injury. Int. Braz. J. Urol. 2018, 44, 617–622. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, W. Drug Metabolism in Drug Discovery and Development. Acta Pharm. Sin. B 2018, 8, 721–732. [Google Scholar] [CrossRef]
- Mohs, R.; Greig, N. Drug Discovery and Development: Role of Basic Biological Research. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Nair, A.; Aly Morsy, M.; Jacob, S. Dose Translation between Laboratory Animals and Human in Preclinical and Clinical Phases of Drug Development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Davison, E.K.; Brimble, M.A. Natural Product Derived Privileged Scaffolds in Drug Discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Joubert, A.; Visagie, M. The Biological Relevance of Papaverine in Cancer Cells. Cells 2022, 11, 3385. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Mabhida, S.E.; Jack, B.U.; Nyambuya, T.M.; Nkambule, B.B.; Basson, A.K.; Tiano, L.; Dludla, P.V. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 2227. [Google Scholar] [CrossRef] [PubMed]
- Emon, N.; Rudra, S.; Alam, S.; Al Haidar, I.; Paul, S.; Richi, F.; Shahriar, S.; Sayeed, M.; Tumpa, N.; Ganguly, A. Chemical, Biological and Protein-Receptor Binding Profiling of Bauhinia Scandens L. Stems Provide New Insights into the Management of Pain, Inflammation. Biomed. Pharmacother. 2021, 143, 112185. [Google Scholar] [CrossRef]
- Ashrafi, S.; Rahman, M.; Ahmed, P.; Alam, S.; Hossain, M.A. Prospective Asian Plants with Corroborated Antiviral Potentials: Position Standing in Recent Years. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Choo, T.M. Nigella Sativa Tea Mitigates Type-2 Diabetes and Edema: A Case Report. Adv. Tradit. Med. 2023, 1–6. [Google Scholar] [CrossRef]
- Akinloye, O.A.; Sulaimon, L.A.; Ogunbiyi, O.E.; Odubiyi, A.E.; Adewale, A.A.; Toriola, M.A.; Salami, O.A.; Boyenle, I.D. Amaranthus Spinosus (Spiny Pigweed) Methanol Leaf Extract Alleviates Oxidative and Inflammation Induced by Doxorubicin in Male Sprague Dawley Rats. Adv. Tradit. Med. 2023, 1–8. [Google Scholar] [CrossRef]
- Alam, S.; Rashid, M.A.; Sarker, M.M.R.; Emon, N.U.; Arman, M.; Mohamed, I.N.; Haque, M.R. Antidiarrheal, Antimicrobial and Antioxidant Potentials of Methanol Extract of Colocasia Gigantea Hook. f. Leaves: Evidenced from in Vivo and in Vitro Studies along with Computer-Aided Approaches. BMC Complement. Med. Ther. 2021, 21, 119. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.R.; Mohamed, I.N. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products against COVID-19: Update on Clinical Trials and Mechanism of Actions. Front. Pharmacol. 2021, 12, 671498. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.; Sultana, T.; Chowdhury, M.; Rashid, M.; Chaity, N.; Zhao, C.; Xiao, J.; Hafez, E.; Khan, S.; et al. Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).