Synthetic Exploration of Bis(phenolate) Aza-BODIPYs and Heavier Group 13 Chelates
Abstract
1. Introduction
2. Results and Discussion
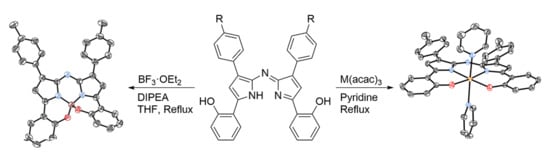
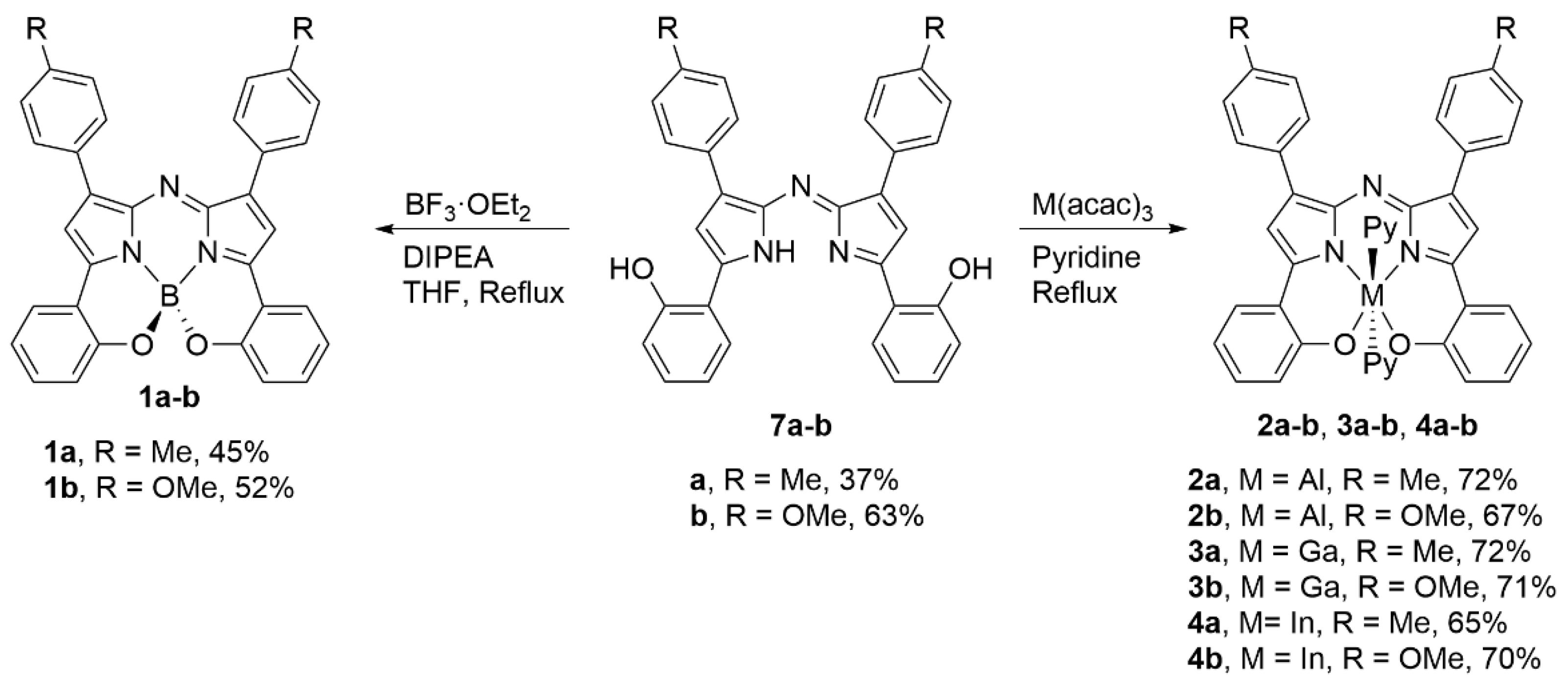
2.1. Synthesis and Structural Characterization
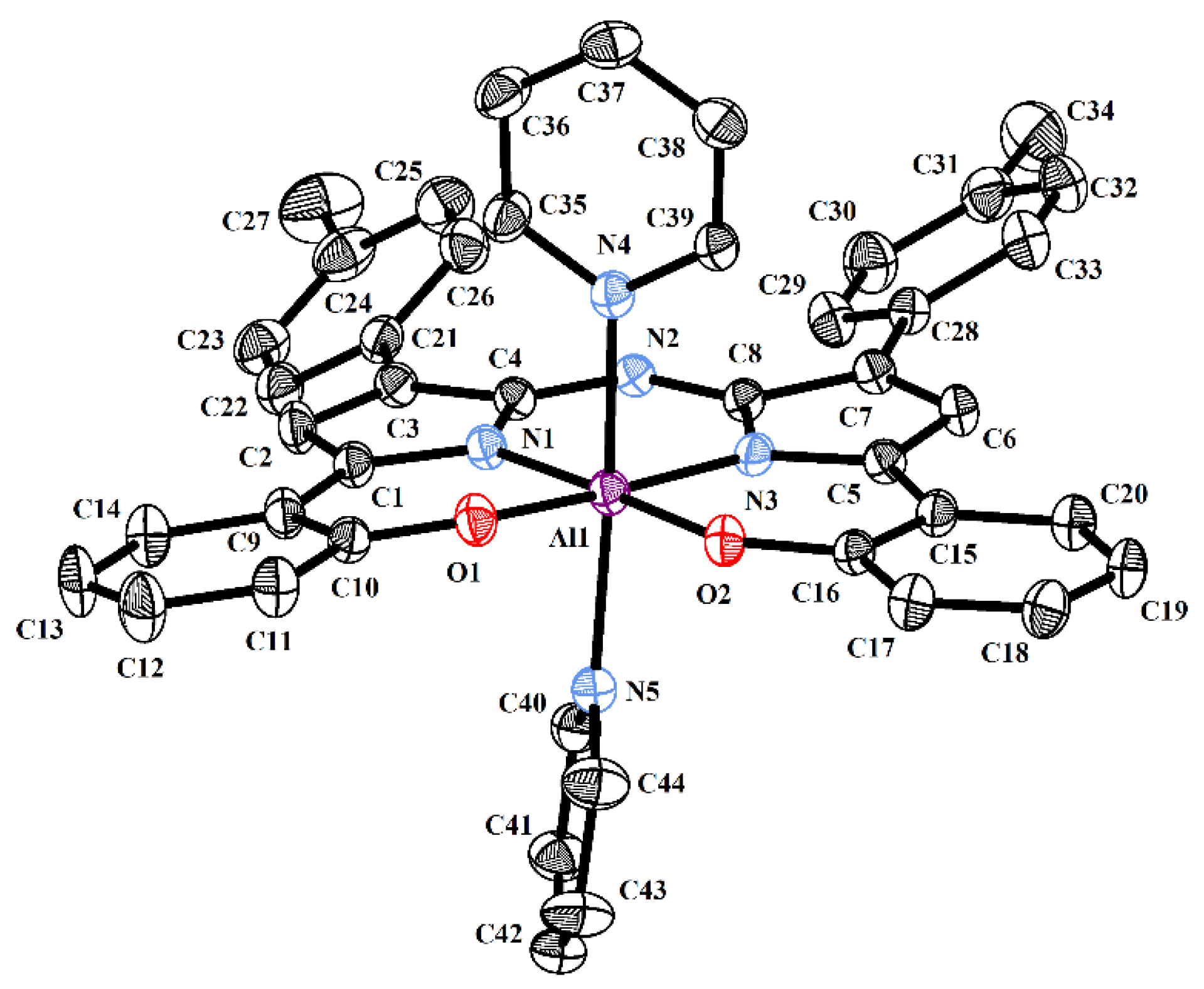
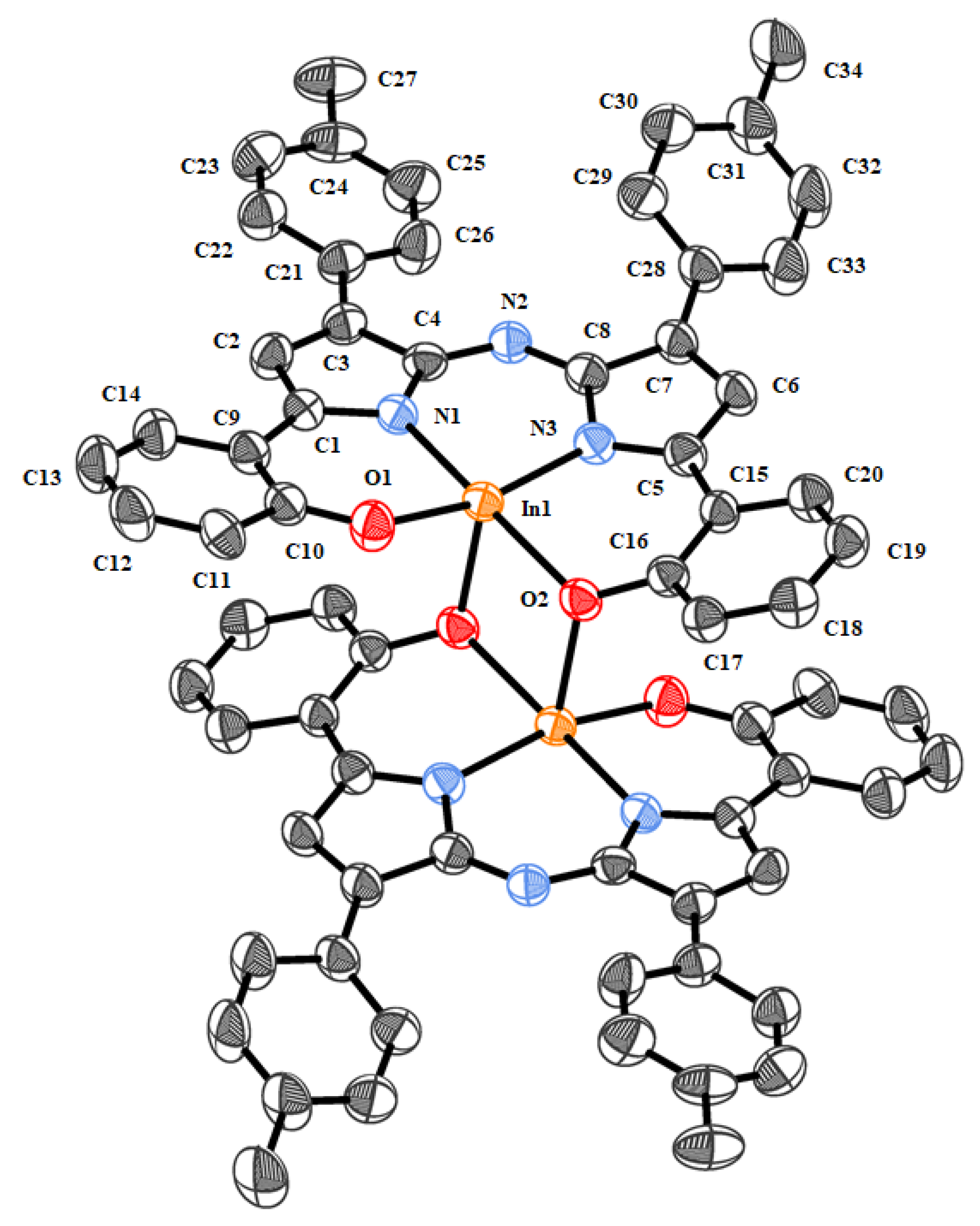
2.2. Crystallography
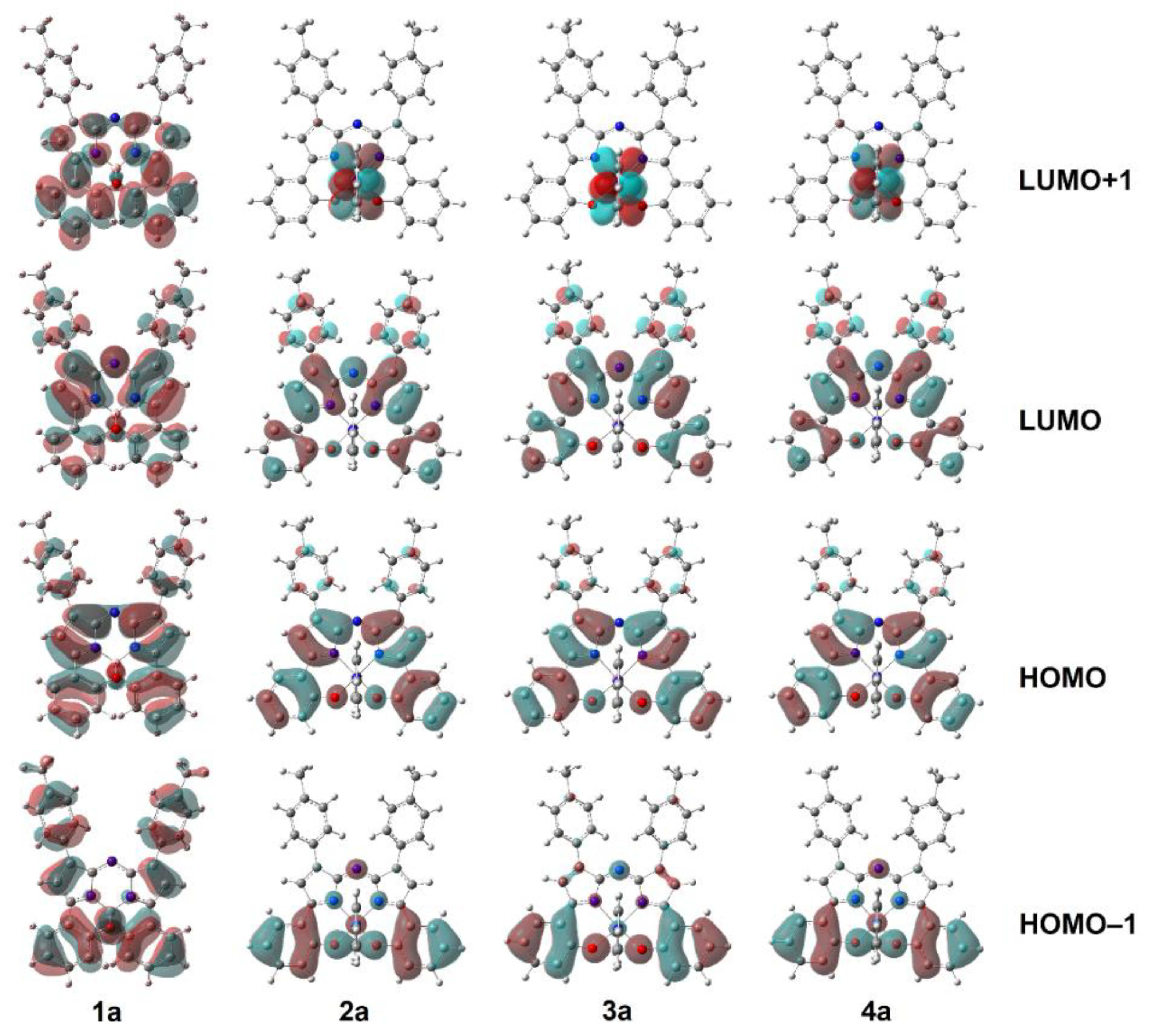
2.3. Computational Measurements
2.4. Photophysical Properties
2.4.1. Electronic Absorption Spectra
2.4.2. Electronic Emission Spectra
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization
3.2.1. 1,4-Product 6a
3.2.2. 1,4-Product 6b
3.2.3. Bis(phenolate) Aza-DIPY 7a
3.2.4. Bis(phenolate) Aza-DIPY 7b
3.2.5. Bis(phenolate) Aza-BODIPY 1a
3.2.6. Bis(phenolate) Aza-BODIPY 1b
3.2.7. Bis(phenolate) Aza-ALDIPY 2a
3.2.8. Bis(phenolate) Aza-ALDIPY 2b
3.2.9. Bis(phenolate) Aza-GADIPY 3a
3.2.10. Bis(phenolate) Aza-GADIPY 3b
3.2.11. Bis(phenolate) Aza-INDIPY 4a
3.2.12. Bis(phenolate) Aza-INDIPY 4b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ge, Y.; O’Shea, D.F. Azadipyrromethenes: From traditional dye chemistry to leading edge applications. Chem. Soc. Rev. 2015, 45, 3846–3864. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Hwuang, W. Bioapplications of small molecule Aza-BODIPY: From rational structural design to in vivo investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Shamova, L.I.; Zatsikha, Y.V.; Nemykin, V.N. Synthesis pathways for the preparation of the BODIPY analogues: Aza-BODIPYs, BOPHYs, and some other pyrrole-based acyclic chromophores. Chem. Soc. Rev. 2021, 50, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Pascal, S.; David, S.; Andraud, C.; Maury, O. Near-infrared dyes for two-photon absorption in the short-wavelength infrared: Strategies toward optical power limiting. Chem. Soc. Rev. 2021, 50, 6613–6658. [Google Scholar] [CrossRef] [PubMed]
- Bellier, Q.; Pégaz, S.; Aronica, C.; Le Guennic, B.; Andraud, C.; Maury, O. Near-infrared nitrofluorene substituted aza-boron-dipyrromethene dyes. Org. Lett. 2011, 13, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Senevirathna, W.; Sauvé, G. Azadipyrromethene-based conjugated oligomers with near-IR absorption and high electron affinity. Org. Lett. 2011, 13, 5354–5357. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Zheng, Y.-Q.; Wu, Q.; Wu, Y.; Yu, C.; Jiao, L.; Hao, E.; Wang, J.-Y.; Pei, J. Synthesis, properties, and semiconducting characteristics of BF2 complexes of β,β-bisphenanthrene-fused azadipyrromethenes. Org. Lett. 2017, 19, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Wu, Y.; Yu, C.; Bobadova-Parvanova, P.; Hao, E.; Jiao, L. Synthesis, crystal structure, and the deep near-infrared absorption/emission of bright azaBODIPY-based organic fluorophores. Org. Lett. 2018, 20, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chang, F.; Wu, Q.; Hao, E.; Jiao, L.; Wang, J.-Y.; Pei, J. Synthesis and semiconducting characteristics of the BF2 complexes of bisbenzothiophene-fused azadipyrromethenes. Org. Lett. 2020, 22, 185–189. [Google Scholar] [CrossRef]
- David, S.; Chang, H.-J.; Lopes, C.; Brännlund, C.; Le Guennic, B.; Berginc, G.; Van Stryland, E.; Bondar, M.V.; Hagan, D.; Jacquemin, D.; et al. Benzothiadiazole-substituted aza-BODIPY dyes: Two-photon absorption enhancement for improved optical limiting performances in the short-wave IR range. Chem. Eur. J. 2021, 27, 3517–3525. [Google Scholar] [CrossRef]
- Zarcone, S.R.; Yarbrough, H.J.; Neal, M.J.; Kelly, J.C.; Kaczynski, K.L.; Bloomfield, A.J.; Bowers, G.M.; Montgomery, T.D.; Chase, D.T. Synthesis and photophysical properties of nitrated aza-BODIPYs. New. J. Chem. 2022, 46, 4483–4496. [Google Scholar] [CrossRef]
- Kovtun, Y.P.; Yakubovskiy, V.P.; Shandura, M.P. Azadipyrromethene dye with a fully chelated boron atom. Chem. Heterocycl. Compd. 2008, 44, 1298–1299. [Google Scholar] [CrossRef]
- Leblebici, S.Y.; Catane, L.; Barclay, D.E.; Olson, T.; Chen, T.L.; Ma, B. Near-infrared azadipyrromethenes as electron donor for efficient planar heterojunction organic solar cells. ACS Appl. Mater. Interfaces 2011, 3, 4469–4474. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, X.; Zhang, S.; Huang, X.; Cheng, Y.; Zhu, C. Near-infrared emission on novel bent-core V-shaped conjugated polymers based on the B,O-chelated azadipyrromethene structure. Polym. Chem. 2013, 4, 4396–4404. [Google Scholar] [CrossRef]
- Zatsikha, Y.V.; Blesener, T.S.; Goff, P.C.; Healy, A.T.; Swedin, R.K.; Herbet, D.E.; Rohde, G.T.; Chanwanno, K.; Ziegler, C.J.; Belosludov, R.V.; et al. 1,7-Dipyrene-containing aza-BODIPYs: Are pyrene groups effective ligands to promote and direct complex formation with common nanocarbon materials? J. Phys. Chem. C 2018, 122, 27893–27916. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, F.; Shao, T.; Jin, G. A near-infrared zadipyrromethene dye: Photophysical properties under different acidity conditions. Inorg. Chem. Commun. 2020, 116, 107942. [Google Scholar] [CrossRef]
- Mori, R.; Asakura, M.; Kobayashi, Y.; Mashimo, H.; Roppongi, M.; Ito, S. Synthesis of π-conjugated asymmetric aza-BODIPYs via nitrosobicyclopyrroles. Tetrahedron Lett. 2022, 96, 153759. [Google Scholar] [CrossRef]
- Loudet, A.; Bandichor, R.; Burgess, K.; Palma, A.; McDonnel, S.O.; Hall, M.J.; O’Shea, D.F. B,O-Chelated azadipyrromethenes as near-IR probes. Org. Lett. 2008, 21, 4771–4774. [Google Scholar]
- Jimenez, J.C.; Zhou, Z.; Rheingold, A.L.; Parker, S.M.; Sauvé, G. Tuning the properties of azadipyrromethene-based near-infrared dyes using intramolecular BO chelation and peripheral substitutions. Inorg. Chem. 2021, 60, 13320–13331. [Google Scholar] [CrossRef]
- Kim, H.; Burghart, A.; Welch, M.B.; Reibenspies, J.; Burgess, K. Synthesis and spectroscopic properties of a new 4-bora-3a,4a-diaza-s-indacene (BODIPY) dye. Chem. Commun. 1999, 35, 1889–1990. [Google Scholar] [CrossRef]
- Ikeda, C.; Ueda, S.; Nabeshima, T. Aluminum complexes of N2O2-type dipyrrins: The first hetero-nuclear complexes of metallo-dipyrrins with high fluorescence quantum yields. Chem. Commun. 2009, 45, 2544–2546. [Google Scholar] [CrossRef]
- Saikawa, M.; Daicho, M.; Nakamura, T.; Uchida, J.; Yamamura, M.; Nabeshima, T. Synthesis of a new family of ionophores based on aluminum-dipyrrin complexes (ALDIPYs) and their strong recognition of alkaline earth ions. Chem. Commun. 2016, 52, 4014–4017. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, A.; Chiba, Y.; Matsuoka, R.; Noda, T.; Nabeshima, T. Efficient luminescent properties and cation recognition ability of heavy group 13 element complexes of N2O2- and N2O4-type dipyrrins. Dalton Trans. 2019, 48, 13169–13175. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ikeda, C.; Yamamura, M.; Nabeshima, T. Structural interconversion and regulation of optical properties of stable hypercoordinate dipyrrin-silicon complexes. J. Am. Chem. Soc. 2011, 133, 4726–4729. [Google Scholar] [CrossRef]
- Yamamura, M.; Takizawa, H.; Sakamoto, N.; Nabeshima, T. Monomeric and dimeric red/NIR-fluorescence dipyrrin-germanium complexes: Facile monomer-dimer interconversion driven by acid/base reactions. Tetrahedron Lett. 2013, 54, 7049–7052. [Google Scholar] [CrossRef]
- Yamamura, M.; Albrecht, M.; Albrecht, M.; Nishimura, Y.; Arai, T.; Nabeshima, T. Red/near-infrared luminescence tuning of group-14 element complexes of dipyrrins based on a central atom. Inorg. Chem. 2014, 53, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, M.; Takizawa, H.; Nabeshima, T. Zweitterionic N2O2-type protonated dipyrrin bearing a phosphonate anionic moiety as a pH-responsive fluorescence indicator. Org. Lett. 2015, 17, 3114–3117. [Google Scholar] [CrossRef]
- Nakano, K.; Kobayashi, K.; Nozaki, K. Tetravalent metal complexes as a new family of catalysts for copolymerization of epoxides with carbon dioxide. J. Am. Chem. Soc. 2011, 133, 10720–10723. [Google Scholar] [CrossRef]
- Rausaria, S.; Kamadulski, A.; Rath, N.P.; Bryant, L.; Chen, Z.; Salvemini, D.; Neumann, W.L. Manganese(III) complexes of bis(hydroxyphenyl)dipyrromethenes are potent orally active peroxynitrite scavengers. J. Am. Chem. Soc. 2011, 133, 4200–4203. [Google Scholar] [CrossRef]
- El Ghachtouli, S.; Wójcik, K.; Copey, L.; Szydlo, F.; Framery, E.; Goux-Henry, C.; Billon, L.; Charlot, M.-F.; Guillot, R.; Andrioletti, B.; et al. Dipyrrinphenol-Mn(III) complex: Synthesis, electrochemistry, spectroscopic characterization and reactvity. Dalton Trans. 2011, 40, 9090–9093. [Google Scholar] [CrossRef]
- Lecarme, L.; Chiang, L.; Moutet, J.; Leconte, N.; Philouze, C.; Jarjayes, O.; Storr, T.; Thomas, F. The structure of a one-electron oxidized Mn(III)-bis(phenolate)dipyrrin radical complex and oxidation catalysis control via ligand-centered redox activity. Dalton Trans. 2016, 45, 16325–16334. [Google Scholar] [CrossRef] [PubMed]
- Kochem, A.; Chiang, L.; Baptiste, B.; Philouze, C.; Leconte, N.; Jarjayes, O.; Storr, T.; Thomas, F. Ligand-centered redox activity of cobalt(II) and nickel(II) bis(phenolate)-dipyrrin complexes. Chem. Eur. J. 2012, 18, 14590–14593. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, M.; Takizawa, H.; Gobo, Y.; Nabeshima, T. Stable neutral radicals of planar N2O2-type dipyrrin platinum complexes: Hybrid radicals of the delocalized organic π-orbital and platinum d-orbital. Dalton Trans. 2016, 45, 6834–6838. [Google Scholar] [CrossRef] [PubMed]
- Moutet, J.; Philouze, C.; du Moulinet d’Hardemare, A.; Leconte, N.; Thomas, F. Ni(II) complexes of the redox-active bis(2-aminophenyl)dipyrrin: Structural, spectroscopic, and theoretical characterization of three members of an electron transfer series. Inorg. Chem. 2017, 56, 6380–6392. [Google Scholar] [CrossRef]
- Kunert, R.; Philouze, C.; Jarjayes, O.; Thomas, F. Stable M(II)-radicals and nickel(III) complexes of a bis(phenol) N-heterocyclic carbene chelated to group 10 metal ions. Inorg. Chem. 2019, 58, 8030–8044. [Google Scholar] [CrossRef]
- Shan, W.; Desbois, N.; Pacquelet, S.; Brandès, S.; Rousselin, Y.; Conradie, J.; Ghosh, A.; Gros, C.P.; Kadish, K.M. Ligand noninnocence in cobalt dipyrrin-bisphenols: Spectroscopic, electrochemical, and theoretical insights indicating an emerging analogy with corroles. Inorg. Chem. 2019, 58, 7677–7689. [Google Scholar] [CrossRef]
- Di Natale, C.; Gros, C.P.; Paolesse, R. Corroles at work: A small macrocycle for great applications. Chem. Soc. Rev. 2022, 51, 1277–1335. [Google Scholar] [CrossRef]
- Verma, P.; Truhlar, D.G. Status and Challenges of Density Functional Theory. Trends Chem. 2020, 2, 302–318. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on Basis Sets Beautiful: Seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Menucci, B. Polarizable continuum model. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 386–404. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 298–310. [Google Scholar] [CrossRef]
- Teets, T.S.; Updegraff III, J.B.; Esswein, A.J.; Gray, T.G. Three-coordinate, phosphine-ligated azadipyrromethene complexes to univalent group 11 metals. Inorg. Chem. 2009, 48, 8134–8144. [Google Scholar] [CrossRef]
- Deligonul, N.; Gray, T.G. Azadipyrromethene complexes of d8 metal centers: Rhodium(I), iridium(I), palladium(II), and platinum(II). Inorg. Chem. 2013, 52, 13048–13057. [Google Scholar] [CrossRef]





| Photophysical a | Computational d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | λabs | log ε | Egap b | λem c | SS c (nm) | SS c (cm−1) | Φc | EHOMO | ELUMO | EGap | λGap |
| 1a | 728 | 4.50 | 1.71 | 747 | 19 | 350 | 13.7 | −5.56 | −3.66 | 1.90 | 652 |
| 1b | 732 | 4.47 | 1.70 | 751 | 19 | 345 | 8.8 | −5.21 | −3.43 | 1.78 | 695 |
| 2a | 731 | 4.48 | 1.70 | 766 | 35 | 625 | 7.5 | −5.00 | −3.20 | 1.80 | 689 |
| 2b | 728 | 4.56 | 1.71 | 762 | 34 | 613 | 7.0 | −4.97 | −3.17 | 1.80 | 689 |
| 3a | 734 | 4.58 | 1.69 | 774 | 40 | 704 | 6.1 | −5.01 | −3.21 | 1.80 | 688 |
| 3b | 732 | 4.71 | 1.70 | 770 | 38 | 674 | 8.4 | −4.98 | −3.17 | 1.81 | 688 |
| 4a | 714 | 4.67 | 1.74 | 752 | 38 | 708 | 0.3 | −5.01 | −3.14 | 1.87 | 661 |
| 4b | 713 | 4.45 | 1.74 | 746 | 37 | 620 | 0.7 | −4.98 | 3.11 | 1.87 | 665 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lane, A.M.; Luong, N.T.C.; Kelly, J.C.; Neal, M.J.; Jamrom, J.; Bloomfield, A.J.; Lummis, P.A.; Montgomery, T.D.; Chase, D.T. Synthetic Exploration of Bis(phenolate) Aza-BODIPYs and Heavier Group 13 Chelates. Molecules 2022, 27, 8256. https://doi.org/10.3390/molecules27238256
Lane AM, Luong NTC, Kelly JC, Neal MJ, Jamrom J, Bloomfield AJ, Lummis PA, Montgomery TD, Chase DT. Synthetic Exploration of Bis(phenolate) Aza-BODIPYs and Heavier Group 13 Chelates. Molecules. 2022; 27(23):8256. https://doi.org/10.3390/molecules27238256
Chicago/Turabian StyleLane, Aiden M., Ny T. C. Luong, Jordan C. Kelly, Martin J. Neal, Jeremiah Jamrom, Aaron J. Bloomfield, Paul A. Lummis, Thomas D. Montgomery, and Daniel T. Chase. 2022. "Synthetic Exploration of Bis(phenolate) Aza-BODIPYs and Heavier Group 13 Chelates" Molecules 27, no. 23: 8256. https://doi.org/10.3390/molecules27238256
APA StyleLane, A. M., Luong, N. T. C., Kelly, J. C., Neal, M. J., Jamrom, J., Bloomfield, A. J., Lummis, P. A., Montgomery, T. D., & Chase, D. T. (2022). Synthetic Exploration of Bis(phenolate) Aza-BODIPYs and Heavier Group 13 Chelates. Molecules, 27(23), 8256. https://doi.org/10.3390/molecules27238256