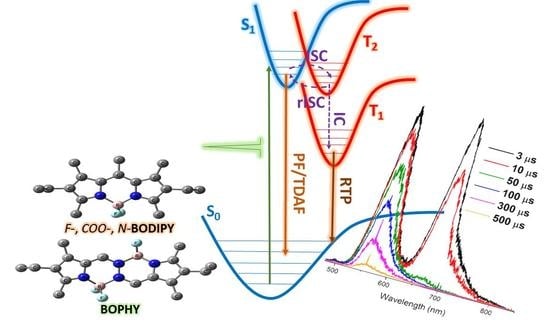
A Computational-Experimental Approach to Unravel the Excited State Landscape in Heavy-Atom Free BODIPY-Related Dyes
Abstract
1. Introduction
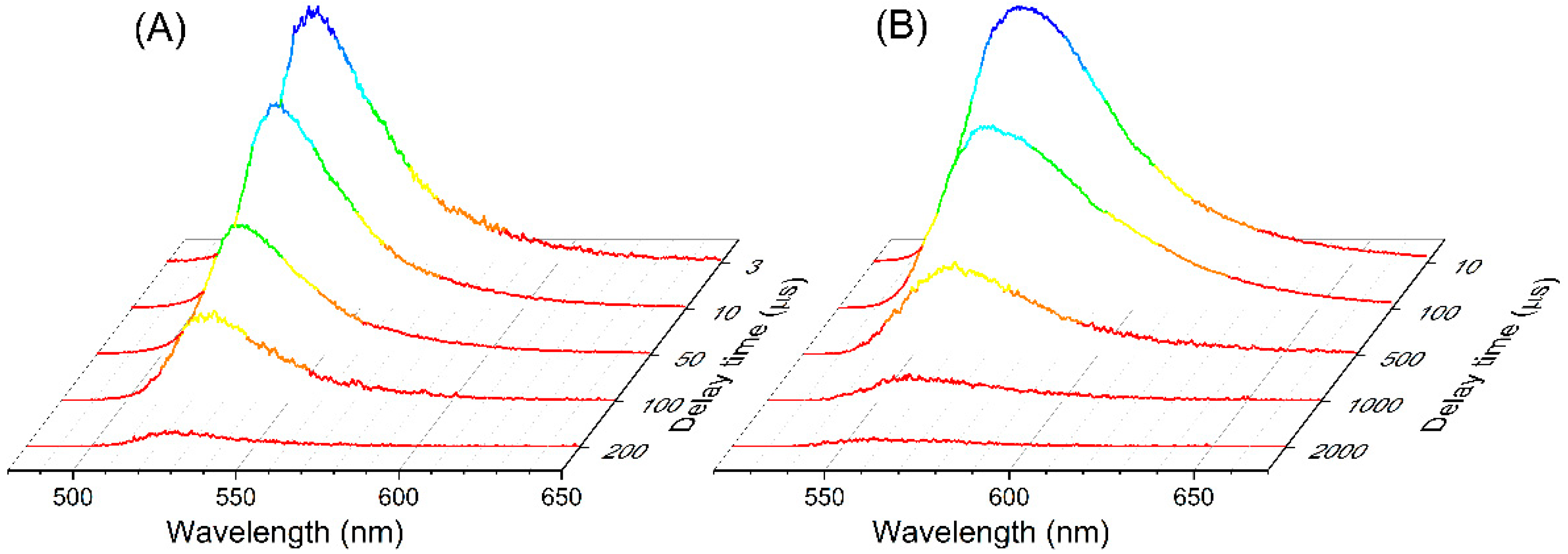
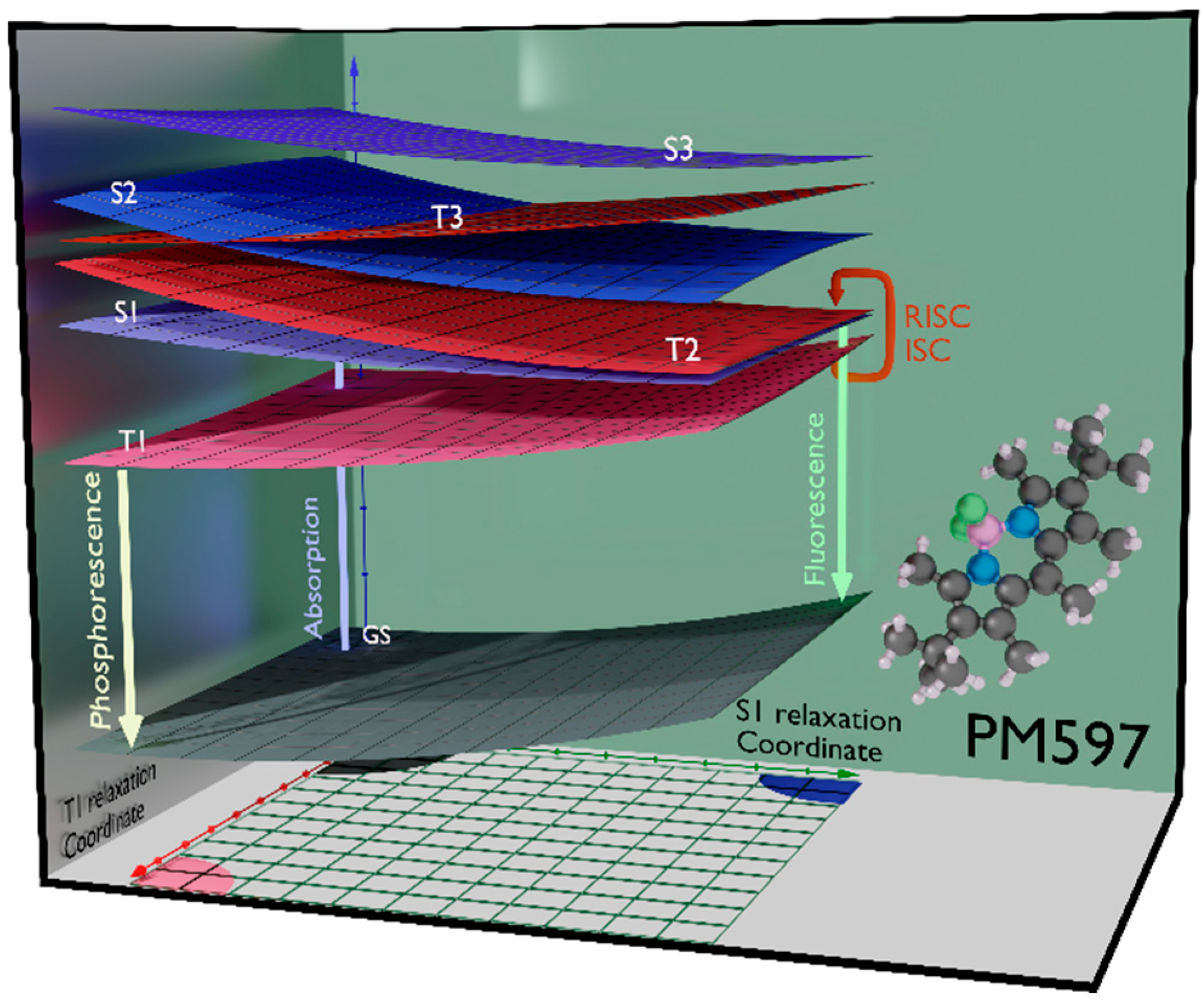
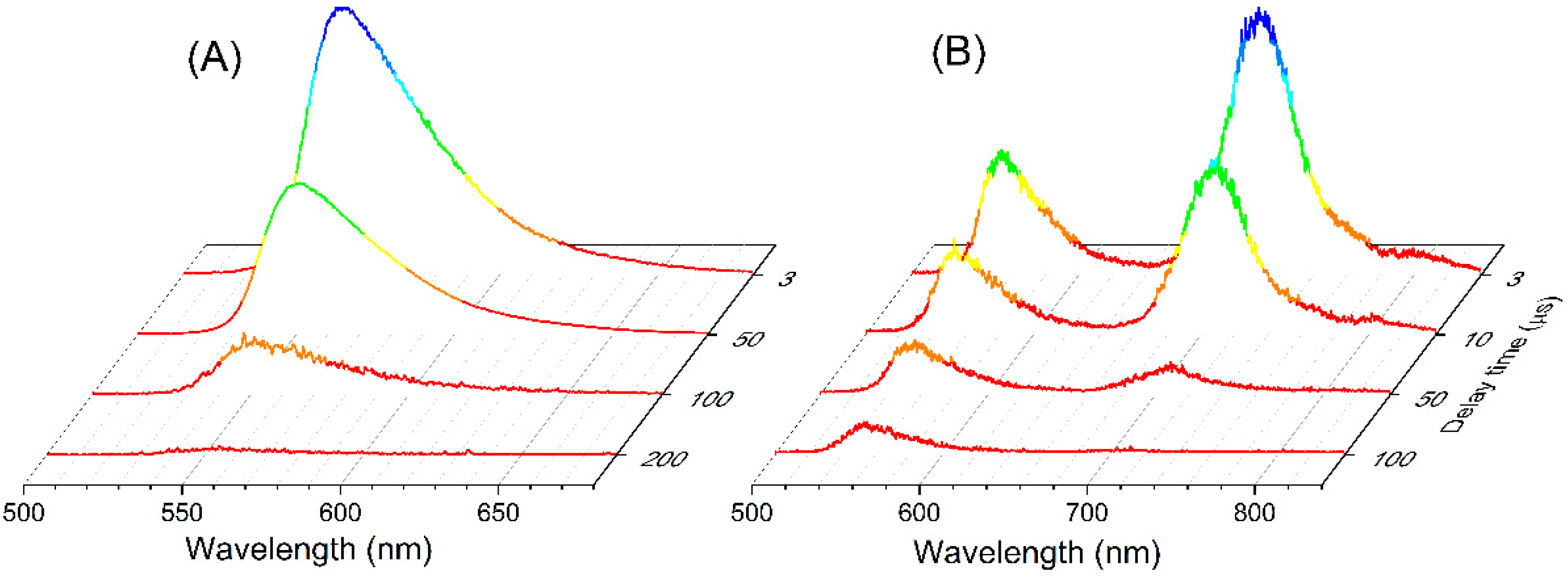
2. Results and Discussion
2.1. Commercial BODIPY Dyes
2.2. At-Boron-Functionalized BODIPY Dye and Related Chromophores
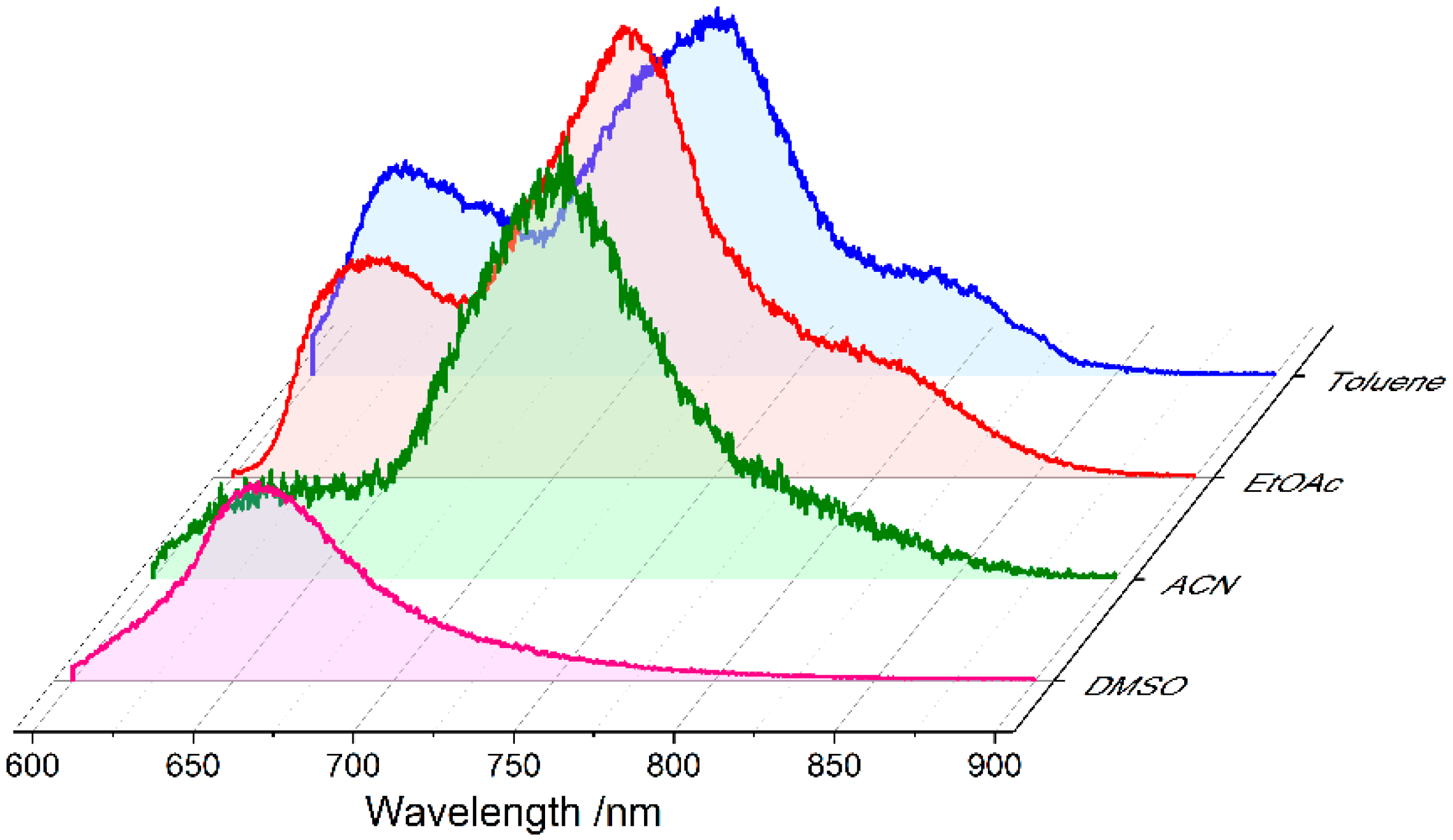
2.3. Charge Transfer Role in the Delayed Emission: The Example of PM650
3. Materials and Methods
3.1. Materials
3.2. Steady-State and Time-Resolved Spectroscopy
3.3. Laser Spectroscopy
3.4. Computational Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent Advances in Organic Thermally Activated Delayed Fluorescence Materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, W.; La, J.W.Y.; Peng, Q.; Ma, H.; Liang, G.; Shuai, Z.; Tang, B.Z. White Light Emission from a Single Organic Molecule with Dual Phosphorescence at Room Temperature. Nat. Commun. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.K.; Kim, D.; Bredas, J.L. Thermally Activated Delayed Fluorescence (TADF) Path toward Efficient Electroluminescence in Purely Organic Materials: Molecular Level Insight. Acc. Chem. Res. 2018, 51, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Forni, A.; Lucenti, E.; Botta, C.; Cariati, E. Metal Free Room Temperature Phosphorescence from Molecular Self-Interactions in the Solid State. J. Mater. Chem. C 2018, 6, 4603–4626. [Google Scholar] [CrossRef]
- Kenry; Chen, C.; Liu, B. Enhancing the Performance of Pure Organic Room-Temperature Phosphorescent Luminophores. Nat. Commun. 2019, 10, 2111. [Google Scholar] [CrossRef]
- Pershin, A.; Hall, D.; Lemaur, V.; Sancho-Garcia, J.C.; Muccioli, L.; Zysman-Colman, E.; Beljonne, D.; Olivier, Y. Highly Emissive Excitons with Reduced Exchange Energy in Thermally Activated Delayed Fluorescent Molecules. Nat. Commun. 2019, 10, 597. [Google Scholar] [CrossRef]
- Wang, Z.; Sukhanov, A.A.; Toffoletti, A.; Sadiq, F.; Zhao, J.; Barbon, A.; Voronkova, V.K.; Dick, B. Insights into the Efficient Intersystem Crossing of Bodipy-Anthracene Compact Dyads with Steady-State and Time-Resolved Optical/Magnetic Spectroscopies and Observation of the Delayed Fluorescence. J. Phys. Chem. C 2019, 123, 265–274. [Google Scholar] [CrossRef]
- Mahmood, Z.; Taddei, M.; Rehmat, N.; Bussotti, L.; Doria, S.; Guan, Q.; Ji, S.; Zhao, J.; Di Donato, M.; Huo, Y.; et al. Color-Tunable Delayed Fluorescence and Efficient Spin–Orbit Charge Transfer Intersystem Crossing in Compact Carbazole-Anthracene-Bodipy Triads Employing the Sequential Electron Transfer Approach. J. Phys. Chem. C 2020, 124, 5944–5957. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gálan, L.A.; Castán, J.M.A.; Dalinot, C.; Marqués, P.S.; Galiana, J.; Blanchard, P.; Andraud, C.; Dumont, E.; Maury, O.; Cabanetos, C.; et al. Exploring the Concept of Dimerization-Induced Intersystem Crossing: At the Origins of Spin-Orbit Coupling Selection Rules. J. Phys. Chem. B 2021, 125, 8572–8580. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Lai, H.Y.; Lee, W.K.; Jiao, M.; Shiu, Y.J.; Zhong, C.; Gong, S.; Zhou, T.; Xie, G.; Sarma, M.; et al. Organic Light-Emitting Diodes: Achieving Nearly 30% External Quantum Efficiency for Orange-Red Organic Light Emitting Diodes by Employing Thermally Activated Delayed Fluorescence Emitters Composed of 1,8-Naphthalimide-Acridine Hybrids. Adv. Mater. 2018, 30, 1704961. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Tao, W.W.; Xiao, Y.F.; Wang, K.; Zhang, M.; Fan, X.C.; Chen, W.C.; Yu, J.; Li, S.; Geng, F.X.; et al. Efficient Orange-Red Thermally Activated Delayed Fluorescence Emitters Feasible for Both Thermal Evaporation and Solution Process. ACS Appl. Mater. Interfaces 2019, 32, 29086–29093. [Google Scholar] [CrossRef]
- Chen, D.; Rajamalli, P.; Tenopala-Carmona, F.; Carpenter-Warren, C.L.; Cordes, D.B.; Keum, C.M.; Slawin, A.M.Z.; Gather, M.C.; Zysman-Colman, E. Bipyridine-Containing Host Materials for High Performance Yellow Thermally Activated Delayed Fluorescence-Based Organic Light Emitting Diodes with Very Low Efficiency Roll-Off. Adv. Opt. Mater. 2020, 8, 1901283. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940. [Google Scholar] [CrossRef]
- Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- Ceroni, P. Design of Phosphorescent Organic Molecules: Old Concepts under a New Light. Chem 2016, 1, 522–530. [Google Scholar] [CrossRef][Green Version]
- Ma, H.; Peng, Q.; An, Z.; Huang, W.; Shuai, Z. Efficient and Long-Lived Room-Temperature Organic Phosphorescence: Theoretical Descriptors for Molecular Designs. J. Am. Chem. Soc. 2019, 141, 1010–1015. [Google Scholar] [CrossRef]
- Lu, A.; Ye, W.; Jiang, X.; Gan, N.; Shi, H.; Yao, W.; Ma, H.; An, Z.; Huang, W. Mechanism of Single-Photon Upconversion Photoluminescence in All-Inorganic Perovskite Nanocrystals: The Role of Self-Trapped Excitons. J. Phys. Chem. Lett. 2019, 10, 1037–1042. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A. Heavy-Atom-Free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, X.; Tuan, H. A Facile Way to Obtain Near-Infrared Room-Temperature Phosphorescent Soft Materials Based on Bodipy Dyes. Chem. Sci. 2020, 11, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Lopez Arbeloa, F.; Bañuelos, J.; Martinez, V.; Arbeloa, T.; Lopez Arbeloa, I. Structural, Photophysical and Lasing Properties of Pyrromethene Dyes. Int. Rev. Phys. Chem. 2005, 24, 339–374. [Google Scholar] [CrossRef]
- Garcia-Moreno, I.; Postils, V.; Rebollar, E.; Ortiz, M.J.; Agarrabeitia, A.R.; Casanova, D. Generation of Multiple Triplet States in an Orthogonal Bodipy Dimer: A Breaktrough Spectroscopic and Theoretical Approach. Phys. Chem. Chem. Phys. 2022, 24, 52929–55938. [Google Scholar] [CrossRef]
- De Vetta, M.; González, L.; Corral, I. The Role of Electronic Triplet States and High-Lying Singlet States in the Deactivation Mechanism of the Parent BODIPY: An ADC(2) and CASPT2 Study. ChemPhotoChem 2019, 3, 727–738. [Google Scholar] [CrossRef]
- Buyuktemiz, M.; Duman, S.; Dede, Y. Luminescence of BODIPY and Dipyrrin: An MCSCF Comparison of Excited States. J. Phys. Chem. A 2013, 117, 1665–1669. [Google Scholar] [CrossRef]
- Postils, V.; Ruipérez, F.; Casanova, D. Mild Open-Shell Character of BODIPY and Its Impact on Singlet and Triplet Excitation Energies. J. Chem. Theory Comput. 2021, 17, 5825–5838. [Google Scholar] [CrossRef] [PubMed]
- Komoto, K.T.; Kowalczyk, T. How Parallel Are Excited State Potential Energy Surfaces from Time-Independent and Time-DFT? A BODIPY Case Study. J. Phys. Chem. A 2016, 102, 8160–8168. [Google Scholar] [CrossRef] [PubMed]
- Prlj, A.; Vannay, L.; Corminboeuf, C. Fluorescence Quenching in BODIPY Dyes: The Role of Intramolecular Interactions and Charge Transfer. Helv. Chim. Acta 2017, 100, e1700093. [Google Scholar] [CrossRef]
- Lin, Z.; Kohn, A.W.; Voorhis, T.V. Toward Prediction of Nonradiative Decay Pathways in Organic Compounds II: Two Internal Conversion Channels in BODIPYs. J. Phys. Chem. C 2020, 124, 3925–3938. [Google Scholar] [CrossRef]
- Boodts, S.; Fron, E.; Hofkens, J.; Dehaen, W. The BOPHY Fluorophore with Double Boron Chelation: Synthesis and Spectroscopy. Coord. Chem. Rev. 2018, 371, 1–10. [Google Scholar] [CrossRef]
- Bismillah, A.N.; Aprahamian, I. Fundamental studies to emerging applications of pyrrole-BF2 (BOPHY) fluorophores. Chem. Soc. Rev. 2021, 50, 5631–5649. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Momeni, M.R.; Brown, A. Why Do TD-DFT Excitation Energies of BODIPY/Aza-BODIPY Families Largely Deviate from Experiment? Answers from Electron Correlated and Multireference Methods. J. Chem. Theory Comput. 2015, 11, 2619–2632. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Schad, C.; Moreno, F.; Maroto, B.L.; Bañuelos, J.; Arbeloa, T.; Garcia-Moreno, I.; Villafuerte, C.; Muller, G.; de la Moya, S. BCl3-Activated Synthesis of COO-BODIPY Laser Dyes: General Scope and High Yields under Mild Conditions. J. Org. Chem. 2020, 85, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Schad, C.; Avellanal, E.; Moreno, F.; Maroto, B.L.; Bañuelos, J.; Garcia-Moreno, I.; de la Moya, S. Multichromophoric COO-BODIPYs: An advantageous design for the development of energy transfer and electron transfer systems. Chem. Commun. 2020, 56, 13025–13028. [Google Scholar] [CrossRef]
- Ray, C.; Diaz, L.; Avellanal, E.; Bañuelos, J.; Cerdan, L.; Garcia-Moreno, I.; Moreno, F.; Maroto, B.L.; Lopez-Arbeloa, I.; de la Moya, S. N-BODIPYs Come into Play: Smart Dyes for Photonic Materials. Chem. Eur. J. 2017, 23, 9383–9390. [Google Scholar] [CrossRef] [PubMed]
- Sola, R.; Jimenez, J.; Avellanal, E.; Johnson, M.; Cabreros, T.A.; Moreno, F.; Maroto, B.L.; Muller, G.; Bañuelos, J.; Cerdan, L.; et al. BOPHYs versus BODIPYs: A comparison of their performance as effective multi-function organic dyes. Dyes Pigments 2019, 170, 107662. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Y.; Huang, J.; Wang, Z.M.; Yu, D.W.; Yang, B.; Ma, Y.G. Computational investigation on the large energy gap between the triplet excited-states in acenes. RSC Adv. 2017, 7, 26697–26703. [Google Scholar] [CrossRef]
- Lopez Arbeloa, F.; Bañuelos, J.; Martinez, V.; Arbeloa, T.; Lopez Arbeloa, I. Intramolecular Charge Transfer in Pyrromethene Laser Dyes: Photophysical Behaviour of PM650. ChemPhysChem 2004, 5, 1762–1771. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with BODIPY dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- TURBOMOLE V7.5.1 2021, A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, Since 2007. Available online: https://www.turbomole.org (accessed on 12 February 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebollar, E.; Bañuelos, J.; de la Moya, S.; Eng, J.; Penfold, T.; Garcia-Moreno, I. A Computational-Experimental Approach to Unravel the Excited State Landscape in Heavy-Atom Free BODIPY-Related Dyes. Molecules 2022, 27, 4683. https://doi.org/10.3390/molecules27154683
Rebollar E, Bañuelos J, de la Moya S, Eng J, Penfold T, Garcia-Moreno I. A Computational-Experimental Approach to Unravel the Excited State Landscape in Heavy-Atom Free BODIPY-Related Dyes. Molecules. 2022; 27(15):4683. https://doi.org/10.3390/molecules27154683
Chicago/Turabian StyleRebollar, Esther, Jorge Bañuelos, Santiago de la Moya, Julien Eng, Thomas Penfold, and Inmaculada Garcia-Moreno. 2022. "A Computational-Experimental Approach to Unravel the Excited State Landscape in Heavy-Atom Free BODIPY-Related Dyes" Molecules 27, no. 15: 4683. https://doi.org/10.3390/molecules27154683
APA StyleRebollar, E., Bañuelos, J., de la Moya, S., Eng, J., Penfold, T., & Garcia-Moreno, I. (2022). A Computational-Experimental Approach to Unravel the Excited State Landscape in Heavy-Atom Free BODIPY-Related Dyes. Molecules, 27(15), 4683. https://doi.org/10.3390/molecules27154683