Highly Dispersed Ni Nanoclusters Spontaneously Formed on Hydrogen Boride Sheets
Abstract
1. Introduction
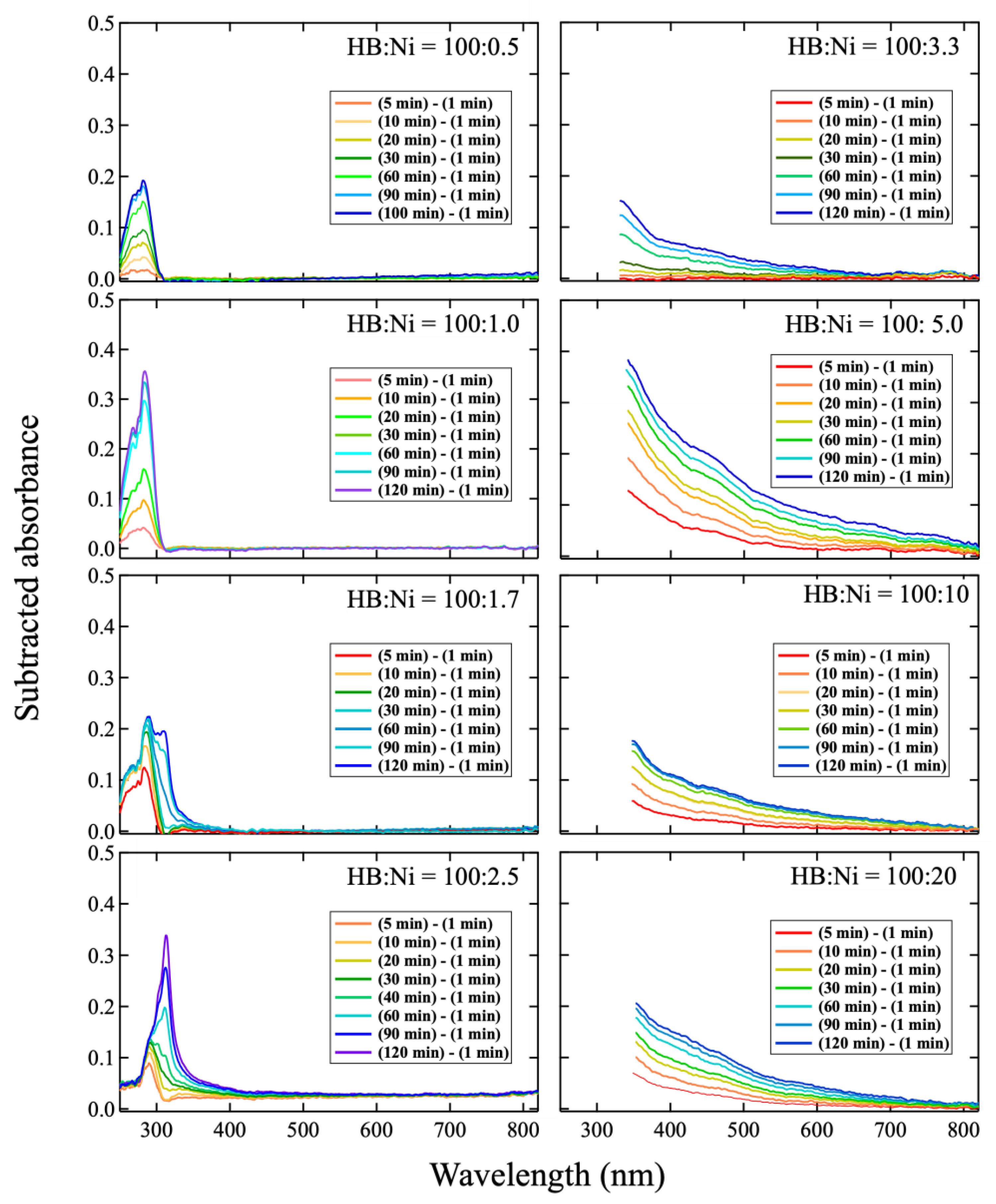
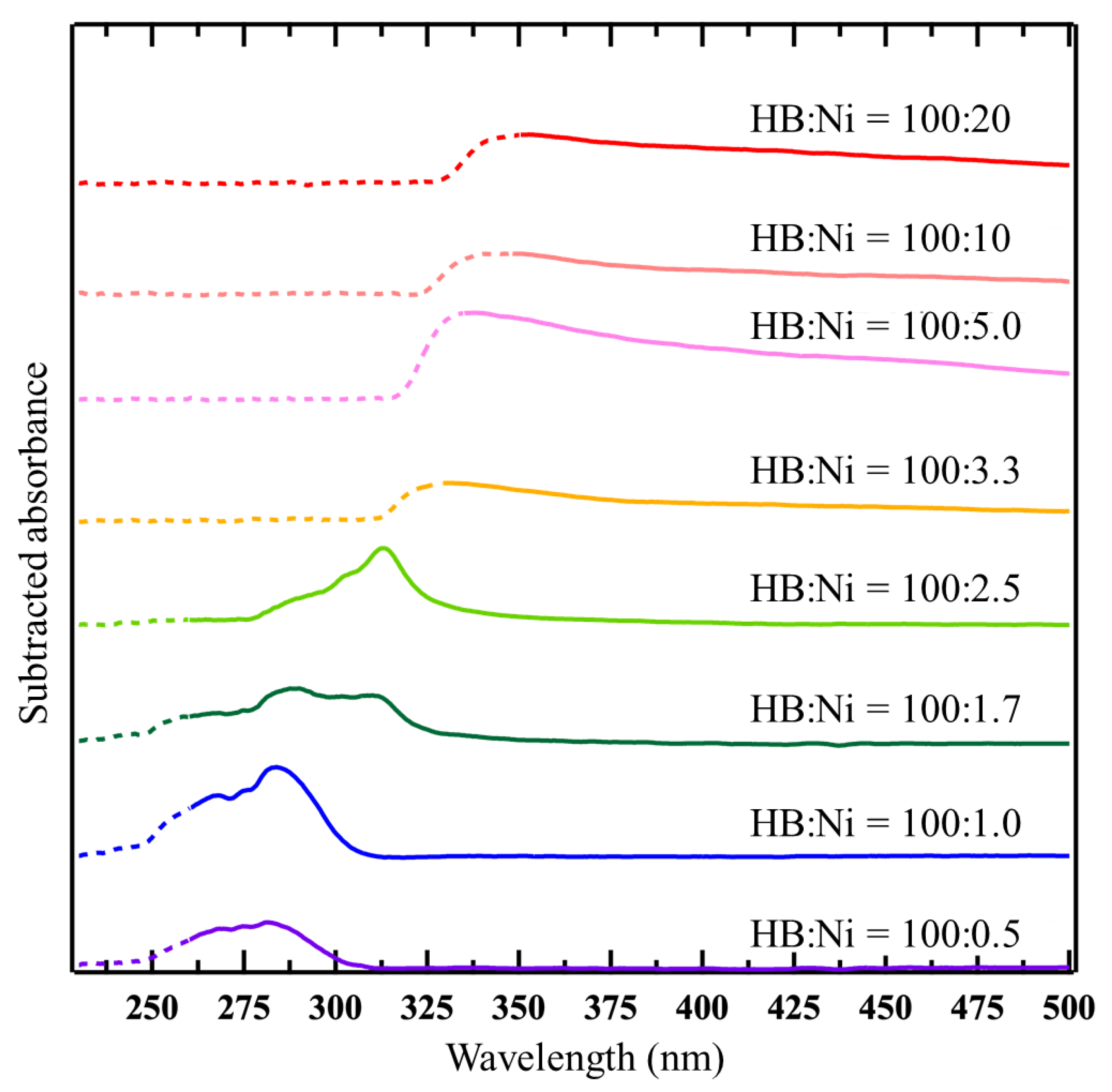
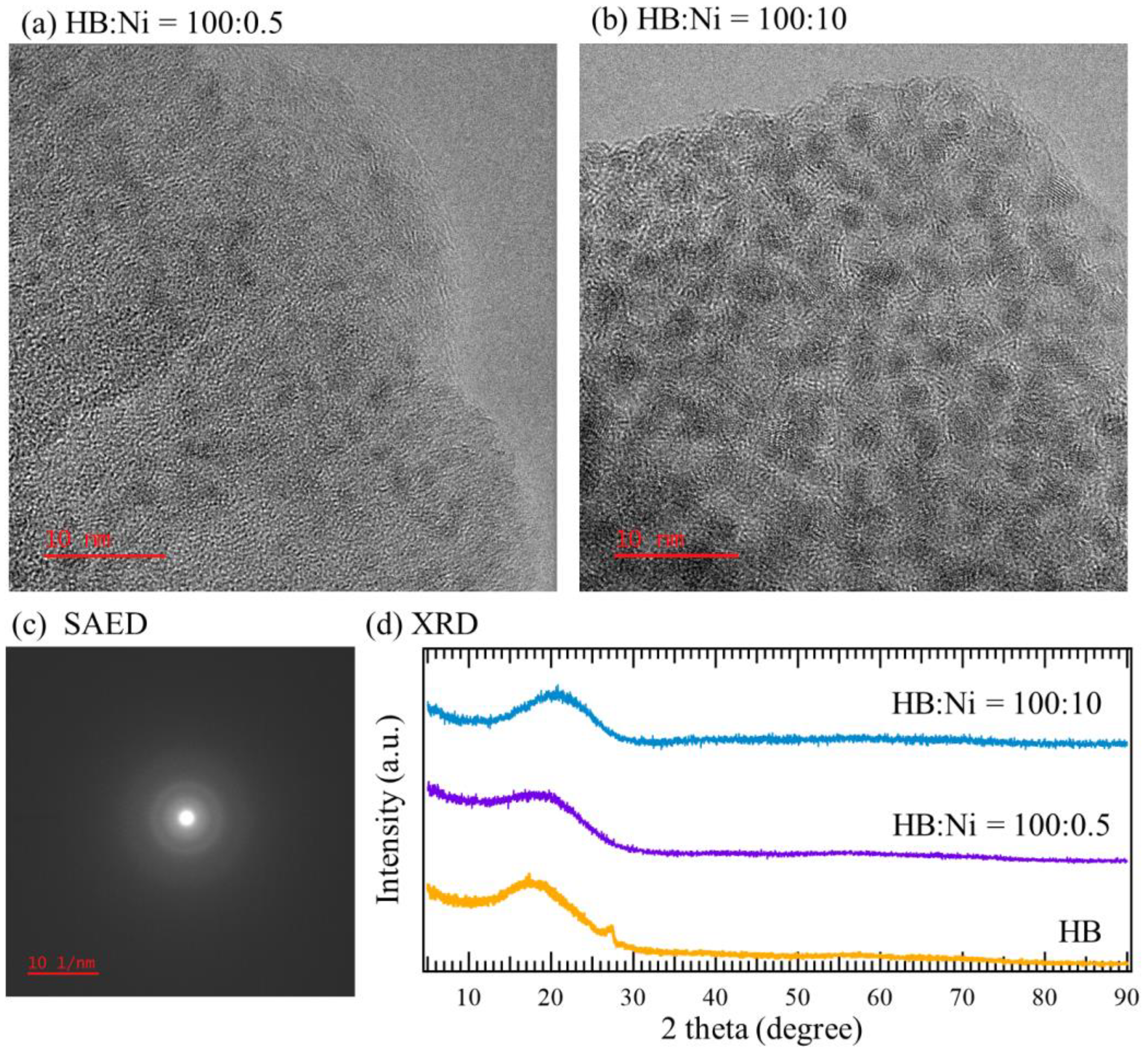
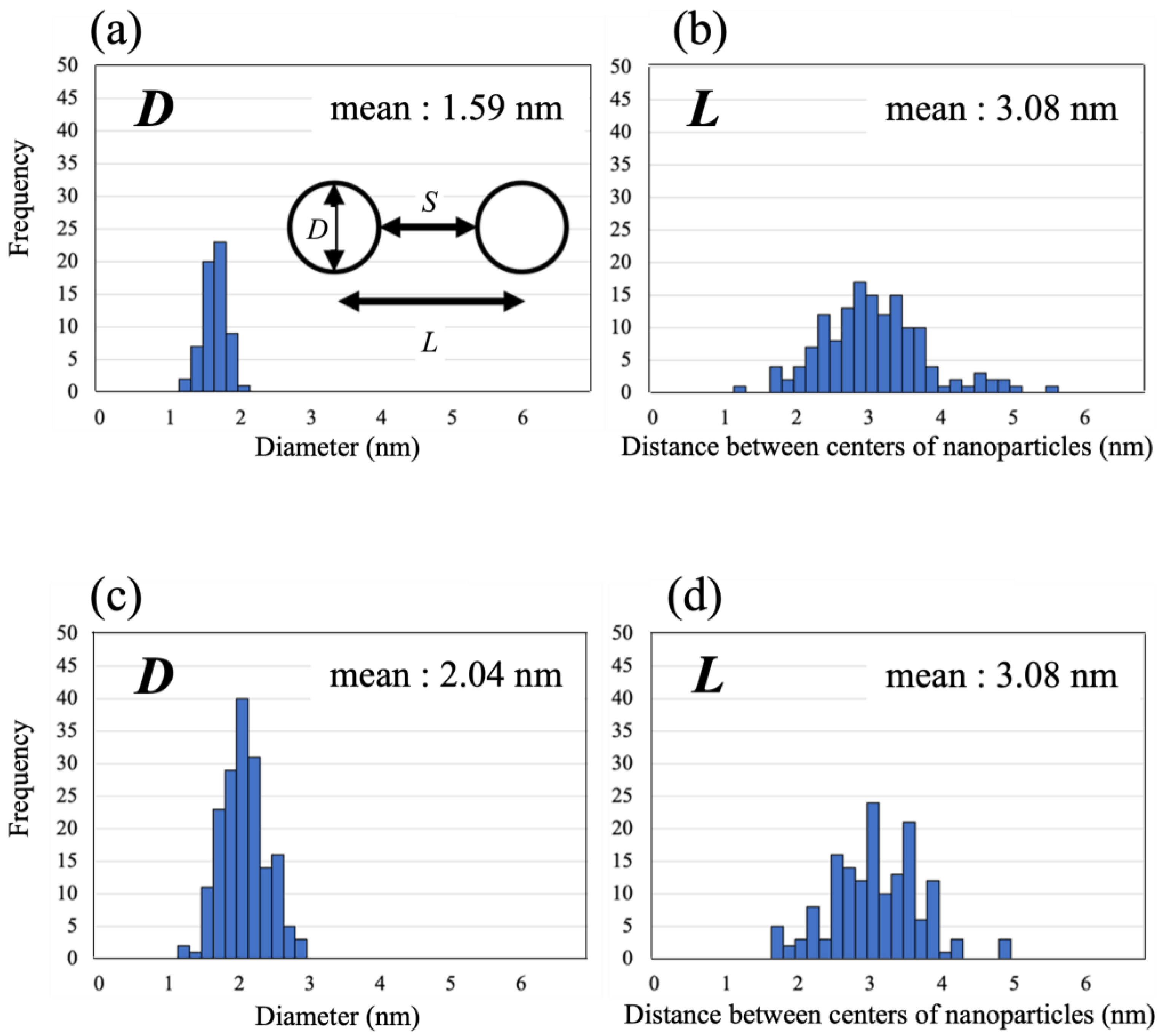
2. Results and Discussion
→ 2H+ + Ni·(n − 2)H+(n − 1)B−·B+
3. Materials and Methods
3.1. Materials
3.2. Synthesis of HB Sheets
3.3. XPS Measurements
3.4. UV-vis Measurements
3.5. TEM, SAED, and EDS Measurements
3.6. XRD Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nishino, H.; Fujita, T.; Cuong, N.T.; Tominaka, S.; Miyauchi, M.; Iimura, S.; Hirata, A.; Umezawa, N.; Okada, S.; Nishibori, E.; et al. Formation and Characterization of Hydrogen Boride Sheets Derived from MgB2 by Cation Exchange. J. Am. Chem. Soc. 2017, 139, 13761–13769. [Google Scholar] [CrossRef] [PubMed]
- Tominaka, S.; Ishibiki, R.; Fujino, A.; Kawakami, K.; Ohara, K.; Masuda, T.; Matsuda, I.; Hosono, H.; Kondo, T. Geometrical Frustration of B-H Bonds in Layered Hydrogen Borides Accessible by Soft Chemistry. Chem 2020, 6, 406–418. [Google Scholar] [CrossRef]
- Rojas, K.I.M.; Cuong, N.T.; Nishino, H.; Ishibiki, R.; Ito, S.I.; Miyauchi, M.; Fujimoto, Y.; Tominaka, S.; Okada, S.; Hosono, H.; et al. Chemical Stability of Hydrogen Boride Nanosheets in Water. Commun. Mater. 2021, 2, 81. [Google Scholar] [CrossRef]
- Fujino, A.; Ito, S.I.; Goto, T.; Ishibiki, R.; Kondo, J.N.; Fujitani, T.; Nakamura, J.; Hosono, H.; Kondo, T. Hydrogenated Borophene Shows Catalytic Activity as Solid Acid. ACS Omega 2019, 4, 14100–14104. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Ito, S.I.; Goto, T.; Ishibiki, R.; Osuga, R.; Kondo, J.N.; Fujitani, T.; Nakamura, J.; Hosono, H.; Kondo, T. Ethanol-Ethylene Conversion Mechanism on Hydrogen Boride Sheets Probed By in Situ infrared Absorption Spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 7724–7734. [Google Scholar] [CrossRef]
- Tateishi, I.; Cuong, N.T.; Moura, C.A.S.; Cameau, M.; Ishibiki, R.; Fujino, A.; Okada, S.; Yamamoto, A.; Araki, M.; Ito, S.; et al. Semimetallicity of Free-Standing Hydrogenated Monolayer Boron from MgB2. Phys. Rev. Mater. 2019, 3, 024004. [Google Scholar] [CrossRef]
- Kawamura, R.; Cuong, N.T.; Fujita, T.; Ishibiki, R.; Hirabayashi, T.; Yamaguchi, A.; Matsuda, I.; Okada, S.; Kondo, T.; Miyauchi, M. Photoinduced Hydrogen Release from Hydrogen Boride Sheets. Nat. Commun. 2019, 10, 4880. [Google Scholar] [CrossRef]
- Goto, T.; Ito, S.; Laxman Shinde, S.; Ishibiki, R.; Hikita, Y.; Matsuda, I.; Hamada, I.; Hosono, H.; Kondo, T. Carbon Dioxide Adsorption and Conversion to Methane and Ethane on Hydrogen Boride Sheets. Commun. Chem. 2022, 5, 118. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, F.; Bell, J.; Bilic, A.; Du, A. Two-Dimensional Boron Hydride Sheets: High Stability, Massless Dirac Fermions, and Excellent Mechanical Properties. Angew. Chem. Int. Ed. 2016, 55, 10292–10295. [Google Scholar] [CrossRef]
- Mortazavi, B.; Makaremi, M.; Shahrokhi, M.; Raeisi, M.; Singh, C.V.; Rabczuk, T.; Pereira, L.F.C. Borophene Hydride: A Stiff 2D Material with High Thermal Conductivity and Attractive Optical and Electronic Properties. Nanoscale 2018, 10, 3759–3768. [Google Scholar] [CrossRef]
- He, J.; Li, D.; Ying, Y.; Feng, C.; He, J.; Zhong, C.; Zhou, H.; Zhou, P.; Zhang, G. Orbitally Driven Giant Thermal Conductance Associated with Abnormal Strain Dependence in Hydrogenated Graphene-like Borophene. NPJ Comput. Mater. 2019, 5, 47. [Google Scholar] [CrossRef]
- Abtew, T.A.; Shih, B.; Dev, P.; Crespi, V.H.; Zhang, P. Prediction of a Multicenter-Bonded Solid Boron Hydride for Hydrogen Storage. Phys. Rev. B 2011, 83, 094108. [Google Scholar] [CrossRef]
- Abtew, T.A.; Zhang, P. Charging-Assisted Hydrogen Release Mechanism in Layered Boron Hydride. Phys. Rev. B 2011, 84, 094303. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Duan, C.; Huang, Y.; Zhang, Q.; Xiao, B. Reversible Hydrogen Storage in Pristine and Li Decorated 2D Boron Hydride. Phys. Chem. Chem. Phys. 2018, 20, 30304–30311. [Google Scholar] [CrossRef]
- An, Y.; Hou, Y.; Wang, H.; Li, J.; Wu, R.; Wang, T.; Da, H.; Jiao, J. Unveiling the Electric-Current-Limiting and Photodetection Effect in Two-Dimensional Hydrogenated Borophene. Phys. Rev. Appl. 2019, 11, 064031. [Google Scholar] [CrossRef]
- Kumawat, R.L.; Jena, M.K.; Pathak, B. Individual Identification of Amino Acids on an Atomically Thin Hydrogen Boride System Using Electronic Transport Calculations. J. Phys. Chem. C 2020, 124, 27194–27202. [Google Scholar] [CrossRef]
- Shukla, V.; Araujo, R.B.; Jena, N.K.; Ahuja, R. Borophene’s Tryst with Stability: Exploring 2D Hydrogen Boride as an Electrode for Rechargeable Batteries. Phys. Chem. Chem. Phys. 2018, 20, 22008–22016. [Google Scholar] [CrossRef]
- Makaremi, M.; Mortazavi, B.; Singh, C.V. 2D Hydrogenated Graphene-like Borophene as a High Capacity Anode Material for Improved Li/Na Ion Batteries: A First Principles Study. Mater. Today Energy 2018, 8, 22–28. [Google Scholar] [CrossRef]
- Xiang, P.; Chen, X.; Xiao, B.; Wang, Z.M. Highly Flexible Hydrogen Boride Monolayers as Potassium-Ion Battery Anodes for Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 8115–8125. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Yasuhara, S.; Shoji, S.; Yamaguchi, A.; Abe, H.; Ueda, S.; Zhu, H.; Kondo, T.; Miyauchi, M. Fabrication of Hydrogen Boride Thin Film by Ion Exchange in MgB2. Molecules 2021, 26, 6212. [Google Scholar] [CrossRef]
- Cuong, N.T.; Tateishi, I.; Cameau, M.; Niibe, M.; Umezawa, N.; Slater, B.; Yubuta, K.; Kondo, T.; Ogata, M.; Okada, S.; et al. Topological Dirac Nodal Loops in Nonsymmorphic Hydrogenated Monolayer Boron. Phys. Rev. B 2020, 101, 195412. [Google Scholar] [CrossRef]
- Zhang, X.; Tsujikawa, Y.; Tateishi, I.; Niibe, M.; Wada, T.; Horio, M.; Hikichi, M.; Ando, Y.; Yubuta, K.; Kondo, T.; et al. Electronic Topological Transition of 2D Boron by the Ion Exchange Reaction. J. Phys. Chem. C 2022, 126, 12802–12808. [Google Scholar] [CrossRef]
- Oliva-Enrich, J.M.; Kondo, T.; Alkorta, I.; Elguero, J.; Klein, D.J. Diborane Concatenation Leads to New Planar Boron Chemistry. ChemPhysChem 2020, 21, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Ploysongsri, N.; Vchirawongkwin, V.; Ruangpornvisuti, V. Hydrogen Boride Nanotubes and Their C, N, O Decoration and Doping Derivatives as Materials for Hydrogen-Containing Gases Storage and Sensing: A SCC–DFTB Study. Vacuum 2021, 187, 110140. [Google Scholar] [CrossRef]
- Lei, B.; Zhang, Y.-Y.; Du, S.-X. Band Engineering of B2H2 Nanoribbons. Chin. Phys. B 2019, 28, 046803. [Google Scholar] [CrossRef]
- Kondo, T. Recent progress in boron nanomaterials. Sci. Technol. Adv. Mater. 2017, 18, 780–804. [Google Scholar] [CrossRef] [PubMed]
- Ozin, G.; Siler, T. Catalyst: New Materials Discovery: Machine-Enhanced Human Creativity. Chem 2018, 4, 1183–1189. [Google Scholar] [CrossRef]
- Niibe, M.; Cameau, M.; Cuong, N.T.; Sunday, O.I.; Zhang, X.; Tsujikawa, Y.; Okada, S.; Yubuta, K.; Kondo, T.; Matsuda, I. Electronic Structure of a Borophene Layer in Rare-Earth Aluminum/Chromium Boride and Its Hydrogenated Derivative Borophane. Phys. Rev. Mater. 2021, 5, 084007. [Google Scholar] [CrossRef]
- Ito, S.I.; Hirabayashi, T.; Ishibiki, R.; Kawamura, R.; Goto, T.; Fujita, T.; Yamaguchi, A.; Hosono, H.; Miyauchi, M.; Kondo, T. Hydrogen Boride Sheets as Reductants and the Formation of Nanocomposites with Metal Nanoparticles. Chem. Lett. 2020, 49, 789–793. [Google Scholar] [CrossRef]
- Saad, A.; Liu, D.; Wu, Y.; Song, Z.; Li, Y.; Najam, T.; Zong, K.; Tsiakaras, P.; Cai, X. Ag Nanoparticles Modified Crumpled Borophene Supported Co3O4 Catalyst Showing Superior Oxygen Evolution Reaction (OER) Performance. Appl. Catal. B 2021, 298, 120529. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Bi, J.; Wang, B.; Li, C.; Liu, J.; Kong, C.; Yang, S.; Yang, S. 2D Hydrogenated Boride as a Reductant and Stabilizer for in Situ Synthesis of Ultrafine and Surfactant-Free Carbon Supported Noble Metal Electrocatalysts with Enhanced Activity and Stability. J. Mater. Chem. A Mater 2020, 8, 18856–18862. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Zhang, Q. Enhanced Hydrogen Desorption Properties of MgH2 by Highly Dispersed Ni: The Role of in-Situ Hydrogenolysis of Nickelocene in Ball Milling Process. J. Alloys Compd. 2022, 900, 163547. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.W.; Khan, D.; Dong, J.J.; Sun, F.Z.; Xu, H.; Zhang, Q.Y.; Zou, J.X. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ooki, W.; Kosaka, Y.R.; Okonogi, A.; Marzun, G.; Wagener, P.; Barcikowski, S.; Kondo, T.; Nakamura, J. Effect of pH on the spontaneous synthesis of palladium nanoparticles on reduced graphene oxide. Appl. Surf. Sci. 2016, 389, 911. [Google Scholar] [CrossRef]
- Itahara, H.; Nakano, H. Formation mechanism of Cu2O particles using layered CaSi2 as a reduction/oxidation mediator. J. Am. CerAm. Soc. 2019, 102, 5738. [Google Scholar] [CrossRef]
- Ohashi, M.; Yaokawa, R.; Takatani, Y.; Nakano, H. Versatile Reducing Reaction Field within Layered Polysilane for Efficient One-Pot Synthesis of Metal Nanoparticles. ChemNanoMat 2017, 3, 534. [Google Scholar] [CrossRef]
- James, A.L.; Khandelwal, S.; Dutta, A.; Jasuja, K. Boron based nanosheets as reducing templates in aqueous solutions: Towards novel nanohybrids with gold nanoparticles and graphene. Nanoscale 2018, 10, 20514. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Theoretical Considerations; Springer: Berlin, Germany, 1995; pp. 13–201. ISBN 978-3-642-08191-0. [Google Scholar]
- Heath, J.R. Size-Dependent Surface-Plasmon Resonances of Bare Silver Particles. Phys. Rev. B 1989, 40, 9982–9985. [Google Scholar] [CrossRef]
- Vaishali, S.S.; Doss, K.; Banu, A.S.; Kurian, G.A. Desmodium Gangeticum Root Aqueous Extract Mediated Synthesis of Ni Nanoparticle and Its Biological Evaluation. Int. J. Pharm. Sci. 2015, 7, 141–146. [Google Scholar]
- Romero, I.; Aizpurua, J.; Bryant, G.W.; de Abajo, F.J.G. Plasmons in Nearly Touching Metallic Nanoparticles: Singular Response in the Limit of Touching Dimers. Opt. Express 2006, 14, 9988–9999. [Google Scholar] [CrossRef]
- Siburian, R.; Kondo, T.; Nakamura, J. Size Control to a Sub-Nanometer Scale in Platinum Catalysts on Graphene. J. Phys. Chem. C 2013, 117, 3635–3645. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Nishino, H.; Fujita, T.; Yamamoto, A.; Fujimori, T.; Fujino, A.; Ito, S.; Nakamura, J.; Hosono, H.; Kondo, T. Formation Mechanism of Boron-Based Nanosheet through the Reaction of MgB2 with Water. J. Phys. Chem. C 2017, 121, 10587–10593. [Google Scholar] [CrossRef]
- Kawamura, R.; Yamaguchi, A.; Shimada, C.; Ishibiki, R.; Fujita, T.; Kondo, T.; Miyauchi, M. Acid Assisted Synthesis of HB Sheets through Exfoliation of MgB2 Bulk in Organic Media. Chem. Lett. 2020, 49, 1194–1196. [Google Scholar] [CrossRef]
- Kusaka, H.; Ishibiki, R.; Toyoda, M.; Fujita, T.; Tokunaga, T.; Yamamoto, A.; Miyakawa, M.; Matsushita, K.; Miyazaki, K.; Li, L.; et al. Crystalline Boron Monosulfide Nanosheets with Tunable Bandgaps. J. Mater. Chem. A 2021, 9, 24631–24640. [Google Scholar] [CrossRef]

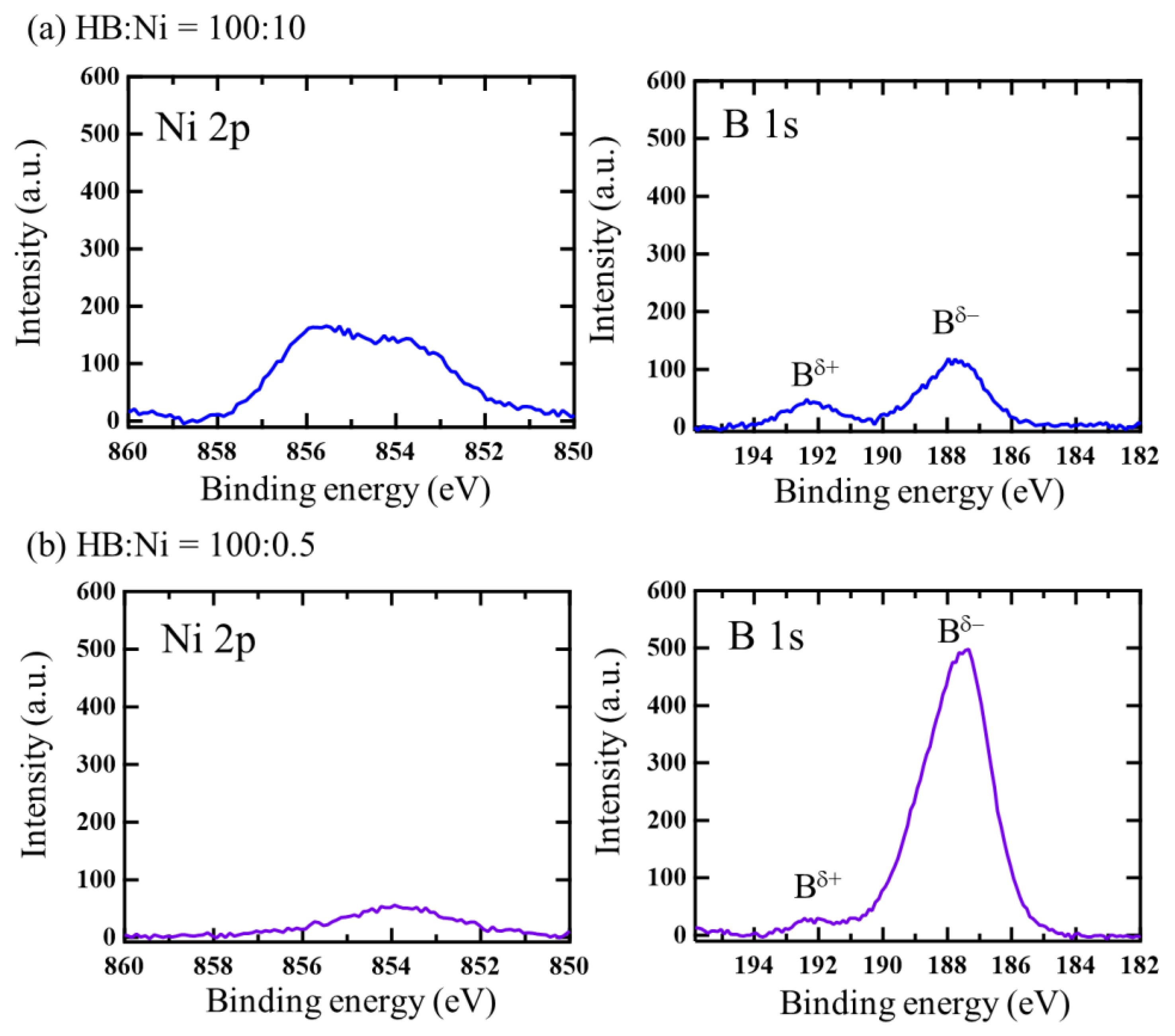
| Atomic Ratio Estimated | B 1s Peak | ||
|---|---|---|---|
| Sample | from Peak Area (B:Ni) | Bδ− | Bδ+ |
| HB:Ni = 100:0.5 | 100:0.5 | 96% | 4% |
| HB:Ni = 100:10 | 100:5.4 | 67% | 33% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, N.; Ito, S.-i.; Hikichi, M.; Cho, Y.; Goto, K.; Kubo, A.; Matsuda, I.; Fujita, T.; Miyauchi, M.; Kondo, T. Highly Dispersed Ni Nanoclusters Spontaneously Formed on Hydrogen Boride Sheets. Molecules 2022, 27, 8261. https://doi.org/10.3390/molecules27238261
Noguchi N, Ito S-i, Hikichi M, Cho Y, Goto K, Kubo A, Matsuda I, Fujita T, Miyauchi M, Kondo T. Highly Dispersed Ni Nanoclusters Spontaneously Formed on Hydrogen Boride Sheets. Molecules. 2022; 27(23):8261. https://doi.org/10.3390/molecules27238261
Chicago/Turabian StyleNoguchi, Natsumi, Shin-ichi Ito, Miwa Hikichi, Yohei Cho, Kazuho Goto, Atsushi Kubo, Iwao Matsuda, Takeshi Fujita, Masahiro Miyauchi, and Takahiro Kondo. 2022. "Highly Dispersed Ni Nanoclusters Spontaneously Formed on Hydrogen Boride Sheets" Molecules 27, no. 23: 8261. https://doi.org/10.3390/molecules27238261
APA StyleNoguchi, N., Ito, S.-i., Hikichi, M., Cho, Y., Goto, K., Kubo, A., Matsuda, I., Fujita, T., Miyauchi, M., & Kondo, T. (2022). Highly Dispersed Ni Nanoclusters Spontaneously Formed on Hydrogen Boride Sheets. Molecules, 27(23), 8261. https://doi.org/10.3390/molecules27238261









