Asperphenyltones A and B: New Phenylfuropyridinone Skeleton from an Endophytic Aspergillus sp. GXNU-A1
Abstract
1. Introduction
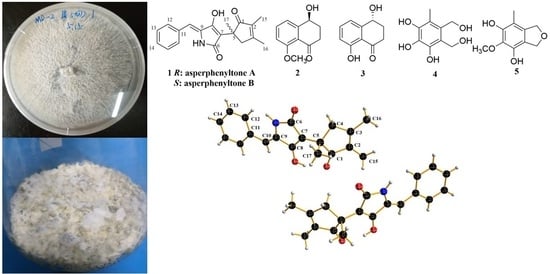
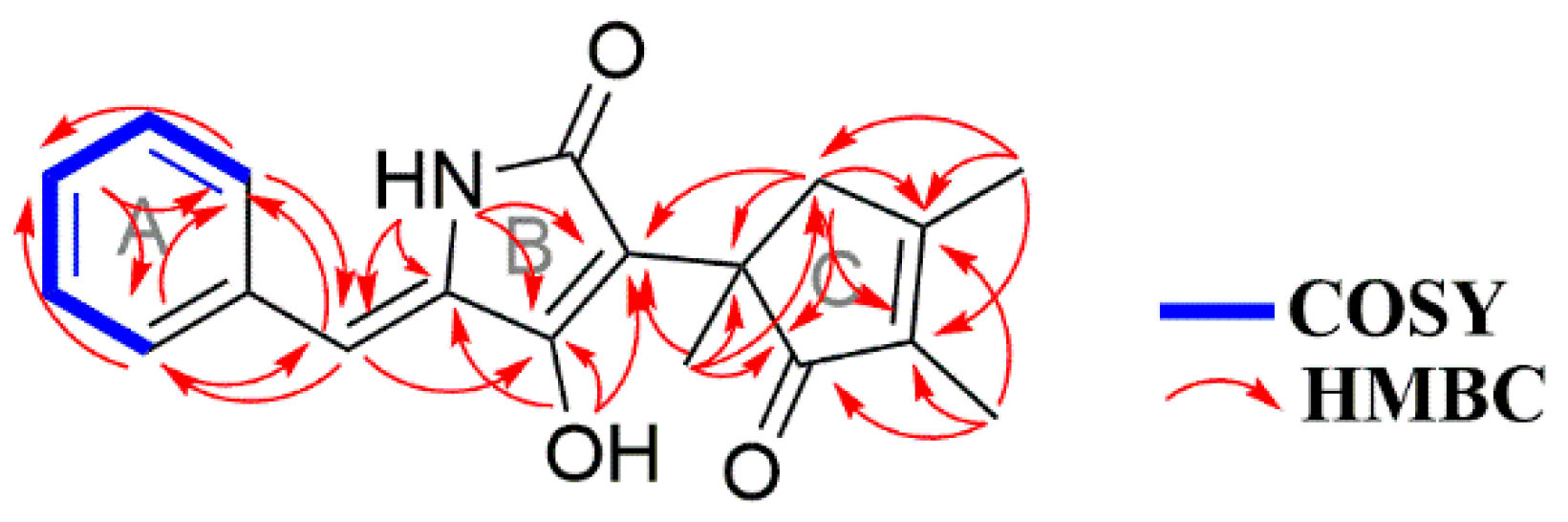
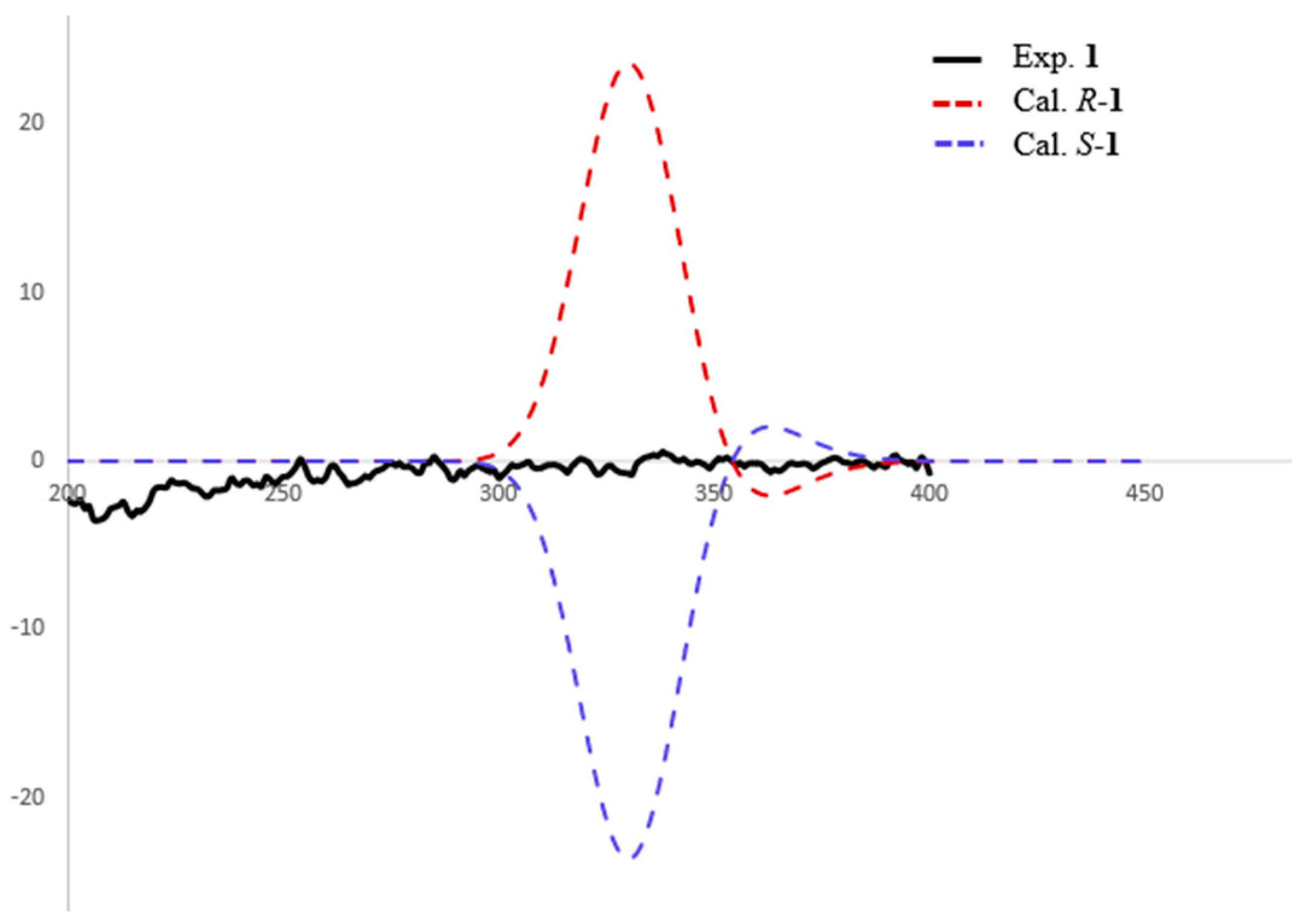
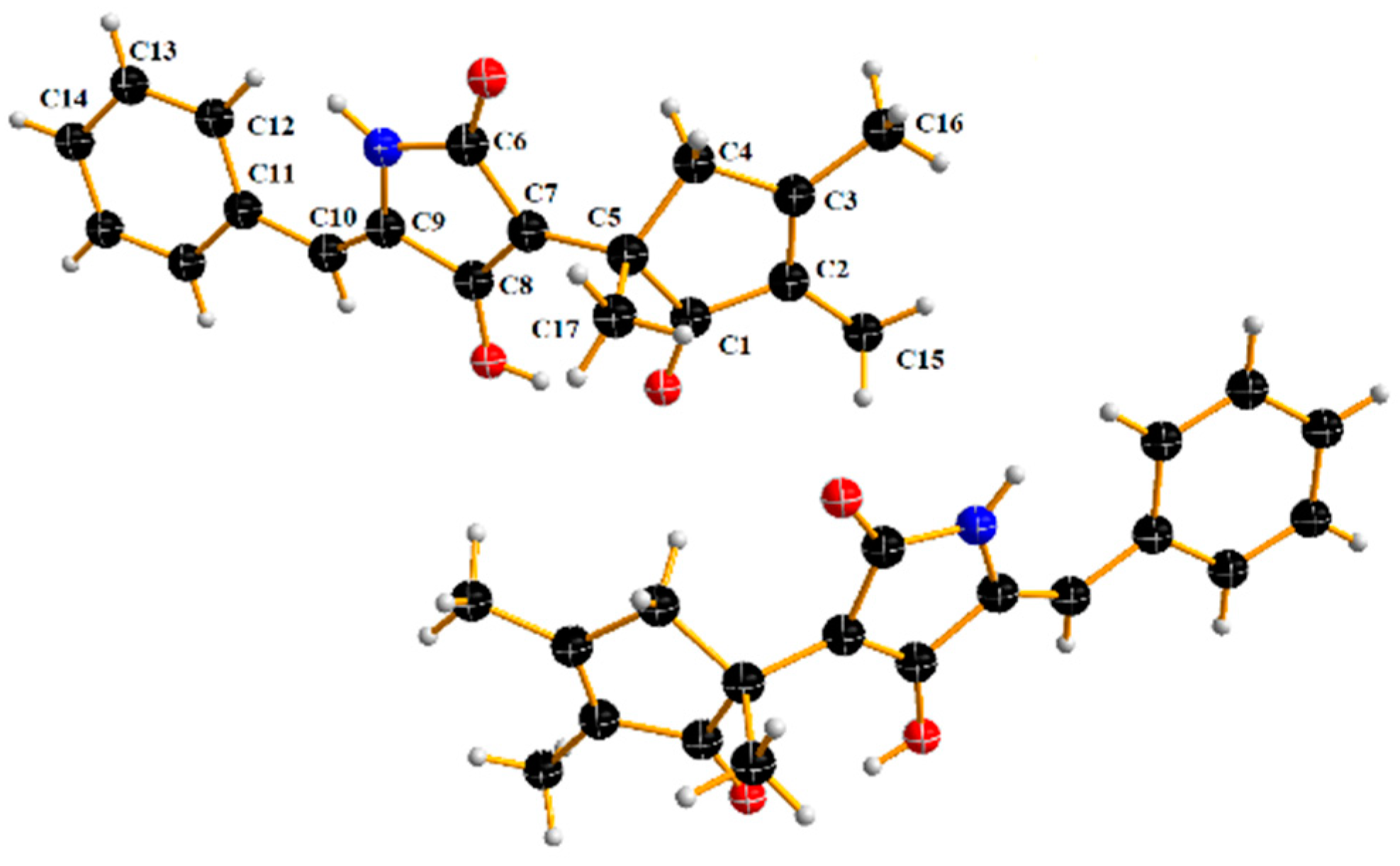
2. Results and Discussion
3. Experimental
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
Physicochemical and Spectral Data
3.4. Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.M.; Yang, W.C.; Cui, H.; Cai, R.L.; Li, C.Y.; Zou, G.; Wang, B.; She, Z.G. Two antimicrobial heterodimeric tetrahydroxanthones with a 7,7′-linkage from mangrove endophytic fungus Aspergillus flavus QQYZ. Molecules 2022, 27, 2691. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Ōmura, S. Citridones, new potentiators of antifungal miconazole activity, produced by Penicillium sp. FKI-1938 I. J. Antibiot. 2005, 58, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Tomoda, H.; Omura, S. Citridones, new potentiators of antifungal miconazole activity, produced by Penicillium sp. FKI-1938 II. J. Antibiot. 2005, 58, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.A.A.; Carvalho, J.M.; dos Santos, D.C.P.; de Oliveira Feitosa, A.; Marinho, P.S.B.; Guilhon, G.M.S.P.; de Souza, A.D.L.; da Silva, F.M.A.; do R. Marinho, A.M. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2012, 27, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ding, W.; Liu, H.; Wang, P.-M.; Zheng, D.-Q.; Xu, J. New pyridone alkaloids from marine-derived fungus Penicillium sp. Tetrahedron Lett. 2020, 61, 151843–151848. [Google Scholar] [CrossRef]
- Chen, S.-C.; Liu, Z.-M.; Tan, H.-B.; Chen, Y.-C.; Li, S.-N.; Li, H.-H.; Guo, H.; Zhu, S.; Liu, H.-X.; Zhang, W.-M. Tersone A-G, new pyridone alkaloids from the deep-sea fungus Phomopsis tersa. Mar. Drugs 2019, 17, 394. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.H.; Cui, H.; Liu, X.L.; Xiao, Z.E.; Wen, S.T.; She, Z.G.; Huang, X.S. Acetylcholinesterase inhibitory meroterpenoid from a mangrove endophytic fungus Aspergillus sp. 16-5c. Molecules 2017, 22, 727. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.L.; Zhou, D.X.; Qin, X.Y. A new depsidone derivative from mangrove endophytic fungus Aspergillus sp. GXNU-A9. Nat. Prod. Res. 2022, 36, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hao, L.; Qin, X.; Huang, J.; Yang, R.; Li, J.; Huang, X. A new lactone from mangrove endophytic fungus Aspergillus sp. GXNU-A9. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Huang, J.; Zhou, D.; Zhang, W.; Zhang, Y.; Li, J.; Yang, R.; Huang, X. Polyketide derivatives, guhypoxylonols A–D from a mangrove endophytic fungus Aspergillus sp. GXNU-Y45 that inhibit nitric oxide production. Mar. Drugs 2022, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Kamisuki, S.; Ishimaru, C.; Onoda, K.; Kuriyama, I.; Ida, N.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Nodulisporol and nodulisporone, novel specific inhibitors of human DNA polymerase λ from a fungus, Nodulisporium sp. Bioorg. Med. Chem. 2007, 15, 3109–3114. [Google Scholar] [CrossRef]
- Yang, N.N.; Ma, Q.Y.; Kong, F.D.; Xie, Q.Y.; Dai, H.F.; Zhou, L.M.; Yu, Z.F.; Zhao, Y.X. Napthrene compounds from mycelial fermentation products of Marasmius berteroi. Molecules 2020, 25, 3898. [Google Scholar] [CrossRef]
- Nishihara, Y.; Takase, S.; Tsujii, E.; Hatanaka, H.; Hashimoto, S. New anti-influenza agents, FR198248 and its derivatives II. characterization of FR198248, its related compounds and some derivatives. J. Antibiot. 2001, 54, 297–303. [Google Scholar] [CrossRef]
- Zou, G.; Tan, Q.; Chen, Y.; Yang, W.; Zang, Z.; Jiang, H.; Chen, S.; Wang, B.; She, Z. Furobenzotropolones A, B and 3-hydroxyepicoccone B with antioxidative activity from mangrove endophytic fungus Epicoccum nigrum MLY-3. Mar. Drugs 2021, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Deng, S.; Zhou, D.; Huang, Y.; Li, C.; Hao, L.; Zhang, G.; Su, S.; Xu, X.; Yang, R.; et al. 3,4-seco-dammarane triterpenoid saponins with anti-inflammatory activity from Cyclocarya paliurus leaves tea. J. Agric. Food Chem. 2020, 68, 2041–2053. [Google Scholar] [CrossRef] [PubMed]


| Position | δC Type | δH, Hz | HMBC |
|---|---|---|---|
| 1 | 213.5, C | ||
| 2 | 131.5, C | ||
| 3 | 171.6, C | ||
| 4 | 46.2, CH2 | 2.56 (1H, d, J = 19.0) 3.16 (1H, dd, J = 19.0, 3.8) | C-2, C-3, C-5, C-7, C-17 |
| 5 | 46.8, C | ||
| 6 | 171.7, C | ||
| 7 | 105.6, C | ||
| 8 | 159.7, C | ||
| 9 | 134.2 | ||
| 10 | 105.5, CH | 6.27 (1H, br s) | C-2, C-8, C-16 |
| 11 | 127.4 | ||
| 12 | 129.1, CH | 7.51 (2H, d, J = 7.4) | C-14, C-16 |
| 13 | 128.8, CH | 7.36 (2H, t, J = 7.4) | C-11, C-15 |
| 14 | 127.5, CH | 7.26 (1H, d, J = 7.4) | C-12, C-16 |
| 15 | 8.0, CH3 | 1.66 (3H, s) | C-1, C-2, C-3 |
| 16 | 17.1, CH3 | 2.07 (3H, s) | C-2, C-3, C-4 |
| 17 | 25.2, CH3 | 1.35 (3H, s) | C-1, C-4, C-5, C-7 |
| -OH -NH | 10.89 (1H, br s) 9.56 (1H, br s) | C-7, C-8, C-9 C-7, C-8, C-9, C-10 |
| Compounds | NO Inhibitory Effects a |
|---|---|
| (±)−1 2 | 21 >80 |
| 3 | 29 |
| 4 | 40 |
| 5 | >80 |
| Dexamethasone | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Bo, X.; Wu, F.; Tan, M.; Wei, Y.; Wang, L.; Zhou, J.; Wu, G.; Huang, X. Asperphenyltones A and B: New Phenylfuropyridinone Skeleton from an Endophytic Aspergillus sp. GXNU-A1. Molecules 2022, 27, 8160. https://doi.org/10.3390/molecules27238160
Huang J, Bo X, Wu F, Tan M, Wei Y, Wang L, Zhou J, Wu G, Huang X. Asperphenyltones A and B: New Phenylfuropyridinone Skeleton from an Endophytic Aspergillus sp. GXNU-A1. Molecules. 2022; 27(23):8160. https://doi.org/10.3390/molecules27238160
Chicago/Turabian StyleHuang, Jiguo, Xianglong Bo, Furong Wu, Meijing Tan, Youquan Wei, Lixia Wang, Junqiang Zhou, Guiming Wu, and Xishan Huang. 2022. "Asperphenyltones A and B: New Phenylfuropyridinone Skeleton from an Endophytic Aspergillus sp. GXNU-A1" Molecules 27, no. 23: 8160. https://doi.org/10.3390/molecules27238160
APA StyleHuang, J., Bo, X., Wu, F., Tan, M., Wei, Y., Wang, L., Zhou, J., Wu, G., & Huang, X. (2022). Asperphenyltones A and B: New Phenylfuropyridinone Skeleton from an Endophytic Aspergillus sp. GXNU-A1. Molecules, 27(23), 8160. https://doi.org/10.3390/molecules27238160