Fluorescence vs. Phosphorescence: Which Scenario Is Preferable in Au(I) Complexes with Benzothiadiazoles?
Abstract
1. Introduction
2. Results and Discussion
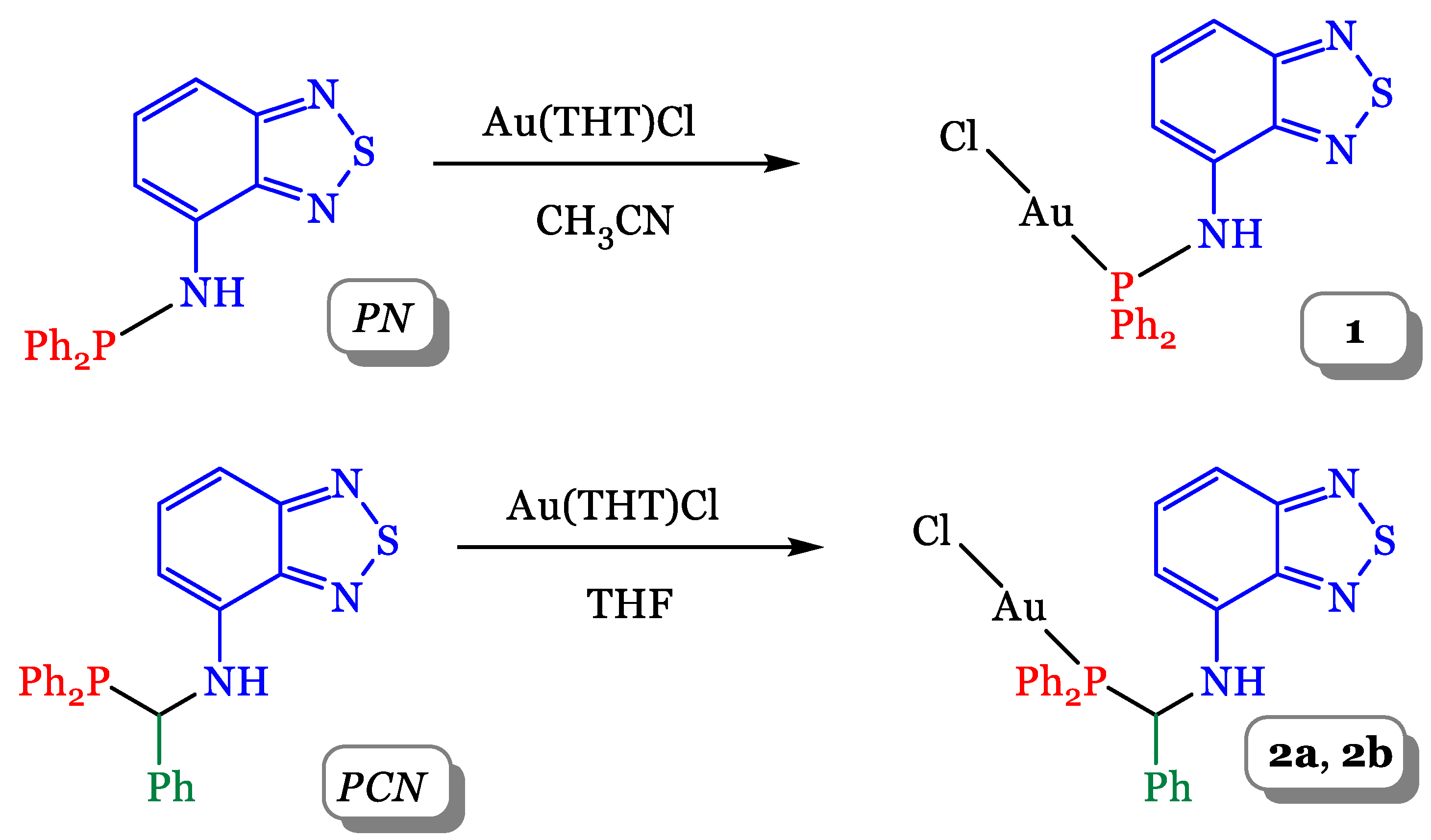
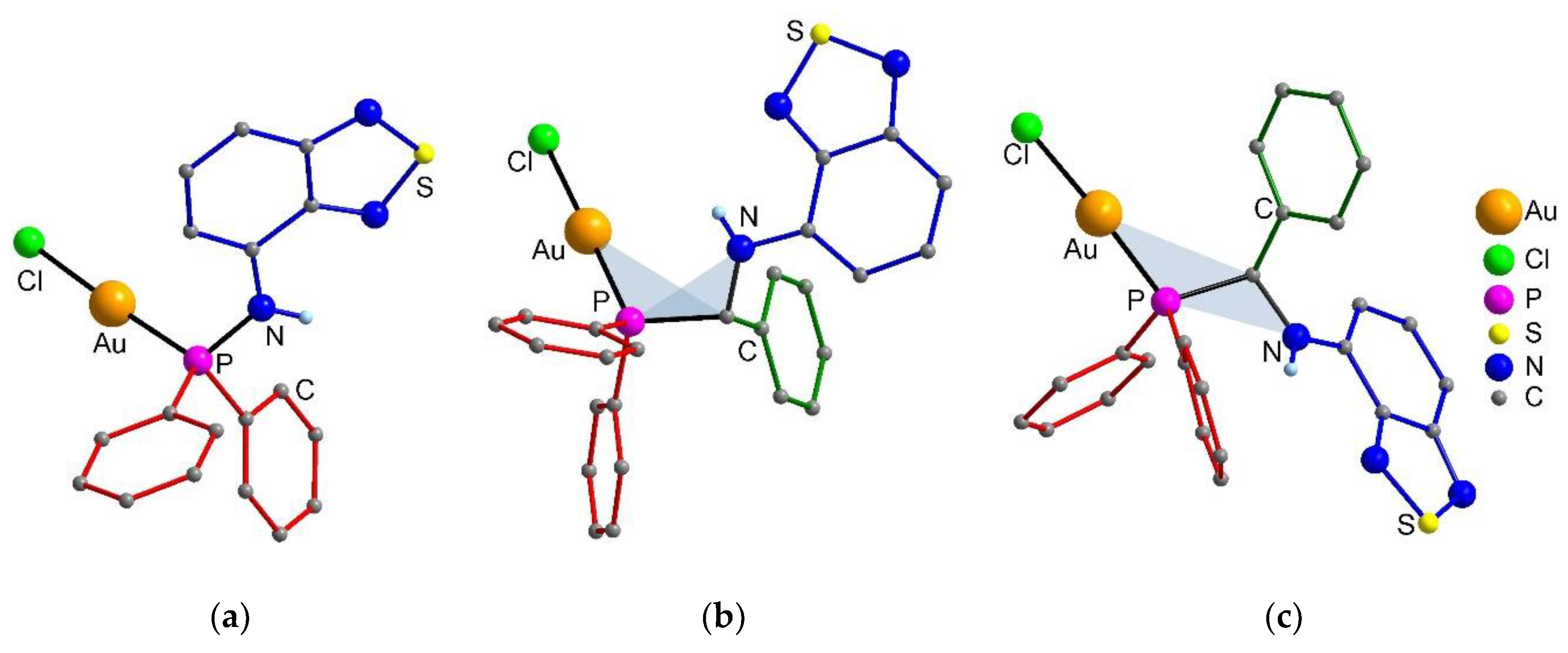
2.1. Synthesis and Crystal Structure of the Compounds
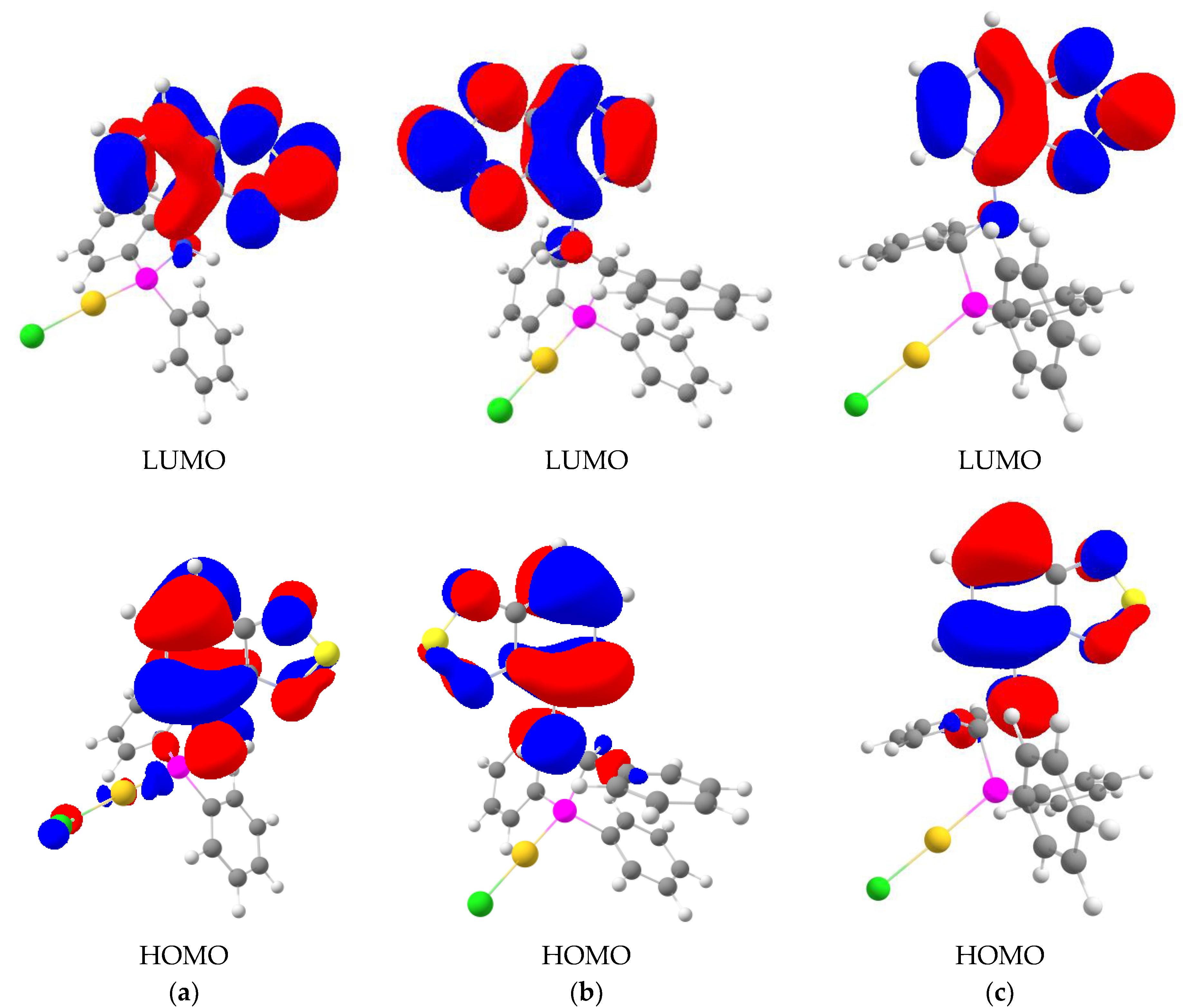
2.2. Photophysical Properties and TD-DFT Calculations
3. Materials and Methods
3.1. General
3.2. Theoretical Calculations
3.3. Syntheses
3.3.1. Synthesis of PN
3.3.2. Synthesis of [Au(PN)Cl] (1)
3.3.3. Synthesis of [Au(PCN)Cl] (2a)
3.3.4. Synthesis of [Au(PCN)Cl] (2b)
3.4. X-ray Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tao, Y.; Wang, Y.; Hu, S.; Young, D.J.; Lu, C.; Li, H.-X.; Ren, Z.-G. A photoluminescent Au(I)/Ag(I)/PNN coordination complex for relatively rapid and reversible alcohol sensing. Dalton Trans. 2021, 50, 6773–6777. [Google Scholar] [CrossRef]
- He, X.; Yam, V.W.-W. Luminescent gold(I) complexes for chemosensing. Coord. Chem. Rev. 2011, 255, 2111–2123. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, Z.; Yin, Y.; Sun, Y.; Liu, S. Progress in mechanochromic luminescence of gold(I) complexes. Chin. Chem. Lett. 2021, 32, 3718–3732. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Grachova, E.V.; Luzyanin, K.V. Tuning the luminescence of transition metal complexes with acyclic diaminocarbene ligands. Inorg. Chem. Front. 2022, 9, 417–439. [Google Scholar] [CrossRef]
- Jin, M.; Ito, H. Solid-state luminescence of Au(I) complexes with external stimuli-responsive properties. J. Photochem. Photobiol. C 2022, 51, 100478. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Daniels, R.; Kozhevnikov, V.N. Unusually fast phosphorescence from Ir(III) complexes via dinuclear molecular design. J. Phys. Chem. Lett. 2019, 10, 7015–7024. [Google Scholar] [CrossRef]
- Petrovskii, S.K.; Paderina, A.V.; Sizova, A.A.; Baranov, A.Y.; Artem’ev, A.A.; Sizov, V.V.; Grachova, E.V. Luminescence behaviour of Au(I)–Cu(I) heterobimetallic coordination polymers based on alkynyl-tris(2-pyridyl)phosphine Au(I) complexes. Dalton Trans. 2020, 49, 13430–13439. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, C.C.; Li, L.-K.; Tang, M.-C.; Lai, S.-L.; Cheung, W.-L.; Ng, M.; Chan, M.-Y.; Yam, V.W.-W. Molecular design of efficient yellow- to red-emissive alkynylgold(III) complexes for the realization of thermally activated delayed fluorescence (TADF) and their applications in solution-processed organic light-emitting devices. Chem. Sci. 2021, 12, 9516–9527. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Slavova, S.O.; Berezin, A.S.; Petrovskii, S.K.; Samsonenko, D.G.; Bagryanskaya, I.Y.; Fedin, V.P.; Grachova, E.V.; Artem’ev, A.V. Controllable synthesis and luminescence behavior of tetrahedral Au@Cu4 and Au@Ag4 clusters supported by tris(2-pyridyl)phosphine. Inorg. Chem. 2022, 61, 10925–10933. [Google Scholar] [CrossRef]
- Shakirova, J.R.; Grachova, E.V.; Gurzhiy, V.V.; Koshevoy, I.O.; Melnikov, A.S.; Sizova, O.V.; Tunik, S.P.; Laguna, A. Luminescent heterometallic gold–copper alkynyl complexes stabilized by tridentate phosphine. Dalton Trans. 2012, 41, 2941–2949. [Google Scholar] [CrossRef]
- Feuerstein, W.; Holzer, C.; Gui, X.; Neumeier, L.; Klopper, W.; Breher, F. Synthesis of new donor-substituted biphenyls: Pre-ligands for highly luminescent (C^C^D) gold(III) pincer complexes. Chem. Eur. J. 2020, 26, 17156–17164. [Google Scholar] [CrossRef]
- Moitra, T.; Karak, P.; Chakraborty, S.; Ruud, K.; Chakrabarti, S. Behind the scenes of spin-forbidden decay pathways in transition metal complexes. Phys. Chem. Chem. Phys. 2021, 23, 59–81. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Demyanov, Y.V.; Rakhmanova, M.I.; Bagryanskaya, I.Y. Pyridylarsine-based Cu(I) complexes showing TADF mixed with fast phosphorescence: A speeding-up emission rate using arsine ligands. Dalton Trans. 2022, 51, 1048–1055. [Google Scholar] [CrossRef]
- Demyanov, Y.V.; Sadykov, E.H.; Rakhmanova, M.I.; Novikov, A.S.; Bagryanskaya, I.Y.; Artem’ev, A.V. Tris(2-pyridyl)arsine as a new platform for design of luminescent Cu(I) and Ag(I) complexes. Molecules 2022, 27, 6059. [Google Scholar] [CrossRef]
- Mońka, M.; Serdiuk, I.E.; Kozakiewicz, K.; Hoffman, E.; Szumilas, J.; Kubicki, A.; Park, S.Y.; Bojarski, P. Understanding the internal heavy-atom effect on thermally activated delayed fluorescence: Application of Arrhenius and Marcus theories for spin–orbit coupling analysis. J. Mater. Chem. C 2022, 10, 7925–7934. [Google Scholar] [CrossRef]
- Tong, G.S.M.; Chow, P.K.; Che, C.-M. Where is the heavy-atom effect? Role of the central ligand in tetragold(I) ethynyl complexes. Angew. Chem. Int. Ed. 2010, 49, 9206–9209. [Google Scholar] [CrossRef]
- Mihaly, J.J.; Stewart, D.J.; Grusenmeyer, T.A.; Phillips, A.T.; Haley, J.E.; Zeller, M.; Gray, T.G. Photophysical properties of organogold(I) complexes bearing a benzothiazole-2,7-fluorenyl moiety: Selection of ancillary ligand influences white light emission. Dalton Trans. 2019, 48, 15917–15927. [Google Scholar] [CrossRef]
- Abramova, E.O.; Paderina, A.V.; Slavova, S.O.; Kostenko, E.A.; Eliseenkov, E.V.; Petrovskii, S.K.; Gitlina, A.Y.; Boyarskiy, V.P.; Grachova, E.V. Just add the gold: Aggregation-induced-emission properties of alkynylphosphinegold(I) complexes functionalized with phenylene–terpyridine subunits. Inorg. Chem. 2021, 60, 18715–18725. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Suo, B.; Zou, W. New Light on an Old Story: Breaking Kasha’s rule in phosphorescence mechanism of organic boron compounds and molecule design. Int. J. Mol. Sci. 2022, 23, 876. [Google Scholar] [CrossRef]
- Shekhovtsov, N.A.; Kokina, T.E.; Vinogradova, K.A.; Panarin, A.Y.; Rakhmanova, M.I.; Naumov, D.Y.; Pervukhina, N.V.; Nikolaenkova, E.B.; Krivopalov, V.P.; Czerwieniec, R.; et al. Near-infrared emitting copper(I) complexes with a pyrazolylpyrimidine ligand: Exploring relaxation pathways. Dalton Trans. 2022, 51, 2898–2911. [Google Scholar] [CrossRef]
- Chan, K.T.; Tong, G.S.M.; To, W.-P.; Yang, C.; Du, L.; Phillips, D.L.; Che, C.-M. The interplay between fluorescence and phosphorescence with luminescent gold(I) and gold(III) complexes bearing heterocyclic arylacetylide ligands. Chem. Sci. 2017, 8, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Moitra, T.; Alam, M.M.; Chakrabarti, S. Intersystem crossing rate dependent dual emission and phosphorescence from cyclometalated platinum complexes: A second order cumulant expansion based approach. Phys. Chem. Chem. Phys. 2018, 20, 23244–23251. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Echeverri, M.; Gómez-Lor, B.; Rodríguez, L. How to achieve near unity fluorescence quantum yields on gold(I) benzothiadiazole-based derivatives. Dyes Pigm. 2022, 202, 110308. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Konchenko, S.N. Luminescent complexes of 2,1,3-benzothiadiazole derivatives. Russ. Chem. Bull. 2019, 68, 651–661. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the coordination and emission properties of 4-amino-2,1,3-benzothiadiazole by introduction of diphenylphosphine group. Cryst. Growth Des. 2020, 20, 5796–5807. [Google Scholar] [CrossRef]
- Pylova, E.K.; Khisamov, R.M.; Bashirov, D.A.; Sukhikh, T.S.; Konchenko, S.N. The effect of halides and coordination mode of 4-amino-2,1,3-benzothiadiazole on the luminescence properties of its Zn complexes. CrystEngComm 2022. accepted manuscript. [Google Scholar] [CrossRef]
- Ceriani, C.; Corsini, F.; Mattioli, G.; Mattiello, S.; Testa, D.; Po, R.; Botta, C.; Griffini, G.; Beverina, L. Sustainable by design, large Stokes shift benzothiadiazole derivatives for efficient luminescent solar concentrators. J. Mater. Chem. C 2021, 9, 14815–14826. [Google Scholar] [CrossRef]
- Khisamov, R.M.; Ryadun, A.A.; Konchenko, S.N.; Sukhikh, T.S. Molecular environment effects that modulate the photophysical properties of novel 1,3-phosphinoamines based on 2,1,3-benzothiadiazole. Molecules 2022, 27, 3857. [Google Scholar] [CrossRef]
- Patrizi, B.; Iagatti, A.; Abbondanza, L.; Bussotti, L.; Zanardi, S.; Salvalaggio, M.; Fusco, R.; Foggi, P. Ultrafast intramolecular and solvation dynamics in 4,7-bis (4,5-dibutylbenzo[1,2-b:4,3-b′]bisthiophene[1,2-b:4,3-b′]bisthiophen-2-yl)-2,1,3-benzothiadiazole. J. Phys. Chem. C 2019, 123, 5840–5852. [Google Scholar] [CrossRef]
- Pritchina, E.A.; Gritsan, N.P.; Rakitin, O.A.; Zibarev, A.V. 2,1,3-Benzochalcogenadiazoles: Regularities and peculiarities over a whole chalcogen pentad O, S, Se, Te and Po. Targets Heterocycl. Syst. 2019, 23, 143–154. [Google Scholar] [CrossRef]
- Plyuta, N.; Cauchy, T.; Hauser, A.; Lloret, F.; Julve, M.; Avarvari, N. Zinc(II) and copper(II) complexes with benzothiadiazole Schiff-base ligands. Polyhedron 2022, 224, 115994. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Kozhevnikov, D.N.; Bodensteiner, M.; Brandl, F.; Czerwieniec, R. Modulation of intersystem crossing rate by minor ligand modifications in cyclometalated platinum(II) complexes. Inorg. Chem. 2016, 55, 7457–7466. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Serrano, A.; Rai-Constapel, V.; Daza, M.C.; Doerr, M.; Marian, C.M. Internal heavy atom effects in phenothiazinium dyes: Enhancement of intersystem crossing via vibronic spin–orbit coupling. Phys. Chem. Chem. Phys. 2015, 17, 11350–11358. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Tozer, D.J. Overcoming low orbital overlap and triplet instability problems in TDDFT. J. Phys. Chem. A 2012, 116, 9783–9789. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F.; Klopper, W. Robust fitting techniques in the chain of spheres approximation to the Fock exchange: The role of the complementary space. J. Chem. Phys. 2013, 139, 094111. [Google Scholar] [CrossRef]
- de Souza, B.; Neese, F.; Izsák, R. On the theoretical prediction of fluorescence rates from first principles using the path integral approach. J. Chem. Phys. 2018, 148, 034104. [Google Scholar] [CrossRef]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

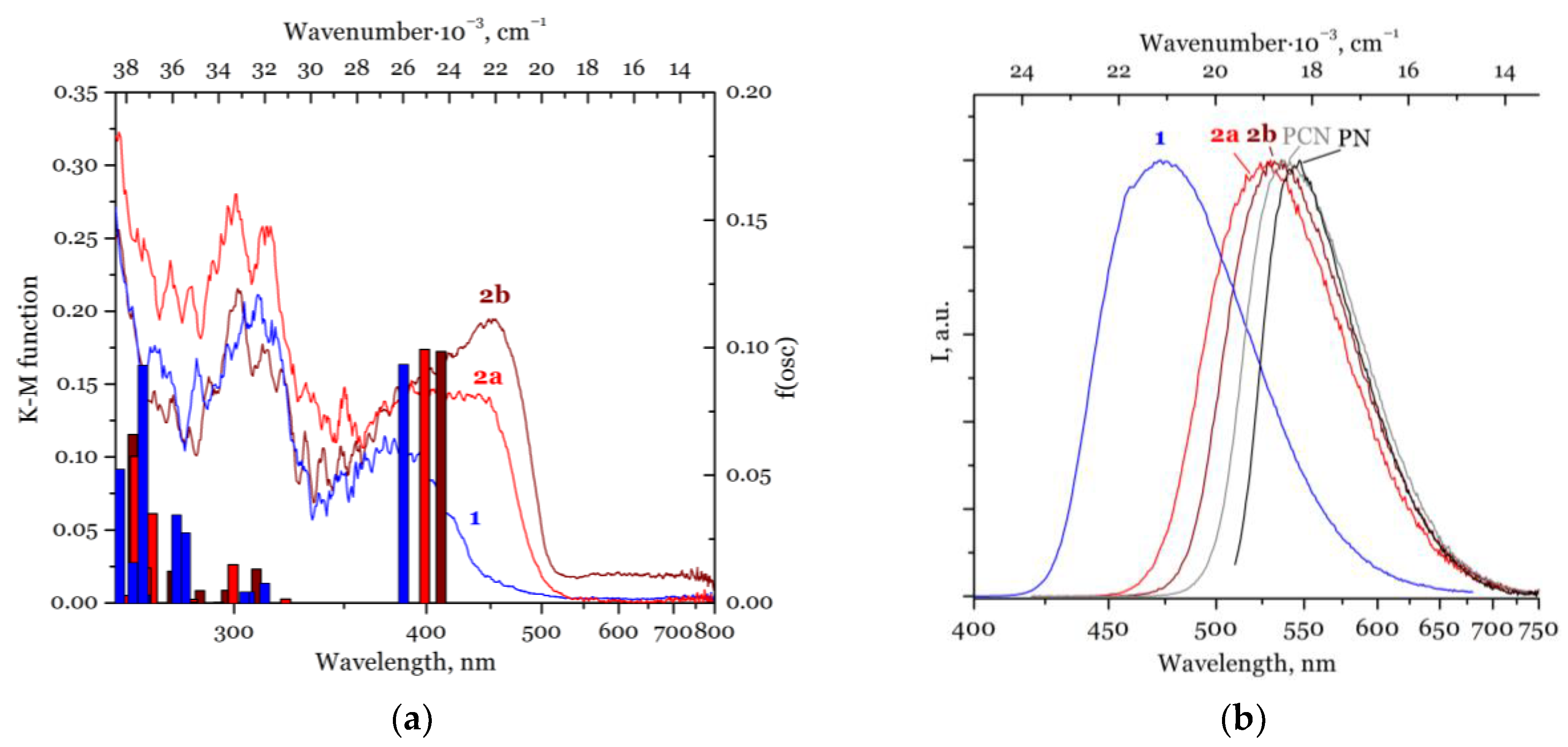
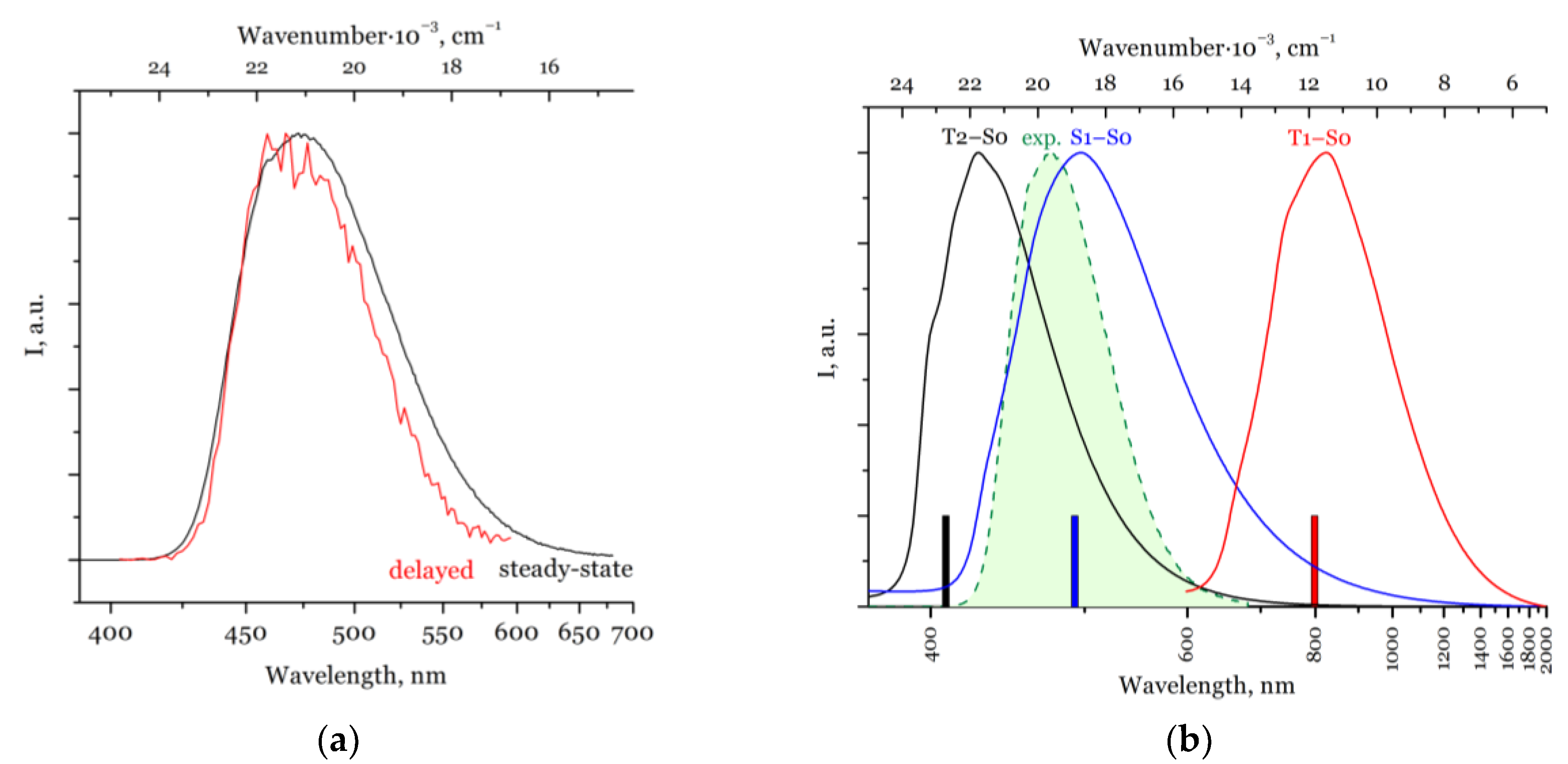
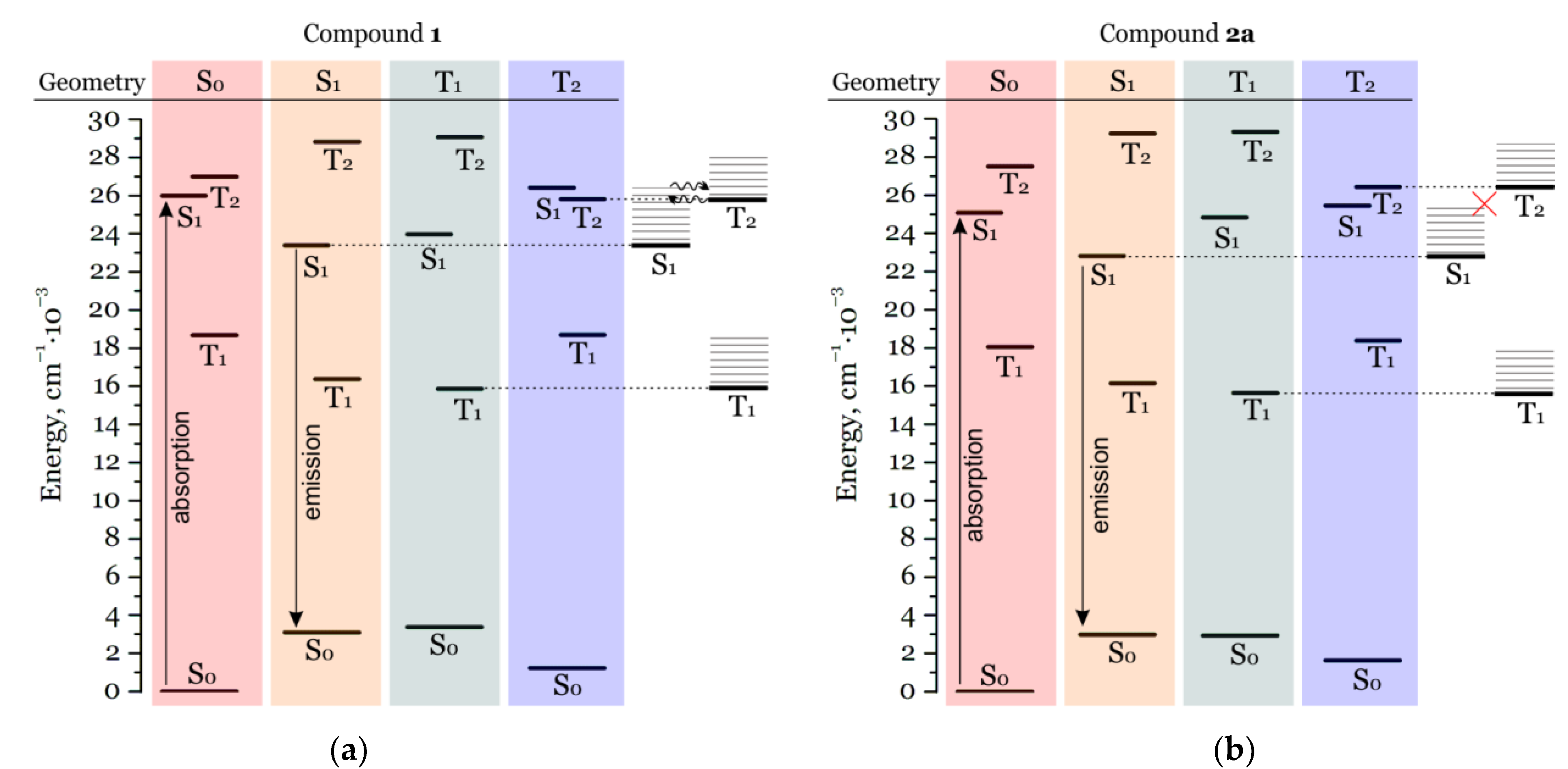
| Compound | λAbs, nm | E(S0→S1), nm | λEm, nm | E(S1→S0), nm | E(T1→S0), nm | E(T2→S0), nm | τ, μs | τ, ns | QY, % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 310, 380 | 384 | 470 | 492 | 798 | 407 | 100 | 10 | 34 |
| 2a | 310, 360–500 (br.) | 399 | 525 | 504 | 785 | 404 | – | 15 | 30 |
| 2b | 310, 360–500 (br.) | 410 | 535 | 512 | 833 | 403 | – | 9, 19 | 33 |
| 1 | 2a | 2b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | λ, nm | f | Electronic States * | λ, nm | f | Electronic States * | λ, nm | f | Electronic States * |
| 1 | 384 | 0.093 | H→L | 399 | 0.099 | H→L | 410 | 0.093 | H→L |
| 2 | 312 | 0.008 | H→L + 1 | 322 | 0.002 | H→L + 1 | 309 | 0.013 | H→L + 1 |
| 3 | 306 | 0.004 | H–1→L | 300 | 0.015 | H→L + 2 | 297 | 0.005 | H→L + 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khisamov, R.M.; Ryadun, A.A.; Konchenko, S.N.; Sukhikh, T.S. Fluorescence vs. Phosphorescence: Which Scenario Is Preferable in Au(I) Complexes with Benzothiadiazoles? Molecules 2022, 27, 8162. https://doi.org/10.3390/molecules27238162
Khisamov RM, Ryadun AA, Konchenko SN, Sukhikh TS. Fluorescence vs. Phosphorescence: Which Scenario Is Preferable in Au(I) Complexes with Benzothiadiazoles? Molecules. 2022; 27(23):8162. https://doi.org/10.3390/molecules27238162
Chicago/Turabian StyleKhisamov, Radmir. M., Alexey A. Ryadun, Sergey N. Konchenko, and Taisiya S. Sukhikh. 2022. "Fluorescence vs. Phosphorescence: Which Scenario Is Preferable in Au(I) Complexes with Benzothiadiazoles?" Molecules 27, no. 23: 8162. https://doi.org/10.3390/molecules27238162
APA StyleKhisamov, R. M., Ryadun, A. A., Konchenko, S. N., & Sukhikh, T. S. (2022). Fluorescence vs. Phosphorescence: Which Scenario Is Preferable in Au(I) Complexes with Benzothiadiazoles? Molecules, 27(23), 8162. https://doi.org/10.3390/molecules27238162







