Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization of A. vulgaris Ethanolic Extract—UHPLC−HRMS
2.1.1. Flavonol and Flavone Glycosides
2.1.2. Phenolic Acids and Their Derivatives
2.1.3. Ellagitannins and Other Phenolic Compounds
2.1.4. Triterpenoids
2.2. Total Phenolics and Flavonoids and Antioxidant Activity
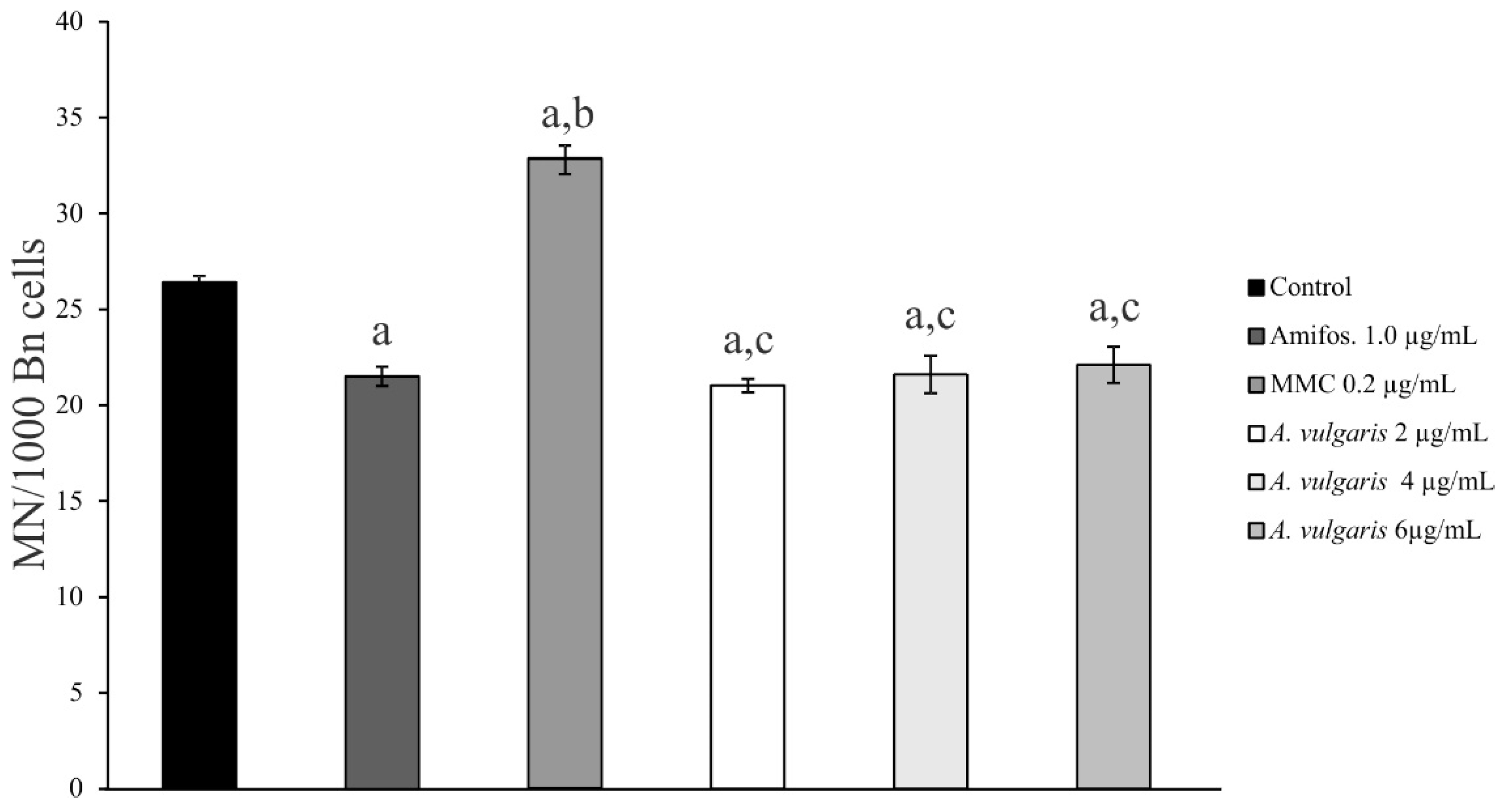
2.3. Genoprotective Effect of A. vulgaris Extract
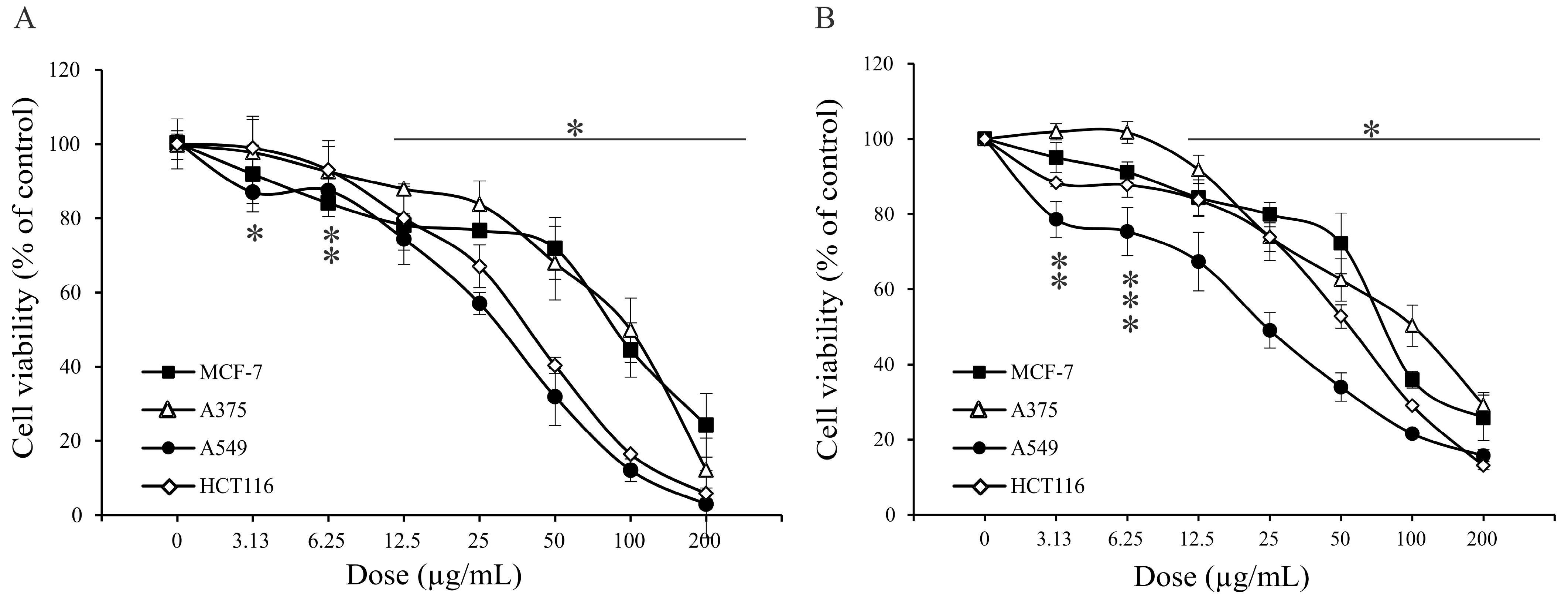
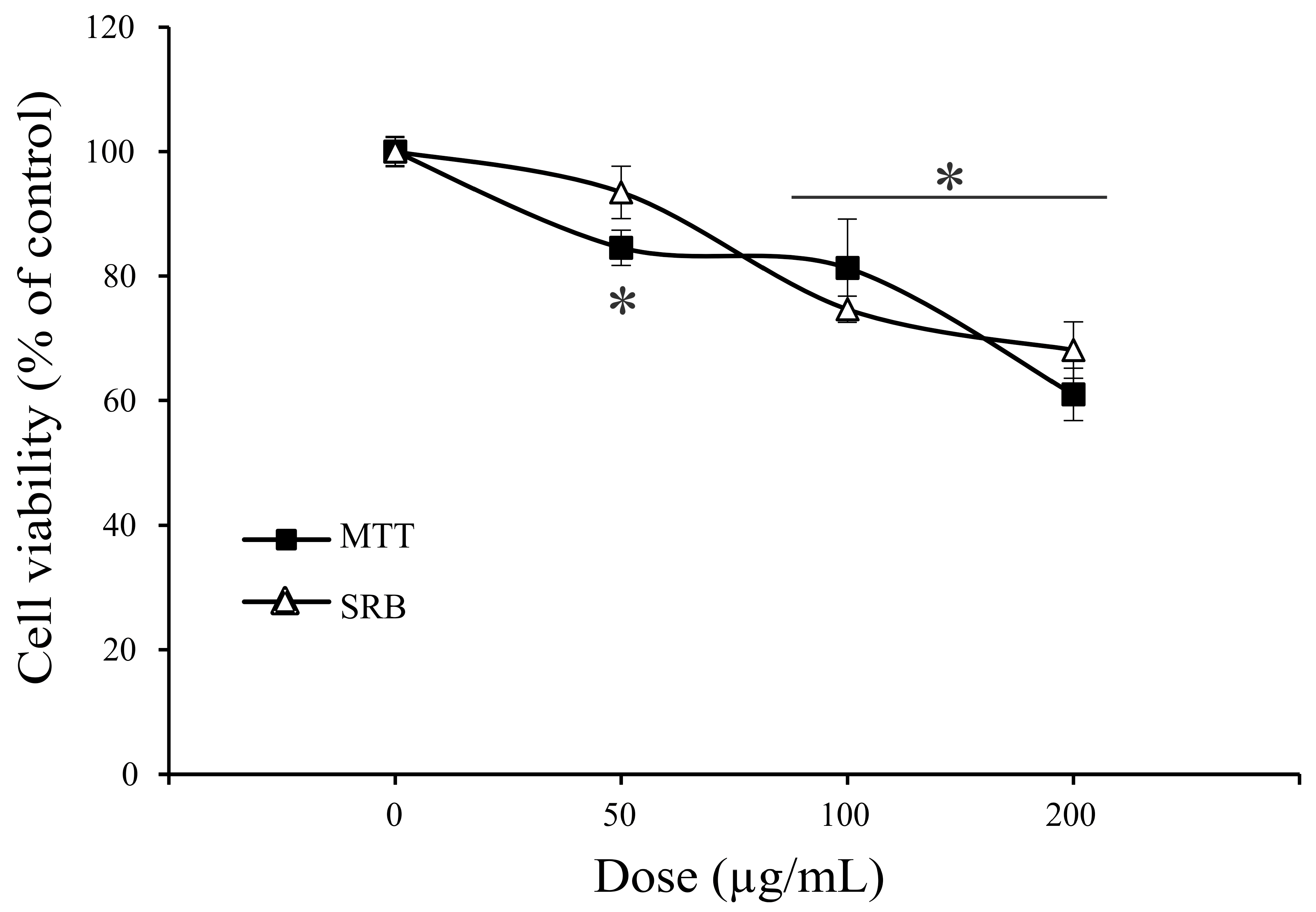
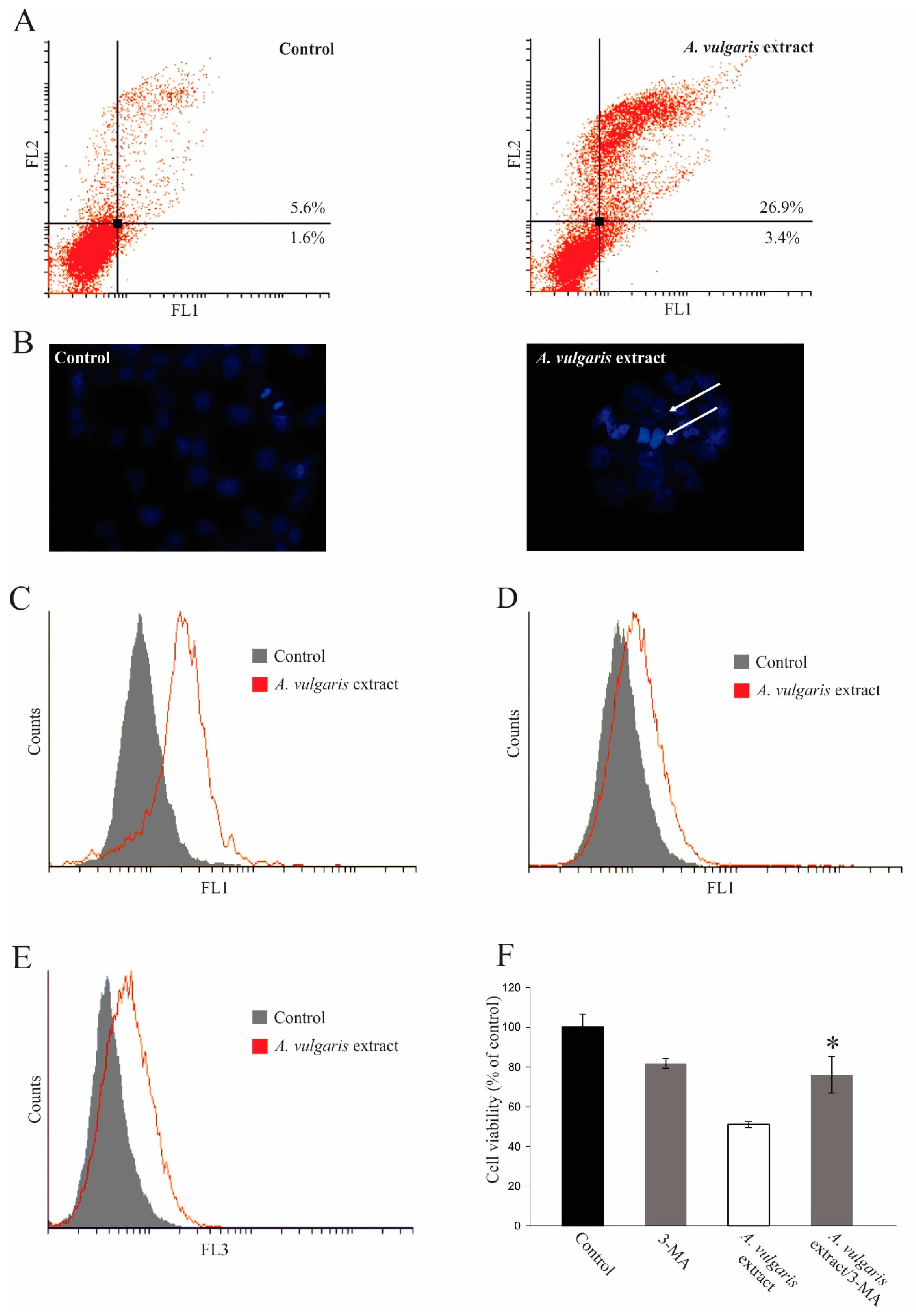
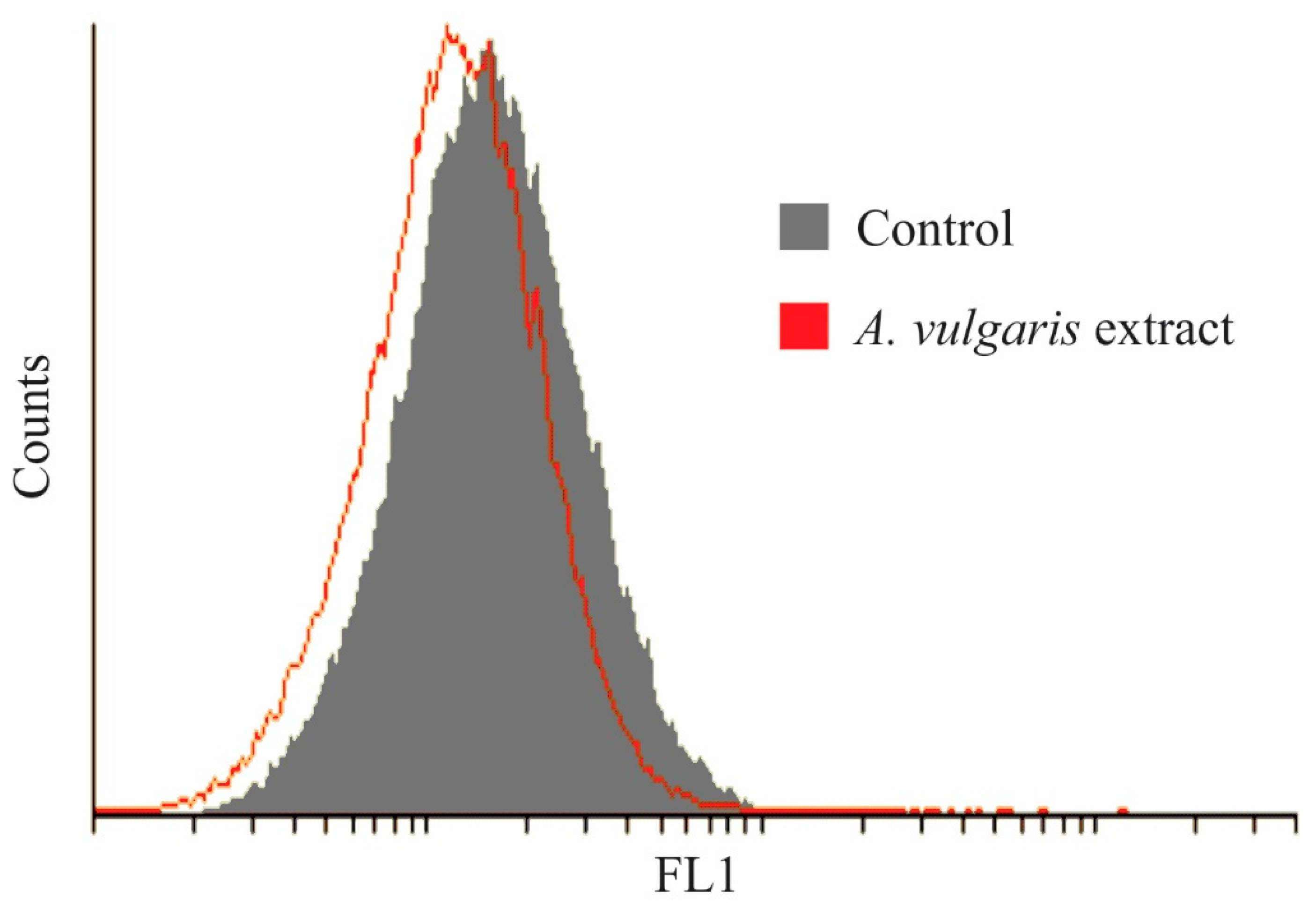
2.4. Antitumor Property of A. vulgaris Extract
3. Materials and Methods
3.1. Plant Material and Extract Preparations
3.2. Phytochemical Analyses
3.2.1. UHPLC–HRMS Analysis
3.2.2. Total Phenolic Content (TPC)
3.2.3. Total Flavonoid Content (TFC)
3.2.4. Total Dihydroxycinnamic Acid Derivative Content (HCA)
3.3. Antioxidant Activity Assays
3.3.1. DPPH (2,2′-Diphenyl-1-picrylhydrazyl Radical) (DPPH) Assay
3.3.2. Ferric Reducing Power (FRP) Assay
3.3.3. Cupric Reducing Antioxidant Activity (CUPRAC) Assay
3.3.4. Total Antioxidant Capacity (TAC) Assay
3.4. In Vitro Antitumor Study
3.4.1. Reagents and Cells
3.4.2. Determination of Cell Viability by SRB and MTT Assays
3.4.3. AnnV/PI, Apostat, and AO Staining
3.4.4. CFSE Staining
3.4.5. Measurement of ROS/RNS Generation
3.4.6. DAPI Staining on Chamber Slides
3.5. Cytokinesis-Blocked Micronucleus (CBMN) Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tadić, V.; Žugić, A.; Arsić, I. Multi-Target Herbal Preparation. In Supercritical CO2 Extractions and Its Application; Edward, R., Ed.; Polish Foundations of the Opportunities Industralization Centers “OIC Poland”: Lublin, Poland, 2014; pp. 99–121. ISBN 9788386499960. [Google Scholar]
- Redzić, S.S. The Ecological Aspect of Ethnobotany and Ethnopharmacology of Population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar] [PubMed]
- Gibbons, S. An Overview of Plant Extracts as Potential Therapeutics. Expert Opin. Ther. Pat. 2003, 13, 489–497. [Google Scholar] [CrossRef]
- Bradley, P. British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Bournemouth, UK, 2006; Volume 2, ISBN 9780903032124. [Google Scholar]
- Gehrke, B.; Bräuchler, C.; Romoleroux, K.; Lundberg, M.; Heubl, G.; Eriksson, T. Molecular Phylogenetics of Alchemilla, Aphanes and Lachemilla (Rosaceae) Inferred from Plastid and Nuclear Intron and Spacer DNA Sequences, with Comments on Generic Classification. Mol. Phylogenetics Evol. 2008, 47, 1030–1044. [Google Scholar] [CrossRef]
- Sepp, S.; Bobrova, V.K.; Troitsky, A.K.; Glazunova, K.P. Genetic Polymorphism Detected with RAPD Analysis and Morpho-logical Variability in Some Microspecies of Apomictic Alchemilla. Ann. Bot. Fenn. 2000, 37, 105–123. [Google Scholar]
- Ergene, B.; Acikara, Ö.B.; Bakar, F.; Saltan, G.; Nebioǧlu, S. Antioxidant Activity and Phytochemical Analysis of Alchemilla persica Rothm. Ank. Univ. Eczaci. Fak. Derg. 2010, 39, 145–154. [Google Scholar] [CrossRef]
- Ghedira, K.; Goetz, P.; Le Jeune, R. Alchemilla vulgaris L.: Alchémille (Rosaceae). Phytothérapie 2012, 10, 263–266. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunić, G.; Menković, N.; Živković, J.; Ćujić, N.; Tereščenko, M.; Bigović, D. Ethnobotanical Study on Traditional Use of Medicinal Plants in South-Western Serbia, Zlatibor District. J. Ethnopharmacol. 2013, 146, 803–810. [Google Scholar] [CrossRef]
- Filippova, E.I. Antiviral Activity of Lady’s Mantle (Alchemilla vulgaris L.) Extracts against Orthopoxviruses. Bull. Exp. Biol. Med. 2017, 163, 374–377. [Google Scholar] [CrossRef]
- Tadić, V.; Krgović, N.; Žugić, A. Lady’s Mantle (Alchemilla vulgaris L., Rosaceae): A Review of Traditional Uses, Phytochemical Profile, and Biological Properties. Lek. Sirovine 2020, 40, 66–74. [Google Scholar] [CrossRef]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of Antimicrobial Activity of Some Plant Extracts Against Antibiotic Susceptible and Resistant Bacterial Strains Causing Wound Infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Shilova, I.V.; Suslov, N.I.; Samylina, I.A.; Baeva, V.M.; Lazareva, N.B.; Mazin, E.V. Neuroprotective Properties of Common Lady’s Mantle Infusion. Pharm. Chem. J. 2020, 53, 1059–1062. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Radu, G.-L. Assessment of Acetylcholinesterase and Tyrosinase Inhibitory and Antioxidant Activity of Alchemilla vulgaris and Filipendula Ulmaria Extracts. J. Taiwan Inst. Chem. Eng. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Stevanović, Z.D. Alchemilla vulgaris Agg. (Lady’s Mantle) from Central Balkan: Antioxidant, Anticancer and Enzyme Inhibition Properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Asiry, K.A.; Alhakamy, N.A.; Mousa, M.A.A. Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines. Plants 2022, 11, 2140. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative Determination of Plant Phenolics in Urtica Dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometric Detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative Analysis of Phenolic Compounds in Apple Pomace Using Liquid Chromatography Coupled to Mass Spectrometry in Tandem Mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic Constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at Different Dates of Harvest. Z. Naturforsch C. J. Biosci. 2012, 67, 529–540. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-turska, M.; Boguszewska, A.; Polz-dacewicz, M.; Senkardes, I.; Guler, G.O.; Sadeer, N.B.; Mahomoodally, M.F.; et al. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural Characterization of Flavonol 3,7-Di-O-Glycosides and Determination of the Glycosylation Position by Using Negative Ion Electrospray Ionization Tandem Mass Spectrometry. Biol. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.). Antioxid. 2020, 9, 398. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumara, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Petsalo, A.; Jalonen, J.; Tolonen, A.A. Identification of Flavonoids of Rhodiola Rosea by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2006, 1112, 224–231. [Google Scholar] [CrossRef]
- Radović, J.; Suručić, R.; Niketić, M.; Kundaković-Vasović, T. Alchemilla viridiflora Rothm.: The Potent Natural Inhibitor of Angiotensin I-Converting Enzyme. Mol. Cell. Biochem. 2022, 477, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z.; et al. Chemical Characterization, Cytotoxic, Antioxidant, Antimicrobial, and Enzyme Inhibitory Effects of Different Extracts from One Sage (Salvia ceratophylla L.) from Turkey: Open a New Window on Industrial Purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Anti-protozoal Potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Yin, S.; Cai, Z.; Chen, C.; Mei, Y.; Wei, L.; Liu, S.; Zou, L.; Wu, N.; Yuan, J.; Liu, X.; et al. Comparative Study on Chemical Constituents of Medicinal and Non-Medicinal Parts of Flos Abelmoschus manihot, Based on Metabolite Profiling Coupled with Multivariate Statistical Analysis. Horticulturae 2022, 8, 317. [Google Scholar] [CrossRef]
- Rane, S.; Hate, M.; Hande, P.; Bs, A.; Datar, A. Development and Validation of LC-MS/MS Method for the Simultaneous Determination of Arjunic Acid, Arjungenin and Arjunetin in Terminalia arjuna (Roxb.) Wight & Arn. Int. J. Sci. Res. 2016, 5, 1097–1101. [Google Scholar] [CrossRef]
- Fraisse, D.; Heitz, A.; Carnat, A.P.; Lamaison, J.-L. Quercetin 3-Arabinopyranoside, a Major Flavonoid Compound from Alchemilla xanthochlora. Fitoterapia 2000, 71, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Todorova, M.; Gavrilova, A.; Vitkova, A. Flavonoid Glycosides from Bulgarian Endemic Alchemilla achtarowii Pawl. Biochem. Syst. Ecol. 2012, 43, 156–158. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Vitkova, A. Flavonoid Glycosides and Free Radical Scavenging Activity of Bulgarian Endemic Alchemilla jumrukczalica Pawl. Bulg. Chem. Commun. 2017, 49, 111–114. [Google Scholar]
- Felser, C.; Schimmer, O. Flavonoid Glycosides from Alchemilla speciosa. Planta Med. 1999, 65, 668–670. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Nikolova, M.; Gavrilova, A.; Vitkova, A. Flavonoid Constituents and Free Radical Scavenging Activity of Alchemilla mollis. Nat. Prod. Commun. 2011, 6, 1851–1854. [Google Scholar] [CrossRef]
- Li, C.; Seeram, N.P. Ultra-Fast Liquid Chromatography Coupled with Electrospray Ionization Time-of-Flight Mass Spectrometry for the Rapid Phenolic Profiling of Red Maple (Acer rubrum) Leaves. J. Sep. Sci. 2018, 41, 2331–2346. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Rocchetti, G.; Lucini, L.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M.; Senkardes, I.; Aktumsek, A.; et al. Chemical Characterization and Bioactive Properties of Different Extracts from Fibigia clypeata, an Unexplored Plant Food. Foods 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.S.; Omarsdottir, S.; Jaroszewski, J.W. Constituents of Three Icelandic Alchemilla Species. Biochem. Syst. Ecol. 2001, 29, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Boroja, T.; Mihailović, V.; Katanić, J.; Pan, S.-P.; Nikles, S.; Imbimbo, P.; Monti, D.M.; Stanković, N.; Stanković, M.S.; Bauer, R. The Biological Activities of Roots and Aerial Parts of Alchemilla vulgaris L. South Afr. J. Bot. 2018, 116, 175–184. [Google Scholar] [CrossRef]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanovic, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a Modern Approach to Traditional Use: In Vitro and in Vivo Evaluation of Alchemilla vulgaris L. Gel Wound Healing Potential. J. Ethnopharmacol. 2019, 238, 111789. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B.; Celiński, R.; Bakalczuk, M.; Dos Santos Szewczyk, K. Phenolic Composition and Antioxidant Activity of Alchemilla Species. Plants 2022, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.P.; Goel, H.C. Iron Chelation and Related Properties of Podophyllum Hexandrum, a Possible Role in Radioprotection. Indian J. Exp. Biol. 2000, 38, 1003–1006. [Google Scholar]
- Girard, P.; Sercombe, R.; Sercombe, C.; Le Lem, G.; Seylaz, J.; Potier, P. A New Synthetic Flavonoid Protects Endothelium-Derived Relaxing Factor-Induced Relaxation in Rabbit Arteries in Vitro: Evidence for Superoxide Scavenging. Biochem. Pharmacol. 1995, 49, 1533–1539. [Google Scholar] [CrossRef]
- Samanta, N.; Kannan, K.; Bala, M.; Goel, H.C. Radioprotective Mechanism of Podophyllum hexandrum during Spermatogenesis. Mol. Cell. Biochem. 2004, 267, 167–176. [Google Scholar] [CrossRef]
- Tominaga, H.; Kodama, S.; Matsuda, N.; Suzuki, K.; Watanabe, M. Involvement of Reactive Oxygen Species (ROS) in the Induction of Genetic Instability by Radiation. J. Radiat. Res. 2004, 45, 181–188. [Google Scholar] [CrossRef]
- Srinivasan, P.; Vadhanam, M.V.; Arif, J.M.; Gupta, R.C. A Rapid Screening Assay for Antioxidant Potential of Natural and Synthetic Agents in Vitro. Int. J. Oncol. 2002, 20, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Orfali, G.d.C.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; Araújo, M.E.M.B.d.; Priviero, F.B.M.; Carvalho, P.O.; Priolli, D.G. Review of Anticancer Mechanisms of Isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bao, B. Gallic Acid Impedes Non-Small Cell Lung Cancer Progression via Suppression of EGFR-Dependent CARM1-PELP1 Complex. Drug Des. Dev. Ther. 2020, 14, 1583–1592. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic Acid Has Anticancer Activity and Enhances the Anticancer Effects of Cisplatin in Non-small Cell Lung Cancer A549 Cells via the JAK/STAT3 Signaling Pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- Xu, J.; Lee, S.S.Y.; Seo, H.; Pang, L.; Jun, Y.; Zhang, R.Y.; Zhang, Z.Y.; Kim, P.; Lee, W.; Kron, S.J.; et al. Quinic Acid-Conjugated Nanoparticles Enhance Drug Delivery to Solid Tumors via Interactions with Endothelial Selectins. Small 2018, 14, e1803601. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Parker, M.L.; Parr, A.J.; Saunders, P.K.; Smith, A.C.; Waldron, K.W. Physicochemical Characteristics of Onion (Allium cepa L.) Tissues. J. Agric. Food Chem. 2000, 48, 5612–5617. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.-J. Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways. Appl. Sci. 2020, 10, 2519. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Królikowska, A.; Pietrzak, M. Antioxidant Activity of Teas Obtained from Leaves of Camellia sinensis (L.) Kuntze in Course of Various Production Processes Available on Polish Market. Herba Pol. 2018, 64, 60–67. [Google Scholar]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative Assessment of Total Polyphenols, Antioxidant and Antimicrobial Activity of Different Tea Varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Yılar, M.; Bayar, Y.; Bayar, A.A.A.; Genc, N. Chemical Composition of the Essential Oil of Salvia Bracteata Banks and the Biological Activity of Its Extracts: Antioxidant, Total Phenolic, Total Flavonoid, Antifungal and Allelopathic Effects. Bot. Serbica 2020, 44, 71–79. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ljubijankić, N.; Stanković, M.; Tešević, V.; Grgurić-Šipka, S.; Jadranin, M.; Begić, S.; Šabanović, E. Cytokinesis Block Micro-nucleus Assay in Human Lymphocytes after Exposure to Ru(III) Thiosemicarbazone Complexes in Vitro. Rasayan J. Chem. 2018, 11, 647–652. [Google Scholar] [CrossRef]
- Zlatanović, I.; Stanković, M.; Ickovski, J.; Dimitrijević, I.; Stojanović, G. Comprehensive Analysis of the Herbal Mixture Made of Juniperus oxycedrus L. Berries, Inner Bark of Betula Pendula Roth., and Grains of Avena sativa L. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
| No. | RT (min) | Tentative Identification | Ion Species | Precursor Ion (m/z) | δ ppm | Molecular Formula | Product Ions (m/z) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.68 | Gallic acid | [M − H]− | 169.01331 | 1.66 | C7H5O5 | 169, 125 | [17] |
| 2 | 6.59 | p-Coumaroyl hexose 1 | [M − H]− | 325.09314 | 4.14 | C15H17O8 | 325, 163, 145, 119, 117 | [18] |
| 3 | 6.69 | Chlorogenic acid | [M − H]− | 353.08835 | 4.64 | C16H17O9 | 191, 173, 179, 135 | [18] |
| 4 | 6.73 | Galloyl-HHDP-hexose | [M − H]− | 633.07430 | 1.52 | C27H21O18 | 633, 463, 301, 275, 229 | [19] |
| 5 | 6.98 | Brevifolin carboxylic acid | [M − H]− | 291.01489 | 4.64 | C13H7O8 | 291, 247, 219, 191 | [20] |
| 6 | 7.08 | p-Coumaroyl hexose 2 | [M − H]− | 325.09314 | 4.54 | C15H17O8 | 325, 163, 145, 119, 117 | [18] |
| 7 | 7.14 | Caffeic acid | [M − H]− | 179.03413 | 1.39 | C9H7O4 | 179, 135, 91 | [17] |
| 8 | 8.06 | Quercetin-hexoside-glucuronide | [M − H]− | 639.12152 | 1.93 | C27H27O18 | 639, 463, 301, 151 | [19] |
| 9 | 8.62 | Quercetin-3-O-arabinoside-7-O-glucoside | [M − H]− | 595.13129 | 1.40 | C26H27O16 | 595, 463, 462, 433, 301, 299, 271 | [21] |
| 10 | 9.10 | p-Coumaroylquinic acid 1 | [M − H]− | 337.09344 | 4.90 | C16H17O8 | 337, 191, 163, 145, 119 | [18] |
| 11 | 9.78 | Syringic acid | [M − H]− | 197.04489 | 2.24 | C9H9O5 | 197, 169, 153, 125 | [22] |
| 12 | 9.93 | Myricetin 3-O-hexoside (glucoside or galactoside) | [M − H]− | 479.08360 | 1.82 | C21H19O13 | 479, 317, 316, 287, 271, 179, 165, 139 | [21] |
| 13 | 10.12 | p-Coumaric acid | [M − H]− | 163.03917 | 1.22 | C9H7O3 | 163, 119 | [17] |
| 14 | 10.24 | Brevifolin | [M − H]− | 247.02469 | 3.94 | C12H7O6 | 247, 219, 191, 173 | [20] |
| 15 | 10.42 | p-Coumaroylquinic acid 2 | [M − H]− | 337.09335 | 4.63 | C16H17O8 | 337, 191, 163, 119 | [18] |
| 16 | 10.43 | Ellagic acid hexose | [M − H]− | 463.05243 | 3.70 | C20H15O13 | 463, 301, 283, 229 | [23] |
| 17 | 10.46 | Gossypetin-7-O-rhamnoside-3-O-hexoside | [M − H]− | 625.14417 | 5.03 | C27H29O17 | 625, 479, 463, 317, 316, 315, 287, 271 | [24] |
| 18 | 10.63 | Gossypetin-7-O-rhamnoside-3-O-deoxyhexoside 1 | [M − H]− | 595.13116 | 1.18 | C26H27O16 | 595, 463, 462, 449, 317, 316, 315, 287, 271 | [24] |
| 19 | 10.65 | Gossypetin-7-O-rhamnoside-3-O-deoxyhexoside 2 | [M − H]− | 595.13116 | 1.18 | C26H27O16 | 595, 463, 462, 449, 317, 316, 315, 287, 271 | [24] |
| 20 | 12.59 | Quercetin-3-O-vicianoside (Quercetin 3-O-α-L-arabinopyranosyl-(1–6)-β-D-glucopyranoside) | [M − H]− | 595.12927 | −1.99 | C26H27O16 | 595, 301, 300, 271, 255, 179 | [21] |
| 21 | 12.86 | Quercetin-3-O-pentoside–7-O-deoxyhexoside | [M − H]− | 579.13635 | 3.29 | C26H27O15 | 579, 447, 446, 433, 301, 299, 271, 179, 151 | [21] |
| 22 | 12.96 | Ellagic acid pentose | [M − H]− | 433.04150 | 3.12 | C19H13O12 | 433, 301, 283, 229, 185 | [23] |
| 23 | 13.61 | Ellagic acid | [M − H]− | 300.99902 | 0.10 | C14H5O8 | 301, 283, 245, 229, 201, 185, 173, 145 | [23] |
| 24 | 13.82 | Rutin | [M − H]− | 609.1473 | 1.96 | C27H30O16 | 609, 300, 271, 255, 179, 151 | [18] |
| 25 | 13.94 | Hyperoside | [M − H]− | 463.08862 | 3.28 | C21H19O12 | 463, 300, 301, 271, 255, 179, 151 | [18] |
| 26 | 14.02 | Miquelianin (Quercetin 3-O-glucuronide) | [M − H]− | 477.06735 | 2.07 | C21H17O13 | 477, 301, 255, 179, 151 | [19] |
| 27 | 14.10 | Agrimoniin | [M − 2H]−2 | 934.0721 | 0.78 | C82H54O52 | 1567, 1265, 1085, 935, 897, 783, 633, 301 | [19] |
| 28 | 14.25 | Kaempferol-3-O-rutinoside | [M − H]− | 593.15198 | 3.17 | C27H29O15 | 593, 285 | [22] |
| 29 | 14.28 | Isoquercitrin (Quercetin 3-O-glucoside) | [M − H]− | 463.08875 | 3.55 | C21H19O12 | 463, 301, 300, 271, 255, 179, 151 | [18] |
| 30 | 14.44 | Cynaroside (luteolin 7-O-glucoside) | [M − H]− | 447.09378 | 3.56 | C21H19O11 | 447, 285, 199 | [22] |
| 31 | 15.05 | Guaiaverin (quercetin 3-O-α-L-arabinopyranoside) | [M − H]− | 433.07797 | 3.31 | C20H17O11 | 433, 300, 301, 271, 255, 179, 151 | [18] |
| 32 | 15.44 | Avicularin (quercetin 3-O-α-L-arabinofuranoside) | [M − H]− | 433.07773 | 2.75 | C20H17O11 | 433, 300, 301, 271, 255, 179, 151 | [18] |
| 33 | 16.16 | Kaempferol 3-O-glucuronide | [M − H]− | 461.07315 | 3.68 | C21H17O12 | 461, 285, 229 | [19] |
| 34 | 16.29 | Astragalin (Kaempferol 3-O-glucoside) | [M − H]− | 447.09375 | 3.49 | C21H19O11 | 447, 300, 285, 284, 255, 227 | [18] |
| 35 | 17.04 | Kaempferol 3-O-xyloside | [M − H]− | 417.08323 | 3.83 | C20H17O10 | 417, 285, 284, 255, 227 | [22] |
| 36 | 18.83 | Quercetin 3-O-(6-O-acetyl-β-D-glucopyranoside | [M − H]− | 505.09930 | 3.24 | C23H21O13 | 505, 301, 300, 271, 255, 179, 151 | [22] |
| 37 | 20.78 | Triterpene acid hexoside | [M + HCOO]− | 711.39728 | 1.64 | C37H59O13 | 503 | [25] |
| 38 | 21.09 | Chrysoeriol 7-O-glucuronide | [M − H]− | 475.08850 | 2.94 | C22H19O12 | 299, 284, 255 | [26] |
| 39 | 21.31 | Kaempferol 3-O-acetylglucoside | [M − H]− | 489.10428 | 3.12 | C23H21O12 | 489, 284, 255, 227 | [27] |
| 40 | 21.45 | Quercetin | [M − H]− | 301.03546 | 3.95 | C15H9O7 | 301, 179, 151 | [28] |
| 41 | 23.21 | Tiliroside (kaempferol 3-O-(6′′-O-p-coumaroyl)-β-D-glucopyranoside) | [M − H]− | 593.13086 | 3.19 | C30H25O13 | 593, 285, 255, 227 | [25] |
| 42 | 27.18 | Triterpene acid hexoside | [M + HCOO]− | 695.40216 | 1.38 | C37H59O12 | 487 | [25] |
| 43 | 27.78 | Triterpene acid hexoside | [M + HCOO]− | 695.40216 | 1.38 | C37H59O12 | 487 | [25] |
| 44 | 27.87 | Triterpene acid hexoside | [M + HCOO]− | 695.40216 | 1.38 | C37H59O12 | 487 | [25] |
| 45 | 29.83 | Arjungenin | [M − H]− | 503.33853 | 3.61 | C30H47O6 | 503, 441, 409 | [29] |
| TPC mg/g GAE | TFC mg/g QE | HCA mg/g CGAE | CUPRAC mg/g AAE | TAC mg/g GAE | DPPH μg/gsr Trolox | FRP mg/g GAE | |
|---|---|---|---|---|---|---|---|
| mean | 7.55 | 6.99 | 14.18 | 2.61 | 326.50 | 18.02 | 31.32 |
| SD | 0.43 | 0.14 | 0.28 | 0.09 | 1.12 | 0.02 | 0.26 |
| Conc. µg/mL | % Bn Cell with MN | MN/Bn Cell | CBPI | Frequency of MN |
|---|---|---|---|---|
| Control | 2.1 ± 0.11 | 1.2 ± 0.06 | 1.6 ± 0.02 | 100% |
| Amifos.—1.0 µg/mL | 1.8 ± 0.14 | 1.2 ± 0.09 | 1.7 ± 0.02 | (81.4%) − 18.6% |
| MMC—0.2 µg/mL | 2.8 ± 0.10 | 1.2 ± 0.02 | 1.6 ± 0.03 | (124.2%) + 24.2% |
| A. vulgaris—2 µg/mL | 1.7 ± 0.09 | 1.2 ± 0.06 | 1.7 ± 0.01 | (79.5%) − 20.5% |
| A. vulgaris—4 µg/mL | 1.6 ± 0.03 | 1.3 ± 0.05 | 1.7 ± 0.06 | (81.8%) − 18.2% |
| A. vulgaris—6 µg/mL | 1.8 ± 0.06 | 1.2 ± 0.04 | 1.6 ± 0.02 | (83.7%) − 16.3% |
| Assays | MCF-7 µg/mL | A375 µg/mL | A549 µg/mL | HCT116 µg/mL |
|---|---|---|---|---|
| MTT | 83.5 ± 8.9 * | 105.8 ± 8.7 | 36.1 ± 5.7 | 36.9 ± 5.6 |
| SRB | 80.3 ± 0.4 | 106.4 ± 8.9 | 30.3 ± 8.3 | 54.9 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelača, S.; Dajić-Stevanović, Z.; Vuković, N.; Kolašinac, S.; Trendafilova, A.; Nedialkov, P.; Stanković, M.; Tanić, N.; Tanić, N.T.; Acović, A.; et al. Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro. Molecules 2022, 27, 8113. https://doi.org/10.3390/molecules27238113
Jelača S, Dajić-Stevanović Z, Vuković N, Kolašinac S, Trendafilova A, Nedialkov P, Stanković M, Tanić N, Tanić NT, Acović A, et al. Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro. Molecules. 2022; 27(23):8113. https://doi.org/10.3390/molecules27238113
Chicago/Turabian StyleJelača, Sanja, Zora Dajić-Stevanović, Nenad Vuković, Stefan Kolašinac, Antoaneta Trendafilova, Paraskev Nedialkov, Miroslava Stanković, Nasta Tanić, Nikola T. Tanić, Aleksandar Acović, and et al. 2022. "Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro" Molecules 27, no. 23: 8113. https://doi.org/10.3390/molecules27238113
APA StyleJelača, S., Dajić-Stevanović, Z., Vuković, N., Kolašinac, S., Trendafilova, A., Nedialkov, P., Stanković, M., Tanić, N., Tanić, N. T., Acović, A., Mijatović, S., & Maksimović-Ivanić, D. (2022). Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro. Molecules, 27(23), 8113. https://doi.org/10.3390/molecules27238113