A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies
Abstract
1. Introduction
2. Experimental
Materials and Methods
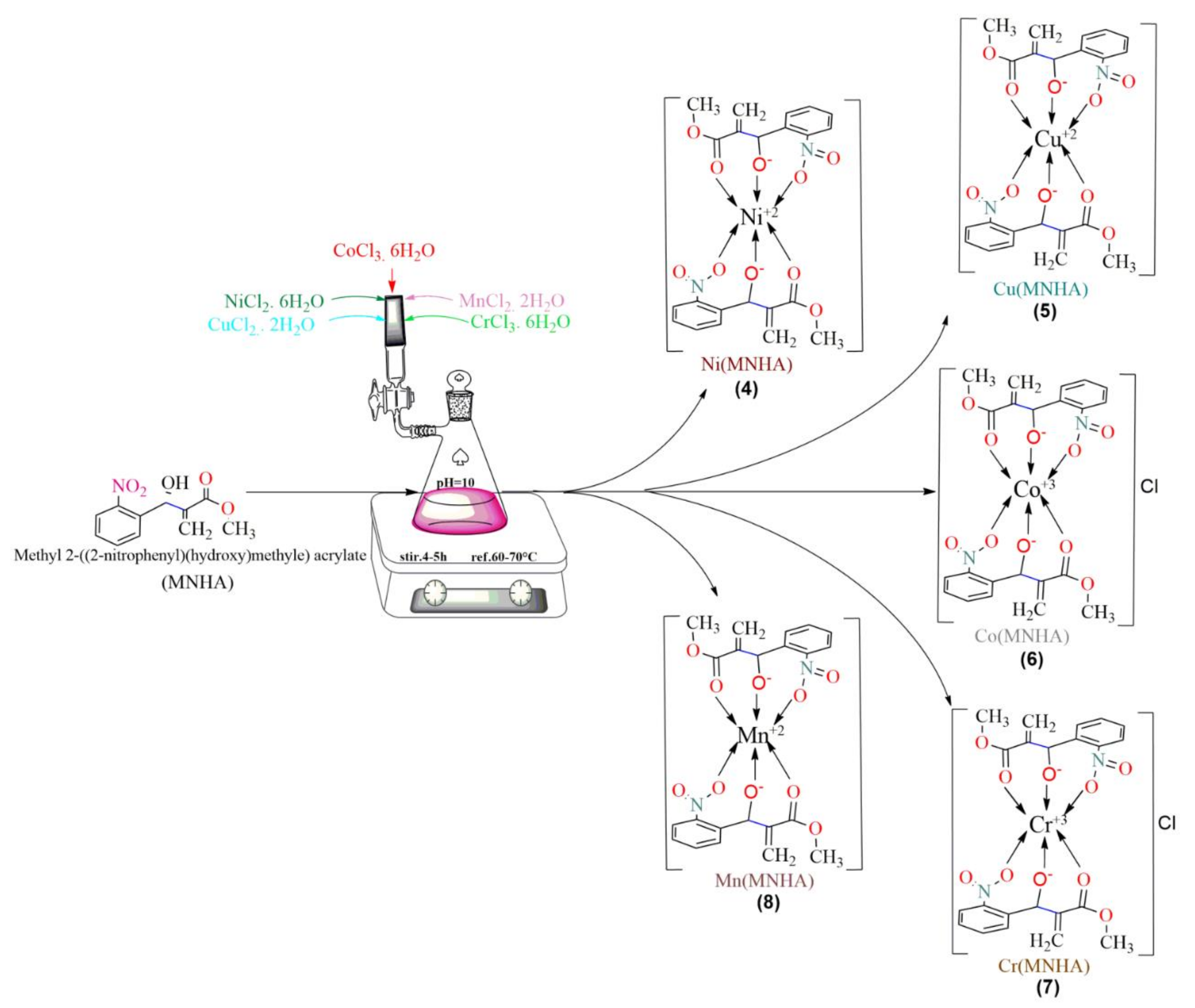
3. Synthesis
3.1. Synthesis of Methyl 2-((2-nitrophenyl)(hydroxy)methyl) Acrylate (MNHA)
3.2. General Procedure for the Synthesis of MNHA Adduct-Based Metal Complexes (Compounds 4–8)
3.2.1. [Ni(MNHA)2] (Compound 4)
3.2.2. [Cu(MNHA)2] (Compound 5)
3.2.3. [Co(MNHA)2] (Compound 6)
3.2.4. [Cr(MNHA)2] (Compound 7)
3.2.5. [Mn(MNHA)2] (Compound 8)
3.3. Antibacterial Assay for Compounds 3–8
3.4. DPPH Radical Scavenging Activity for Compounds 3–8
3.5. Antifungal assay of Compounds 3–8
4. Results and Discussion
4.1. Physical Characteristics and Elemental Analysis
4.2. UV–Visible Spectroscopy
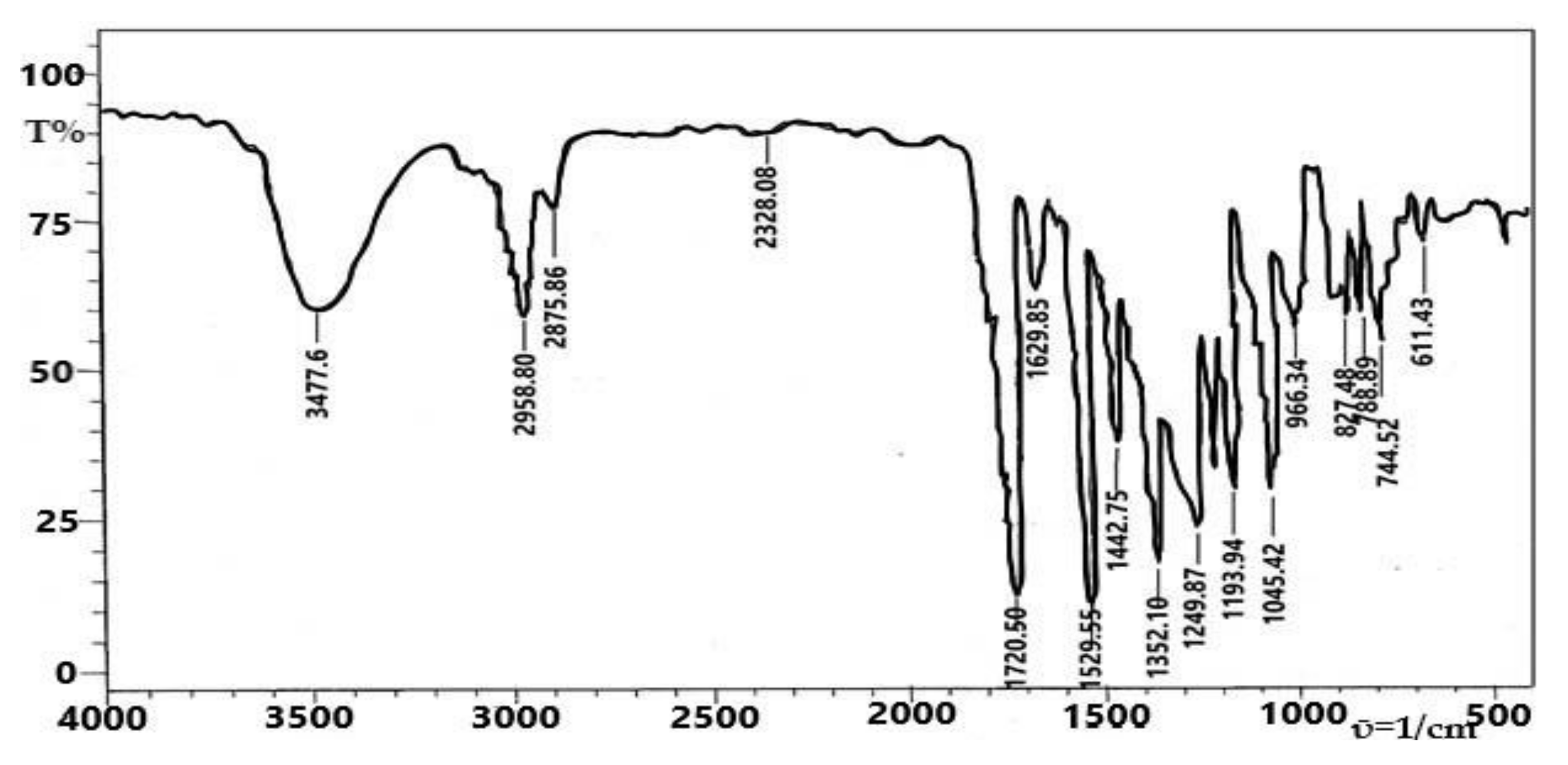
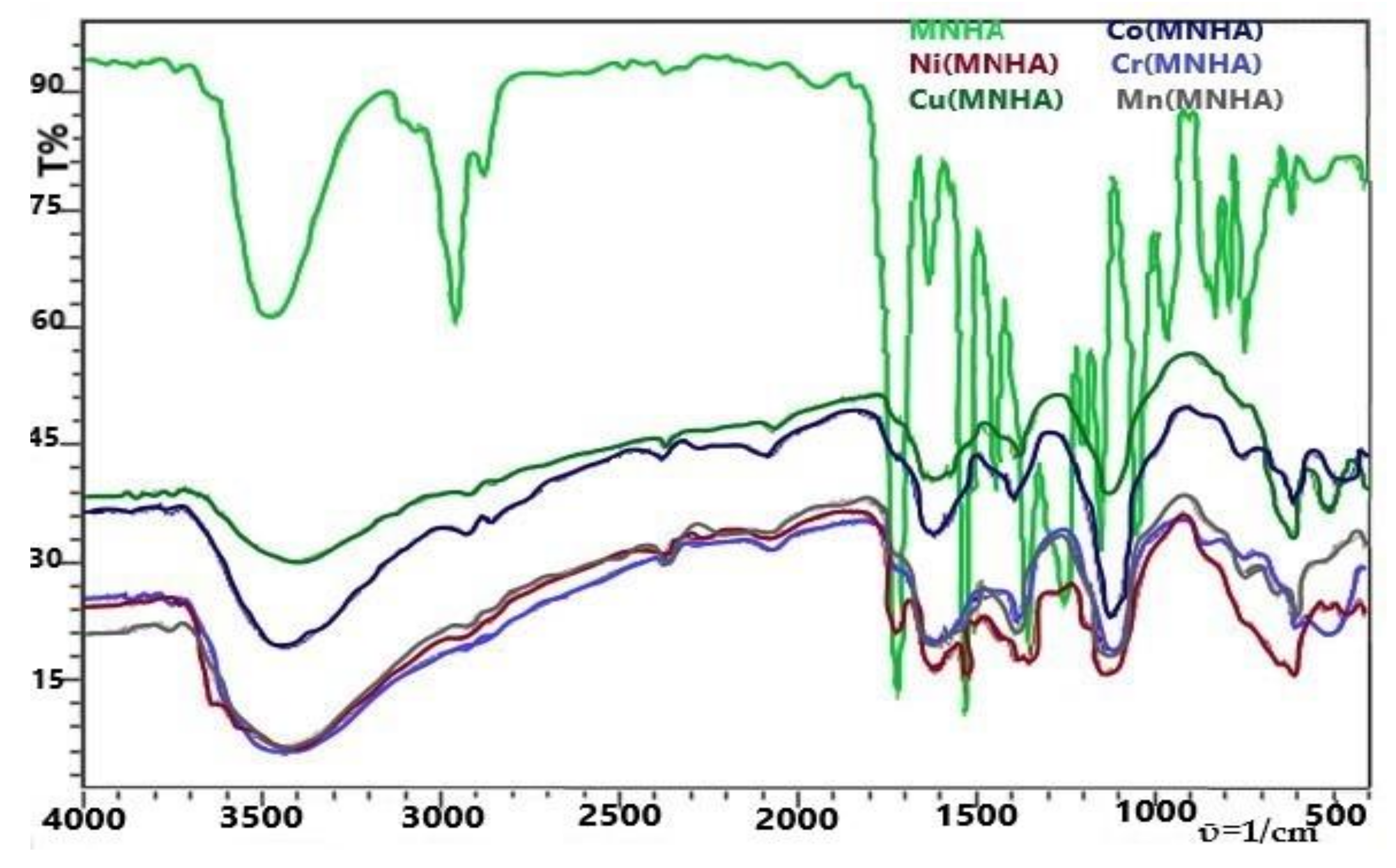
4.3. FT-IR Spectroscopy
4.4. Mass Spectrometry
4.5. 1H NMR Spectroscopy of Compound 3 (MNHA)
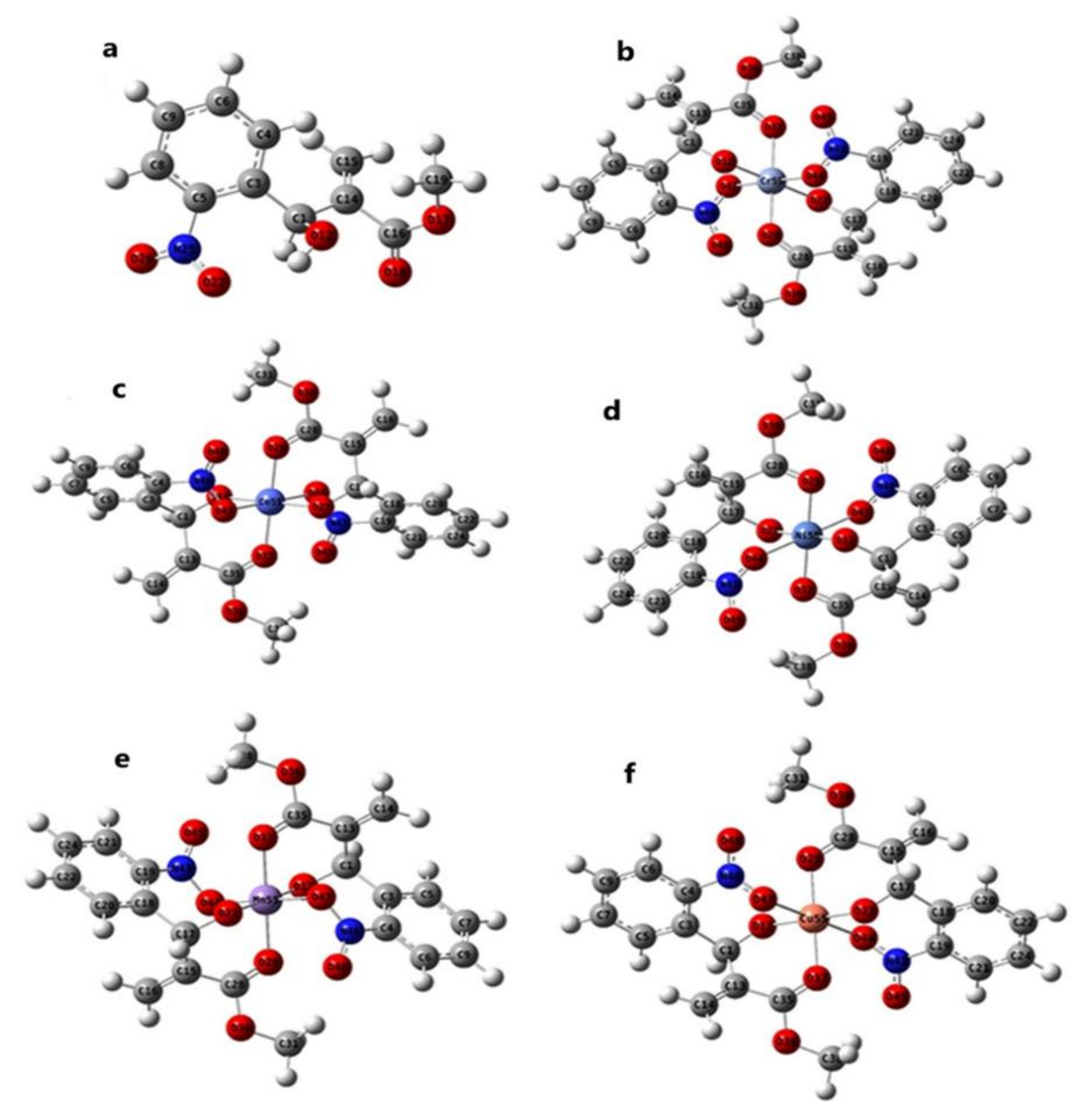
4.6. Geometrical Optimization
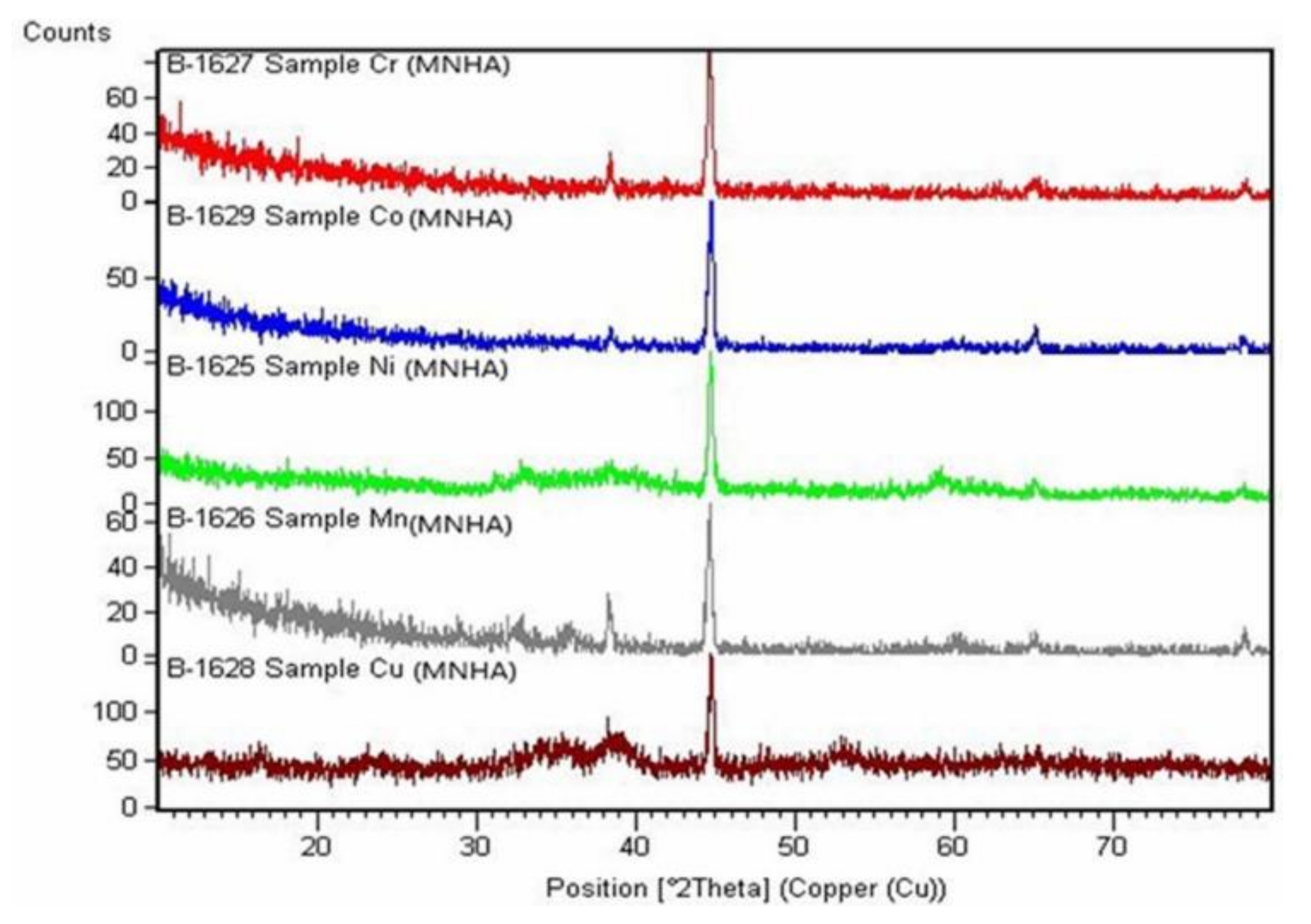
4.7. X-ray Powder Diffraction
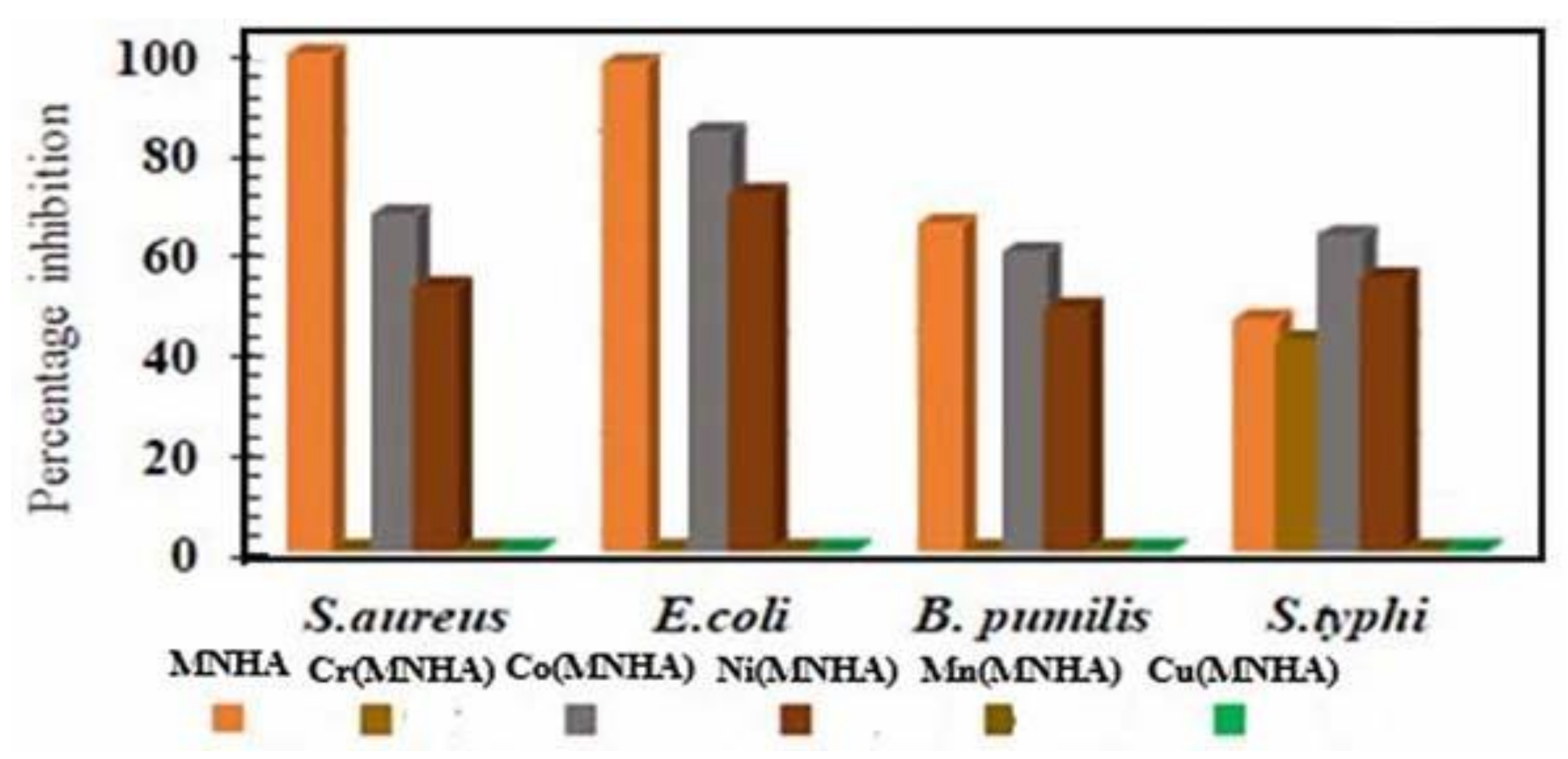
4.8. Antibacterial Assay
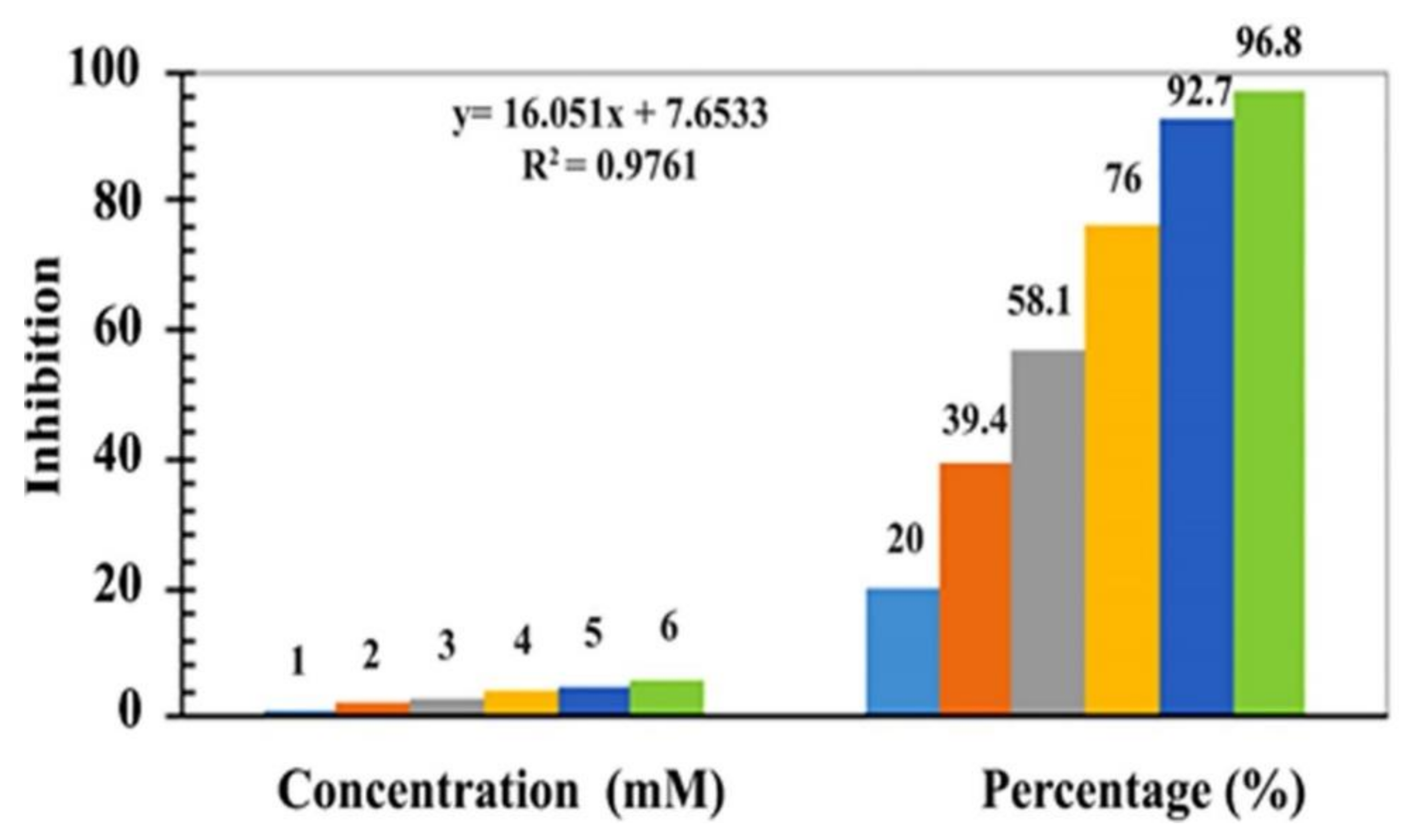
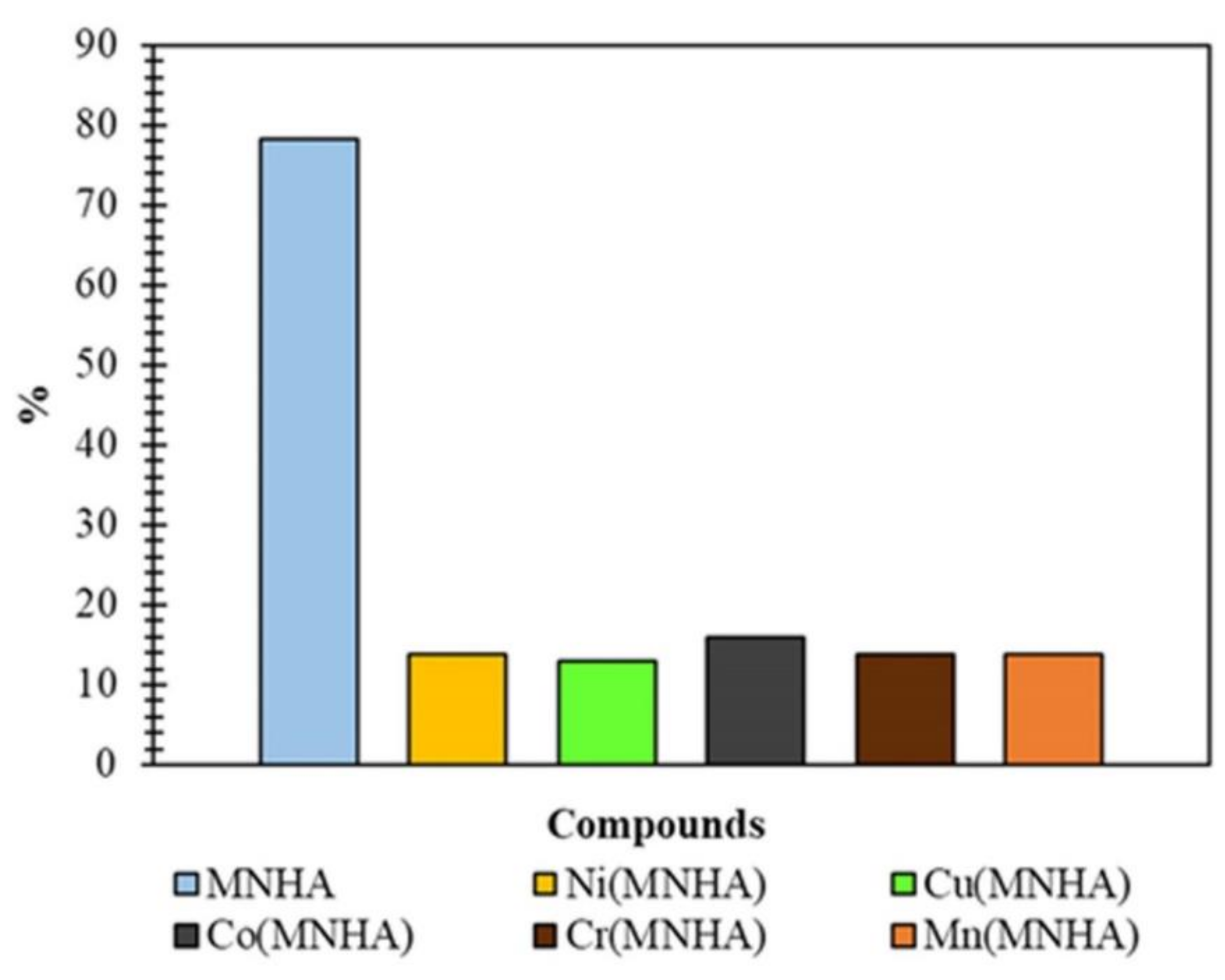
4.9. Antioxidant Activity
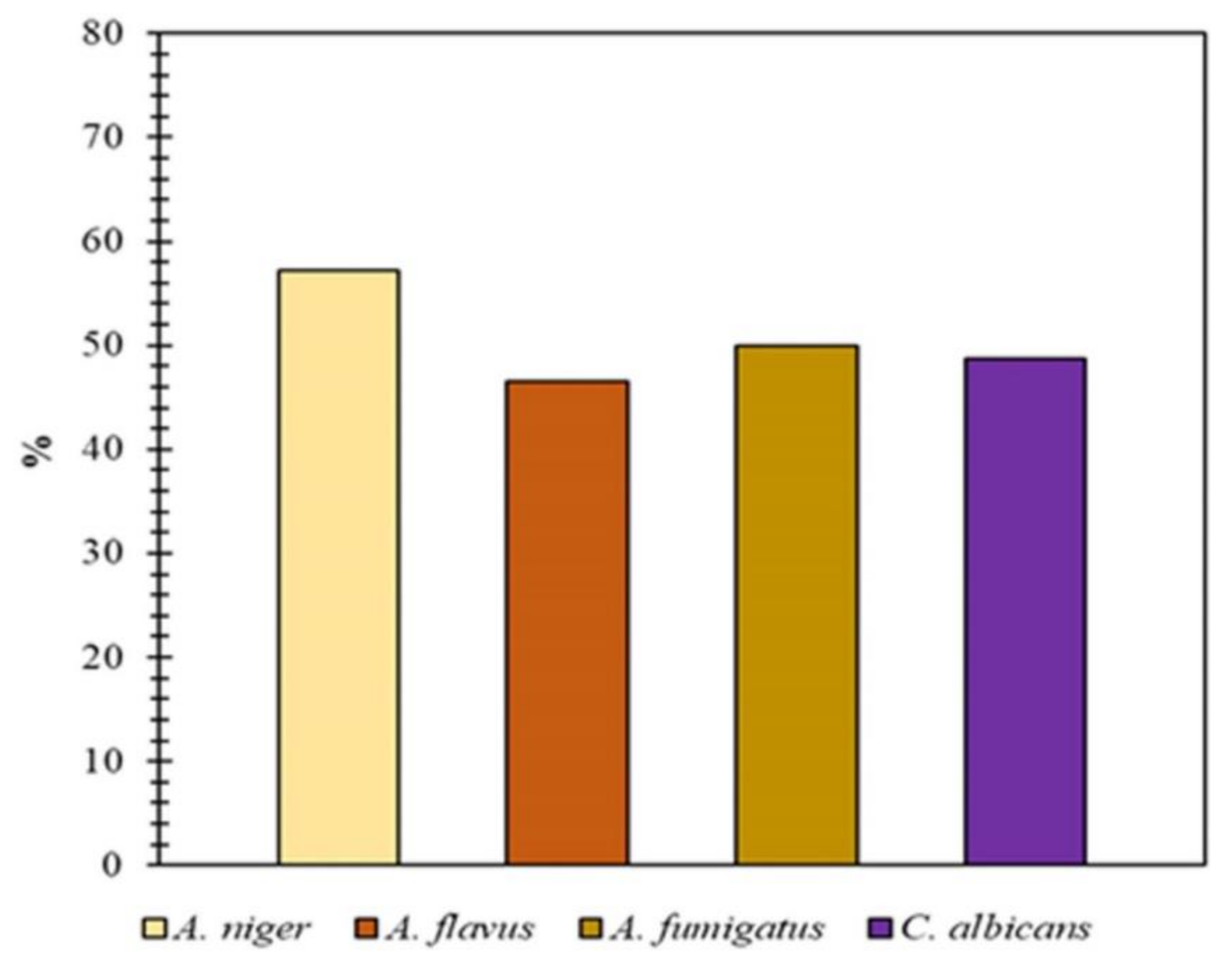
4.10. Antifungal Activity
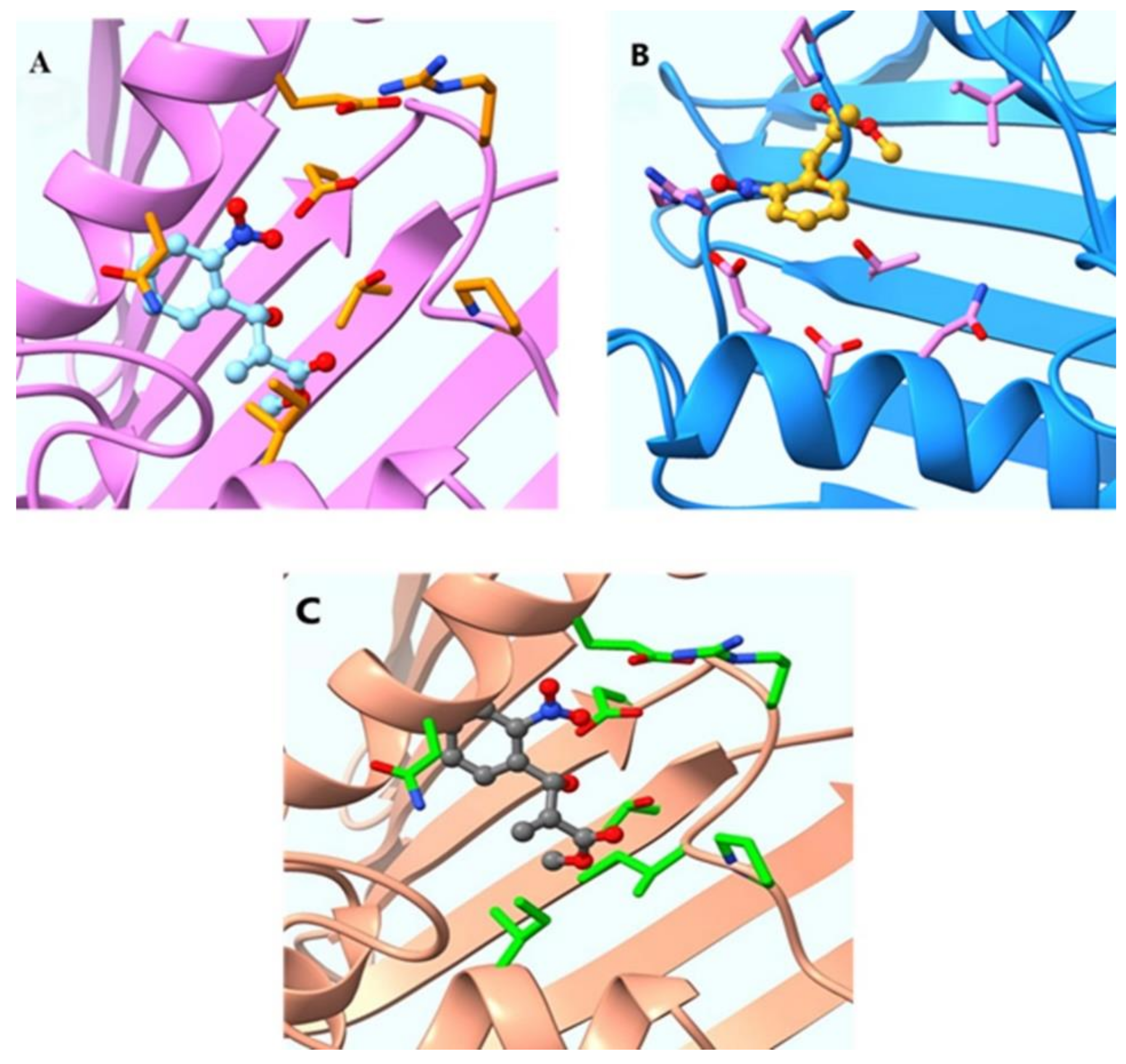
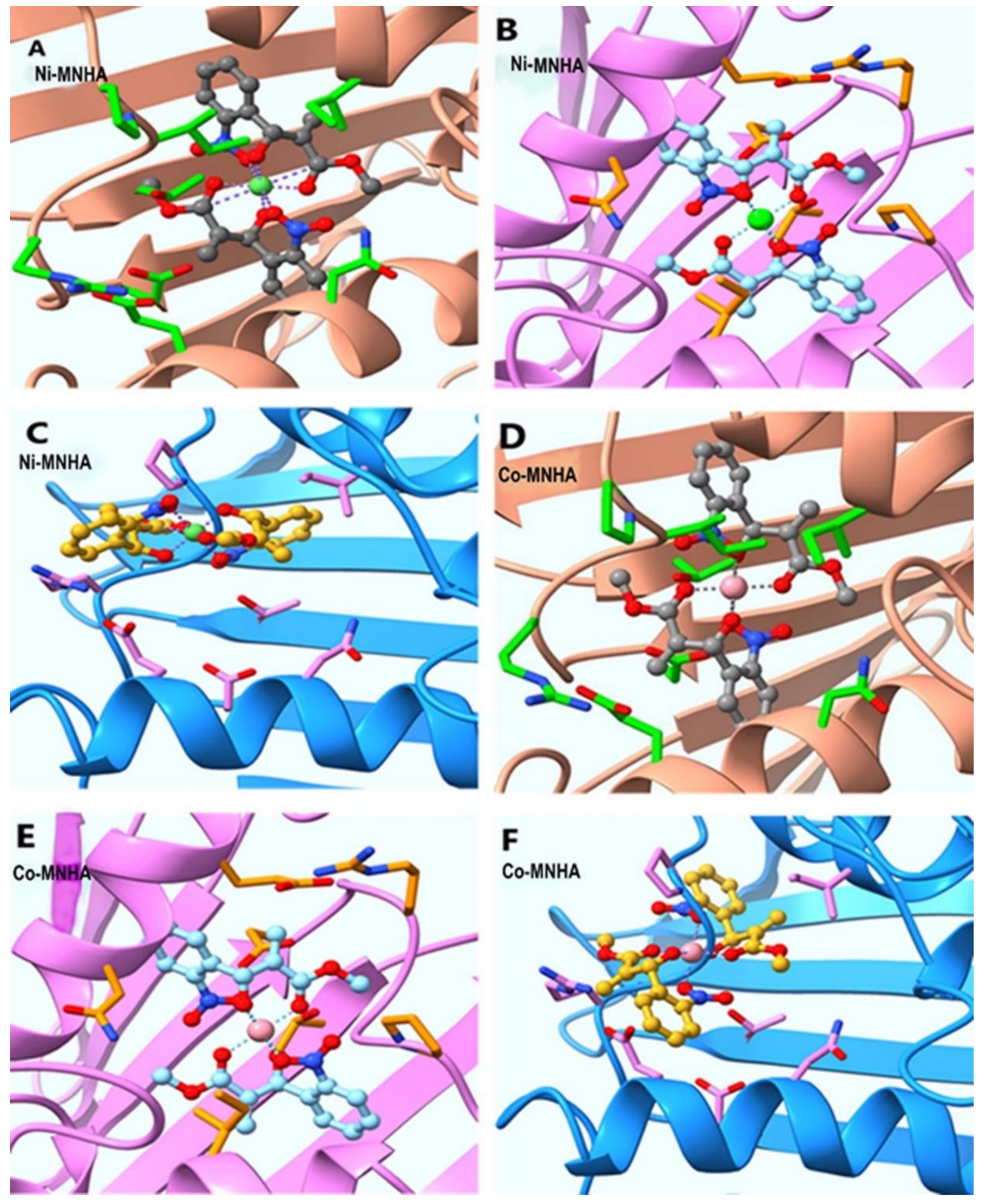
4.11. Molecular Docking of Compounds 3–8
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sousa, B.A.; Dos Santos, A.A. A Facile, Versatile, and Mild Morita-Baylis-Hillman-Type Reaction for the Modular One-Pot Synthesis of Highly Functionalized MBH Adducts. European J. Org. Chem. 2012, 3431–3436. [Google Scholar] [CrossRef]
- Du, J.Y.; Ma, Y.H.; Meng, F.X.; Zhang, R.R.; Wang, R.N.; Shi, H.L.; Wang, Q.; Fan, Y.X.; Huang, H.L.; Cui, J.C.; et al. Lewis Base-Catalyzed [4 + 3] Annulation of Ortho-Quinone Methides and MBH Carbonates: Synthesis of Functionalized Benzo[ b]Oxepines Bearing Oxindole Scaffolds. Org. Lett. 2019, 21, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Ketata, E.; Elleuch, H.; Neifar, A.; Mihoubi, W.; Ayadi, W.; Marrakchi, N.; Rezgui, F.; Gargouri, A. Anti-Melanogenesis Potential of a New Series of Morita-Baylis-Hillman Adducts in B16F10 Melanoma Cell Line. Bioorg. Chem. 2019, 84, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Huang, Y. Morita-Baylis-Hillman Adduct Derivatives (MBHADs): Versatile Reactivity in Lewis Base-Promoted Annulation. Org. Biomol. Chem. 2015, 13, 8578–8595. [Google Scholar] [CrossRef]
- Kaye, P.T. Applications of the Morita–Baylis–Hillman Reaction in the Synthesis of Heterocyclic Systems, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 127, ISBN 9780128171493. [Google Scholar]
- Takizawa, S. Organocatalytic Synthesis of Highly Functionalized Heterocycles by Enantioselective Aza-Morita–Baylis–Hillman-Type Domino Reactions. Chem. Pharm. Bull. 2020, 68, 299–315. [Google Scholar] [CrossRef] [PubMed]
- da Câmara Rocha, J.; da Franca Rodrigues, K.A.; do Nascimento Néris, P.L.; da Silva, L.V.; Almeida, F.S.; Lima, V.S.; Peixoto, R.F.; da Câmara Rocha, J.; de Azevedo, F. de L.A.A.; Veras, R.C.; et al. Biological Activity of Morita-Baylis-Hillman Adduct Homodimers in L. Infantum and L. Amazonensis: Anti-Leishmania Activity and Cytotoxicity. Parasitol. Res. 2019, 118, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, Y.G.; Pinho Júnior, W.; De Souza, A.A.; Costa, C.O.; Silva, F.P.L.; Lima-Junior, C.G.; Vasconcellos, M.L.A.A.; Goulart, M.O.F. Electrochemical and Computational Studies, in Protic Medium, of Morita-Baylis-Hillman Adducts and Correlation with Leishmanicidal Activity. Electrochim. Acta 2014, 140, 557–563. [Google Scholar] [CrossRef]
- Lima-Junior, C.G.; Vasconcellos, M.L.A.A. Morita-Baylis-Hillman Adducts: Biological Activities and Potentialities to the Discovery of New Cheaper Drugs. Bioorganic Med. Chem. 2012, 20, 3954–3971. [Google Scholar] [CrossRef] [PubMed]
- Filho, E.B.A.; Moraes, I.A.; Weber, K.C.; Rocha, G.B.; Vasconcellos, M.L.A.A. DFT/PCM, QTAIM, 1H NMR Conformational Studies and QSAR Modeling of Thirty-Two Anti-Leishmania Amazonensis Morita-Baylis-Hillman Adducts. J. Mol. Struct. 2012, 1022, 72–80. [Google Scholar] [CrossRef]
- Lima-Junior, G.C.; V. Faheina-Martins, G.; C. B. Bomfim, C.; B. Dantas, B.; P. Silva, E.; A. M. de Araújo, D.; B. A. Filho, E.; L. A., A. Vasconcellos, M. Synthesis, Cytotoxic Activity on Leukemia Cell Lines and Quantitative Structure-Activity Relationships (QSAR) Studies of Morita-Baylis-Hillman Adducts. Med. Chem. 2016, 12, 602–612. [Google Scholar] [CrossRef]
- Jeslin Kanaga Inba, P.; Annaraj, B.; Thalamuthu, S.; Neelakantan, M.A. Cu(II), Ni(II), and Zn(II) Complexes of Salan-Type Ligand Containing Ester Groups: Synthesis, Characterization, Electrochemical Properties, and in Vitro Biological Activities. Bioinorg. Chem. Appl. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A.M. Characterization, Thermal and Fluorescence Study of Mn(II) and Pd(II) Schiff Base Complexes. J. Therm. Anal. Calorim. 2017, 128, 1579–1590. [Google Scholar] [CrossRef]
- Mohan, R.; Rastogi, N.; Namboothiri, I.N.N.; Mobin, S.M.; Panda, D. Synthesis and Evaluation of α-Hydroxymethylated Conjugated Nitroalkenes for Their Anticancer Activity: Inhibition of Cell Proliferation by Targeting Microtubules. Bioorganic Med. Chem. 2006, 14, 8073–8085. [Google Scholar] [CrossRef]
- Dadwal, M.; Mohan, R.; Panda, D.; Mobin, S.M.; Namboothiri, I.N.N. The Morita-Baylis-Hillman Adducts of β-Aryl Nitroethylenes with Other Activated Alkenes: Synthesis and Anticancer Activity Studies. Chem. Commun. 2006, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xian, D.M.; Li, H.H.; Zhang, J.C.; You, Z.L. Synthesis and Structures of Halo-Substituted Aroylhydrazones with Antimicrobial Activity. Aust. J. Chem. 2012, 65, 343–350. [Google Scholar] [CrossRef]
- Qian, H.Y. Synthesis, Characterization, X-Ray Crystal Structures and Antibacterial Activities of Oxidovanadium(V) Complexes with Hydrazone and Hydroxamate Ligands. Acta Chim. Slov. 2019, 66, 995–1001. [Google Scholar] [CrossRef]
- Asraf, M.A.; Rahman, M.M.; Kabiraz, D.C.; Ansary, R.H.; Hossen, M.F.; Haque, M.F.; Zakaria, C.M. Structural Elucidation, 3D Molecular Modeling and Antibacterial Activity of Ni (II), Co (II), Cu (II) and Mn (II) Complexes Containing Salophen Ligand. Asian J. Appl. Chem. Res. 2019, 3, 1–15. [Google Scholar] [CrossRef]
- Amarante, G.W.; Benassi, M.; Pascoal, R.N.; Eberlin, M.N.; Coelho, F. Mechanism and Synthesis of Pharmacologically Active Quinolones from Morita-Baylis-Hillman Adducts. Tetrahedron 2010, 66, 4370–4376. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal−Organic Frameworks against Toxic Chemicals. Chem. Rev. 2020, 120, 8130–8160. [Google Scholar] [CrossRef]
- Outis, M.; Rosa, V.; Laia, C.A.T.; Lima, C.; Barroso, S. Synthesis, Crystal Structure and DFT Study of Two New Dinuclear Copper (I) Synthesis, Crystal Structure, and DFT Study of Two New Dinuclear Copper (I) Complexes Bearing Ar-BIAN Ligands Functionalized with NO 2 Groups. Eur. J. Inorg. Chem. 2020, 30, 2900–2911. [Google Scholar] [CrossRef]
- Ring, P. Tuning the Reactivity and Bonding Properties of Metal Square- Planar Complexes by the Substitution(s) on the Trans-Coordinated Pyridine Ring. ACS Omega 2020, 5, 11768–11783. [Google Scholar] [CrossRef]
- Karmakar, A.; Bandyopadhyay, P.; Banerjee, S.; Chandra, N.; Singh, B. Synthesis, Spectroscopic, Theoretical and Antimicrobial Studies on Molecular Charge-Transfer Complex of 4-(2-Thiazolylazo) Resorcinol (TAR) with 3, 5-Dinitrosalicylic Acid, Picric Acid, and Chloranilic Acid. J. Mol. Liq. 2020, 299, 112217. [Google Scholar] [CrossRef]
- Junior, C.G.L.; De Assis, P.A.C.; Silva, F.P.L.; Sousa, S.C.O.; De Andrade, N.G.; Barbosa, T.P.; Nerís, P.L.N.; Segundo, L.V.G.; Anjos, Í.C.; Carvalho, G.A.U.; et al. Efficient Synthesis of 16 Aromatic Morita-Baylis-Hillman Adducts: Biological Evaluation on Leishmania Amazonensis and Leishmania Chagasi. Bioorg. Chem. 2010, 38, 279–284. [Google Scholar] [CrossRef]
- Solaiselvi, R.; Mandal, A.B.; Shanmugam, P. Nucleophilic Substitution Reaction of Morita-Baylis-Hillman Adducts of Isatin with X, S, N, and O-Nucleophiles: A Facile and Efficient Synthesis of Highly Functionalized Tetrasubstituted Alkene Appended Oxindoles. Tetrahedron Lett. 2012, 53, 90–94. [Google Scholar] [CrossRef]
- Carrasco-Sanchez, V.; Simirgiotis, M.J.; Santos, L.S. The Morita-Baylis-Hillman Reaction: Insights into Asymmetry and Reaction Mechanisms by Electrospray Ionization Mass Spectrometry. Molecules 2009, 14, 3989–4021. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Su, Y.Q.; Dong, C.L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.C.; Li, F.M.; You, B.; Guo, W.; et al. Scalable Molten Salt Synthesis of Platinum Alloys Planted in Metal–Nitrogen–Graphene for Efficient Oxygen Reduction. Angew. Chemie -Int. Ed. 2022, 61, e202115835. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Hills, I.D.; Fu, G.C. The First General Method for the Synthesis of Transition-Metal π Complexes of an Electronically Diverse Family of 1,2-Azaborolyls. Organometallics 2002, 21, 4323–4325. [Google Scholar] [CrossRef]
- Aiyelabola, T.; Akinkunmi, E.; Ojo, I.; Obuotor, E.; Adebajo, C.; Isabirye, D. Syntheses, Characterization, Resolution, and Biological Studies of Coordination Compounds of Aspartic Acid and Glycine. Bioinorg. Chem. Appl. 2017, 2017, 2956145. [Google Scholar] [CrossRef]
- Anacona, J.R.; Santaella, J.; Al-shemary, R.K.R.; Amenta, J.; Otero, A.; Ramos, C.; Celis, F. Ceftriaxone-Based Schiff Base Transition Metal(II) Complexes. Synthesis, Characterization, Bacterial Toxicity, and DFT Calculations. Enhanced Antibacterial Activity of a Novel Zn(II) Complex against S. Aureus and E. Coli. J. Inorg. Biochem. 2021, 223, 111519. [Google Scholar] [CrossRef]
- Saqib, S.; Faryad, S.; Afridi, M.I.; Arshad, B.; Younas, M.; Naeem, M.; Zaman, W.; Ullah, F.; Nisar, M.; Ali, S.; et al. Bimetallic Assembled Silver Nanoparticles Impregnated in Aspergillus Fumigatus Extract Damage the Bacterial Membrane Surface and Release Cellular Contents. Coatings 2022, 12, 1505. [Google Scholar] [CrossRef]
- Kirbag, S.; Zengin, F.; Fac, S.; Processing, F. 4 1 305. Antimicrob. Act. Some Euphorbia Species 2013, 10, 305–309. [Google Scholar]
- Narang, R.; Narasimhan, B.; Sharma, S. A Review on Biological Activities and Chemical Synthesis of Hydrazide Derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.S.; Vikraman, D. Schiff Base Rare Earth Metal Complexes: Studies on Functional, Optical and Thermal Properties and Assessment of Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 403–410. [Google Scholar] [CrossRef]
- Sinthuja, S.A.; Shaji, Y.C.; Rose, G.L. Synthesis, Characterization and Evaluation of Biological Properties of Transition Metal Chelates with Schiff Base Ligands Derived from Glutaraldehyde with L-Leucine. Int. J. Sci. Res. Sci. Technol 2018, 4, 587–593. [Google Scholar]
- Selvaganapathy, M.; Raman, N. Pharmacological Activity of a Few Transition Metal Complexes: A Short Review. J. Chem. Biol. Ther. 2016, 01, 108. [Google Scholar] [CrossRef]
- Elleuch, H.; Mihoubi, W.; Mihoubi, M.; Ketata, E.; Gargouri, A.; Rezgui, F. Potential Antioxidant Activity of Morita-Baylis-Hillman Adducts. Bioorg. Chem. 2018, 78, 24–28. [Google Scholar] [CrossRef]
- Tyurin, V.Y.; Moiseeva, A.A.; Shpakovsky, D.B.; Milaeva, E.R. The electrochemical approach to antioxidant activity assay of metal complexes with dipicolylamine ligand, containing 2,6-di-tert-butylphenol groups, based on electrochemical DPPH-test. J. Electroanal. Chem. 2015, 756, 212–221. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, S.; Wu, W.; Yang, K.; Zhang, Y.F.; Tumukunde, E.; Wang, S.; Wang, Y. Functional Analysis of Peptidyl-Prolyl Cis-Trans Isomerase from Aspergillus Flavus. Int. J. Mol. Sci. 2019, 20, 2206. [Google Scholar] [CrossRef]
- El-Halim, H.F.A.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational Behaviour of Lomefloxacin Drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO 2(VI) Ions: Synthesis, Structural Characterization and Biological Activity Studies. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2011, 82, 8–19. [Google Scholar] [CrossRef]
- Devi, J.; Batra, N. Synthesis, Characterization and Antimicrobial Activities of Mixed Ligand Transition Metal Complexes with Isatin Monohydrazone Schiff Base Ligands and Heterocyclic Nitrogen Base. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2015, 135, 710–719. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-Based Catalyst Supports for Oxygen Reduction in Proton-Exchange Membrane Fuel Cells. Trends Chem. 2022, 4, 886–906. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Tombesi, A.; Duan, F.; Zhou, L.; Messori, L.; Giacomelli, C.; Marchetti, L.; Trincavelli, M.L.; Marzo, T.; et al. Ruthenium(II) 1,4,7-Trithiacyclononane Complexes of Curcumin and Bisdemethoxycurcumin: Synthesis, Characterization, and Biological Activity. J. Inorg. Biochem. 2021, 218, 111387. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, B.B.; Mishra, R.R.; Sarangi, A.K. Synthesis, Characterisation, XRD, Molecular Modelling and Potential Antibacterial Studies of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) Complexes with Bidentate Azodye Ligand. J. Saudi Chem. Soc. 2016, 20, 635–643. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdelhamid, A.A.; Marzouk, A.A.; Shehata, M.R.; Bakheet, M.A.; Almaghrabi, O.A.; Nafady, A. Synthesis and Characterization of New Cr(III), Fe(III) and Cu(II) Complexes Incorporating Multi-Substituted Aryl Imidazole Ligand: Structural, DFT, DNA Binding, and Biological Implications. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2020, 228, 117700. [Google Scholar] [CrossRef]
- Köse, D.A.; Necefo, H.È. Synthesis and Characterization of Bis(Nicotinamide) m-Hydroxybenzoate Complexes of Co(II), Ni(II), Cu(II) and Zn(II). J. Therm. Anal. Calorim. 2008, 93, 509–514. [Google Scholar] [CrossRef]
- Bursal, E.; Turkan, F.; Buldurun, K.; Turan, N.; Aras, A.; Çolak, N.; Murahari, M.; Yergeri, M.C. Transition Metal Complexes of a Multidentate Schiff Base Ligand Containing Pyridine: Synthesis, Characterization, Enzyme Inhibitions, Antioxidant Properties, and Molecular Docking Studies. BioMetals 2021, 34, 393–406. [Google Scholar] [CrossRef]
- Yeğiner, G.; Gülcan, M.; Işık, S.; Ürüt, G.Ö.; Özdemir, S.; Kurtoğlu, M. Transition Metal (II) Complexes with a Novel Azo-Azomethine Schiff Base Ligand: Synthesis, Structural and Spectroscopic Characterization, Thermal Properties and Biological Applications. J. Fluoresc. 2017, 27, 2239–2251. [Google Scholar] [CrossRef]
- Saad, F.A. Synthesis, Spectral, Electrochemical and X-Ray Single Crystal Studies on Ni(II) and Co(II) Complexes Derived from 1-Benzoyl-3-(4-Methylpyridin-2-Yl) Thiourea. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2014, 128, 386–392. [Google Scholar] [CrossRef]
- Şahin, Ö.; Özdemir, Ü.Ö.; Seferoğlu, N.; Genc, Z.K.; Kaya, K.; Aydıner, B.; Tekin, S.; Seferoğlu, Z. New Platinum (II) and Palladium (II) Complexes of Coumarin-Thiazole Schiff Base with a Fluorescent Chemosensor Properties: Synthesis, Spectroscopic Characterization, X-ray Structure Determination, in Vitro Anticancer Activity on Various Human Carcinoma Ce. J. Photochem. Photobiol. B Biol. 2018, 178, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.; Al-Bairmani, H.K.; Sabri Abbas, Z.; Mahdi Rheima, A. Nano Metal-Complexes of Theophylline Derivative: Synthesis, Characterization, Molecular Structure Studies, and Antibacterial Activity. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 052028. [Google Scholar] [CrossRef]
- Coelho, F.; Almeida, W.P.; Veronese, D.; Mateus, C.R.; Silva Lopes, E.C.; Rossi, R.C.; Silveira, G.P.C.; Pavam, C.H. Ultrasound in Baylis-Hillman Reactions with Aliphatic and Aromatic Aldehydes: Scope and Limitations. Tetrahedron 2002, 58, 7437–7447. [Google Scholar] [CrossRef]
- Saikia, M.; Sarma, J.C. Baylis-Hillman Reaction under Solvent-Free Conditions - Remarkable Rate Acceleration and Yield Enhancement. Can. J. Chem. 2010, 88, 1271–1276. [Google Scholar] [CrossRef]
- Abdi, Y.; Bensouilah, N.; Siziani, D.; Hamdi, M.; Silva, A.M.S.; Boutemeur-Kheddis, B. New Complexes of Manganese (II) and Copper (II) Derived from the Two New Furopyran-3, 4-Dione Ligands: Synthesis, Spectral Characterization, ESR, DFT Studies and Evaluation of Antimicrobial Activity. J. Mol. Struct. 2020, 1202, 127307. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Nicolova, I.; Konstantinov, S.; Karaivanova, M. New Lanthanide Complexes of 4-Methyl-7-Hydroxycoumarin and Their Pharmacological Activity. Eur. J. Med. Chem. 2001, 36, 339–347. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, A.; Hussain, I.; Khan, M.A.; Gul, S.; Iqbal, M.; Inayat-Ur-Rahman; Khuda, F. Spectral, XRD, SEM and Biological Properties of New Mononuclear Schiff Base Transition Metal Complexes. Inorg. Chem. Commun. 2013, 35, 104–109. [Google Scholar] [CrossRef]
- Bouhdada, M.; Amane, M.E.L.; El Hamzaoui, N. Synthesis, Spectroscopic Studies, X-Ray Powder Diffraction Data and Antibacterial Activity of Mixed Transition Metal Complexes with Sulfonate Azo Dye, Sulfamate and Caffeine Ligands. Inorg. Chem. Commun. 2019, 101, 32–39. [Google Scholar] [CrossRef]
- Alkış, M.E.; Buldurun, K.; Turan, N.; Alan, Y.; Yılmaz, Ü.K.; Mantarcı, A. Synthesis, Characterization, Antiproliferative of Pyrimidine Based Ligand and Its Ni(II) and Pd(II) Complexes and Effectiveness of Electroporation. J. Biomol. Struct. Dyn. 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Bingham, A.L.; Drake, J.E.; Hursthouse, M.B.; Light, M.E.; Nirwan, M.; Ratnani, R. Synthesis, Characterization and Spectral Studies of Nitrogen Base Adducts of Bis(O,O′-Ditolyldithiophosphato)Nickel(II). Crystal Structures of Ni[S2P(OC6H4Me-p)2] 2 · C10H8N2 and Ni[S2P(OC6H4Me-o)2] 2 · C14H12N. Polyhedron 2007, 26, 2672–2678. [Google Scholar] [CrossRef]
- Mahapatra, B.B.; Mishra, R.R.; Sarangi, A.K. Synthesis, Spectral, Thermogravimetric, XRD, Molecular Modelling and Potential Antibacterial Studies of Dimeric Complexes with Bis Bidentate on-No Donor Azo Dye Ligands. J. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Rahaman, F.; Mruthyunjayaswamy, B.H.M. Synthesis, Spectral Characterization and Biological Activity Studies of Transition Metal Complexes of Schiff Base Ligand Containing Indole Moiety. Complex Met. 2014, 1, 88–95. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K.; Abou El-Enein, S.A.; El-Sheikh-Amer, M.M. Design of New Metal Complexes of 2-(3-Amino-4,6-Dimethyl-1H-Pyrazolo[3,4-b]Pyridin-1-Yl)Aceto-Hydrazide: Synthesis, Characterization, Modelling and Antioxidant Activity. J. Mol. Struct. 2017, 1127, 386–396. [Google Scholar] [CrossRef]
- Kantcheva, M.; Vakkasoglu, A.S. Cobalt Supported on Zirconia and Sulfated Zirconia I. FT-IR Spectroscopic Characterization of the NOx Species Formed upon NO Adsorption and NO/O2 Coadsorption. J. Catal. 2004, 223, 352–363. [Google Scholar] [CrossRef]
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic Activity of Cerium Complexes with Coumarin Derivatives. Molecular Modeling of the Ligands. Arch. Pharm. 2000, 333, 93–98. [Google Scholar] [CrossRef]
- Szabó, P.T.; Kele, Z. Electrospray Mass Spectrometry of Hydrophobic Compounds Using Dimethyl Sulfoxide and Dimethylformamide as Solvents. Rapid Commun. Mass Spectrom. 2001, 15, 2415–2419. [Google Scholar] [CrossRef]
- Castro, M.E.; Percino, M.J.; Chapela, V.M.; Ceron, M.; Soriano-Moro, G.; Lopez-Cruz, J.; Melendez, F.J. Theoretical and Experimental Spectroscopic Analysis of Cyano-Substituted Styrylpyridine Compounds. Int. J. Mol. Sci. 2013, 14, 4005–4029. [Google Scholar]
- Enisoğlu Atalay, V.; Barış, Ö.; Karahan, M. Modeling of BSA–Metal Ion-Acrylic Acid Complex by Theoretical Methods: Semi-Empirical PM6 and Docking Study. Acta Phys. Pol. A 2018, 134, 1200–1203. [Google Scholar] [CrossRef]
- Pourjavid, M.R.; Sehat, A.A.; Arabieh, M.; Yousefi, S.R.; Hosseini, M.H.; Rezaee, M. Column Solid Phase Extraction and Flame Atomic Absorption Spectrometric Determination of Manganese(II) and Iron(III) Ions in Water, Food and Biological Samples Using 3-(1-Methyl-1H-Pyrrol-2-Yl)-1H-Pyrazole-5-Carboxylic Acid on Synthesized Graphene Oxide. Mater. Sci. Eng. C 2014, 35, 370–378. [Google Scholar] [CrossRef]
- Nisa, B.; Ullah, F.; Nisa, I.; Ahmad, M.; Zafar, M.; Munir, M.; Sultana, S.; Zaman, W.; Manghwar, H.; Ullah, F.; et al. Biodiesel Production Using Wild Apricot (Prunus Aitchisonii) Seed Oil via Heterogeneous Catalysts. Molecules 2022, 27, 4752. [Google Scholar] [CrossRef]
- Neelakantan, M.A.; Marriappan, S.S.; Dharmaraja, J.; Jeyakumar, T.; Muthukumaran, K. Spectral, XRD, SEM and Biological Activities of Transition Metal Complexes of Polydentate Ligands Containing Thiazole Moiety. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2008, 71, 628–635. [Google Scholar] [CrossRef]
- Martí-Rujas, J. Structural Elucidation of Microcrystalline MOFs from Powder X-Ray Diffraction. Dalt. Trans. 2020, 49, 13897–13916. [Google Scholar] [CrossRef]
- Hankare, P.P.; Naravane, S.R.; Bhuse, M.V.; Delekar, S.D.; Jagtap, A.H. Synthesis and Characterization of Mn(II), Co(II), Ni(II), Cu(II) and Zn (II) Azo Coumarin Complexes. Indian J. Chem. - Sect. A Inorganic, Phys. Theor. Anal. Chem. 2004, 43, 1464–1467. [Google Scholar]
- Roller, J. X-Ray Diffraction. PEM Fuel Cell Diagnostic Tools 2011, 289–313. [Google Scholar] [CrossRef]
- Zaman, S.; Tian, X.; Su, Y.Q.; Cai, W.; Yan, Y.; Qi, R.; Douka, A.I.; Chen, S.; You, B.; Liu, H.; et al. Direct Integration of Ultralow-Platinum Alloy into Nanocarbon Architectures for Efficient Oxygen Reduction in Fuel Cells. Sci. Bull. 2021, 66, 2207–2216. [Google Scholar] [CrossRef]
- Borders, B.; Adinehnia, M.; Chilukuri, B.; Ruf, M.; Hipps, K.W.; Mazur, U. Tuning the Optoelectronic Characteristics of Ionic Organic Crystalline Assemblies. J. Mater. Chem. C 2018, 6, 4041–4056. [Google Scholar]
- Ramachandra, M.; Abhishek, A.; Siddeshwar, P.; Bharathi, V. Hardness and Wear Resistance of ZrO2 Nano Particle Reinforced Al Nanocomposites Produced by Powder Metallurgy. Procedia Mater. Sci. 2015, 10, 212–219. [Google Scholar]
- Cid, A.; Simal-Gandara, J. Synthesis, Characterization, and Potential Applications of Transition Metal Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1011–1032. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis and Antimicrobial Activity of Copper(II) and Silver(I) Complexes of Hydroxynitrocoumarins: X-Ray Crystal Structures of [Cu(Hnc) 2(H2O)2] · 2H2O and [Ag(Hnc)] (HncH = 4-Hydroxy-3-Nitro-2H-Chromen-2-One). Polyhedron 2005, 24, 949–957. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Wu, W.Z.; Ahmad, S.; Wang, S.; Zhang, Y.F.; Yang, H.; Wang, S.H.; Wang, Y. Expression and Antibody Preparation of Small Ubiquitin-like Modifier (SUMO) from Aspergillus Flavus. IOP Conf. Ser. Earth Environ. Sci. 2019, 346. [Google Scholar] [CrossRef]
- Tavares, E.C.; Rubinger, M.M.M.; Zacchi, C.H.C.; Silva, S.A.; Oliveira, M.R.L.; Guilardi, S.; Alcântara, A.F.D.C.; Piló-Veloso, D.; Zambolim, L. Synthesis, Characterization, and Antifungal Activity of Novel (Z)-N-(2-Cyano-3-Phenylprop-2-En-1-Yl)-Alkyl/Aryl-Sulfonamides Derived from a Morita-Baylis-Hillman Adduct. J. Mol. Struct. 2014, 1067, 43–51. [Google Scholar] [CrossRef]

| Compound | Molecular Formula | M. Wt (g/mol) | Color | M.P(°C) | Analysis (%) Calc. (Found) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | |||||
| MNHA (3) | C11H11NO5 | 237.06 | Orange brown | 124.3–126.5 | 55.70 (55.20) | 4.67 (4.56) | 5.90 (6.1) | 33.72 (32.98) |
| Ni(MNHA) (4) | C22H20N2NiO10 | 531.10 | Penny brown | >300 | 49.56 (50.20) | 3.80 (4.35) | 5.27 (5.9) | 30.13 (31.05) |
| Cu(MNHA) (5) | C22H20CuN2O10 | 535.95 | Shamrock green | >300 | 49.30 (50.12) | 3.12 (4.54) | 5.23 (5.38) | 29.85 (28.96) |
| Co(MNHA) (6) | C22H20CoN2O10+ | 531.33 | Dark gray | >300 | 49.73 (49.54) | 3.79 (4.05) | 5.27 (5.03) | 30.11 (30.84) |
| Cr(MNHA) (7) | C22H20CrN2O10+ | 524.05 | Caramel brown | >300 | 50.39 (50.89) | 3.84 (4.16) | 5.34 (5.07) | 30.51 (30.10) |
| Mn(MNHA) (8) | C22H20MnN2O10 | 527.05 | Amber brown | >300 | 50.11 (50.65) | 3.82 (3.53) | 5.31 (5.97) | 30.34 (30.16) |
| Sample (s) | Concentration 1 × 10−3 (M) | λmax (nm) | ɛ | Absorbance |
|---|---|---|---|---|
| (mol−1cm−1L) | (A) | |||
| MNHA | 1.051 | 250 | 676 | 0.71 |
| 266 | 770.69 | 0.81 | ||
| 275 | 808.76 | 0.85 | ||
| 300 | 57.088 | 0.06 | ||
| Ni(MNHA) | 1.049 | 264 | 2694 | 0.132 |
| 283 | 108.67 | 0.114 | ||
| 370 | 74.36 | 0.078 | ||
| Cu(MNHA) | 1.047 | 266 | 209.17 | 0.219 |
| 290 | 169.05 | 0.177 | ||
| 371 | 108.88 | 0.114 | ||
| Co(MNHA) | 1.05 | 268 | 42.86 | 0.045 |
| 308 | 60.95 | 0.064 | ||
| 371 | 115.23 | 0.121 | ||
| Cr(MNHA) | 1.049 | 265 | 27.65 | 0.029 |
| 308 | 20.97 | 0.022 | ||
| 371 | 65.78 | 0.069 | ||
| Mn(MNHA) | 1.048 | 268 | 432.25 | 0.453 |
| 286 | 317.75 | 0.333 | ||
| 366 | 110.69 | 0.116 |
| Functional Group | (MNHA) Exp. | (MNHA) Calc. | Ni (MNHA) | Cu (MNHA) | Co( MNHA) | Cr (MNHA) | Mn (MNHA) |
|---|---|---|---|---|---|---|---|
| ῡ (-OH) | 3477 | 3659 | 3450–3300 | 3480–3380 | 3500–3350 | 3500–3400 | 3450–3350 |
| ῡ (=C-H) | 2958 | 3136 | 2931 | 2926 | 2929 | 2950 | 2926 |
| ῡ (sp3 C-H stretch) | 2875 | 3037 | 2860 | 2856 | 2858 | 2800 | 2856 |
| ῡ (ester C=O) | 1720 | 1677, 1697 | 1716 | - | - | - | - |
| ῡ (aromatic C=C) | 1629 | 1612 | 1610 | 1622 | 1597 | 1625 | 1610 |
| ῡ (-N=O) | 1529, 1552 | 1434 | 1525 | - | 1573 | 1527 | 1560 |
| ῡ (-CH3) | 1442 | 1483, 1521 | 1431 | - | - | - | - |
| ῡ (C-O) | 1249 | 1235 | 1384 | 1388 | 1390 | 1390 | 1388 |
| ῡ (C-OCH3) | 1149 | 1159 | 1141 | 1114 | 1114 | 1177 | 1128 |
| ῡ (-OH bend) | 1045 | 980, 438 | 1111 | - | - | 1107 | - |
| ῡ (=C-H aromatic) | 744 | 748 | 651 | - | - | 750 | - |
| ῡ (M-O) | - | 609 | 607 | 651 | 603 | 611 | |
| ῡ (M-OH) | - | 790 | 782 | 748 | 842 | 750 | |
| ῡ (M-NO2) | - | 464 | 472 | 607 | 514 | 509 |
| Compound | S. Group (G. No.) | Volume (10−6 pm3) | Density (g/cm3) | Unit Cell Dimensions (Å) | Crystal Class | No. of Unit Cells (Z) | Reference Intensity Ratio (RIR) | S (nm) |
|---|---|---|---|---|---|---|---|---|
| Ni(MNHA) | P6322 (182) | 79.31 | 7.96 | a = b ≠ c α = β = 90° γ = 120° | Hexagonal | 2 | 6.40 | 9.9876 |
| Cu(MNHA) | Bmm2 (38) | 307.56 | 5.66 | a ≠ b ≠ c α = β = γ = 90° | Orthorhombic | 2 | 4.97 | 6.9064 |
| Co(MNHA) | Fd-3m (227) | 528.30 | 6.05 | a = b = c α = β = γ = 90° | Cubic | 8 | 5.32 | 4.6041 |
| Cr(MNHA) | Pm-3n (223) | 93.82 | 6.09 | a = b = c α = β = γ = 90° | Cubic | 2 | 6.39 | 5.9128 |
| Mn(MNHA) | Fm-3n (225) | 42.51 | 8.58 | a = b = c α = β = γ = 90° | Cubic | 4 | 7.53 | 5.9015 |
| Complex Compound | Miller Indices | d [Å] | 2° 𝜃 | Intensity (%) | ||
|---|---|---|---|---|---|---|
| H | K | L | ||||
| [Ni(MNHA)2] | 1 | 1 | 0 | 2.308 | 38.993 | 18.8 |
| 0 | 0 | 2 | 2.149 | 42.01 | 23.5 | |
| 1 | 1 | 1 | 2.0333 | 44.522 | 100 | |
| 1 | 1 | 2 | 1.572 | 58.615 | 14.7 | |
| [Cu(MNHA)2] | −1 | 1 | 1 | 2.524 | 35.539 | 100 |
| 1 | 1 | 1 | 2.323 | 38.731 | 100 | |
| 2 | 0 | 0 | 2.311 | 38.941 | 100 | |
| 0 | 0 | 2 | 2.431 | 35.438 | 60 | |
| [Co(MNHA)2]Cl | 2 | 2 | 0 | 2.86 | 31.249 | 40 |
| 3 | 1 | 1 | 2.438 | 36.837 | 100 | |
| 5 | 1 | 1 | 1.555 | 59.35 | 35 | |
| 4 | 4 | 0 | 1.4293 | 65.222 | 45 | |
| [Cr(MNHA)2]Cl | 1 | 1 | 0 | 3.213 | 27.742 | 27.2 |
| 2 | 0 | 0 | 2.272 | 39.637 | 16 | |
| 2 | 1 | 0 | 2.0321 | 45.551 | 100 | |
| 2 | 1 | 1 | 1.855 | 49.069 | 28.6 | |
| [Mn(MNHA)2] | 0 | 1 | 1 | 3.08 | 28.967 | 40 |
| 1 | 0 | 3 | 2.76 | 32.412 | 60 | |
| 2 | 1 | 0 | 2.49 | 36.041 | 80 | |
| 1 | 2 | 4 | 2.03 | 44.6 | 100 | |
| Compound | S. aureus (%) | E. coli (%) | B. pumilis (%) | S.typhi (%) |
|---|---|---|---|---|
| MNHA | 98.6 | 96.8 | 64.8 | 45.8 |
| Ni(MNHA) | 52.3 | 70.8 | 48.1 | 54.1 |
| Cu(MNHA) | - | - | - | - |
| Co(MNHA) | 66.6 | 83.0 | 59.2 | 62.5 |
| Cr(MNHA) | - | - | - | 41.6 |
| Mn(MNHA) | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishfaq, S.; Nisar, S.; Iqbal, S.; Ali, S.; Ali, S.T.; Din, E.; Alsaiari, N.S.; Dahlous, K.A.; Javed, M.S.; Bocchetta, P. A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies. Molecules 2022, 27, 8150. https://doi.org/10.3390/molecules27238150
Ishfaq S, Nisar S, Iqbal S, Ali S, Ali ST, Din E, Alsaiari NS, Dahlous KA, Javed MS, Bocchetta P. A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies. Molecules. 2022; 27(23):8150. https://doi.org/10.3390/molecules27238150
Chicago/Turabian StyleIshfaq, Shazia, Shazia Nisar, Sadaf Iqbal, Saqib Ali, Syed Tariq Ali, ElSayed Din, Norah Salem Alsaiari, Kholood A. Dahlous, Muhammad Sufyan Javed, and Patrizia Bocchetta. 2022. "A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies" Molecules 27, no. 23: 8150. https://doi.org/10.3390/molecules27238150
APA StyleIshfaq, S., Nisar, S., Iqbal, S., Ali, S., Ali, S. T., Din, E., Alsaiari, N. S., Dahlous, K. A., Javed, M. S., & Bocchetta, P. (2022). A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies. Molecules, 27(23), 8150. https://doi.org/10.3390/molecules27238150









