Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae
Abstract
1. Introduction
2. Results
2.1. Preliminary Chemical Characterization
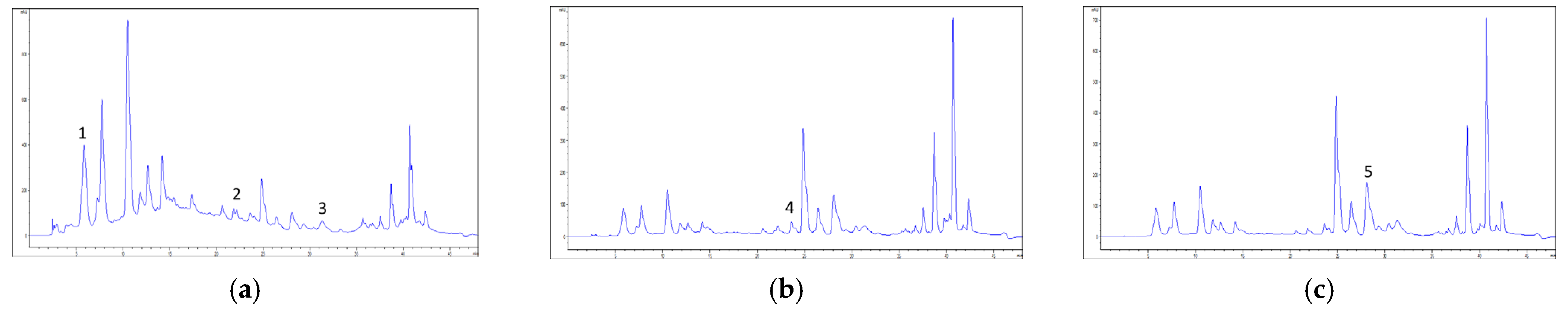
2.2. High-Performance Liquid Chromatography Coupled to Diode Array Detector (HPLC-DAD) Chemical Profiling
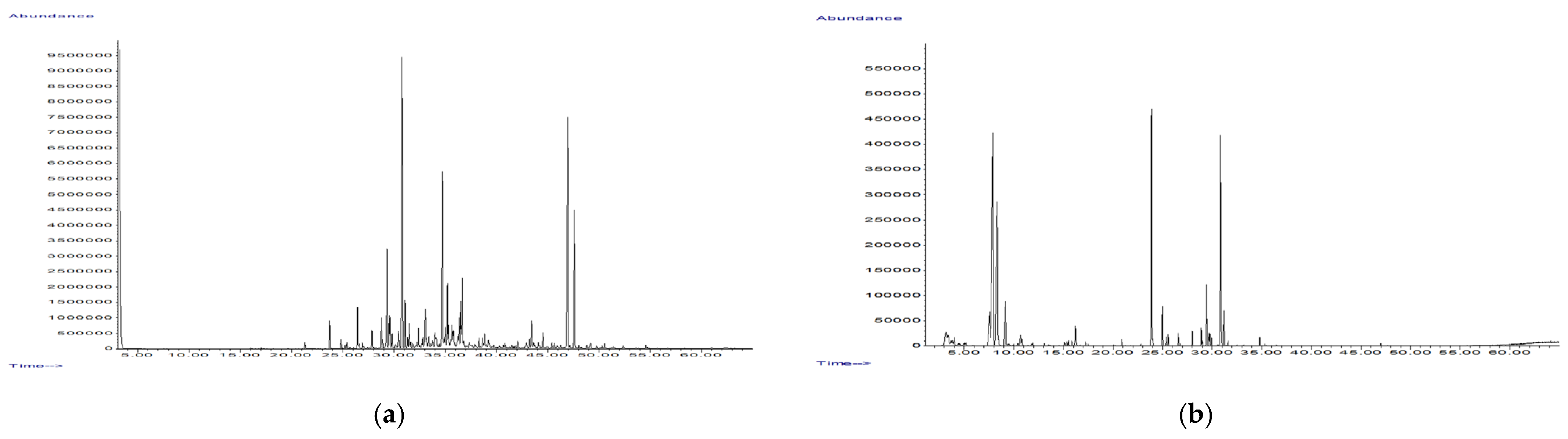
2.3. Chemical Analysis of Volatile Organic Compounds (VOCs)
2.4. Antioxidant Potential
2.5. Antihyperglycemic Potential
2.6. Cistus Biological Activity–Chemometric Approach
3. Discussion
4. Materials and Methods
4.1. Herbal Material
4.2. Preparation of Samples for Further Analysis
4.3. Chemical Characterization
4.3.1. Preliminary Chemical Characterization
4.3.2. HPLC-DAD Chemical Profiling
4.4. Chemical Analysis of Volatile Organic Compounds
4.5. Antioxidant Potential
4.6. Antihyperglicemic Potential
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Martí Bruñá, N.; Saura López, D.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus sp.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 649–658. [Google Scholar]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- Abreu, M.M.; Santos, E.S.; Ferreira, M.; Magalhães, M.C.F. Cistus salviifolius a promising species for mine wastes remediation. J. Geochem. Explor. 2012, 113, 86–93. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vieira, C.; Abreu, M.M.; Magalhaes, M.C.F. Physiological response of Cistus salviifolius L. to high arsenic concentrations. Environ. Geochem. Health 2020, 42, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, M.A.; Rodriguez-Echeverria, S. Effect of smoke, charred wood, and nitrogenous compounds on seed germination of ten species from woodland in central-western Spain. J. Chem. Ecol. 2003, 29, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Thanos, C.A.; Georghiou, K.; Kadis, C.; Pantazi, C. Cistaceae: A plant family with hard seeds. Israel J. Bot. 1992, 41, 251–263. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B 2019, 193, 51–88. [Google Scholar] [CrossRef]
- Frazao, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabaris, C.; Goncalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Sayah, K.; Mrabti, H.N.; Belarj, B.; Kichou, F.; Cherrah, Y.; El Abbes Faouzi, M. Evaluation of antidiabetic effect of Cistus salviifolius L. (Cistaceae) in streptozotocin-nicotinamide induced diabetic mice. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 121–127. [Google Scholar] [CrossRef]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Faouzi, M.E. Antioxidant Activity and Inhibitory Potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. BioMed Res. Int. 2017, 2017, 2789482. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Al-Jaber, H.I.; Tashtoush, H.I.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical composition, biological and cytotoxic activities of Cistus salviifolius flower buds and leaves extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- Zeouk, I.; Balouiri, M.; Bekhti, K. Antistaphylococcal Activity and Phytochemical Analysis of Crude Extracts of Five Medicinal Plants Used in the Center of Morocco against Dermatitis. Int. J. Microbiol. 2019, 2019, 1803102. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Roldan, C.; Guillen, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Carev, I.; Maravic, A.; Ilic, N.; Cikes Culic, V.; Politeo, O.; Zoric, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavaric, N.; Bozin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Fanouriou, E.; Kalivas, D.; Daferera, D.; Tarantilis, P.; Trigas, P.; Vahamidis, P.; Economou, G. Hippocratic medicinal flora on the Greek Island of Kos: Spatial distribution, assessment of soil conditions, essential oil content and chemotype analysis. J. Appl. Res. Med. Aromat. Plants 2018, 9, 97–109. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Ben Jemia, M.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Demetzos, C.; Angelopoulou, D.; Perdetzoglou, D. A comparative study of the essential oils of Cistus salviifolius in several populations of Crete (Greece). Biochem. Syst. Ecol. 2002, 30, 651–665. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Usai, M. Comparison of essential oils from Cistus species growing in Sardinia. Nat. Prod. Res. 2017, 31, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Maravić, A.; Burčula, F.; Carev, I.; Kamenjarin, J. Phytochemical Composition and Antimicrobial Activity of Essential Oils of Wild Growing Cistus species in Croatia. Nat. Prod. Commun. 2018, 13, 771–774. [Google Scholar] [CrossRef]
- Pistelli, L.; Bandeira Reidel, R.V.; Parri, F.; Morelli, E.; Pistelli, L. Chemical composition of essential oil from plants of abandoned mining site of Elba island. Nat. Prod. Res. 2019, 33, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Kladar, N.; Grujic, N.; Anackov, G.; Samojlik, I.; Gavaric, N.; Conic, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar]
- Kuhn, C.; Arapogianni, N.E.; Halabalaki, M.; Hempel, J.; Hunger, N.; Wober, J.; Skaltsounis, A.L.; Vollmer, G. Constituents from Cistus salvifolius (Cistaceae) activate peroxisome proliferator-activated receptor-gamma but not -delta and stimulate glucose uptake by adipocytes. Planta Med. 2011, 77, 346–353. [Google Scholar] [CrossRef]
- Essential oils in herbal drugs. In European Pharmacopoeia (Ph.Eur.), 10th ed.; European Directorate for the Quality of Medicines & Healthcare, Council of Europe: Strasbourg, France, 2019; pp. 307–308.
- Kladar, N.; Srđenović, B.; Grujić, N.; Bokić, B.; Rat, M.; Anačkov, G.; Božin, B. Ecologically and ontogenetically induced variations in phenolic compounds and biological activities of Hypericum maculatum subsp. maculatum, Hypericaceae. Braz. J. Bot. 2015, 38, 703–715. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Srđenović Čonić, B.; Jeremić, K.; Barjaktarović, J.; Hitl, M.; Gavaric, N.; Božin, B. Stabilization of sunflower and olive oils with savory (Satureja kitaibelii, Lamiaceae). J. Food Nutr. Res.-Slov. 2020, 59, 259–271. [Google Scholar]
- Adams, R.P.; Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3rd ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2004. [Google Scholar]
- Kladar, N.; Mrdanovic, J.; Anackov, G.; Solajic, S.; Gavaric, N.; Srdenovic, B.; Bozin, B. Hypericum perforatum: Synthesis of Active Principles during Flowering and Fruitification-Novel Aspects of Biological Potential. Evid. Based Complement. Alternat. Med. 2017, 2017, 2865610. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Anačkov, G.T.; Balog, K.J.; Francišković, M.M.; Mimica-Dukić, N.M. Juniperus sibirica Burgsdorf as a novel source of antioxidant and antiinflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Parmar, H.S.; Jain, P.; Chauhan, D.S.; Bhinchar, M.K.; Munjal, V.; Yusuf, M.; Choube, K.; Tawani, A.; Tiwari, V.; Manivannan, E.; et al. DPP-IV inhibitory potential of naringin: An in silico, in vitro and in vivo study. Diabetes Res. Clin. Pract. 2012, 97, 105–111. [Google Scholar] [CrossRef]


| P-Et | E-Et | P-Aq | E-Aq | |
|---|---|---|---|---|
| yield of extraction (%) | 13.45 ± 0.95 a,b | 14.84 ± 0.76 c | 37.04 ± 1.22 a,c | 33.32 ± 1.11 b,c |
| content of total phenolics (mg GAE/g DE) | 352.88 ± 3.56 d | 379.58 ± 0.88 d,e | 352.37 ± 3.27 e | 279.32 ± 0.33 d,e |
| content of total tannins (mg GAE/g DE) | 301.30 ± 4.44 f | 329.49 ± 2.78 f | 211.18 ± 8.11 f | 159.22 ± 6.03 f |
| content of total flavonoids (mg QE/g DE) | 26.33 ± 0.87 g,h | 29.41 ± 1.43 i | 36.20 ± 2.98 g,j | 45.37 ± 4.02 h,i,j |
| P-Et | E-Et | P-Aq | E-Aq | |
|---|---|---|---|---|
| gallic acid (mg/g DE) | 14.96 ± 2.244 | 21.18 ± 3.177 | 15.09 ± 2.263 | 27.67 ± 4.15 |
| trans-cinnamic acid (mg/g DE) | 0.14 ± 0.015 | 0.24 ± 0.026 | n.d. | n.d. |
| p-coumaric acid (mg/g DE) | n.d. | n.d. | n.d. | n.d. |
| caffeic acid (mg/g DE) | 0.43 ± 0.021 | 0.46 ± 0.023 | 0.63 ± 0.032 | 0.41 ± 0.021 |
| ferulic acid (mg/g DE) | n.d. | n.d. | n.d. | n.d. |
| chlorogenic acid (mg/g DE) | 0.95 ± 0.047 | 2.20 ± 0.11 | 0.89 ± 0.045 | 1.28 ± 0.064 |
| rosmarinic acid (mg/g DE) | n.d. | n.d. | n.d. | n.d. |
| quercetin (mg/g DE) | n.d. | n.d. | n.d. | n.d. |
| quercitrin (mg/g DE) | n.d. | n.d. | n.d. | n.d. |
| rutin (mg/g DE) | 8.97 ± 0.718 | 5.59 ± 0.447 | 4.09 ± 0.237 | 2.58 ± 0.206 |
| Peak No. | Compound | RI | RIl | P-HSS | E-HSS | P-EO | E-EO |
|---|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 5.54 | 6.45 | 0.42 | 0.90 | |||
| 1 | α-Pinene | 939 | 933 | 0.86 ± 0.04 | 0.65 ± 0.03 | 0.47 ± 0.02 | 0.62 ± 0.03 |
| 2 | β-Pinene | 979 | 973 | 2.91 ± 0.14 | 3.3 ± 0.16 | 0.42 ± 0.02 | 0.28 ± 0.01 |
| 4 | o-Cymene | 1025 | 1025 | 0.96 ± 0.05 | 1.39 ± 0.07 | n.i. | n.i. |
| 5 | Limonene | 1029 | 1023 | 1.67 ± 0.08 | 1.76 ± 0.09 | n.i. | n.i |
| Oxygenated monoterpenes | 5.27 | 7.82 | 3.05 | 1.14 | |||
| 7 | Camphor | 1146 | 1129 | 5.02 ± 0.25 | 7.63 ± 0.38 | 1.11 ± 0.05 | 0.88 ± 0.04 |
| 8 | Carvenone | 1258 | 1248 | 0.25 ± 0.01 | 0.19 ± 0.01 | 1.94 ± 0.10 | 0.26 ± 0.01 |
| Aromatic oxygenated monoterpenes | 2.62 | 2.63 | 0.96 | 3.69 | |||
| 9 | Thymol | 1291 | 1275 | 2.62 ± 0.13 | 2.63 ± 0.13 | 0.96 ± 0.05 | 3.69 ± 0.18 |
| Sesquiterpene hydrocarbons | 67.82 | 65.30 | 79.00 | 77.02 | |||
| 10 | α-Cubebene | 1351 | 1530 | n.i. | n.i. | 0.55 ± 0.03 | 0.34 ± 0.02 |
| 11 | α-Copaene | 1377 | 1376 | n.i. | n.i. | 0.63 ± 0.03 | 0.8 ± 0.04 |
| 12 | β-Elemene | 1395 | 1374 | 0.91 ± 0.04 | 0.85 ± 0.04 | 0.95 ± 0.05 | 0.63 ± 0.03 |
| 13 | Longifolene | 1408 | 1401 | 1.74 ± 0.09 | 1.81 ± 0.09 | 2.18 ± 0.11 | 0.35 ± 0.02 |
| 14 | E-caryophyllene | 1419 | 1419 | 2.19 ± 0.11 | 2.48 ± 0.12 | 2.55 ± 0.13 | 3.52 ± 0.18 |
| 15 | β-Copaene | 1432 | 1434 | 0.92 ± 0.05 | 0.46 ± 0.02 | 4.53 ± 0.23 | 4.66 ± 0.23 |
| 16 | (E)-β-Farnesene | 1456 | 1448 | 19.67 ± 0.98 | 18.23 ± 0.91 | 16.39 ± 0.82 | 17.34 ± 0.87 |
| 17 | Alloaromadendrene | 1461 | 1460 | n.i. | 0.11 ± 0.01 | 0.85 ± 0.04 | 0.36 ± 0.02 |
| 18 | β-Selinene | 1486 | 1481 | 0.89 ± 0.04 | 0.62 ± 0.03 | 0.89 ± 0.04 | 0.79 ± 0.04 |
| 19 | Germacrene D | 1488 | 1485 | 40.92 ± 2.04 | 39.82 ± 1.99 | 41.092.05 | 39.78 ± 1.99 |
| 20 | epi-Bicyclosesquiphellandrene | 1496 | 1490 | n.i. | 0.12 ± 0.01 | 1.25 ± 0.06 | 1.77 ± 0.09 |
| 21 | α-Muurolene | 1499 | 1495 | n.i. | n.i. | 0.83 ± 0.04 | 0.81 ± 0.04 |
| 22 | Bicyclogermacrene | 1502 | 1497 | 0.23 ± 0.01 | 0.36 ± 0.02 | 4.47 ± 0.22 | 3.45 ± 0.17 |
| 23 | β-Bisabolene | 1511 | 1505 | 0.35 ± 0.02 | 0.44 ± 0.02 | 0.65 ± 0.03 | 0.83 ± 0.04 |
| 24 | (-)-β-Cadinene | 1518 | 1522 | n.i. | n.i. | 1.19 ± 0.04 | 1.59 ± 0.08 |
| Aromatic sesquiterpene hydrocarbons | 1.46 | 1.47 | 3.39 | 3.06 | |||
| 25 | cis-Calamenene | 1531 | 1531 | 1.24 ± 0.06 | 1.33 ± 0.07 | 2.11 ± 0.10 | 1.52 ± 0.08 |
| 26 | α-Calacorene | 1542 | 1540 | 0.22 ± 0.01 | 0.14 ± 0.01 | 1.28 ± 0.06 | 1.54 ± 0.08 |
| Oxygenated sesquiterpenes | 10.71 | 11.53 | 11.16 | 9.95 | |||
| 27 | (-)-Spathulenol | 1577 | 1568 | 0.32 ± 0.02 | 0.23 ± 0.01 | 1.54 ± 0.08 | 1.44 ± 0.11 |
| 28 | Caryophyllene oxide | 1581 | 1574 | 8.29 ± 0.41 | 9.77 ± 0.49 | 6.28 ± 0.31 | 5.66 ± 0.28 |
| 30 | α-Bisabolol oxide | 1744 | 1722 | 1.98 ± 0.10 | 1.53 ± 0.08 | 2.66 ± 0.13 | 2.19 ± 0.11 |
| 31 | b-Bisabolenol | 1790 | 1774 | 0.12 ± 0.01 | n.i. | 0.68 ± 0.03 | 0.66 ± 0.03 |
| Aliphatic compounds | 2.00 | 2.80 | n.i. | n.i. | |||
| 3 | n-Decane | 1000 | 1015 | 0.87 ± 0.04 | 0.97 ± 0.05 | n.i. | n.i. |
| 6 | Undecane | 1100 | 1115 | 0.98 ± 0.05 | 1.52 ± 0.08 | n.i. | n.i. |
| 29 | Heneicosane | 1600 | 2100 | 1.02 ± 0.05 | 1.28 ± 0.06 | n.i. | n.i. |
| TOTAL OF INDENTIFIED COMPOUNDS | 95.42 | 98.00 | 97.98 | 95.76 | |||
| P-Et | E-Et | P-Aq | E-Aq | BHT | AA | PG | |
|---|---|---|---|---|---|---|---|
| DPPH (RSC50, in μg/mL) | 1.98 ± 0.08 a | 1.78 ± 0.1 b | 1.54 ± 0.04 a,b,c | 1.92 ± 0.06 c,d | / | / | 0.59 ± 0.04 a,b,c,d |
| OH (RSC50, in μg/mL) | n.d. | n.d. | 145.17 ± 5.92 e | 66.27 ± 3.33 e,f | 0.04 ± 0.01 e,f | 2.09 ± 0.11 e,f | 9.1 ± 0.52 e,f |
| NO (RSC50, in μg/mL) | 11.14 ± 0.98 | 10.07 ± 0.85 | 11.48 ± 0.44 | 11.24 ± 1.01 | / | / | 9.23 ± 0.39 |
| LP (IC50, in μg/mL) | 26.56 ± 1.11 g | 38.66 ± 2.83 g,h | 33.45 ± 2.84 i | 68.71 ± 5.55 g,h,i,j | 7.45 ± 0.55 g,h,i,j | / | / |
| FRAP (mg AAE/g DE) | 628.10 ± 3.76 k | 679.74 ± 4.67 k,l | 631.15 ± 2.33 l | 643.75 ± 2.94 k,l | / | / | / |
| P-Et | E-Et | P-Aq | E-Aq | Acarbose | Sitagliptin | |
|---|---|---|---|---|---|---|
| α-amylase (IC50, in μg/mL) | 3.46 ± 0.22 a | 5.44 ± 0.42 b | 17.59 ± 0.99 a,b,c | 23.79 ± 1.09 a,b,c,d | 4.23 ± 0.33 c,d | / |
| α-glucosidase (IC50, in μg/mL) | 0.098 ± 0.01 e | 0.066 ± 0.01 f | 0.33 ± 0.02 g | 0.12 ± 0.04 h | 44.67 ± 1.22 e,f,g,h | / |
| DPP-4 (IC50, in μg/mL) | 1124.58 ± 5.67 i | 313.30 ± 4.02 i,j | 316.98 ± 8.41 i,k | 651.56 ± 4.38 i,l | / | 0.0211 ± 0.0016 i,j,k,l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitl, M.; Bijelić, K.; Stilinović, N.; Božin, B.; Srđenović-Čonić, B.; Torović, L.; Kladar, N. Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae. Molecules 2022, 27, 8003. https://doi.org/10.3390/molecules27228003
Hitl M, Bijelić K, Stilinović N, Božin B, Srđenović-Čonić B, Torović L, Kladar N. Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae. Molecules. 2022; 27(22):8003. https://doi.org/10.3390/molecules27228003
Chicago/Turabian StyleHitl, Maja, Katarina Bijelić, Nebojša Stilinović, Biljana Božin, Branislava Srđenović-Čonić, Ljilja Torović, and Nebojša Kladar. 2022. "Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae" Molecules 27, no. 22: 8003. https://doi.org/10.3390/molecules27228003
APA StyleHitl, M., Bijelić, K., Stilinović, N., Božin, B., Srđenović-Čonić, B., Torović, L., & Kladar, N. (2022). Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae. Molecules, 27(22), 8003. https://doi.org/10.3390/molecules27228003










