Chemical Composition of Tobacco Seed Oils and Their Antioxidant, Anti-Inflammatory, and Whitening Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Chemical Constituents by GC-MS
2.2. Composition of Fatty Acid Profiles
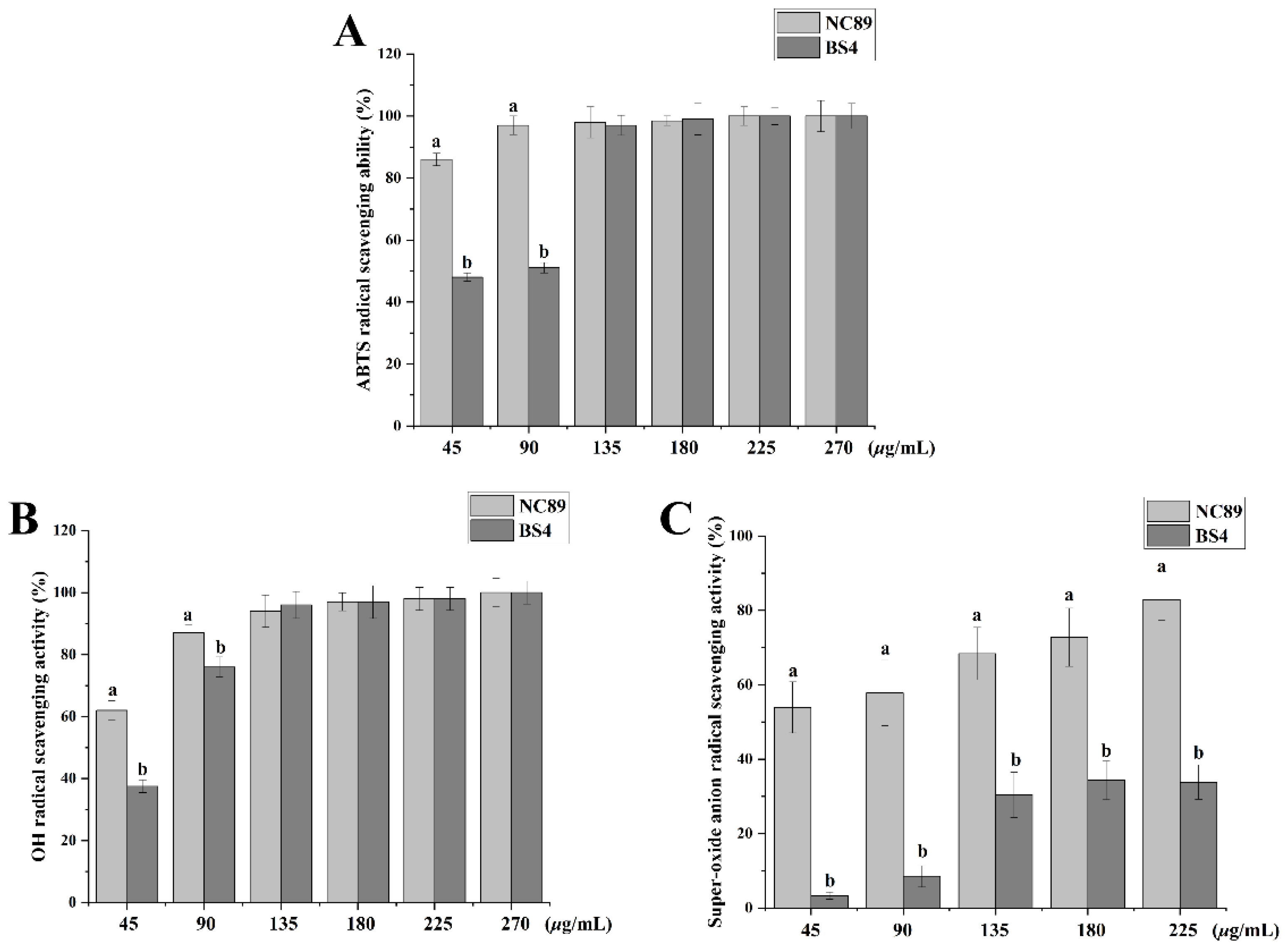
2.3. Ability of Tobacco Seed Oil on Scavenging Free Radicals
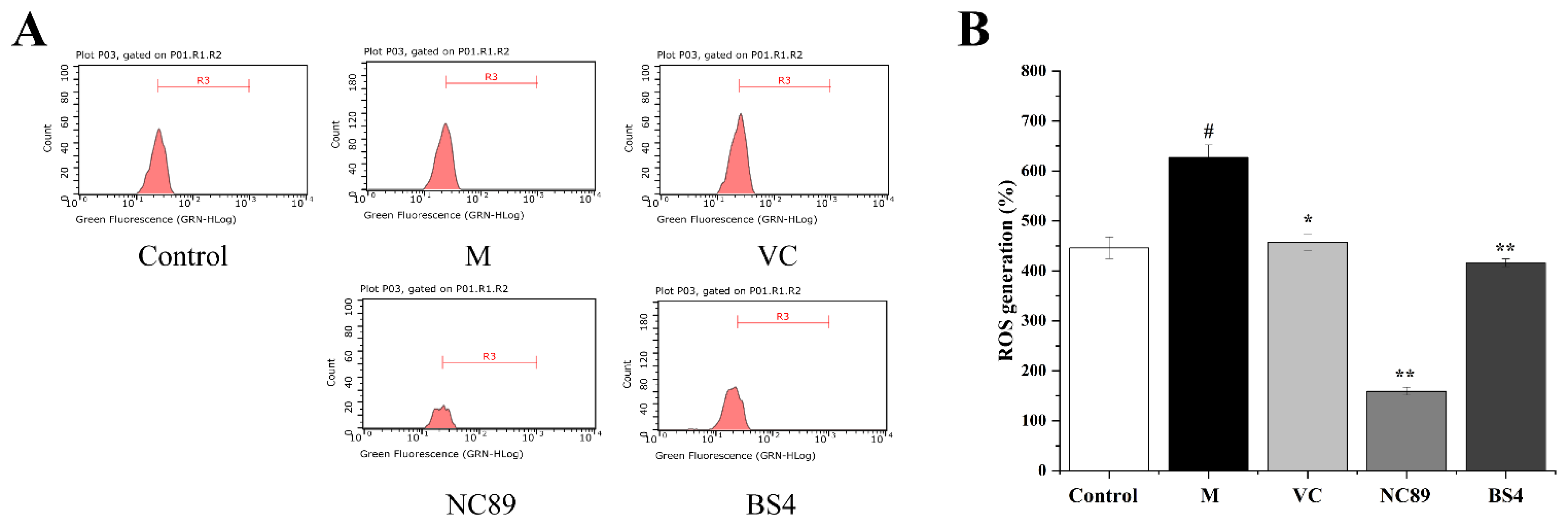
2.4. Inhibitory Effect of Tobacco Seed Oil on Intracellular ROS Generation
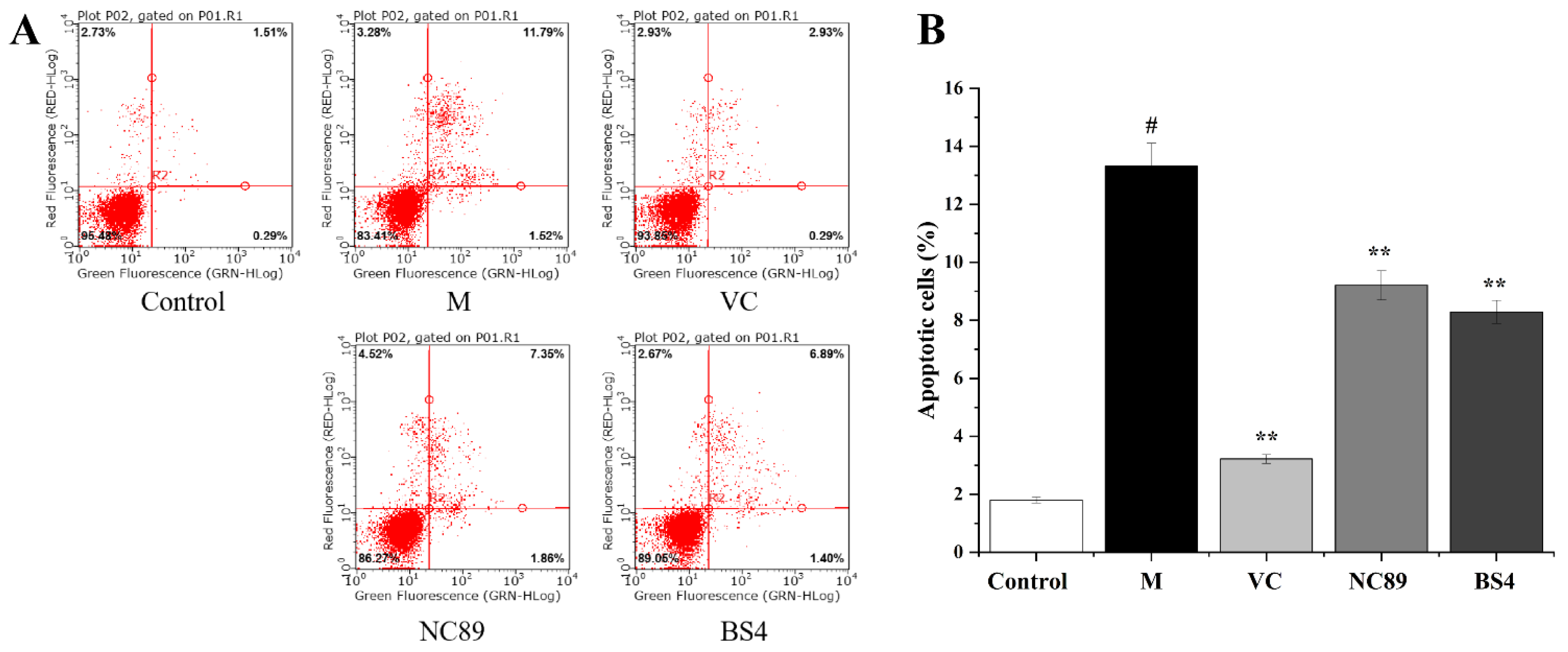
2.5. Cytoprotective Activity against H2O2-Induced Cell Apoptosis
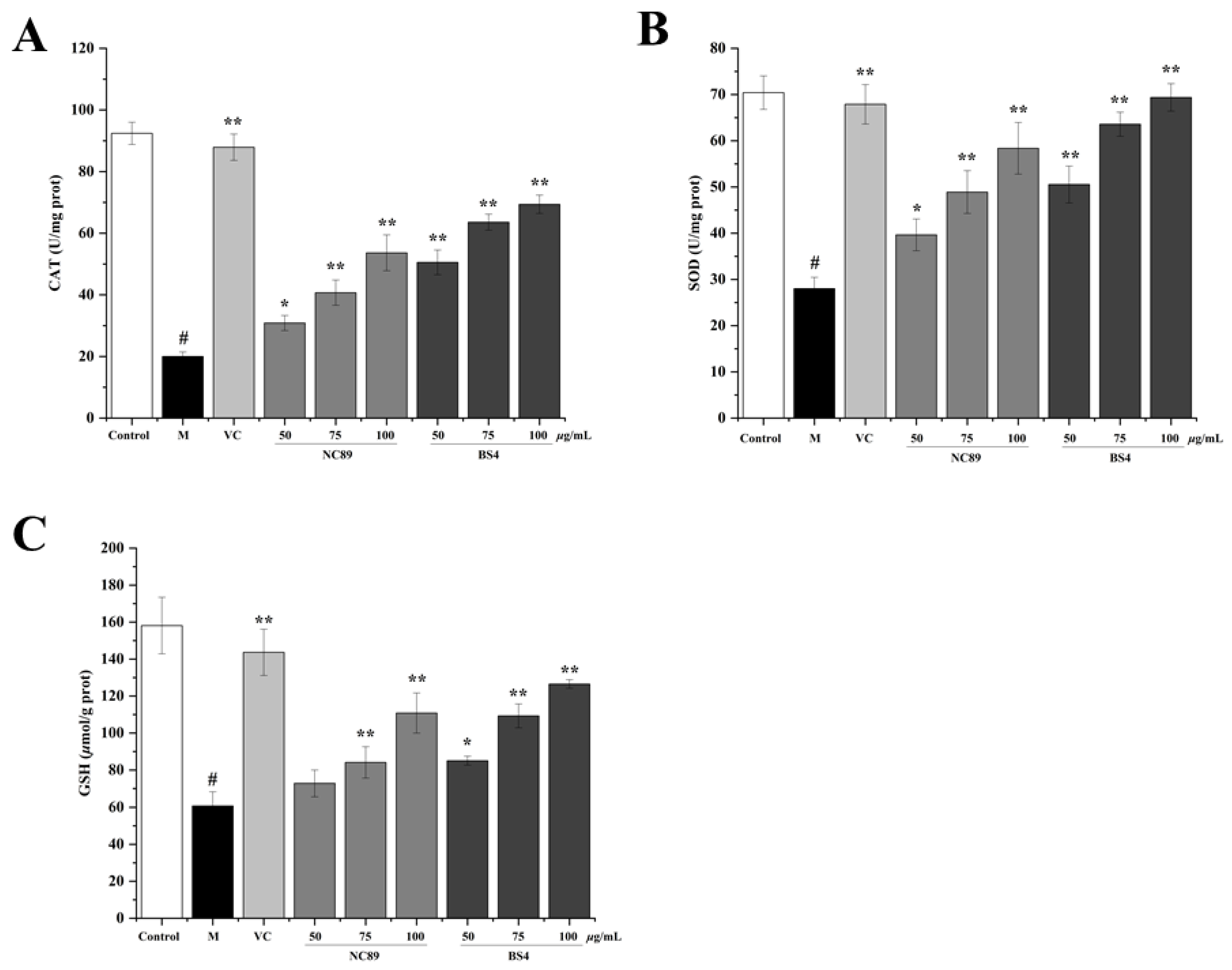
2.6. Antioxidant Ability of Tobacco Seed Oil on H2O2-Induced HepG2 Cells
2.7. Anti-Inflammatory Effect of Tobacco Seed Oil
2.8. The Inhibitory Effect of Tobacco Seed Oil on the MAPK Pathway
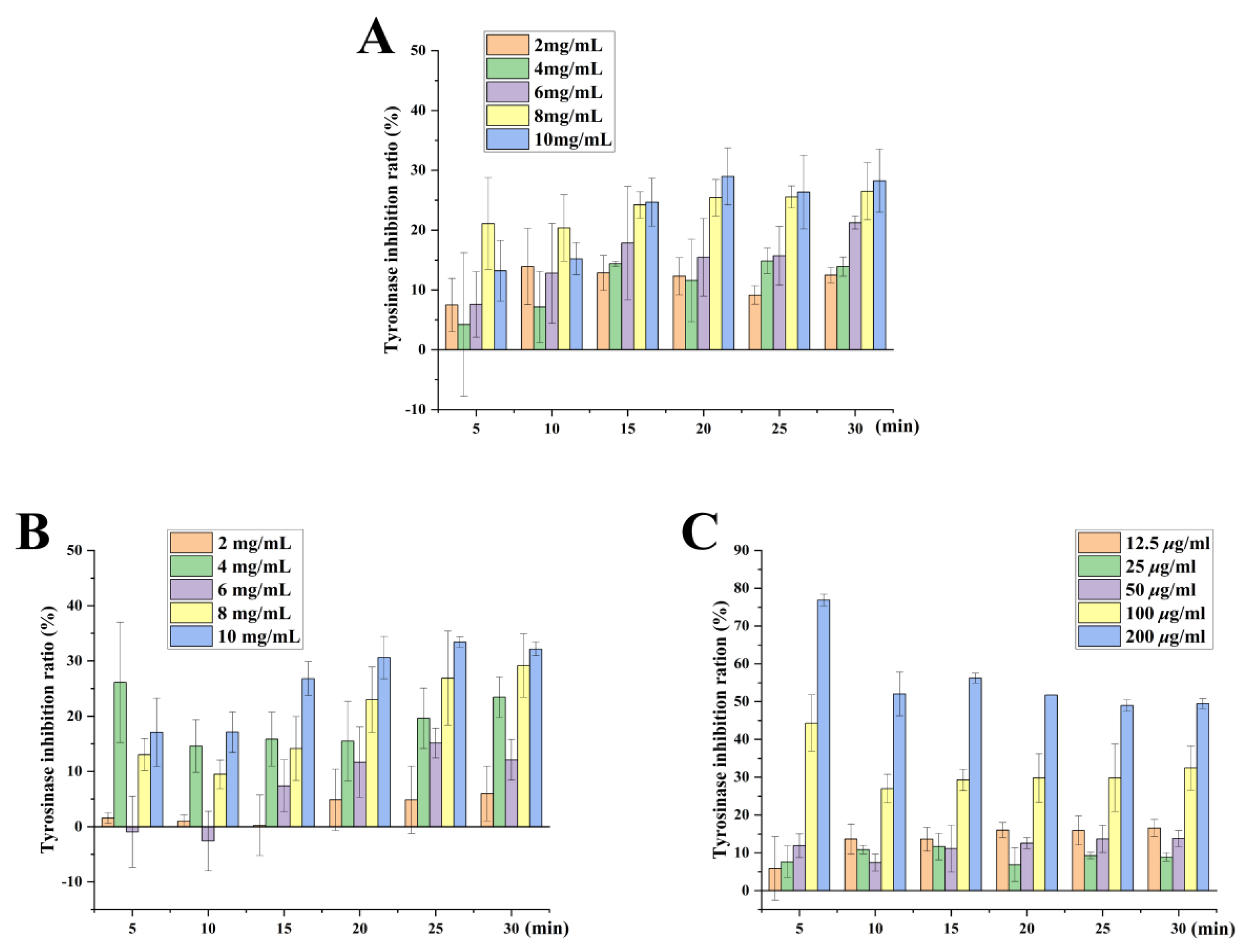
2.9. Whitening Effect of Tobacco Seed Oils on Inhibition Rate of Tyrosinase Activity
2.10. Effect of Tobacco Seed Oil on the Proliferation in B16 Cells
2.11. Inhibitory Effect of Tobacco Seed Oil on Tyrosinase in B16 Cells
2.12. Inhibitory Effect of Tobacco Seed Oil on Melanin Production in B16 Cells
3. Material and Methods
3.1. Chemical and Reagents
3.2. Preparation of Tobacco Seed Oil
3.3. Chemical Composition of Tobacco Seed Oil Using GC/MS Analysis
3.4. Determination of Fatty Acid Composition in Tobacco Seed Oil
3.5. Antioxidant Activity Assessment
3.5.1. Scavenging Effect on ABTS Radicals
3.5.2. Scavenging Effects on OH- Radicals
3.5.3. Scavenging Effects on O2− Radicals
3.6. Cytoprotective Effect on H2O2-Induced HepG2 Cells
3.6.1. HepG2 Cell Culture and Cell Viability Assay
3.6.2. Inhibitory Effects on the ROS Generation
3.6.3. Inhibitory Effects on Cell Apoptosis
3.6.4. Analysis of Intracellular SOD, CAT Activities and GSH Content
3.7. Anti-Inflammatory Activity of Tobacco Seed Oil
3.7.1. Determination of NO, IL-1β, IL-6, and TNF-α Levels in RAW264.7 Cells
3.7.2. Determination of MAPK Signaling Pathway Proteins
3.8. Whitening Effect of Tobacco Seed Oils
3.8.1. Inhibitory Effect on Tyrosinase Activity
3.8.2. The Cytotoxicity of Tobacco Seed Oils on B16 Melanoma Cells
3.8.3. Inhibitory Effect on Tyrosinase Level in B16 Melanoma Cells
3.8.4. Inhibitory Effects on Melanin Synthesis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Wu, X.; Liu, G.; Zhang, Z.; Chao, J.; Li, Z.; Guo, Y.; Sun, Y. Characterization and mapping of a novel premature leaf senescence mutant in common tobacco (Nicotiana tabacum L.). Plants 2019, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, C.; Valadier, M.H.; Brugière, N.; Morot-Gaudry, J.F.; Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 2000, 211, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Lakicevic, S.; Stamenkovic, O.; Todorovic, Z.; Lazic, M. Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 2006, 85, 2671–2675. [Google Scholar] [CrossRef]
- Rao, P.U. Nutrient composition of some less-familiar oil seeds. Food Chem. 1994, 50, 379–382. [Google Scholar] [CrossRef]
- Wang, L.; Du, X.; Feng, Y.; Liu, P.; Zhu, J.; Zhang, L.; Du, H.; Ma, M.; Li, F. Ectopic expression of EuWRI1, encoding a transcription factor in E. ulmoides, changes the seeds oil content in transgenic tobacco. Biotechnol. Progr. 2018, 34, 337–346. [Google Scholar] [CrossRef]
- Zheng, C.; Yoo, J.; Lee, T.; Cho, H.; Kim, Y.; Kim, W. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Im, D.S. FFA4 (GPR120) as a fatty acid sensor involved in appetite control, insulin sensitivity and inflammation regulation. Mol. Asp. Med. 2017, 64, 92–108. [Google Scholar] [CrossRef]
- Gasque, P.; Jaffar-Bandjee, M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, T.; Liu, Y.; Wang, Y.; Cheng, G.; Cao, J. Phenolic constituents, antioxidant activity and neuroprotective effects of ethanol extracts of fruits, leaves and flower buds from Vaccinium dunalianum Wight. Food Chem. 2021, 374, 131752. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Santiago, J.L. Anti-inflammatory andskin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef]
- Panda, C.; Varadharaj, S.; Voruganti, V.S. PUFA, genotypes and risk for cardiovascular disease. Prostaglandins Leukot. Essent. Fat. Acids 2021, 176, 102377. [Google Scholar] [CrossRef] [PubMed]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.d.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Batkowska, J.; Drabik, K.; Brodacki, A.; Czech, A.; Adamczuk, A. Fatty acids profile, cholesterol level and quality of table eggs from hens fed with the addition of linseed and soybean oil. Food Chem. 2021, 334, 127612. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Jayasooriya, A.P.; Madhujith, T. Optimization of the production of structured lipid by enzymatic interesterification from coconut (Cocos nucifera) oil and sesame (Sesamum indicum) oil using response surface methodology. LWT-Food Sci. Technol. 2018, 101, 723–730. [Google Scholar] [CrossRef]
- Biswas, S.; Das, R.; Ray Banerjee, E. Role of free radicals in human inflammatory diseases. AIMS. Biophys. 2017, 4, 596–614. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kesen, S.; Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. LC-DAD-ESI-MS/MS and GC-MS profiling of phenolic and aroma compounds of high oleic sunflower oil during deep-fat frying. J. Food Process. Preserv. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [PubMed]
- Zhang, J.; Wang, Y.; Xue, Q.; Zhao, T.; Khan, A.; Wang, Y.; Liu, Y.; Cao, J.; Cheng, G. The effect of ultra-high pretreatment on free, esterified and insoluble-bound phenolics from mango leaves and their antioxidant and cytoprotective activities. Food Chem. 2021, 368, 130864. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Sheng, Z.; Li, X.; Chang, Y.; Dai, W.; Chang, S.K.; Liu, J.; Yang, Y. Effect of pinolenic acid on oxidative stress injury in HepG2 cells induced by H2O2. Food Sci. Nutr. 2021, 9, 5689–5697. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, H.; Wu, Q.; Tang, H. The anti-oxidation and mechanism of essential oil of Paederia scandens in the NAFLD model of chicken. Animals 2019, 9, 850. [Google Scholar] [CrossRef]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S. Afr. J. Bot. 2017, 112, 70–78. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; Núñez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 2000, 32, 595–603. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: In vivo ROS detection and alterations in antioxidant enzymes. Biochem. Syst. Ecol. 2012, 44, 390–399. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar]
- Kurokawa, I.; Layton, A.M.; Ogawa, R. Updated treatment for acne: Targeted therapy based on pathogenesis. Dermatol. Ther. 2021, 11, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, J.; Guo, L.; Xu, Y.; Hu, L.; Mao, H.; Miao, L.; Zhang, H.; Chai, L. Neobavaisoflavone ameliorates LPS-induced RAW264.7 cell inflammations by suppressing the activation of NF-κB and MAPKs signaling pathways. Iran. J. Basic. Med. Sci. 2022, 25, 1021–1027. [Google Scholar] [PubMed]
- Truong, V.L.; Manochai, B.; Pham, T.-T.; Jeong, W.S. Antioxidant and Anti-Inflammatory Activities of Zingiber montanum Oil in HepG2 Cells and Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Med. Food 2021, 24, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Gao, C.; Jiang, Q.; Xu, J.; Xiong, L.; Liu, K.; Sun, D.; Li, H.; Chen, L. Diterpenoid alkaloids from Delphinium forrestii var. viride and their anti-inflammation activity. Phytochemistry 2021, 192, 112971. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Apaza Ticona, L.; Thiebaut Estrada, C.; Rumbero Sánchez, Á. Inhibition of melanin production and tyrosinase activity by flavonoids isolated from Loranthus acutifolius. Nat. Prod. Res. 2020, 35, 4690–4693. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kuge, S.; Nishiyama, S.; Tomita, Y.; Ito, S.; Wakamatsu, K.; Hearing, V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell. Res. 1995, 8, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Katsuyama, Y.; Yoshioka, M.; Okano, Y.; Masaki, H. 3-O-Glyceryl-2-O-hexyl ascorbate suppresses melanogenesis by interfering with intracellular melanosome transport and suppressing tyrosinase protein synthesis. J. Cosmet. Dermatol. 2017, 17, 1209–1215. [Google Scholar] [CrossRef]
- Seo, S.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Lee, I.J.; Yun, S.K.; Kim, H.U.; Park, B.H.; Park, J.W. Saponified evening primrose oil reduces melanogenesis in B16 melanoma cells and reduces UV-induced skin pigmentation in humans. Lipids 2010, 45, 401–407. [Google Scholar] [CrossRef]
- Wang, L.X.; Qian, J.; Zhao, L.N.; Zhao, S.H. Effects of volatile oil from ginger on the murine B16 melanoma cells and its mechanism. Food Funct. 2018, 9, 1058–1069. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G.; Liu, Y.; Yi, Y.; Chen, D.; Zhang, L.; Wang, X.; Cao, J. Correlation between microorganisms and flavor of Chinese fermented sour bamboo shoot: Roles of Lactococcus and Lactobacillus in flavor formation. Food. Biosci. 2022, 50, 101994. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Domínguez, G.; Campos, D. Comparison of the physico-chemical and phytochemical characteristics of the oil of two Plukenetia species. Food Chem. 2014, 173, 1203–1206. [Google Scholar] [CrossRef]
- Moukette, B.M.; Pieme, C.A.; Njimou, J.R.; Biapa, C.P.N.; Marco, B.; Ngogang, J.Y. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol. Res. 2015, 48, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Antioxidant, anti-aging and organ protective effects of total saponins from Aralia taibaiensis. Drug Des. Dev. Ther. 2021, 15, 4025–4042. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crop. Prod 2019, 143, 111433. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Fan, Z.; Xue, Q.; Njateng, G.S.S.; Liu, Y.; Cao, J.; Khan, A.; Cheng, G. Chemical constituents and anti-inflammatory activity of the total alkaloid extract from Melodinus cochinchinensis (Lour.) Merr. and its inhibition of the NF-κB and MAPK signaling pathways. Phytomedicine 2021, 91, 153684. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, Y.; Tian, L.; Yang, M.; He, S.; Liu, Y.; Khan, A.; Li, Y.; Cao, J.; Cheng, G. Anneslea fragrans Wall. ameliorates ulcerative colitis via inhibiting NF-κB and MAPK activation and mediating intestinal barrier integrity. J. Ethnopharmacol. 2021, 278, 114304. [Google Scholar] [CrossRef]
- Vanjare, B.D.; Choi, N.G.; Mahajan, P.G.; Raza, H.; Hassan, M.; Han, Y.; Yu, S.-M.; Kim, S.J.; Seo, S.-Y.; Lee, K.H. Novel 1,3,4-oxadiazole compounds inhibit the tyrosinase and melanin level: Synthesis, in-vitro, and in-silico studies. Bioorgan. Med. Chem. 2021, 41, 116222. [Google Scholar] [CrossRef]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive effect of a combination of artocarpus lakoocha and glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals 2020, 13, 310. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, T.; Hong, M.; Zhou, Y.; Huang, H.; Xiao, X.; Zheng, J.; Zhou, H.; Lu, Z. Quantitative proteomic analysis uncovers inhibition of melanin synthesis by silk fibroin via MITF/tyrosinase axis in B16 melanoma cells. Life Sci. 2021, 284, 119930. [Google Scholar] [CrossRef]



| Peak | tR (min) | CAS | Molecular Formula | Molecular Weight | Compounds |
|---|---|---|---|---|---|
| 1 | 2.355 | 32749-94-3 | C7H14O | 114.19 | 2,3-Dimethylpentanal |
| 2 | 2.655 | 107-84-6 | C5H11Cl | 106.59 | Chloroisopentane |
| 3 | 2.870 | 58735-67-4 | C8H16O | 128.212 | 2-Ethyl-hexanal |
| 4 | 5.040 | 123-51-3 | C5H12O | 88.15 | 3-Methyl-1-butanol |
| 5 | 5.165 | 15250-22-3 | C10H22O | 158.2811 | 2,7-Dimethyl-1-octanol |
| 6 | 5.985 | 108-88-3 | C7H8 | 92.14 | Toluene |
| 7 | 6.155 | 71-41-0 | C5H12O | 88.15 | 1-Pentanol |
| 8 | 7.420 | 66-25-1 | C6H12O | 100.16 | Hexanal |
| 9 | 12.62 | 111-27-3 | C6H14O | 102.17 | Hexyl alcohol |
| Peak | tR (min) | CAS | Molecular Formula | Molecular Weight | Compounds |
|---|---|---|---|---|---|
| 1 | 2.340 | 16630-91-4 | C8H16O | 128.212 | 2-methyl heptanal |
| 2 | 2.845 | 3010-96-6 | C8H16O2 | 144.2114 | 2,2,4,4-Tetramethyl 1,3-cyclobutanediol |
| 3 | 3.910 | 543-75-9 | C4H6O2 | 86.0892 | 2,3-dihydro-1,4-Dioxin |
| 4 | 14.350 | 110-43-0 | C7H14O | 114.19 | 2-Heptanone |
| Fatty Acids | NC89 | BS4 |
|---|---|---|
| butyric acid | 0.32 | 0.14 |
| myristic acid | 0.03 | 0.03 |
| palmitic acid | 8.18 | 8.83 |
| palmitoleic acid | 0.10 | 0.12 |
| heptadecanoic acid | 0.13 | 0.12 |
| 10-heptadecenoic acid | 0.06 | 0.06 |
| stearic acid | 3.21 | 3.30 |
| oleic acid | 12.45 | 14.04 |
| linolelaidic | 0.42 | 0.39 |
| linoleic acid | 73.53 | 71.55 |
| α-linoleic acid | 0.93 | 0.82 |
| arachidic acid | 0.21 | 0.21 |
| eicosenoic acid | 0.13 | 0.13 |
| 11,14-eicosadienoic acid | 0.10 | 0.08 |
| heneicosanoic acid | 0.02 | 0.01 |
| behenic acid | 0.08 | 0.10 |
| carnaubic acid | 0.08 | 0.05 |
| tetracosenic acid | 0.05 | 0.03 |
| SFA | 12.24 | 12.79 |
| MUFA | 12.78 | 14.37 |
| PUFA | 74.98 | 72.84 |
| Sample | Concentration | Viability (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| NC89 | 4.2 mg/mL | 110.18 ± 1.72 * | 104.14 ± 4.06 | 114.95 ± 4.96 |
| BS4 | 4.2 mg/mL | 105.43 ± 6.96 * | 112.24 ± 3.5 | 108.8 ± 4.78 |
| Arbutin | 31 μg/mL | 124.20 ± 3.84 | 96.9 ± 2.41 | 90.38 ± 3.93 |
| NC89 | 5 mg/mL | 105.43 ± 2.66 * | 102.91 ± 1.83 | 106.25 ± 2.77 |
| BS4 | 5 mg/mL | 107.93 ± 3.86 | 106.02 ± 2.02 | 108.38 ± 3.44 |
| Arbutin | 62.5 μg/mL | 113.15 ± 6.23 | 93.54 ± 3.27 | 88.98 ± 7.35 |
| NC89 | 6 mg/mL | 108.58 ± 5.06 * | 102.2 ± 2.48 | 110.08 ± 2.88 |
| BS4 | 6 mg/mL | 117.07 ± 6.7 | 113.22 ± 1.83 | 95.15 ± 3.98 |
| Arbutin | 125 μg/mL | 124.77 ± 3.71 | 92.8 ± 1.22 | 92.31 ± 1.26 |
| NC89 | 8.3 mg/mL | 119.00 ± 2.32 | 103.34 ± 1.81 | 94.12 ± 1.95 |
| BS4 | 8.3 mg/mL | 125.07 ± 3.57 | 106.59 ± 2.51 | 84.43 ± 2.83 * |
| Arbutin | 250 μg/mL | 112.22 ± 6.27 | 92.41 ± 0.51 | 93.11 ± 2.32 |
| NC89 | 11.1 mg/mL | 126.32 ± 3.65 | 90.15 ± 1.52 | 89.73 ± 2.63 |
| BS4 | 11.1 mg/mL | 127.25 ± 1.71 | 91.78 ± 2.25 | 81.1 ± 3.08 * |
| Arbutin | 500 μg/mL | 107.36 ± 3.23 | 90.38 ± 1.37 | 93.12 ± 2.16 |
| Sample | Concentration | Inhibition Ratio (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| NC89 | 4.2 mg/mL | −5.58 ± 4.46 | −1.79 ± 2.05 | 0.47 ± 1.26 |
| BS4 | 4.2 mg/mL | −5.58 ± 2.41 | −4.77 ± 2.67 | −1.04 ± 2.58 |
| Arbutin | 31 μg/mL | −5.63 ± 2.39 | 3.19 ± 1.12 | 9.41 ± 3.19 |
| NC89 | 5 mg/mL | −3.7 ± 4.28 | −0.05 ± 1.91 | 2.32 ± 1.8 |
| BS4 | 5 mg/mL | −1.82 ± 4.19 | 0.42 ± 2.92 | 1.48 ± 1.54 |
| Arbutin | 62.5 μg/mL | 0.58 ± 1.86 | 6.75 ± 2.02 | 15.27 ± 3.22 |
| NC89 | 6 mg/mL | −1.5 ± 1.98 | 1.19 ± 2.28 | 4.17 ± 1.81 |
| BS4 | 6 mg/mL | 1.94 ± 2.41 | 3.25 ± 3.35 | 4.34 ± 1.46 |
| Arbutin | 125 μg/mL | 1.82 ± 1.66 | 12.53 ± 2.81 | 22.88 ± 2.49 |
| NC89 | 8.3 mg/mL | −1.19 ± 3.69 | 6.25 ± 3.14 | 8.88 ± 3.21 |
| BS4 | 8.3 mg/mL | 5.39 ± 2.26 | 6.65 ± 3.06 | 7.87 ± 1.84 |
| Arbutin | 250 μg/mL | 9.27 ± 2.81 | 24.09 ± 3.5 | 28.26 ± 2.8 |
| NC89 | 11.1 mg/mL | 4.76 ± 3.65 | 10.13 ± 2.28 | 12.91 ± 2.63 |
| BS4 | 11.1 mg/mL | 7.89 ± 1.88 | 10.63 ± 1.57 | 12.58 ± 1.71 |
| Arbutin | 500 μg/mL | 15.17 ± 1.25 | 33.87 ± 1.06 | 38.1 ± 2.33 |
| Sample | Concentration | Inhibition Ratio (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| NC89 | 4.2 mg/mL | −8.3 ± 2.13 | 1.91 ± 1.45 | 2.14 ± 1.82 |
| BS4 | 4.2 mg/mL | −3.15 ± 0.84 | 1.5 ± 2.83 | 1.43 ± 2.76 |
| Arbutin | 31 μg/mL | 5.85 ± 1.08 | 5.64 ± 1.94 | 3.23 ± 1.67 |
| NC89 | 5 mg/mL | −5.55 ± 3.4 | 4.12 ± 1.22 | 3.69 ± 2.28 |
| BS4 | 5 mg/mL | −1.95 ± 1.7 | 3.76 ± 2.85 | 3.93 ± 0.82 |
| Arbutin | 62.5 μg/mL | 6.95 ± 1.59 | 14.36 ± 2.6 | 10.05 ± 2.91 |
| NC89 | 6 mg/mL | −3.67 ± 2.04 | 4.93 ± 1.88 | 6.33 ± 3.6 |
| BS4 | 6 mg/mL | 1.88 ± 1.31 | 5.52 ± 3.32 | 4.78 ± 3.06 |
| Arbutin | 125 μg/mL | 14.59 ± 1.44 | 26.81 ± 1.61 | 24.99 ± 1.24 |
| NC89 | 8.3 mg/mL | −0.72 ± 1.14 | 5.8 ± 1.08 | 7.05 ± 3.41 |
| BS4 | 8.3 mg/mL | 3.25 ± 1.08 | 5.21 ± 1.08 | 5.88 ± 1.38 |
| Arbutin | 250 μg/mL | 23.25 ± 0.43 | 35.09 ± 1.23 | 37.43 ± 1.85 |
| NC89 | 11.1 mg/mL | 6.31 ± 1.86 | 6.42 ± 2.33 | 9.78 ± 1.12 |
| BS4 | 11.1 mg/mL | 5.63 ± 1.06 | 7.81 ± 2.99 | 9.14 ± 2.19 |
| Arbutin | 500 μg/mL | 35.49 ± 1.25 | 39.71 ± 1.66 | 42.82 ± 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Zhang, X.; Song, B.; Zhou, D.; Niu, Y.; Cheng, G.; Zheng, Y.; Wang, Y. Chemical Composition of Tobacco Seed Oils and Their Antioxidant, Anti-Inflammatory, and Whitening Activities. Molecules 2022, 27, 8516. https://doi.org/10.3390/molecules27238516
Gu J, Zhang X, Song B, Zhou D, Niu Y, Cheng G, Zheng Y, Wang Y. Chemical Composition of Tobacco Seed Oils and Their Antioxidant, Anti-Inflammatory, and Whitening Activities. Molecules. 2022; 27(23):8516. https://doi.org/10.3390/molecules27238516
Chicago/Turabian StyleGu, Ji, Xiaoyu Zhang, Biqing Song, Dongjie Zhou, Yongzhi Niu, Guiguang Cheng, Yunye Zheng, and Yudan Wang. 2022. "Chemical Composition of Tobacco Seed Oils and Their Antioxidant, Anti-Inflammatory, and Whitening Activities" Molecules 27, no. 23: 8516. https://doi.org/10.3390/molecules27238516
APA StyleGu, J., Zhang, X., Song, B., Zhou, D., Niu, Y., Cheng, G., Zheng, Y., & Wang, Y. (2022). Chemical Composition of Tobacco Seed Oils and Their Antioxidant, Anti-Inflammatory, and Whitening Activities. Molecules, 27(23), 8516. https://doi.org/10.3390/molecules27238516







